Cancer Metastases to the Liver: Mechanisms of Tumor Cell Colonization
Abstract
1. Introduction
2. Neovascularization
3. The Recruitment to the Liver
4. Cell Adhesion
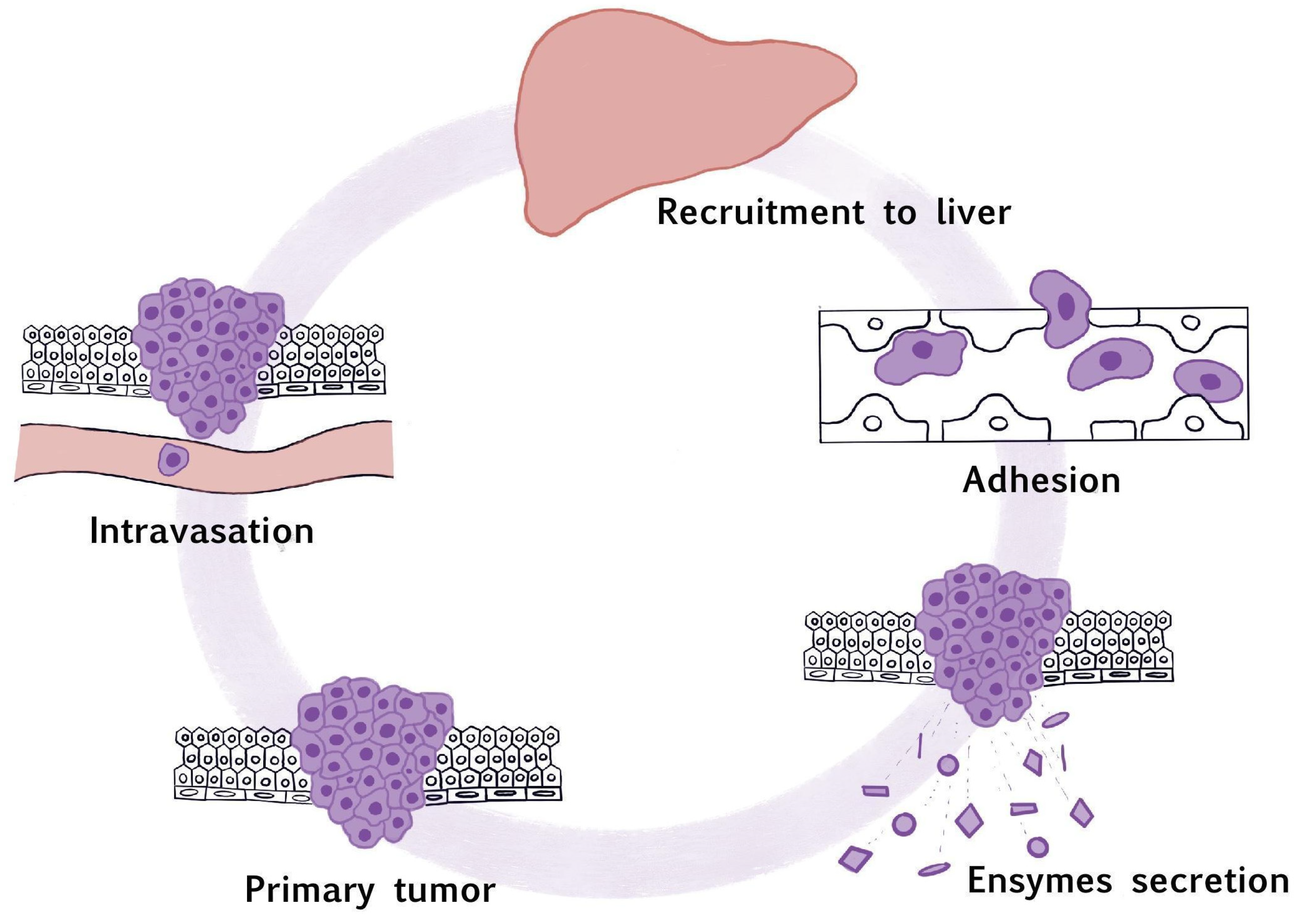

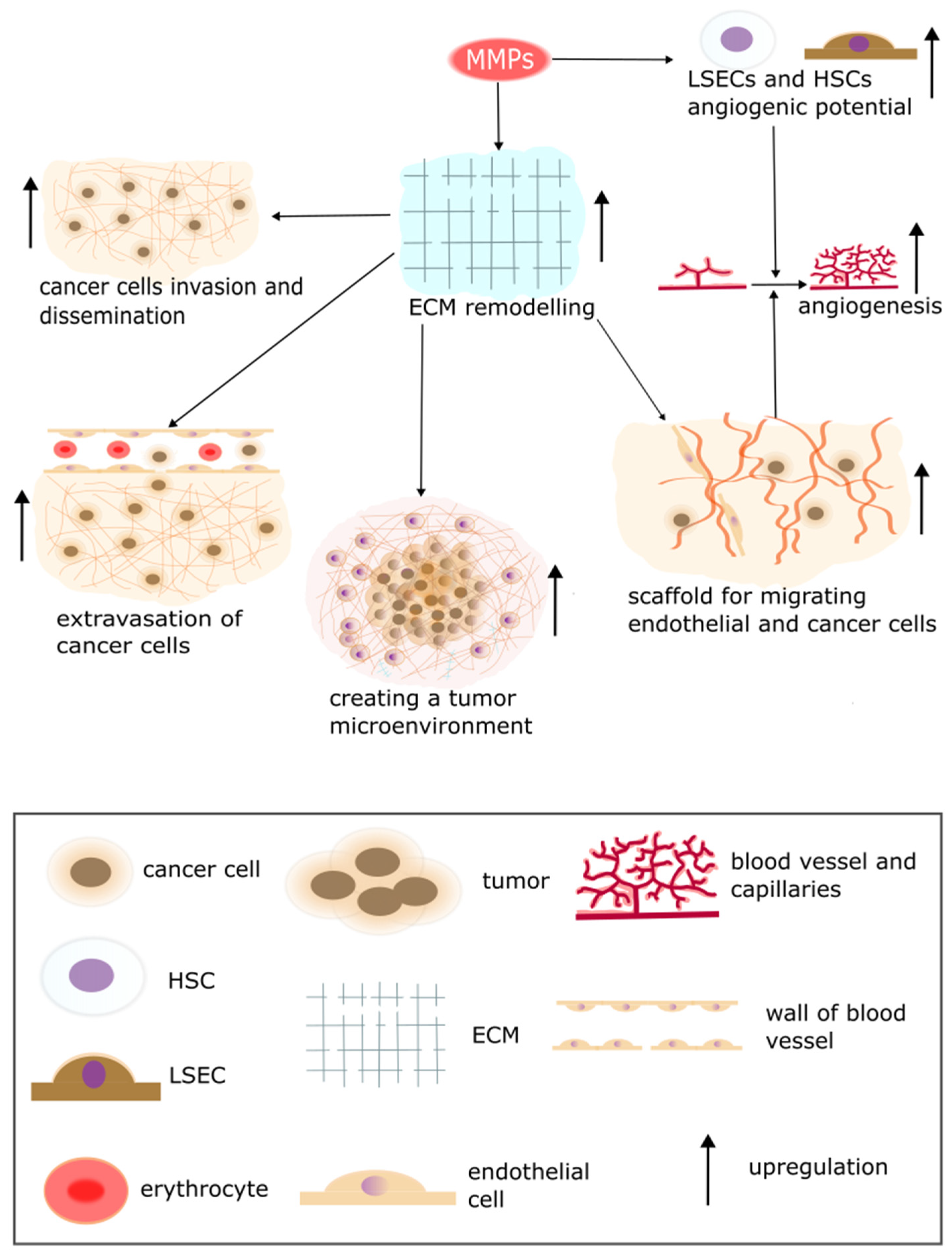
5. The Secretion of Proteolytic Enzymes
6. Methods for Inhibiting Liver Metastasis
6.1. Pharmacological Methods
6.2. Other Liver Metastases Treatment Approaches
7. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Gerstberger, S.; Jiang, Q.; Ganesh, K. Metastasis. Cell 2023, 186, 1564–1579. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.M.; Ma, B.; Taylor, D.L.; Griffith, L.; Wells, A. Liver Metastases: Microenvironments and Ex-Vivo Models. Exp. Biol. Med. 2016, 241, 1639. [Google Scholar] [CrossRef] [PubMed]
- Keirsse, J.; Van Damme, H.; Geeraerts, X.; Beschin, A.; Raes, G.; Van Ginderachter, J.A. The Role of Hepatic Macrophages in Liver Metastasis. Cell Immunol. 2018, 330, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Beatty, G.L. Inflammatory Networks Cultivate Cancer Cell Metastasis to the Liver. Cell Cycle 2020, 19, 642. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.G. Cell Adhesion Molecules in the Formation of Liver Metastasis. J. Hepatobiliary Pancreat. Surg. 1998, 5, 375–382. [Google Scholar] [CrossRef]
- Brodt, P. Role of the Microenvironment in Liver Metastasis: From Pre- to Prometastatic Niches. Clin. Cancer Res. 2016, 22, 5971–5982. [Google Scholar] [CrossRef]
- Castaneda, M.; den Hollander, P.; Kuburich, N.A.; Rosen, J.M.; Mani, S.A. Mechanisms of Cancer Metastasis. Semin. Cancer Biol. 2022, 87, 17–31. [Google Scholar] [CrossRef]
- Kim, K.J.; Li, B.; Winer, J.; Armanini, M.; Gillett, N.; Phillips, H.S.; Ferrara, N. Inhibition of Vascular Endothelial Growth Factor-Induced Angiogenesis Suppresses Tumour Growth in Vivo. Nature 1993, 362, 841–844. [Google Scholar] [CrossRef]
- Zhang, T.; Wahib, R.; Zazara, D.E.; Lücke, J.; Shiri, A.M.; Kempski, J.; Zhao, L.; Agalioti, T.; Machicote, A.P.; Giannou, O.; et al. CD4+ T Cell-Derived IL-22 Enhances Liver Metastasis by Promoting Angiogenesis. Oncoimmunology 2023, 12, 2269634. [Google Scholar] [CrossRef]
- Dvorak, H.F. Tumors: Wounds That Do Not Heal—Redux. Cancer Immunol. Res. 2015, 3, 1–11. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, C. The Role of Liver Sinusoidal Endothelial Cells in Cancer Liver Metastasis. Am. J. Cancer Res. 2021, 11, 1845–1860. [Google Scholar] [PubMed]
- Svistounov, D.; Warren, A.; McNerney, G.P.; Owen, D.M.; Zencak, D.; Zykova, S.N.; Crane, H.; Huser, T.; Quinn, R.J.; Smedsrød, B.; et al. The Relationship between Fenestrations, Sieve Plates and Rafts in Liver Sinusoidal Endothelial Cells. PLoS ONE 2012, 7, e46134. [Google Scholar] [CrossRef] [PubMed]
- Huu Hoang, T.; Sato-Matsubara, M.; Yuasa, H.; Matsubara, T.; Thuy, L.T.T.; Ikenaga, H.; Phuong, D.M.; Hanh, N.V.; Hieu, V.N.; Hoang, D.V.; et al. Cancer Cells Produce Liver Metastasis via Gap Formation in Sinusoidal Endothelial Cells through Proinflammatory Paracrine Mechanisms. Sci. Adv. 2022, 8, eabo5525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, W.J.; Cai, H.Q.; Liu, H.L.; Peng, L.; Li, C.H.; Ye, L.Y.; Xu, S.Q.; Yang, Z.H.; Lou, J.N. Platelet Adhesion and Fusion to Endothelial Cell Facilitate the Metastasis of Tumor Cell in Hypoxia-Reoxygenation Condition. Clin. Exp. Metastasis 2011, 28, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Yin, S.; Yang, S.; Jiang, X.; Wang, J.; Zhang, M.; Zhang, L. Roles of Platelets in Tumor Invasion and Metastasis: A Review. Heliyon 2022, 8, e12072. [Google Scholar] [CrossRef]
- Haemmerle, M.; Stone, R.L.; Menter, D.G.; Afshar-Kharghan, V.; Sood, A.K. The Platelet Lifeline to Cancer: Challenges and Opportunities. Cancer Cell 2018, 33, 965–983. [Google Scholar] [CrossRef]
- Kopp, H.G.; Placke, T.; Salih, H.R. Platelet-Derived Transforming Growth Factor-β down-Regulates NKG2D Thereby Inhibiting Natural Killer Cell Antitumor Reactivity. Cancer Res. 2009, 69, 7775–7783. [Google Scholar] [CrossRef]
- Lutz, M.S.; Klimovich, B.; Maurer, S.; Heitmann, J.S.; Märklin, M.; Zekri, L.; Jung, G.; Salih, H.R.; Hinterleitner, C. Platelets Subvert Antitumor Efficacy of T Cell-Recruiting Bispecific Antibodies. J. Immunother. Cancer 2022, 10, 3655. [Google Scholar] [CrossRef]
- Rachidi, S.; Metelli, A.; Riesenberg, B.; Wu, B.X.; Nelson, M.H.; Wallace, C.; Paulos, C.M.; Rubinstein, M.P.; Garrett-Mayer, E.; Hennig, M.; et al. Platelets Subvert T Cell Immunity Against Cancer via GARP-TGFβ Axis. Sci. Immunol. 2017, 2, eaai7911. [Google Scholar] [CrossRef]
- Bambace, N.M.; Levis, J.E.; Holmes, C.E. The Effect of P2Y-Mediated Platelet Activation on the Release of VEGF and Endostatin from Platelets. Platelets 2010, 21, 85–93. [Google Scholar] [CrossRef]
- Terayama, N.; Terada, T.; Nakanuma, Y. An Immunohistochemical Study of Tumour Vessels in Metastatic Liver Cancers and the Surrounding Liver Tissue. Histopathology 1996, 29, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Shiga, K.; Hara, M.; Nagasaki, T.; Sato, T.; Takahashi, H.; Takeyama, H. Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth. Cancers 2015, 7, 2443–2458. [Google Scholar] [CrossRef] [PubMed]
- Birgani, M.T.; Carloni, V. Tumor Microenvironment, a Paradigm in Hepatocellular Carcinoma Progression and Therapy. Int. J. Mol. Sci. 2017, 18, 405. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, T.; Hara, M.; Shiga, K.; Takeyama, H. Relationship between Inflammation and Cancer Progression: Recent Advances in Interleukin-6 Signaling and Its Blockage in Cancer Therapy. Recept. Clin. Investig. 2014, 1, 202. [Google Scholar] [CrossRef]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal Fibroblasts Present in Invasive Human Breast Carcinomas Promote Tumor Growth and Angiogenesis through Elevated SDF-1/CXCL12 Secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef]
- Vong, S.; Kalluri, R. The Role of Stromal Myofibroblast and Extracellular Matrix in Tumor Angiogenesis. Genes. Cancer 2011, 2, 1139. [Google Scholar] [CrossRef]
- Cirri, P.; Chiarugi, P. Cancer Associated Fibroblasts: The Dark Side of the Coin. Am. J. Cancer Res. 2011, 1, 482. [Google Scholar]
- Rossetto, A.; De Re, V.; Steffan, A.; Ravaioli, M.; Miolo, G.; Leone, P.; Racanelli, V.; Uzzau, A.; Baccarani, U.; Cescon, M. Carcinogenesis and Metastasis in Liver: Cell Physiological Basis. Cancers 2019, 11, 1731. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Z.; Wang, Y.; Wen, X.; Amador, E.H.; Yuan, L.; Ran, X.; Xiong, L.; Ran, Y.; Chen, W.; et al. Colorectal Liver Metastasis: Molecular Mechanism and Interventional Therapy. Signal Transduct. Target. Ther. 2022, 7, 70. [Google Scholar] [CrossRef]
- Lukacs-Kornek, V. The Role of Lymphatic Endothelial Cells in Liver Injury and Tumor Development. Front. Immunol. 2016, 7, 29. [Google Scholar] [CrossRef]
- Roy, S.; Banerjee, P.; Ekser, B.; Bayless, K.; Zawieja, D.; Alpini, G.; Glaser, S.S.; Chakraborty, S. Targeting Lymphangiogenesis and Lymph Node Metastasis in Liver Cancer. Am. J. Pathol. 2021, 191, 2052. [Google Scholar] [CrossRef] [PubMed]
- Nyström, H. Extracellular Matrix Proteins in Metastases to the Liver—Composition, Function and Potential Applications. Semin. Cancer Biol. 2021, 71, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Yuzhalin, A.E.; Gordon-Weeks, A.N.; Tognoli, M.L.; Jones, K.; Markelc, B.; Konietzny, R.; Fischer, R.; Muth, A.; O’Neill, E.; Thompson, P.R.; et al. Colorectal Cancer Liver Metastatic Growth Depends on PAD4-Driven Citrullination of the Extracellular Matrix. Nat. Commun. 2018, 9, 4783. [Google Scholar] [CrossRef] [PubMed]
- van Huizen, N.A.; Coebergh van den Braak, R.R.J.; Doukas, M.; Dekker, L.J.M.; IJzermans, J.N.M.; Luider, T.M. Up-Regulation of Collagen Proteins in Colorectal Liver Metastasis Compared with Normal Liver Tissue. J. Biol. Chem. 2019, 294, 281–289. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Ntanasis-Stathopoulos, I.; Pawlik, T.M. Molecular Mechanisms of Colorectal Liver Metastases. Cells 2023, 12, 1657. [Google Scholar] [CrossRef] [PubMed]
- Taura, K.; De Minicis, S.; Seki, E.; Hatano, E.; Iwaisako, K.; Osterreicher, C.H.; Kodama, Y.; Miura, K.; Ikai, I.; Uemoto, S.; et al. Hepatic Stellate Cells Secrete Angiopoietin 1 That Induces Angiogenesis in Liver Fibrosis. Gastroenterology 2008, 135, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic Cancer Exosomes Initiate Pre-Metastatic Niche Formation in the Liver. Nat. Cell Biol. 2015, 17, 816. [Google Scholar] [CrossRef]
- Matsumoto, K.; Nakamura, T. Hepatocyte Growth Factor: Renotropic Role and Potential Therapeutics for Renal Diseases. Kidney Int. 2001, 59, 2023–2038. [Google Scholar] [CrossRef]
- Matsumoto, K.; Umitsu, M.; De Silva, D.M.; Roy, A.; Bottaro, D.P. Hepatocyte Growth Factor/MET in Cancer Progression and Biomarker Discovery. Cancer Sci. 2017, 108, 296. [Google Scholar] [CrossRef]
- Parikh, R.A.; Wang, P.; Beumer, J.H.; Chu, E.; Appleman, L.J. The Potential Roles of Hepatocyte Growth Factor (HGF)-MET Pathway Inhibitors in Cancer Treatment. Onco Targets Ther. 2014, 7, 969. [Google Scholar] [CrossRef]
- Stoker, M.; Gherardi, E.; Perryman, M.; Gray, J. Scatter Factor Is a Fibroblast-Derived Modulator of Epithelial Cell Mobility. Nature 1987, 327, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Extracellular Signal-Regulated Kinase and Akt Activation Play a Critical Role in the Process of Hepatocyte Growth Factor-Induced Epithelial-Mesenchymal Transition. Available online: https://www.spandidos-publications.com/ijo/42/2/556 (accessed on 20 February 2024).
- Perdomo, G.; Martinez-Brocca, M.A.; Bhatt, B.A.; Brown, N.F.; O’Doherty, R.M.; Garcia-Ocaña, A. Hepatocyte Growth Factor Is a Novel Stimulator of Glucose Uptake and Metabolism in Skeletal Muscle Cells. J. Biol. Chem. 2008, 283, 13700. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zhang, Z.; Zhu, C.; Ni, B.; Wang, S.; Yang, S.; Yu, F.; Zhao, E.; Li, Q.; Zhao, G. Neutrophil Extracellular Traps Promote Metastasis in Gastric Cancer Patients with Postoperative Abdominal Infectious Complications. Nat. Commun. 2022, 13, 1017. [Google Scholar] [CrossRef] [PubMed]
- Spicer, J.; Brodt, P.; Ferri, L. Role of Inflammation in the Early Stages of Liver Metastasis. Cancer Metastasi Biol. Treat. 2011, 16, 155–185. [Google Scholar] [CrossRef]
- Bendas, G.; Borsig, L. Cancer Cell Adhesion and Metastasis: Selectins, Integrins, and the Inhibitory Potential of Heparins. Int. J. Cell Biol. 2012, 2012, 676731. [Google Scholar] [CrossRef]
- Fang, C.; Fan, C.; Wang, C.; Huang, Q.; Meng, W.; Yu, Y.; Yang, L.; Hu, J.; Li, Y.; Mo, X.; et al. Prognostic Value of CD133+ Circulating Tumor Cells in Colorectal Cancer with Liver Metastasis. Cancer Med. 2017, 6, 2850. [Google Scholar] [CrossRef]
- Benedicto, A.; Romayor, I.; Arteta, B. Role of Liver ICAM-1 in Metastasis. Oncol. Lett. 2017, 14, 3883. [Google Scholar] [CrossRef]
- Sun, J.J.; Zhou, X.D.; Liu, Y.K.; Tang, Z.Y.; Feng, J.X.; Zhou, G.; Xue, Q.; Chen, J. Invasion and Metastasis of Liver Cancer: Expression of Intercellular Adhesion Molecule 1. J. Cancer Res. Clin. Oncol. 1999, 125, 28–34. [Google Scholar] [CrossRef]
- Metastasis and Cell Adhesion Pathway|Abcam. Available online: https://www.abcam.com/en-dk/technical-resources/pathways/cell-adhesion-and-metastasis-pathway (accessed on 20 February 2024).
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The Biology and Role of CD44 in Cancer Progression: Therapeutic Implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Brodt, P.; Clavien, P.A.; Muschel, R.J.; D’Angelica, M.I.; Endo, I.; Parks, R.W.; Doyle, M.; de Santibañes, E.; Pawlik, T.M. Liver Metastases. Nat. Rev. Dis. Primers 2021, 7, 27. [Google Scholar] [CrossRef]
- Chandra, R.; Karalis, J.D.; Liu, C.; Murimwa, G.Z.; Park, J.V.; Heid, C.A.; Reznik, S.I.; Huang, E.; Minna, J.D.; Brekken, R.A. The Colorectal Cancer Tumor Microenvironment and Its Impact on Liver and Lung Metastasis. Cancers 2021, 13, 6206. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Obenauf, A.C. Metastatic Colonization by Circulating Tumour Cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, X. Characteristics and Significance of the Pre-Metastatic Niche. Cancer Cell 2016, 30, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, R.; Kawada, K.; Itatani, Y.; Ogawa, R.; Kiyasu, Y.; Sakai, Y. The Role of Tumor-Associated Neutrophils in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 529. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of Extracellular Matrix Remodelling in Tumour Progression and Metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Paolillo, M.; Schinelli, S. Extracellular Matrix Alterations in Metastatic Processes. Int. J. Mol. Sci. 2019, 20, 4947. [Google Scholar] [CrossRef]
- Weidle, U.H.; Birzele, F.; Krüger, A. Molecular Targets and Pathways Involved in Liver Metastasis of Colorectal Cancer. Clin. Exp. Metastasis 2015, 32, 623–635. [Google Scholar] [CrossRef]
- Walker, C.; Mojares, E.; Del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef]
- Najafi, M.; Farhood, B.; Mortezaee, K. Extracellular Matrix (ECM) Stiffness and Degradation as Cancer Drivers. J. Cell Biochem. 2019, 120, 2782–2790. [Google Scholar] [CrossRef]
- Golán-Cancela, I.; Caja, L. The TGF-β Family in Glioblastoma. Int. J. Mol. Sci. 2024, 25, 1067. [Google Scholar] [CrossRef]
- Poltavets, V.; Kochetkova, M.; Pitson, S.M.; Samuel, M.S. The Role of the Extracellular Matrix and Its Molecular and Cellular Regulators in Cancer Cell Plasticity. Front. Oncol. 2018, 8, 378027. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Baber, J.; Fujii, T.; Coito, A.J. Matrix Metalloproteinases in Liver Injury, Repair and Fibrosis. Matrix Biol. 2015, 44–46, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Said, A.H.; Raufman, J.P.; Xie, G. The Role of Matrix Metalloproteinases in Colorectal Cancer. Cancers 2014, 6, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Fang, Y.; Su, C. Research Progress on the Microenvironment and Immunotherapy of Advanced Non-Small Cell Lung Cancer With Liver Metastases. Front. Oncol. 2022, 12, 893716. [Google Scholar] [CrossRef]
- Yuzhalin, A.E.; Lim, S.Y.; Kutikhin, A.G.; Gordon-Weeks, A.N. Dynamic Matrisome: ECM Remodeling Factors Licensing Cancer Progression and Metastasis. Biochim. Et Biophys. Acta (BBA) Rev. Cancer 2018, 1870, 207–228. [Google Scholar] [CrossRef]
- Wernicke, A.K.; Churin, Y.; Sheridan, D.; Windhorst, A.; Tschuschner, A.; Gattenlöhner, S.; Roderfeld, M.; Roeb, E. Matrix Metalloproteinase-13 Refines Pathological Staging of Precancerous Colorectal Lesions. Oncotarget 2016, 7, 73552. [Google Scholar] [CrossRef][Green Version]
- Houg, D.S.; Bijlsma, M.F. The Hepatic Pre-Metastatic Niche in Pancreatic Ductal Adenocarcinoma. Mol. Cancer 2018, 17, 95. [Google Scholar] [CrossRef]
- Peltonen, R.; Hagström, J.; Tervahartiala, T.; Sorsa, T.; Haglund, C.; Isoniemi, H. High Expression of MMP-9 in Primary Tumors and High Preoperative MPO in Serum Predict Improved Prognosis in Colorectal Cancer with Operable Liver Metastases. Oncology 2021, 99, 144–160. [Google Scholar] [CrossRef]
- Kopitz, C.; Gerg, M.; Bandapalli, O.R.; Ister, D.; Pennington, C.J.; Hauser, S.; Flechsig, C.; Krell, H.W.; Antolovic, D.; Brew, K.; et al. Tissue Inhibitor of Metalloproteinases-1 Promotes Liver Metastasis by Induction of Hepatocyte Growth Factor Signaling. Cancer Res. 2007, 67, 8615–8623. [Google Scholar] [CrossRef]
- Cruz-Munoz, W.; Sanchez, O.H.; Di Grappa, M.; English, J.L.; Hill, R.P.; Khokha, R. Enhanced Metastatic Dissemination to Multiple Organs by Melanoma and Lymphoma Cells in Timp-3−/− Mice. Oncogene 2006, 25, 6489–6496. [Google Scholar] [CrossRef] [PubMed]
- Hawinkels, L.J.A.C.; Paauwe, M.; Verspaget, H.W.; Wiercinska, E.; Van Der Zon, J.M.; Van Der Ploeg, K.; Koelink, P.J.; Lindeman, J.H.N.; Mesker, W.; Ten Dijke, P.; et al. Interaction with Colon Cancer Cells Hyperactivates TGF-β Signaling in Cancer-Associated Fibroblasts. Oncogene 2012, 33, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Mendonsa, A.M.; VanSaun, M.N.; Ustione, A.; Piston, D.W.; Fingleton, B.M.; Gorden, D.L. Host and Tumor Derived MMP13 Regulate Extravasation and Establishment of Colorectal Metastases in the Liver. Mol. Cancer 2015, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Kow, A.W.C. Hepatic Metastasis from Colorectal Cancer. J. Gastrointest. Oncol. 2019, 10, 1274. [Google Scholar] [CrossRef]
- Kondo, T.; Okabayashi, K.; Hasegawa, H.; Tsuruta, M.; Shigeta, K.; Kitagawa, Y. The Impact of Hepatic Fibrosis on the Incidence of Liver Metastasis from Colorectal Cancer. Br. J. Cancer 2016, 115, 34–39. [Google Scholar] [CrossRef]
- Liu, J.; Geng, X.; Hou, J.; Wu, G. New Insights into M1/M2 Macrophages: Key Modulators in Cancer Progression. Cancer Cell Int. 2021, 21, 389. [Google Scholar] [CrossRef]
- Wood, S.L.; Pernemalm, M.; Crosbie, P.A.; Whetton, A.D. The Role of the Tumor-Microenvironment in Lung Cancer-Metastasis and Its Relationship to Potential Therapeutic Targets. Cancer Treat. Rev. 2014, 40, 558–566. [Google Scholar] [CrossRef]
- Su, H.; Yang, F.; Fu, R.; Trinh, B.; Sun, N.; Liu, J.; Kumar, A.; Baglieri, J.; Siruno, J.; Le, M.; et al. Collagenolysis-Dependent DDR1 Signalling Dictates Pancreatic Cancer Outcome. Nature 2022, 610, 366–372. [Google Scholar] [CrossRef]
- Popper, H.H. Progression and Metastasis of Lung Cancer. Cancer Metastasis Rev. 2016, 35, 75–91. [Google Scholar] [CrossRef]
- Grossniklaus, H.E.; Zhang, Q.; You, S.; McCarthy, C.; Heegaard, S.; Coupland, S.E. Metastatic Ocular Melanoma to the Liver Exhibits Infiltrative and Nodular Growth Patterns. Hum. Pathol. 2016, 57, 165–175. [Google Scholar] [CrossRef]
- Jin, K.; Gao, W.; Lu, Y.; Lan, H.; Teng, L.; Cao, F. Mechanisms Regulating Colorectal Cancer Cell Metastasis into Liver (Review). Oncol. Lett. 2012, 3, 11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yan, C.Y.; Zhao, M.L.; Wei, Y.N.; Zhao, X.H. Mechanisms of Drug Resistance in Breast Cancer Liver Metastases: Dilemmas and Opportunities. Mol. Ther. Oncolytics 2023, 28, 212. [Google Scholar] [CrossRef] [PubMed]
- Kazazi-Hyseni, F.; Beijnen, J.H.; Schellens, J.H.M. Bevacizumab. Oncologist 2010, 15, 819. [Google Scholar] [CrossRef] [PubMed]
- Milette, S.; Sicklick, J.K.; Lowy, A.M.; Brodt, P. Molecular Pathways: Targeting the Microenvironment of Liver Metastases. Clin. Cancer Res. 2017, 23, 6390–6399. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Tabernero, J.; Lakomy, R.; Prenen, H.; Prausová, J.; Macarulla, T.; Ruff, P.; Van Hazel, G.A.; Moiseyenko, V.; Ferry, D.; et al. Addition of Aflibercept to Fluorouracil, Leucovorin, and Irinotecan Improves Survival in a Phase III Randomized Trial in Patients with Metastatic Colorectal Cancer Previously Treated with an Oxaliplatin-Based Regimen. J. Clin. Oncol. 2012, 30, 3499–3506. [Google Scholar] [CrossRef]
- Tabernero, J.; Yoshino, T.; Cohn, A.L.; Obermannova, R.; Bodoky, G.; Garcia-Carbonero, R.; Ciuleanu, T.E.; Portnoy, D.C.; Van Cutsem, E.; Grothey, A.; et al. Ramucirumab versus Placebo in Combination with Second-Line FOLFIRI in Patients with Metastatic Colorectal Carcinoma That Progressed during or after First-Line Therapy with Bevacizumab, Oxaliplatin, and a Fluoropyrimidine (RAISE): A Randomised, Double-Blind, Multicentre, Phase 3 Study. Lancet Oncol. 2015, 16, 499–508. [Google Scholar] [CrossRef]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib Monotherapy for Previously Treated Metastatic Colorectal Cancer (CORRECT): An International, Multicentre, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Rapid Induction of Cytokine and E-Selectin Expression in the Liver in Response to Metastatic Tumor Cells1|Cancer Research|American Association for Cancer Research. Available online: https://aacrjournals.org/cancerres/article/59/6/1356/505916/Rapid-Induction-of-Cytokine-and-E-Selectin (accessed on 10 September 2024).
- Inhibition of Hepatic Endothelial E-Selectin Expression by C-Raf Antisense Oligonucleotides Blocks Colorectal Carcinoma Liver Metastasis1|Cancer Research|American Association for Cancer Research. Available online: https://aacrjournals.org/cancerres/article/62/19/5393/509271/Inhibition-of-Hepatic-Endothelial-E-Selectin (accessed on 22 August 2024).
- Wen, S.W.; Ager, E.I.; Christophi, C. Bimodal Role of Kupffer Cells during Colorectal Cancer Liver Metastasis. Cancer Biol. Ther. 2013, 14, 606–613. [Google Scholar] [CrossRef]
- Rivoltini, L.; Mazzaferro, V. Exploiting Liver Immunity for the Prevention of Hepatic Metastases. J. Hepatol. 2010, 53, 596–598. [Google Scholar] [CrossRef]
- Immunotherapy for Liver Metastasis from Colorectal Cancer—HSR Research. Available online: https://research.hsr.it/en/news/immunotherapy-for-liver-metastasis-from-colorectal-cancer.html (accessed on 22 August 2024).
- Xu, Q.; Guo, L.; Gu, X.; Zhang, B.; Hu, X.; Zhang, J.; Chen, J.; Wang, Y.; Chen, C.; Gao, B.; et al. Prevention of Colorectal Cancer Liver Metastasis by Exploiting Liver Immunity via Chitosan-TPP/Nanoparticles Formulated with IL-12. Biomaterials 2012, 33, 3909–3918. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.P.; Trinchieri, G. Interleukin-12 in Anti-Tumor Immunity and Immunotherapy. Cytokine Growth Factor. Rev. 2002, 13, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Boutaud, O.; Sosa, I.R.; Amin, T.; Oram, D.; Adler, D.; Hwang, H.S.; Crews, B.C.; Milne, G.; Harris, B.K.; Hoeksema, M.; et al. Inhibition of the Biosynthesis of Prostaglandin E2 by Low-Dose Aspirin: Implications for Adenocarcinoma Metastasis. Cancer Prev. Res. 2016, 9, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Leadbeater, P.D.M.; Kirkby, N.S.; Thomas, S.; Dhanji, A.R.; Tucker, A.T.; Milne, G.L.; Mitchell, J.A.; Warner, T.D. Aspirin Has Little Additional Anti-platelet Effect in Healthy Volunteers Receiving Prasugrel. J. Thromb. Haemost. 2011, 9, 2050–2056. [Google Scholar] [CrossRef]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Vinca Alkaloids. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547989/ (accessed on 12 September 2020).
- Gezici, S.; Şekeroğlu, N. Current Perspectives in the Application of Medicinal Plants Against Cancer: Novel Therapeutic Agents. Anticancer. Agents Med. Chem. 2019, 19, 101–111. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, N.H.; Son, C.G. A Review of Herbal Resources Inducing Anti-Liver Metastasis Effects in Gastrointestinal Tumors via Modulation of Tumor Microenvironments in Animal Models. Cancers 2023, 15, 3415. [Google Scholar] [CrossRef]
- Mariani, P.; Piperno-Neumann, S.; Servois, V.; Berry, M.G.; Dorval, T.; Plancher, C.; Couturier, J.; Levy-Gabriel, C.; Lumbroso-Le Rouic, L.; Desjardins, L.; et al. Surgical Management of Liver Metastases from Uveal Melanoma: 16 Years’ Experience at the Institut Curie. Eur. J. Surg. Oncol. 2009, 35, 1192–1197. [Google Scholar] [CrossRef]
- Agarwala, S.S.; Eggermont, A.M.M.; O’Day, S.; Zager, J.S. Metastatic Melanoma to the Liver: A Contemporary and Comprehensive Review of Surgical, Systemic, and Regional Therapeutic Options. Cancer 2014, 120, 781–789. [Google Scholar] [CrossRef]
- Kaštelan, S.; Gverović Antunica, A.; Beketić Oresković, L.; Kasun, B.; Hat, K. Uveal Melanoma: An Overview of Management and Prognosis. Libr. Oncol. Croat. J. Oncol. 2018, 46, 95–104. [Google Scholar] [CrossRef]
- Alagusundaramoorthy, S.S.; Gedaly, R. Role of Surgery and Transplantation in the Treatment of Hepatic Metastases from Neuroendocrine Tumor. World J. Gastroenterol. 2014, 20, 14348–14358. [Google Scholar] [CrossRef]
- Izzo, F.; Granata, V.; Grassi, R.; Fusco, R.; Palaia, R.; Delrio, P.; Carrafiello, G.; Azoulay, D.; Petrillo, A.; Curley, S.A. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist 2019, 24, e990–e1005. [Google Scholar] [CrossRef] [PubMed]
- Muttillo, E.M.; Mazzarella, G.; Picardi, B.; Rossi, S.; Cinelli, L.; Diana, M.; Baiocchini, A.; Felli, E.; Pessaux, P.; Felli, E.; et al. Treatment Strategies for Neuroendocrine Liver Metastases: A Systematic Review. HPB 2022, 24, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, F.H.; Solomon, S.B. Treatment of Primary Liver Tumors and Liver Metastases, Part 2: Non–Nuclear Medicine Techniques. J. Nucl. Med. 2018, 59, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Bala, M.M.; Riemsma, R.P.; Wolff, R.; Pedziwiatr, M.; Mitus, J.W.; Storman, D.; Swierz, M.J.; Kleijnen, J. Cryotherapy for Liver Metastases. Cochrane Database Syst. Rev. 2019, 2019, CD009058. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental Regulation of Tumor Progression and Metastasis. Nat. Med. 2013, 19, 1423. [Google Scholar] [CrossRef]
- Liu, L.X.; Zhang, W.H.; Jiang, H.C. Current Treatment for Liver Metastases from Colorectal Cancer. World J. Gastroenterol. 2003, 9, 193. [Google Scholar] [CrossRef]

| Medication | Target of the Medication | The Pharmacological Effect |
|---|---|---|
| Bevacizumab | VEGF | Suppression of neovascularization |
| Ziv-aflibercept | Limited blood supply to cancer cells | |
| Regorafenib | VEGFR | Suppression of neovascularization |
| ramucirumab | Limited blood supply to cancer cells | |
| Antisense oligonucleotides (ASO) C-raf treatment | E-selectin, | Reduction in metastases formation |
| TNFα mRNA | ||
| Gadolinium chloride | KCs | Decreased number of VEGF-expressing infiltrating cells |
| Tumor-specific monoclonal antibodies (Ab) | Potential cancer cell elimination | |
| INF-α | Liver endothelial cells | Reduction in metastases formation |
| IL-12 | T helper cell 1 (Th1) NK-cells | Antitumor immunity stimulation |
| Aspirin | COX1 | Inhibition of platelet aggregation |
| COX2 | ||
| Vinblastine | Tumor cells | Antineoplastic effect |
| Vincristine | ||
| Curcumin | IL-6 | Proinflammatory effect |
| Emodin | Nuclear factor-κB (NF-κB) | Suppression of tumor stimulation |
| Honokiol | Macrophages | Antitumor immunity stimulation |
| Dendritic cells | ||
| MMPs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andryszkiewicz, W.; Misiąg, P.; Karwowska, A.; Resler, Z.; Wojno, A.; Kulbacka, J.; Szewczyk, A.; Rembiałkowska, N. Cancer Metastases to the Liver: Mechanisms of Tumor Cell Colonization. Pharmaceuticals 2024, 17, 1251. https://doi.org/10.3390/ph17091251
Andryszkiewicz W, Misiąg P, Karwowska A, Resler Z, Wojno A, Kulbacka J, Szewczyk A, Rembiałkowska N. Cancer Metastases to the Liver: Mechanisms of Tumor Cell Colonization. Pharmaceuticals. 2024; 17(9):1251. https://doi.org/10.3390/ph17091251
Chicago/Turabian StyleAndryszkiewicz, Wiktoria, Piotr Misiąg, Anna Karwowska, Zofia Resler, Aleksandra Wojno, Julita Kulbacka, Anna Szewczyk, and Nina Rembiałkowska. 2024. "Cancer Metastases to the Liver: Mechanisms of Tumor Cell Colonization" Pharmaceuticals 17, no. 9: 1251. https://doi.org/10.3390/ph17091251
APA StyleAndryszkiewicz, W., Misiąg, P., Karwowska, A., Resler, Z., Wojno, A., Kulbacka, J., Szewczyk, A., & Rembiałkowska, N. (2024). Cancer Metastases to the Liver: Mechanisms of Tumor Cell Colonization. Pharmaceuticals, 17(9), 1251. https://doi.org/10.3390/ph17091251









