The Anti-Vitiligo Effects of Feshurin In Vitro from Ferula samarcandica and the Mechanism of Action
Abstract
1. Introduction
2. Results
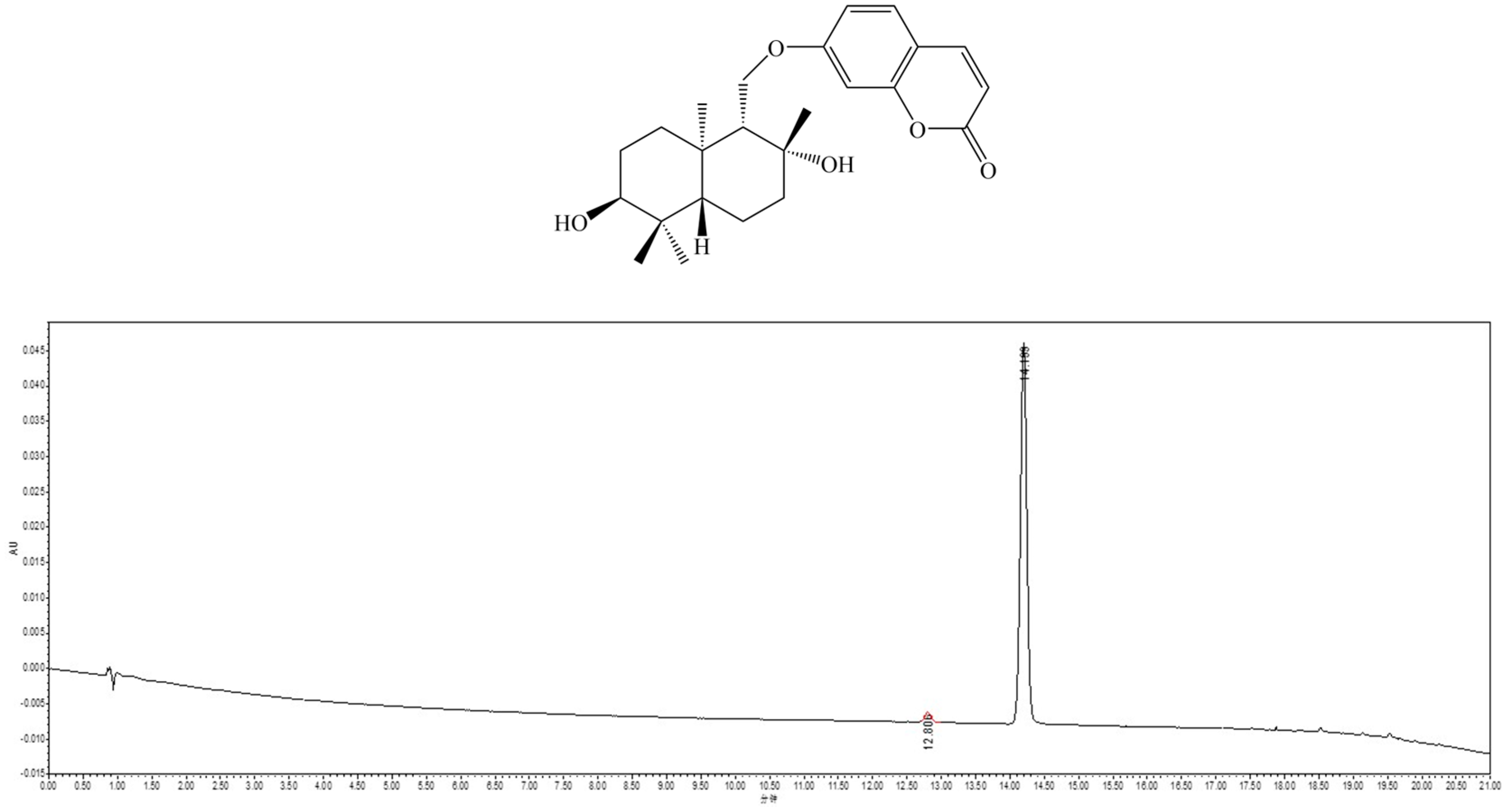
2.1. Isolation and Identification of Feshurin
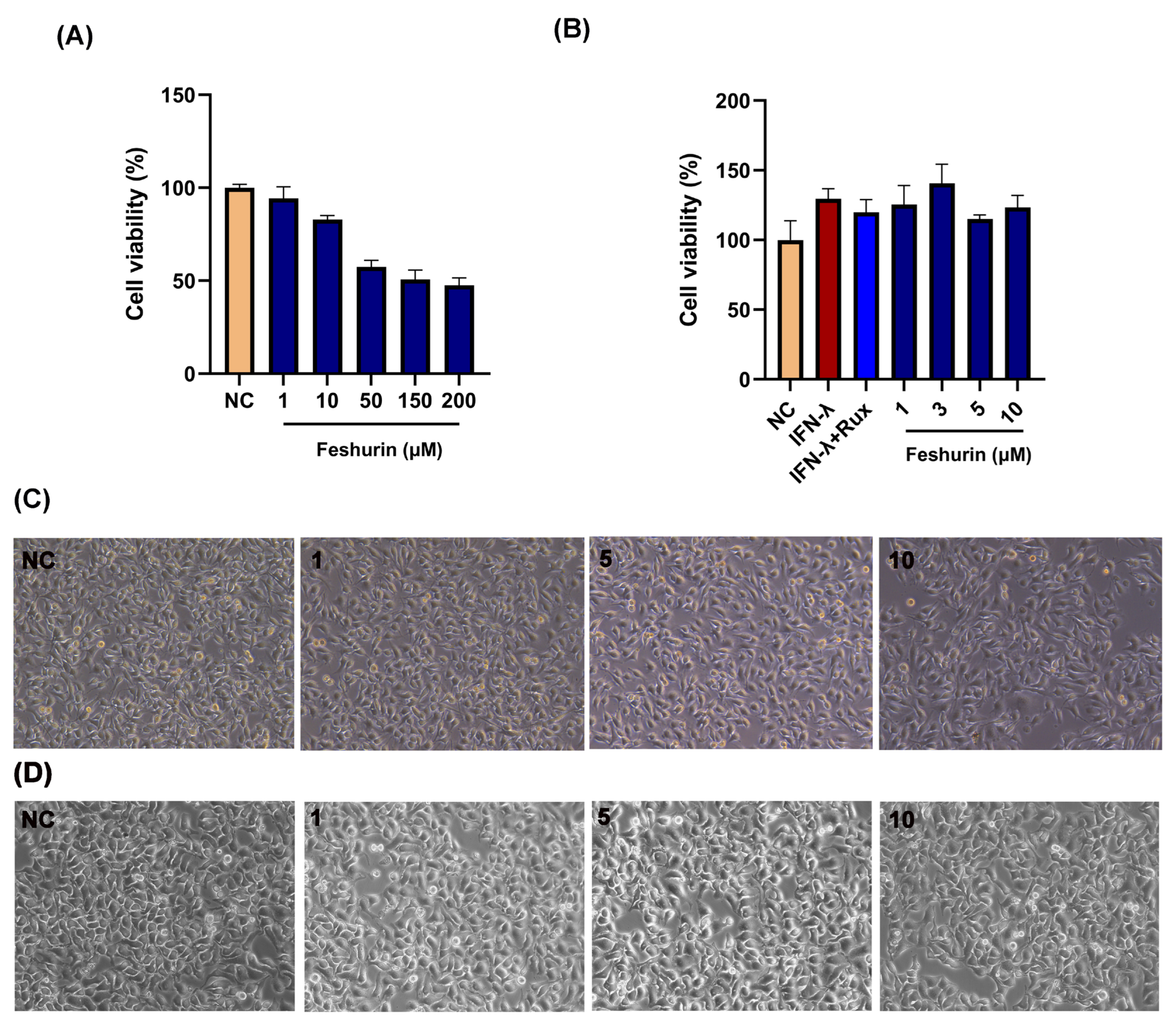
2.2. Effects of Feshurin on B16 and HaCaT Cell Viability
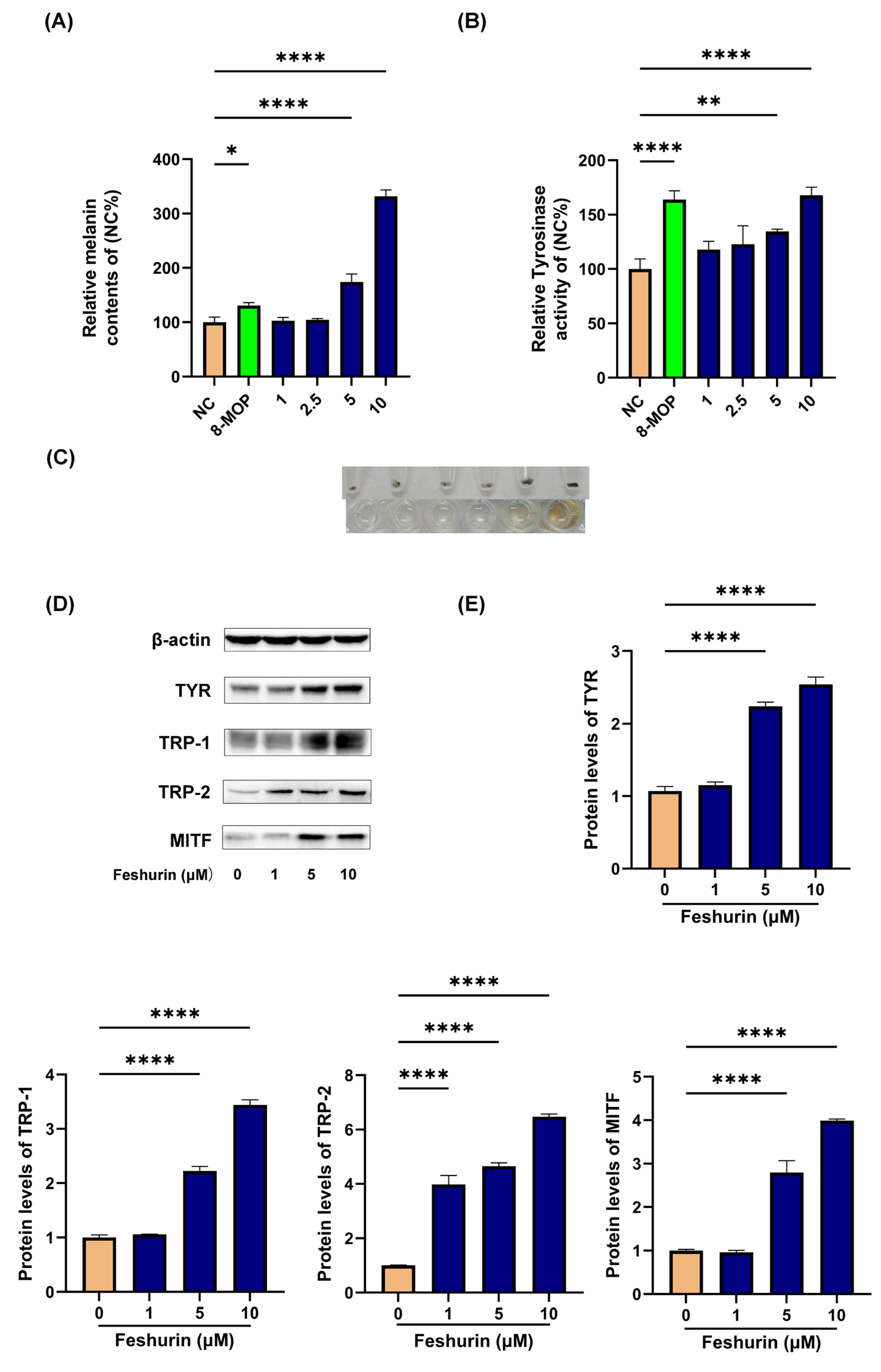
2.3. Intracellular Melanin Content and TYR Activity
2.4. Feshurin Promotes the Expression Level of Proteins Related to Melanogenesis
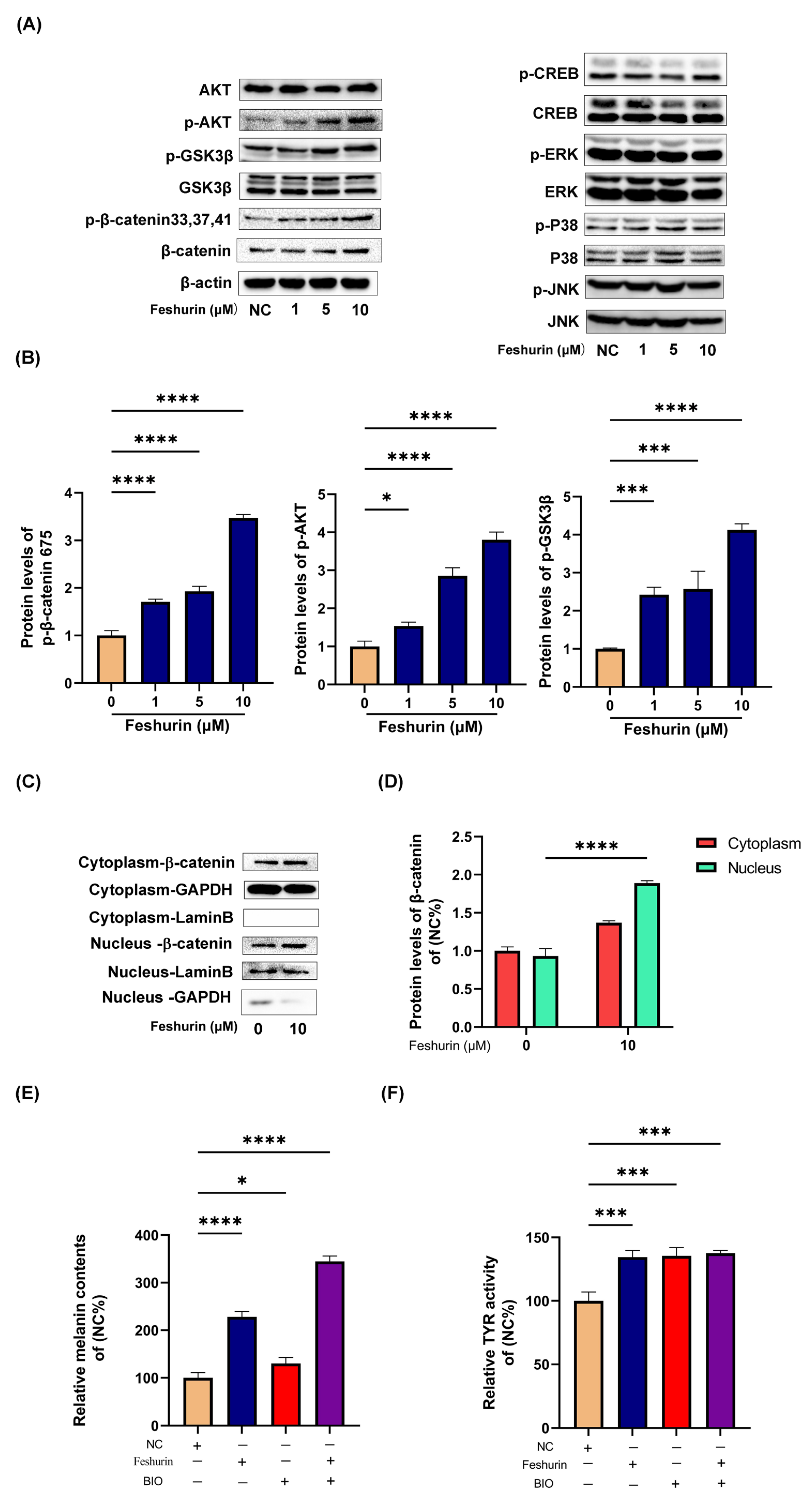
2.5. Feshurin Mediates Melanogenesis via the Wnt Signaling Pathway
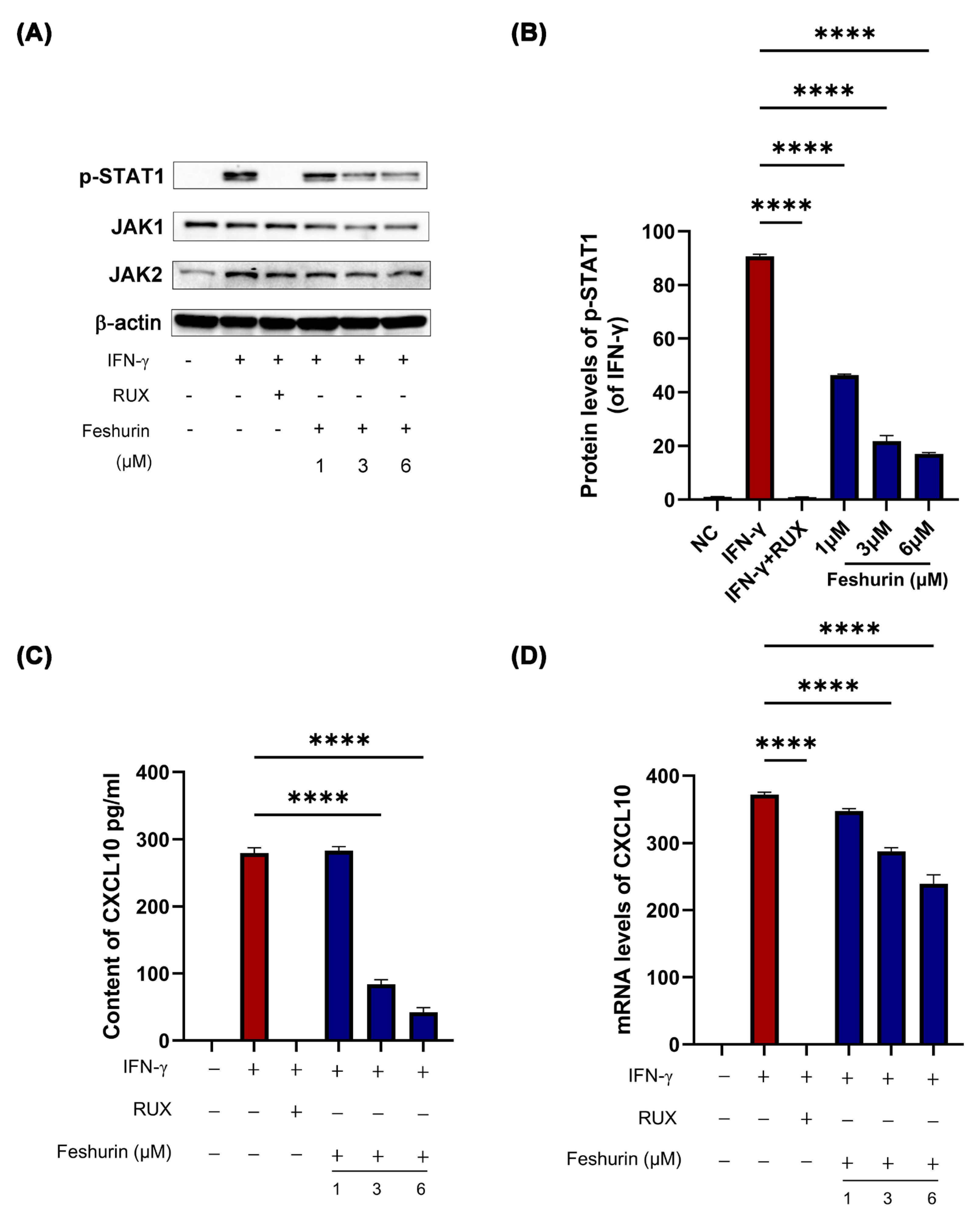
2.6. Feshurin Inhibits the IFN-γ-Induced CXCL10 Production in HaCaT Cells via the JAK-STAT Pathway
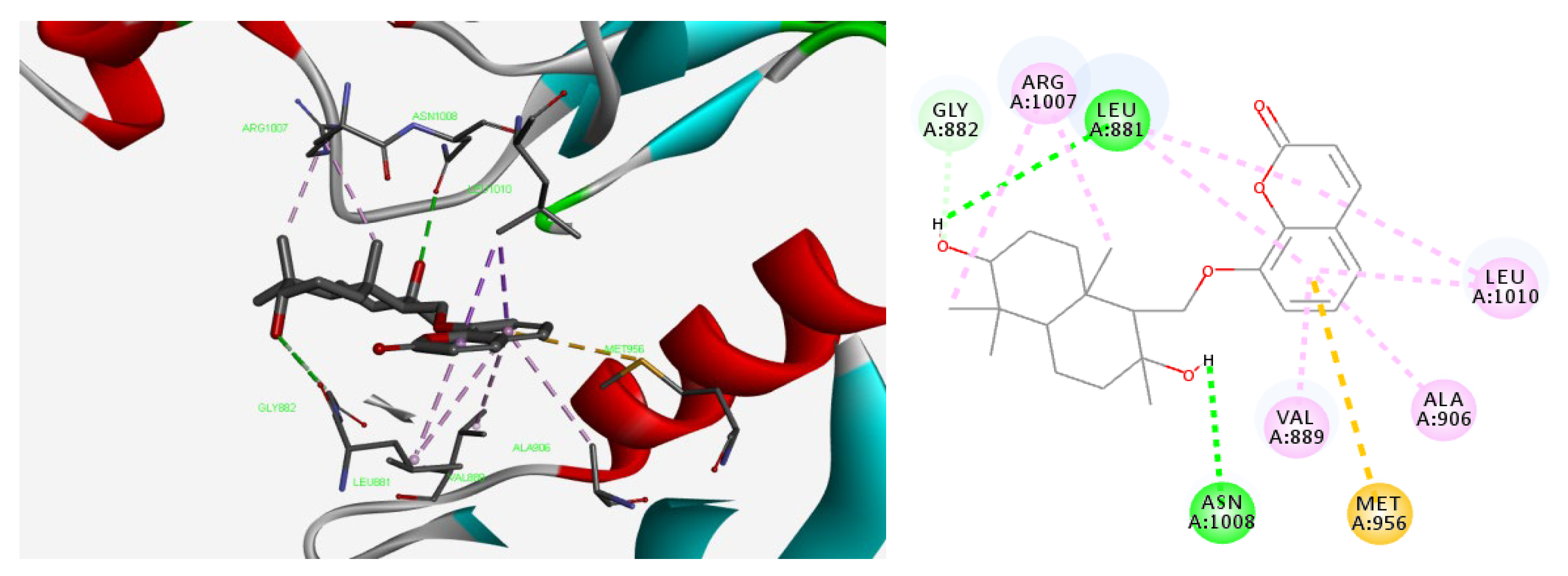
2.7. Molecular Docking Studies of Feshurin on JAK1
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Reagents
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. Determination of Relative Intracellular Melanin Content
4.6. Determination of Intracellular TYR Activity
4.7. Western Blotting
4.8. Real-Time Reverse Transcription-Quantitative PCR (RT-qPCR) (TaqMan) ™ Analysis
4.9. Enzyme-Linked Immunosorbent Assay (ELISA)
4.10. Molecular Docking
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kundu, R.V.; Mhlaba, J.M.; Rangel, S.M.; Le Poole, I.C. The convergence theory for vitiligo: A reappraisal. Exp. Dermatol. 2019, 28, 647–655. [Google Scholar] [CrossRef]
- Shah, F.; Patel, S.; Begum, R.; Dwivedi, M. Emerging role of Tissue Resident Memory T cells in vitiligo: From pathogenesis to therapeutics. Autoimmun. Rev. 2021, 20, 102868. [Google Scholar] [CrossRef] [PubMed]
- De Castro, C.C.S.; Miot, H.A. Prevalence of vitiligo in Brazil-A population survey. Pigment. Cell Melanoma Res. 2018, 31, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Catucci Boza, J.; Giongo, N.; Machado, P.; Horn, R.; Fabbrin, A.; Cestari, T. Quality of Life Impairment in Children and Adults with Vitiligo: A Cross-Sectional Study Based on Dermatology-Specific and Disease-Specific Quality of Life Instruments. Dermatology 2016, 232, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Kubelis-Lopez, D.E.; Zapata-Salazar, N.A.; Said-Fernandez, S.L.; Sanchez-Dominguez, C.N.; Salinas-Santander, M.A.; Martinez-Rodriguez, H.G.; Vazquez-Martinez, O.T.; Wollina, U.; Lotti, T.; Ocampo-Candiani, J. Updates and new medical treatments for vitiligo (Review). Exp. Ther. Med. 2021, 22, 797. [Google Scholar] [CrossRef] [PubMed]
- Touni, A.A.; Shivde, R.S.; Echuri, H.; Abdel-Aziz, R.T.A.; Abdel-Wahab, H.; Kundu, R.V.; Le Poole, I.C. Melanocyte-keratinocyte cross-talk in vitiligo. Front. Med. 2023, 10, 1176781. [Google Scholar] [CrossRef]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Jang, E.J.; Shin, Y.; Park, H.J.; Kim, D.; Jung, C.; Hong, J.Y.; Kim, S.; Lee, S.K. Anti-melanogenic activity of phytosphingosine via the modulation of the microphthalmia-associated transcription factor signaling pathway. J. Dermatol. Sci. 2017, 87, 19–28. [Google Scholar] [CrossRef]
- Zang, D.; Niu, C.; Aisa, H.A. Amine derivatives of furocoumarin induce melanogenesis by activating Akt/GSK-3β/β-catenin signal pathway. Drug Des. Devel Ther. 2019, 13, 623–632. [Google Scholar] [CrossRef]
- Lu, Y.; Tonissen, K.F.; Di Trapani, G. Modulating skin colour: Role of the thioredoxin and glutathione systems in regulating melanogenesis. Biosci. Rep. 2021, 41, BSR20210427. [Google Scholar] [CrossRef]
- Cavalié, M.; Ezzedine, K.; Fontas, E.; Montaudié, H.; Castela, E.; Bahadoran, P.; Taïeb, A.; Lacour, J.P.; Passeron, T. Maintenance therapy of adult vitiligo with 0.1% tacrolimus ointment: A randomized, double blind, placebo-controlled study. J. Investig. Dermatol. 2015, 135, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Prieux, R.; Ferrara, F.; Cervellati, F.; Guiotto, A.; Benedusi, M.; Valacchi, G. Inflammasome involvement in CS-induced damage in HaCaT keratinocytes. In Vitro Cell. Dev. Biol. Anim. 2022, 58, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Roberts, G.H.L.; Ferrara, T.M.; Ben, S.; van Geel, N.; Wolkerstorfer, A.; Ezzedine, K.; Siebert, J.; Neff, C.P.; Palmer, B.E.; et al. Early-onset autoimmune vitiligo associated with an enhancer variant haplotype that upregulates class II HLA expression. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.E.; Harris, T.H.; Weninger, W.; John Wherry, E.; Hunter, C.A.; Turka, L.A. A Mouse Model of Vitiligo with Focused Epidermal Depigmentation Requires IFN-γ for Autoreactive CD8+ T-Cell Accumulation in the Skin. J. Investig. Dermatol. 2012, 132, 1869–1876. [Google Scholar] [CrossRef]
- Banerjee, S.; Biehl, A.; Gadina, M.; Hasni, S.; Schwartz, D.M. JAK-STAT Signaling as a Target for Inflammatory and Autoimmune Diseases: Current and Future Prospects. Drugs 2017, 77, 521–546. [Google Scholar] [CrossRef]
- Bergqvist, C.; Ezzedine, K. Vitiligo: A focus on pathogenesis and its therapeutic implications. J. Dermatol. 2021, 48, 252–270. [Google Scholar] [CrossRef]
- White, C.; Miller, R. A Literature Review Investigating the Use of Topical Janus Kinase Inhibitors for the Treatment of Vitiligo. J. Clin. Aesthet. Dermatol. 2022, 15, 20–25. [Google Scholar]
- Speeckaert, R.; Caelenberg, E.V.; Belpaire, A.; Speeckaert, M.M.; Geel, N.v. Vitiligo: From Pathogenesis to Treatment. J. Clin. Med. 2024, 13, 5225. [Google Scholar] [CrossRef]
- Qi, F.; Liu, F.; Gao, L. Janus Kinase Inhibitors in the Treatment of Vitiligo: A Review. Front. Immunol. 2021, 12, 790125. [Google Scholar] [CrossRef]
- Motai, T.; Kitanaka, S. Sesquiterpene phenylpropanoids from Ferula fukanensis and their nitric oxide production inhibitory effects. J. Nat. Prod. 2005, 68, 365–368. [Google Scholar] [CrossRef]
- Su, B.N.; Takaishi, Y.; Honda, G.; Itoh, M.; Takeda, Y.; Kodzhimatov, O.K.; Ashurmetov, O. Sesquiterpene phenylpropanoid and sesquiterpene chromone derivatives from Ferula pallida. J. Nat. Prod. 2000, 63, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Miski, M.; Mabry, T.J.; Bohlmann, F. Fercoperol, an unusual cyclic-endoperoxynerolidol derivative from Ferula communis subsp. communis. J. Nat. Prod. 1986, 49, 916–918. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Razek, M.H.; Ohta, S.; Ahmed, A.A.; Hirata, T. Sesquiterpene coumarins from the roots of Ferula assa-foetida. Phytochemistry 2001, 58, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.M.; Janani, M. A new ester coumarin from Ferula Persica wild, indigenous to Iran. Nat. Product. Res. 2014, 29, 717–721. [Google Scholar] [CrossRef]
- Iranshahi, M.; Arfa, P.; Ramezani, M.; Jaafari, M.R.; Sadeghian, H.; Bassarello, C.; Piacente, S.; Pizza, C. Sesquiterpene coumarins from Ferula szowitsiana and in vitro antileishmanial activity of 7-prenyloxycoumarins against promastigotes. Phytochemistry 2007, 68, 554–561. [Google Scholar] [CrossRef]
- Tayeboon, G.S.; Tavakoli, F.; Hassani, S.; Khanavi, M.; Sabzevari, O.; Ostad, S.N. Effects of Cymbopogon citratus and Ferula assa-foetida extracts on glutamate-induced neurotoxicity. In Vitro Cell. Dev. Biol. Anim. 2013, 49, 706–715. [Google Scholar] [CrossRef]
- Kojima, K.; Isaka, K.; Ondognii, P.; Zevgeegiin, O.; Gombosurengyin, P.; Davgiin, K.; Mizukami, H.; Ogihara, Y. Sesquiterpenoid derivatives from Ferula ferulaeoides [correction of ferulioides]. IV. Chem. Pharm. Bull. 2000, 48, 353–356. [Google Scholar] [CrossRef]
- Motai, T.; Kitanaka, S. Sesquiterpene coumarins from Ferula fukanensis and nitric oxide production inhibitory effects. Chem. Pharm. Bull. 2004, 52, 1215–1218. [Google Scholar] [CrossRef]
- Jabrane, A.; Ben Jannet, H.; Mighri, Z.; Mirjolet, J.F.; Duchamp, O.; Harzallah-Skhiri, F.; Lacaille-Dubois, M.A. Two new sesquiterpene derivatives from the Tunisian endemic Ferula tunetana Pom. Chem. Biodivers. 2010, 7, 392–399. [Google Scholar] [CrossRef]
- Zhou, Y.; Xin, F.; Zhang, G.; Qu, H.; Yang, D.; Han, X. Recent Advances on Bioactive Constituents in Ferula. Drug Dev. Res. 2017, 78, 321–331. [Google Scholar] [CrossRef]
- Kamoldinov, K.; Li, J.; Eshbakova, K.; Sagdullaev, S.; Xu, G.; Zhou, Y.; Li, J.; Aisa, H.A. Sesquiterpene coumarins from Ferula samarkandica Korovin and their bioactivity. Phytochemistry 2021, 187, 112705. [Google Scholar] [CrossRef] [PubMed]
- Migayron, L.; Boniface, K.; Seneschal, J. Vitiligo, From Physiopathology to Emerging Treatments: A Review. Dermatol. Ther. 2020, 10, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Frisoli, M.L.; Essien, K.; Harris, J.E. Vitiligo: Mechanisms of Pathogenesis and Treatment. Annu. Rev. Immunol. 2020, 38, 621–648. [Google Scholar] [CrossRef] [PubMed]
- Domaszewska-Szostek, A.; Polak, A.; Slupecka-Ziemilska, M.; Krzyzanowska, M.; Puzianowska-Kuznicka, M. Current Status of Cell-Based Therapies for Vitiligo. Int. J. Mol. Sci. 2023, 24, 3357. [Google Scholar] [CrossRef] [PubMed]
- Morelli, M.; Scarponi, C.; Mercurio, L.; Facchiano, F.; Pallotta, S.; Madonna, S.; Girolomoni, G.; Albanesi, C. Selective Immunomodulation of Inflammatory Pathways in Keratinocytes by the Janus Kinase (JAK) Inhibitor Tofacitinib: Implications for the Employment of JAK-Targeting Drugs in Psoriasis. J. Immunol. Res. 2018, 2018, 7897263. [Google Scholar] [CrossRef] [PubMed]
- Marchioro, H.Z.; de Castro, C.C.S.; Fava, V.M.; Sakiyama, P.H.; Dellatorre, G.; Miot, H.A. Update on the pathogenesis of vitiligo. An. Bras. Dermatol. 2022, 97, 478–490. [Google Scholar] [CrossRef]
- Chang, Y.; Kang, P.; Cui, T.; Guo, W.; Zhang, W.; Du, P.; Yi, X.; Guo, S.; Gao, T.; Li, C.; et al. Pharmacological inhibition of demethylzeylasteral on JAK-STAT signaling ameliorates vitiligo. J. Transl. Med. 2023, 21, 1–19. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Y.; Shen, Q.; Xiong, R.; Song, X.; Guan, C. DCUN1D1, a new molecule involved in depigmentation via upregulating CXCL10. Exp. Dermatol. 2023, 32, 457–468. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, X.; Chen, S.; Kang, Y.; Wang, X.; Zhang, C.; Xiang, L. Increased Circulating CXCL10 in Non-Segmental Vitiligo Concomitant with Autoimmune Thyroid Disease and Alopecia Areata. Ann. Dermatol. 2019, 31, 393–402. [Google Scholar] [CrossRef]
- Porro, C.; Cianciulli, A.; Trotta, T.; Lofrumento, D.D.; Panaro, M.A. Curcumin Regulates Anti-Inflammatory Responses by JAK/STAT/SOCS Signaling Pathway in BV-2 Microglial Cells. Biology 2019, 8, 51. [Google Scholar] [CrossRef]
- Hlača, N.; Žagar, T.; Kaštelan, M.; Brajac, I.; Prpić-Massari, L. Current Concepts of Vitiligo Immunopathogenesis. Biomedicines 2022, 10, 1639. [Google Scholar] [CrossRef]
- Zhou, J.; Ling, J.; Song, J.; Wang, Y.; Feng, B.; Ping, F. Interleukin 10 protects primary melanocyte by activation of Stat-3 and PI3K/Akt/NF-κB signaling pathways. Cytokine 2016, 83, 275–281. [Google Scholar] [CrossRef]
| Primer | Forward(5′-3′) | Reverse(5′-3′) |
|---|---|---|
| CXCL-10 | GTGGCATTCAAGGAGTACCTC | TGATGGCCTTCGATTCTGGATT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nueraihemaiti, M.; Deng, Z.; Kamoldinov, K.; Chao, N.; Habasi, M.; Aisa, H.A. The Anti-Vitiligo Effects of Feshurin In Vitro from Ferula samarcandica and the Mechanism of Action. Pharmaceuticals 2024, 17, 1252. https://doi.org/10.3390/ph17091252
Nueraihemaiti M, Deng Z, Kamoldinov K, Chao N, Habasi M, Aisa HA. The Anti-Vitiligo Effects of Feshurin In Vitro from Ferula samarcandica and the Mechanism of Action. Pharmaceuticals. 2024; 17(9):1252. https://doi.org/10.3390/ph17091252
Chicago/Turabian StyleNueraihemaiti, Mayire, Zang Deng, Khamidulla Kamoldinov, Niu Chao, Maidina Habasi, and Haji Akber Aisa. 2024. "The Anti-Vitiligo Effects of Feshurin In Vitro from Ferula samarcandica and the Mechanism of Action" Pharmaceuticals 17, no. 9: 1252. https://doi.org/10.3390/ph17091252
APA StyleNueraihemaiti, M., Deng, Z., Kamoldinov, K., Chao, N., Habasi, M., & Aisa, H. A. (2024). The Anti-Vitiligo Effects of Feshurin In Vitro from Ferula samarcandica and the Mechanism of Action. Pharmaceuticals, 17(9), 1252. https://doi.org/10.3390/ph17091252







