Tight Junctions and Cancer: Targeting Claudin-1 and Claudin-4 in Thyroid Pathologies
Abstract
1. Introduction
2. Results
2.1. Basic Characteristics of the Research Group
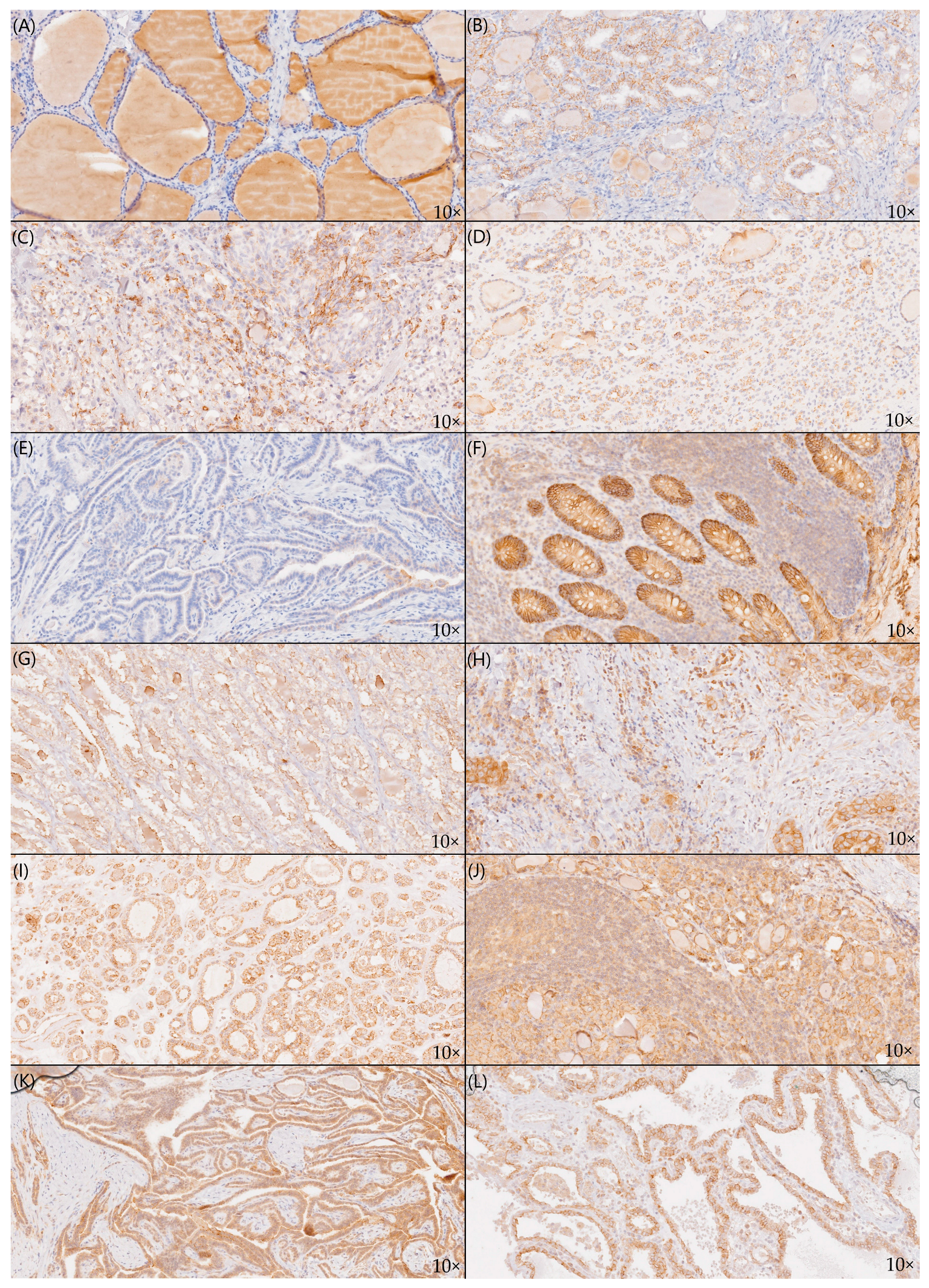
2.2. Staining Patterns of Claudin-1 and Claudin-4
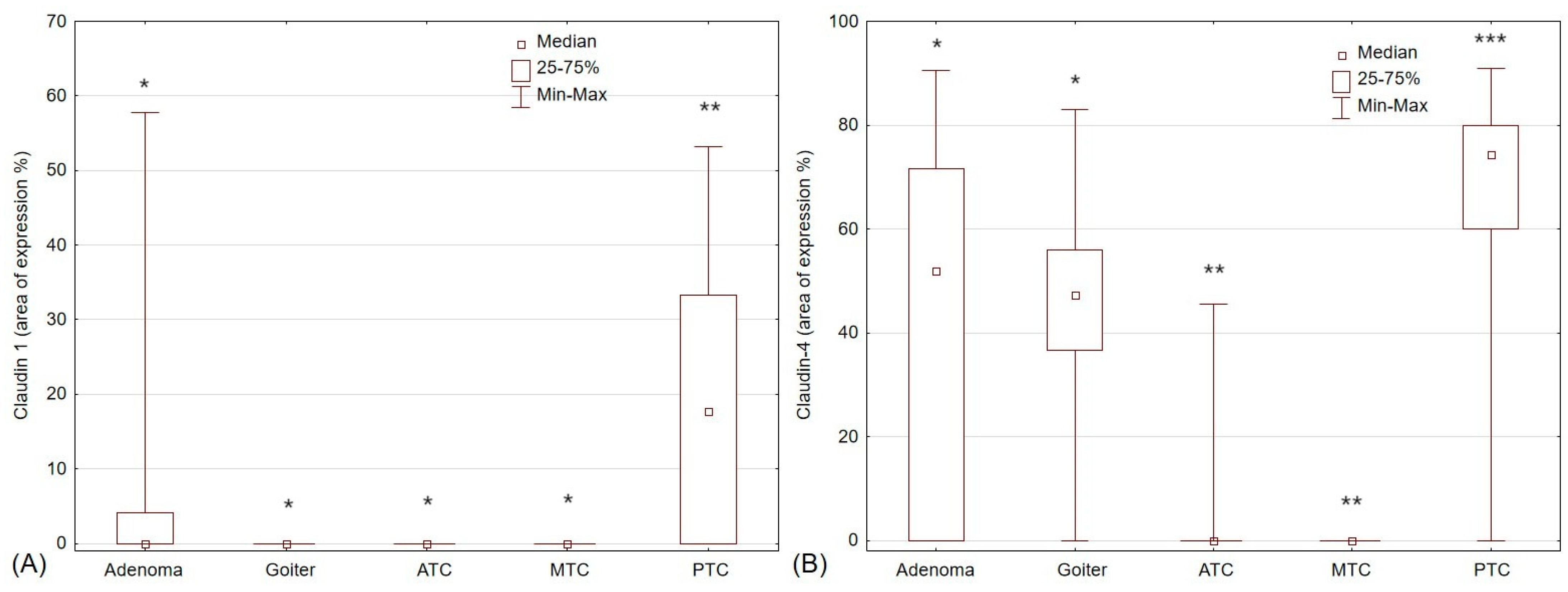
2.3. Claudin-1 and Claudin-4 Expression in Thyroid Pathologies
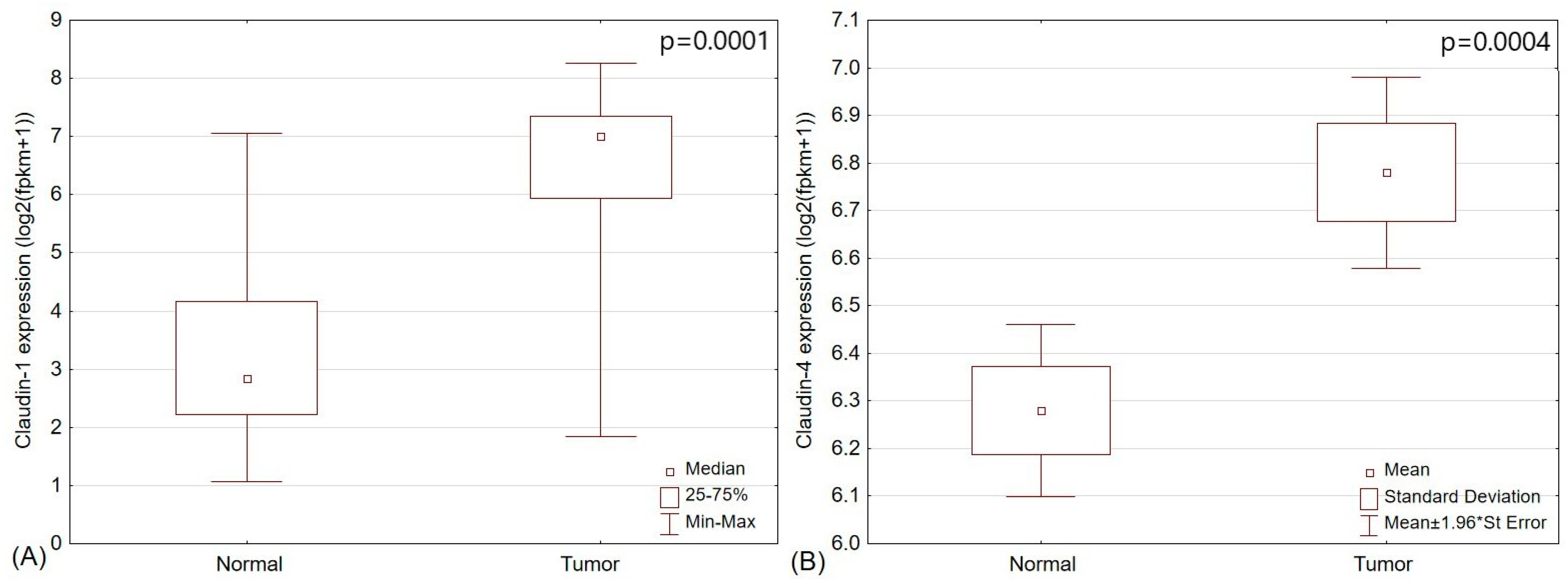
2.4. The Analysis of TCGA Papillary Thyroid Cancer Cohort
3. Discussion
3.1. The Role of Claudins in Thyroid Pathologies
3.2. Targeting Claudin 1 and Claudin 4 in Thyroid Cancer
| Agent/Intervention | Settings | Biological Effects | Impact on Claudin-1 | Ref. |
|---|---|---|---|---|
| PMTPV (short peptide) | Lung adenocarcinoma; A549 cell line | -PMTPV increases the chemosensitivity to doxorubicin | -PMTPV selectively decreased claudin-1 protein level by promoting its endocytosis and degradation | [58] |
| Tranilast and zoledronic acid (EMT inhibitors) | Small cell lung cancer; SBC-3 cell line | -Co-treatment with both agents increased the chemosensitivity of claudin-1-overexpressing SBC-3 cancer cells to doxorubicin | N/A | [59] |
| SCLC-derived exosomal miR-375-3p inhibitor (anti-miR-375) | Small cell lung cancer; cell lines and murine xenograft | -Decreased permeability of the vascular barrier and SCLC metastasis in vivo | -Reversed claudin-1 downregulation in endothelium | [60] |
| Claudin-1 specific siRNA | Head and neck squamous cell carcinoma; FaDu and SNU1041 cell lines; murine xenograft | -Inhibition of tumor progression through the activation of the AMPK signaling pathway -Suppression of cancer cell proliferation and migration -Negative regulation of the EMT | -Claudin-1 downregulation | [61] |
| Claudin-1 specific monoclonal antibody (mAb) | Hepatocellular carcinoma; cell lines and murine xenograft | -Anti-cancer effect on HCC cells, including sorafenib-and nivolumab-resistant cells; suppression of HCC growth in vivo through the promotion of apoptosis -Promotion of tumor metabolism reprogramming -Potential enhancement of the antitumor immunity | -Targeting claudin-1 | [62] |
| Claudin-1-RNA interference lentivirus (LV-CLDN1-RNAi) | Gallbladder cancer; SGC996 and GBC-SD cell lines | -Significant decrease in the proportion of cancer cells in the S phase of the cell cycle -Promotion of apoptosis and inhibition of the invasion capacity of cancer cells | -Claudin-1 downregulation | [63] |
| siRNA targeting GALNT5 (siGALNT5) expression | Cholangiocarcinoma; KKU-213 and KKU-214 cell lines | -Suppression of the growth, migration, and invasion of ChCC cells -Suppression of GALNT5 may inhibit the EMT in ChCC through upregulation of claudin-1 | -siGALNT5 increased claudin-1 expression -GALNT5 decreased the expression of claudin-1 through the activation of Akt and Erk signaling | [64] |
| Lycopene | Cutaneous squamous cell carcinoma; COLO-16 cell line | N/A | -Downregulation of claudin-1 expression through activation of ERK, JKN, and MTORC1 pathways and inhibition of autophagy in cancer cells | [65] |
| MIGR1 plasmid expressing p63 isotype ΔNp63α (MIGR1-ΔNp63α) | Cervical squamous cell carcinoma; SiHa cell line | -Suppression of tumor growth in vivo -Increase in K5 and involucrin expression in CSCC cells -Promotion of epithelial differentiation -Inhibition of angiogenesis -Reduction of cisplatin resistance in CSCC | -Upregulation of claudin-1 expression both in vitro and in vivo -Claudin-1 correlates positively with ΔNp63α in CSCC tissues | [66] |
| pMIGR1 plasmid expressing claudin-1 (pMIGR1-CLDN1) | Cervical squamous cell carcinoma; SiHa cell line and murine xenograft | -Claudin-1 overexpression suppressed cancer cell proliferation and migration and promoted their apoptosis -Claudin-1 promoted cell cycle arrest and inhibited CSCC growth in vivo | -Overexpression of claudin-1 in CSCC cells | [67] |
|
YM201636 (PIKfyve
inhibitor) | Ovarian cancer; HEYA8 and DOV13 cell lines | -Disruption of spheroid formation, inhibition of cell proliferation and migration -YM201636 may limit ovarian cancer spread and relapse | -Inhibition of claudin-1 recycling to the cellular membrane | [68] |
| Withaferin A | Oral squamous cell carcinoma; Ca9.22, HSC-4 and HN22 cell lines; murine xenograft | -Impair of cancer cells’ motility via downregulation of claudin-1 -Suppression of OSCC distant metastasis in vivo (murine model) with no systemic toxicity | -Claudin-1 downregulation | [69] |
| 3,3′-Diindolylmethane (DIM) | Esophageal squamous cell carcinoma; TE1 and KYSE150 cell lines; murine xenograft | -Suppression of the EMT process through the upregulation of claudin-1 in vivo -The anti-EMT effect of DIM is associated with the modulation of AHR through repression of the COX2/PGE2 pathway | - Upregulation of claudin-1 in vivo | [70] |
| MiR-27b-3p expressing pcDNA3.1 vector | Esophageal squamous cell carcinoma; TE-1 and EC9706 cell lines | -Suppression of proliferation and migration in EC9706 cells -Decrease of Nrf2 expression; -Potential inhibition of EMT through targeting Nrf2 | - Downregulation of claudin-1 | [71] |
| Resveratrol | Gastric cancer; SGC7901, GES-1, MGC803 and AGS cell lines | -Inhibition of the proliferation, migration, and invasion together with the promotion of apoptosis of gastric cancer cells through downregulation of miR-155-5p -Downregulation of c-Myc, cyclin D1, and Bcl-2 and upregulation of caspase-3 expression | -Downregulation of claudin-1 | [72] |
|
European Olive (Olea europaea L.) leaf extract (OLE) | Gastric cancer; AGS cell line | -Sensitization of gastric cancer to chemotherapy by targeting claudin-1 -Inhibition of the EMT process and suppression of AGS cell migration and stem-like phenotype | -Downregulation of claudin-1 in AGS cells; more prominent in cells treated with OLE+5-FU and OLE+Cisplatinum | [73] |
| MiR-633 lentivirus vector (LV-miR-633) | Gastric cancer; SGC-7901 and HGC-27 cell lines | -Inhibition of cancer cell proliferation, induction of cell cycle arrest at G0/G1, and promotion of cancer cell apoptosis -Suppression of cancer cell migration and invasion -MiR-633 exhibits anti-cancer effects through targeting MAPK1 | -MiR-633 may target the claudin-1 gene and negatively regulate its expression | [74] |
| shRNA targeting Ephrin-A2 | Prostate cancer; LNCaP, PC-3, and DU145 cell lines; murine xenograft | -Suppression of tumor growth, metastasis to lymph nodes and lungs, and angiogenesis in vivo -Targeting ephrin-A2 may decrease prostate cancer metastasis by targeting EMT-related markers | -Upregulation of claudin-1 | [75] |
|
Glutamine (Gln)
deprivation, Diazo-O-norleucine ( glutaminase inhibitor) (DON) | Breast cancer; MDA-MB-231 and MCF-7 cell lines | -Gln-deprivation and DON treatment induced epithelial differentiation of breast cancer stem cells and reduced their stemness -Targeting glutamine may decrease breast cancer invasion and metastasis | -Upregulation of claudin-1 | [76] |
| Brahma (BRM) overexpression through transient transfection | Breast cancer; MCF-7 and MDA-231 cell lines | -Suppression of migration and invasion of cancer cells -TGF-β treatment disrupted the BRM-induced upregulation of claudin1/4 | -Upregulation of claudin-1 and claudin-4 through acetylation of histones surrounding the claudin1/4 promoters | [77] |
| C150 (2-[2-(5-nitro-2-thienyl)vinyl]quinoline) | Pancreatic ductal adenocarcinoma; PANC-1 cell line; murine xenograft | -Reduction of tumor growth in vivo -C150 treatment is associated with a statistically significant loss of mean body weight | -Upregulation of claudin-1 and ZO-1 in vivo through the promotion of Snail proteasomal degradation | [78] |
| miRNA-193b-5p mimic transfection | Pancreatic ductal adenocarcinoma; PANC-1 cell line and murine xenograft | -Suppression of cancer cell proliferation, migration, invasion, and EMT process through targeting eEF2K signaling pathways -Lipid-nanoparticles-based delivery of miR-193b in vivo significantly reduces tumor growth and eEF2K expression | -Upregulation of claudin-1 and E-cadherin | [79] |
| N-(phenylcarbamothioyl)-2-napthamides as claudin-1 inhibitors | Colorectal carcinoma; SW620 cell line | -Compound VM-A-155B is a promising anti-CRC claudin-1 inhibitor (best activity, in vitro and in vivo properties) | -Targeting claudin-1 in CRC cells | [80] |
| Lactobacillus plantarum— derived metabolites (LDMs) | Colorectal carcinoma; HCT-116 and HCT-116/5FUR cell lines | -LDMs enhance the sensitivity and cytotoxicity of 5-FU in resistant cancer cells -LDMs inhibit the metastatic potential of resistant cancer cells -LDMs’ anti-cancer activity is associated with the downregulation of claudin-1 | -LDMs alone and in combination with 5-FU downregulate the claudin-1 in HCT-116/5FUR | [81] |
| tRNA-derived fragment tRF-20-M0NK5Y93 | Colorectal carcinoma; RKO and SW480 cell lines | -Inhibition of CRC cell migration, invasion, and metastatic properties through inhibition of claudin-1 -Hypoxic conditions downregulate tRF-20-M0NK5Y93 expression | - Direct and negative regulation of claudin-1 expression | [82] |
| PDS0330, a first-generation inhibitor of claudin-1 | Colorectal carcinoma; HCT116, SW480cld1, and SW620 cell lines; in silico; murine xenograft | -PDS-0330 is characterized by favorable pharmacodynamics and pharmacokinetics in vitro and in vivo -PDS-0330 has higher affinity and specificity binding to claudin-1 compared to other claudins -Reduction in anoikis and therapy resistance of cancer cells -Inhibition of tumor growth in vivo with no major cytotoxicity -Synergistic effect with 5-FU in vivo -Reduction of claudin-1-dependent CRC chemoresistance | -Inhibition of claudin1-mediated signaling through interferential with claudin-1-Src binding | [83] |
| Oxaliplatin and anti-CLDN1 antibody-drug conjugate (6F6-ADC) | Colorectal carcinoma; SW620 and HCT116 cell lines; murine xenograft | -Oxaliplatin-induced claudin-1 expression is associated with p38/GSK3β/Wnt-β-catenin pathway -Oxaliplatin resistance is mediated by claudin-1-induced apoptosis resistance -6F6-ADC suppresses CRC growth in vivo -Sequential oxaliplatin +6F6-ADC exhibits a stronger anti-cancer effect and increased survival than oxaliplatin monotherapy | -Standard CRC chemotherapy upregulates the claudin-1 expression in CRC in vitro and in vivo -6F6-ADC targets extracellular part of claudin-1 in CRC | [84] |
3.3. Targeting Claudin-1 and Claudin-4 in Other Malignancies
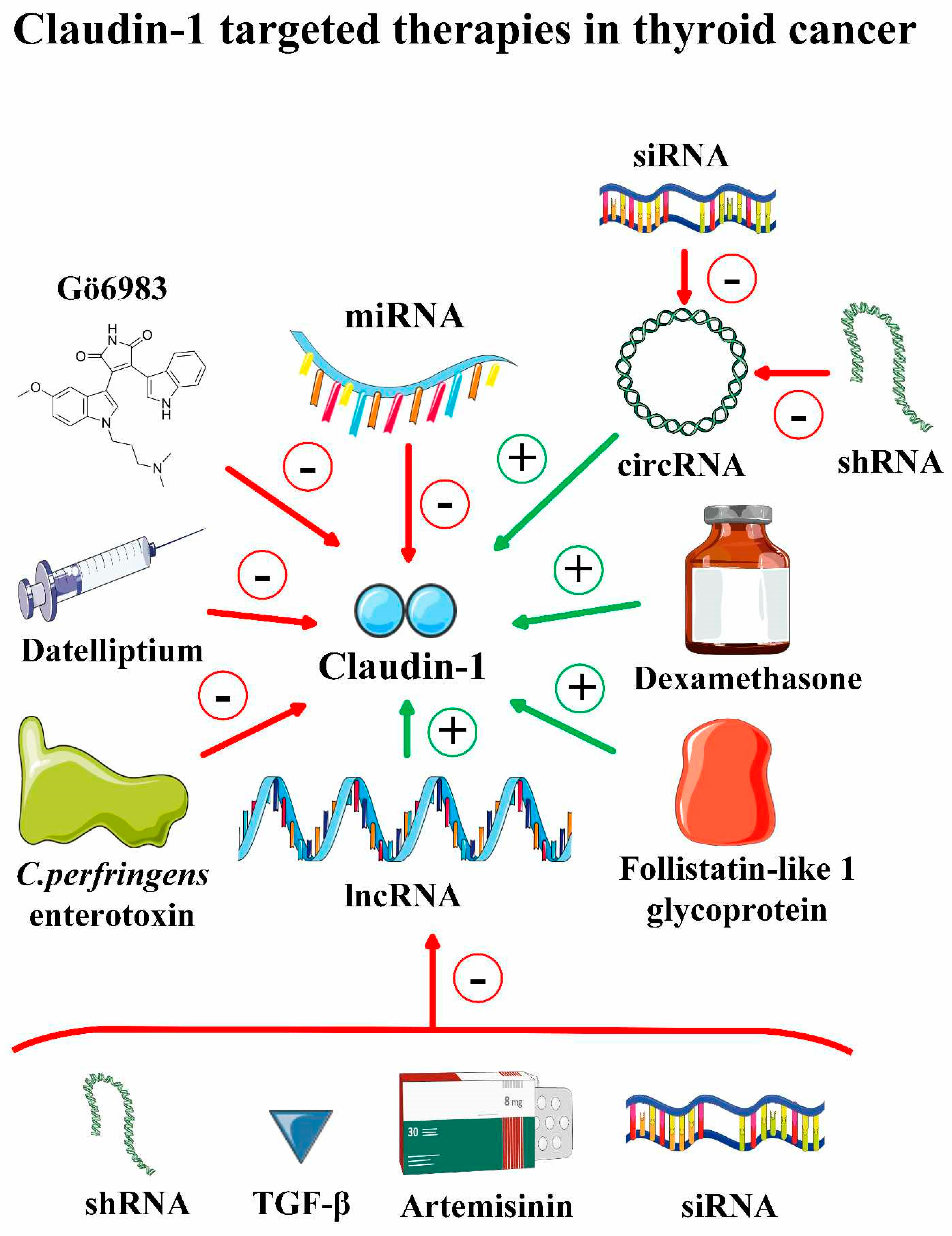
| Agent/Intervention | Malignancy | Biological Effects | Impact on Claudin-4 | Ref. |
|---|---|---|---|---|
| Clostridium perfringens enterotoxin Obstructing Protein (COP-1), synthetic antibody fragment (sFab) | Structural, biophysical, and biochemical analysis with the use of single-particle cryogenic electron microscopy (cryo-EM) | -COP-1 is a monovalent and stable molecule and binds with high affinity and selectivity to claudin-4 | -COP-1 binds with the extracellular surface of human claudin-4 | [93] |
| siRNA targeting PVT1 exon 9 expression (siPVT1e9) | Triple-negative breast cancer; MDA MB 231 cell line | -Suppression of migratory capacity of MDA MB 231 cancer cells | -Knockdown of PVT1 exon 9 results in re-expression of claudin-4 protein with no impact on claudin-4 mRNA level | [88] |
| Fusion toxin secreted by Bifidobacterium longum (B. longum-C-CPE-PE23) | Breast cancer; MCF-7, MDA-MB-468 and HCC1937 cell lines; murine xenograft | -Suppression of tumor growth in vivo -Treatment did not affect body weight, ALT, AST, and BUN levels | -C-CPE-PE23 is cytotoxic to cells expressing claudin-4 | [94] |
| Anti-claudin-4 extracellular domain antibody (4D3) | Breast cancer; MCF-7 and MDA-468 cell lines; murine xenograft | -4D3 enhances paclitaxel-induced anti-cancer effect in vitro -4D3 facilities paclitaxel intracellular penetration -4DC exhibits immunomodulatory effect on TME in breast cancer -Three-way combination of paclitaxel, tamoxifen, and 4D3 significantly prolonged survival of mice -Combined treatment of paclitaxel, zoledronic acid, and 4D3 reduced the growth of breast cancer bone metastasis | -Targeting cells expressing claudin-4 | [85] |
| Claudin-4 targeting short hairpin RNA (shCLDN4); claudin-4 expressing pLenti-C-mGFP vector (CLDN4-GFP); claudin-4-disrupting peptide (CMP) | Ovarian cancer; OVCAR3, PEO4, OVCAR8, and OVCAR4 cell lines | -Claudin-4 overexpression in ovarian cancer drives paclitaxel resistance -Claudin-4 expressing cells exhibited a higher response to paclitaxel, but no cisplatin, after cotreatment with CMP or shCLDN4 -Cancer cells lacking claudin-4 are characterized by inhibition of G2-M progression of cell cycle and abundant of mitotic figures -Claudin-4 regulates the polymerization of microtubules, which may be a potential paclitaxel-resistance mechanism | -Claudin-4 knockdown after shCLDN4 -Claudin-4 overexpression in cells transfected with CLDN4-GFP -CMP inhibits claudin-4 activity | [95] |
| Claudin-4 short hairpin RNA lentiviral vector (shCLDN4); CMP | Ovarian cancer; in vitro studies | -Claudin-4 expression correlates with DNA damage repair -Claudin-4 KO increases sensitivity to PARP inhibitors by affecting nonhomologous end joining DNA repair -Olaparib/CMP cotreatment inhibited proliferation regardless of the claudin-4 expression in tumor | -Claudin-4 knockdown after shCLDN4 -CMP inhibits claudin-4 activity | [90] |
| Celastrol | Gastric cancer; SGC7901 and BGC823 cell lines | -Celastrol inhibits proliferation, migration, and invasion of cancer cells by targeting the FOXA1/CLDN4 axis and later inhibition of PI3K/AKT signaling | -Celastrol targets FOXA1 which results in claudin-4 downregulation | [89] |
| Anti-CLDN4 antibody; 4D3 | Gastric cancer; TMK-1 and MKN74 cell lines; murine xenograft | -4D3 treatment downregulates claudin-4, EGFR, and VEGF in cancer cells and inhibits cell proliferation -4D3 decreases gastric cancer stemness markers -Combined 4D3 and cis-diamminedichloroplatinum (CDDP) have a synergic anti-cancer effect -4D3 increases CDDP intracellular concentration -4D3 monotherapy inhibited the growth of well-differentiated gastric cancer in vivo but not poorly differentiated one -Concurrent CDDP and 4D3 exhibit higher anti-cancer activity compared to monotherapy in both tumors and improve mice survival | -4D3 decreases claudin-4 expression | [86] |
| 4D3 | Pancreatic ductal adenocarcinoma; MIA-PaCa-2 cell line; murine xenograft | -4D3 decreased HIF-1α expression in PDAC cells -4D3 increased intracellular concentration of 5-FU -Simultaneous treatment of 4D3 increased the antitumoral effect of the FFX regimen, i.e., oxaliplatin (L-OHP), irinotecan (CPT-11), and 5-FU -4D3 and FFX suppressed tumor growth in vivo and prolonged mice survival -A half dose of FFX is a significantly safer approach than a full dose of FFX with a similar anti-cancer effect | -4D3 inhibits claudin-4 | [87] |
| Doxorubicin-loaded- Clostridium perfringens enterotoxin peptide-conjugated polysialic acid nanoparticles (DOX-C-SNPs) | Pancreatic ductal adenocarcinoma; KPC960 murine PDAC cell lines; murine allograft | -DOX-C-SNPs release specifically and effectively doxorubicin in cancer cells -DOX-C-SNPs provide low non-specific/off-target uptake of doxorubicin and high stability -DOX-C-SNPs exhibited a higher anti-cancer effect compared to DOX-SNP and free doxorubicin in vivo -DOX-C-SNPs were associated with reduced doxorubicin-related systemic toxicity and prolonged mice survival | -DOX-C-SNPs bind with the extracellular domain of claudin-4 expressed on the surface of cancer cells | [91] |
| Doxorubicin (Dox)-loaded, CPE17-conjugated liposomes (D@C-LPs) | Pancreatic ductal adenocarcinoma; ASPC-1 and KPC960 cell lines; murine allograft and xenograft | -D@C-LPs bind specifically with claudin-4 expressing cancer cells -Claudin-4 in case of PDAC is exposed superficially, whereas access to claudin-4 in case of normal pancreatic tissue is hindered -D@C-LP resulted in significant suppression of tumor growth in vivo and prolonged mice survival compared to free doxorubicin -D@C-LP was associated with no major systemic toxicities | -D@C-LPs targets claudin-4 positive cancer cells | [92] |
| Y364947, TGFβ1 inhibitor | Colorectal cancer; HT29 and HCT116 cell lines | -LY364947 suppressed claudin-4 mRNA and protein levels in both CRC cell lines | -Y364947 downregulates claudin-4 expression through TGFβ1 signaling inhibition | [96] |
3.4. Limitations
4. Materials and Methods
4.1. Patients and Tissue Samples
4.2. Tissue Microarray (TMA) Preparation
4.3. Immunohistochemical Staining
4.4. Image Acquisition and Analysis
4.5. In Silico Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farquhar, M.G.; Palade, G.E. Junctional Complexes in Various Epithelia. J. Cell Biol. 1963, 17, 375–412. [Google Scholar] [CrossRef] [PubMed]
- Van Itallie, C.M.; Anderson, J.M. Claudin Interactions in and out of the Tight Junction. Tissue Barriers 2013, 1, e25247. [Google Scholar] [CrossRef] [PubMed]
- Lal-Nag, M.; Morin, P.J. The Claudins. Genome Biol. 2009, 10, 235. [Google Scholar] [CrossRef]
- Shakib, H.; Rajabi, S.; Dehghan, M.H.; Mashayekhi, F.J.; Safari-Alighiarloo, N.; Hedayati, M. Epithelial-to-Mesenchymal Transition in Thyroid Cancer: A Comprehensive Review. Endocrine 2019, 66, 435–455. [Google Scholar] [CrossRef]
- Wodarz, A.; Näthke, I. Cell Polarity in Development and Cancer. Nat. Cell Biol. 2007, 9, 1016–1024. [Google Scholar] [CrossRef]
- Singh, A.B.; Dhawan, P. Claudins and Cancer: Fall of the Soldiers Entrusted to Protect the Gate and Keep the Barrier Intact. Semin. Cell Dev. Biol. 2015, 42, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Vasko, V.; Espinosa, A.V.; Scouten, W.; He, H.; Auer, H.; Liyanarachchi, S.; Larin, A.; Savchenko, V.; Francis, G.L.; de la Chapelle, A.; et al. Gene Expression and Functional Evidence of Epithelial-to-Mesenchymal Transition in Papillary Thyroid Carcinoma Invasion. Proc. Natl. Acad. Sci. USA 2007, 104, 2803–2808. [Google Scholar] [CrossRef]
- Runkle, E.A.; Mu, D. Tight Junction Proteins: From Barrier to Tumorigenesis. Cancer Lett. 2013, 337, 41–48. [Google Scholar] [CrossRef]
- Krause, G.; Protze, J.; Piontek, J. Assembly and Function of Claudins: Structure-Function Relationships Based on Homology Models and Crystal Structures. Semin. Cell Dev. Biol. 2015, 42, 3–12. [Google Scholar] [CrossRef]
- Capaldo, C.T.; Nusrat, A. Claudin Switching: Physiological Plasticity of the Tight Junction. Semin. Cell Dev. Biol. 2015, 42, 22–29. [Google Scholar] [CrossRef]
- Furuse, M.; Hata, M.; Furuse, K.; Yoshida, Y.; Haratake, A.; Sugitani, Y.; Noda, T.; Kubo, A.; Tsukita, S. Claudin-Based Tight Junctions Are Crucial for the Mammalian Epidermal Barrier. J. Cell Biol. 2002, 156, 1099–1111. [Google Scholar] [CrossRef]
- Shen, L. Tight Junctions on the Move: Molecular Mechanisms for Epithelial Barrier Regulation. Ann. N. Y. Acad. Sci. 2012, 1258, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Van Itallie, C.M.; Anderson, J.M. Claudins and Epithelial Paracellular Transport. Annu. Rev. Physiol. 2006, 68, 403–429. [Google Scholar] [CrossRef]
- Anderson, J.M.; Van Itallie, C.M. Physiology and Function of the Tight Junction. Cold Spring Harb. Perspect. Biol. 2009, 1, a002584. [Google Scholar] [CrossRef]
- Piontek, A.; Eichner, M.; Zwanziger, D.; Beier, L.-S.; Protze, J.; Walther, W.; Theurer, S.; Schmid, K.W.; Führer-Sakel, D.; Piontek, J.; et al. Targeting Claudin-Overexpressing Thyroid and Lung Cancer by Modified Clostridium Perfringens Enterotoxin. Mol. Oncol. 2020, 14, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Zwanziger, D.; Badziong, J.; Ting, S.; Moeller, L.C.; Schmid, K.W.; Siebolts, U.; Wickenhauser, C.; Dralle, H.; Fuehrer, D. The Impact of CLAUDIN-1 on Follicular Thyroid Carcinoma Aggressiveness. Endocr. Relat. Cancer 2015, 22, 819–830. [Google Scholar] [CrossRef]
- Ma, H.; Yan, J.; Zhang, C.; Qin, S.; Qin, L.; Liu, L.; Wang, X.; Li, N. Expression of Papillary Thyroid Carcinoma-Associated Molecular Markers and Their Significance in Follicular Epithelial Dysplasia with Papillary Thyroid Carcinoma-like Nuclear Alterations in Hashimoto’s Thyroiditis. Int. J. Clin. Exp. Pathol. 2014, 7, 7999–8007. [Google Scholar] [PubMed]
- Lin, J.; Qiu, Y.; Zheng, X.; Dai, Y.; Xu, T. The MiR-199a-5p/PD-L1 Axis Regulates Cell Proliferation, Migration and Invasion in Follicular Thyroid Carcinoma. BMC Cancer 2022, 22, 756. [Google Scholar] [CrossRef]
- Miskad, U.A.; Aswidah, A.; Dahlan, H.; Ikram, D.; Cangara, M.H.; Djimahit, T.; Kaelan, C. The Role of Claudin-1 Expression in Follicular and Papillary Thyroid Neoplasm. Asian Pac. J. Cancer Prev. 2022, 23, 4023–4027. [Google Scholar] [CrossRef]
- Hess, J.; Thomas, G.; Braselmann, H.; Bauer, V.; Bogdanova, T.; Wienberg, J.; Zitzelsberger, H.; Unger, K. Gain of Chromosome Band 7q11 in Papillary Thyroid Carcinomas of Young Patients Is Associated with Exposure to Low-Dose Irradiation. Proc. Natl. Acad. Sci. USA 2011, 108, 9595–9600. [Google Scholar] [CrossRef]
- Németh, J.; Németh, Z.; Tátrai, P.; Péter, I.; Somorácz, A.; Szász, A.M.; Kiss, A.; Schaff, Z. High Expression of Claudin-1 Protein in Papillary Thyroid Tumor and Its Regional Lymph Node Metastasis. Pathol. Oncol. Res. 2010, 16, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Gowrikumar, S.; Ahmad, R.; Uppada, S.B.; Washington, M.K.; Shi, C.; Singh, A.B.; Dhawan, P. Upregulated Claudin-1 Expression Promotes Colitis-Associated Cancer by Promoting β-Catenin Phosphorylation and Activation in Notch/p-AKT-Dependent Manner. Oncogene 2019, 38, 5321–5337. [Google Scholar] [CrossRef]
- Tzelepi, V.N.; Tsamandas, A.C.; Vlotinou, H.D.; Vagianos, C.E.; Scopa, C.D. Tight Junctions in Thyroid Carcinogenesis: Diverse Expression of Claudin-1, Claudin-4, Claudin-7 and Occludin in Thyroid Neoplasms. Mod. Pathol. 2008, 21, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, Y.; Zhang, W.; Zhang, W.; Fan, L.; Wang, L.; Liu, Y.; Liu, S.; Guo, Y.; Wang, Y.; et al. MicroRNA-142-5p Contributes to Hashimoto’s Thyroiditis by Targeting CLDN1. J. Transl. Med. 2016, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Zake, T.; Skuja, S.; Kalere, I.; Konrade, I.; Groma, V. Heterogeneity of Tissue IL-17 and Tight Junction Proteins Expression Demonstrated in Patients with Autoimmune Thyroid Diseases. Medicine 2018, 97, e11211. [Google Scholar] [CrossRef]
- Sayar, I.; Gurbuzel, M. Potentially Important Markers in Thyroid Neoplasia: Claudin-1 and MMP-7. Niger. J. Clin. Pract. 2023, 26, 412–416. [Google Scholar] [CrossRef]
- Rehman, A.U.; Ehsan, M.; Javed, H.; Ameer, M.Z.; Mohsin, A.; Aemaz Ur Rehman, M.; Nawaz, A.; Amjad, Z.; Ameer, F. Solitary and Multiple Thyroid Nodules as Predictors of Malignancy: A Systematic Review and Meta-Analysis. Thyroid. Res. 2022, 15, 22. [Google Scholar] [CrossRef]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A Pathology Atlas of the Human Cancer Transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef]
- Leech, A.O.; Cruz, R.G.B.; Hill, A.D.K.; Hopkins, A.M. Paradigms Lost-an Emerging Role for over-Expression of Tight Junction Adhesion Proteins in Cancer Pathogenesis. Ann. Transl. Med. 2015, 3, 184. [Google Scholar] [CrossRef]
- Thiery, J.P.; Sleeman, J.P. Complex Networks Orchestrate Epithelial-Mesenchymal Transitions. Nat. Rev. Mol. Cell Biol. 2006, 7, 131–142. [Google Scholar] [CrossRef]
- Latorre, I.J.; Frese, K.K.; Javier, R.T. Tight Junction Proteins and Cancer. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Süren, D.; Yildirim, M.; Sayiner, A.; Alikanoğlu, A.S.; Atalay, I.; Gündüz, U.R.; Kaya, V.; Gündüz, Ş.; Oruç, M.T.; Sezer, C. Expression of Claudin 1, 4 and 7 in Thyroid Neoplasms. Oncol. Lett. 2017, 13, 3722–3726. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bolin, J. Thyroid Follicular Epithelial Cell–Derived Cancer: New Approaches and Treatment Strategies. J. Nucl. Med. Technol. 2021, 49, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Abd El Atti, R.M.; Shash, L.S. Potential Diagnostic Utility of CD56 and Claudin-1 in Papillary Thyroid Carcinoma and Solitary Follicular Thyroid Nodules. J. Egypt. Natl. Canc. Inst. 2012, 24, 175–184. [Google Scholar] [CrossRef]
- Ma, H.; Xu, S.; Yan, J.; Zhang, C.; Qin, S.; Wang, X.; Li, N. The Value of Tumor Markers in the Diagnosis of Papillary Thyroid Carcinoma Alone and in Combination. Pol. J. Pathol. 2014, 65, 202–209. [Google Scholar] [CrossRef]
- Tao, D.; Guan, B.; Li, H.; Zhou, C. Expression Patterns of Claudins in Cancer. Heliyon 2023, 9, e21338. [Google Scholar] [CrossRef]
- Suzuki, M.; Kato-Nakano, M.; Kawamoto, S.; Furuya, A.; Abe, Y.; Misaka, H.; Kimoto, N.; Nakamura, K.; Ohta, S.; Ando, H. Therapeutic Antitumor Efficacy of Monoclonal Antibody against Claudin-4 for Pancreatic and Ovarian Cancers. Cancer Sci. 2009, 100, 1623–1630. [Google Scholar] [CrossRef]
- Ding, L.; Lu, Z.; Lu, Q.; Chen, Y.-H. The Claudin Family of Proteins in Human Malignancy: A Clinical Perspective. Cancer Manag. Res. 2013, 5, 367–375. [Google Scholar] [CrossRef]
- Kominsky, S.L.; Tyler, B.; Sosnowski, J.; Brady, K.; Doucet, M.; Nell, D.; Smedley, J.G., 3rd; McClane, B.; Brem, H.; Sukumar, S. Clostridium Perfringens Enterotoxin as a Novel-Targeted Therapeutic for Brain Metastasis. Cancer Res. 2007, 67, 7977–7982. [Google Scholar] [CrossRef] [PubMed]
- Bajracharya, R.; Song, J.G.; Patil, B.R.; Lee, S.H.; Noh, H.-M.; Kim, D.-H.; Kim, G.-L.; Seo, S.-H.; Park, J.-W.; Jeong, S.H.; et al. Functional Ligands for Improving Anticancer Drug Therapy: Current Status and Applications to Drug Delivery Systems. Drug Deliv. 2022, 29, 1959–1970. [Google Scholar] [CrossRef]
- Alqahtani, T.; Alswied, A.; Sun, D. Selective Antitumor Activity of Datelliptium toward Medullary Thyroid Carcinoma by Downregulating RET Transcriptional Activity. Cancers 2021, 13, 3288. [Google Scholar] [CrossRef]
- Landa, I.; Cabanillas, M.E. Genomic Alterations in Thyroid Cancer: Biological and Clinical Insights. Nat. Rev. Endocrinol. 2024, 20, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Melnik, D.; Cortés-Sánchez, J.L.; Sandt, V.; Kahlert, S.; Kopp, S.; Grimm, D.; Krüger, M. Dexamethasone Selectively Inhibits Detachment of Metastatic Thyroid Cancer Cells during Random Positioning. Cancers 2023, 15, 1641. [Google Scholar] [CrossRef] [PubMed]
- Orlandella, F.M.; Imperlini, E.; Pane, K.; Luciano, N.; Braile, M.; De Stefano, A.E.; Iervolino, P.L.C.; Ruocco, A.; Orrù, S.; Franzese, M.; et al. MiR-331-5p Affects Motility of Thyroid Cancer Cell Lines and Regulates BID Expression. Biomedicines 2024, 12, 658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, Q.; Jiang, J. Circ_0027446 Induces CLDN1 Expression to Promote Papillary Thyroid Cancer Cell Malignancy by Binding to MiR-129-5p. Pathol. Res. Pract. 2022, 238, 154095. [Google Scholar] [CrossRef]
- He, A.T.; Liu, J.; Li, F.; Yang, B.B. Targeting Circular RNAs as a Therapeutic Approach: Current Strategies and Challenges. Signal Transduct. Target. Ther. 2021, 6, 185. [Google Scholar] [CrossRef]
- Ji, Y.; Ji, J.; Yin, H.; Chen, X.; Zhao, P.; Lu, H.; Wang, T. Exosomes Derived from MicroRNA-129-5p-Modified Tumor Cells Selectively Enhanced Suppressive Effect in Malignant Behaviors of Homologous Colon Cancer Cells. Bioengineered 2021, 12, 12148–12156. [Google Scholar] [CrossRef]
- Menon, A.; Abd-Aziz, N.; Khalid, K.; Poh, C.L.; Naidu, R. MiRNA: A Promising Therapeutic Target in Cancer. Int. J. Mol. Sci. 2022, 23, 1502. [Google Scholar] [CrossRef]
- Chen, J.; Chen, R.; Huang, S.; Zu, B.; Zhang, S. Atezolizumab Alleviates the Immunosuppression Induced by PD-L1-positive Neutrophils and Improves the Survival of Mice during Sepsis. Mol. Med. Rep. 2021, 23, 144. [Google Scholar] [CrossRef]
- Du, Y.-L.; Liang, Y.; Cao, Y.; Liu, L.; Li, J.; Shi, G.-Q. LncRNA XIST Promotes Migration and Invasion of Papillary Thyroid Cancer Cell by Modulating MiR-101-3p/CLDN1 Axis. Biochem. Genet. 2021, 59, 437–452. [Google Scholar] [CrossRef]
- Zheng, M.; Xu, L.; Wei, C.; Guan, W. CircRTN1 Stimulates HMGB1 to Regulate the Malignant Progression of Papillary Thyroid Cancer by Sponging MiR-101-3p. Hormones 2023, 22, 281–293. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, J.; Zhao, Z.; Liu, Y.; Zhao, Z.; Fu, K.; Li, B.; Jin, J. Artemisinin Suppresses Aerobic Glycolysis in Thyroid Cancer Cells by Downregulating HIF-1a, Which Is Increased by the XIST/MiR-93/HIF-1a Pathway. PLoS ONE 2023, 18, e0284242. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Deng, H.; Zhao, Y.; Li, C.; Liang, Y. LncRNA XIST/MiR-34a Axis Modulates the Cell Proliferation and Tumor Growth of Thyroid Cancer through MET-PI3K-AKT Signaling. J. Exp. Clin. Cancer Res. 2018, 37, 279. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; He, Y.; Peng, B. IncRNA XIST Stimulates Papillary Thyroid Cancer Development through the MiR-330-3p/PDE5A Axis. Crit. Rev. Eukaryot. Gene Expr. 2023, 33, 13–26. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, J.; Wang, J. Long Noncoding RNA XIST Promotes Proliferation and Invasion by Targeting MiR-141 in Papillary Thyroid Carcinoma. Onco. Targets. Ther. 2018, 11, 5035–5043. [Google Scholar] [CrossRef]
- Xin, Y.; Sun, X.; Chi, J.; Zhang, W.; Wang, Y.; Zhao, S. The TGF-β-Regulated X-Inactive Specific Transcript Inhibits Papillary Thyroid Cancer Migration and Invasion. Transl. Cancer Res. 2018, 7, 958–968. [Google Scholar] [CrossRef]
- Park, J.S.; Choi, A.; Kim, J.H.; Park, K.; Lee, S.B.; Kim, Y.; Nam, J.S.; Kang, S.; Ahn, C.W. Follistain-like 1 (Fstl1) May Reduce Metastastic Activity of Anaplastic Thyroid Cancer by Increasging Thyroid Transcription Factor 1 (TTF1) and Epithelial Gene Markers. In Proceedings of the Endocrine Abstracts, Lyon, France, 18–21 May 2019; Bioscientifica: Bristol, UK, 2019; Volume 63. [Google Scholar]
- Nasako, H.; Takashina, Y.; Eguchi, H.; Ito, A.; Ishikawa, Y.; Matsunaga, T.; Endo, S.; Ikari, A. Increase in Toxicity of Anticancer Drugs by PMTPV, a Claudin-1-Binding Peptide, Mediated via Down-Regulation of Claudin-1 in Human Lung Adenocarcinoma A549 Cells. Int. J. Mol. Sci. 2020, 21, 5909. [Google Scholar] [CrossRef]
- Nagaoka, Y.; Oshiro, K.; Yoshino, Y.; Matsunaga, T.; Endo, S.; Ikari, A. Activation of the TGF-Β1/EMT Signaling Pathway by Claudin-1 Overexpression Reduces Doxorubicin Sensitivity in Small Cell Lung Cancer SBC-3 Cells. Arch. Biochem. Biophys. 2024, 751, 109824. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Zheng, S.; Lu, Z.; Wang, X.; Wang, Y.; Zhang, G.; Xu, H.; Huang, J.; Lei, Y.; Liu, C.; et al. Exosomal MiR-375-3p Breaks Vascular Barrier and Promotes Small Cell Lung Cancer Metastasis by Targeting Claudin-1. Transl. Lung Cancer Res. 2021, 10, 3155–3172. [Google Scholar] [CrossRef]
- Chang, J.W.; Seo, S.T.; Im, M.A.; Won, H.-R.; Liu, L.; Oh, C.; Jin, Y.L.; Piao, Y.; Kim, H.J.; Kim, J.T.; et al. Claudin-1 Mediates Progression by Regulating EMT through AMPK/TGF-β Signaling in Head and Neck Squamous Cell Carcinoma. Transl. Res. 2022, 247, 58–78. [Google Scholar] [CrossRef]
- Roehlen, N.; Muller, M.; Nehme, Z.; Crouchet, E.; Jühling, F.; Zompo, F.D.; Cherradi, S.; Duong, F.H.T.; Almeida, N.; Saviano, A.; et al. Treatment of HCC with Claudin-1-Specific Antibodies Suppresses Carcinogenic Signaling and Reprograms the Tumor Microenvironment. J. Hepatol. 2023, 78, 343–355. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, Q.; Zhang, S.; Liu, H.; Man, Z.; Wang, Y. Effects of Claudin-1 Downregulation on the Physiological Processes of Gallbladder Cancer SGC996 Cells. Oncol. Lett. 2019, 17, 1688–1694. [Google Scholar] [CrossRef]
- Detarya, M.; Sawanyawisuth, K.; Aphivatanasiri, C.; Chuangchaiya, S.; Saranaruk, P.; Sukprasert, L.; Silsirivanit, A.; Araki, N.; Wongkham, S.; Wongkham, C. The O-GalNAcylating Enzyme GALNT5 Mediates Carcinogenesis and Progression of Cholangiocarcinoma via Activation of AKT/ERK Signaling. Glycobiology 2020, 30, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Li, L.; Gu, H.; Li, M.; Xu, S.; Bu, W.; Zhang, M.; Zhou, Z.; Chen, X. Lycopene Upregulates ZO-1 and Downregulates Claudin-1 through Autophagy Inhibition in the Human Cutaneous Squamous Cell Carcinoma Cell Line COLO-16. J. Cancer 2019, 10, 510–521. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, H.; Wang, J.; Wang, X.; Qian, L.; Xu, F.; Song, W.; Wu, D.; Shen, Z.; Feng, D.; et al. ΔNp63α Exerts Antitumor Functions in Cervical Squamous Cell Carcinoma. Oncogene 2020, 39, 905–921. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Song, W.; Qian, L.; Zhu, J.; Li, Y.; Li, M.; Zhang, T.; Zhao, W.; Zhou, Y.; Yang, X. Effect of Claudin 1 on Cell Proliferation, Migration and Apoptosis in Human Cervical Squamous Cell Carcinoma. Oncol. Rep. 2021, 45, 606–618. [Google Scholar] [CrossRef]
- Visco, Z.R.; Sfakianos, G.; Grenier, C.; Boudreau, M.-H.; Simpson, S.; Rodriguez, I.; Whitaker, R.; Yao, D.Y.; Berchuck, A.; Murphy, S.K.; et al. Epigenetic Regulation of Claudin-1 in the Development of Ovarian Cancer Recurrence and Drug Resistance. Front. Oncol. 2021, 11, 620873. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-A.; Kim, L.-H.; Ryu, M.H.; Choi, S.-Y.; Jin, B.; Lee, W.; Jung, Y.C.; Ahn, C.-H.; Ahn, M.-H.; Hong, K.-O.; et al. Withaferin A Mitigates Metastatic Traits in Human Oral Squamous Cell Carcinoma Caused by Aberrant Claudin-1 Expression. Cell Biol. Toxicol. 2022, 38, 147–165. [Google Scholar] [CrossRef]
- Zhu, P.; Yu, H.; Zhou, K.; Bai, Y.; Qi, R.; Zhang, S. 3,3′-Diindolylmethane Modulates Aryl Hydrocarbon Receptor of Esophageal Squamous Cell Carcinoma to Reverse Epithelial-Mesenchymal Transition through Repressing RhoA/ROCK1-Mediated COX2/PGE2 Pathway. J. Exp. Clin. Cancer Res. 2020, 39, 113. [Google Scholar] [CrossRef]
- Han, M.; Li, N.; Li, F.; Wang, H.; Ma, L. MiR-27b-3p Exerts Tumor Suppressor Effects in Esophageal Squamous Cell Carcinoma by Targeting Nrf2. Human. Cell 2020, 33, 641–651. [Google Scholar] [CrossRef]
- Su, N.; Li, L.; Zhou, E.; Li, H.; Wu, S.; Cao, Z. Resveratrol Downregulates MiR-155-5p to Block the Malignant Behavior of Gastric Cancer Cells. Biomed. Res. Int. 2022, 2022, 6968641. [Google Scholar] [CrossRef]
- Tekin, C.; Ercelik, M.; Dunaev, P.; Galembikova, A.; Tezcan, G.; Aksoy, S.A.; Budak, F.; Isık, O.; Ugras, N.; Boichuk, S.; et al. Leaf Extract from European Olive (Olea europaea L.) Post-Transcriptionally Suppresses the Epithelial-Mesenchymal Transition and Sensitizes Gastric Cancer Cells to Chemotherapy. Biochem. Mosc. 2024, 89, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-L.; Song, Y.-H.; Du, Z.-P.; Hu, Y.-H.; Wang, Z.-X.; Chen, X.; Lu, X.-M.; Chen, Y.-X.; Duan, Y.-Q.; Zhu, X.-D. Overexpression of MiR-633 Suppresses the Tumorigenicity of Gastric Cancer Cells and Induces Apoptosis by Targeting MAPK1. Curr. Med. Sci. 2022, 42, 1033–1045. [Google Scholar] [CrossRef]
- Zhao, Y.; Cai, C.; Zhang, M.; Shi, L.; Wang, J.; Zhang, H.; Ma, P.; Li, S. Ephrin-A2 Promotes Prostate Cancer Metastasis by Enhancing Angiogenesis and Promoting EMT. J. Cancer Res. Clin. Oncol. 2021, 147, 2013–2023. [Google Scholar] [CrossRef]
- Jariyal, H.; Gupta, C.; Andhale, S.; Gadge, S.; Srivastava, A. Comparative Stemness and Differentiation of Luminal and Basal Breast Cancer Stem Cell Type under Glutamine-Deprivation. J. Cell Commun. Signal 2021, 15, 207–222. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, L.; Fang, M.; Bai, H.; Xu, Y. The Chromatin Remodeling Protein BRM Regulates the Transcription of Tight Junction Proteins: Implication in Breast Cancer Metastasis. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, P.; Dong, R.; Weir, S.; Baltezor, M.; Schoenen, F.J.; Chen, Q. Novel Compound C150 Inhibits Pancreatic Cancer Cell Epithelial-to-Mesenchymal Transition and Tumor Growth in Mice. Front. Oncol. 2021, 11, 773350. [Google Scholar] [CrossRef]
- Gurbuz, N.; Kahraman, N.; Sonmez, H.E.; Mokhlis, H.A.; Kosar, P.A.; Ozpolat, B. MiRNA-193b-5p Suppresses Pancreatic Cancer Cell Proliferation, Invasion, Epithelial Mesenchymal Transition, and Tumor Growth by Inhibiting EEF2K. Anticancer. Agents Med. Chem. 2022, 22, 2607–2618. [Google Scholar] [CrossRef] [PubMed]
- Mashinson, V.; Webster, T.M.; Vadukoot, A.K.; Tolentino, K.T.; Simeon, P.; Fatima, I.; Dhawan, P.; Hopkins, C.R. Discovery, Synthesis and Biological Evaluation of a Series of N-(Phenylcarbamothioyl)-2-Napthamides as Inhibitors of Claudin-1. Bioorg Med. Chem. 2023, 92, 117416. [Google Scholar] [CrossRef]
- An, J.; Ha, E.-M. Lactobacillus-Derived Metabolites Enhance the Antitumor Activity of 5-FU and Inhibit Metastatic Behavior in 5-FU-Resistant Colorectal Cancer Cells by Regulating Claudin-1 Expression. J. Microbiol. 2020, 58, 967–977. [Google Scholar] [CrossRef]
- Luan, N.; Chen, Y.; Li, Q.; Mu, Y.; Zhou, Q.; Ye, X.; Deng, Q.; Ling, L.; Wang, J.; Wang, J. TRF-20-M0NK5Y93 Suppresses the Metastasis of Colon Cancer Cells by Impairing the Epithelial-to-Mesenchymal Transition through Targeting Claudin-1. Am. J. Transl. Res. 2021, 13, 124–142. [Google Scholar]
- Fatima, I.; Uppada, J.P.; Chhonker, Y.S.; Gowrikumar, S.; Barman, S.; Roy, S.; Tolentino, K.T.; Palermo, N.; Natarajan, A.; Beauchamp, D.R.; et al. Identification and Characterization of a First-Generation Inhibitor of Claudin-1 in Colon Cancer Progression and Metastasis. Biomed. Pharmacother. 2023, 159, 114255. [Google Scholar] [CrossRef] [PubMed]
- Cherradi, S.; Garambois, V.; Marines, J.; Andrade, A.F.; Fauvre, A.; Morand, O.; Fargal, M.; Mancouri, F.; Ayrolles-Torro, A.; Vezzo-Vié, N.; et al. Improving the Response to Oxaliplatin by Targeting Chemotherapy-Induced CLDN1 in Resistant Metastatic Colorectal Cancer Cells. Cell Biosci. 2023, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Kishi, S.; Sasaki, T.; Ohmori, H.; Fujiwara-Tani, R.; Mori, S.; Goto, K.; Nishiguchi, Y.; Mori, T.; Kawahara, I.; et al. Targeting Claudin-4 Enhances Chemosensitivity in Breast Cancer. Cancer Sci. 2020, 111, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, Y.; Fujiwara-Tani, R.; Sasaki, T.; Luo, Y.; Ohmori, H.; Kishi, S.; Mori, S.; Goto, K.; Yasui, W.; Sho, M.; et al. Targeting Claudin-4 Enhances CDDP-Chemosensitivity in Gastric Cancer. Oncotarget 2019, 10, 2189–2202. [Google Scholar] [CrossRef]
- Sasaki, T.; Fujiwara-Tani, R.; Kishi, S.; Mori, S.; Luo, Y.; Ohmori, H.; Kawahara, I.; Goto, K.; Nishiguchi, Y.; Mori, T.; et al. Targeting Claudin-4 Enhances Chemosensitivity of Pancreatic Ductal Carcinomas. Cancer Med. 2019, 8, 6700–6708. [Google Scholar] [CrossRef]
- Levine, F.; Ogunwobi, O.O. Targeting PVT1 Exon 9 Re-Expresses Claudin 4 Protein and Inhibits Migration by Claudin-Low Triple Negative Breast Cancer Cells. Cancers 2021, 13, 1046. [Google Scholar] [CrossRef]
- Peng, W.; Chen, L.; Liu, J. Celastrol Inhibits Gastric Cancer Cell Proliferation, Migration, and Invasion via the FOXA1/CLDN4 Axis. Toxicol. Res. 2023, 12, 392–399. [Google Scholar] [CrossRef]
- Yamamoto, T.M.; Webb, P.G.; Davis, D.M.; Baumgartner, H.K.; Woodruff, E.R.; Guntupalli, S.R.; Neville, M.; Behbakht, K.; Bitler, B.G. Loss of Claudin-4 Reduces DNA Damage Repair and Increases Sensitivity to PARP Inhibitors. Mol. Cancer Ther. 2022, 21, 647–657. [Google Scholar] [CrossRef]
- Shim, M.K.; Na, J.; Cho, I.K.; Jang, E.H.; Park, J.; Lee, S.; Kim, J.-H. Targeting of Claudin-4 by Clostridium Perfringens Enterotoxin-Conjugated Polysialic Acid Nanoparticles for Pancreatic Cancer Therapy. J. Control Release 2021, 331, 434–442. [Google Scholar] [CrossRef]
- Bang, C.; Park, M.G.; Cho, I.K.; Lee, D.-E.; Kim, G.L.; Jang, E.H.; Shim, M.K.; Yoon, H.Y.; Lee, S.; Kim, J.-H. Liposomes Targeting the Cancer Cell-Exposed Receptor, Claudin-4, for Pancreatic Cancer Chemotherapy. Biomater. Res. 2023, 27, 53. [Google Scholar] [CrossRef]
- Erramilli, S.K.; Dominik, P.K.; Ogbu, C.P.; Kossiakoff, A.A.; Vecchio, A.J. Structural and Biophysical Insights into Targeting of Claudin-4 by a Synthetic Antibody Fragment. Commun. Biol. 2024, 7, 733. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Isoda, K.; Taira, Y.; Taira, I.; Kondoh, M.; Ishida, I. Anti-Tumor Effect of a Recombinant Bifidobacterium Strain Secreting a Claudin-Targeting Molecule in a Mouse Breast Cancer Model. Eur. J. Pharmacol. 2020, 887, 173596. [Google Scholar] [CrossRef] [PubMed]
- Breed, C.; Hicks, D.A.; Webb, P.G.; Galimanis, C.E.; Bitler, B.G.; Behbakht, K.; Baumgartner, H.K. Ovarian Tumor Cell Expression of Claudin-4 Reduces Apoptotic Response to Paclitaxel. Mol. Cancer Res. 2019, 17, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, W.; Li, M.; Dam, J.; Huang, K.; Wang, Y.; Qiu, Z.; Sun, T.; Chen, P.; Zhang, Z.; et al. Evaluation of the Prognostic Relevance of Differential Claudin Gene Expression Highlights Claudin-4 as Being Suppressed by TGFβ1 Inhibitor in Colorectal Cancer. Front. Genet. 2022, 13, 783016. [Google Scholar] [CrossRef]
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simões, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr. Pathol. 2022, 33, 27–63. [Google Scholar] [CrossRef]
- Janiszewska, J.; Bodnar, M.; Paczkowska, J.; Ustaszewski, A.; Smialek, M.J.; Szylberg, L.; Marszalek, A.; Kiwerska, K.; Grenman, R.; Szyfter, K.; et al. Loss of the MAF Transcription Factor in Laryngeal Squamous Cell Carcinoma. Biomolecules 2021, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Oksvold, P.; Fagerberg, L.; Lundberg, E.; Jonasson, K.; Forsberg, M.; Zwahlen, M.; Kampf, C.; Wester, K.; Hober, S.; et al. Towards a Knowledge-Based Human Protein Atlas. Nat. Biotechnol. 2010, 28, 1248–1250. [Google Scholar] [CrossRef]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repečka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N.; et al. Visualizing and Interpreting Cancer Genomics Data via the Xena Platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef]
- Expression of CLDN1 in Thyroid Cancer—The Human Protein Atlas; Ver. 21.1. Available online: https://www.proteinatlas.org/ENSG00000163347-CLDN1/pathology/thyroid+cancer (accessed on 7 September 2022).
- Expression of CLDN4 in Thyroid Cancer—The Human Protein Atlas; Ver 21.1. Available online: https://www.proteinatlas.org/ENSG00000189143-CLDN4/pathology/thyroid+cancer (accessed on 7 September 2022).
- Budczies, J.; Klauschen, F.; Sinn, B.V.; Győrffy, B.; Schmitt, W.D.; Darb-Esfahani, S.; Denkert, C. Cutoff Finder: A Comprehensive and Straightforward Web Application Enabling Rapid Biomarker Cutoff Optimization. PLoS ONE 2012, 7, e51862. [Google Scholar] [CrossRef]

| Variables | n (%) | |
|---|---|---|
| Sex | Female | 70 (86.42%) |
| Male | 11 (13.58%) | |
| Histological diagnosis | Goiter | 62 (38.27%) |
| Adenoma | 52 (32.10%) | |
| Oncocytic adenoma | 6 (3.7%) | |
| Papillary thyroid cancer | 22 (13.58%) | |
| Medullary thyroid cancer | 10 (6.17%) | |
| Anaplastic thyroid cancer | 10 (6.17%) | |
| Cancer stage | pT1 | 10 (23.18%) |
| pT2 | 8 (19.05%) | |
| pT3 | 4 (9.52%) | |
| pT4 | 6 (14.29%) | |
| Unknown | 14 (33.33%) | |
| Type of Tissue | Total (n) | Claudin-1 | Claudin-4 | ||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | p-Value | Positive | Negative | p-Value | ||
| Goiter | 62 | 0 | 62 | p < 0.0007 *↟ | 60 | 2 | p = 0.816 |
| Adenoma | 58 | 18 | 40 | 42 | 16 | ||
| Cancer | 42 | 16 | 26 | 22 | 20 | ||
| Papillary cancer | 22 | 16 | 6 | p < 0.0055 *● | 20 | 2 | p < 0.011 *● |
| Medullary cancer | 10 | 0 | 10 | 0 | 10 | ||
| Anaplastic cancer | 10 | 0 | 10 | 2 | 8 | ||
| Clinical Data | n (%) | Claudin-1 | Claudin-4 | |||||
|---|---|---|---|---|---|---|---|---|
| Low | High | p-Value | Low | High | p-Value | |||
| Age | ≤45 | 235 | 106 (45.11%) | 129 (54.89%) | p = 0.01 | 77 (32.77%) | 158 (67.23%) | p = 0.008 |
| >45 | 266 | 149 (54.14%) | 117 (45.86%) | 59 (22.18%) | 207 (77.82%) | |||
| Sex | Female | 366 | 180 (49.18%) | 186 (50.82%) | p = 0.2 | 105 (28.69%) | 261 (71.31%) | p = 0.2 |
| Male | 135 | 75 (55.56%) | 60 (44.44%) | 31 (22.96%) | 104 (77.04%) | |||
| Race | White | 332 | 159 (47.89%) | 173 (52.11%) | p = 0.34 | 116 (34.94%) | 216 (65.06%) | p = 0.196 |
| Asian | 51 | 20 (39.22%) | 31 (60.78%) | 9 (17.65%) | 42 (82.35%) | |||
| Black or African American | 27 | 15 (55.56%) | 12 (44.44%) | 10 (37.04%) | 17 (62.96%) | |||
| Clinical stage | I | 289 | 142 (49.13%) | 147 (50.87%) | p = 0.001 | 89 (30.80%) | 200 (69.20%) | p = 0.027 |
| II | 52 | 36 (69.23%) | 16 (30.77%) | 6 (11.54%) | 46 (88.46%) | |||
| III | 111 | 53 (47.75%) | 58 (52.25%) | 31 (27.93%) | 80 (72.07%) | |||
| IV | 47 | 23 (48.94%) | 24 (51.06%) | 9 (9.15%) | 38 (80.85%) | |||
| Lymph node invasion | N0 | 223 | 131 (58.74%) | 92 (41.26%) | p = 0.0001 | 46 (20.63%) | 177 (79.37%) | p = 0.01 |
| N1 | 216 | 87 (40.29%) | 129 (59.72%) | 67 (31.02%) | 149 (68.98%) | |||
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age (≤45 vs. >45) | N/A | N/A | 0.98 | - | - | - |
| Sex (F vs. M) | 0.51 | 0.18–1.4 | 0.19 | - | - | - |
| Tumor stage (T1 vs. T2-T4) | 0.47 | 0.11–2.06 | 0.097 | - | - | - |
| Clinical stage (I vs II-IV) | 0.29 | 0.1–0.84 | 0.023 | 0.3 | 0.1–0.86 | 0.025 |
| Nodes (N0 vs. N1) | 0.7 | 0.23–2.16 | 0.54 | - | - | - |
| CLDN-1 (Low vs. High) | 8.06 | 1.82–35.62 | 0.006 | 7.91 | 1.79–35 | 0.006 |
| CLDN-4 (Low vs. High) | 0.3 | 0.07–1.31 | 0.11 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borowczak, J.; Łaszczych, D.; Olejnik, K.; Michalski, J.; Gutowska, A.; Kula, M.; Bator, A.; Sekielska-Domanowska, M.; Makarewicz, R.; Marszałek, A.; et al. Tight Junctions and Cancer: Targeting Claudin-1 and Claudin-4 in Thyroid Pathologies. Pharmaceuticals 2024, 17, 1304. https://doi.org/10.3390/ph17101304
Borowczak J, Łaszczych D, Olejnik K, Michalski J, Gutowska A, Kula M, Bator A, Sekielska-Domanowska M, Makarewicz R, Marszałek A, et al. Tight Junctions and Cancer: Targeting Claudin-1 and Claudin-4 in Thyroid Pathologies. Pharmaceuticals. 2024; 17(10):1304. https://doi.org/10.3390/ph17101304
Chicago/Turabian StyleBorowczak, Jędrzej, Dariusz Łaszczych, Katarzyna Olejnik, Jakub Michalski, Anna Gutowska, Monika Kula, Anita Bator, Marta Sekielska-Domanowska, Roman Makarewicz, Andrzej Marszałek, and et al. 2024. "Tight Junctions and Cancer: Targeting Claudin-1 and Claudin-4 in Thyroid Pathologies" Pharmaceuticals 17, no. 10: 1304. https://doi.org/10.3390/ph17101304
APA StyleBorowczak, J., Łaszczych, D., Olejnik, K., Michalski, J., Gutowska, A., Kula, M., Bator, A., Sekielska-Domanowska, M., Makarewicz, R., Marszałek, A., Szylberg, Ł., & Bodnar, M. (2024). Tight Junctions and Cancer: Targeting Claudin-1 and Claudin-4 in Thyroid Pathologies. Pharmaceuticals, 17(10), 1304. https://doi.org/10.3390/ph17101304






