TAIII Suppresses the Growth of T790M-Mutant Non-Small-Cell Lung Cancer by Targeting the EGFR/ERK Signaling Pathway
Abstract
1. Introduction
2. Results
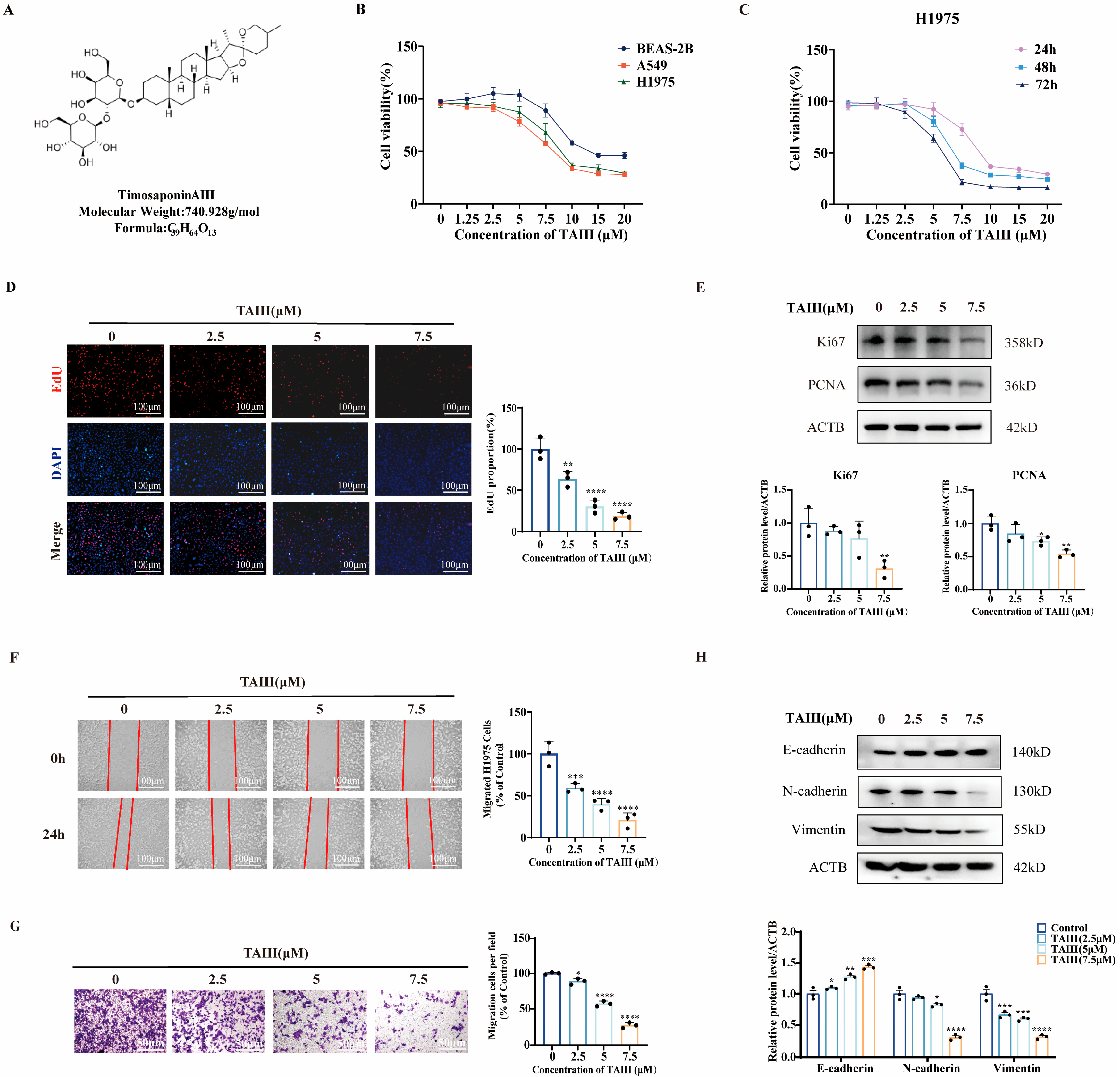
2.1. TAIII Suppresses the Viability, Proliferation, and Migration of T790M-Mutant H1975 Cells
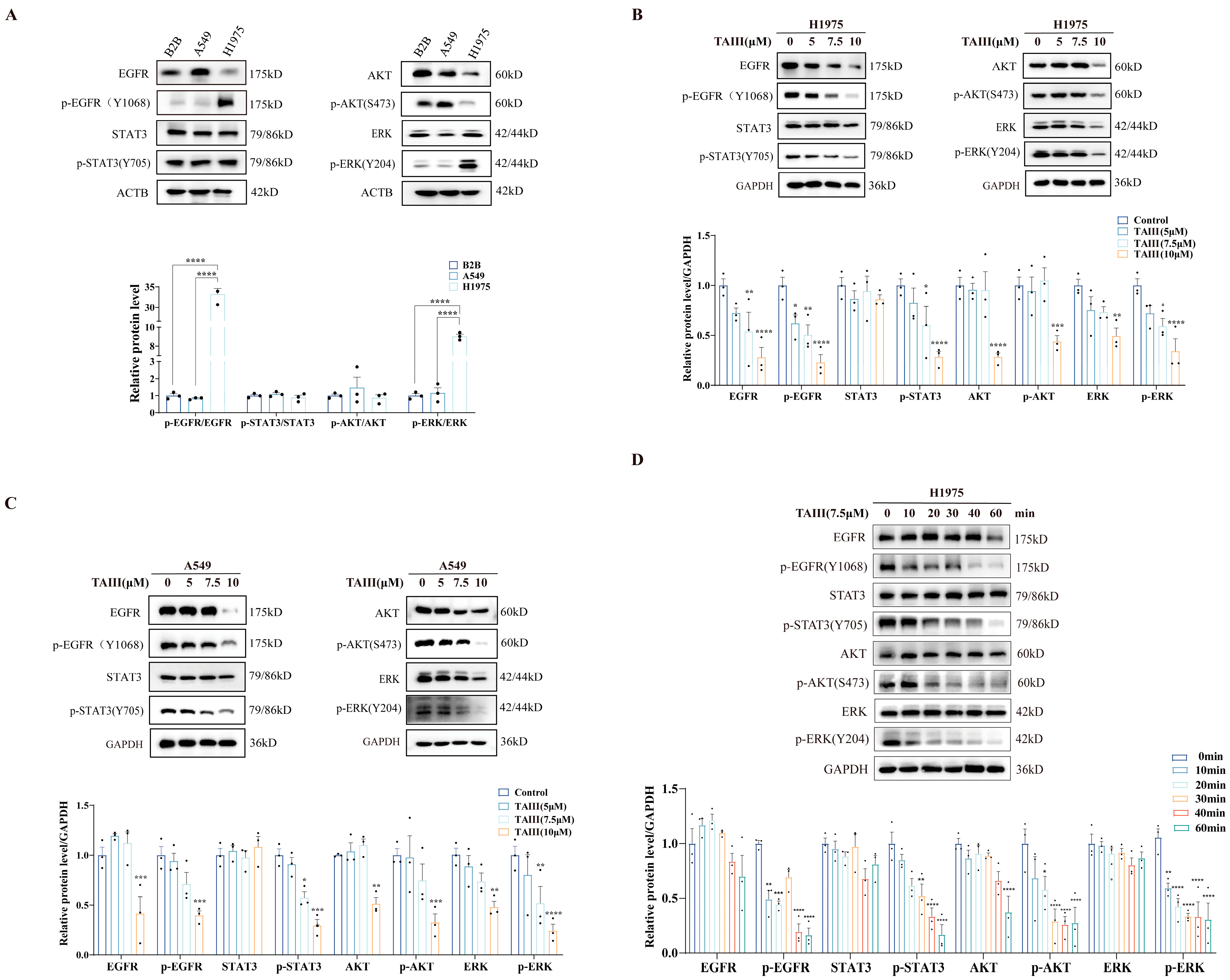
2.2. TAIII Potently Suppresses EGFR Phosphorylation and Its Downstream Signaling Cascades in T790M-Mutant H1975 Cells
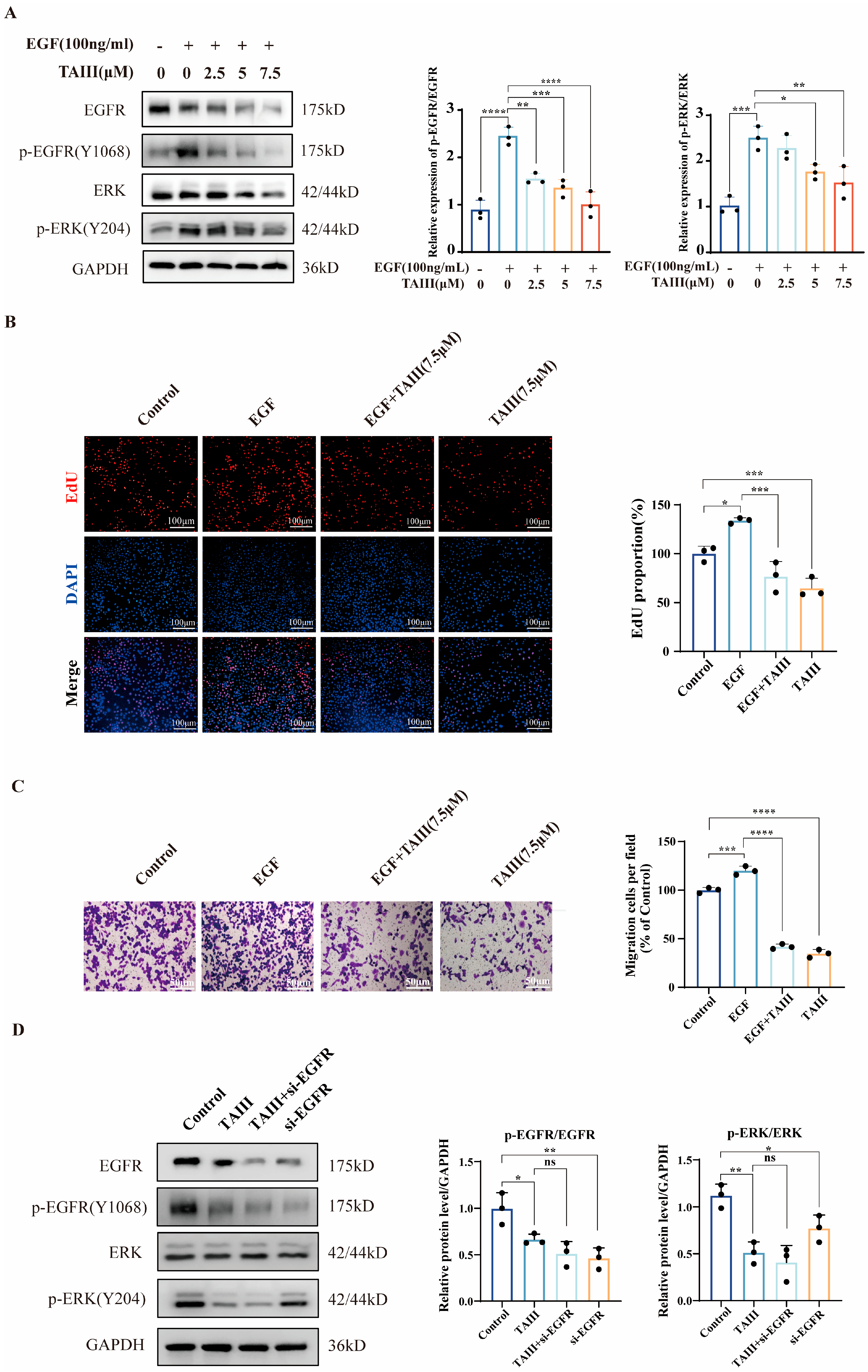
2.3. TAIII Acts as an EGFR Inhibitor
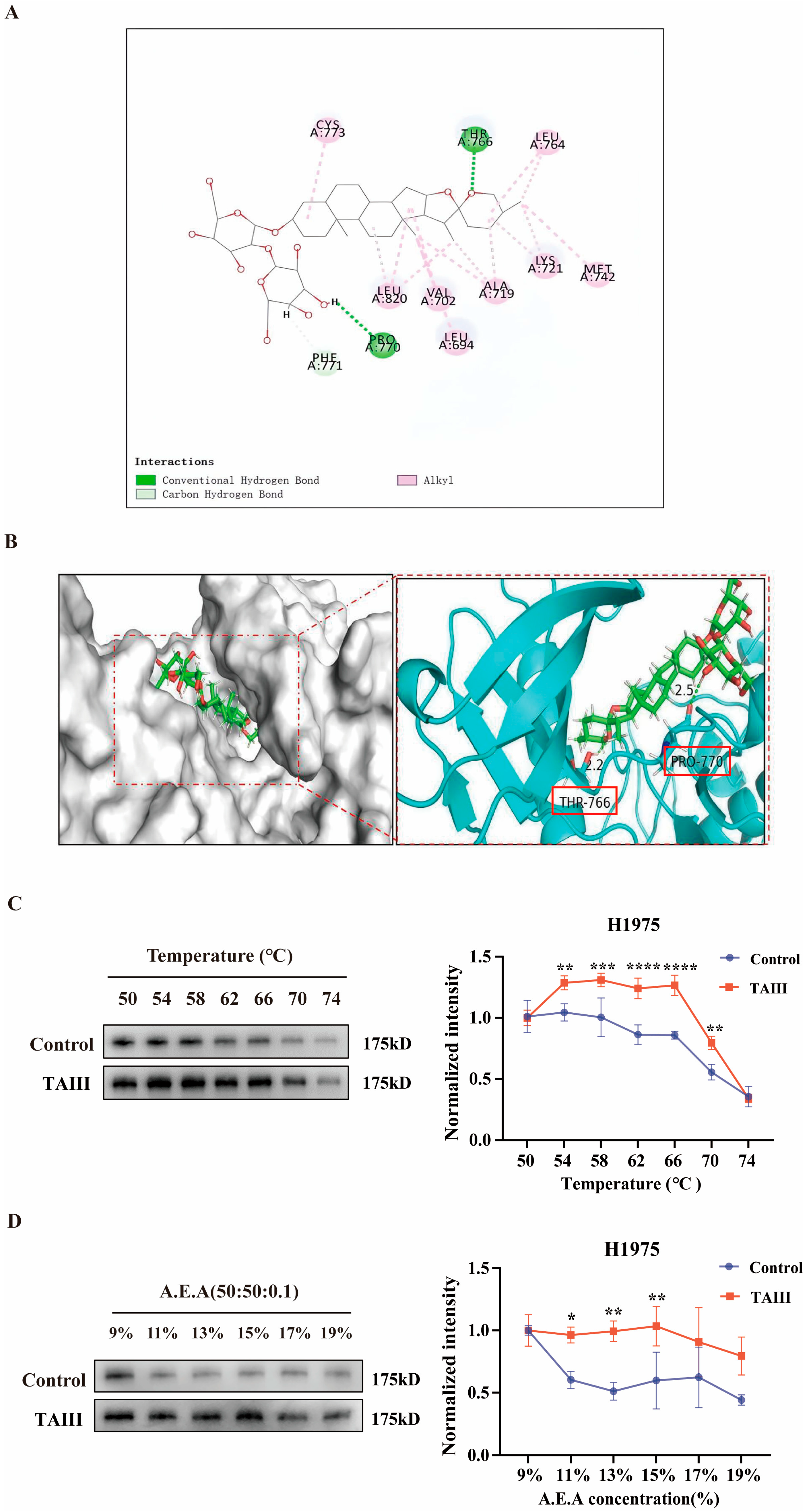
2.4. Molecular Interaction and Binding Stability of TAIII with EGFR
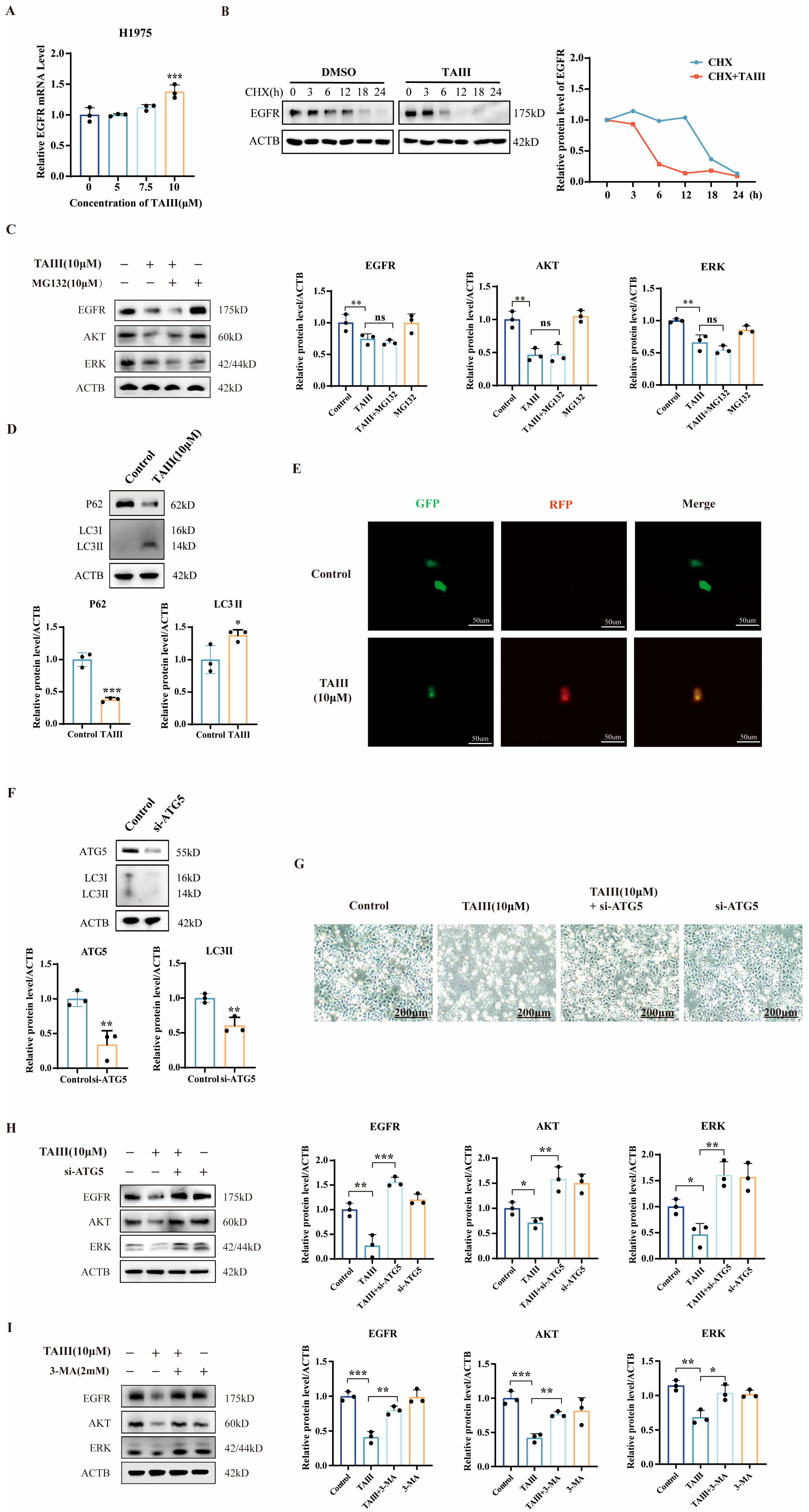
2.5. Mechanism of EGFR Degradation by High Concentration of TAIII
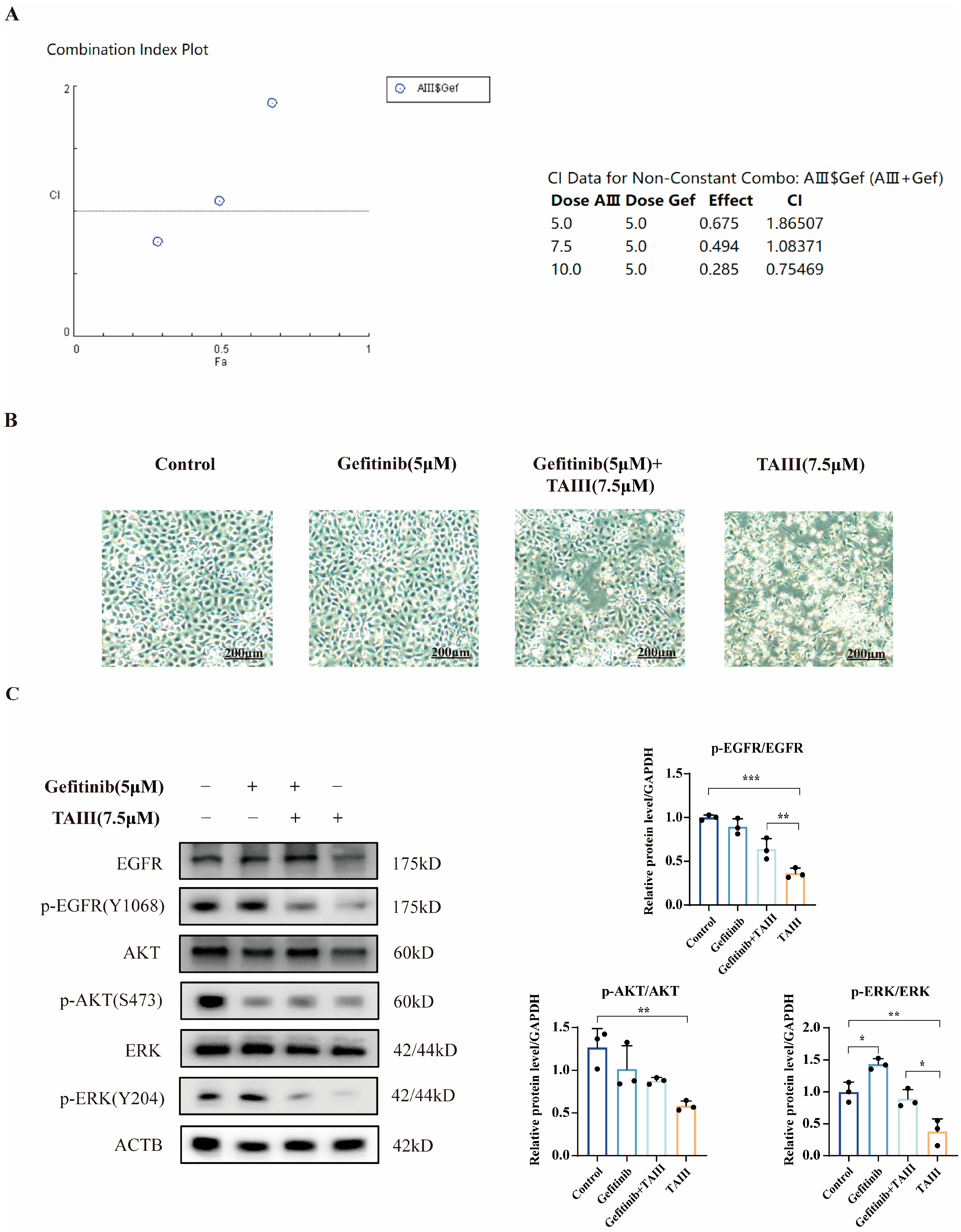
2.6. Combination Treatment of TAIII and Gefitinib
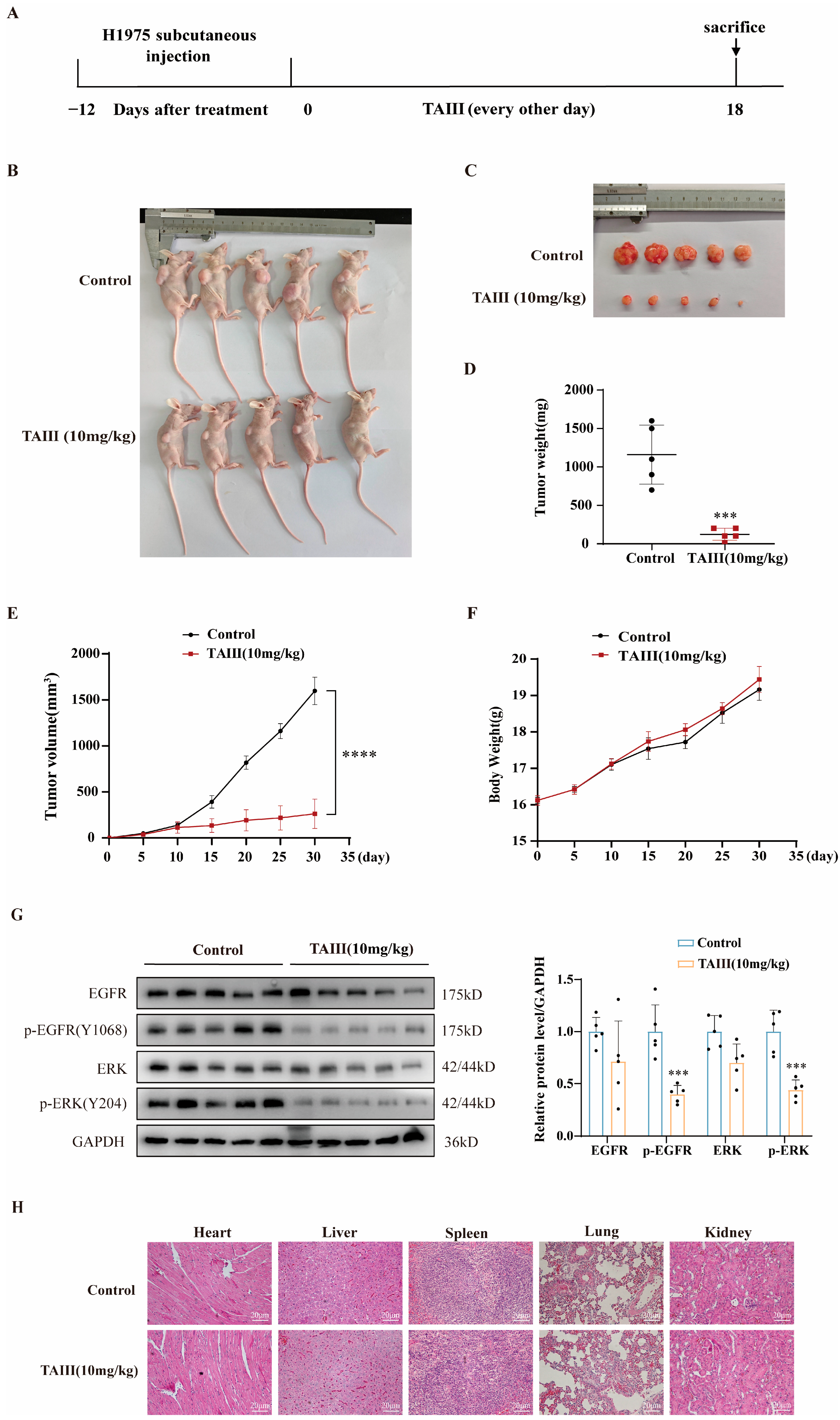
2.7. In Vivo Efficacy of TAIII Against H1975 Xenografts
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Chemicals and Reagents
4.3. CCK8 Assay
4.4. EdU Staining Assay
4.5. Transwell Migration Assay
4.6. Scratch Assay
4.7. Molecular Docking
4.8. Cellular Thermal Shift Assay (CETSA)
4.9. Solvent-Induced Protein Precipitation (SIP)
4.10. Quantitative Reverse Transcription-PCR (qRT-PCR)
4.11. Transfection
4.12. GFP-RFP-LC3 Dual Fluorescent Labeling Assay
4.13. Western Blotting
4.14. H&E Staining
4.15. Tumor Xenograft
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TAIII | Timosaponin AIII |
| NSCLC | Non-small-cell lung cancer |
| EGFR | Epidermal growth factor receptor |
| EGF | Epidermal growth factor |
| ATG5 | Autophagy-related gene 5 |
| DMSO | Dimethyl sulfoxide |
| PCNA | Proliferating cell nuclear antigen |
| Ki67 | Marker of proliferation |
| Bcl2 | B-cell lymphoma-2 |
| ERK | Extracellular signal-regulated kinase |
| AKT | Protein Kinase B |
| CCK8 | Cell Counting Kit8 |
| BCA | Bicinchoninic Acid |
| PCR | Polymerase Chain Reaction |
| RT-PCR | Reverse Transcription-Polymerase Chain Reaction |
| WB | Western blot |
| FBS | Fetal bovine serum |
| PBS | Phosphate buffer saline |
| APS | Ammonium acid |
| DAPI | 4′,6-Diamidino-2′-phenylindole |
| CQ | Chloroquine |
| EdU | 5-Ethynyl-2′-deoxyuridine |
| ECL | Enhanced chemiluminescence |
| EDTA | Ethylene diamine tetraacetic acid |
| DEPC | Diethyl pyrocarbonate |
| CHX | Cycloheximide |
| MG132 | Z-Leu-Leu-Leu-al |
| ACTB | Beta-actin |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
References
- Zhang, S.; Sun, K.; Zheng, R.; Zeng, H.; Wang, S.; Chen, R.; Wei, W.; He, J. Cancer incidence and mortality in China, 2015. J. Natl. Cancer Cent. 2021, 1, 2–11. [Google Scholar] [CrossRef]
- Chen, P.; Liu, Y.; Wen, Y.; Zhou, C. Non-small cell lung cancer in China. Cancer Commun. 2022, 42, 937–970. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): A multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022, 399, 1607–1617. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Vicente, D.; Tafreshi, A.; Robinson, A.; Parra, H.S.; Mazières, J.; Hermes, B.; Cicin, I.; Medgyasszay, B.; Rodríguez-Cid, J.; et al. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients with Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407. J. Thorac. Oncol. 2020, 15, 1657–1669. [Google Scholar] [CrossRef]
- Levy, A.; Adebahr, S.; Hurkmans, C.; Ahmed, M.; Ahmad, S.; Guckenberger, M.; Geets, X.; Lievens, Y.; Lambrecht, M.; Pourel, N.; et al. Stereotactic Body Radiotherapy for Centrally Located Inoperable Early-Stage NSCLC: EORTC 22113-08113 LungTech Phase II Trial Results. J. Thorac. Oncol. 2024, 19, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Jänne, P.A.; Riely, G.J.; Gadgeel, S.M.; Heist, R.S.; Ou, S.-H.I.; Pacheco, J.M.; Johnson, M.L.; Sabari, J.K.; Leventakos, K.; Yau, E.; et al. Adagrasib in Non-Small-Cell Lung Cancer Harboring a KRAS(G12C) Mutation. N. Engl. J. Med. 2022, 387, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Passaro, A.; Jänne, P.A.; Mok, T.; Peters, S. Overcoming therapy resistance in EGFR-mutant lung cancer. Nat. Cancer 2021, 2, 377–391. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, X.; Wang, Y.; Zheng, D.; Meng, Q.; Jiang, L.; Yang, S.; Zhang, S.; Zhang, X.; Liu, Y.; et al. Mechanisms of resistance to targeted therapy and immunotherapy in non-small cell lung cancer: Promising strategies to overcoming challenges. Front. Immunol. 2024, 15, 1366260. [Google Scholar] [CrossRef]
- Jiang, Z.; Gu, Z.; Yu, X.; Cheng, T.; Liu, B. Research progress on the role of bypass activation mechanisms in resistance to tyrosine kinase inhibitors in non-small cell lung cancer. Front. Oncol. 2024, 14, 1447678. [Google Scholar] [CrossRef]
- Murphrey, M.B.; Quaim, L.; Rahimi, N.; Varacallo, M.A. Biochemistry, Epidermal Growth Factor Receptor; StatPearls LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Levantini, E.; Maroni, G.; Del Re, M.; Tenen, D.G. EGFR signaling pathway as therapeutic target in human cancers. Semin. Cancer Biol. 2022, 85, 253–275. [Google Scholar] [CrossRef]
- Ayoup, M.S.; Shawki, I.; Abdel-Hamid, H.; Ghareeb, D.A.; Masoud, A.; Harras, M.F.; El-Atawy, M.; Alharbi, N.S.; Ismail, M.M.F. Targeting EGFR/PI3K/AKT/mTOR signaling in lung and colon cancers: Synthesis, antitumor evaluation of new 1,2,4-oxdiazoles tethered 1,2,3-triazoles. RSC Adv. 2024, 14, 16713–16726. [Google Scholar] [CrossRef]
- Chen, C.; Zheng, X.; Wang, C.; Zhou, H.; Zhang, Y.; Ye, T.; Yang, Y. CTHRC1 Attenuates Tendinopathy via Enhancing EGFR/MAPK Signaling Pathway. Adv. Sci. 2024, 11, e2406611. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Wu, J.; Liu, L.; Tian, Y.; Buettner, R.; Hsieh, M.-Y.; Horne, D.; Dellinger, T.H.; Han, E.S.; Jove, R.; et al. Synergistic anti-tumor effect of combined inhibition of EGFR and JAK/STAT3 pathways in human ovarian cancer. Mol. Cancer 2015, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sadhukhan, S.; Sonawane, A. 20 years since the approval of first EGFR-TKI, gefitinib: Insight and foresight. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188967. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Schuler, M.; Popat, S.; Miura, S.; Heeke, S.; Park, K.; Märten, A.; Kim, E.S. Afatinib for the Treatment of NSCLC Harboring Uncommon EGFR Mutations: A Database of 693 Cases. J. Thorac. Oncol. 2020, 15, 803–815. [Google Scholar] [CrossRef]
- Chen, R.L.; Sun, L.L.; Cao, Y.; Chen, H.R.; Zhou, J.X.; Gu, C.Y.; Zhang, Y.; Wang, S.Y.; Hou, W.; Lin, L.Z. Adjuvant EGFR-TKIs for Patients with Resected EGFR-Mutant Non-Small Cell Lung Cancer: A Meta-Analysis of 1,283 Patients. Front. Oncol. 2021, 11, 629394. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Syn, N.L.; Cho, B.C.; Soo, R.A. Acquired resistance to EGFR targeted therapy in non-small cell lung cancer: Mechanisms and therapeutic strategies. Cancer Treat. Rev. 2018, 65, 1–10. [Google Scholar] [CrossRef]
- Passaro, A.; Guerini-Rocco, E.; Pochesci, A.; Vacirca, D.; Spitaleri, G.; Catania, C.M.; Rappa, A.; Barberis, M.; de Marinis, F. Targeting EGFR T790M mutation in NSCLC: From biology to evaluation and treatment. Pharmacol. Res. 2017, 117, 406–415. [Google Scholar] [CrossRef]
- Liao, W.L.; Lin, H.; Li, Y.H.; Yang, T.Y.; Chen, M.C. RAGE potentiates EGFR signaling and interferes with the anticancer effect of gefitinib on NSCLC cells. Am. J. Physiol. Cell Physiol. 2023, 325, C1313–C1325. [Google Scholar]
- Chen, N.; Tyler, L.C.; Le, A.T.; Welsh, E.A.; Fang, B.; Elliott, A.; Davies, K.D.; Danhorn, T.; Riely, G.J.; Ladanyi, M.; et al. MIG6 Mediates Adaptive and Acquired Resistance to ALK/ROS1 Fusion Kinase Inhibition through EGFR Bypass Signaling. Mol. Cancer Ther. 2024, 23, 92–105. [Google Scholar] [CrossRef]
- Xue, Y.; Hou, S.; Ji, H.; Han, X. Evolution from genetics to phenotype: Reinterpretation of NSCLC plasticity, heterogeneity, and drug resistance. Protein Cell 2017, 8, 178–190. [Google Scholar]
- Yun, C.H.; Mengwasser, K.E.; Toms, A.V.; Woo, M.S.; Greulich, H.; Wong, K.K.; Meyerson, M.; Eck, M.J. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc. Natl. Acad. Sci. USA 2008, 105, 2070–2075. [Google Scholar] [CrossRef]
- Rivera-Concepcion, J.; Uprety, D.; Adjei, A.A. Challenges in the Use of Targeted Therapies in Non-Small Cell Lung Cancer. Cancer Res. Treat. 2022, 54, 315–329. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, L.; Chen, Z.; Fan, Y.; Zhou, Y.; Yuan, Z.; Zhang, W. Current treatments for non-small cell lung cancer. Front. Oncol. 2022, 12, 945102. [Google Scholar] [CrossRef]
- Song, X.; Cao, L.; Ni, B.; Wang, J.; Qin, X.; Sun, X.; Xu, B.; Wang, X.; Li, J. Challenges of EGFR-TKIs in NSCLC and the potential role of herbs and active compounds: From mechanism to clinical practice. Front. Pharmacol. 2023, 14, 1090500. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Zhao, B.; Kim, J.; Sui, G.; Shi, J. Small Molecule Compounds of Natural Origin Target Cellular Receptors to Inhibit Cancer Development and Progression. Int. J. Mol. Sci. 2022, 23, 2672. [Google Scholar] [CrossRef]
- Wang, Y.; Dan, Y.; Yang, D.; Hu, Y.; Zhang, L.; Zhang, C.; Zhu, H.; Cui, Z.; Li, M.; Liu, Y. The genus Anemarrhena Bunge: A review on ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2014, 153, 42–60. [Google Scholar] [CrossRef]
- Lin, Y.; Zhao, W.R.; Shi, W.T.; Zhang, J.; Zhang, K.Y.; Ding, Q.; Chen, X.L.; Tang, J.Y.; Zhou, Z.Y. Pharmacological Activity, Pharmacokinetics, and Toxicity of Timosaponin AIII, a Natural Product Isolated from Anemarrhena asphodeloides Bunge: A Review. Front. Pharmacol. 2020, 11, 764. [Google Scholar] [CrossRef]
- Tu, H.; Zhou, X.; Zhou, H.; Luo, Z.; Yan, Y.; Luo, Z.; Qi, Q. Anti-tumor effect and mechanisms of Timosaponin AIII across diverse cancer progression. Biochem. Pharmacol. 2024, 228, 116080. [Google Scholar] [CrossRef]
- Jung, O.; Lee, J.; Lee, Y.J.; Yun, J.-M.; Son, Y.-J.; Cho, J.Y.; Ryou, C.; Lee, S.Y. Timosaponin AIII inhibits migration and invasion of A549 human non-small-cell lung cancer cells via attenuations of MMP-2 and MMP-9 by inhibitions of ERK1/2, Src/FAK and β-catenin signaling pathways. Bioorg Med. Chem. Lett. 2016, 26, 3963–3967. [Google Scholar] [CrossRef]
- Liu, J.; Deng, X.; Sun, X.; Dong, J.; Huang, J. Inhibition of autophagy enhances timosaponin AIII-induced lung cancer cell apoptosis and anti-tumor effect in vitro and in vivo. Life Sci. 2020, 257, 118040. [Google Scholar] [CrossRef]
- Zhou, C.; Yu, T.; Zhu, R.; Lu, J.; Ouyang, X.; Zhang, Z.; Chen, Q.; Li, J.; Cui, J.; Jiang, F.; et al. Timosaponin AIII promotes non-small-cell lung cancer ferroptosis through targeting and facilitating HSP90 mediated GPX4 ubiquitination and degradation. Int. J. Biol. Sci. 2023, 19, 1471–1489. [Google Scholar] [CrossRef]
- Hafiz, M.Z.; Pan, J.; Gao, Z.; Huo, Y.; Wang, H.; Liu, W.; Yang, J. Timosaponin AIII induces drug-metabolizing enzymes by activating constitutive androstane receptor (CAR) via dephosphorylation of the EGFR signaling pathway. J. Biomed. Res. 2024, 38, 382–396. [Google Scholar] [CrossRef]
- Santos, E.D.S.; Nogueira, K.A.B.; Fernandes, L.C.C.; Martins, J.R.P.; Reis, A.V.F.; de Brito Vieira Neto, J.; da Silva Júnior, I.J.; Pessoa, C.; Petrilli, R.; Eloy, J.O. EGFR targeting for cancer therapy: Pharmacology and immunoconjugates with drugs and nanoparticles. Int. J. Pharm. 2021, 592, 120082. [Google Scholar] [CrossRef]
- Dhillon, S. Gefitinib: A review of its use in adults with advanced non-small cell lung cancer. Target. Oncol. 2015, 10, 153–170. [Google Scholar] [CrossRef]
- Chen, L.Y.; Molina-Vila, M.A.; Ruan, S.Y.; Su, K.Y.; Liao, W.Y.; Yu, K.L.; Ho, C.C.; Shih, J.Y.; Yu, C.J.; Yang, J.C.H.; et al. Coexistence of EGFR T790M mutation and common activating mutations in pretreatment non-small cell lung cancer: A systematic review and meta-analysis. Lung Cancer 2016, 94, 46–53. [Google Scholar] [CrossRef]
- Lu, S.; Shih, J.Y.; Jang, T.W.; Liam, C.K.; Yu, Y. Afatinib as First-Line Treatment in Asian Patients with EGFR Mutation-Positive NSCLC: A Narrative Review of Real-World Evidence. Adv. Ther. 2021, 38, 2038–2053. [Google Scholar] [CrossRef]
- Cheng, Z.; Cui, H.; Wang, Y.; Yang, J.; Lin, C. The advance of the third-generation EGFR-TKI in the treatment of non-small cell lung cancer (Review). Oncol. Rep. 2024, 51, 16. [Google Scholar] [CrossRef]
- Lin, Y.T.; Chen, J.S.; Liao, W.Y.; Ho, C.C.; Hsu, C.L.; Yang, C.Y.; Chen, K.Y.; Lee, J.H.; Lin, Z.Z.; Shih, J.Y.; et al. Clinical outcomes and secondary epidermal growth factor receptor (EGFR) T790M mutation among first-line gefitinib, erlotinib and afatinib-treated non-small cell lung cancer patients with activating EGFR mutations. Int. J. Cancer 2019, 144, 2887–2896. [Google Scholar] [CrossRef]
- Park, J.; McDonald, J.J.; Petter, R.C.; Houk, K.N. Molecular Dynamics Analysis of Binding of Kinase Inhibitors to WT EGFR and the T790M Mutant. J. Chem. Theory Comput. 2016, 12, 2066–2078. [Google Scholar] [CrossRef]
- Godin-Heymann, N.; Ulkus, L.; Brannigan, B.W.; McDermott, U.; Lamb, J.; Maheswaran, S.; Settleman, J.; Haber, D.A. The T790M “gatekeeper” mutation in EGFR mediates resistance to low concentrations of an irreversible EGFR inhibitor. Mol. Cancer Ther. 2008, 7, 874–879. [Google Scholar] [CrossRef]
- Ciardiello, F.; Hirsch, F.R.; Pirker, R.; Felip, E.; Valencia, C.; Smit, E.F. The role of anti-EGFR therapies in EGFR-TKI-resistant advanced non-small cell lung cancer. Cancer Treat. Rev. 2024, 122, 102664. [Google Scholar] [CrossRef]
- Song, Z.; Shi, Y.; Han, Q.; Dai, G. Endothelial growth factor receptor-targeted and reactive oxygen species-responsive lung cancer therapy by docetaxel and resveratrol encapsulated lipid-polymer hybrid nanoparticles. Biomed. Pharmacother. 2018, 105, 18–26. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Li, W.; Shi, Z.; Liu, Z.; Zhou, J.; Tang, J.; Ren, Z.; Qiao, Y.; Liu, D. Mechanism of luteolin against non-small-cell lung cancer: A study based on network pharmacology, molecular docking, molecular dynamics simulation, and in vitro experiments. Front. Oncol. 2024, 14, 1471109. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, J.; Zou, H.; Zhang, J.; Zhang, T. In vitro and in silico evaluation of EGFR targeting activities of curcumin and its derivatives. Food Funct. 2021, 12, 10667–10675. [Google Scholar] [CrossRef]
- Lin, Y.J.; Liang, W.M.; Chen, C.J.; Tsang, H.; Chiou, J.S.; Liu, X.; Cheng, C.F.; Lin, T.H.; Liao, C.C.; Huang, S.M.; et al. Network analysis and mechanisms of action of Chinese herb-related natural compounds in lung cancer cells. Phytomedicine 2019, 58, 152893. [Google Scholar] [CrossRef]
- Li, L.B.; Yang, L.X.; Liu, L.; Liu, F.R.; Li, A.H.; Zhu, Y.L.; Wen, H.; Xue, X.; Tian, Z.X.; Sun, H.; et al. Targeted inhibition of the HNF1A/SHH axis by triptolide overcomes paclitaxel resistance in non-small cell lung cancer. Acta Pharmacol. Sin. 2024, 45, 1060–1076. [Google Scholar] [CrossRef]
- Sim, E.H.; Yang, I.A.; Wood-Baker, R.; Bowman, R.V.; Fong, K.M. Gefitinib for advanced non-small cell lung cancer. Cochrane Database Syst. Rev. 2018, 1, Cd006847. [Google Scholar] [CrossRef]
- Imyanitov, E.N.; Iyevleva, A.G.; Levchenko, E.V. Molecular testing and targeted therapy for non-small cell lung cancer: Current status and perspectives. Crit. Rev. Oncol. Hematol. 2021, 157, 103194. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhang, Y.; Chen, R.; Li, L.; Huang, C.; Zhou, Z.; Cao, Y.; Li, H.; Li, J.; Zhang, Y.; et al. A novel regulatory axis of MSI2-AGO2/miR-30a-3p-CGRRF1 drives cancer chemoresistance by upregulating the KRAS/ERK pathway. Neoplasia 2025, 59, 101082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, S.; Slimani, S.; Lv, Y.; Nie, Q.; Cao, Z.; Yang, S.; Bao, H.; Wang, C.; Chen, X.; et al. Solid-state screening and dry powder inhaler development of ziyuglycoside II sodium salt for enhanced bioavailability. Int. J. Pharm. 2025, 681, 125868. [Google Scholar] [CrossRef]
- Liu, Y.; Pu, Y.; Zhang, T.; Ding, Y.; Wang, B.; Cai, Z. Rapid and Sensitive Determination of Timosaponin AIII in Rat Plasma by LC-MS/MS and Its Pharmacokinetic Application. Int. J. Mol. Sci. 2013, 14, 3656–3670. [Google Scholar] [CrossRef]
- Lu, L.; Ding, Y.; Zhang, Y.; Ho, R.J.; Zhao, Y.; Zhang, T.; Guo, C. Antibody-modified liposomes for tumor-targeting delivery of timosaponin AIII. Int. J. Nanomedicine 2018, 13, 1927–1944. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Luan, Y.; Yu, X.; Wang, L.; Huang, X.; Yang, J.; Liu, W. TAIII Suppresses the Growth of T790M-Mutant Non-Small-Cell Lung Cancer by Targeting the EGFR/ERK Signaling Pathway. Pharmaceuticals 2025, 18, 1431. https://doi.org/10.3390/ph18101431
Gao S, Luan Y, Yu X, Wang L, Huang X, Yang J, Liu W. TAIII Suppresses the Growth of T790M-Mutant Non-Small-Cell Lung Cancer by Targeting the EGFR/ERK Signaling Pathway. Pharmaceuticals. 2025; 18(10):1431. https://doi.org/10.3390/ph18101431
Chicago/Turabian StyleGao, Shang, Ying Luan, Xinhao Yu, Ludan Wang, Xuefeng Huang, Jian Yang, and Wei Liu. 2025. "TAIII Suppresses the Growth of T790M-Mutant Non-Small-Cell Lung Cancer by Targeting the EGFR/ERK Signaling Pathway" Pharmaceuticals 18, no. 10: 1431. https://doi.org/10.3390/ph18101431
APA StyleGao, S., Luan, Y., Yu, X., Wang, L., Huang, X., Yang, J., & Liu, W. (2025). TAIII Suppresses the Growth of T790M-Mutant Non-Small-Cell Lung Cancer by Targeting the EGFR/ERK Signaling Pathway. Pharmaceuticals, 18(10), 1431. https://doi.org/10.3390/ph18101431




