Efficacy of Diterpene Polyalthic Acid Combined with Amphotericin B against Leishmania amazonensis In Vitro
Abstract
1. Introduction
2. Results
2.1. PA Exhibits Leishmanicidal Activity against Both Promastigote and Amastigote Forms of L. amazonensis without Causing Cytotoxic or Hemolytic Effects In Vitro
2.2. The Combination of PA and AmpB Displays Synergistic Leishmanicidal Activity, While It Results in Indifferent Cytotoxic and Hemolytic Effects In Vitro
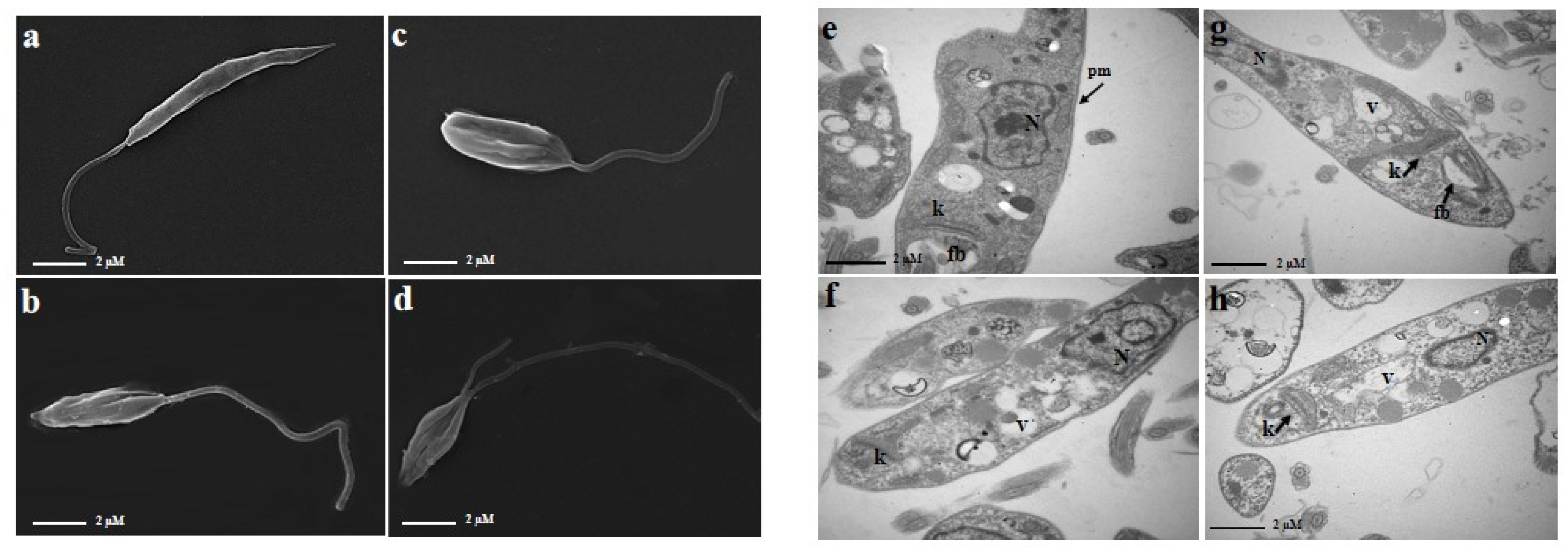
2.3. The Combination of PA and AmpB Induces Morphological and Ultrastructural Damages in Promastigote Forms of L. amazonensis
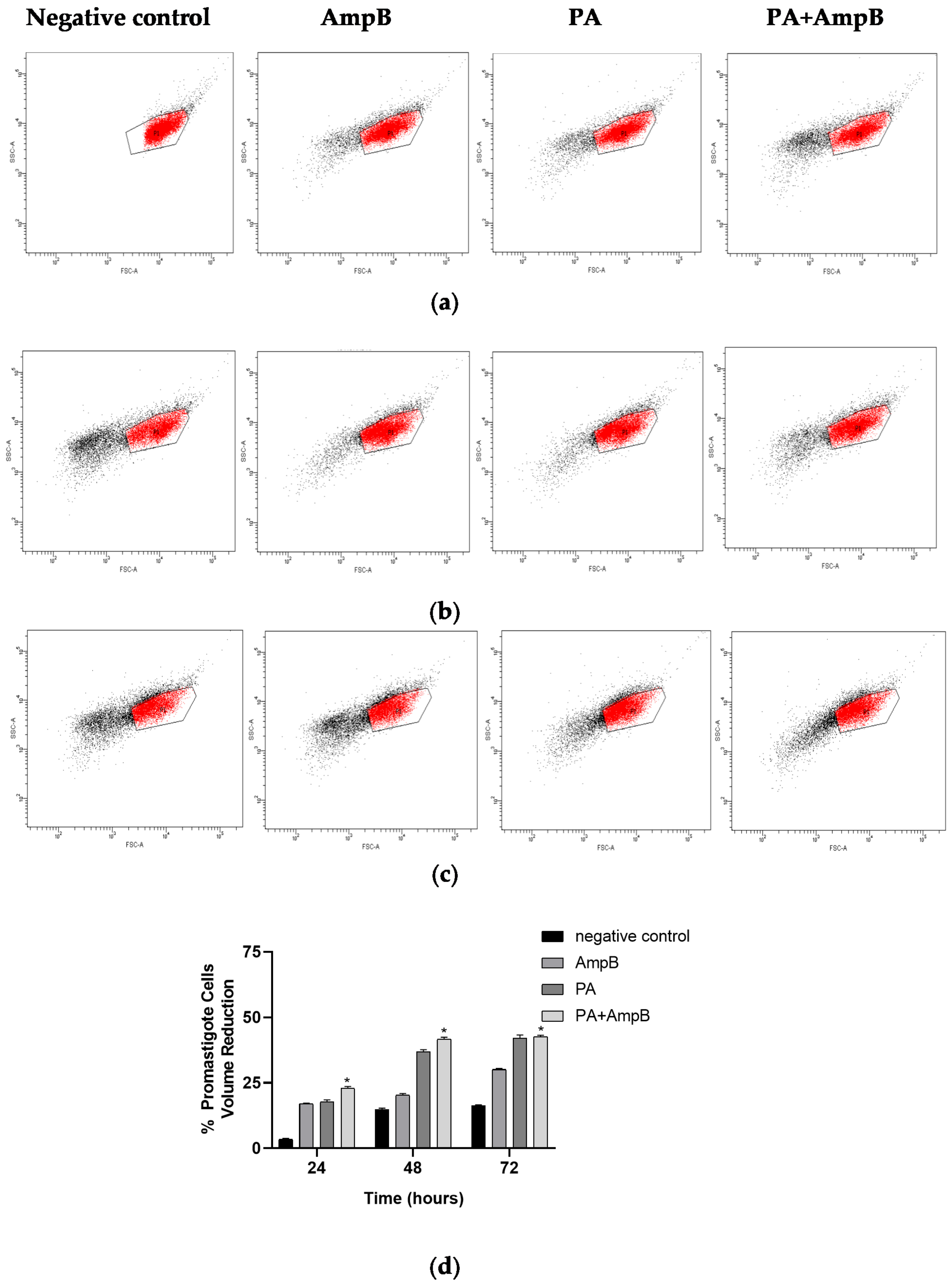
2.4. PA and Combination Decrease Cell Volume
3. Discussion
4. Materials and Methods
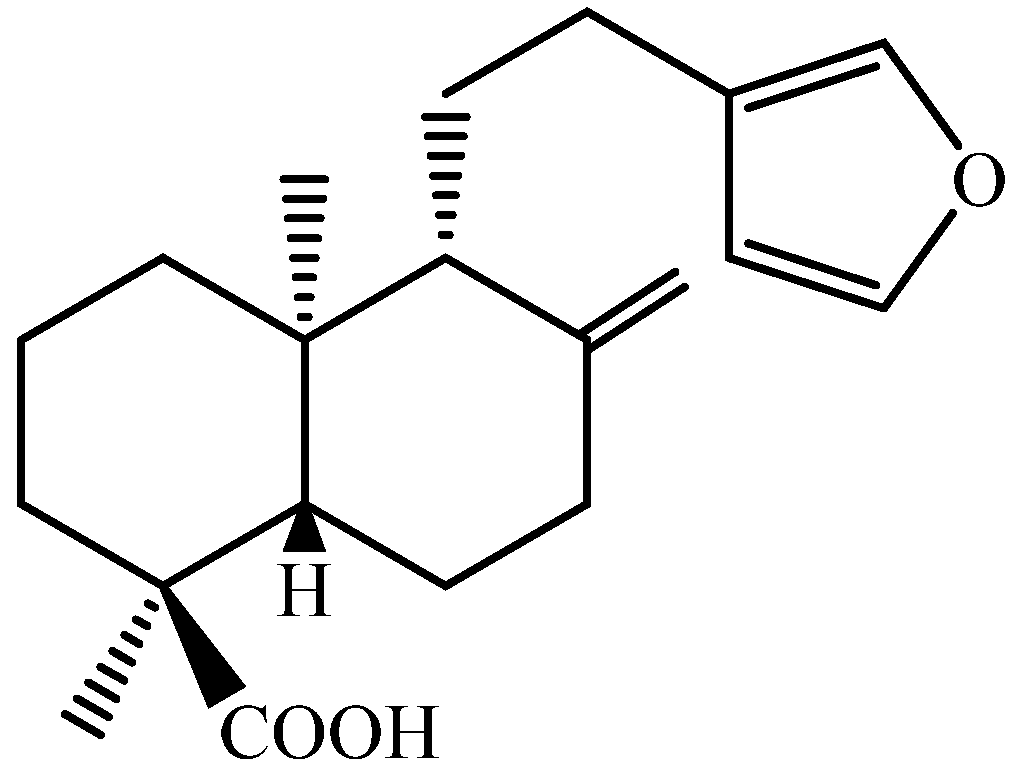
4.1. Compounds
4.2. Parasites and Macrophages
4.3. In Vitro Leishmanicidal Activity
4.4. Cytotoxicity and Hemolytic Activities
4.5. Drug Combination and Determination of Fractional Inhibitory Concentration (FIC) Index
4.6. Electron Microscopy
4.7. Cell Volume Analysis
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathison, B.A.; Bradley, B.T. Review of the clinical presentation, pathology, diagnosis, and treatment of leishmaniasis. Lab. Med. 2023, 54, 363–371. [Google Scholar] [CrossRef] [PubMed]
- WHO. Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 19 August 2024).
- Sheikh, S.Y.; Hassan, F.; Shukla, D.; Bala, S.; Faruqui, T.; Akhter, Y.; Khan, A.R.; Nasibullah, M. A review on potential therapeutic targets for the treatment of leishmaniasis. Parasitol. Int. 2024, 100, 102863. [Google Scholar] [CrossRef] [PubMed]
- Shmueli, M.; Ben-Shimol, S. Review of leishmaniasis treatment: Can we see the forest through the trees? Pharmacy 2024, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- van Griensven, J.; Dorlo, T.P.; Diro, E.; Costa, C.; Burza, S. The status of combination therapy for visceral leishmaniasis: An updated review. Lancet Infect. Dis. 2024, 24, e36–e46. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, T.F.; Cunha-Junior, E.F.; Wanderley, J.L.M.; Pacheco, J.D.S.; Pinto-da-Silva, L.H.; Andrade-Neto, V.V.; Chaves, S.P. Editorial: Strategies in the drug discovery and development for leishmaniasis: Immunomodulators, natural products, synthetic compounds, and drug repositioning. Front. Cell Infect. Microbiol. 2024, 14, 1384244. [Google Scholar] [CrossRef]
- Santos, A.O.; Izumi, E.; Ueda-Nakamura, T.; Dias-Filho, B.P.; Veiga-Júnior, V.F.; Nakamura, C.V. Antileishmanial activity of diterpene acids in copaiba oil. Memórias Inst. Oswaldo Cruz 2013, 108, 59–64. [Google Scholar] [CrossRef]
- Mizuno, C.S.; Souza, A.B.; Tekwani, B.L.; Ambrósio, S.R.; Veneziani, R.C. Synthesis and biological evaluation of polyalthic acid derivatives for the treatment of neglected diseases. Bioorg. Med. Chem. Lett. 2015, 25, 5529–5531. [Google Scholar] [CrossRef]
- Izumi, E.; Ueda-Nakamura, T.; Veiga-Júnior, V.F.; Pinto, A.C.; Nakamura, C.V. Terpenes from Copaifera demonstrated in vitro antiparasitic and synergic activity. J. Med. Chem. 2012, 55, 2994–3001. [Google Scholar] [CrossRef]
- Borges, C.H.G.; Cruz, M.G.; Carneiro, L.J.; da Silva, J.J.M.; Bastos, J.K.; Tavares, D.C.; de Oliveira, P.F.; Rodrigues, V.; Veneziani, R.C.S.; Parreira, R.L.T.; et al. Copaifera duckei oleoresin and its main nonvolatile terpenes: In vitro schistosomicidal properties. Chem. Biodiver. 2016, 13, 1348–1356. [Google Scholar] [CrossRef]
- Dos Santos, A.O.; Ueda-Nakamura, T.; Dias Filho, B.P.; Veiga-Júnior, V.F.; Nakamura, C.V. Copaiba oil: An alternative to development of new drugs against leishmaniasis. Evid. Based Complement. Alternat. Med. 2012, 2012, 898419. [Google Scholar] [CrossRef]
- Afonso, R.C.; Yien, R.M.K.; de Siqueira, L.B.O.; Simas, N.K.; Dos Santos, A.P.M.; Ricci-Júnior, E. Promising natural products for the treatment of cutaneous leishmaniasis: A review of in vitro and in vivo studies. Exp. Parasitol. 2023, 251, 108554. [Google Scholar] [CrossRef] [PubMed]
- Nweze, J.A.; Mbaoji, F.N.; Li, Y.M.; Yang, L.Y.; Huang, S.S.; Chigor, V.N.; Eze, E.A.; Pan, L.X.; Zhang, Y.; Yang, D.F. Potentials of marine natural products against malaria, leishmaniasis, and trypanosomiasis parasites: A review of recent articles. Infect. Dis. Poverty 2021, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.K.; Yang, K.H.; Bryant, K.; Li, J.; Joice, A.C.; Werbovetz, K.A.; Narayan, R.J. Microneedle-based delivery of amphotericin B for treatment of cutaneous leishmaniasis. Biomed. Microdevices 2019, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Katsuno, K.; Burrows, J.N.; Duncan, K.; Hooft van Huijsduijnen, R.; Kaneko, T.; Kita, K.; Mowbray, C.E.; Schmatz, D.; Warner, P.; Slingsby, B.T. Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nat. Rev. Drug Discov. 2015, 14, 751–758. [Google Scholar] [CrossRef]
- Hadighi, R.; Boucher, P.; Khamesipour, A.; Meamar, A.R.; Roy, G.; Ouellette, M.; Mohebali, M. Glucantime-resistant Leishmania tropica isolated from Iranian patients with cutaneous leishmaniasis are sensitive to alternative antileishmania drugs. Parasitol. Res. 2007, 101, 1319–1322. [Google Scholar] [CrossRef]
- Shokri, A.; Sharifi, I.; Khamesipour, A.; Nakhaee, N.; Fasihi Harandi, M.; Nosratabadi, J.; Hakimi Parizi, M.; Barati, M. The effect of verapamil on in vitro susceptibility of promastigote and amastigote stages of Leishmania tropica to meglumine antimoniate. Parasitol. Res. 2012, 110, 1113–1117. [Google Scholar] [CrossRef]
- Rodrigues, R.R.L.; Nunes, T.A.L.; de Araújo, A.R.; Marinho Filho, J.D.B.; da Silva, M.V.; Carvalho, F.A.A.; Pessoa, O.D.L.; Freitas, H.P.S.; Rodrigues, K.A.D.F.; Araújo, A.J. Antileishmanial activity of cordiaquinone E towards Leishmania (Leishmania) amazonensis. Int. Immunopharmacol. 2021, 90, 107124. [Google Scholar] [CrossRef]
- Zauli-Nascimento, R.C.; Miguel, D.C.; Yokoyama-Yasunaka, J.K.; Pereira, L.I.; Pelli de Oliveira, M.A.; Ribeiro-Dias, F.; Dorta, M.L.; Uliana, S.R. In vitro sensitivity of Leishmania (Viannia) braziliensis and Leishmania (Leishmania) amazonensis Brazilian isolates to meglumine antimoniate and amphotericin B. Trop. Med. Int. Health 2010, 15, 68–76. [Google Scholar] [CrossRef]
- Ferreira, B.A.; Coser, E.M.; de la Roca, S.; Aoki, J.I.; Branco, N.; Soares, G.H.C.; Lima, M.I.S.; Coelho, A.C. Amphotericin B resistance in Leishmania amazonensis: In vitro and in vivo characterization of a Brazilian clinical isolate. PLoS Negl. Trop. Dis. 2024, 18, e0012175. [Google Scholar] [CrossRef]
- Field, M.C.; Horn, D.; Fairlamb, A.H.; Ferguson, M.A.; Gray, D.W.; Read, K.D.; De Rycker, M.; Torrie, L.S.; Wyatt, P.G.; Wyllie, S.; et al. Anti-trypanosomatid drug discovery: An ongoing challenge and a continuing need. Nat. Rev. Microbiol. 2017, 15, 217–231, Erratum in Nat. Rev. Microbiol. 2017, 15, 447. https://doi.org/10.1038/nrmicro.2017.69. Erratum in Nat. Rev. Microbiol. 2018, 16, 714. https://doi.org/10.1038/s41579-018-0085-1. [Google Scholar] [CrossRef] [PubMed]
- Jamal, F.; Altaf, I.; Ahmed, G.; Asad, S.; Ahmad, H.; Zia, Q.; Azhar, A.; Farheen, S.; Shafi, T.; Karim, S.; et al. Amphotericin B nano-assemblies circumvent intrinsic toxicity and ensure superior protection in experimental visceral leishmaniasis with feeble toxic manifestation. Vaccines 2023, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Borges, B.S.; Bueno, G.P.; Tomiotto-Pellissier, F.; Figueiredo, F.B.; Soares, L.C.M. In vitro anti-Leishmania activity of triclabendazole and its synergic effect with amphotericin B. Front. Cell Infect. Microbiol. 2023, 12, 1044665. [Google Scholar] [CrossRef] [PubMed]
- Trinconi, C.T.; Reimão, J.Q.; Yokoyama-Yasunaka, J.K.; Miguel, D.C.; Uliana, S.R. Combination therapy with tamoxifen and amphotericin B in experimental cutaneous leishmaniasis. Antimicrob. Agents Chemother. 2014, 58, 2608–2613. [Google Scholar] [CrossRef] [PubMed]
- Valentim-Silva, J.R.; de Barros, N.B.; Macedo, S.R.A.; Ferreira, A.D.S.; Silva, R.S.; Dill, L.S.M.; Zanchi, F.B.; do Nascimento, J.R.; do Nascimento, F.R.F.; Lourenzoni, M.R.; et al. Antileishmanial activity, cytotoxicity and cellular response of amphotericin B in combination with crotamine derived from Crotalus durissus terrificus venom using in vitro and in silico approaches. Toxicon 2022, 217, 96–106. [Google Scholar] [CrossRef]
- Kobayashi, S.; Hamashima, S.; Homma, T.; Sato, M.; Kusumi, R.; Bannai, S.; Fujii, J.; Sato, H. Cystine/glutamate transporter, system xc−, is involved in nitric oxide production in mouse peritoneal macrophages. Nitric Oxide. 2018, 78, 32–40. [Google Scholar] [CrossRef]
- Braun, G.H.; Ramos, H.P.; Candido, A.C.B.B.; Pedroso, R.C.N.; Siqueira, K.A.; Soares, M.A.; Dias, G.M.; Magalhães, L.G.; Ambrósio, S.R.; Januário, A.H.; et al. Evaluation of antileishmanial activity of harzialactone a isolated from the marine-derived fungus Paecilomyces sp. Nat. Prod. Res. 2021, 35, 1644–1647. [Google Scholar] [CrossRef]
- de Moraes, A.R.D.P.; Tavares, G.D.; Rocha, F.J.S.; de Paula, E.; Giorgio, S. Effects of nanoemulsions prepared with essential oils of copaiba and andiroba against Leishmania infantum and Leishmania amazonensis infections. Exp Parasitol. 2018, 187, 12–21. [Google Scholar] [CrossRef]
- Scariot, D.B.; Britta, E.A.; Moreira, A.L.; Falzirolli, H.; Silva, C.C.; Ueda-Nakamura, T.; Dias-Filho, B.P.; Nakamura, C.V. Induction of early autophagic process on Leishmania amazonensis by synergistic effect of miltefosine and innovative semi-synthetic thiosemicarbazone. Front. Microbiol. 2017, 8, 255. [Google Scholar] [CrossRef]
- Fivelman, Q.L.; Adagu, I.S.; Warhurst, D.C. Modified fixed-ratio isobologram method for studying in vitro interactions between atovaquone and proguanil or dihydroartemisinin against drug-resistant strains of Plasmodium falciparum. Antimicrob. Agents Chemother. 2004, 48, 4097–4102. [Google Scholar] [CrossRef]
- Seifert, K.; Croft, S.L. In vitro and in vivo interactions between miltefosine and other antileishmanial drugs. Antimicrob. Agents Chem. 2006, 50, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
| EC50 | CC50 | CH50 | SI | ||
|---|---|---|---|---|---|
| Promastigotes (24 h) | Amastigotes (48 h) | Peritoneal Macrophages (48 h) | Erythrocytes (30 min) | ||
| PA | 2.01 ± 0.24 | 3.22 ± 0.35 | ≥100 | ≥50 | ND |
| AmpB | ≤0.025 | 0.095 ± 0.012 | 22.41 ± 0.44 | ≤0.195 | 235.89 |
| PA + AmpB | ||
|---|---|---|
| x∑FICI | Profile | |
| Promastigotes | 0.36 | Synergistic |
| Amastigotes | 0.39 | Synergistic |
| Peritoneal macrophages | 1.39 | Indifferent |
| Erythrocytes | 0.68 | Indifferent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candido, A.C.B.B.; Pagotti, M.C.; Santos, D.A.d.; Paula, L.A.d.L.; Veneziani, R.C.S.; Bastos, J.K.; Ambrósio, S.R.; Magalhães, L.G. Efficacy of Diterpene Polyalthic Acid Combined with Amphotericin B against Leishmania amazonensis In Vitro. Pharmaceuticals 2024, 17, 1243. https://doi.org/10.3390/ph17091243
Candido ACBB, Pagotti MC, Santos DAd, Paula LAdL, Veneziani RCS, Bastos JK, Ambrósio SR, Magalhães LG. Efficacy of Diterpene Polyalthic Acid Combined with Amphotericin B against Leishmania amazonensis In Vitro. Pharmaceuticals. 2024; 17(9):1243. https://doi.org/10.3390/ph17091243
Chicago/Turabian StyleCandido, Ana Carolina Bolela Bovo, Mariana Cintra Pagotti, Daiane Albino dos Santos, Lucas Antonio de Lima Paula, Rodrigo Cássio Sola Veneziani, Jairo Kenupp Bastos, Sérgio Ricardo Ambrósio, and Lizandra Guidi Magalhães. 2024. "Efficacy of Diterpene Polyalthic Acid Combined with Amphotericin B against Leishmania amazonensis In Vitro" Pharmaceuticals 17, no. 9: 1243. https://doi.org/10.3390/ph17091243
APA StyleCandido, A. C. B. B., Pagotti, M. C., Santos, D. A. d., Paula, L. A. d. L., Veneziani, R. C. S., Bastos, J. K., Ambrósio, S. R., & Magalhães, L. G. (2024). Efficacy of Diterpene Polyalthic Acid Combined with Amphotericin B against Leishmania amazonensis In Vitro. Pharmaceuticals, 17(9), 1243. https://doi.org/10.3390/ph17091243









