2.1. Method Development and Optimization
During the method development, different chromatographic conditions were tested, considering the chemical structure and physicochemical properties of ritlecitinib. It is a pyrrolopyrimidine that contains three rings, three rotatable bonds, and six heteroatoms (one oxygen and five nitrogen atoms). Four of these are hydrogen acceptors, while two are hydrogen donors. Ritlecitinib is a white to off-white solid that is insoluble in water. Based on data calculated by the ALOGPS v2.1 program, its water solubility is 0.46 mg/mL. It is moderately lipophilic, with predicted log P data in the range of 1.27 (ACD/Labs v9.0 program) to 1.95 (Mcule v1.0 program). Ritlecitinib is a multiprotic compound with dissociation constant (pKa) values ranging from 6.6 (strongest basic) to 13.6 (strongest acidic).
This part of the study aimed to develop a chromatographic method that would allow for fast separation of ritlecitinib and its degradation products, as well as the quantitation of ritlecitinib in stressed samples. As a result, Core-Shell technology was employed as the stationary phase to achieve higher chromatographic efficiencies at substantially lower system backpressures. Furthermore, chromatographic columns packed with smaller particle size (sub-3 µm) were chosen to explore the benefits offered by these columns. Finally, the C18 column showed higher hydrophobic retention suitable for ritlecitinib and its non-polar degradation products in comparison to the C8 column. The reversed-phase C18 columns were also used by Bauman and co-workers [
10] for ritlecitinib metabolite profiling, as well as by Salva and Galla [
11] for the determination of ritlecitinib in rat plasma samples.
After the selection of the stationary phase, the mobile phase composition was optimized to achieve the required retention and selectivity of the chromatographic procedure. The mobile phase modifiers led to different eluent viscosity and UV cut-offs, and these factors were considered during the design of eluent systems as well. Since the volatility and the solvent’s ability to donate a proton are important in the electrospray ionization (ESI) technique, they were also scrutinized when designing eluent systems. Experimental trials revealed that using acetonitrile, compared to methanol and ethanol, resulted in improved peak shapes for ritlecitinib and its degradation products. Different mobile phase modifiers covering acidic to neutral pH were screened. Using lower pH values, solvents offered better separation and enhanced peak resolution. We observed that the detection sensitivity of ritlecitinib and its degradation products was improved using formic acid (p
Ka 3.75) as an additive of the mobile phase for UHPLC-DAD-MS/MS analysis. Overall, these findings follow analytical methods reported by Bauman and co-workers [
10] as well as Salva and Galla [
11].
By analyzing the stressed samples, it became evident that fewer polar degradation products had suitable retention factor values with a lower percentage of aqueous mobile phase, while polar degradation products had appropriate retention factor values with a lower percentage of organic mobile phase. Therefore, the use of a gradient system for increasing eluting power was necessary.
Ritlecitinib showed good absorption between 200 and 300 nm; however, the wavelength of 280 nm was selected for analysis to achieve superior sensitivity and peak shape.
By applying optimized chromatographic conditions, parameters defining the system’s suitability were determined. The system suitability parameters for the proposed method were established at the target concentration of ritlecitinib (10 µg/mL). The proposed analytical method meets ICH and USP standards for system appropriateness. The observed retention time of ritlecitinib was 3.77 min with a method total run time of 13 min, making the method both time- and cost-effective. According to
Table 1, the relative standard deviation (RSD) values did not surpass the recommended value of 2%. Compliance with these factors is crucial in verifying the system’s appropriateness for its intended purpose.
2.2. Method Validation
Using guidelines (Validation of analytical procedures, ICH Q2(R2)), various parameters for the developed stability-indicating method were validated, including linearity, limit of detection (LOD), limit of quantitation (LOQ), precision, accuracy, and standard solution stability [
12].
The ritlecitinib solutions, ranging from 2 to 100 µg/mL, were utilized to create the linearity curve.
Table 2 contains the results of the linear regression study. With a correlation coefficient (
r) value of 0.999, the results demonstrate a significant correlation between observed responses and ritlecitinib concentrations. In addition, the accuracy of the back-calculated concentrations of each calibration standard was evaluated, revealing relative errors within ±2.24% of the theoretical concentration at all investigated levels. Furthermore, residual analysis was conducted to assess the assumptions of linearity and homoscedasticity (
Figure S1). The visual evaluation of the pattern of the residual versus fits plot showed that the residuals appeared to be randomly dispersed around the zero, with no recognizable patterns in the points, revealing that the proposed linear model approximates the data points well without favoring certain inputs.
The LOD and LOQ values were determined experimentally, the method based on signal-to-noise (S/N) ratio was applied. The LOD (S/N = 3) and LOQ (S/N = 10) values were found to be 0.04 and 0.14 µg/mL, indicating the sensitivity of the method (
Table 2).
Method precision was determined by repeatability (intraday), where the analysis of samples at the same concentrations was evaluated on the same day at different time intervals, and by intermediate precision (interday), where the samples were evaluated by comparing the assays on three separate days. Each day six sample solutions (10 µg/mL) were prepared and analyzed; the assay value was expressed in terms of RSD values and the box-and-whisker plot (
Table 2,
Figure S2). Low values of RSDs, ranging from 0.12 to 0.15%, demonstrate acceptable method precision.
Method accuracy was calculated using the regression equation based on percentage recoveries at three concentration levels (low-level 2 µg/mL, medium-level 10 µg/mL, and high-level 100 µg/mL) crossing the linearity curve. All solutions prepared and analyzed in triplicate were experimentally recovered (99.9–100.3%) with low values of RSD (lower than 0.7%) during analysis (
Table 2).
The experimental results demonstrate the high stability of the standard solution. Despite being stored for 8 h at room temperature, for 3 days in a laboratory refrigerator (4 °C), and for 7 days in a laboratory freezer (−20 °C), the stability testing solutions showed excellent agreement with the freshly prepared samples. All stability tests showed no significant difference in ritlecitinib concentrations, with recoveries ranging from 99.2 to 99.7% (
Table 2).
2.3. Forced Degradation Study
Intentional degradation was attempted at stress conditions of acidic (using 0.01–1 M HCl), basic (using 0.01–1 M NaOH), oxidative (using 3–33% H
2O
2), photolytic (daylight degradation), and thermal degradation (heated at 40 and 70 °C) to evaluate the ability of the proposed analytical method to separate ritlecitinib and its degradation products [
13,
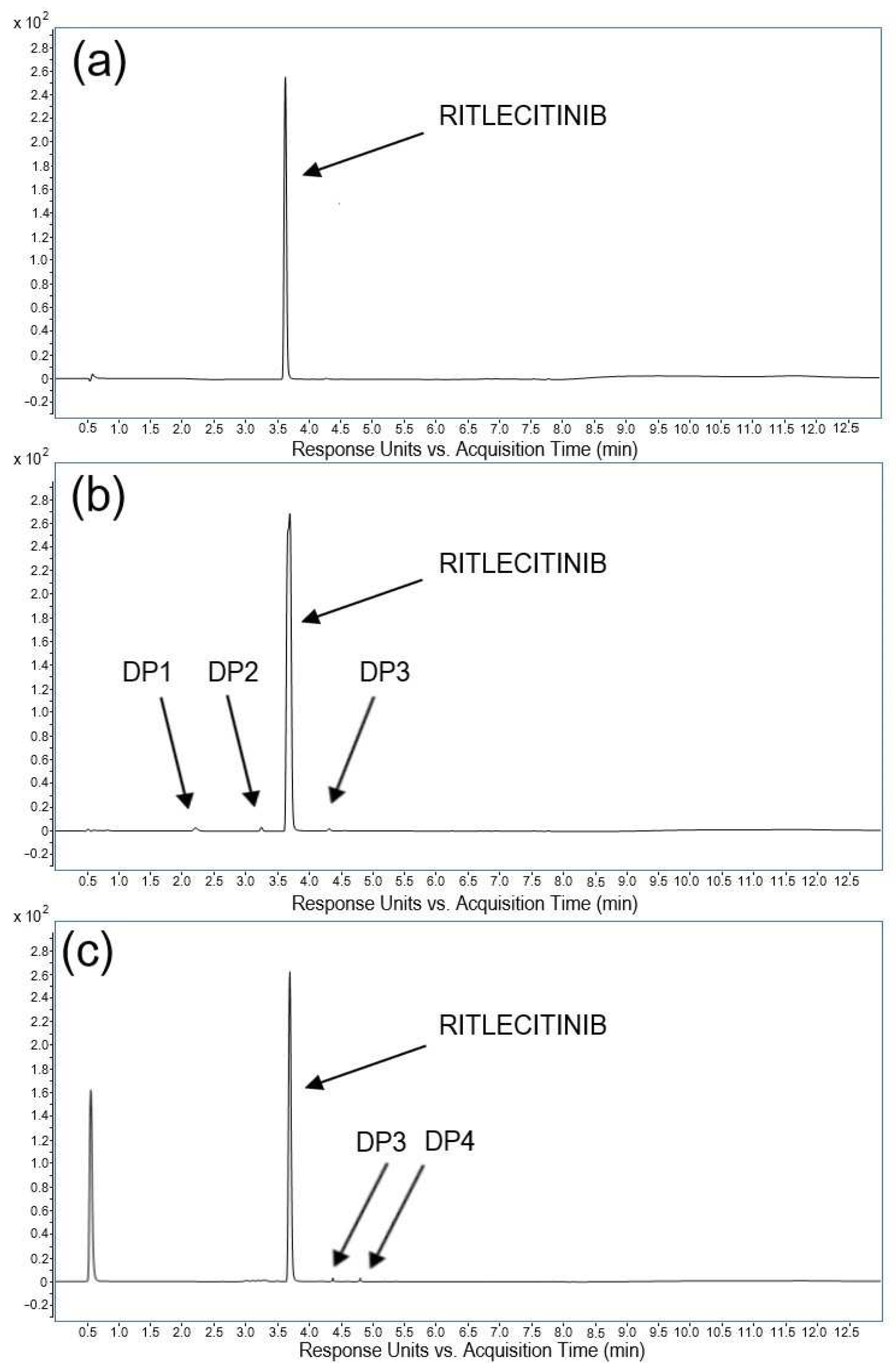
14]. A critical aspect of designing well-structured forced degradation experiments was defining the severity and exposure time for each condition, along with providing meaningful endpoints. The two endpoints, the degradation of the drug molecule up to 10–20% or an exposure time of no more than 10 days, were selected to ensure thorough coverage of pharmaceutically-relevant degradation. The analysis of all stress samples revealed that the retention times for ritlecitinib remained unaltered compared to the control solution (
tR(average) = 3.74 min; RSD = 1.1%). The peak purity of ritlecitinib in all stress samples was above 999.1, with the resolution between the peaks of ritlecitinib and degradation products higher than 6.5. Thus, the concentration of ritlecitinib in stress samples was reliably quantified, and the degradation products were separated, indicating the high specificity of the proposed method as well as its stability-indicating power. The chromatograms depicting the degradation behavior of the ritlecitinib drug substance under different stress conditions are shown in
Figure 2.
The results for the acidic, basic, oxidative, thermal, and photolytic degradation of ritlecitinib are presented in
Table 3. Four novel degradation products, which have not been previously reported, were identified under the applied stress conditions. As per the elution pattern, the degradation products were denoted as DP1 to DP4 (
Table 4).
Over 72 h, ritlecitinib significantly degraded through basic hydrolysis at room temperature. This stress condition resulted in a degradation of 12.6% of ritlecitinib with the appearance of three degradation peaks. Relative retention times (RRTs) of DP1 and DP2 were lower than 1.0, indicating lower hydrophobicity of these degradation products, while the RRT value of DP3 was higher than 1.0, indicating its higher hydrophobicity compared to ritlecitinib.
The degradation of ritlecitinib under oxidative stress, using 33% hydrogen peroxide solution, occurred rapidly, achieving a degradation of 9.5% of ritlecitinib within 48 h. The chromatogram obtained from analyzing oxidative stress illustrates a distinct separation between ritlecitinib and two more hydrophobic degradation products, namely DP3 and DP4.
The acidic degradation study was carried out in 1 M HCl for 10 days under reflux conditions, at room temperature and 70 °C. It was revealed that ritlecitinib is stable to acidic hydrolysis, with a loss of ritlecitinib of less than 1%. For thermal degradation, the ritlecitinib standard solution was kept at 40 and 70 °C for 10 days, but no appreciable degradation occurred under these conditions (less than 1%). The photolytic degradation of ritlecitinib resulted in a loss of analyte of 1% without the formation of a degradant peak.
Our results underscored ritlecitinib’s vulnerability to basic and oxidizing conditions. It was noticed that it was not sensitive to acidic conditions, temperature, and light. These data provide invaluable insights into the stability profile of ritlecitinib, guiding the optimization of synthesis conditions, formulation development, package development of pharmaceutical products, and the development of storage protocols. Moreover, by addressing the stability-related challenges early in the development process, the production of high-quality ritlecitinib formulations with innovative excipients can be ensured.
After testing for accelerated degradation, a kinetic study of basic and oxidative degradation of ritlecitinib was performed. For this purpose, samples were collected at predefined time intervals (at least ten data points were collected before reaching 50% drug degradation). Various kinetic models were used and compared to examine the rate of the basic and oxidative degradation processes. The reaction order was determined by plotting the drug concentration (zero-order), the logarithm of drug concentration (first-order), and the reciprocal of drug concentration (second-order) versus time (all equations are given in
Table S1). The regression equations were computed, the correlation coefficients were calculated, and the reaction order was identified based on the best-fitting model.
Based on the correlation coefficients obtained for zero-order (r = 0.983), first-order (r = 0.989), and second-order model fits (r = 0.994) in the basic degradation kinetic study, it was concluded that the basic degradation of ritlecitinib follows second-order kinetics. Accordingly, basic degradation of ritlecitinib mainly depends on the concentration of the drug substance and stressor.
The correlation coefficient for the zero-order kinetics model in the oxidative degradation kinetic study was 0.999. It was higher than the coefficients derived from first-order (
r = 0.997) and second-order model fits (
r = 0.993). This suggests that the oxidative degradation process followed the zero-order kinetics model, indicating that the oxidative degradation of ritlecitinib does not depend on the concentration of the drug and stressor. It is by now generally accepted that zero-order reactions primarily include rearrangement or radical-mediated cleavage of chemical bonds under oxidative stress conditions [
15].
Rate constant, half-life (
t50), and shelf-life (
t90) are the primary parameters measured in degradation kinetics, presented in
Table 5. Both
t50 and
t90 values were found to be lower for oxidative degradation than basic hydrolysis.
2.4. Mass Spectrometry of Ritlectinib and Its Degradation Products
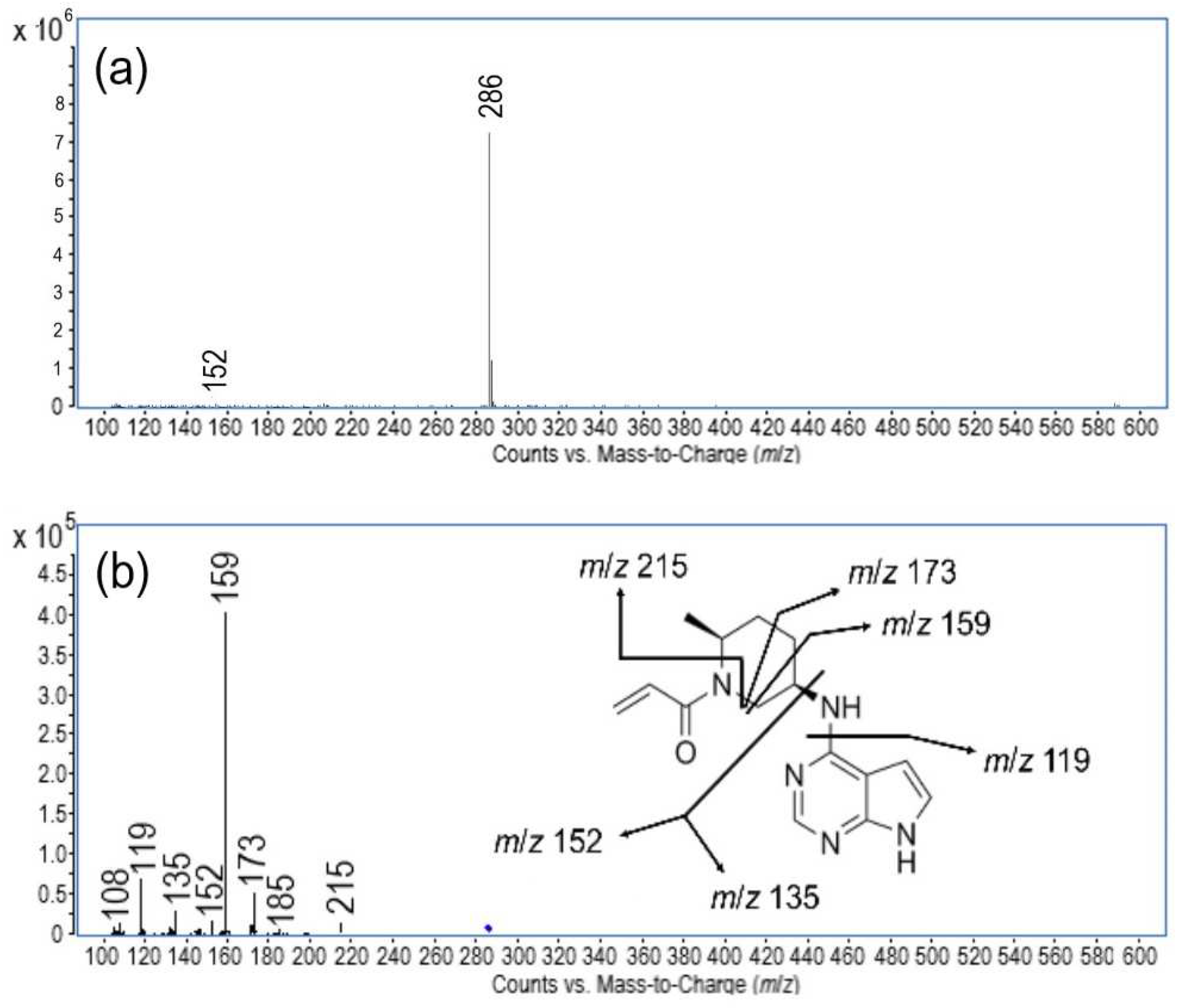
The mass spectrometric experiments were conducted for the mass identification of newly generated degradation products (DP1–DP4). Monitoring was carried out following a detailed analysis of the mass spectrum of ritlecitinib and its fragmentation products, enabling the identification of the characteristic fragmentation processes involved (
Figure 3). Under positive ESI-MS conditions, the prominent molecular peak [M + H]
+ at
m/
z 286 was observed, confirming that the molecular formula for ritlecitinib is C
15H
19N
5O. Thereafter, MS/MS experiments were conducted to acquire a thorough understanding of its fragmentation behavior, facilitating the establishment of a defined fragmentation pattern for ritlecitinib and its degradation products. The fragmentation pattern observed for ritlecitinib is shown in
Figure 3. A total of six fragments were distinguished. The first fragment, having
m/
z 215, was obtained due to the neutral loss of the prop-2-enamide. Further cleavage of the piperidine ring resulted in the formation of the fragment at
m/
z 173. The loss of the methyl group gave rise to the prominent fragment having
m/
z 159. The next fragments, with
m/
z 152 and 135, were observed due to cleavage around the central nitrogen. The fragment ion at
m/
z 119 was generated due to the removal of the amino group from the fragment at
m/
z 135, forming 7H-pyrrolo [2,3-d]pyrimidine. To our knowledge, only one report on the pharmacokinetics, metabolism, and clearance mechanism of ritlecitinib, including its mass spectrometric data, is available in the literature [
10]. Our proposed fragmentation pattern of ritlecitinib bears a close resemblance to the previously published one. However, due to the careful optimization of the mass spectrometry parameters, our procedure revealed three new fragments.
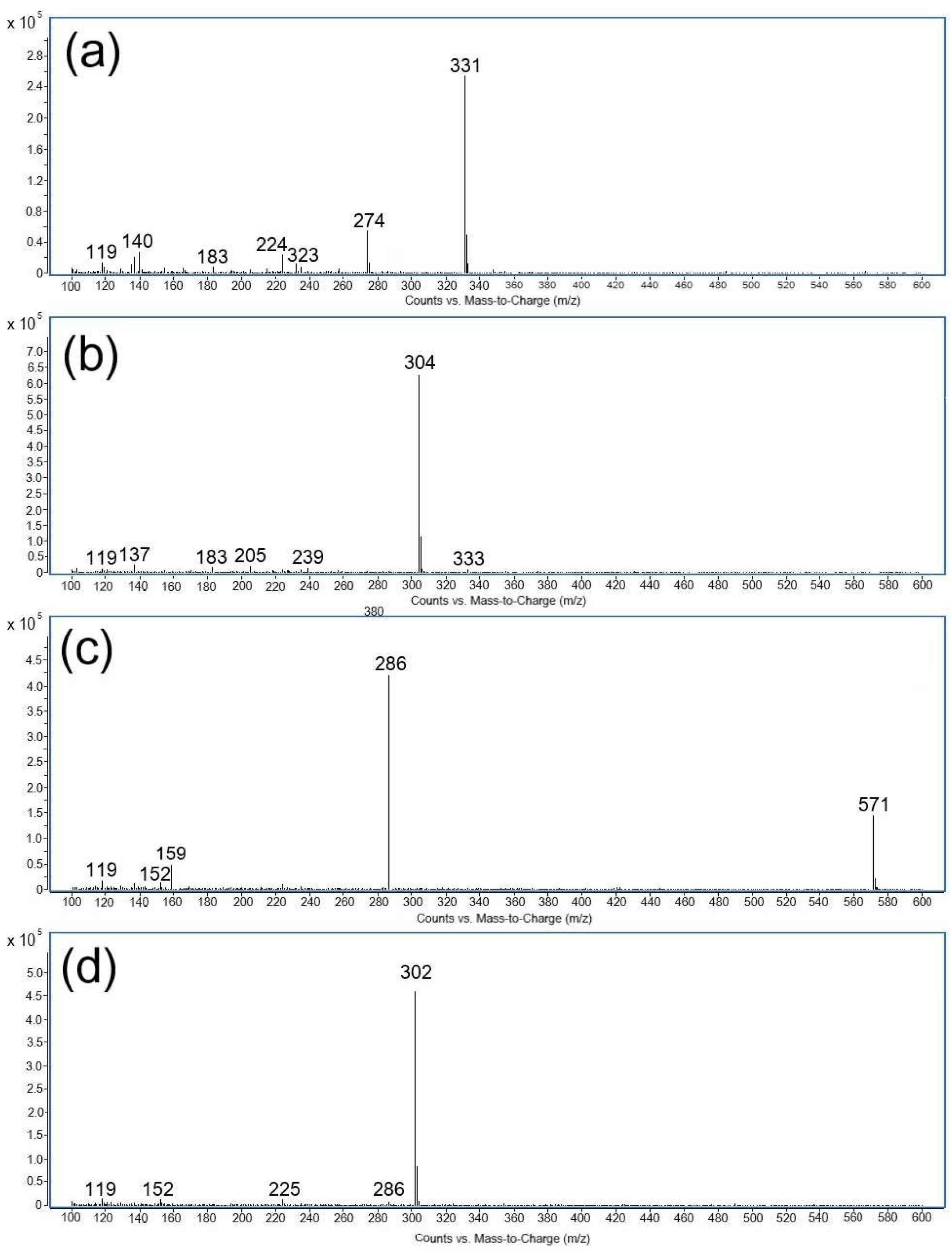
The formation of degradation products was evaluated based on the mass fragmentation pattern of ritlecitinib as well as its degradation products, identifying the underlying chemical transformations. Furthermore, this was complemented by the EPFL Mass Spectrometry online tool, chemical knowledge, and the conditions of impurity formation. The mass spectra of all four degradation products (DP1-DP4) are shown in
Figure 4. The retention times, relative retention times (RRTs), main peaks, along with major fragments of all the degradation products, are summarized in
Table 4.
DP1 (base peak at m/z 331) and DP2 (base peak at m/z 304), derived from the basic hydrolysis of ritlecitinib, showed RRT values lower than 1. Both degradation products generated a simple MS spectrum with the formation of one base peak and several minor fragment ions. In the case of DP2, the experimental m/z value of the base peak was 304, which is 18 Da higher than the molecular ion of ritlecitinib [M + H]+. It is presumed that this polar product is generated under basic conditions due to the 1,4 addition of a hydroxyl group across the alkene bond of the α, β-unsaturated carbonyl present in the acrylamide moiety of ritlecitinib.
DP3 is produced under both stress conditions, basic hydrolysis and oxidative stress. The most important peak of DP3 was found to be at
m/
z 571, as shown in
Figure 4 and
Table 4. Due to its apolar nature, it elutes after ritlecitinib with an RRT of 1.16. Three ions were also observed for DP3, having
m/
z 286, 159, 152, and 119. Ritlecitinib’s structure contains an acrylamide moiety, which is prone to the formation of dimers featuring either C-C or C-N linked chains, with the latter arising from Michael addition. Based on the aforementioned data on the MS spectrum of DP3 and its apolar nature, a possible structure of DP3 could be proposed as the ritlecitinib dimer. Recent work of Chum and co-workers [
16] highlighted the importance of determining ritlecitinib dimers, as this impurity may affect the crystallization of the drug.
The second apolar degradation product of oxidative stress (DP4) was observed at the highest retention time (RRT = 1.27). In the MS spectrum, the base peak of DP4 was found to be at
m/
z 302 in positive mode, indicating a molecular weight of 301 Da, which is 16 Da higher than the molecular weight of ritlecitinib. As described in the literature, hydrogen peroxide can react with tertiary amines, which are susceptible to electron transfer oxidation, producing N-oxides [
17]. The difference of 16 Da corresponds to oxygen and may be related to the formation of an N-oxide in the piperidine ring of ritlecitinib. Other peaks obtained in the MS spectrum of DP4 showed three characteristic peaks at
m/
z 286, 152, and 119. The formation of the peak at
m/
z 286 could be attributed to the loss of an oxygen atom [M + H − O]
+. This fragmentation pattern has already been described for N-oxides [
18]. Two other peaks at
m/
z 152 and 119 were also observed in the MS spectrum of ritlecitinib.
2.5. Assessment of Sustainability of Forced Degradation Procedure
We used seven green metric tools to evaluate the green performance of the developed forced degradation procedure and its subsequent impact on the environment.
The Analytical Method Greenness Score (AMGS) tool was used in the greenness assessment because it is a composite of several metrics, including the operator’s safety and procedure’s environmental impact [
19]. The assigned greenness score of the forced degradation procedure of ritlecitinib, using the developed UHPLC/DAD/MS/MS method, was 711.85. Based on the calculated data, the highest contribution to the greenness score came from instrument energy (436.25, or 61.3% of overall score), followed by solvent influence (182.05, or 25.6% of overall score) and environment, health, and safety (99.55, or 13.1% of overall score). The scoring also includes a color-coding system that indicates the contribution of each category to the overall score. A green color highlights the green parameters of the method, while yellow and red colors indicate areas where the method can be improved. For the ritlecitinib forced degradation study, the category of instrument energy contributed over 50% of the overall AMGS score and is thus indicated in red. According to the AMGS score, the weakest point of our method is the energy consumption by the LC/MS technique; therefore, we attempted to reduce the instrument energy by shortening the method run time as much as possible.
The Analytical Eco-Scale [
20] assigns a score out of 100, and our procedure accrued a total of 16 penalty points. Eleven penalty points were assigned due to the reagents used for the promotion of ritlecitinib degradation, two penalty points due to energy consumption of the LC/MS technique, and three points due to waste generation. Nonetheless, a score of 84 demonstrates the procedure’s strong ecological sustainability and environmental friendliness.
Płotka-Wasylka and co-workers [
21,
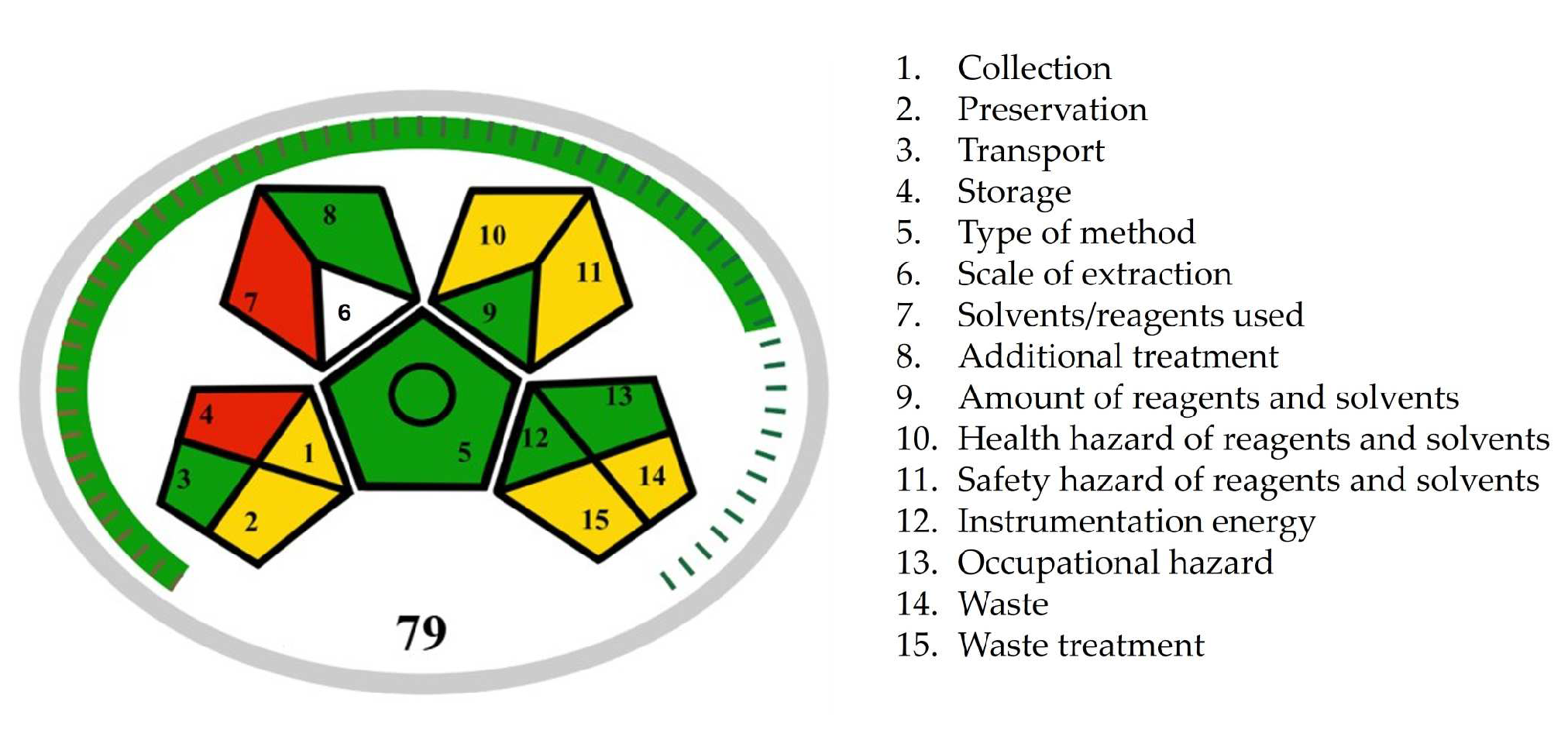
22] developed complementary metric tools Green Analytical Procedure Index (GAPI), along with its recent versions Modified Green Analytical Procedure Index (MoGAPI), and Complementary Green Analytical Procedure Index (ComplexGAPI). MoGAPI evaluated the environmental hazards of our procedure using a central pentagon surrounded by four additional pentagons mirroring each of its sides, excluding the bottom. Each pentagon assessed specific aspects of the methodology using three colors (green, yellow, and red), where green represents the most eco-friendly method aspects and red indicates non-ecofriendly method aspects (
Figure 5). According to the MoGAPI pictogram, the ritlecitinib forced degradation study showed six green, six yellow, and two red areas. The yellow areas (fields 10, 11, 14, and 15) are related to the usage of organic solvents and formic acid as mobile phase modifiers, leading to chemical waste generation. The red areas (fields 4 and 7) are attributed to the preparation of forced degraded samples, particularly the use of stressors and high temperatures. We need to be aware that method development and validation, as well as the evaluation of degradation kinetics for ritlecitinib, included numerous sample analyses that needed to be performed. Furthermore, the use of stress conditions in forced degradation studies is inevitable. Therefore, during the method development, we prioritized the minimization of sample size and method run time and, alongside that, the minimization of waste production. The MoGAPI tool also offered an overall assessment of the method’s greenness by calculating a total score. The total score of 79 for the ritlecitinib forced degradation study appeared on the chart, and the green color of the scale around the pentagrams indicated a high overall greenness score.
ComplexGAPI expands the pictogram by adding a hexagonal field at the bottom to represent the environmental impact of pre-analysis processes [
22]. The ComplexGAPI pictogram indicated six green areas, two yellow areas, and one red area of the hexagonal grid (
Figure 6). Again, the red area (field II) is attributed to the use of a high temperature for the thermal stress study, while the two yellow areas (fields IV
a and IV
b) are attributed to the use of acids, bases, and oxidants for the induction of chemical stress, although low amounts of these reagents were used.
The Analytical GREEnness Metric Approach and Software (AGREE) v1.0 tool is based on twelve consecutive assessment steps that correspond to the ten principles of green analytical chemistry [
23]. The result is presented as a round green pictogram with a circle in the center that shows an overall score of 0.71, indicating that the developed method complies with the principles of green analytical chemistry, despite several limitations (
Figure 7). The segments display scores in the range of 0.33–1.00. A score of 1, indicating high greenness and depicted by the deepest shade of green, is assigned for three criteria (criteria 4, 6, and 9). Scores in the range of 0.60–0.80, with the color transitions gradually from dark green to light green, are assigned for five criteria (criteria 2 and 5 — score 0.75; criterion 8 — score 0.68; criterion 11 — score 0.80; and criterion 12 — score 0.60). As previously mentioned, one of the most critical points of our procedure is related to the usage of hazardous chemicals, making criteria 1 and 10 highlighted in yellow with scores of 0.48 and 0.50, respectively. Each sample analysis generates approximately 10 mL of waste, placing criterion 7 at the bottom of the scale (score 0.39, highlighted in orange). The main limitation regarding greenness is associated with the sampling procedure. Since in situ sampling cannot be applied in a forced degradation study, criterion 3 is scored with 0.33 and highlighted in orange. Despite these constraints, the overall methodology was considered environmentally friendly, as indicated by the high overall score, eight out of twelve green areas, and the absence of red areas in the AGREE pictogram.
Sample preparation in the forced degradation study has been identified as one of the most critical steps from the perspective of green analytical chemistry [
8,
9], primarily due to the typical requirements for reagents and energy consumption. Thus, we used the Analytical GREEnness Metric for Sample Preparation (AGREEprep) tool, which emphasizes sample preparation. It predicted, as well as identified, aspects of the developed forced degradation study that could be improved for greening the critical step of sample preparation. The key strength of the AGREEprep tool lies in its comprehensive coverage of all ten green sample preparation principles, offering a detailed evaluation of each aspect through a diverse range of colors [
24].
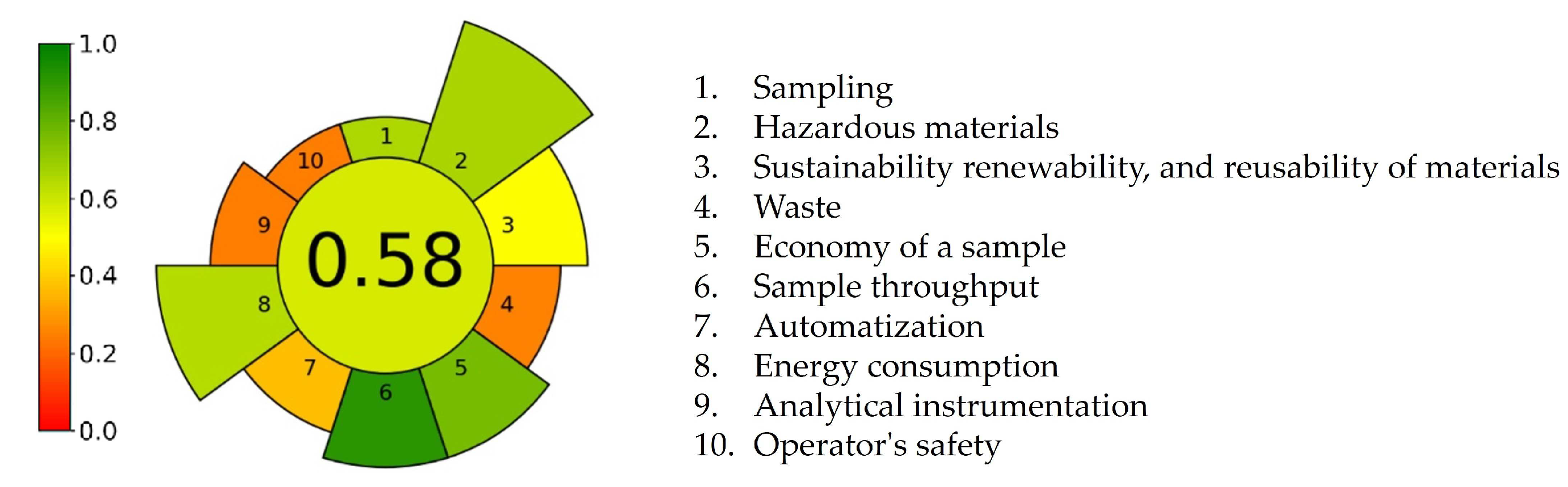
Figure 8 depicts a round AGREEprep pictogram with a circle in the middle that shows an overall score of 0.58, colored in light green. Ten trapezoid bars correspond to the ten criteria. Five out of ten criteria are marked with various shades of green, with scores between 0.64 and 0.92. These green points were obtained due to high sample preparation throughput, low sample size, and moderate consumption of hazardous reagents. The third principle of green sample preparation promotes the use of reusable, renewable, and sustainable resources. Since we utilized recycled materials and reusable laboratory glassware whenever possible, the corresponding bar is highlighted in yellow with a score of 0.5. Four bars, representing the waste generation (criterion 4, score 0.26), automatization of procedure (criterion 7, score 0.38), analytical technique used for sample analysis (criterion 9, score 0.25), and operator safety (criterion 10, score 0.25), are shown in orange, denoting the weakest points of the developed procedure.
In recent years, the idea of white analytical chemistry has also been promoted as an extension and complement to green analytical chemistry. It combines the ecological, analytical, and practical perspectives of an analytical method [
25]. To obtain a more comprehensive approach to the sustainability of the proposed procedure, the Blue Applicability Grade Index (BAGI) approach was applied [
26].
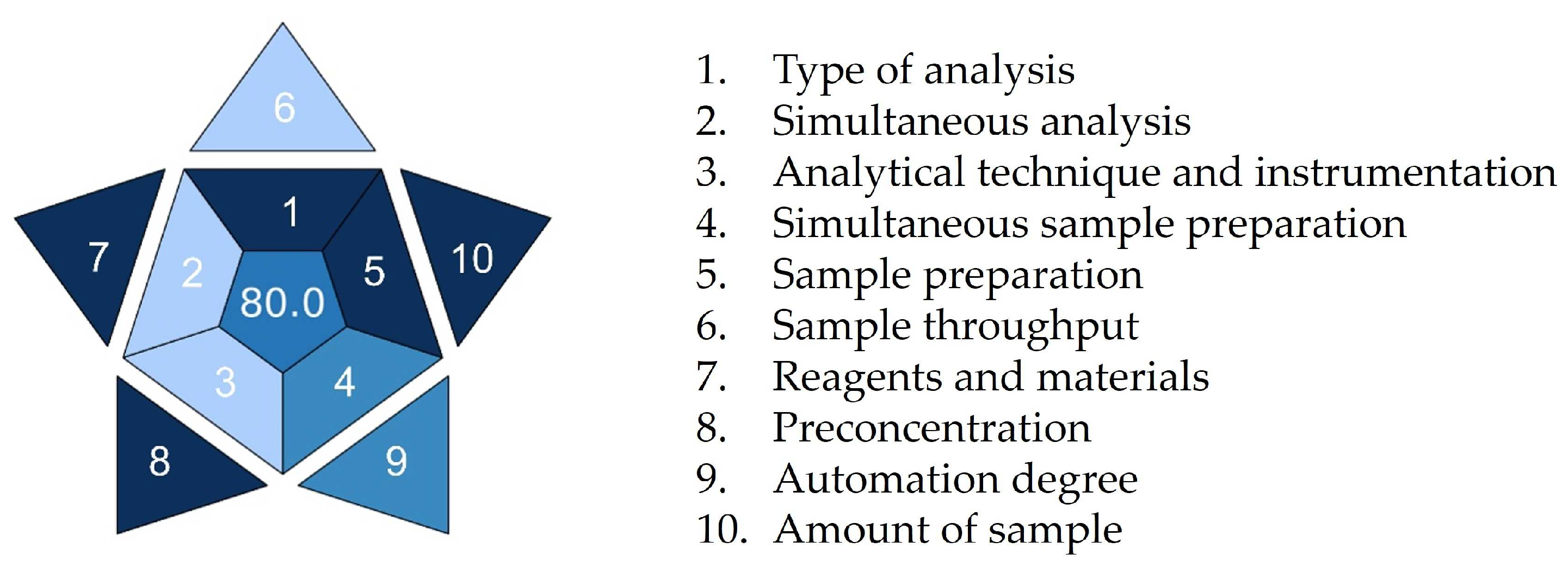
Figure 9 shows an asteroid pictogram demonstrating the practicability of our procedure. A BAGI score of 80 was assigned to the procedure, demonstrating its superiority in terms of practicality and applicability. The shades of blue assigned to the individual pictogram fields indicate the weak and strong points of the analytical procedure. The biggest advantages of the proposed procedure are undemanding sample preparation, low sample quantity, and usage of commonly commercially available reagents (criteria 1, 5, 7, 8, and 10 highlighted in dark blue for high compliance). Semi-automated devices were used for sample preparation, making criteria 4 and 9 highlighted in blue for medium compliance. On the other hand, due to limited sample analysis capacity per hour, concurrent analyte determination capacity, and instrumentation requirements, criteria 2, 3, and 6 are highlighted in light blue for low compliance. It is encouraging that none of the procedure’s aspects were highlighted in white, demonstrating no compliance.