Radiolabeling and Preliminary In Vivo Evaluation of the Candidate CCR2 Targeting PET Radioligand [11C]AZD2423
Abstract
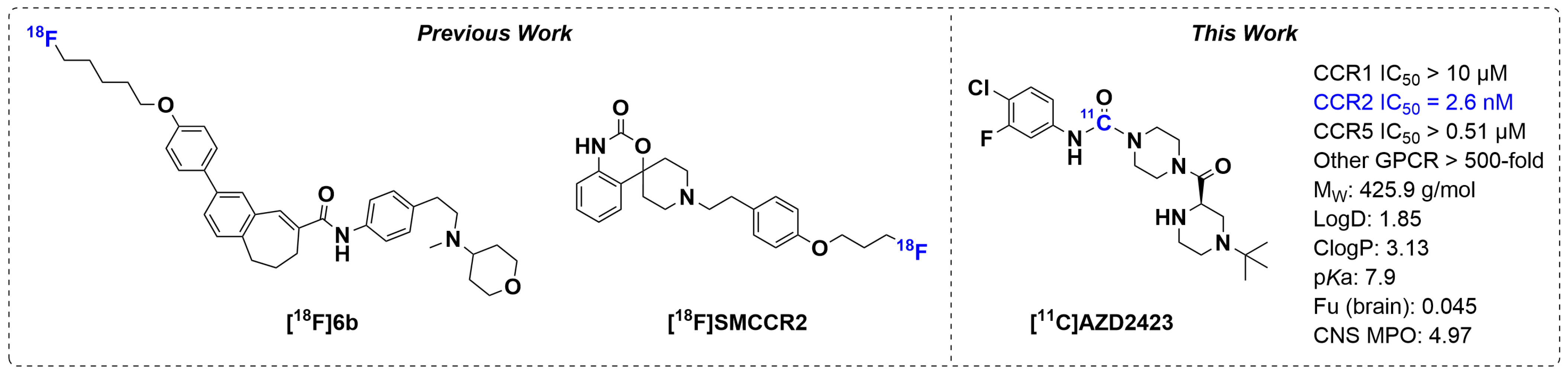
1. Introduction
2. Results and Discussion
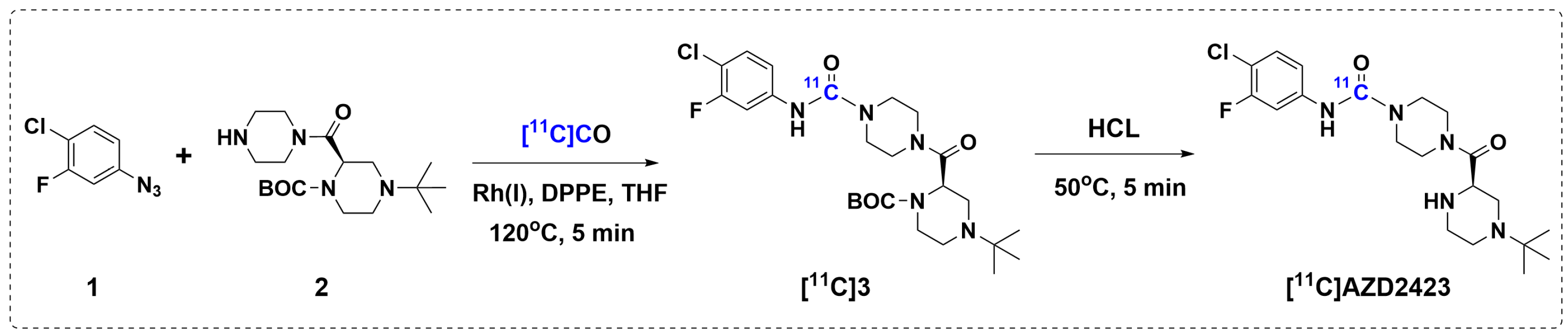
2.1. Radiolabeling of [11C]AZD2423 and Automation
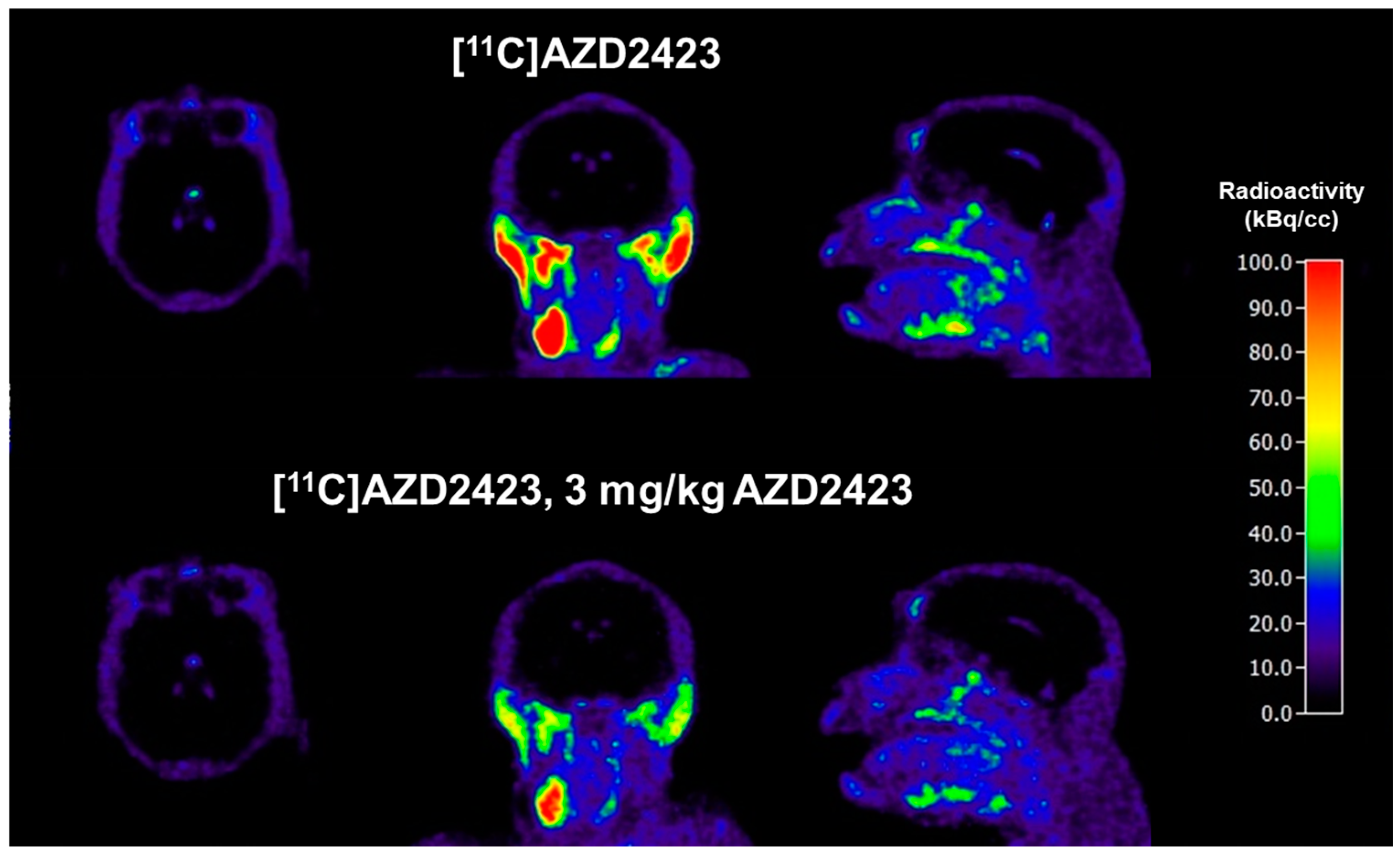
2.2. PET Imaging Studies in Non-Human Primates
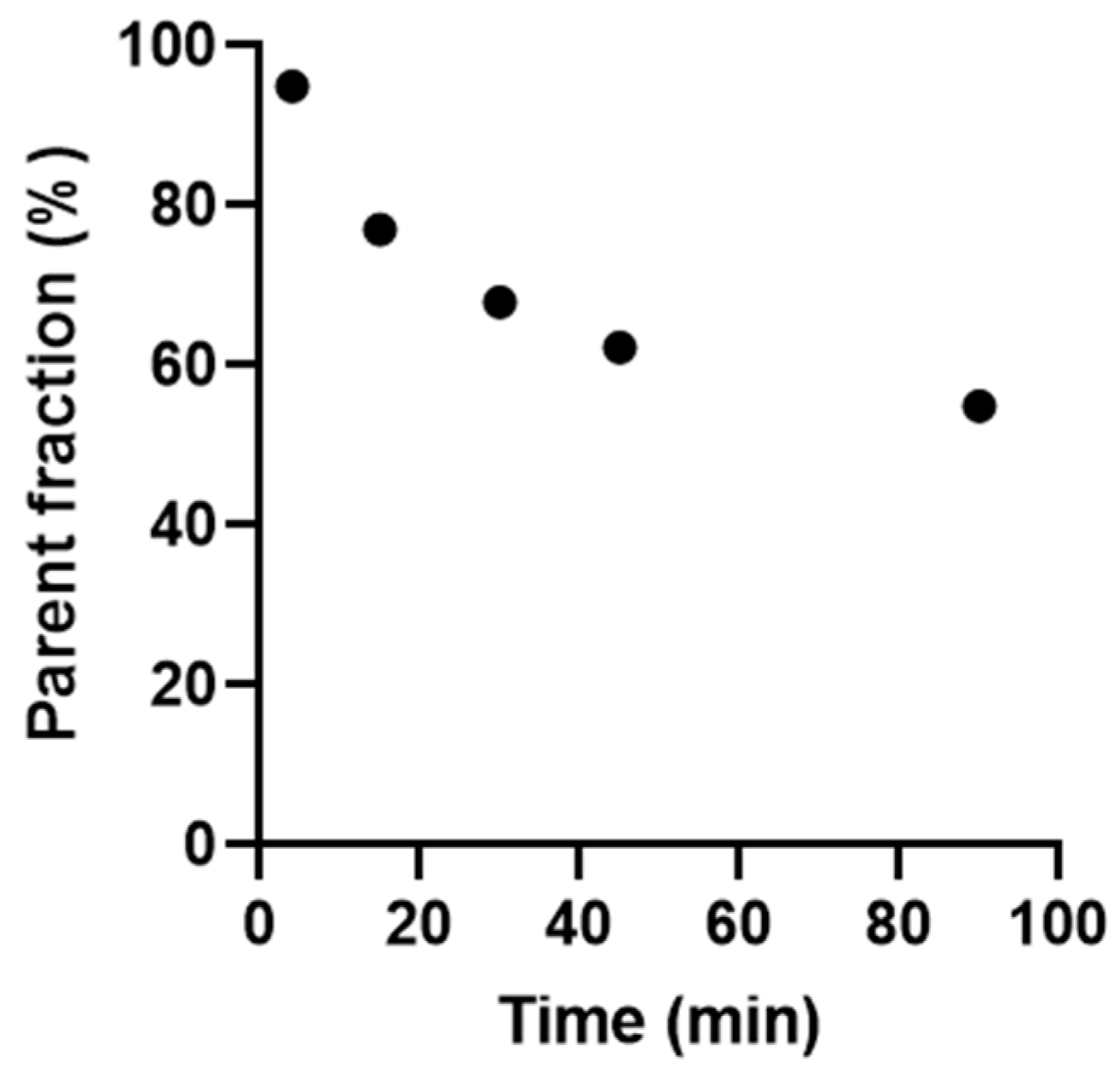
2.3. Radiometabolite Analysis
3. Materials and Methods
3.1. Radiochemistry
3.2. PET Imaging in Non-Human Primates
3.3. Kinetic Model Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, H.X.; Arumugam, T.V.; Gelderblom, M.; Magnus, T.; Drummond, G.R.; Sobey, C.G. Role of CCR2 in inflammatory conditions of the central nervous system. J. Cereb. Blood Flow Metab. 2014, 34, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Ren, X.; Yu, H.; Zhan, Y. Targeting the CCL2/CCR2 Axis in Cancer Immunotherapy: One Stone, Three Birds? Front. Immunol. 2021, 12, 771210. [Google Scholar] [CrossRef]
- Shao, Z.; Tan, Y.; Shen, Q.; Hou, L.; Yao, B.; Qin, J.; Xu, P.; Mao, C.; Chen, L.-N.; Zhang, H.; et al. Molecular insights into ligand recognition and activation of chemokine receptors CCR2 and CCR3. Cell Discov. 2022, 8, 44–55. [Google Scholar] [CrossRef]
- Naert, G.; Rivest, S. CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2011, 31, 6208–6220. [Google Scholar] [CrossRef]
- Fife, B.T.; Huffnagle, G.B.; Kuziel, W.A.; Karpus, W.J. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J. Exp. Med. 2000, 192, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic, O.B.; Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A.V. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke 2007, 38, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Ametamey, S.M.; Honer, H.; Schubiger, P.A. Molecular imaging with PET. Chem. Rev. 2008, 108, 1501–1516. [Google Scholar] [CrossRef]
- Miller, P.W.; Long, N.J.; Vilar, R.; Gee, A.D. Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew. Chem. Int. Ed. 2008, 47, 8998–9033. [Google Scholar] [CrossRef]
- Deng, X.; Rong, J.; Wang, L.; Vasdev, N.; Zhang, L.; Josephson, L.; Liang, S.H. Chemistry for Positron Emission Tomogra-phy: Recent Advances in 11C-, 18F-, 13N-, and 15O-Labeling Reactions. Angew. Chem. Int. Ed. 2018, 58, 2580–2605. [Google Scholar] [CrossRef]
- Alluri, S.R.; Higashi, Y.; Kil, K.-E. PET Imaging Radiotracers of Chemokine Receptors. Molecules 2021, 26, 5174. [Google Scholar] [CrossRef]
- Liu, Y.; Li, W.; Luehmann, H.P.; Zhao, Y.; Detering, L.; Sultan, D.H.; Hsiao, H.M.; Krupnick, A.S.; Gelman, A.E.; Combadiere, C.; et al. No invasive imaging of CCR2+ cells in ischemia-reperfusion injury after lung transplantation. Am. J. Transplant. 2016, 16, 3016–3023. [Google Scholar] [CrossRef]
- Liu, Y.; Gunsten, S.P.; Sultan, D.H.; Luehmann, H.P.; Zhao, Y.; Blackwell, T.S.; Bollermann-Nowlis, Z.; Pan, J.H.; Byers, D.E.; Atkinson, J.J.; et al. PET-based imaging of chemokine receptor 2 in experimental and disease-related lung inflammation. Radiology 2017, 283, 758–768. [Google Scholar] [CrossRef] [PubMed]
- English, S.J.; Sastriques, S.E.; Detering, L.; Sultan, D.; Luehmann, H.; Arif, B.; Heo, G.S.; Zhang, X.; Laforest, R.; Zheng, J.; et al. CCR2 Positron Emission Tomography for the Assessment of Abdominal Aortic Aneurysm Inflammation and Rupture Prediction. Circ. Cardiovasc. Imaging 2020, 13, e009889. [Google Scholar] [CrossRef]
- Heo, G.S.; Lou, L.; Sultan, D.; Liu, Y. The Latest Advances in Imaging Crosstalk Between the Immune System and Fibrosis in Cardiovascular Disease. J. Nucl. Med. 2021, 62, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Gatti, F.D.M.; Silva, D.G.; Zacarias, N.V.O.; Zweemer, A.J.M.; Hermann, S.; De Maria, M.; Koch, M.; Weiss, C.; Schepmann, D.; et al. Development of the First Potential Nonpeptidic Positron Emission Tomography Tracer for the Imaging of CCR2 Receptors. ChemMedChem 2021, 16, 640–645. [Google Scholar] [CrossRef]
- Zhang, X.; Qiu, L.; Sultan, D.H.; Luehmann, H.P.; Yu, Y.; Zhang, X.; Heo, G.S.; Li, A.; Lahad, D.; Rho, S.; et al. Development of a CCR2 targeted 18F-labeled radiotracer for atherosclerosis imaging with PET. Nucl. Med. Biol. 2024, 130–131, 108893. [Google Scholar] [CrossRef] [PubMed]
- Kalliomäki, J.; Attal, N.; Jonzon, B.; Bach, F.W.; Huizar, K.; Ratcliffe, S.; Eriksson, B.; Janecki, M.; Danilov, A.; Bouhassira, D. A randomized, double-blind, placebo-controlled trial of a chemokine receptor 2 (CCR2) antagonist in posttraumatic neuralgia. PAIN 2013, 154, 761–767. [Google Scholar] [CrossRef]
- Kalliomäki, J.; Jonzon, B.; Huizar, K.; O’Malley, M.; Andersson, A.; Simpson, D.M. Evaluation of a novel chemokine receptor 2 (CCR2)-antagonist in painful diabetic polyneuropathy. Scand. J. Pain 2013, 4, 77–83. [Google Scholar] [CrossRef]
- Cumming, J.G. CCR2 antagonists for the treatment of neuropathic pain. In Proceedings of the 17th RSC/SCI Medicinal Chemistry Symposium, Cambridge, UK, 8–11 September 2013. [Google Scholar]
- Wager, T.T.; Hou, X.; Verhoest, P.R.; Villalobos, A. Central Nervous System Multiparameter Optimization Desirability: Application in Drug Discovery. ACS Chem. Neurosci. 2016, 7, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Rotstein, B.H.; Liang, S.H.; Placzek, M.S.; Hooker, J.M.; Gee, A.D.; Dolle, F.; Wilson, A.A.; Vasdev, N. 11CO bonds made easily for positron emission tomography radiopharmaceuticals. Chem. Soc. Rev. 2016, 45, 4708–4726. [Google Scholar] [CrossRef]
- Dahl, K.; Halldin, C.; Schou, M. New methodologies for the preparation of carbon-11 labeled radiopharmaceuticals. Clin. Transl. Imag. 2017, 5, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.; Antoni, G.; Långström, B.; Itsenko, O. The development of 11C-carbonylation chemistry: A systematic view. Nucl. Med. Biol. 2021, 92, 115–137. [Google Scholar] [CrossRef] [PubMed]
- Doi, H.; Barletta, J.; Suzuki, M.; Noyori, R.; Watanabe, Y.; Långström, B. Synthesis of 11C-labelled N,N′-diphenylurea and ethyl phenylcarbamate by a rhodium-promoted carbonylation via [11C]isocyanatobenzene using phenyl azide and [11C]carbon monoxide. Org. Biomol. Chem. 2004, 2, 3063–3066. [Google Scholar] [CrossRef] [PubMed]
- Dahl, K.; Itsenko, O.; Rahman, O.; Ulin, J.; Sjöberg, C.; Sandblom, P.; Larsson, L.; Schou, M.; Halldin, C. An evaluation of a high-pressure 11CO carbonylation apparatus. J. Label. Compd. Radiopharm. 2015, 58, 220–225. [Google Scholar] [CrossRef]
- Fridén, M.; Wennerberg, M.; Antonsson, M.; Sandberg-Ställ, M.; Farde, L.; Schou, M. Identification of positron emission tomography (PET) tracer candidates by prediction of the target-bound fraction in the brain. EJNMMI Res. 2014, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Dahl, K.; Johnström, P.; Forsberg-Morén, A.; Gustafsson, B.; Miranda-Azpiazu, P.; Khani, Y.; Halldin, C.; Farde, L.; Elmore, C.S.; Schou, M. Synthesis and Preclinical Evaluation of [11C]AZ11895530 for PET Imaging of the Serotonin 1A Receptor. ACS Chem. Neurosci. 2022, 13, 2078–2083. [Google Scholar] [CrossRef] [PubMed]
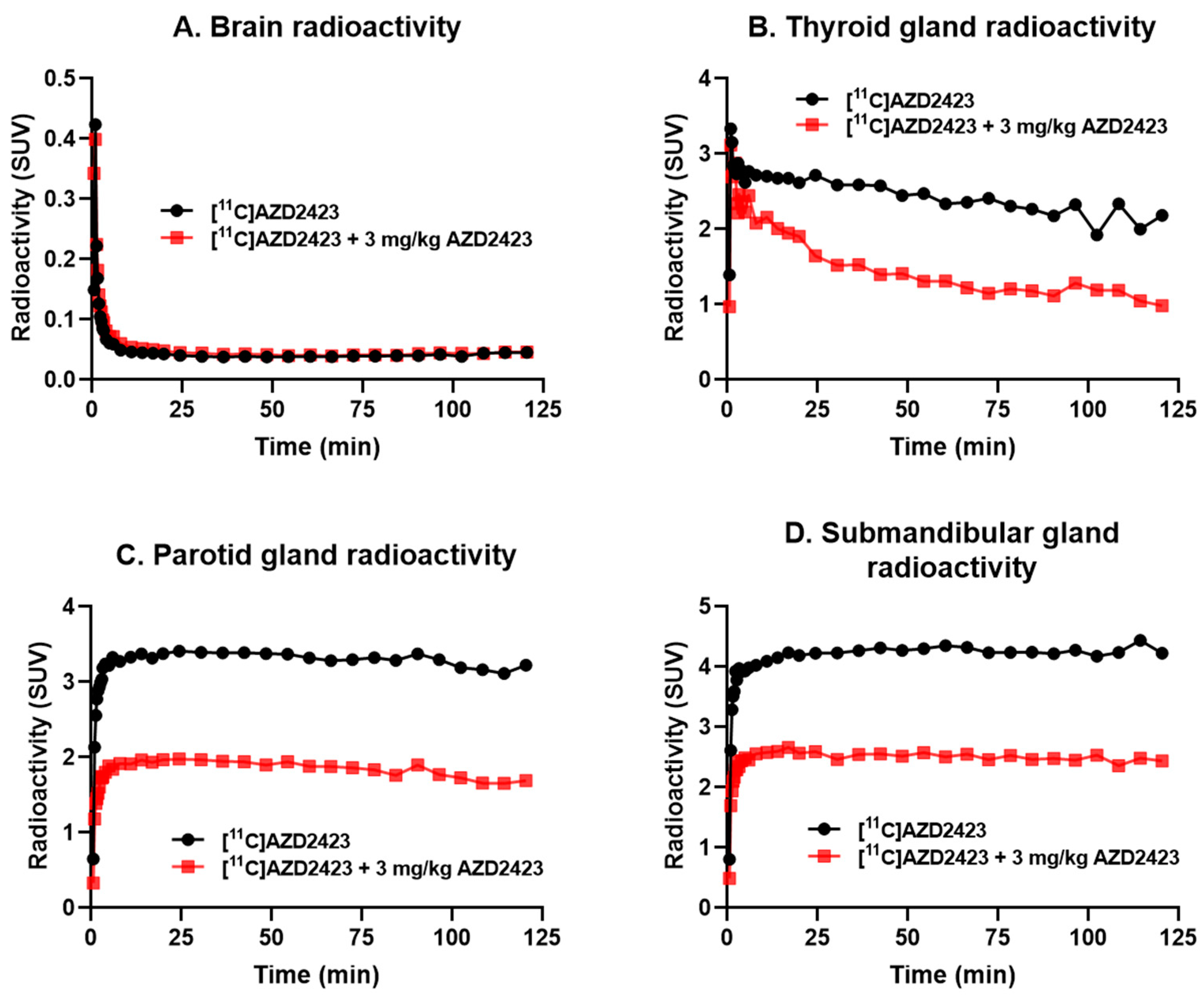
| Periphel Organ | Baseline | Pretreatment | Percentage Decrease (%) |
|---|---|---|---|
| Thyroid gland | 53.1 | 19.4 | 63.4 |
| Parotid gland | 111.1 | 41.6 | 62.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahl, K.; Johnström, P.; Tóth, M.; Bolin, M.; Varnäs, K.; Nakao, R.; Takano, A.; Khani Meynaq, Y.; Bayrakdarian, M.; Cselényi, Z.; et al. Radiolabeling and Preliminary In Vivo Evaluation of the Candidate CCR2 Targeting PET Radioligand [11C]AZD2423. Pharmaceuticals 2025, 18, 135. https://doi.org/10.3390/ph18020135
Dahl K, Johnström P, Tóth M, Bolin M, Varnäs K, Nakao R, Takano A, Khani Meynaq Y, Bayrakdarian M, Cselényi Z, et al. Radiolabeling and Preliminary In Vivo Evaluation of the Candidate CCR2 Targeting PET Radioligand [11C]AZD2423. Pharmaceuticals. 2025; 18(2):135. https://doi.org/10.3390/ph18020135
Chicago/Turabian StyleDahl, Kenneth, Peter Johnström, Miklós Tóth, Martin Bolin, Katarina Varnäs, Ryuji Nakao, Akihiro Takano, Yasir Khani Meynaq, Malken Bayrakdarian, Zsolt Cselényi, and et al. 2025. "Radiolabeling and Preliminary In Vivo Evaluation of the Candidate CCR2 Targeting PET Radioligand [11C]AZD2423" Pharmaceuticals 18, no. 2: 135. https://doi.org/10.3390/ph18020135
APA StyleDahl, K., Johnström, P., Tóth, M., Bolin, M., Varnäs, K., Nakao, R., Takano, A., Khani Meynaq, Y., Bayrakdarian, M., Cselényi, Z., Halldin, C., Farde, L., & Schou, M. (2025). Radiolabeling and Preliminary In Vivo Evaluation of the Candidate CCR2 Targeting PET Radioligand [11C]AZD2423. Pharmaceuticals, 18(2), 135. https://doi.org/10.3390/ph18020135









