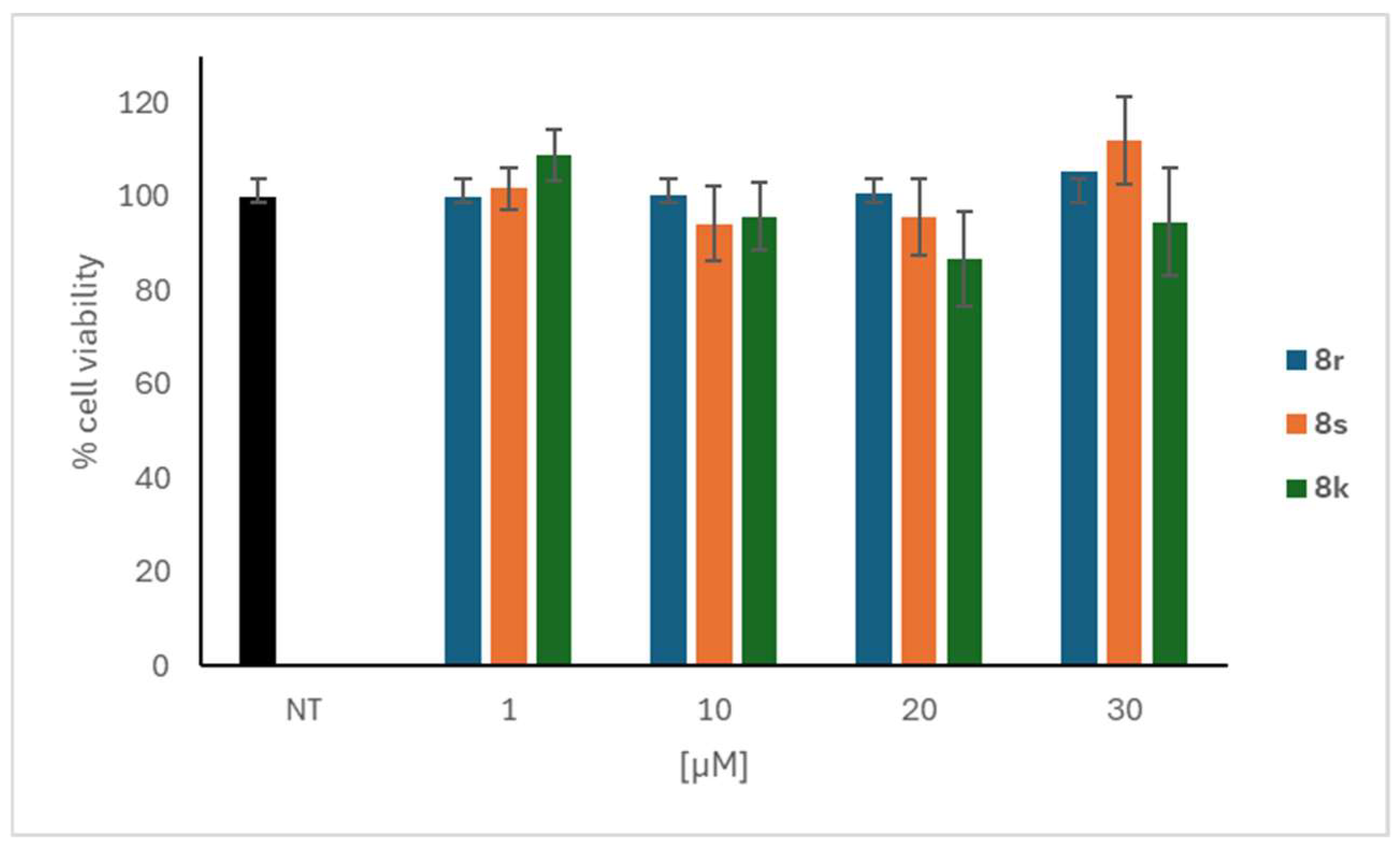
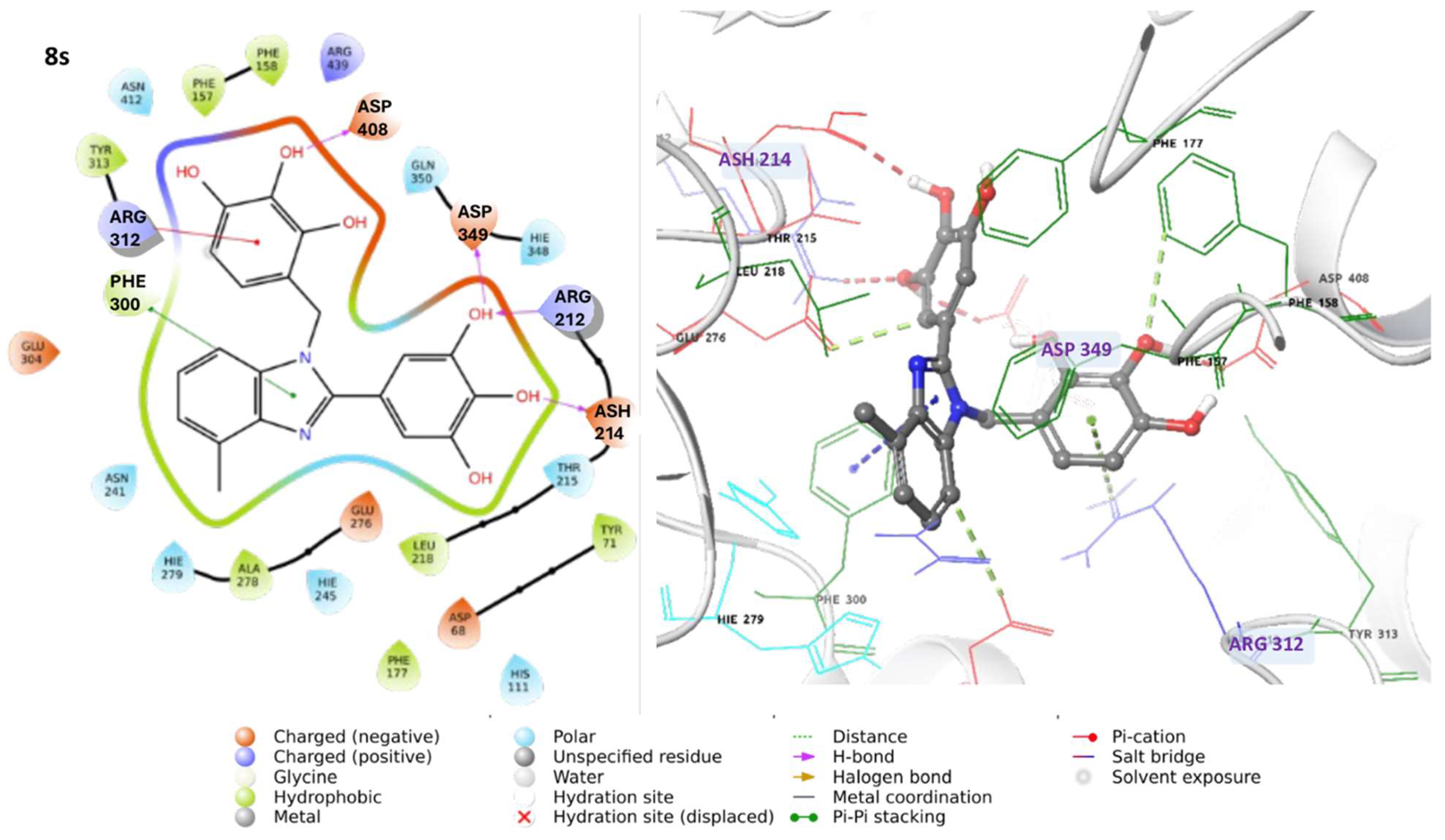
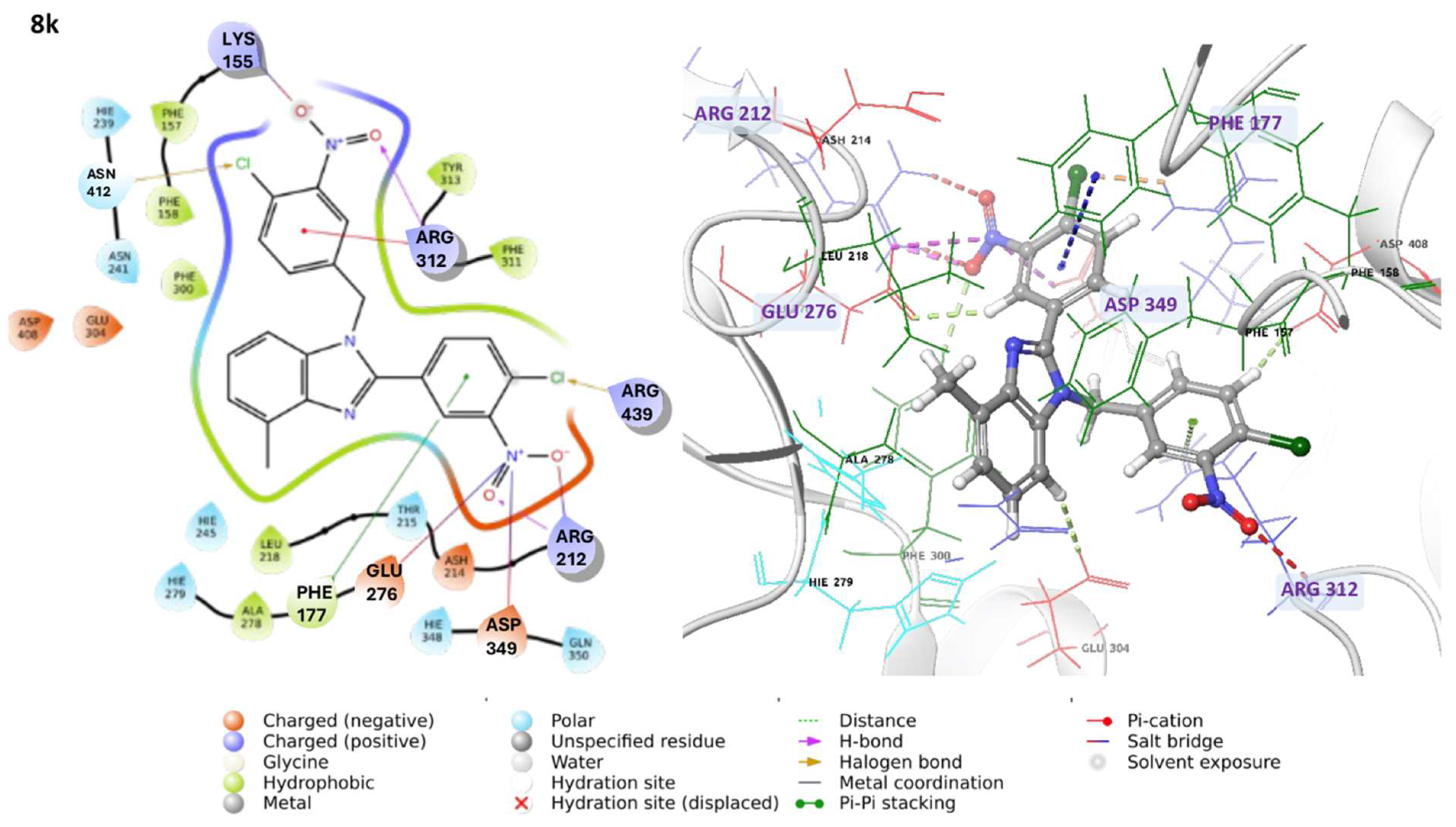
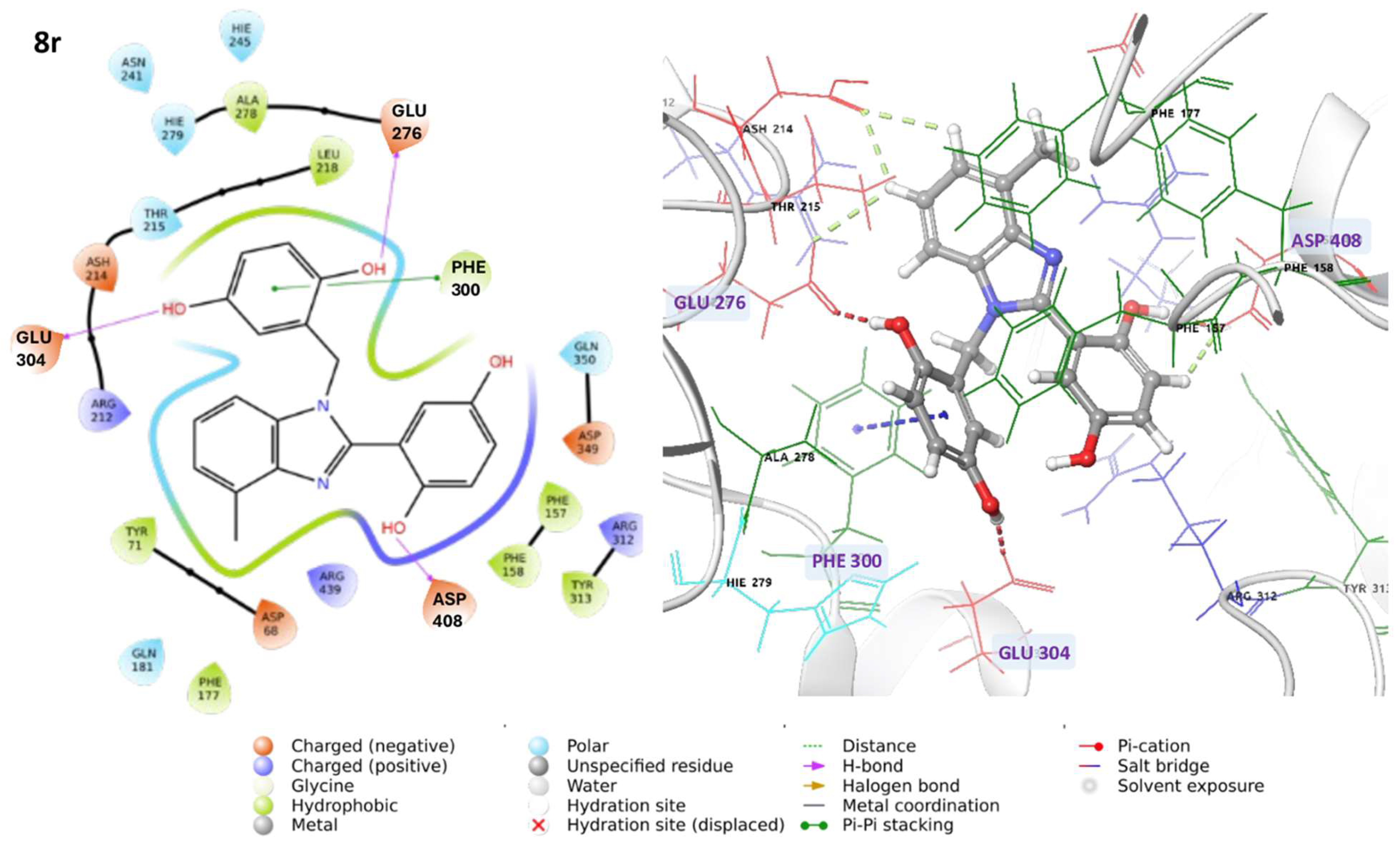
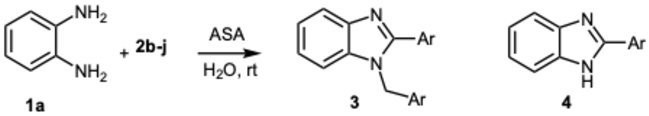
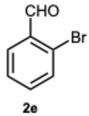
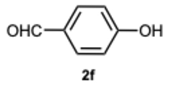
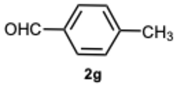
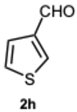
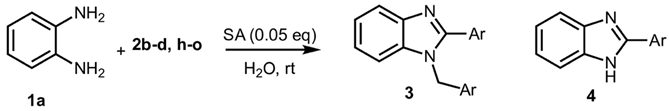
3.1.3. Characterization Data of All the Tested Compounds
1-Benzyl-2-phenyl-1H-benzo[d]imidazole 3a. White solid. M.p.130–131 °C. NMR spectra are consistent with those previously reported [
38].
1H NMR (600 MHz, CDCl
3): δ 7.85 (d, J = 8.0 Hz, 1H), 7.59 (dd, J = 8.2, 2.0 Hz, 2H), 7.30 (d, J = 7.2 Hz, 1H), 7.29–7.17 (m, 6H), 7.13 (d, J = 7.5 Hz, 2H), 7.00 (d, J = 7.6 Hz, 2H), 5.41 (s, 2H) ppm.
13C NMR (151 MHz, CDCl
3): δ 154.50, 143.36, 140.16, 137.58, 136.26, 133.62, 129.84, 129.57, 129.32, 127.37, 126.05, 122.96, 122.67, 119.99, 110.64, 48.35ppm. HRMS(ESI): m/z cal. for C
20H
16N
2: 284.1313, found [M + H]
+ 285.1323.
1-((1H-Indol-3-yl)methyl)-2-(1H-indol-3-yl)-1H-benzo[d]imidazole 3b. Pale yellow solid. M.p. 233–235 °C. NMR spectra are consistent with those previously reported [
39].
1H NMR (600 MHz, DMSO): δ 11.66 (s, 1H), 10.98 (d,
J = 2.6 Hz, 1H), 8.33 (dt,
J = 7.9, 1.1 Hz, 1H), 7.86 (s, 1H), 7.70–7.65 (m, 1H), 7.57–7.53 (m, 1H), 7.49 (dt,
J = 8.0, 1.0 Hz, 1H), 7.32 (dd,
J = 8.1, 1.0 Hz, 1H), 7.25 (t,
J = 8.0 Hz, 1H), 7.24–7.20 (m, 1H), 7.17 (dtd,
J = 16.4, 7.5, 1.3 Hz, 3H), 7.06–7.01 (m, 2H), 6.84 (ddd,
J = 8.0, 7.0, 1.0 Hz, 1H), 5.84 (s, 2H) ppm.
13C NMR (600 MHz, DMSO): δ 149.64, 143.35, 136.34, 136.06, 135.62, 126.51, 126.36, 125.55, 123.52, 122.32, 121.42, 121.41, 121.39, 121.33, 120.26, 118.82, 118.37, 118.21, 111.80, 111.70, 110.45, 110.40, 105.14, 40.71 ppm. HRMS(ESI): m/z cal. for C
24H
18N
4: 362.1531, found [M + H]
+ 363.1533.
2-(Benzo[d][1,3]dioxol-5-yl)-1-(benzo[d][1,3]dioxol-5-ylmethyl)-1H-benzo[d]imidazole 3c. Beige solid. M.p. 175–177 °C. NMR spectra are consistent with those previously reported [
40].
1H NMR (600 MHz, CDCl
3): δ 7.85 (dt,
J = 8.0, 1.0 Hz, 1H), 7.62 (d,
J = 1.3 Hz, 1H), 7.33 (ddd,
J = 8.1, 5.9, 2.5 Hz, 1H), 7.30–7.24 (m, 2H), 7.19–7.14 (m, 2H), 6.85 (dd,
J = 7.5, 0.9 Hz, 1H), 6.61–6.56 (m, 2H), 6.01 (s, 2H), 5.96 (s, 2H), 5.37 (s, 2H) ppm.
13C NMR (151 MHz, CDCl
3): δ 153.91, 149.27, 148.52, 143.03, 136.07, 123.72, 123.14, 122.86, 119.88, 119.40, 110.60, 109.73, 108.80, 108.71, 106.66, 101.68, 101.59, 101.40, 48.29.ppm. HRMS(ESI): m/z cal. for C
22H
16N
2O
4: 372.1183, found [M + H]
+ 373.1187.
1-(2-Nitrobenzyl)-2-(2-nitrophenyl)-1H-benzo[d]imidazole 3d. Pale yellow solid. M.p. 172–174 °C. NMR spectra are consistent with those previously reported [
41].
1H NMR (600 MHz, CD
3CN): δ 8.10 (ddt,
J = 7.6, 4.1, 2.3 Hz, 2H), 7.77–7.74 (m, 1H), 7.74–7.70 (m, 2H), 7.59–7.55 (m, 1H), 7.54–7.47 (m, 2H), 7.35–7.29 (m, 3H), 6.88 (ddt, J = 7.7, 2.3, 1.0 Hz, 1H), 5.74 (s, 1H) ppm.
13C NMR (151 MHz, CD
3CN): δ 150.94, 148.44, 144.12, 136.31, 135.15, 134.54, 132.90, 132.63, 132.49, 129.91, 129.90, 129.37, 126.33, 125.99, 124.54, 123.81, 120.76, 118,30, 111.93, 46.52 ppm. HRMS(ESI): m/z cal. for C
20H
14N
4O
4 [M + H]
+: 374.1088, found 375.1086.
1-(2-Bromobenzyl)-2-(2-bromophenyl)-1H-benzo[d]imidazole 3e. Pale yellow solid. M.p. 148–150 °C. NMR spectra are consistent with those previously reported [
19].
1H NMR (600 MHz, CDCl
3): δ 7.89 (d,
J = 8.0 Hz, 1H), 7.70 (dq,
J = 5.7, 2.9, 2.0 Hz, 1H), 7.52 (dt,
J = 6.5, 1.9 Hz, 1H), 7.42–7.37 (m, 1H), 7.37–7.31 (m, 3H), 7.30–7.24 (m, 1H), 7.20 (d,
J = 8.1 Hz, 1H), 7.12–7.06 (m, 2H), 6.60 (dt,
J = 6.7, 1.9 Hz, 1H), 5.32 (s, 2H).ppm.
13C NMR (151 MHz, CDCl
3): δ 152.71, 143.09, 135.02, 134.82, 133.20, 133.01, 132.25, 131.88, 131.68, 129.37, 127.97, 127.89, 127.58, 124.08, 123.56, 122.90, 122.31, 120.58, 110.67, 48.34ppm. HRMS(ESI): m/z cal. for C
20H
14Br
2N
2: 442.9597, found [M + H]
+ 4413.9597.
4-(1-(4-Hydroxybenzyl)-1H-benzo[d]imidazol-2-yl)phenol 3f. Beige solid. M.p. 223–225 °C. NMR spectra are consistent with those previously reported [
39].
1H NMR (600 MHz, DMSO): δ 7.57–7.52 (m, 2H), 7.43–7.38 (m, 1H), 7.23–7.11 (m, 2H), 6.92 (dd,
J = 8.6, 6.8 Hz, 2H), 6.85–6.80 (m, 2H), 6.70–6.65 (m, 2H), 5.41 (s, 2H) ppm.
13C NMR (151 MHz, DMSO): δ 159.25, 156.84, 153.67, 142.74, 135.86, 131.29, 131.12, 130.51, 127.44, 124.72, 122.47, 122.03, 121.79, 120.48, 118.78, 115.68, 115.61, 115.50, 110.91, 59.73 ppm. HRMS(ESI): m/z cal. for C
20H
16N
2O
2: 316.1285, found [M + H]
+ 317.2584.
1-(4-Methylbenzyl)-2-(p-tolyl)-1H-benzo[d]imidazole 3g. Pale brown solid. M.p. 129–131 °C. NMR spectra are consistent with those previously reported [
42].
1H NMR (600 MHz, CDCl
3): δ 7.88–7.83 (m, 1H), 7.61–7.57 (m, 2H), 7.29 (ddd,
J = 8.2, 6.5, 1.8 Hz, 1H), 7.25 (s, 2H), 7.24–7.18 (m, 2H), 7.13 (d,
J = 7.9 Hz, 2H), 7.00 (d,
J = 7.9 Hz, 2H), 5.41 (s, 2H), 2.41 (s, 3H), 2.34 (s, 3H) ppm.
13C NMR (151 MHz, CDCl
3): δ 154.53, 143.22, 138.94, 136.64, 136.28, 130.81, 130.40, 130.06, 129.06, 128.66, 128.64, 126.81, 126.19, 123.25, 123.10, 122.75, 120.03, 110.68, 48.58, 21.58, 21.48 ppm. HRMS(ESI): m/z cal. for C
22H
20N
2: 312.1699, found [M + H]
+ 313.1700.
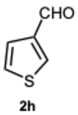
2-(Thiophen-3-yl)-1-(thiophen-3-ylmethyl)-1H-benzo[d]imidazole 3h. Yellow solid. M.p. 190–192 °C. Pale yellow solid. M.p. 148-150 °C. NMR spectra are consistent with those previously reported [
43].
1H NMR (600 MHz, CDCl
3): δ 7.83 (dt,
J = 7.9, 1.0 Hz, 1H), 7.64 (dd,
J = 2.9, 1.3 Hz, 1H), 7.52 (dd,
J = 5.0, 1.3 Hz, 1H), 7.42 (dd,
J = 5.1, 2.9 Hz, 1H), 7.34 (dd,
J = 4.9, 3.1 Hz, 1H), 7.32–7.27 (m, 2H), 7.25 (ddd,
J = 8.0, 7.0, 1.2 Hz, 1H), 6.95–6.90 (m, 2H), 5.47 (d,
J = 1.2 Hz, 2H) ppm.
13C NMR (151 MHz, CDCl
3): δ 149.48, 143.08, 137.61, 135.89, 131.00, 128.29, 127.55, 126.65, 126.47, 125.73, 123.19, 122.84, 121.70, 119.95, 110.07, 44.74 ppm. HRMS(ESI): m/z cal. for C
16H
12N
2S
2: 296.0515, found [M + H]
+ 297.0607.
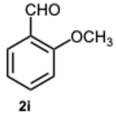
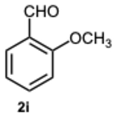
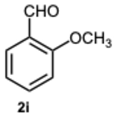
1-(2-Methoxybenzyl)-2-(2-methoxyphenyl)-1H-benzo[d]imidazole 3i. Beige solid. M.p. 131–133 °C. NMR spectra are consistent with those previously reported [
44].
1H NMR (600 MHz, CDCl
3): δ 7.84 (d,
J = 8.0 Hz, 1H), 7.55–7.50 (m, 1H), 7.45 (t,
J = 8.1 Hz, 1H), 7.23–7.16 (m, 4H), 7.07–7.02 (m, 1H), 6.95 (d,
J = 8.4 Hz, 1H), 6.83 (d,
J = 8.2 Hz, 1H), 6.76 (t,
J = 7.6 Hz, 1H), 6.69 (d,
J = 7.6 Hz, 1H), 5.23 (s, 2H), 3.78 (s, 3H), 3.59 (s, 3H). ppm.
13C NMR (151 MHz, CDCl
3): δ 157.77, 156.67, 132.55, 131.53, 128.52, 127.92, 124.74, 122.59, 122.08, 120.94, 120.55, 119.97, 110.98, 110.89, 110.08, 55.36, 55.29, 43.66 ppm. HRMS(ESI): m/z calc. for C
22H
20N
2O
2: 344.1598, found [M + H]
+ 345.1598.
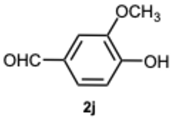
4-(1-(4-Hydroxy-3-methoxybenzyl)-1H-benzo[d]imidazol-2-yl)-2-methoxyphenol 3j. Pale yellow solid. M.p. 186–188 °C. NMR spectra are consistent with those previously reported [
39].
1H NMR (600 MHz, DMSO): δ 9.60 (s, 1H), 9.00 (s, 1H), 7.68–7.62 (m, 1H), 7.50–7.44 (m, 1H), 7.24 (d,
J = 2.0 Hz, 1H), 7.23–7.20 (m, 2H), 7.17 (dd,
J = 8.1, 2.0 Hz, 1H), 6.91 (d,
J = 8.2 Hz, 1H), 6.67 (d,
J = 2.0 Hz, 1H), 6.64 (d,
J = 8.1 Hz, 1H), 6.36 (dd,
J = 8.1, 2.0 Hz, 1H), 5.43 (s, 2H), 3.71 (s, 3H), 3.62 (s, 3H) ppm.
13C NMR (151 MHz, DMSO): δ 153.66, 148.32, 147.81, 147.72, 145.94, 142.60, 136.06, 127.95, 122.38, 122.16, 122.10, 121.15, 118.91, 118.64, 115.80, 115.69, 115.65, 113.12, 111.00, 110.82,, 55.61, 55.58, 47.43.ppm. HRMS(ESI): m/z calc. for C
22H
20N
2O
4: 376.1496, found [M + H]
+ 377.1523.
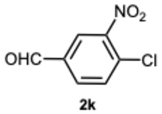
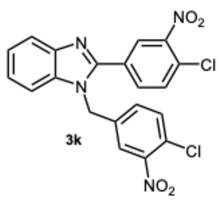
1-(4-Chloro-3-nitrobenzyl)-2-(4-chloro-3-nitrophenyl)-1H-benzo[d]imidazole 3k. Pale yellow solid. M.p. 192–194 °C. NMR spectra are consistent with those previously reported [
30].
1H NMR (600 MHz, DMSO): δ 8.39 (s, 1H), 8.03–7.98 (m, 1H), 7.93 (dd,
J = 8.4, 2.1 Hz, 1H), 7.84 (s, 1H), 7.79 (dq,
J = 5.7, 3.0, 2.4 Hz, 1H), 7.68 (dd,
J = 8.5, 2.0 Hz, 1H), 7.58 (dt,
J = 6.0, 2.4 Hz, 1H), 7.33 (dq,
J = 5.6, 3.1, 2.4 Hz, 2H), 7.25 (dd,
J = 8.4, 2.5 Hz, 1H), 5.74 (s, 2H)) ppm.
13C NMR (151 MHz, DMSO): δ 150.03, 147.55, 147.52, 142.48, 137.82, 135.87, 134.04, 132.28, 132.10, 131.59, 130.04, 126.55, 126.19, 124.05, 123.81, 123.68, 122.91, 119.73, 111.21, 46.40 ppm. HRMS(ESI): m/z calc. for C
20H
12 Cl
2N
4O
4: 442.0308, found [M + H]
+ 443.0308.
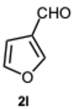
2-(Furan-3-yl)-1-(furan-3-ylmethyl)-1H-benzo[d]imidazole 3l. Beige solid. M.p. 190–192 °C. NMR spectra are consistent with those previously reported [
30].
1H NMR (600 MHz, CDCl
3): δ 7.78 (dd,
J = 6.5, 3.6 Hz, 1H), 7.65 (s, 1H), 7.50 (dd,
J = 6.2, 3.4 Hz, 1H), 7.35–7.24 (m, 4H), 7.22 (t,
J = 2.6 Hz, 1H), 6.61 (q,
J = 1.9 Hz, 1H), 6.28 (q,
J = 2.3 Hz, 1H), 6.24 (t,
J = 2.7 Hz, 1H), 5.65 (d,
J = 2.0 Hz, 2H) ppm.
13C NMR (151 MHz, CDCl
3): δ 149.74, 145.54, 144.12, 144.07, 143.10, 142.81, 135.64, 123.40, 123.08, 119.96, 113.08, 112.21, 110.68, 110.13, 108.50, 41.84 ppm. HRMS(ESI): m/z calc. for C
16H
12N
2O
2: 264.0972, found [M + H]
+ 265.0980.
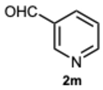
2-(Pyridin-3-yl)-1-(pyridin-3-ylmethyl)-1H-benzo[d]imidazole 3m. Beige solid. M.p. 129–130 °C. NMR spectra are consistent with those previously reported [
41].
1H NMR (600 MHz, CDCl
3): δ 8.86 (d,
J = 2.2 Hz, 1H), 8.68 (dd,
J = 4.9, 1.7 Hz, 1H), 8.51 (dd,
J = 4.8, 1.6 Hz, 1H), 8.41 (d,
J = 2.4 Hz, 1H), 7.96 (dt,
J = 7.9, 2.0 Hz, 1H), 7.85 (d,
J = 8.1 Hz, 1H), 7.38 (ddd,
J = 7.9, 4.9, 0.9 Hz, 1H), 7.31 (ddd,
J = 8.2, 7.1, 1.2 Hz, 1H), 7.28–7.23 (m, 2H), 7.23–7.17 (m, 2H), 5.45 (s, 2H).ppm.
13C NMR (151 MHz, CDCl
3): δ 150.91, 150.77, 149.47, 149.41, 147.64, 143.11, 135.66, 133.64, 131.52, 126.18, 123.91, 123.88, 123.65, 123.28, 120.34, 110.24, 46.05 ppm. HRMS(ESI): m/z calc. for C
18H
14N
4: 286.1291, found [M + H]
+ 287.1357.
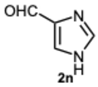
1-((1H-Imidazol-4-yl)methyl)-2-(1H-imidazol-4-yl)-1H-benzo[d]imidazole 3n. Pale yellow solid. M.p. 248–250 °C. 1H NMR (600 MHz, DMSO): δ 7.89 (dd, J = 11.3, 1.2 Hz, 2H), δ 7.89 (dd, J = 11.3, 1.2 Hz, 2H), 7.67–7.61 (m, 1H), 7.57–7.54 (m, 1H), 7.52 (d, J = 1.2 Hz, 1H), 7.19–7.12 (m, 2H), 6.94 (s, 1H), 5.87 (s, 2H) ppm.13C NMR (151 MHz, DMSO): δ 147.17, 142.76, 136.58, 135.53, 135.06, 121.56, 121.38, 121.25, 118.08, 110.91, 41.21 ppm. HRMS(ESI): m/z calc. for C14H12N6: 264.1196, found [M + H]+ 265.1224.
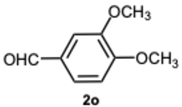
1-(3,4-Dimethoxybenzyl)-2-(3,4-dimethoxyphenyl)-1H-benzo[d]imidazole 3o. Brown solid. M.p. 131–133 °C. NMR spectra are consistent with those previously reported [
41].
1H NMR (600 MHz, CDCl
3): δ 7.86 (dt,
J = 8.0, 1.0 Hz, 1H), 7.31 (ddd,
J = 8.1, 5.8, 2.5 Hz, 1H), 7.28 (d,
J = 2.0 Hz, 1H), 7.26–7.22 (m, 3H), 6.92 (d,
J = 8.3 Hz, 1H), 6.81 (d,
J = 8.1 Hz, 1H), 6.68–6.62 (m, 2H), 5.40 (s, 2H), 3.92 (s, 3H), 3.86 (s, 3H), 3.77 (d,
J = 8.5 Hz, 6H) ppm.
13C NMR (151 MHz, CDCl
3): δ 154.14, 150.50, 149.52, 149.10, 148.58, 143.04, 136.30, 129.10, 122.93, 122.67, 122.55, 121.86, 119.71, 118.13, 112.35, 111.57, 111.03, 110.30, 109.09, 55.97, 55.95, 55.91, 55.82, 48.14 ppm. HRMS(ESI): m/z calc. for C
24H
24N
2O
4: 404.1809, found [M + H]
+ 405.1925.
2-(Pyridin-3-yl)-1H-benzo[d]imidazole 4m. Pale yellow solid. M.p. 245–247 °C. NMR spectra are consistent with those previously reported [
44].
1H NMR (600 MHz, DMSO): δ 13.10 (s, 1H), 9.35 (d,
J = 2.2 Hz, 1H), 8.68 (dd,
J = 4.8, 1.6 Hz, 1H), 8.50 (dt,
J = 8.0, 2.0 Hz, 1H), 7.71 (d,
J = 7.9 Hz, 1H), 7.63–7.55 (m, 2H), 7.29–7.19 (m, 2H) ppm.
13C NMR (151 MHz, DMSO): δ 150.49, 148.85, 147.51, 133.74, 126.15, 124.00, 122.97, 121.96, 119.07, 111.52 ppm. HRMS(ESI): m/z calc. for C
12H
9N
3: 195.0869, found [M + H]
+ 196.0923.
1-Benzyl-5-nitro-2-phenyl-1H-benzo[d]imidazole 5a. Pale yellow solid. M.p. 149–151 °C. NMR spectra are consistent with those previously reported [
44].
1H NMR (600 MHz, CDCl
3): δ 8.24 (dd,
J = 8.9, 2.2 Hz, 1H), 8.17 (d,
J = 2.2 Hz, 1H), 7.90 (d,
J = 8.9 Hz, 1H), 7.75–7.69 (m, 2H), 7.58–7.52 (m, 1H), 7.52–7.47 (m, 2H), 7.39–7.30 (m, 3H), 7.11–7.05 (m, 2H), 5.55 (s, 2H) ppm.
13C NMR (151 MHz, CDCl
3): δ 158.97, 147.74, 143.84, 135.56, 135.29, 131.04, 129.41, 129.18, 129.08, 128.49, 126.00, 120.13, 118.83, 107.57, 48.94 ppm. HRMS(ESI): m/z calc. for C
20H
15N
3O
2: 329.1164, found [M + H]
+ 330.1237.
5-Nitro-2-phenyl-1H-benzo[d]imidazole 6a. Beige solid. M.p. 205–207 °C. NMR spectra are consistent with those previously reported [
43].
1H NMR (600 MHz, DMSO): δ 13.60 (s, 1H), 8.48 (s, 1H), 8.22 (dt,
J = 8.0, 1.3 Hz, 2H), 8.14 (dt,
J = 8.7, 1.7 Hz, 1H), 7.78 (d,
J = 8.8 Hz, 1H), 7.64–7.55 (m, 3H) ppm.
13C NMR (151 MHz, DMSO): δ 142.72, 130.97, 129.16, 129.02, 126.97, 117.99 ppm. HRMS(ESI): m/z calc. for C
13H
9N
3O
2: 239.0768, found [M + H]
+ 240.0821.
2-(2-Methoxyphenyl)-5-nitro-1H-benzo[d]imidazole 6i. Light brown. M.p. 205–207 °C. NMR spectra are consistent with those previously reported [
45].
1H NMR (600 MHz, DMSO): δ 12.66 (s, 1H), 8.53 (d,
J = 2.3 Hz, 1H), 8.37 (d,
J = 7.9 Hz, 1H), 8.15–8.10 (m, 1H), 7.80 (d,
J = 8.8 Hz, 1H), 7.56 (ddd,
J = 8.7, 7.2, 1.8 Hz, 1H), 7.30 (d,
J = 8.4 Hz, 1H), 7.16 (td,
J = 7.5, 1.0 Hz, 1H), 4.06 (s, 3H) ppm.
13C NMR (151 MHz, DMSO): δ 157.32, 157.08, 142.23, 132.69, 132.44, 130.12, 121.06, 118.47, 117.85, 117.46, 114.44, 112.35, 108.69, 55.95 ppm. HRMS(ESI): m/z calc. for C
14H
11N
3O
3: 269.0800, found [M + H]
+ 270.0767.
2-(4-Chloro-3-nitrobenzylidene)amino)-5-nitroaniline 7. Light orange solid. M.p. 218–220 °C. 1H NMR (600 MHz, DMSO): δ 8.97 (s, 1H), 8.82 (d, J = 1.9 Hz, 1H), 8.34 (dd, J = 8.4, 1.9 Hz, 1H), 8.14 (d, J = 2.6 Hz, 1H), 7.98–7.92 (m, 2H), 6.96 (s, 1H), 6.79 (d, J = 9.1 Hz, 1H) ppm. 13C NMR (151 MHz, DMSO): δ 156.28, 151.48, 148.18, 136.54, 135.82, 133.84, 132.39, 132.35, 131.96, 126.86, 125.00, 124.75, 113.31 ppm. HRMS(ESI): m/z calc. for C13H9ClN4O4: 320.0385, found [M + H]+ 321.0385.
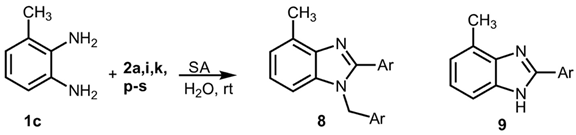
1-Benzyl-4-methyl-2-phenyl-1H-benzo[d]imidazole 8a. White solid. M.p. 169–171 °C. NMR spectra are consistent with those previously reported [
46].
1H NMR (600 MHz, CDCl
3): δ 7.72–7.65 (m, 2H), 7.49–7.42 (m, 3H), 7.35–7.26 (m, 3H), 7.18–7.04 (m, 5H), 5.41 (s, 2H), 2.78 (s, 3H) ppm.
13C NMR (151 MHz, CDCl
3): δ 153.57, 142.62, 136.65, 135.70, 130.48, 130.17, 129.83, 129.51, 129.07, 128.79, 127.77, 126.12, 123.08, 123.00, 108.09, 48.44, 16.94 ppm. HRMS(ESI): m/z calc. for C
21H
18N
2: 298.1470, found [M + H]
+ 299.1465.
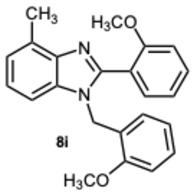
1-(2-Methoxybenzyl)-2-(2-methoxyphenyl)-4-methyl-1H-benzo[d]imidazole 8i. Light brown solid. M.p. 188–190 °C. 1H NMR (600 MHz, CDCl3): δ 7.54 (dd, J = 7.5, 1.8 Hz, 1H), 7.43 (ddd, J = 8.4, 7.4, 1.8 Hz, 1H), 7.18 (ddd, J = 8.2, 7.4, 1.7 Hz, 1H), 7.14–7.01 (m, 4H), 6.94 (dd, J = 8.4, 1.0 Hz, 1H), 6.82 (dd, J = 8.3, 1.1 Hz, 1H), 6.76 (td, J = 7.5, 1.1 Hz, 1H), 6.71–6.67 (m, 1H), 5.22 (s, 2H), 3.78 (s, 3H), 3.58 (s, 3H), 2.76 (s, 3H) ppm. 13C NMR (151 MHz, CDCl3): δ 150.49, 149.51, 149.09, 148.57, 136.29, 129.09, 122.92, 122.66, 121.85, 119.70, 118.12, 111.56, 111.02, 110.29, 109.89, 55.96, 55.94, 48.13, 21.03 ppm. HRMS(ESI): m/z calc. for C23H22N2O2: 358.1754, found [M + H]+ 359.1919.
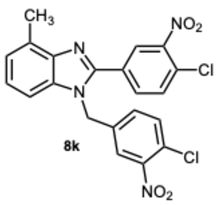
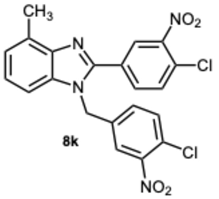
1-(4-Chloro-3-nitrobenzyl)-2-(4-chloro-3-nitrophenyl)-4-methyl-1H-benzo[d]imidazole 8k. Pale yellow solid. M.p. 166–168 °C. NMR spectra are consistent with those previously reported [
30].
1H NMR (600 MHz, CDCl
3): δ 8.39 (d,
J = 1.9 Hz, 1H), 7.94 (d,
J = 8.3 Hz, 1H), 7.79 (d,
J = 8.2 Hz, 1H), 7.73 (d,
J = 8.4 Hz, 1H), 7.57 (d,
J = 8.3 Hz, 1H), 7.33 (t,
J = 7.8 Hz, 1H), 7.28 (d,
J = 8.3 Hz, 2H), 7.20 (dd,
J = 8.4, 2.2 Hz, 1H), 7.13 (d,
J = 8.1 Hz, 1H), 5.56 (s, 2H), 2.77 (s, 3H) ppm.
13C NMR (151 MHz, CDCl
3): δ 154.13, 149.09, 148.57, 143.03, 129.09, 122.92, 122.66, 122.54, 121.85, 118.12, 111.56, 111.02, 110.29, 109.08, 48.13, 21.03 ppm. HRMS: m/z calc. for C
21H
14Cl
2N
4O
4: 456.0470, found [M + H]
+ 457.0437.
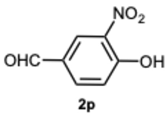
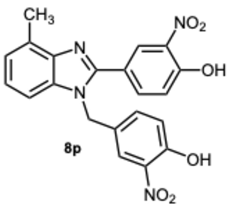
4-(1-(4-Hydroxy-3-nitrobenzyl)-4-methyl-1H-benzo[d]imidazol-2-yl)-2-nitrophenol 8p. Yellow solid. M.p. 195–197 °C. NMR spectra are consistent with those previously reported [
30].
1H NMR (600 MHz, CDCl
3): δ 8.41 (s, 1H), 8.01 (dd,
J = 8.7, 2.2 Hz, 1H), 7.94–7.91 (m, 1H), 7.34 (d,
J = 4.6 Hz, 1H), 7.28 (d,
J = 7.6 Hz, 1H), 7.22 (dd,
J = 6.3, 1.0 Hz, 1H), 7.14 (d,
J = 8.0 Hz, 2H), 7.08 (dt,
J = 9.4, 0.9 Hz, 1H), 5.45 (s, 2H), 2.79 (s, 3H) ppm.
13C NMR (151 MHz, CDCl
3): δ 157.77, 132.55, 131.53, 128.52, 127.92, 122.59, 122.08, 120.94, 120.55, 119.97, 110.98, 110.89, 110.08, 43.66, 29.85 ppm. HRMS(ESI): m/z calc. for C
21H
16N
4O
6: 420.1070, found [M + H]
+ 421.1059.
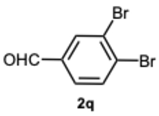
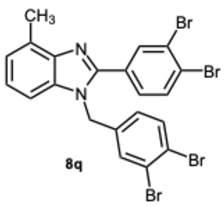
1-(3,4-Dibromobenzyl)-2-(3,4-dibromophenyl)-4-methyl-1H-benzo[d]imidazole 8q. Pale yellow solid. M.p. 152–154 °C. 1H NMR (600 MHz, CDCl3): δ 7.95 (d, J = 2.1 Hz, 1H), 7.70 (d, J = 8.3 Hz, 1H), 7.56 (d, J = 8.3 Hz, 1H), 7.38 (d, J = 2.1 Hz, 1H), 7.35 (dd, J = 8.2, 2.1 Hz, 1H), 7.21 (t, J = 7.6 Hz, 1H), 7.15 (d, J = 7.2 Hz, 1H), 7.04 (d, J = 8.0 Hz, 1H), 6.82 (dd, J = 8.3, 2.1 Hz, 1H), 5.31 (s, 2H), 2.74 (s, 3H) ppm. 13C NMR (151 MHz, CDCl3): δ 151.42, 143.06, 136.95, 135.89, 134.49, 134.30, 134.06, 131.20, 130.36, 128.56, 127.17, 126.00, 125.90, 125.70, 124.64, 124.03, 123.44, 120.51, 110.16, 47.39, 22.28 ppm. HRMS(ESI): m/z calc. for C21H14Br4N2 [M + H]+: 609.7963, found 610.7980.
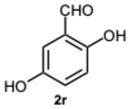
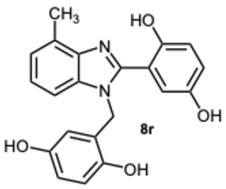
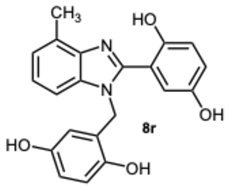
2-(1-(2,5-Dihydroxybenzyl)-4-methyl-1H-benzo[d]imidazol-2-yl)benzene-1,4-diol 8r. Beige solid. M.p. 195–198 °C. 1HNMR (600 MHz, DMSO): δ 10.56 (s, 1H), 9.11 (s, 1H), 8.99 (s, 1H), 8.52 (s, 1H), 7.14–7.08 (m, 2H), 7.08–7.04 (m, 1H), 6.88–6.83 (m, 2H), 6.79 (dd, J = 8.6, 2.9 Hz, 1H), 6.65 (d, J = 8.6 Hz, 1H), 6.43 (dd, J = 8.6, 2.9 Hz, 1H), 5.79 (d, J = 2.9 Hz, 1H), 5.32 (s, 2H), 2.58 (s, 3H) ppm. 13C NMR (151 MHz, DMSO): δ 148.28, 141.86, 140.30, 137.02, 133.03, 131.10, 127.12, 122.85, 112.52, 109.66, 46.26, 24.62 ppm. HRMS: m/z calc. for C21H18N2O4: 362.1339, found [M − H]+ 361.1195.
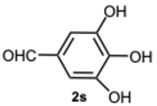
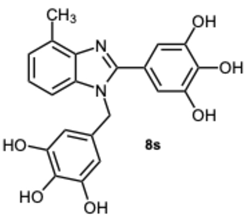
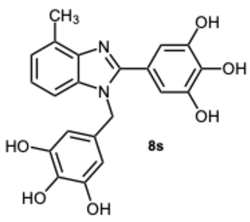
5-(4-Methyl-1-(3,4,5-trihydroxybenzyl)-1H-benzo[d]imidazol-2-yl)benzene-1,2,3-triol 8s. Pale yellow solid. M.p. 200–202 °C. 1H NMR (600 MHz, DMSO): δ 7.04–6.98 (m, 3H), 6.72 (s, 2H), 5.98 (s, 2H), 5.27 (s, 2H), 2.56 (s, 3H) ppm. 13C NMR (151 MHz, DMSO): δ 153.52, 146.73, 146.61, 146.45, 140.69, 135.94, 135.69, 135.45, 128.63, 127.94, 127.54, 121.19, 109.24, 109.00, 108.75, 105.32, 60.23, 21.23 ppm. HRMS(ESI): m/z calc. for C21H18N2O6 : 394.1165, found [M + H]+ 395.1206.
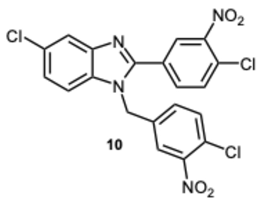
5-Chloro-1-(4-chloro-3-nitrobenzyl)-2-(4-chloro-3-nitrophenyl)-1H-benzo[d]imidazole 10 [30]. Yellow solid. M.p. 183–185 °C.
1H NMR (600 MHz, DMSO): δ 8.83 (d,
J = 2.1 Hz, 1H), 8.45 (dd,
J = 8.5, 2.1 Hz, 1H), 8.05–7.96 (m, 1H), 7.85–7.79 (m, 3H), 7.73 (d,
J = 2.1 Hz, 1H), 7.68 (dd,
J = 8.5, 6.5 Hz, 1H), 7.30 (dd,
J = 8.6, 2.0 Hz, 1H), 5.75 (s, 2H) ppm.
13C NMR (151 MHz, DMSO): δ 148.72, 147.52, 140.34, 140.31, 133.19, 131.87, 130.41, 129.95, 126.60, 124.02, 119.62, 111.64, 108.25, 105.46 ppm. HRMS(ESI): m/z calc. for C
20H
12Cl
3N
4O
4 [M + H]
+: 476.9924, found: 476.9922.