Production and Quality Control of [68Ga]Ga-FAPI-46: Development of an Investigational Medicinal Product Dossier for a Bicentric Clinical Trial
Abstract
1. Introduction
2. Results
2.1. Drug Substances
2.1.1. FAPI-46
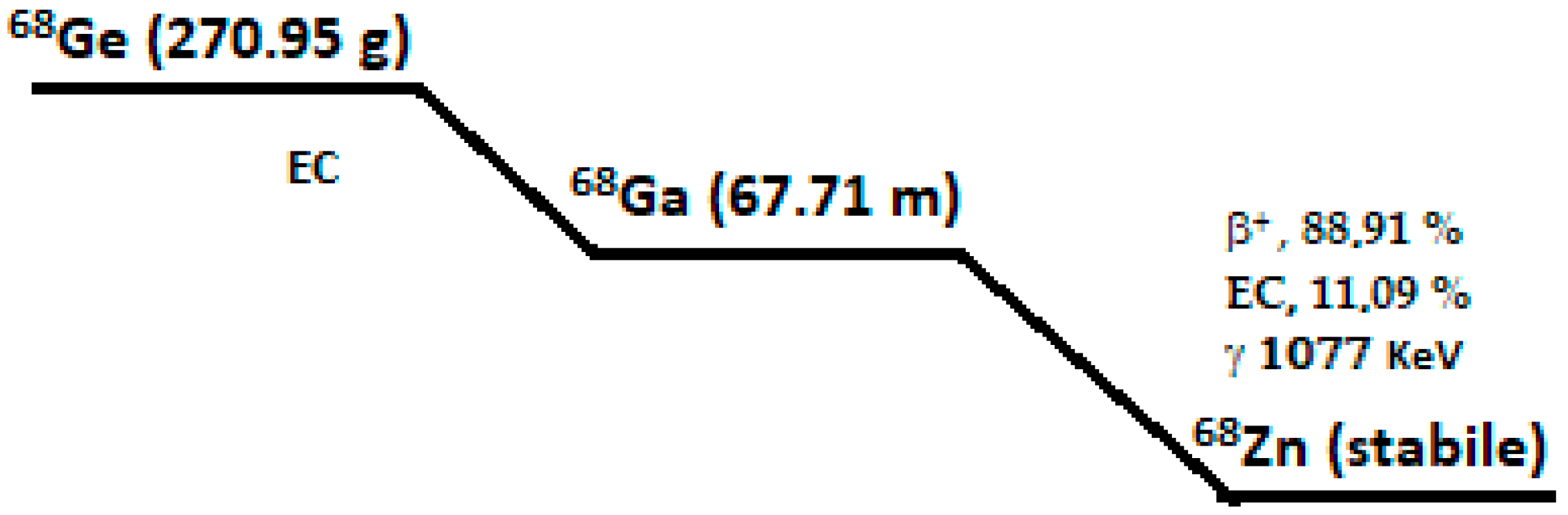
2.1.2. Gallium-68
2.2. Investigational Medicinal Product Under Test (IMP)
2.2.1. Description and Composition of the IMP
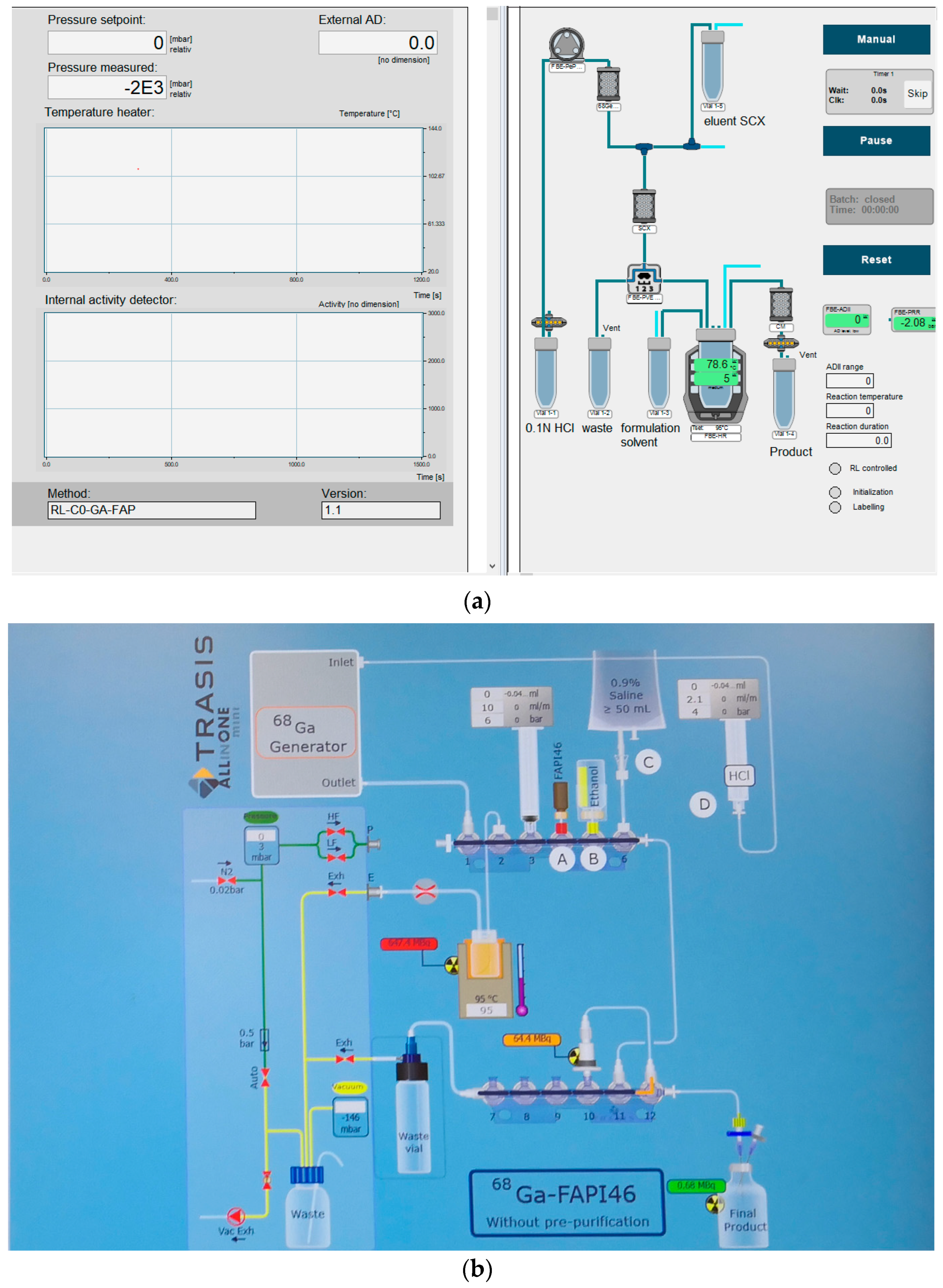
2.2.2. Description of the Manufacturing Process and Process Controls
2.3. Quality Controls
2.3.1. Acceptance Criteria
2.3.2. Validation of the Analytical Procedures
2.3.3. Bioburden
- Total aerobic microbial count (TMAC) < 1 cfu/mL.
- Total yeast and mold count (TYMC) < 1 cfu/mL.
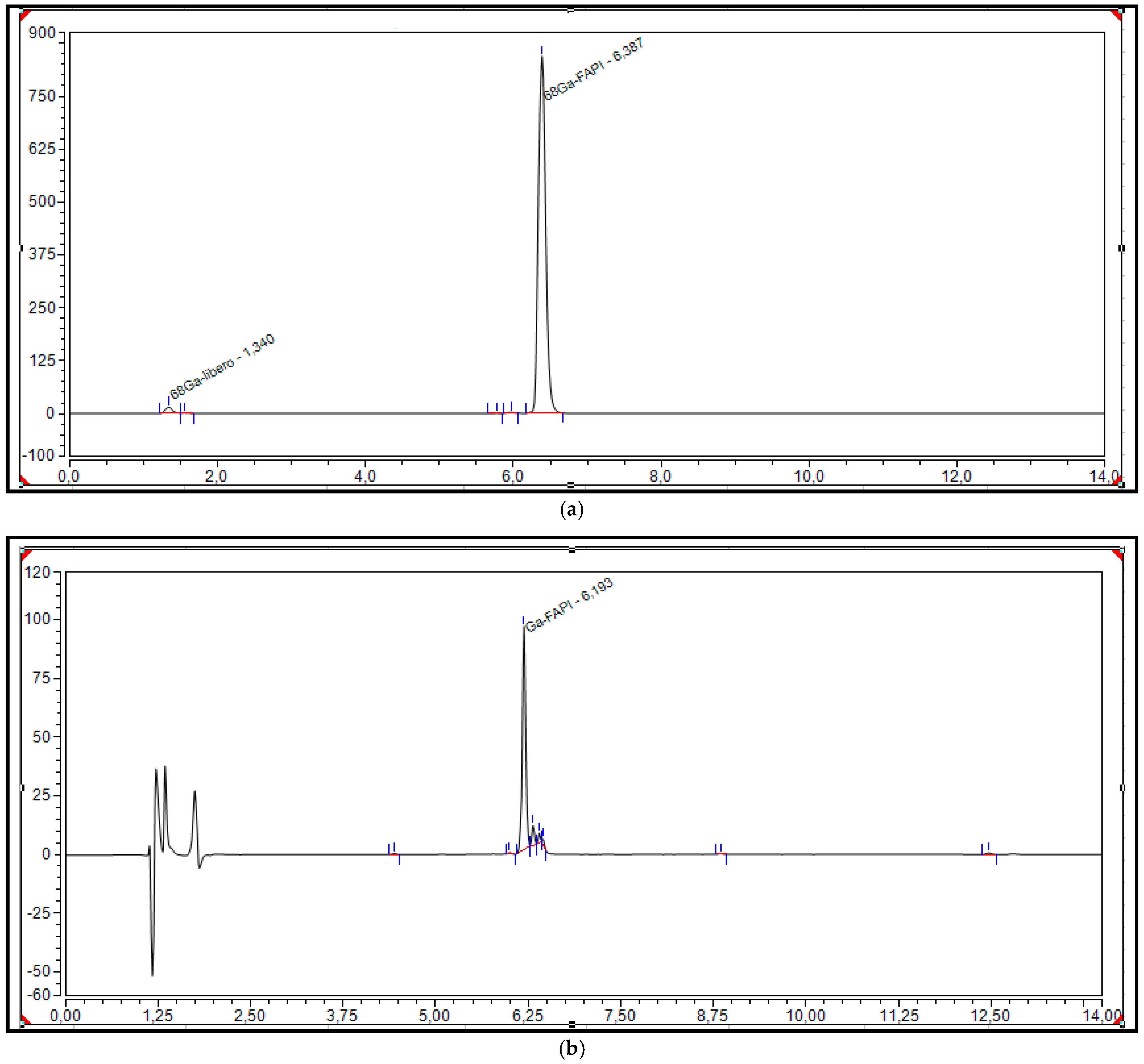
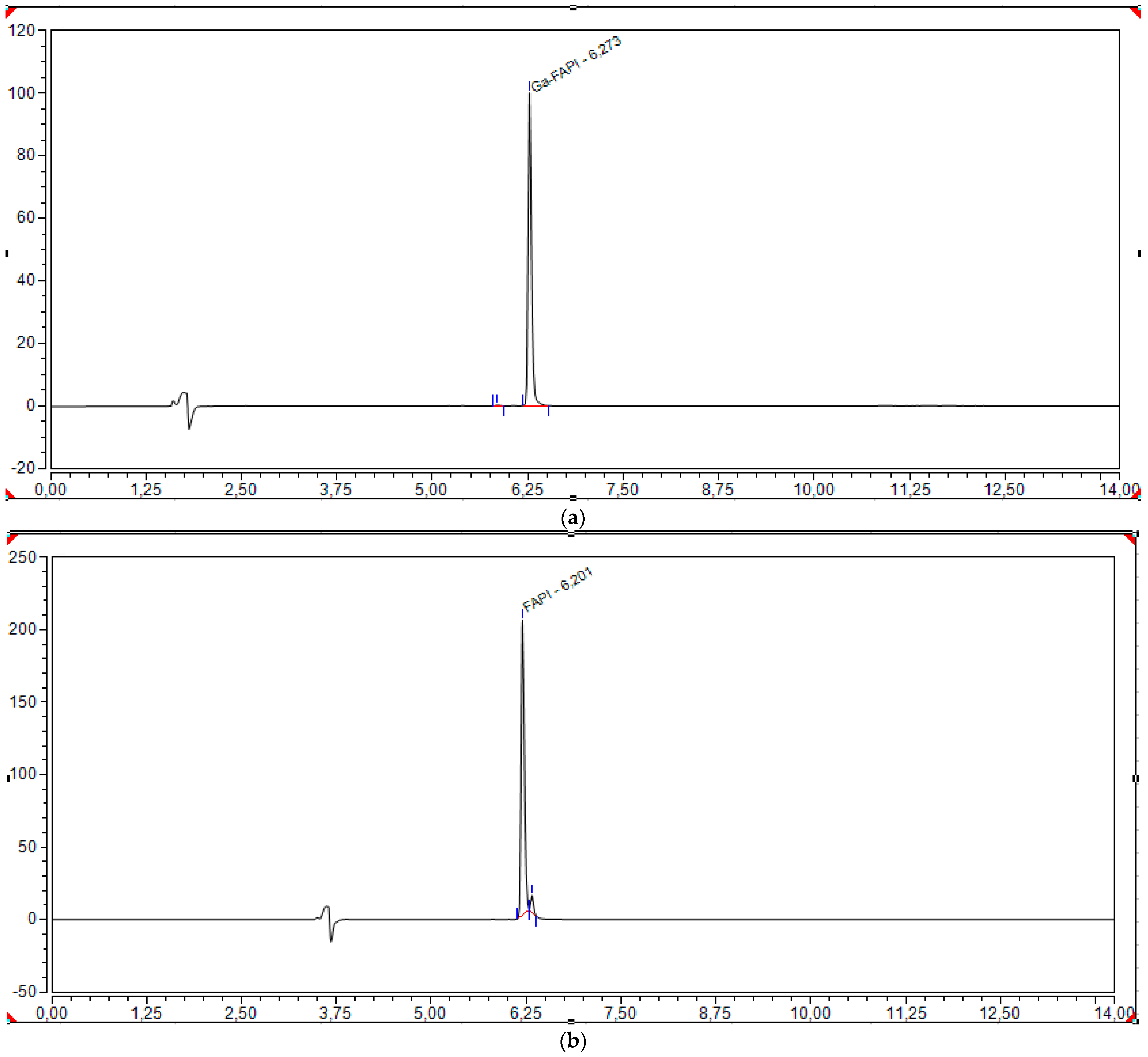
2.3.4. Batch Analysis and Process Validation
2.3.5. Stability
3. Discussion
4. Materials and Methods
4.1. Description of [68Ga]Ga-FAPI-46 Manufacturing Process
4.1.1. Set up of Radiosynthesizer
4.1.2. Reagents
- Reagent set product by ABX (Heinrich-Glaeser- Straße 10-14, 01454 Radeberg, Germany), composed of the following:
- ○
- Vial 1 (EZ-102-V1) containing 5 mL of NaCl 5 M/HCl 30%;
- ○
- Vial 2 (EZ-102-V2) containing 680 mg of sodium acetate trihydrate;
- ○
- Vial 5 (EZ-102-V5) containing 3 mg of ascorbic acid.
- Water for injectable preparations (100 mL bottles) with MA was purchased from Monico S.p.A., Venezia/Mestre, Italy.
- Sodium chloride 0,9% 100 mL with an MA was purchased from Fresenius Kabi S.r.l., Isola della Scala, Italy.
- Single-use sterile cassettes were produced by Eckert & Ziegler Eurotope GmbH (Robert Rossle- Straße 10, Berlin, Germany) and provided by Radius (RADIUS s.r.l. Via Luigi Menarini, 31, 40054 Budrio, BO, Italy).
- Sterilizing filter 0.22 mm (product code: SY25PL-S-MDI Advanced Microdevices PVT LTD 21 Ind., Area Ambala Canti 133006, India).
- Reagent set produced by Trasis (Rue Gilles Magnée 90, 4430 Ans, Belgium), composed of the following:
- ○
- Part 1: Syringe containing E&Z Eluent; syringe containing acetate buffer; ethanol vial; and sodium Chloride 0.9% (BBraun-Melsungen AG, 34209 Melsungen, Germany).
- ○
- Part 2: Sodium ascorbate.
- Single-use sterile cassette product by Medline Liége Science Park-Rue des Gardes-Frontiére 5, 4031 Angleur Belgium, distributed by Trasis (Rue Gilles Magnée 90, 4430 Ans, Belgium). The cassette includes a solid phase extraction (SPE) cartridge (Oasis HLB Plus Short Cartridge, 225 mg sorbent per cartridge, 60 mm, 50/pk).
- Sterilizing filter 0.22 mm (product code: 6,764,192 PALL Medical Avenue de Tivoli 3, CH-1700 Fribourg, Switzerland).
4.1.3. Process Description
4.2. Quality Control
4.2.1. Standard Procedures
- The pH value of the formulation was determined using pH strips (Merck pH indicator strip, Acilit, increment 0,5 pH unit).
- The endotoxin test was performed using the Limulus Amebocyte Lysate test (LAL test), on an Endosafe Nexgen-PTSTM (Charles River Laboratories, 26866 Sant’Angelo Lodigiano (LO), Italy).
- As required by national quality assurance regulations (NBP MN), since this preparation cannot proceed to terminal sterilization, it must be sterilized by filtration using a sterile disposable membrane with 0.22 µm pores. Filter integrity must be checked by the bubble point test before release:
- ○
- For Laboratory 1, the bubble point test was performed on an Integritest 4 system (Merck Millipore KGaA, Darmstadt, Germany).
- ○
- For Laboratory 2, the bubble point test was performed automatically by the synthesis module (Mini All-in-One Trasis—Hot Cell H700).
- The sterility test was performed by an external laboratory for both Laboratory 1 and Laboratory 2.
- Radionuclidic purity was assessed in both laboratories by measuring the half-life and identifying characteristic emission peaks for gallium-68, in accordance with the current Ph. Eur. Monograph 2.2.66 “Detection and Measurement of Radioactivity.”
4.2.2. HPLC Analysis
4.2.3. Thin Layer Chromatography (TLC)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edosada, C.Y.; Quan, C.; Wiesmann, C.; Tran, T.; Sutherlin, D.; Reynolds, M.; Elliott, J.M.; Raab, H.; Fairbrother, W.; Wolf, B.B. Selective inhibition of fibroblast activation protein protease based on dipeptide substrate specificity. J. Biol. Chem. 2006, 281, 7437–7444. [Google Scholar] [CrossRef]
- Park, J.E.; Lenter, M.C.; Zimmermann, R.N.; Garin-Chesa, P.; Old, L.J.; Rettig, W.J. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J. Biol. Chem. 1999, 274, 36505–36512. [Google Scholar] [CrossRef]
- Hamson, E.J.; Keane, F.M.; Tholen, S.; Schilling, O.; Gorrell, M.D. Understanding fibroblast activation protein (FAP): Substrates, activities, expression and targeting for cancer therapy. Proteom. Clin. Appl. 2014, 8, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Zi, F.; He, J.; He, D.; Li, Y.; Yang, L.; Cai, Z. Fibroblast activation protein α in tumor microenvironment: Recent progression and implications. Mol. Med. Rep. 2015, 11, 3203–3211. [Google Scholar] [CrossRef]
- Lindner, T.; Loktev, A.; Giesel, F.; Kratochwil, C.; Altmann, A.; Haberkorn, U. Targeting of activated fibroblasts for imaging and therapy. EJNMMI Radiopharm. Chem. 2019, 4, 16. [Google Scholar] [CrossRef]
- Altmann, A.; Haberkorn, U.; Siveke, J. The Latest Developments in Imaging of Fibroblast Activation Protein. J. Nucl. Med. 2021, 62, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Gascard, P.; Tlsty, T.D. Carcinoma-associated fibroblasts: Orchestrating the composition of malignancy. Genes Dev. 2016, 30, 1002–1019. [Google Scholar] [CrossRef] [PubMed]
- Brennen, W.N.; Isaacs, J.T.; Denmeade, S.R. Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol. Cancer Ther. 2012, 11, 257–266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wikberg, M.L.; Edin, S.; Lundberg, I.V.; Van Guelpen, B.; Dahlin, A.M.; Rutegård, J.; Stenling, R.; Oberg, A.; Palmqvist, R. High intratumoral expression of fibroblast activation protein (FAP) in colon cancer is associated with poorer patient prognosis. Tumour Biol. 2013, 34, 1013–1020. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bauer, S.; Jendro, M.C.; Wadle, A.; Kleber, S.; Stenner, F.; Dinser, R.; Reich, A.; Faccin, E.; Gödde, S.; Dinges, H.; et al. Fibroblast activation protein is expressed by rheumatoid myofibroblast-like synoviocytes. Arthritis Res. Ther. 2006, 8, R171. [Google Scholar] [CrossRef] [PubMed]
- Windisch, P.; Zwahlen, D.R.; Koerber, S.A.; Giesel, F.L.; Debus, J.; Haberkorn, U.; Adeberg, S. Clinical Results of Fibroblast Activation Protein (FAP) Specific PET and Implications for Radiotherapy Planning: Systematic Review. Cancers 2020, 12, 2629. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, G.M.; Xu, W.; Du, J.; Zhang, K.S.; Zhang, Q.G.; Wang, X.W.; Liu, Z.G.; Liu, S.Q.; Xie, W.Y.; Liu, H.F.; et al. The application of the fibroblast activation protein α-targeted immunotherapy strategy. Oncotarget 2016, 7, 33472–33482. [Google Scholar] [CrossRef]
- Puré, E.; Lo, A. Can Targeting Stroma Pave the Way to Enhanced Antitumor Immunity and Immunotherapy of Solid Tumors? Cancer Immunol. Res. 2016, 4, 269–278. [Google Scholar] [CrossRef]
- Meletta, R.; Müller Herde, A.; Chiotellis, A.; Isa, M.; Rancic, Z.; Borel, N.; Ametamey, S.M.; Krämer, S.D.; Schibli, R. Evaluation of the radiolabeled boronic acid-based FAP inhibitor MIP-1232 for atherosclerotic plaque imaging. Molecules 2015, 20, 2081–2099. [Google Scholar] [CrossRef]
- Imlimthan, S.; Moon, E.S.; Rathke, H.; Afshar-Oromieh, A.; Rösch, F.; Rominger, A.; Gourni, E. New Frontiers in Cancer Imaging and Therapy Based on Radiolabeled Fibroblast Activation Protein Inhibitors: A Rational Review and Current Progress. Pharmaceuticals 2021, 14, 1023. [Google Scholar] [CrossRef]
- Jansen, K.; Heirbaut, L.; Cheng, J.D.; Joossens, J.; Ryabtsova, O.; Cos, P.; Maes, L.; Lambeir, A.M.; De Meester, I.; Augustyns, K.; et al. Selective Inhibitors of Fibroblast Activation Protein (FAP) with a (4-Quinolinoyl)-glycyl-2-cyanopyrrolidine Scaffold. ACS Med. Chem. Lett. 2013, 4, 491–496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Syed, M.; Flechsig, P.; Liermann, J.; Windisch, P.; Staudinger, F.; Akbaba, S.; Koerber, S.A.; Freudlsperger, C.; Plinkert, P.K.; Debus, J.; et al. Fibroblast activation protein inhibitor (FAPI) PET for diagnostics and advanced targeted radiotherapy in head and neck cancers. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2836–2845. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Terry, S.Y.; Koenders, M.I.; Franssen, G.M.; Nayak, T.K.; Freimoser-Grundschober, A.; Klein, C.; Oyen, W.J.; Boerman, O.C.; Laverman, P. Monitoring Therapy Response of Experimental Arthritis with Radiolabeled Tracers Targeting Fibroblasts, Macrophages, or Integrin αvβ3. J. Nucl. Med. 2016, 57, 467–472. [Google Scholar] [CrossRef] [PubMed]
- van der Geest, T.; Roeleveld, D.M.; Walgreen, B.; Helsen, M.M.; Nayak, T.K.; Klein, C.; Hegen, M.; Storm, G.; Metselaar, J.M.; van den Berg, W.B.; et al. Imaging fibroblast activation protein to monitor therapeutic effects of neutralizing interleukin-22 in collagen-induced arthritis. Rheumatology 2018, 57, 737–747. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Burger, E.M.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Marmé, F.; Jäger, D.; Mier, W.; et al. Development of Fibroblast Activation Protein-Targeted Radiotracers with Improved Tumor Retention. J. Nucl. Med. 2019, 60, 1421–1429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meyer, C.; Dahlbom, M.; Lindner, T.; Vauclin, S.; Mona, C.; Slavik, R.; Czernin, J.; Haberkorn, U.; Calais, J. Radiation Dosimetry and Biodistribution of 68Ga-FAPI-46 PET Imaging in Cancer Patients. J. Nucl. Med. 2020, 61, 1171–1177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, P.; Singh, S.S.; Gayana, S. Fibroblast Activation Protein Inhibitor PET/CT: A Promising Molecular Imaging Tool. Clin. Nucl. Med. 2021, 46, e141–e150. [Google Scholar] [CrossRef] [PubMed]
- Rubira, L.; Torchio, J.; Fouillet, J.; Vanney, J.; Fersing, C. GMP-Compliant Automated Radiolabeling and Quality Controls of [68Ga]Ga-FAPI-46 for Fibroblast Activation Protein-Targeted PET Imaging in Clinical Settings. Chem. Pharm. Bull. 2024, 72, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.J.; Cheung, Y.Y.; Liu, F.; Sollert, C.; Peterson, T.E.; Kropski, J.A. Fully automated radiosynthesis of [68Ga]Ga-FAPI-46 with cyclotron produced gallium. EJNMMI Radiopharm. Chem. 2023, 8, 29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meisenheimer, M.; Saenko, Y.; Eppard, E. Gallium-68: Radiolabeling of Radiopharmaceuticals for PET Imaging—A Lot to Consider. In Medical Isotopes; Naqvi, S.A.R., Imrani, M.B., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Boschi, S.; Malizia, C.; Lodi, F. Overview and perspectives on automation strategies in 68Ga radiopharmaceutical preparations. Recent Results Cancer Res. 2013, 194, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Bauwens, M.; Chekol, R.; Vanbilloen, H.; Bormans, G.; Verbruggen, A. Optimal buffer choice of the radiosynthesis of 68Ga–Dotatoc for clinical application. Nucl. Med. Commun. 2010, 31, 753–758. [Google Scholar] [CrossRef]
- Rubira, L.; Donzé, C.; Fouillet, J.; Algudo, B.; Kotzki, P.O.; Deshayes, E.; Fersing, C. [68Ga]Ga-FAPI-46 synthesis on a GAIA® module system: Thorough study of the automated radiolabeling reaction conditions. Appl. Radiat. Isot. 2024, 206, 111211. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I. 68Ga-Based radiopharmaceuticals: Production and application relationship. Molecules 2015, 20, 12913–12943. [Google Scholar] [CrossRef]
- Available online: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2010-11-23&atto.codiceRedazionale=10A13706&elenco30giorni=false (accessed on 19 July 2025).
- European Medicines Agency (EMA). Requirements to the Chemical and Pharmaceutical Quality Documentation Concerning Investigational Medicinal Products in Clinical Trials—Scientific Guideline. 31 March 2006. Available online: https://www.ema.europa.eu/en/requirements-chemical-pharmaceutical-quality-documentation-concerning-investigational-medicinal-products-clinical-trials-scientific-guideline (accessed on 19 July 2025).
- Abbasi, S.; Dehghani, M.; Khademi, S.; Irajirad, R.; Parizi, Z.P.; Sahebi, M.; Sadeghi, M.; Montazerabadi, A.; Tavakoli, M. Revolutionizing cancer diagnosis and dose biodistribution: A meta-analysis of [68Ga] FAPI- 46 vs. [18F] FDG imaging. Syst. Rev. 2025, 14, 109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peppicelli, S.; Andreucci, E.; Ruzzolini, J.; Bianchini, F.; Calorini, L. FDG uptake in cancer: A continuing debate. Theranostics 2020, 10, 2944–2948. [Google Scholar] [CrossRef] [PubMed]
- Alavi, A.; Saboury, B.; Nardo, L.; Zhang, V.B.; Wang, M.; Li, H.; Raynor, W.Y.; Werner, T.J.M.; Høilund-Carlsen, P.F.M.; Revheim, M.-E.M. Potential and Most Relevant Applications of Total Body PET/CT Imaging. Clin. Nucl. Med. 2022, 47, 43–55. [Google Scholar] [CrossRef]
- Naka, S.; Watabe, T.; Lindner, T.; Cardinale, J.; Kurimoto, K.; Moore, M.; Tatsumi, M.; Mori, Y.; Shimosegawa, E.; Valla FJr Kato, H.; et al. One-pot and one-step automated radio-synthesis of [18F]AlF-FAPI-74 using a multi purpose synthesizer: A proof-of-concept experiment. EJNMMI Radiopharm. Chem. 2021, 6, 28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, L.; Niu, B.; Fang, J.; Pang, Y.; Li, S.; Xie, C.; Sun, L.; Zhang, X.; Guo, Z.; Lin, Q.; et al. Synthesis, Preclinical Evaluation, and a Pilot Clinical PET Imaging Study of 68Ga-Labeled FAPI Dimer. J. Nucl. Med. 2022, 63, 862–868. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Parameters | Acceptance Criteria |
|---|---|
| Appearance | Clear and colorless solution |
| Radionuclidic purity | ≥99.9% |
| 68Ge breakthrough | ≤0.001% |
| Non-radioactive metals (ICP-EOS) | Iron: <10 μg/GBq Zinc: <10 μg/GBq |
| Identity | Gamma spectrometry: 0.511; 1.077 MeV (a sum peak may be observed at 1.022 MeV) |
| Half-life | 62–74 min |
| Radiochemical purity | ITLC-SG; mobile phase: methanol/ammonium acetate (1:1) ≥ 95% |
| pH | ≤2 |
| Endotoxin level | ≤175 EU/V |
| Components | Function | Amount/Activity |
|---|---|---|
| [68Ga]Ga-FAPI-46 | Active Pharmaceutical Ingredient | 620–697 MBq ART |
| Sodium Chloride NaCl ≥ 99.99% Suprapur | Eluent | 312.4 mg |
| Chloridric Acid HCl 30% TraceSELECT Ultra | 15 µL | |
| Ultrapure Water TraceSELECT Ultra, ACS Reagent | 1.08 mL | |
| Chloridric Acid HCl 30% TraceSELECT Ultra | Reaction Buffer | 8.8 µL |
| Acetic Acid ≥ 99.5% | 20 µL | |
| Sodium Acetate Trihydrate BioUltra ≥ 99.5% | 60.4 mg | |
| Ultrapure Water TraceSELECT Ultra, ACS Reagent | 0.37 mL | |
| Ascorbic Acid | Radical Scavenger | 0.3 mg |
| Sodium Chloride 0.9% | Diluent/Excipient | 7.5 mL |
| Components | Function | Amount/Activity |
|---|---|---|
| [68Ga]Ga-FAPI-46 | Active Pharmaceutical Ingredient | 500–700 MBq ART |
| Ethanol Absolute 100%, EMSURE ACS, ISO, Eur Ph Reag. | Eluent | 0.7 mL |
| Sodium Ascorbate Ph Eur | Radical Scavenger | 0.1 g |
| Sodium Chloride 0.9% | Diluent/Excipient | 9.5 mL |
| Parameter | Method | Acceptance Criteria | Pre/Post Release |
|---|---|---|---|
| [68Ga]Ga-FAPI-46 activity | Dose calibrator | Lab 1: 620–697 MBq Lab 2: 500–700 MBq | Pre |
| Radioactive concentration | Dose calibrator | Lab 1: 68–78 MBq/mL Lab 2: 50–70 MBq/ml | Pre |
| Appearance | Visual inspection | Clear and colorless solution | Pre |
| Identification | γ-spectrometry | Peaks at 0.511 and 1022 Mev | Pre |
| Half-life | 62–74 min. | ||
| Identification | HPLC | TR [68Ga]Ga-FAPI-46 ± 0.2 min TR natGa-FAPI-46 | Pre |
| Radiochemical purity | TLC | [68Ga]Ga-FAPI-46 ≥ 95%—[68Ga]Ga3+ ≤ 3% | Pre |
| Radiochemical purity | HPLC | [68Ga]Ga-FAPI-46 ≥ 95%—[68Ga]Ga3+ and other radiolysis products ≤ 5% of which [68Ga]Ga3+ ≤ 2% | Pre |
| System suitability | HPLC | Symmetry factor [68Ga]Ga-FAPI-46 ≤ 2.5 | Pre |
| pH | pH strips | 4.0–8.0 | Pre |
| Filter integrity | Bubble point test | ≥50 psi | Pre |
| Radionuclidic purity | γ-spectrometry | ≤0.001% | Post |
| Sterility | Sterility test (Ph. Eur.) | Sterile | Post |
| Bacterial endotoxin | Ph. Eur. | ≤175 EU/V | Pre |
| Chemical Purity UV Detector | |||
|---|---|---|---|
| Parameters | Acceptance Criteria | Lab 1 Results | Lab 2 Results |
| Specificity | Rs natGa-FAPI-46 and FAPI-46 Rs ≥ 1.5 | Comply | Comply |
| Precision | CV% FAPI-46 ≤ 5% CV% natGa-FAPI-46 ≤ 5% | 2% 2.6% | 2.3% 2.4% |
| Linearity | R2 FAPI-46 ≥ 0.99 R2 natGa-FAPI-46 ≥ 0.99 | 0.999 0.998 | 0.998 0.999 |
| Limit of quantification LOQ (μg/mL) | Experimental | FAPI-46 = 0.39 natGa-FAPI-46 = 0.74 | FAPI-46 = 1.91 natGa-FAPI-46 = 0.58 |
| Limit of detection LOD (μg/mL) | Experimental | FAPI-46 = 0.13 natGa-FAPI-46 = 0.39 | FAPI-46 = 0.63 natGa-FAPI-46 = 0.19 |
| Range accuracy | Average bias < 5% | Comply | Comply |
| Radiochemical Purity Radiodetector | |||
| Parameters | Acceptance Criteria | Lab 1 Results | Lab 2 Results |
| Specificity | Not applicable | NA | NA |
| Precision | CV% ≤ 5% | 3.1% | 3.1% |
| Linearity | R2 ≥ 0.99 | 0.9983 | 0.992 |
| Limit of quantification LOQ (MBq/mL) | Experimental | 30.01 | 4.5 |
| Limit of detection LOD (MBq/mL) | Experimental | 9.9 | 1.5 |
| Range accuracy | Average bias < 5% | Comply | Comply |
| Parameters | Method | Acceptance Criteria | Laboratory 1 | Laboratory 2 | ||||
|---|---|---|---|---|---|---|---|---|
| Batch 1 8 Apr. 2022 | Batch 2 12 May 2022 | Batch 3 18 May 2022 | Batch 1 8 Aug. 2023 | Batch 2 10 Aug. 2023 | Batch 3 11 Aug. 2023 | |||
| [68Ga]Ga-FAPI-46 activity | Dose calibrator | Lab 1: 620–697 MBq Lab 2: 500–700 MBq | 628 MBq | 662 MBq | 697 MBq | 644 MBq | 662 MBq | 593 MBq |
| Radioactive concentration | Dose calibrator | Lab 1: 68–78 MBq Lab 2:50–70 MBq | 69.7 MBq/mL | 73.5 MBq/mL | 77.4 MBq/mL | 64.4 MBq/mL | 66.2 MBq/mL | 59.3 MBq/mL |
| Volume | - | Lab 1: 9 mL Lab 2: 10 ml | Comply | Comply | Comply | Comply | Comply | Comply |
| Appearance | Visual test | Clear and colorless solution | Comply | Comply | Comply | Comply | Comply | Comply |
| Identification | HPLC | TR [68Ga]Ga-FAPI-46 ± 0.2 min TR natGa-FAPI-46 | 0.157 min | 0.172 min | 0.144 min | 0.141 min | 0.160 min | 0.156 min |
| Radionuclidic identity | γ-spectrometry | Peaks at 0.511 and 1022 Mev | Comply | Comply | Comply | Comply | Comply | Comply |
| Half-life | 62–74 min. | 67.85 min | 69.39 min | 67.83 min | 68.2 min | 68.9 min. | 66.9 min. | |
| Radiochemical purity | TLC | [68Ga]Ga-FAPI-46 ≥ 95%—[68Ga]Ga3+ ≤ 3% | 99.6% | 99.9% | 99.2% | 99.2% | 99.7% | 99.6% |
| 0.4% | 0.1% | 0.8% | 0.8% | 0.3% | 0.4% | |||
| Radiochemical purity | HPLC | [68Ga]Ga-FAPI-46 ≥ 95% | 98.1% | 99.0% | 97.1% | 99.9% | 99.7% | 99.9% |
| [68Ga]Ga3+ and other radiolysis products ≤ 5% | 1.9% | 1.0% | 2.9% | 0.1% | 0.3% | 0.0% | ||
| [68Ga]Ga3+ ≤ 2% | 1.6% | 0.7% | 2.0% | 0.1% | 0.3% | 0.0% | ||
| System suitability | HPLC | Symmetry factor [68Ga]Ga-FAPI-46 ≤ 2.5% | Comply | Comply | Comply | Comply | Comply | Comply |
| pH | pH strips | 4.0–8.0 | 4.5 | 4.5 | 4.5 | 5.8 | 6.0 | 5.9 |
| Filter integrity | Bubble point test | ≥50 psi | ≥50 psi | ≥50 psi | ≥50 psi | ≥50 psi | ≥50 psi | ≥50 psi |
| Radionuclidic purity | γ-spectrometry | ≤0.001% | 0.000035% | 0.000018% | 0.000034% | <2 × 10−5% | <2 × 10−5% | <2 × 10−5% |
| Sterility | Sterility test (Ph. Eur.) | Sterile | Comply | Comply | Comply | Comply | Comply | Comply |
| Bacterial endotoxin | Ph. Eur. | ≤175 EU/V | Comply | Comply | Comply | Comply | Comply | Comply |
| 1 h Stability Test | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Method | Acceptance Criteria | Laboratory 1 | Laboratory 2 | ||||
| Batch 1 8 Apr. 2022 | Batch 2 12 May 2022 | Batch 3 18 May 2022 | Batch 1 8 Aug. 2023 | Batch 2 10 Aug. 2023 | Batch 3 11 Aug. 2023 | |||
| Appearance | Visual test | Clear and colorless solution | Comply | Comply | Comply | Comply | Comply | Comply |
| Radiochemical purity | TLC | [68Ga]Ga-FAPI-46 ≥ 95%—[68Ga]Ga3+ ≤ 5% | 99.6% | 99.9% | 99.6% | 98.9% | 99.6% | 99.5% |
| 0.4% | 0.1% | 0.4% | 1.1% | 0.4% | 0.5% | |||
| Radiochemical purity | HPLC | [68Ga]Ga-FAPI-46 ≥ 95% | 98.0% | 98.7% | 97.0% | 99.8% | 99.7% | 99.8% |
| [68Ga]Ga3+ and other radiolysis products ≤ 5% | 2.0% | 1.3% | 3.0% | 0.2% | 0.3% | 0.2% | ||
| [68Ga]Ga3+ ≤ 2% | 1.7% | 0.8% | 2.0% | 0.1% | 0.3% | 0.1% | ||
| pH | pH strips | 4.0–8.0 | 4.5 | 4.5 | 4.5 | 5.8 | 5.9 | 5.9 |
| 2 h Stability Test | ||||||||
| Parameters | Method | Acceptance Criteria | Laboratory 1 | Laboratory 2 | ||||
| Batch 1 8 Apr. 2022 | Batch 2 12 May 2022 | Batch 3 18 May 2022 | Batch 1 8 Aug. 2023 | Batch 2 10 Aug. 2023 | Batch 3 11 Aug. 2023 | |||
| Appearance | Visual test | Clear and colorless solution | Comply | Comply | Comply | Comply | Comply | Comply |
| Radiochemical purity | TLC | [68Ga]Ga-FAPI-46 ≥ 95%—[68Ga]Ga3+ ≤ 5% | 99.5% | 99.7% | 99.5% | 98.6% | 99.5% | 99.3% |
| 0.5% | 0.3% | 0.5% | 1.3% | 0.5% | 0.6% | |||
| Radiochemical purity | HPLC | [68Ga]Ga-FAPI-46 ≥ 95% | 97.5% | 98.5% | 96.7% | 99.3% | 98.5% | 99.3% |
| [68Ga]Ga3+ and other radiolysis products ≤ 5% | 2.5% | 1.5% | 3.3% | 0.7% | 0.6% | 0.7% | ||
| [68Ga]Ga3+ ≤ 2% | 1.7% | 0.8% | 2.0% | 0.6% | 0.1% | 0.1% | ||
| pH | pH strips | 4.0–8.0 | 4.5 | 4.5 | 4.5 | 5.8 | 5.8 | 5.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cafaro, A.; Cuni, C.; Boschi, S.; Landi, E.; Foschi, G.; Monti, M.; Caroli, P.; Matteucci, F.; Masini, C.; Di Iorio, V. Production and Quality Control of [68Ga]Ga-FAPI-46: Development of an Investigational Medicinal Product Dossier for a Bicentric Clinical Trial. Pharmaceuticals 2025, 18, 1475. https://doi.org/10.3390/ph18101475
Cafaro A, Cuni C, Boschi S, Landi E, Foschi G, Monti M, Caroli P, Matteucci F, Masini C, Di Iorio V. Production and Quality Control of [68Ga]Ga-FAPI-46: Development of an Investigational Medicinal Product Dossier for a Bicentric Clinical Trial. Pharmaceuticals. 2025; 18(10):1475. https://doi.org/10.3390/ph18101475
Chicago/Turabian StyleCafaro, Alessandro, Cristina Cuni, Stefano Boschi, Elisa Landi, Giacomo Foschi, Manuela Monti, Paola Caroli, Federica Matteucci, Carla Masini, and Valentina Di Iorio. 2025. "Production and Quality Control of [68Ga]Ga-FAPI-46: Development of an Investigational Medicinal Product Dossier for a Bicentric Clinical Trial" Pharmaceuticals 18, no. 10: 1475. https://doi.org/10.3390/ph18101475
APA StyleCafaro, A., Cuni, C., Boschi, S., Landi, E., Foschi, G., Monti, M., Caroli, P., Matteucci, F., Masini, C., & Di Iorio, V. (2025). Production and Quality Control of [68Ga]Ga-FAPI-46: Development of an Investigational Medicinal Product Dossier for a Bicentric Clinical Trial. Pharmaceuticals, 18(10), 1475. https://doi.org/10.3390/ph18101475










