Silver N-Heterocyclic Carbene (NHC) Complexes as Antimicrobial and/or Anticancer Agents
Abstract
1. Introduction
2. Ag–NHC Complexes as Antimicrobials
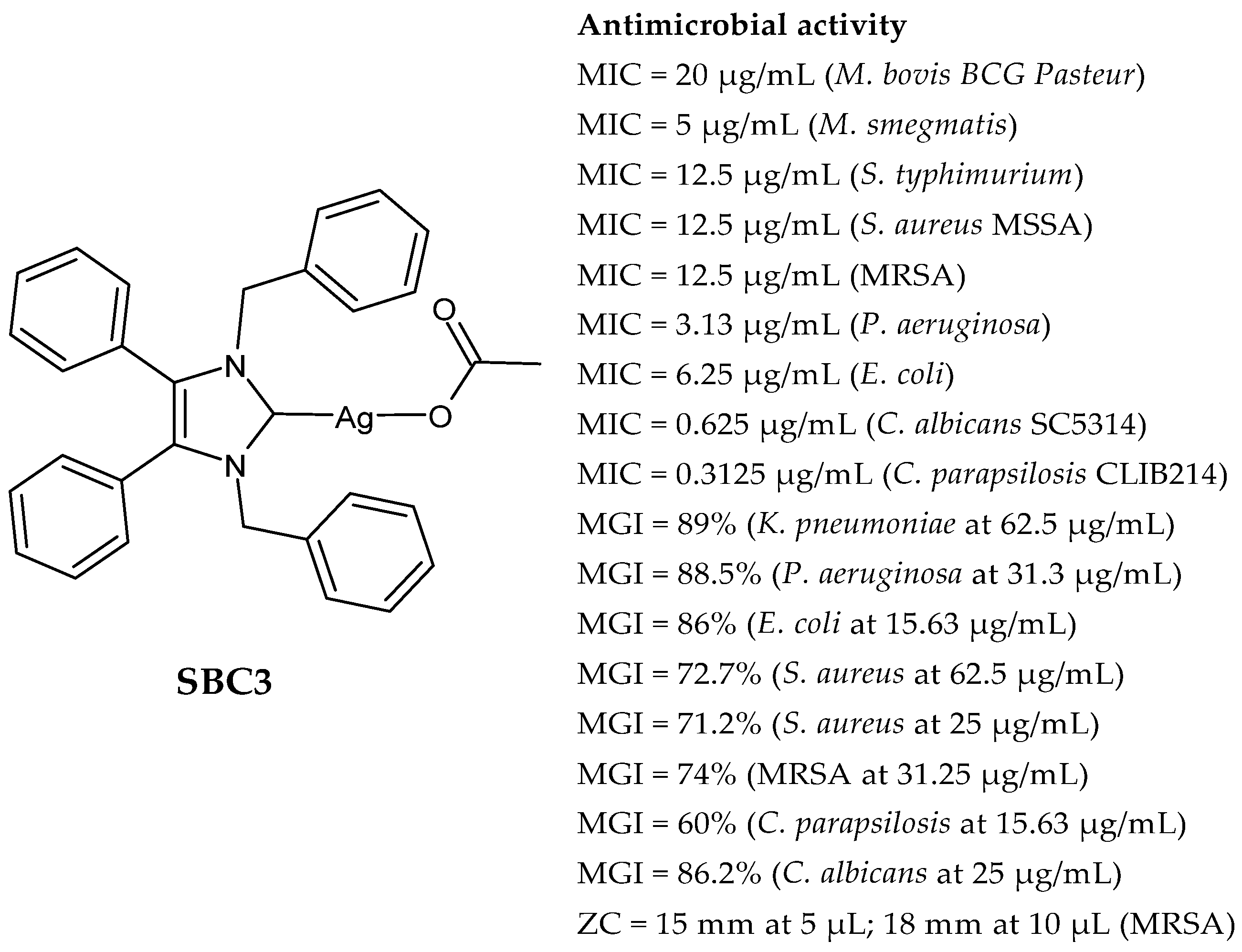
2.1. SBC3 as an Antimicrobial Agent
2.2. Other Ag–NHC Complexes as Antimicrobial Agents
| Compd | Antibacterial Activity | Ref | |
|---|---|---|---|
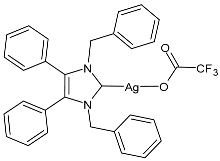 | 1 | MGI = 85% (K. pneumoniae at 15.63 μg/mL) MGI not given (P. aeruginosa) MGI = 86% (E. coli at 7.8 μg/mL) MGI = 73% (S. aureus at 15.63 μg/mL) MGI = 79% (MRSA at 31.25 μg/mL) MGI = 77% (C. parapsilosis at 7.8 μg/mL) MGI = No inhibition (C. albicans) | [72] |
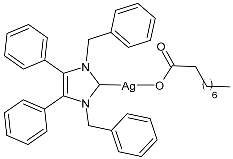 | 2 | MGI = 87% (K. pneumoniae at 15.63 µg/mL) MGI = 84% (P. aeruginosa at 15.63 µg/mL) MGI = 86% (E. coli at 7.8 μg/mL) MGI = ∼ 50% (S. aureus at 15.63 μg/mL) MGI = ∼ 50% (MRSA at 15.63 μg/mL) MGI = 77% (C. parapsilosis at 15.63 μg/mL) MGI = 95% (C. albicans at 62.5 µg/mL) | [72] |
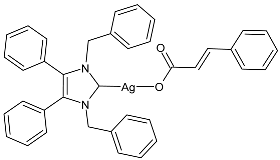 | 3 | MGI not given (K. pneumoniae) MGI not given (P. aeruginosa) MGI = 82% (E. coli at 15.63 µg/mL) MGI = not given (S. aureus) MGI = 76% (MRSA at 62.5 µg/mL) MGI = not given (C. parapsilosis) MGI = 94% (C. albicans at 125 µg/mL) | [72] |
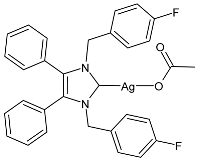 | 4 | ZC = 15 mm at 5 µL; 18 mm at 10 µL (MRSA) | [64] |
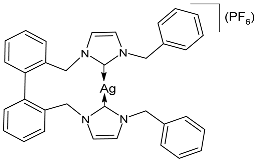 | 5 | MIC = 50 mg/L (S. aureus IE903) MIC = 200 mg/L (S. aureus E321) MIC = 10 mg/L (P. aeruginosa E322) | [73] |
 | 6 | MIC = 50 mg/L (S. aureus IE903) MIC = 100 mg/L (S. aureus E321) MIC = 10 mg/L (P. aeruginosa E322) | [73] |
 | 7 | MIC ≤ 1 µM (E. coli DH5α) MIC ≤ 1 µM (P. aeruginosa PAOI) MIC ≤ 1 µM (B. subtilis PY79) MIC ≤ 1 µM (S. aureus 6538P) | [74] |
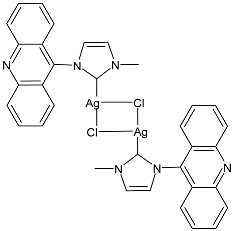 | 8 | MIC ≤ 1 µM (E. coli DH5α) MIC ≤ 1 µM (P. aeruginosa PAOI) MIC ≤ 1 µM (B. subtilis PY79) MIC ≤ 1 µM (S. aureus 6538P) | [74] |
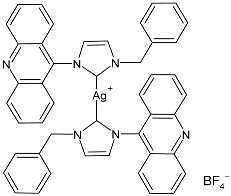 | 9 | MIC ≤ 1 µM (E. coli DH5α) MIC ≤ 1 µM (P. aeruginosa PAOI) MIC ≤ 1 µM (B. subtilis PY79) MIC ≤ 1 µM (S. aureus 6538P) | [74] |
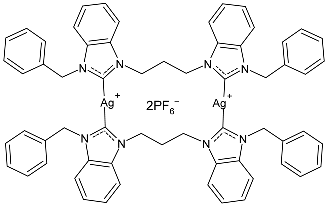 | 10 | IZD = 18.67 ± 0.58 mm (S. aureus) IZD = 12.5 ± 0.5 mm (E. coli) | [77] |
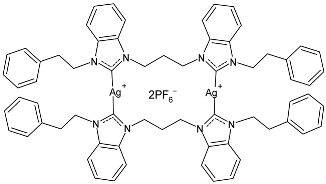 | 11 | IZD = 19.83 ± 0.29 mm (S. aureus) IZD = 14.33 ± 0.58 mm (E. coli) | [77] |
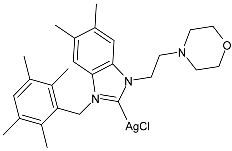 | 12 | MIC = 26 ± 0.7 µmol/L (E. coli ATCC 25988) MIC = 80 ± 1.8 µmol/L (P. aeruginosa ATCC 27853) MIC = 22 ± 0.6 µmol/L (K. pneumoniae ATCC 700603) MIC = 15 ± 0.3 µmol/L (S. aureus ATCC 29213) MIC = 11 ± 0.2 µmol/L (MRSA ATCC 43300) MIC = 8 ± 0.6 µmol/L (C. albicans ATCC 14053) | [78] |
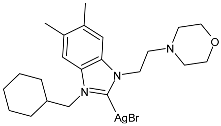 | 13 | MIC = 26 ± 0.3 µmol/L (E. coli ATCC 25988) MIC = 51 ± 1.2 µmol/L (P. aeruginosa ATCC 27853) MIC = 14 ± 0.2 µmol/L (K. pneumoniae ATCC 700603) MIC = 14 ± 0.6 µmol/L (S. aureus ATCC 29213) MIC = 13 ± 0.4 µmol/L (MRSA ATCC 43300) MIC = 14 ± 0.3 µmol/L (C. albicans ATCC 14053) | [78] |
 | 14 | MIC = 23 ± 0.8 µmol/L (E. coli ATCC 25988) MIC = 26 ± 1.3 µmol/L (P. aeruginosa ATCC 27853) MIC = 24 ± 0.8 µmol/L (K. pneumoniae) ATCC 700603 MIC = 12 ± 0.4 µmol/L (S. aureus ATCC 29213) MIC = 27 ± 1.1 µmol/L (MRSA ATCC 43300) MIC = 15 ± 0.2 µmol/L (C. albicans ATCC 14053) | [78] |
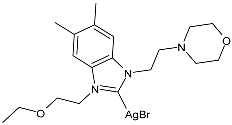 | 15 | MIC = 26 ± 0.8 µmol/L (E. coli ATCC 25988) MIC = 27 ± 0.8 µmol/L (P. aeruginosa ATCC 27853) MIC = 26 ± 0.9 µmol/L (K. pneumoniae ATCC 700603) MIC = 12 ± 0.4 µmol/L (S. aureus ATCC 29213) MIC = 26 ± 0.7 µmol/L (MRSA ATCC 43300) MIC = 14 ± 0.2 µmol/L (C. albicans ATCC 14053) | [78] |
 | 16 | MIC = 27 ± 0.9 µmol/L (E. coli ATCC 25988) MIC = 24 ± 0.9 µmol/L (P. aeruginosa ATCC 27853) MIC = 24 ± 0.8 µmol/L (K. pneumoniae ATCC 700603) MIC = 17 ± 0.2 µmol/L (S. aureus ATCC 29213) MIC = 27 ± 0.5 µmol/L (MRSA ATCC 43300) MIC = 13 ± 0.2 µmol/L (C. albicans ATCC 14053) | [78] |
3. Ag–NHC Complexes as Anticancer Agents
4. Ag–NHC as Dual Anticancer and Antibacterial Agents
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations and Cell Lines
| A-2780, ovarian cancer cells |
| A549, lung cancer cells |
| A. baumanii = Acinetobacter baumanii |
| BEAS-2B, bronchial epithelial cells |
| C. albicans = Candida albicans |
| C. parapsilosis = Candida parapsilosis |
| DU-145, prostate cancer cells |
| E. faecium = Enterococcus faecium |
| HCT-116, human colorectal cancer cells |
| Hek-293, normal embryonic kidney cells |
| HeLa, human cervical cancer cells |
| Hs27, normal human skin fibroblasts |
| IC50 = half-maximal (50%) inhibitory concentration |
| IMR-90, lung fibroblasts |
| IZD = inhibitory zone diameter |
| K. pneumoniae = Klebsiella pneumoniae |
| K-562, erythromyeloblastoid leukemia cells |
| M. bovis = Mycobacterium bovis |
| MDA-MB-231, breast cancer cells |
| MGI = Maximum growth inhibition |
| MIC = minimum inhibitory concentration |
| MRSA = methicillin-resistant S. aureus |
| MSSA = methicillin-sensitive S. aureus |
| M. smegmatis = Mycobacterium smegmatis |
| MCF-7, breast cancer cells |
| MCF-10A, normal breast cells |
| MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) |
| NHC = N-heterocyclic carbene |
| P. aeruginosa = Pseudomonas aeruginosa |
| RMS, rhabdomyosarcoma cells |
| S. aureus = Staphylococcus aureus |
| SDS = sodium dodecyl sulfate |
| S. epidermidis = Staphylococcus epidermidis |
| SH-SY5Y, neuroblastoma cancer cells |
| S. typhi = Salmonella typhi |
| S. typhimurium = Salmonella typhimurium |
| SW480, colon cells |
| ZC = zone of clearance |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef] [PubMed]
- Gaidai, O.; Yan, P.; Xing, Y. Future World Cancer Death Rate Prediction. Sci. Rep. 2023, 13, 303. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial Resistance: Impacts, Challenges, and Future Prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Pellegrino, M.; Giuzio, F.; Marra, M.; Rosano, C.; Saturnino, C.; Sinicropi, M.S.; Aquaro, S. Antibiotic-Resistant ESKAPE Pathogens and COVID-19: The Pandemic beyond the Pandemic. Viruses 2023, 15, 1843. [Google Scholar] [CrossRef]
- Nanayakkara, A.K.; Boucher, H.W.; Fowler, V.G.; Jezek, A.; Outterson, K.; Greenberg, D.E. Antibiotic Resistance in the Patient with Cancer: Escalating Challenges and Paths Forward. CA Cancer J. Clin. 2021, 71, 488–504. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Ceramella, J.; Iacopetta, D.; Catalano, A.; Mariconda, A.; Rosano, C.; Saturnino, C.; El-Kashef, H.; Longo, P. Metal Complexes with Schiff bases: Data Collection and Recent Studies on Biological Activities. Int. J. Mol. Sci. 2022, 23, 14840. [Google Scholar] [CrossRef]
- Parveen, S.; Naseem, H.A.; Ahmad, K.; Shah, H.-U.-R.; Tariq, A.; Muhammad, A.; Rauf, A. Design, Synthesis and Spectroscopic Characterizations of Medicinal Hydrazide Derivatives and Metal Complexes of Malonic Ester. Curr. Bioact. Compd. 2023, 19, 31–46. [Google Scholar] [CrossRef]
- Aziz, M.; Nasim, T.; Ahmad, H.A.; Shah, K.; Parveen, H.U.R.; Ahmad, S.; Majeed, M.M.; Galal, H.; Rauf, A.M.; Ashfaq, A. Rational Synthesis, Biological Screening of Azo Derivatives of Chloro-Phenylcarbonyl Diazenyl Hydroxy Dipyrimidines/Thioxotetrahydropyrimidines and their Metal Complexes. Heliyon 2023, 9, e12492. [Google Scholar] [CrossRef] [PubMed]
- Liscano, Y.; Oñate-Garzón, J.; Delgado, J.P. Peptides with Dual Antimicrobial–Anticancer Activity: Strategies to Overcome Peptide Limitations and Rational Design of Anticancer Peptides. Molecules 2020, 25, 4245. [Google Scholar] [CrossRef] [PubMed]
- Thankappan, B.; Sivakumar, J.; Asokan, S.; Ramasamy, M.; Pillai, M.M.; Selvakumar, R.; Angayarkanni, J. Dual Antimicrobial and Anticancer Activity of a Novel Synthetic Alpha-helical Antimicrobial Peptide. Eur. J. Pharm. Sci. 2021, 161, 105784. [Google Scholar] [CrossRef] [PubMed]
- Polinário, G.; Primo, L.M.D.G.; Rosa, M.A.B.C.; Dett, F.H.M.; Barbugli, P.A.; Roque-Borda, C.A.; Pavan, F.R. Antimicrobial Peptides as Drugs with Double Response against Mycobacterium tuberculosis Coinfections in Lung Cancer. Front. Microbiol. 2023, 14, 1183247. [Google Scholar] [CrossRef]
- Yang, W.; Choi, J.; Choi, S.H.; Shin, S.; Park, S.M.; Lee, Y.; Seo, J. A Conjugate of Chlorin e6 and Cationic Amphipathic Peptoid: A Dual Antimicrobial and Anticancer Photodynamic Therapy Agent. Photochem. Photobiol. Sci. 2023, 22, 655–667. [Google Scholar] [CrossRef]
- Mackeviciute, M.; Vaickelioniene, R.; Anusevicius, K.; Siugzdaite, J.; Lelesius, R.; Kavaliauskas, P.; Mickevicius, V. Synthesis and Characterization of Sulphanilamide and Benzimidazole Pharmacophores Containing γ-Amino Acid Derivatives as Dual Antimicrobial and Anticancer Agents. Arkivoc 2023, 7, 202312015. [Google Scholar] [CrossRef]
- Ugalde-Arbizu, M.; Aguilera-Correa, J.J.; Garcia-Almodovar, V.; Ovejero-Paredes, K.; Diaz-Garcia, D.; Esteban, J.; Paez, P.L.; Prashar, S.; San Sebastian, E.; Filice, M.; et al. Dual Anticancer and Antibacterial Properties of Silica-Based Theranostic Nanomaterials Functionalized with Coumarin, Folic Acid and a Cytotoxic Organotin(IV) Metallodrug. Pharmaceutics 2023, 15, 560. [Google Scholar] [CrossRef]
- D’Amato, A.; Troiano, R.; Iacopetta, D.; Mariconda, A.; Ceramella, J.; Catalano, A.; Sinicropi, M.S.; Longo, P. N-Heterocyclic Carbene Complexes RuII (arene) and their Application as ROMP Catalysts, Antioxidant and Anticancer Agents. Eur. J. Inorg. Chem. 2024, e202400402. [Google Scholar] [CrossRef]
- D’Amato, A.; Sirignano, M.; Russo, S.; Troiano, R.; Mariconda, A.; Longo, P. Recent Advances in N-Heterocyclic Carbene Coinage Metal Complexes in A3-Coupling and Carboxylation Reaction. Catalysts 2023, 13, 811. [Google Scholar] [CrossRef]
- Hobbs, M.S.; Chen, W.Y.; Youngs, W.J.; Ziegler, C.J. 2-Ethenyl Imidazolium Salts: Synthesis, Characterization, and Evaluation as Potential Chemotherapeutics. ChemistrySelect 2024, 9, e202403213. [Google Scholar] [CrossRef]
- Hussaini, S.Y.; Haque, R.A.; Razali, M.R. Recent Progress in Silver(I)-, Gold(I)/(III)- and Palladium(II)-N-Heterocyclic Carbene Complexes: A Review towards Biological Perspectives. J. Organomet. Chem. 2019, 882, 96–111. [Google Scholar] [CrossRef]
- Donmez, M.; Turkyilmaz, M. The New Pincer-type NHCs Obtained by Synthesizing Ag(I)-NHC Complexes with Various Tails Containing Hydroxyl or Acetate Derivatives: Structural Properties and In Vitro Antibacterial Activities. J. Mol. Liq. 2024, 415, 126270. [Google Scholar] [CrossRef]
- Sahu, P.; Mandal, S.M.; Biswas, R.; Chakraborty, S.; Natarajan, R.; Isab, A.A.; Dinda, J. Design, Synthesis and Bioactivity Evaluation of Ag(I)-, Au(I)- and Au(III)-Quinoxaline-Wingtip N-Heterocyclic Carbene Complexes Against Antibiotic Resistant Bacterial Pathogens. ChemMedChem 2024, 19, e202400236. [Google Scholar] [CrossRef] [PubMed]
- Frei, A.; Verderosa, A.D.; Elliott, A.G.; Zuegg, J.; Blaskovich, M.A.T. Metals to Combat Antimicrobial Resistance. Nat. Rev. Chem. 2023, 7, 202–224. [Google Scholar] [CrossRef]
- Waters, J.E.; Stevens-Cullinane, L.; Siebenmann, L.; Hess, J. Recent Advances in the Development of Metal Complexes as Antibacterial Agents with Metal-Specific Modes of Action. Curr. Opin. Microbiol. 2023, 75, 102347. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Yin, Y.K.; Zhang, H.; Han, Y.F. Metal N-Heterocyclic Carbene Complexes as Potential Metallodrugs in Antitumor Therapy. Coord. Chem. Rev. 2024, 514, 215941. [Google Scholar] [CrossRef]
- Iacopetta, D.; Costabile, C.; La Chimia, M.; Mariconda, A.; Ceramella, J.; Scumaci, D.; Catalano, A.; Rosano, C.; Cuda, G.; Sinicropi, M.S.; et al. NHC-Ag(I) and NHC-Au(I) Complexes with N-Boc-Protected α-Amino Acidate Counterions Powerfully Affect the Growth of MDA-MB-231 Cells. ACS Med. Chem. Lett. 2023, 14, 1567–1575. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, X.; Chang, X.; Liang, Z.; Lv, L.; Shan, M.; Lu, Q.; Wen, Z.; Gust, R.; Liu, W. Recent Development of Gold(I) and Gold(III) Complexes as Therapeutic Agents for Cancer Diseases. Chem. Soc. Rev. 2022, 51, 5518–5556. [Google Scholar] [CrossRef]
- Alsalhi, A. N-Heterocyclic Carbene Complexes of Au(I), Ag(I), and Cu(I) as Potential Anticancer Agents: A Review. J. Coord. Chem. 2023, 76, 847–861. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Rosano, C.; Mariconda, A.; Pellegrino, M.; Sirignano, M.; Saturnino, C.; Catalano, A.; Aquaro, S.; Longo, P.; et al. N-Heterocyclic Carbene-Gold(I) Complexes Targeting Actin Polymerization. Appl. Sci. 2021, 11, 5626. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Mariconda, A.; Rosano, C.; Scumaci, D.; Saturnino, C.; Longo, P.; Sinicropi, M.S. New Achievements for the Treatment of Triple-Negative Breast Cancer. Appl. Sci. 2022, 12, 5554. [Google Scholar] [CrossRef]
- Adhikari, S.; Nath, P.; Das, A.; Datta, A.; Baildya, N.; Duttaroy, A.K.; Pathak, S. A Review on Metal Complexes and its Anti-cancer Activities: Recent Updates from In Vivo Studies. Biomed. Pharmacother. 2024, 171, 116211. [Google Scholar] [CrossRef] [PubMed]
- Ceramella, J.; Mariconda, A.; Sirignano, M.; Iacopetta, D.; Rosano, C.; Catalano, A.; Saturnino, C.; Sinicropi, M.S.; Longo, P. Novel Au Carbene Complexes as Promising Multi-Target Agents in Breast Cancer Treatment. Pharmaceuticals 2022, 15, 507. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.A.; Hoagland, P.; Patil, S.A.; Bugarin, A. N-Heterocyclic Carbene-Metal Complexes as Bio-Organometallic Antimicrobial and Anticancer Drugs, an Update (2015–2020). Future Med. Chem. 2020, 12, 2239–2275. [Google Scholar] [CrossRef]
- Catalano, A.; Mariconda, A.; Sinicropi, M.S.; Ceramella, J.; Iacopetta, D.; Saturnino, C.; Longo, P. Biological Activities of Ruthenium NHC Complexes: An Update. Antibiotics 2023, 12, 365. [Google Scholar] [CrossRef]
- D’Amato, A.; Iacopetta, D.; Ceramella, J.; Troiano, R.; Mariconda, A.; Catalano, A.; Marra, M.; Saturnino, C.; Rosano, C.; Sinicropi, M.S.; et al. Design, Synthesis and Biological Evaluation of Multitarget Hybrid Molecules Containing NHC-Au(I) Complexes and Carbazole Moieties. Eur. J. Med. Chem. 2024, 277, 116757. [Google Scholar] [CrossRef]
- Ceramella, J.; Troiano, R.; Iacopetta, D.; Mariconda, A.; Pellegrino, M.; Catalano, A.; Saturnino, C.; Aquaro, S.; Sinicropi, M.S.; Longo, P. Synthesis of Novel N-Heterocyclic Carbene-Ruthenium (II) Complexes, “Precious” Tools with Antibacterial, Anticancer and Antioxidant Properties. Antibiotics 2023, 12, 693. [Google Scholar] [CrossRef]
- D’Amato, A.; Mariconda, A.; Iacopetta, D.; Ceramella, J.; Catalano, A.; Sinicropi, M.S.; Longo, P. Complexes of Ruthenium(II) as Promising Dual-Active Agents against Cancer and Viral Infections. Pharmaceuticals 2023, 16, 1729. [Google Scholar] [CrossRef]
- Tutar, U.; Celik, C.; Sahin, N. Allyl Functionalized Benzimidazolium-Derived Ag(I)-N-Heterocyclic Carbene Complexes: Anti-Biofilm and Antimicrobial Properties. Pharm. Chem. J. 2022, 56, 54–60. [Google Scholar] [CrossRef]
- Turkyilmaz, M.; Dönmez, M.; Ates, M. Synthesis of Pincer Type Carbene and their Ag (I)-NHC Complexes, and their Antimicrobial Activities. J. Sustain. Constr. Mater. Technol. 2022, 7, 53–61. [Google Scholar] [CrossRef]
- Napoli, M.; Saturnino, C.; Cianciulli, E.I.; Varcamonti, M.; Zanfardino, A.; Tommonaro, G.; Longo, P. Silver(I) N-heterocyclic carbene complexes: Synthesis, characterization and antibacterial activity. J. Organomet. Chem. 2013, 725, 46–53. [Google Scholar] [CrossRef]
- Slimani, I.; Mansour, L.; Abutaha, N.; Harrath, A.H.; Al-Tamimi, J.; Gürbüz, N.; Özdemir, I.; Hamdi, N. Synthesis, Structural Characterization of Silver(I)-NHC Complexes and their Antimicrobial, Antioxidant and Antitumor Activities. J. King Saud Univ. Sci. 2020, 32, 1544–1554. [Google Scholar] [CrossRef]
- Johnson, N.A.; Southerland, M.R.; Youngs, W.J. Recent Developments in the Medicinal Applications of Silver-NHC Complexes and Imidazolium Salts. Molecules 2017, 22, 1263. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Luan, S.; Yin, Z.; He, M.; He, C.; Yin, L.; Zou, Y.; Yuan, Z.; Li, L.; Song, X.; et al. Recent Advances in the Medical Use of Silver Complex. Eur. J. Med. Chem. 2018, 157, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Raju, S.K.; Karunakaran, A.; Kumar, S.; Sekar, P.; Murugesan, M.; Karthikeyan, M. Silver Complexes as Anticancer Agents: A Perspective Review. Ger. J. Pharm. Biomater. 2022, 1, 6–28. [Google Scholar] [CrossRef]
- Ataş, A.D.; Akın-Polat, Z.; Gülpınar, D.G.; Şahin, N. The First Evaluation of the In Vitro Effects of Silver(I)-N-Heterocyclic Carbene Complexes on Encephalitozoon intestinalis and Leishmania major Promastigotes. J. Biol. Inorg. Chem. 2024, 29, 499–509. [Google Scholar] [CrossRef]
- Şahin-Bölükbaşi, S.; Cantürk-Kiliçkaya, P.; Kiliçkaya, O. Silver(I)-N-Heterocyclic Carbene Complexes Challenge Cancer; Evaluation of their Anticancer Properties and In Silico Studies. Drug Dev. Res. 2021, 82, 907–926. [Google Scholar] [CrossRef]
- Șahin-Bölükbaşi, S.; Șahin, N. Novel Silver-NHC Complexes: Synthesis and Anticancer Properties. J. Organomet. Chem. 2019, 891, 78–84. [Google Scholar] [CrossRef]
- Wang, Z.; Tzouras, N.V.; Nolan, S.P.; Bi, X. Silver N-Heterocyclic Carbenes: Emerging Powerful Catalysts. Trends Chem. 2021, 3, 674–685. [Google Scholar] [CrossRef]
- Costabile, C.; Mariconda, A.; Sirignano, M.; Crispini, A.; Scarpelli, F.; Longo, P. A Green Approach for A3-Coupling Reactions: An Experimental and Theoretical Study on NHC Silver and Gold Catalysts. N. J. Chem. 2021, 45, 18509–18517. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, T.; Yu, X.; Szostak, M. Ag–NHC Complexes in the π-Activation of Alkynes. Molecules 2023, 28, 950. [Google Scholar] [CrossRef] [PubMed]
- Frith, A.; Clarke, A.K.; Heyam, A.; Lynam, J.M.; Newman, P.D.; Unsworth, W.P.; Willans, C.E. Silver–N-Heterocyclic Carbenes in π–Activation: Synergistic Effects between the Ligand Ring Size and the Anion. Organometallics 2024, 43, 598–604. [Google Scholar] [CrossRef]
- Sirignano, M.; Mariconda, A.; Vigliotta, G.; Ceramella, J.; Iacopetta, D.; Sinicropi, M.S.; Longo, P. Catalytic and Biological Activity of Silver and Gold Complexes Stabilized by NHC with Hydroxy Derivatives on Nitrogen Atoms. Catalysts 2022, 12, 18. [Google Scholar] [CrossRef]
- Hao, H.J.; Chen, D.F.; Zhang, W.; Li, X.; Wang, K.F.; Lei, J.Y.; Tang, L.F. Synthesis and catalytic Activity of Silver N-Heterocyclic Carbene Complexes Based on Bis(3,5-Dimethylpyrazol-1-yl)methyl-substituted Pyridylimidazole. J. Coord. Chem. 2024, 77, 375–391. [Google Scholar] [CrossRef]
- Muqeed, M.; Manchal, R.; Botla, V.; Azam, M.; Vasam, C.S.; Thirukovela, N.S. New Ag(I)−NHC/TBHP System for Oxidative Coupling of α-Hydroxy Acetophenones with Amines: A Base-Free and Efficient Synthesis of α-Ketoamides in Water. Asian J. Org. Chem. 2023, 12, e202300356. [Google Scholar] [CrossRef]
- D’Amato, A.; Sirignano, M.; Viceconte, F.; Longo, P.; Mariconda, A. Catalytic Behaviour of NHC-Silver Complexes in the Carboxylation of Terminal Alkynes with CO2. Inorganics 2024, 12, 283. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, A.; Singh, H.; Sonawane, P.; Pathak, P.; Grishina, M.; Yadav, J.P.; Verma, A.; Kumar, P. Metal Complexes in Cancer Treatment: Journey so Far. Chem. Biodivers. 2023, 20, e202300061. [Google Scholar] [CrossRef]
- Pueyo, J.; Joven-Sancho, D.; Martín, A.; Menjón, B.; Baya, M. The Fluoride Method: Access to Silver(III) NHC Complexes. Chemistry 2024, 30, e202303937. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Ronga, L.; Varcamonti, M.; Tesauro, D. Structure-activity Relationships in NHC-Silver Complexes as Antimicrobial Agents. Molecules 2023, 28, 4435. [Google Scholar] [CrossRef]
- Isbel, S.R.; Patil, S.A.; Bugarin, A. NHCs Silver Complexes as Potential Antimicrobial Agents. Inorg. Chim. Acta 2023, 563, 121899. [Google Scholar] [CrossRef] [PubMed]
- Shahini, C.R.; Achar, G.; Budagumpi, S.; Tacke, M.; Patil, S.A. Synthesis, Structural Investigation and Antibacterial Studies of Non–symmetrically p–Nitrobenzyl Substituted Benzimidazole N–Heterocyclic Carbene–Silver(I) Complexes. Inorg. Chim. Acta 2017, 466, 432–441. [Google Scholar] [CrossRef]
- Gök, Y.; Akkoç, S.; Albayrak, S.; Akkurt, M.; Tahir, M.N. N-Phenyl-substituted Carbene Precursors and their Silver Complexes: Synthesis, Characterization and Antimicrobial Activities. Appl. Organometal. Chem. 2014, 28, 244–251. [Google Scholar] [CrossRef]
- Beato, Z.; Ryan, B.; Mueller-Bunz, H.; Baumann, M.; Tacke, M. Synthesis and Biological Evaluation of Fluoro-Substituted Cationic and Neutral Antibiotic NHC* Silver Derivatives of SBC3. J. Organomet. Chem. 2022, 976, 122436. [Google Scholar] [CrossRef]
- Sharkey, M.; O’Gara, J.; Gordon, S.; Hackenberg, F.; Healy, C.; Paradisi, F.; Patil, S.; Schaible, B.; Tacke, M. Investigations into the Antibacterial Activity of the Silver-Based Antibiotic Drug Candidate SBC3. Antibiotics 2012, 1, 25–28. [Google Scholar] [CrossRef]
- Browne, N.; Hackenberg, F.; Streciwilk, W.; Tacke, M.; Kavanagh, K. Assessment of In Vivo Antimicrobial Activity of the Carbene Silver(I) Acetate Derivative SBC3 using Galleria mellonella Larvae. Biometals 2014, 27, 745–752. [Google Scholar] [CrossRef]
- O’Loughlin, J.; Napolitano, S.; Alkhathami, F.; O’Beirne, C.; Marhöfer, D.; O’Shaughnessy, M.; Howe, O.; Tacke, M.; Rubini, M. The Antibacterial Drug Candidate SBC3 is a Potent Inhibitor of Bacterial Thioredoxin Reductase. ChemBioChem 2021, 22, 1093–1098. [Google Scholar] [CrossRef]
- O’Beirne, C.; Alhamad, N.F.; Ma, Q.; Müller-Bunz, H.; Kavanagh, K.; Buder, G.; Zhu, X.; Tacke, M. Synthesis, Structures and Antimicrobial Activity of Novel NHC* and Ph3P-Ag(I)-Benzoate Derivatives. Inorg. Chim. Acta 2019, 486, 294–303. [Google Scholar] [CrossRef]
- Piatek, M.; O’Beirne, C.; Beato, Z.; Tacke, M.; Kavanagh, K. Exposure of Candida parapsilosis to the Silver(I) Compound SBC3 Induces Alterations in the Proteome and Reduced Virulence. Metallomics 2022, 14, mfac046. [Google Scholar] [CrossRef]
- Piatek, M.; O’Beirne, C.; Beato, Z.; Tacke, M.; Kavanagh, K. Pseudomonas aeruginosa and Staphylococcus aureus Display Differential Proteomic Responses to the Silver(I) Compound, SBC3. Antibiotics 2023, 12, 348. [Google Scholar] [CrossRef]
- Chen, H.; Lu, Q.; An, H.; Li, J.; Shen, S.; Zheng, X.; Chen, W.; Wang, L.; Li, J.; Du, Y.; et al. The synergistic activity of SBC3 in combination with Ebselen against Escherichia coli infection. Front. Pharmacol. 2022, 13, 1080281. [Google Scholar] [CrossRef] [PubMed]
- O’Beirne, C.; Piatek, M.E.; Fossen, J.; Müller-Bunz, H.; Andes, D.R.; Kavanagh, K.; Patil, S.A.; Baumann, M.; Tacke, M. Continuous Flow Synthesis and Antimicrobial Evaluation of NHC* Silver Carboxylate Derivatives of SBC3 In Vitro and In Vivo. Metallomics 2021, 13, mfaa011. [Google Scholar] [CrossRef] [PubMed]
- Muniyappan, N.; Advaya, G.R.; Sujitha, E.; Sabiah, S. Picolyl and Benzyl Functionalized Biphenyl NHC Carbenes and their Silver Complexes: Sigma Donating and Antimicrobial Properties. J. Organomet. Chem. 2021, 954–955, 122075. [Google Scholar] [CrossRef]
- Prencipe, F.; Zanfardino, A.; Di Napoli, M.; Rossi, F.; D’Errico, S.; Piccialli, G.; Mangiatordi, G.F.; Saviano, M.; Ronga, L.; Varcamonti, M.; et al. Silver(I) N-Heterocyclic Carbene Complexes: A Winning and Broad Spectrum of Antimicrobial Properties. Int. J. Mol. Sci. 2021, 22, 2497. [Google Scholar] [CrossRef]
- Concepción Gimeno, M.; Laguna, A.; Visbal, R.N. Heterocyclic Carbene Coinage Metal Complexes as Intense Blue-Green Emitters. Organometallics 2012, 31, 7146–7157. [Google Scholar] [CrossRef]
- Patil, S.A.; Patil, S.A.; Patil, R.; Keri, R.S.; Budagumpi, S.; Balakrishna, G.R.; Tacke, M. N-Heterocyclic Carbene Metal Complexes as Bio-Organometallic Antimicrobial and Anticancer Drugs. Future Med. Chem. 2015, 7, 1305–1333. [Google Scholar] [CrossRef]
- Nadeem, R.Y.; Yaqoob, M.; Yam, W.; Haque, R.A.; Iqbal, M.A. Synthesis, Characterization and Biological Evaluation of Bis-benzimidazolium Salts and their Silver(I)-N-Heterocyclic Carbene Complexes. Med. Chem. Res. 2022, 31, 1783–1791. [Google Scholar] [CrossRef]
- Bensalah, D.; Gurbuz, N.; Özdemir, I.; Gatri, R.; Mansour, L.; Hamdi, N. Synthesis, Characterization, Antimicrobial Properties, and Antioxidant Activities of Silver-N-Heterocyclic Carbene Complexes. Bioinorg. Chem. Appl. 2023, 2023, 3066299. [Google Scholar] [CrossRef]
- Guarra, F.; Busto, N.; Guerri, A.; Marchetti, L.; Marzo, T.; García, B.; Biver, T.; Gabbiani, C. Cytotoxic Ag(I) and Au(I) NHC-carbenes bind DNA and show TrxRInhibition. J. Inorg. Biochem. 2020, 205, 110998. [Google Scholar] [CrossRef]
- Çevik-Yıldız, E.; Şahin, N.; Şahin-Bölükbaşı, S. Synthesis, Characterization, and Investigation of Antiproliferative Activity of Novel Ag (I)-N-Heterocyclic Carbene (NHC) Compounds. J. Mol. Struct. 2020, 1199, 126987. [Google Scholar] [CrossRef]
- Atif, M.; Bhatti, H.N.; Haque, R.A.; Iqbal, M.A.; Ahamed Khadeer, M.B.; Majid, A.M.S.A. Synthesis, Structure, and Anticancer Activity of Symmetrical and Non-symmetrical Silver(I)-N-Heterocyclic Carbene Complexes. Appl. Biochem. Biotechnol. 2020, 191, 1171–1189. [Google Scholar] [CrossRef] [PubMed]
- Md Zin, N.F.H.; Ooi, S.Y.S.; Khor, B.-K.; Chear, N.J.-Y.; Tang, W.K.; Siu, C.-K.; Razali, M.R.; Haque, R.A.; Yam, W. Cytotoxicity of Asymmetric Mononuclear Silver(I)-N-Heterocyclic Carbene Complexes against Human Cervical Cancer: Synthesis, Crystal Structure, DFT Calculations and Effect of Substituents. J. Organomet. Chem. 2022, 976, 122439. [Google Scholar] [CrossRef]
- Hobsteter, A.W.; Irazoqui, A.P.; Gonzalez, A.; Picco, A.S.; Rubert, A.A.; Buitrago, C.G.; Lo Fiego, M.J.; Silbestri, G.F. Acetylated Galactopyranosyl N-Heterocyclic Monocarbene Complexes of Silver(I) as Novel Anti-proliferative Agents in a Rhabdomyosarcoma Cell Line. Bioorg. Med. Chem. 2024, 107, 117756. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, R.; Akram, S.; El-Naggar, M.; Kanwal, A.; Tok, T.T.; Iqbal, M.A.; Bhatti, H.N.; Amin, M.A.; El-Bahy, S.M.; El-Bahy, Z.M. In Vitro and In Silico Anticancer Potential of Binuclear Silver(I) N-Heterocyclic Carbene (NHCs) Complexes: Investigation of Interaction with Surfactants. Inorg. Chim. Acta 2024, 571, 122181. [Google Scholar] [CrossRef]
- Ben Salah, D.; Zouaghi, M.O.; Hassen, S.; Arfaoui, Y.; Mansour, L.; Al-Quraishy, S.; Özdemir, N.; Gurbuz, N.; Özdemir, I.; Sauthier, M.; et al. Novel Nonsymmetrically Benzimidazolium Salts and their Silver(I)-N-heterocyclic Carbene Complexes: Synthesis, Crystal Structure, DFTstudies and Anticancer Activities. Inorg. Chim. Acta 2025, 574, 122398. [Google Scholar] [CrossRef]
- Rahali, E.; Boubakri, L.; Gürbüz, N.; Özdemir, İ.; Mansour, L.; Arfaoui, Y.; Sauthier, M.; Hamdi, N. Novel Nonsymmetrically Benzimidazolium Salts and their Silver(I)-N-heterocyclic Carbene Complexes: Synthesis, DFT Study and Anticancer Activities. J. Mol. Struct. 2025, 1321, 139947. [Google Scholar] [CrossRef]
- Sharhan, O.; Heidelberg, T.; Hashim, N.M.; Al-Madhagi, W.M.; Ali, H.M. Benzimidazolium-Acridine-Based Silver N-HeteroCyclic Carbene Complexes as Potential Anti-Bacterial and Anti-Cancer Drug. Inorganica Chim. Acta 2020, 504, 119462. [Google Scholar] [CrossRef]
- Jakob, C.H.G.; Muñoz, A.W.; Schlagintweit, J.F.; Weiß, V.; Reich, R.M.; Sieber, S.A.; Correia, J.D.G.; Kühn, F.E. Anticancer and Antibacterial Properties of Trinuclear Cu(I), Ag(I) and Au(I) Macrocyclic NHC/urea Complexes. J. Organomet. Chem. 2021, 932, 121643. [Google Scholar] [CrossRef]
- Mariconda, A.; Iacopetta, D.; Sirignano, M.; Ceramella, J.; Costabile, C.; Pellegrino, M.; Rosano, C.; Catalano, A.; Saturnino, C.; El-Kashef, H.; et al. N-Heterocyclic Carbene (NHC) Silver Complexes as Versatile Chemotherapeutic Agents Targeting Human Topoisomerases and Actin. ChemMedChem 2022, 17, e202200345. [Google Scholar] [CrossRef]
- Sarfraz, A.; Ashraf, R.; Ali, S.; Taskin-Tok, T.; Khalid, Z.; Ullah, S.; Kahlid, T.; Mushtaq, M.; El-Bahy, S.M.; El-Bahy, Z.M. Synthesis, In silico and In Vitro Studies of Silver(I)-N Heterocyclic Carbene Complexes. J. Mol. Struct. 2022, 1251, 131946. [Google Scholar] [CrossRef]
- Ulu, Ö.D.; Kuruçay, A.; Ateş, B.; Özdemir, İ. Synthesis, Characterization, In Vitro Antibacterial, and Anticancer Studies of Ag(I)-N-heterocyclic Carbene (NHC) Complexes. Chem. Pap. 2023, 77, 423–435. [Google Scholar] [CrossRef]
- Mariconda, A.; Iacopetta, D.; Sirignano, M.; Ceramella, J.; D’Amato, A.; Marra, M.; Pellegrino, M.; Sinicropi, M.S.; Aquaro, S.; Longo, P. Silver and Gold Complexes with NHC-Ligands Derived from Caffeine: Catalytic and Pharmacological Activity. Int. J. Mol. Sci. 2024, 25, 2599. [Google Scholar] [CrossRef] [PubMed]
- Karci, H.; Dündar, M.; Nawaz, Z.; Özdemir, İ.; Gürbüz, N.; Koç, A.; Özdemir, İ.; Mansour, L.; Hamdi, N. Synthesis, Characterisation, Anticancer and Antimicrobial Activity of Ag-N-Heterocyclic Carbene Complexes Containing Benzimidazole Derivatives. Inorg. Chim. Acta 2024, 565, 121992. [Google Scholar] [CrossRef]
| Structure | Compd | Anticancer Activity | Ref. |
|---|---|---|---|
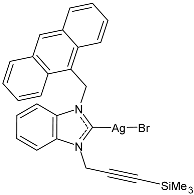 | 17 | IC50 = 7 ± 2 µM (SW480) IC50 = 10 ± 1 µM (A549) IC50 = 8 ± 1 µM (HepG2) | [79] |
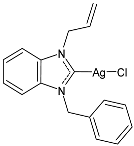 | 18 | IC50 = <1 µM (MCF-7) IC50 = 2.74 ± 0.32 µM (DU-145) IC50 = <1 µM (MDA-MB-231) | [80] |
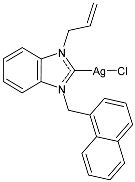 | 19 | IC50 = <1 µM (MCF-7) IC50 = 2.11 ± 0.05 µM (DU-145) IC50 = 1.26 ± 0.02 µM (MDA-MB-231) | [80] |
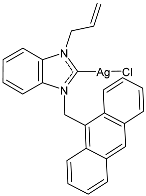 | 20 | IC50 = 1.02 ± 0.05 µM (MCF-7) IC50 = 1.74 ± 0.21 µM (DU-145) IC50 = <1 µM (MDA-MB-231) | [80] |
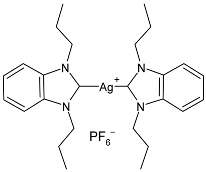 | 21 | IC50 = 0.31 µM (HCT-116) IC50 = 15.1 µM (MCF-7) IC50 = 7.0 µM (K-562) | [81] |
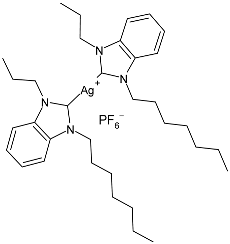 | 22 | IC50 = 15.1 µM (HCT-116) IC50 = 16.1 µM (MCF-7) IC50 = 17.9 µM (K-562) | [81] |
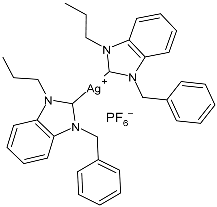 | 23 | IC50 = 1.99 µM (HCT-116) IC50 = 35.2 µM (MCF-7) IC50 = 10.7 µM (K-562) | [81] |
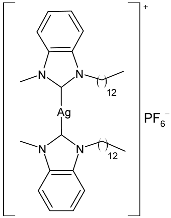 | 24 | IC50 = 1.04 ± 0.21 µg/mL; 1.22 µM (HeLa) | [82] |
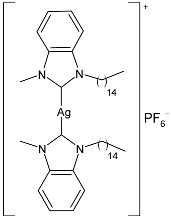 | 25 | IC50 = 1.07 ± 0.08 µg/mL; 1.18 µM (HeLa) | [82] |
 | 26 | IC50 = 1.14 ± 0.18 µg/mL; 1.18 µM (HeLa) | [82] |
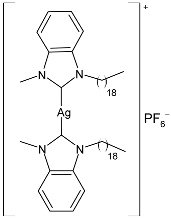 | 27 | IC50 = 2.27 ± 0.04 µg/mL; 2.22 µM (HeLa) | [82] |
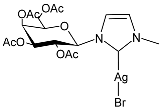 | 28 | IC50 = 34.5 µM (RMS cell line, RD) | [83] |
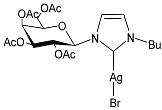 | 29 | IC50 = 28.2 µM (RMS cell line, RD) | [83] |
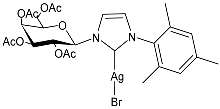 | 30 | IC50 = 26.7 µM (RMS cell line, RD) | [83] |
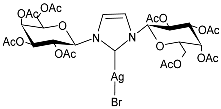 | 31 | IC50 = 20.4 µM (RMS cell line, RD) | [83] |
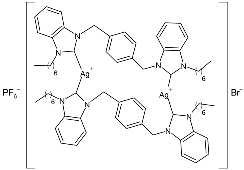 | 32 | IC50 = 1.087 ± 0.05 µg/mL (MCF-7) IC50 = 0.986 ± 0.05 µg/mL (A549) IC50 = 0.962 ± 0.08 µg/mL (A-2780) IC50 = 1.023 ± 0.07 µg/mL (HCT-116) | [84] |
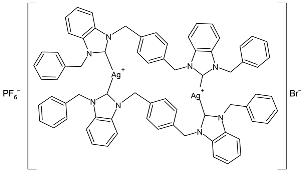 | 33 | IC50 = 0.996 ± 0.04 µg/mL (MCF-7) IC50 = 0.975 ± 0.04 µg/mL (A549) IC50 = 0.872 ± 0.05 µg/mL (A-2780) IC50 = 0.987 ± 0.05 µg/mL (HCT-116) | [84] |
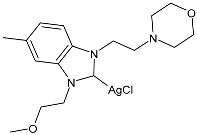 | 34 | IC50 = 6.26 ± 0.30 µM (HepG2) IC50 = 3.25 ± 0.12 µM (A549) IC50 = 5.74 ± 0.12 µM (MCF-7) | [85] |
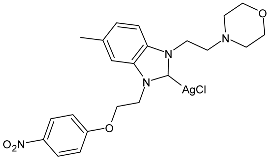 | 35 | IC50 = 6.21 ± 0.08 µM (HepG2) IC50 = 1.65 ± 0.05 µM (A549) IC50 = 5.46 ± 0.16 µM (MCF-7) | [85] |
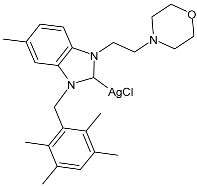 | 36 | IC50 = 12.62 ± 0.55 µM (HepG2) IC50 = 6.49 ± 0.06 µM (A549) IC50 = 8.43 ± 0.27 µM (MCF-7) | [85] |
 | 37 | IC50 = 5.37 ± 0.20 µM (HepG2) IC50 = 3.34 ± 0.13 µM (A549) IC50 = 5.83 ± 0.11 µM (MCF-7) | [86] |
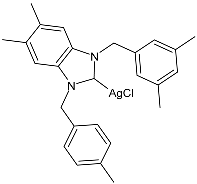 | 38 | IC50 = 4.44 ± 0.07 µM (HepG2) IC50 = 1.77 ± 0.04 µM (A549) IC50 = 4.58 ± 0.14 µM (MCF-7) | [86] |
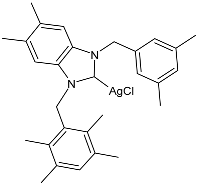 | 39 | IC50 = 11.82 ± 0.59 µM (HepG2) IC50 = 6.09 ± 0.08 µM (A549) IC50 = 9.54 ± 0.28 µM (MCF-7) | [86] |
| Structure | Compd | Antibacterial Activity | Anticancer Activity | Ref. |
|---|---|---|---|---|
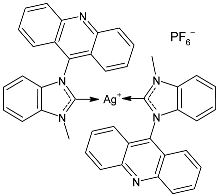 | 40 | MIC = 250 mg/mL (S. aureus ATCC 25923) MIC = 250 mg/mL (S. epidermidis clinical isolate) MIC = 250 mg/mL (P. aeruginosa ATCC27853) MIC = 250 mg/mL (E. coli clinical isolate) MIC = 250 mg/mL (S. typhi clinical isolate) | IC50 = 20 ± 3 mg/mL; 22 µM (MCF-7) | [87] |
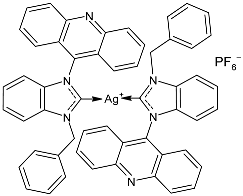 | 41 | MIC = 250 mg/mL (S. aureus ATCC 25923 MIC = 250 mg/mL (S. epidermidis clinical isolate) MIC = 250 mg/mL (P. aeruginosa ATCC27853) MIC = 250 mg/mL (E. coli clinical isolate) MIC = 250 mg/mL (S. typhi clinical isolate) | IC50 = 22 ± 3 mg/mL; 21 µM (MCF-7) | [87] |
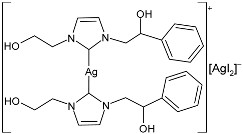 | 42 | MIC = 15 µg/mL; 32.1 µM (E. coli) MIC = 50 µg/mL; 107.0 µM (S. aureus) | IC50 > 200 µM (MDA-MB-231) IC50 = 20.3 ± 1.1 µM (MCF-7) IC50 = 12.2 ± 1.0 µM (HeLa) | [53] |
 | 43 | MIC = 15 µg/mL; 27.9 µM (E. coli) MIC > 150 µg/mL; > 279.8 µM (S. aureus) | IC50 > 200 µM (MDA-MB-231) IC50 = 19.5 ± 0.9 µM (MCF-7) IC50 11.9 ± 0.4 µM (HeLa) | [53] |
 | 44 | MIC = 30 µM (S. aureus) MIC = 25–50 µM (E. coli) | IC50 = 3.61 ± 1.04 µM (HeLa) IC50 = 3.03 ± 1.06 µM (MCF-7) | [88] |
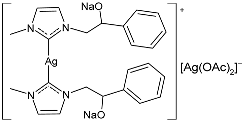 | 45 | MIC = 5 µg/mL (E. coli) MIC = 5 µg/mL (S. aureus) MIC = 10 µg/mL (E. faecalis) | IC50 = 7.0 ± 0.4 µM (MDA-MB-231) IC50 = 18.3 ± 0.8 µM (MCF-7) | [89] |
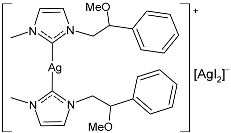 | 46 | MIC = 5 µg/mL (E. coli) MIC = 5 µg/mL (S. aureus) MIC = 10 µg/mL (E. faecalis) | IC50 = 52.6 ± 0.3 µM (MDA-MB-231) IC50 = 31.8 ± 0.8 µM (MCF-7) | [89] |
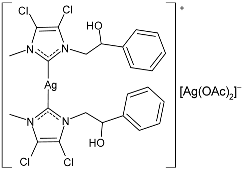 | 47 | MIC = 10 µg/mL (E. coli) MIC = 5 µg/mL (S. aureus) MIC = 10 µg/mL (E. faecalis) | IC50 = 38.1 ± 0.8 µM (MDA-MB-231) IC50 = 13.2 ± 0.3 µM (MCF-7) | [89] |
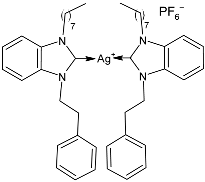 | 48 | MIC = 0.15 ± 0.03 µg/mL (S. aureus) MIC = 0.073 ± 0.02 µg/mL (M. luteus) MIC = 0.12 ± 0.05 µg/mL (E. coli) MIC = 0.095 ± 0.02 µg/mL (S. typhimurium) | IC50 = 1.1691 ± 0.12 µM (MCF-7) IC50 = 1.116 ± 0.09 µM (HCT-116) IC50 = 0.9850 ± 0.08 µM (A549) | [90] |
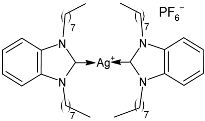 | 49 | MIC = 0.035 ± 0.01 µg/mL (S. aureus) MIC = 0.11 ± 0.05 µg/mL (M. luteus) MIC = 0.21 ± 0.03 µg/mL (E. coli) MIC = 0.15 ± 0.06 µg/mL (S. typhimurium) | IC50 = 0.9813 ± 0.09 µM (MCF-7) IC50 = 1.101 ± 0.14 µM (HCT-116) IC50 = 0.9730 ± 0.12 µM (A549) | [90] |
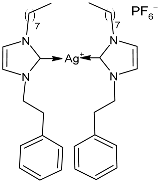 | 50 | MIC = 0.046 ± 0.01 µg/mL (S. aureus) MIC = 0.035 ± 0.04 µg/mL (M. luteus) MIC = 0.13 ± 0.08 µg/mL (E. coli) MIC = 0.054 ± 0.01 µg/mL (S. typhimurium) | IC50 = 1.3787 ± 0.17 µM (MCF-7) IC50 = 1.299 ± 0.13 µM (HCT-116) IC50 = 1.1773 ± 0.17 µM (A549) | [90] |
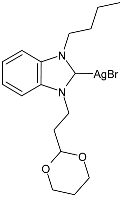 | 51 | IZD = not active (B. subtilis) IZD = not active (E. coli) | IC50 = 6.01 ± 2.39 µg/mL (MCF-7) | [91] |
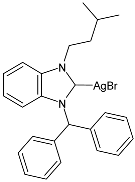 | 52 | IZD = 7.33 ± 0.57 mm (B. subtilis) IZD = 9.33 ± 1.15 mm (E. coli) | IC50 = 11.58 ± 2.58 µg/mL (MCF-7) | [91] |
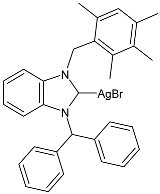 | 53 | IZD = 10.33 ± 1.52 mm (B. subtilis) IZD = 16.00 ± 2.00 mm (E. coli) | IC50 = 3.40 ± 0.87 µg/mL (MCF-7) | [91] |
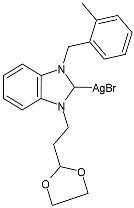 | 54 | IZD = 5.33 ± 1.15 mm (B. subtilis) IZD = 8.67 ± 1.15 mm (E. coli) | IC50 = 9.08 ± 2.91 µg/mL (MCF-7) | [91] |
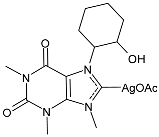 | 55 | MIC = 100 µg/mL (S. aureus) MIC = 150 µg/mL (E. faecalis) MIC = 100 µg/mL (S. epidermidis) MIC = 100 µg/mL (E. coli) MIC = 125 µg/mL (K. pneumoniae) MIC = 100 µg/mL (P. aeruginosa) MIC = 150 µg/mL (S. typhimurium) | IC50 = 19.4 ± 1 µM (MDA-MB-231) IC50 = 28.7 ± 1 µM (MCF-7) | [92] |
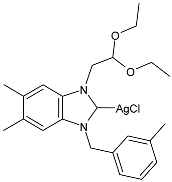 | 56 | MIC = 12.5 µg/mL (C. albicans) MIC = 6.25 µg/mL (C. glabrata) MIC = 25 µg/mL (E. coli) MIC = 25 µg/mL (P. aeruginosa) MIC = 35 µg/mL (S. aureus) | IC50 = 35.82 ± 0.43 µM (A549) IC50 = 40.29 ± 1.26 µM (MCF-7) IC50 = 32.58 ± 0.63 µM (HCT-116) IC50 = 47.75 ± 0.63 µM (SH-SY5Y) | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceramella, J.; Catalano, A.; Mariconda, A.; D’Amato, A.; Aquila, S.; Saturnino, C.; Rosano, C.; Sinicropi, M.S.; Longo, P. Silver N-Heterocyclic Carbene (NHC) Complexes as Antimicrobial and/or Anticancer Agents. Pharmaceuticals 2025, 18, 9. https://doi.org/10.3390/ph18010009
Ceramella J, Catalano A, Mariconda A, D’Amato A, Aquila S, Saturnino C, Rosano C, Sinicropi MS, Longo P. Silver N-Heterocyclic Carbene (NHC) Complexes as Antimicrobial and/or Anticancer Agents. Pharmaceuticals. 2025; 18(1):9. https://doi.org/10.3390/ph18010009
Chicago/Turabian StyleCeramella, Jessica, Alessia Catalano, Annaluisa Mariconda, Assunta D’Amato, Saveria Aquila, Carmela Saturnino, Camillo Rosano, Maria Stefania Sinicropi, and Pasquale Longo. 2025. "Silver N-Heterocyclic Carbene (NHC) Complexes as Antimicrobial and/or Anticancer Agents" Pharmaceuticals 18, no. 1: 9. https://doi.org/10.3390/ph18010009
APA StyleCeramella, J., Catalano, A., Mariconda, A., D’Amato, A., Aquila, S., Saturnino, C., Rosano, C., Sinicropi, M. S., & Longo, P. (2025). Silver N-Heterocyclic Carbene (NHC) Complexes as Antimicrobial and/or Anticancer Agents. Pharmaceuticals, 18(1), 9. https://doi.org/10.3390/ph18010009