Lilium brownii/Baihe as Nutraceuticals: Insights into Its Composition and Therapeutic Properties
Abstract
1. Introduction
2. Lilium/Baihe: The Brief Introduction
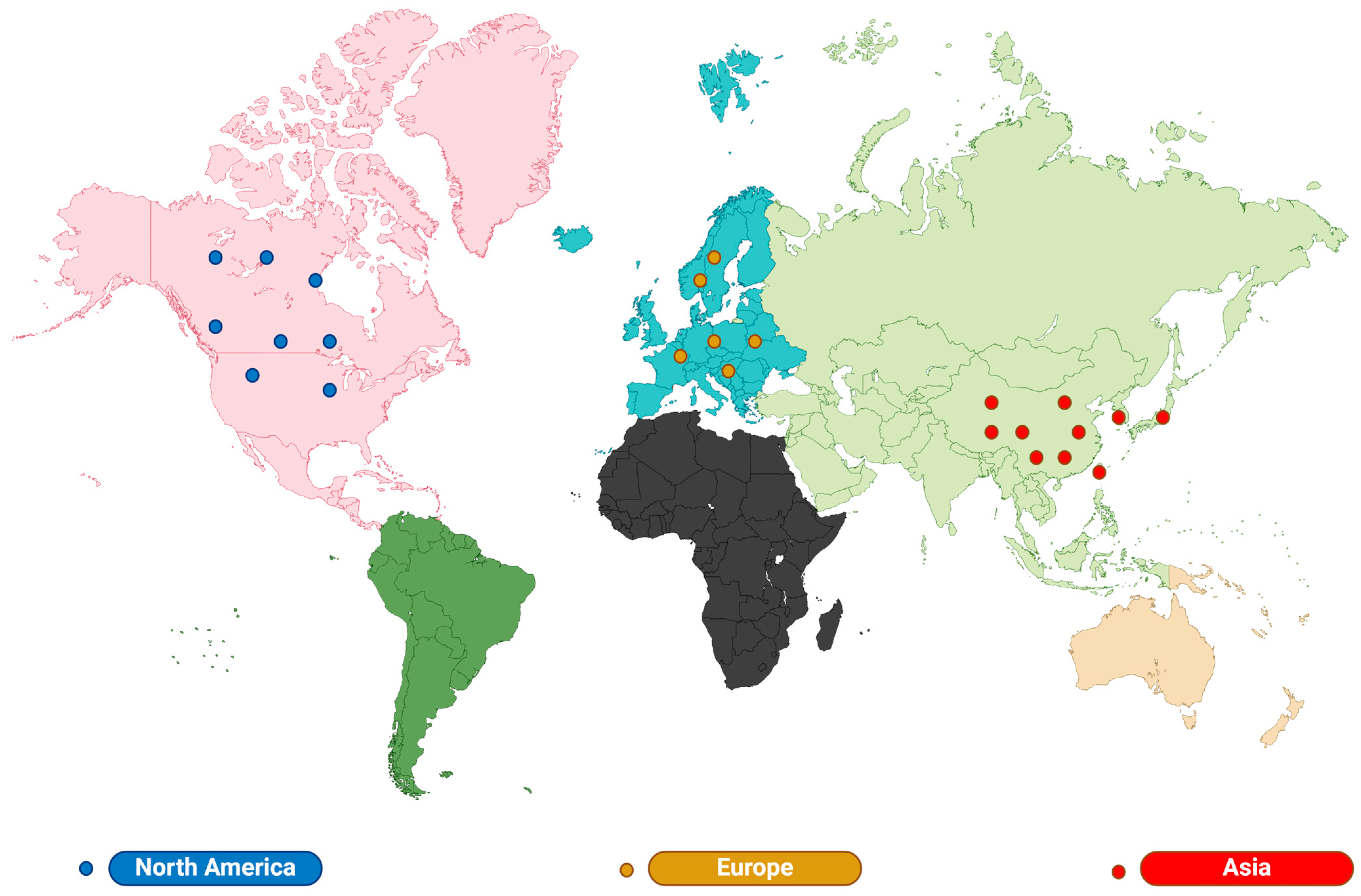
2.1. Lilium: Distribution and Habit
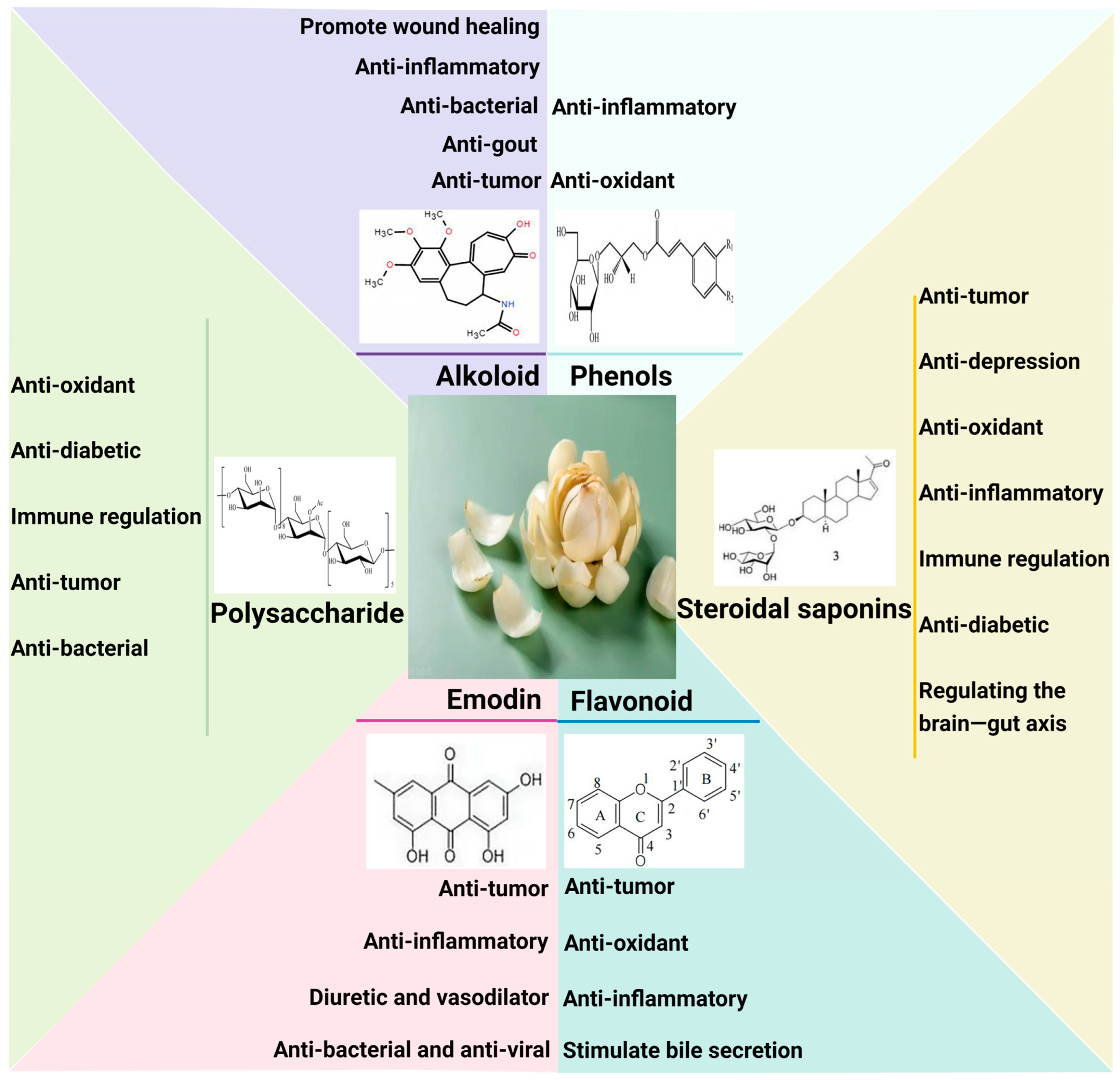
2.2. Lilium: Complex and Diverse Bioactive Components
2.2.1. Steroidal Saponins
2.2.2. Polysaccharides
2.2.3. Phenols
2.2.4. Alkaloid
2.2.5. Emodin
2.2.6. β Sitosterol
2.2.7. Other Components
| Distribution | Species and Quantity | Representative Category | Growth Characteristics | Main Use | References |
|---|---|---|---|---|---|
| Asia | 70 | Martagon, Leucolirion, Archelirion, Daurolirion, Martagon | Well-drained slopes, mountain meadows, forest margins, rock crevices, forest slopes, thickets, grasses, and valleys | Antitussive and expectorant, diuretic, antipyretic. | [33,34,35,36] |
| North America | 24 | Pseudolirium | Streams and swamps | food | [37,38] |
| Europe | 22 | Martagon, Liriotypus | A place with high mineral coverage | Wound healing; treatment of mastitis, liver disease, and herpes zoster. | [29,30,31,32] |
2.3. Lilium: Both Food and Medicine
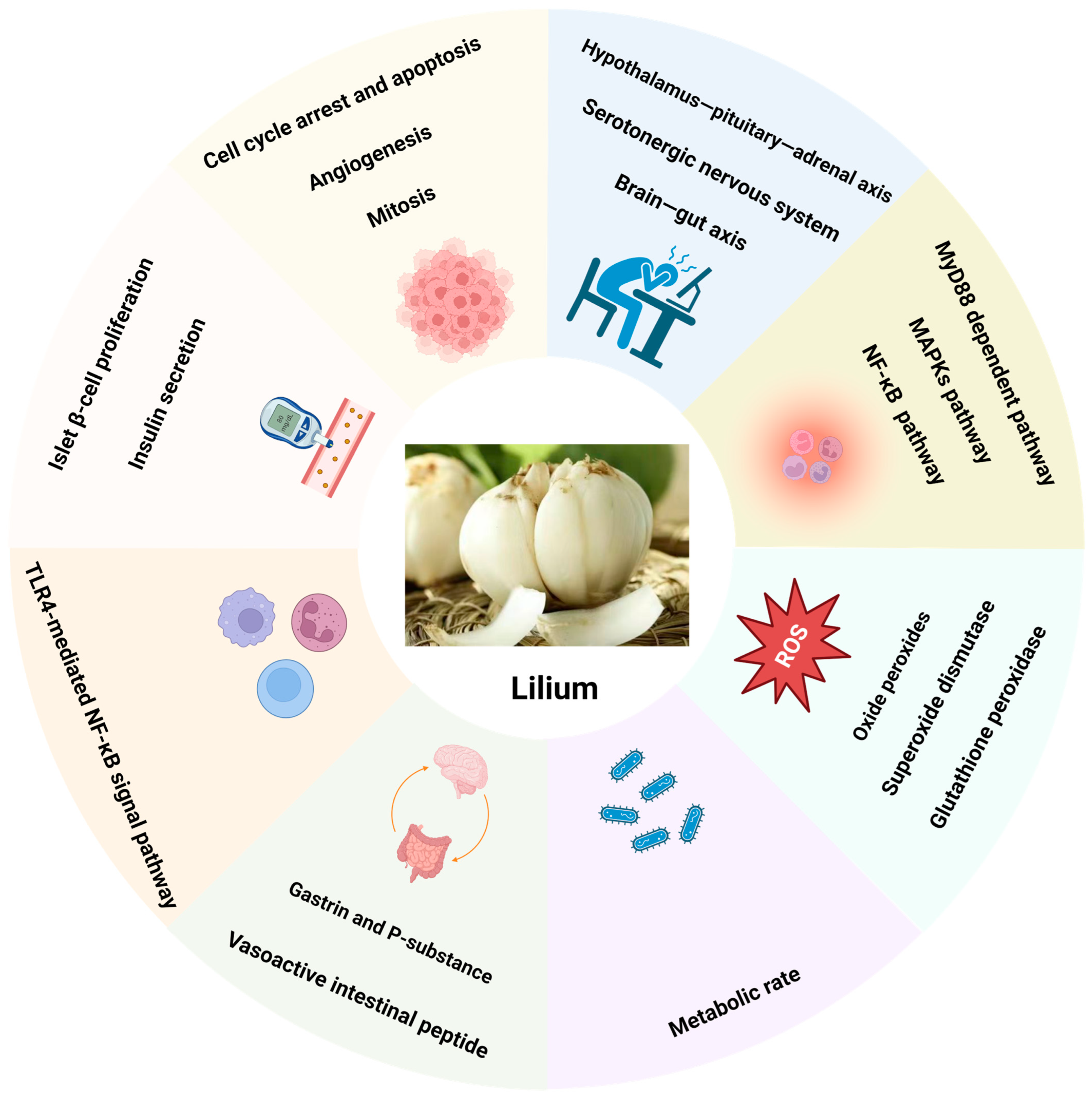
3. Lilium: Pharmacological Effects and Mechanisms of Action
3.1. Anti-Tumor
3.1.1. Anti-Tumor Effect of Polysaccharide
3.1.2. Anti-Tumor Effect of Saponins
3.1.3. Anti-Tumor Effect of Alkaloids
3.1.4. Anti-Tumor Effect of Flavonoids
3.1.5. Anti-Tumor Effect of Emodin
3.2. Anti-Depressant
3.3. Anti-Diabetic
3.4. Anti-Oxidation and Scavenging Free Radicals
3.5. Immunomodulation
3.6. Anti-Inflammatory
3.7. Regulation of Brain–Gut Axis
3.8. Anti-Fatigue and Hypoxia Tolerance
3.9. Anti-Bacterial
3.10. Other Pharmacological Effects
3.11. Security
3.12. Lilium Acts through the Interaction between Multiple Organs
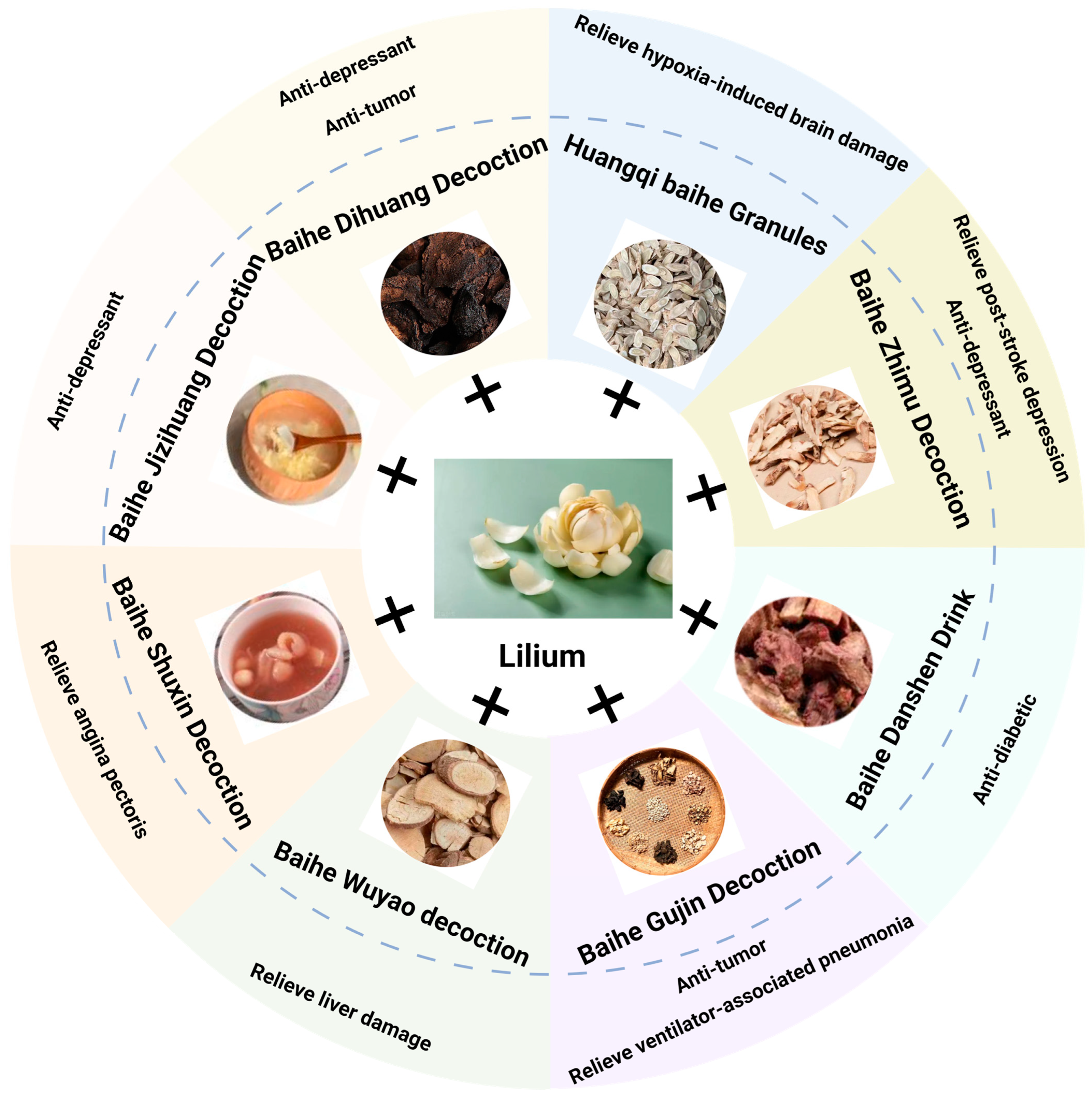
4. Baihe: The Application of Traditional Chinese Medicine
4.1. Baihe Dihuang Decoction (BDD)
4.2. Baihe Zhimu Decoction (BZD)
4.3. Baihe Gujin Decoction (BGD)
4.4. Other Compound Preparations Based on Lilium
5. Summary and Prospect
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lu, Y.Q.; Zhang, C. Knowledge of Medicine and Food Homology in Ancient China. J. Sci. Educ. (Mid-Term Issue) 2019, 14, 190–192. [Google Scholar] [CrossRef]
- Mao, X.Y.; Jin, M.Z.; Chen, J.F.; Zhou, H.H.; Jin, W.L. Live or let die: Neuroprotective and anti-cancer effects of nutraceutical antioxidants. Pharmacol. Ther. 2018, 183, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.M. Bei Ji Qian Jin Yao Fang. Prepared for Emergency; Liaoning Science and Technology Press: Shenyang, China, 1997. [Google Scholar]
- Meng, X. Shi liao ben cao; Renmin Health Publishing House: Beijing, China, 1984. [Google Scholar]
- Pu, Z.G. Bao Sheng Yao Lu; Shanghai Ancient Books Publishing House: Shanghai, China, 1990. [Google Scholar]
- Hu, S.H. Yin Shan Zheng Yao; China Traditional Chinese Medicine Press: Beijing, China, 2019. [Google Scholar]
- Cheng, B. Biological characteristics and application of Lilium. Contemp. Hortic. 2015, 14, 160. [Google Scholar] [CrossRef]
- Li, Y.P.; Gong, Y.C.; Wu, G.J.; Li, Z.L.; Liu, J.T.; Xiong, X.Y. Application Values, Distribution and Development Prospect of Lilium. J. Anhui Agric. 2010, 38, 3395–3396+3399. [Google Scholar] [CrossRef]
- Du, F. Origin, classification and germplasm diversity in Lilium. J. China Agric. Univ. 2023, 28, 68–79. [Google Scholar]
- Comber, H.F. A new classification of the genus Lilium. In Lily Year Book of RHS; RHS: London, UK, 1949; pp. 86–105. [Google Scholar]
- Wang, T.; Huang, H.; Zhang, Y.; Li, X.; Li, H.; Jiang, Q.; Gao, W. Role of effective composition on antioxidant, anti-inflammatory, sedative-hypnotic capacities of 6 common edible Lilium varieties. J. Food Sci. 2015, 80, H857–H868. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.C.; Ma, Y.L.; Li, X.L.; Li, D. Research Progress on Chemical constituents and Application of Lilium. Agric. Technol. 2022, 42, 5–8. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Wei, M.Z.; Xie, X.N. Determination of saponins and polysaccharides in different varieties of Lilium. Light Ind. Sci. Technol. 2017, 33, 41–42. [Google Scholar]
- Hu, Y.; Du, Y.P.; Tian, C.J.; Zhang, X.H.; Ren, J.W. A Review of Chemical Components and Their Bioactivities from the Genus Lilium. J. Food Sci. 2018, 39, 323–332. [Google Scholar] [CrossRef]
- Zhang, X.L. Analysis of Polysaccharide Content in Different Varieties of Lilium brownii and Their Inner and Outer Scales. Mod. Food 2021, 03, 195–197. [Google Scholar] [CrossRef]
- Su, Q.; Wu, P.; Xia, B.H.; Li, Y.M.; Lin, Y.; Lin, L.M.; Liao, D. Advance in Research on Chemical Constituents and Pharmacological Activities of Lilium. Chin. Pharm. J. 2021, 56, 875–882. [Google Scholar] [CrossRef]
- Li, Z.N. The Study of Flavone in Lilium; Northwest A& F university of China: Xianyang, China, 2008. [Google Scholar]
- Munafo, J.P., Jr.; Ramanathan, A.; Jimenez, L.S.; Gianfagna, T.J. Isolation and structural determination of steroidal glycosides from the bulbs of easter lily (Lilium longiflorum Thunb.). J. Agric. Food Chem. 2010, 58, 8806–8813. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Shuang, Q.; Liu, Y.; Zeng, L.; Su, W. Baihe Extracts Reduce the Activation and Apoptosis of Microglia in the Hippocampus of Mice with Depression-like Behaviors by Downregulating MYC. ACS Chem. Neurosci. 2022, 13, 587–598. [Google Scholar] [CrossRef]
- Feng, S.L.; He, L.; Wang, M.; Jiao, K. Studies on the Chemical constituents of Lilium. Chin. J. Tradit. Chin. Med. 1994, 10, 611–612+639. [Google Scholar]
- Matsuo, Y.; Takaku, R.; Mimaki, Y. Novel Steroidal Glycosides from the Bulbs of Lilium pumilum. Molecules 2015, 20, 16255–16265. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, Y.; Yan, L.; Guo, Y.; Niu, L. Phenolic compounds and antioxidant activity of bulb extracts of six Lilium species native to China. Molecules 2012, 17, 9361–9378. [Google Scholar] [CrossRef]
- Liu, X.D.; Chen, X.; Liu, C.Y.; Pei, G.; Zhou, R.B.; Wang, Z.; Chen, N.H. Quality evaluation and analysis of lilIum herbs from different sources. J. Hunan Univ. Chin. Med. 2019, 39, 480–484. [Google Scholar]
- Xie, J.; Tang, X.Y.; Hao, J.; Zhong, C.; Liu, H.; Huang, J.H.; Zhang, S.H.; Tang, Z.P. Correlation Analysis between Climate Factors and Main Components of Lilium with Different Areas. Mod. Chin. Med. 2020, 22, 2053–2058. [Google Scholar] [CrossRef]
- Zhang, W.X.; Zhu, F.; Hu, R.; Zhang, C.H. Progress in modern study of total saponin in lilIum. J. Hebei North Univ. (Nat. Sci. Ed.) 2017, 33, 42–43+46. [Google Scholar] [CrossRef]
- Mao, Y.F.; Li, Z.L.; Duan, Q.; Du, W.W.; Cui, G.F. A Comparative Study on the Nutritional Components of Four Types of Lilium. J. Yunnan Agric. Univ. (Nat. Sci.) 2017, 32, 366–370. [Google Scholar] [CrossRef]
- Han, X.X.; Li, J.; Qiu, G.Y.; Ma, X. Determination of polysaccharides in Lanzhou lily from different origin. Gansu Sci. Technol. 2020, 36, 55–57. [Google Scholar]
- Alexandre, J.; Foucault, A.; Coutance, G.; Scanu, P.; Milliez, P. Digitalis intoxication induced by an acute accidental poisoning by lily of the valley. Circulation 2012, 125, 1053–1055. [Google Scholar] [CrossRef] [PubMed]
- Pieroni, A. Medicinal plants and food medicines in the folk traditions of the upper Lucca Province, Italy. J. Ethnopharmacol. 2000, 70, 235–273. [Google Scholar] [CrossRef] [PubMed]
- Pieroni, A.; Dibra, B.; Grishaj, G.; Grishaj, I.; Gjon Maçai, S. Traditional phytotherapy of the Albanians of Lepushe, Northern Albanian Alps. Fitoterapia 2005, 76, 379–399. [Google Scholar] [CrossRef]
- Bruni, A.; Ballero, M.; Poli, F. Quantitative ethnopharmacological study of the Campidano Valley and Urzulei district, Sardinia, Italy. J. Ethnopharmacol. 1997, 57, 97–124. [Google Scholar] [CrossRef]
- Fontaine, N.; Gauthier, P.; Casazza, G.; Thompson, J.D. Niche Variation in Endemic Lilium pomponium on a Wide Altitudinal Gradient in the Maritime Alps. Plants 2022, 11, 833. [Google Scholar] [CrossRef]
- Mimaki, Y.; Satou, T.; Kuroda, M.; Sashida, Y.; Hatakeyama, Y. Steroidal saponins from the bulbs of Lilium candidum. Phytochemistry 1999, 51, 567–573. [Google Scholar] [CrossRef]
- Lee, E.; Yun, N.; Jang, Y.P.; Kim, J. Lilium lancifolium Thunb. extract attenuates pulmonary inflammation and air space enlargement in a cigarette smoke-exposed mouse model. J. Ethnopharmacol. 2013, 149, 148–156. [Google Scholar] [CrossRef]
- Warrier, P.; Nambiar, V.; Ramankutty, C. Indian Medicinal Plants: A Compendium of 500 Medicinal Plants; Arya Vaidya Sala Oriental Longman: Chennai, India, 1997. [Google Scholar]
- Du, Y.; Di Zhang, S.W.; Wang, L.; Yan, X.; Tang, Z. Sexual system characteristics of Lilium concolor var. megalanthum in peatland. Biodivers. Sci. 2021, 29, 1321. [Google Scholar] [CrossRef]
- Munafo, J.P., Jr.; Gianfagna, T.J. Chemistry and biological activity of steroidal glycosides from the Lilium genus. Nat. Prod. Rep. 2015, 32, 454–477. [Google Scholar] [CrossRef]
- Relatives, W.C. Genomic and Breeding Resources; Legume Crop and Forages; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Downer, S.; Berkowitz, S.A.; Harlan, T.S.; Olstad, D.L.; Mozaffarian, D. Food is medicine: Actions to integrate food and nutrition into healthcare. BMJ 2020, 369, m2482. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lin, Z.J.; Zhang, B. Research Progress on Chemical Constituents and Pharmacological Effect of Lilii Bulbus. Chin. J. Exp. Tradit. Med. Formulae 2017, 23, 201–211. [Google Scholar] [CrossRef]
- Hossain, R.; Quispe, C.; Herrera-Bravo, J.; Islam, S.; Sarkar, C.; Islam, M.T.; Martorell, M.; Cruz-Martins, N.; Al-Harrasi, A.; Al-Rawahi, A.; et al. Lasia spinosa Chemical Composition and Therapeutic Potential: A Literature-Based Review. Oxid. Med. Cell. Longev. 2021, 2021, 1602437. [Google Scholar] [CrossRef] [PubMed]
- Astani, A.; Reichling, J.; Schnitzler, P. Melissa officinalis extract inhibits attachment of herpes simplex virus in vitro. Chemotherapy 2012, 58, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Bouyahya, A.; El Menyiy, N.; El Omari, N.; Shahinozzaman; Ovey, M.A.H.; Koirala, N.; Panthi, M.; Ertani, A.; et al. Ethnobotany, Phytochemistry, Biological Activities, and Health-Promoting Effects of the Genus Bulbophyllum. Evid. Based Complement. Alternat. Med. 2022, 2022, 6727609. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Kumar, M.; Akram, M.; Amin, M.; Iqbal, M.; Koirala, N.; Sytar, O.; Kregiel, D.; Nicola, S.; et al. Hyssopus Essential Oil: An Update of Its Phytochemistry, Biological Activities, and Safety Profile. Oxid. Med. Cell. Longev. 2022, 2022, 8442734. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Dey, A.; Koirala, N.; Shaheen, S.; El Omari, N.; Salehi, B.; Goloshvili, T.; Silva, N.C.C.; Bouyahya, A.; Vitalini, S.; et al. Cinnamomum Species: Bridging Phytochemistry Knowledge, Pharmacological Properties and Toxicological Safety for Health Benefits. Front. Pharmacol. 2021, 12, 600139. [Google Scholar] [CrossRef]
- Sun, X.; Gao, R.L.; Xiong, Y.K.; Huang, Q.C.; Xu, M. Antitumor and immunomodulatory effects of a water-soluble polysaccharide from Lilii Bulbus in mice. Carbohydr. Polym. 2014, 102, 543–549. [Google Scholar] [CrossRef]
- Yang, Y.; Li, F. Effects of neutral polysaccharide from lily on enhancing efficacy and reducing toxicity of 5-FU and proliferation of gastric arcinoma cell line SGC-7901 in vitro. J. Yanan Univ. 2013, 11, 8–11. [Google Scholar]
- Hou, J.; Li, F.; Li, X.H.; Mei, Q.B.; Mi, M. Lily polysaccharide 1 enhances the effect of metformin on proliferation and apoptosis of human breast carcinoma cells. Chin. J. Cell. Mol. Immunol. 2016, 32, 780–783+788. [Google Scholar] [CrossRef]
- Hou, J.; Li, F.; Li, X.H.; Mei, Q.B.; Mi, M. Effect of lily polysaccharide combined genistein on proliferation and cell cycle in human breast carcinoma cells. Mod. Oncol. 2015, 23, 12–14. [Google Scholar] [CrossRef]
- Luo, L.M.; Qin, L.; Zhan, J.H.; Pei, G.; Zhou, X.J.; Chen, N.H. [Study on effects of total saponins from Lilii Bulbus on proliferation, apoptosis, invasion and metastasis of lung cancer cells and its preliminary mechanism]. China J. Chin. Mater. Medica 2018, 43, 4498–4505. [Google Scholar] [CrossRef]
- Mimaki, Y.; Nakamura, O.; Sashida, Y.; Satomi, Y.; Nishino, A.; Nishino, H. Steroidal saponins from the bulbs of Lilium longiflorum and their antitumour-promoter activity. Phytochemistry 1994, 37, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Mimaki, Y.; Sashida, Y.; Kuroda, M.; Nishino, A.; Satomi, Y.; Nishino, H. Inhibitory effects of steroidal saponins on 12-O-tetradecanoylphorbol-13-acetate (TPA)-enhanced 32P-incorporation into phospholipids of HeLa cells and proliferation of human malignant tumor cells. Biol. Pharm. Bull. 1995, 18, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Mimaki, Y.; Sashida, Y. Steroidal saponins and alkaloids from the bulbs of Lilium brownii var. colchesteri. Chem. Pharm. Bull. 1990, 38, 3055–3059. [Google Scholar] [CrossRef]
- Lei, L.H. Antioxidant Capacity And Lung Cancer Cell Rejection Characteristic of Lilium Lancifolium Bulbs from Different Populations; Northwest A&F University of China: Xianyang, China, 2016. [Google Scholar]
- Wei, Q.Q.; Zhang, Z.L. Research progress of colchicine in anti-inflammatory, anti-fibrosis and anti-cancer. China J. Mod. Med. 2020, 30, 76–81. [Google Scholar]
- Melidou, M.; Riganakos, K.; Galaris, D. Protection against nuclear DNA damage offered by flavonoids in cells exposed to hydrogen peroxide: The role of iron chelation. Free Radic. Biol. Med. 2005, 39, 1591–1600. [Google Scholar] [CrossRef]
- Hafeez, B.B.; Siddiqui, I.A.; Asim, M.; Malik, A.; Afaq, F.; Adhami, V.M.; Saleem, M.; Din, M.; Mukhtar, H. A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer PC3 cells in vitro and in vivo: Involvement of nuclear factor-kappaB signaling. Cancer Res. 2008, 68, 8564–8572. [Google Scholar] [CrossRef]
- Dong, X.; Fu, J.; Yin, X.; Cao, S.; Li, X.; Lin, L.; Huyiligeqi; Ni, J. Emodin: A Review of its Pharmacology, Toxicity and Pharmacokinetics. Phytother. Res. PTR 2016, 30, 1207–1218. [Google Scholar] [CrossRef]
- Jesulola, E.; Micalos, P.; Baguley, I.J. Understanding the pathophysiology of depression: From monoamines to the neurogenesis hypothesis model—Are we there yet? Behav. Brain Res. 2018, 341, 79–90. [Google Scholar] [CrossRef]
- Guo, Q.P. Studies on Quality Standard and Antidepression Effect of Lilium Brownii; Guangzhou University of Chinese Medicine: Guangzhou, China, 2009. [Google Scholar]
- Huang, J.J. Studies on Quality Standard and Antidepression Effect of the Effective Parts in Bulbus Lilii; Guangzhou University of Chinese Medicine: Guangzhou, China, 2011. [Google Scholar]
- Gao, S.Y. Quality Standard Research of Total Saponins of Lily and Discussing on Anti-Depression Accompanied with IBS; Guangzhou University of Chinese Medicine: Guangzhou, China, 2013. [Google Scholar]
- Li, Y.P.; Pi, X.F.; Liu, C.M.; Gong, Y.C.; Li, Z.L. Study on the hypoglycemic mechanism of lily polysaccharide in vitro. Li Shi zhen Med. Mater. Med. Res. 2012, 23, 1964–1966. [Google Scholar] [CrossRef]
- Liu, C.M.; Fu, G.M.; Tu, Z.C.; Wan, Y. Study on hypoglycemic function of Lily Polysaccharide. Food Sci. 2002, 6, 113–114. [Google Scholar]
- Xiao, X.; Wu, X.; He, C.L. Hypoglycemic Effect of Lily Polysaccharides in Type I Diabetic Rats. Food Sci. 2014, 35, 209–213. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, J.; Jin, Z.Y.; Xu, X.M.; Chen, H.Q. Protective effects of polysaccharides from Lilium lancifolium on streptozotocin-induced diabetic mice. Int. J. Biol. Macromol. 2014, 65, 436–440. [Google Scholar] [CrossRef] [PubMed]
- He, C.L.; Chen, L.S.; Ren, F.L. Study of Scavenging Effect from Lilium Saponins on Hydroxyl Radical. J. Hunan Normal Univ. (Med. Sci.) 2004, 1, 56–58. [Google Scholar]
- Francis, J.A.; Rumbeiha, W.; Nair, M.G. Constituents in Easter lily flowers with medicinal activity. Life Sci. 2004, 76, 671–683. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, D.; Chen, K.; Zhang, H.; Wang, M. Research Progress on the Effect of Lilium Polysaccharide on Immunity. Chin. J. Anim. Infect. Dis. 2021, 29, 114–118. [Google Scholar] [CrossRef]
- Li, X.H.; Mi, M.; Li, F.; Ren, L.J.; Wang, D.N.; Yang, Y. Experimental Research of the Immunoregulatory Action of Polysaccha Rides of LILY. Mod. Prev. Med. 2010, 37, 2708–2709. [Google Scholar]
- Pan, G.; Xie, Z.; Huang, S.; Tai, Y.; Cai, Q.; Jiang, W.; Sun, J.; Yuan, Y. Immune-enhancing effects of polysaccharides extracted from Lilium lancifolium Thunb. Int. Immunopharmacol. 2017, 52, 119–126. [Google Scholar] [CrossRef]
- Hou, R.; Chen, J.; Yue, C.; Li, X.; Liu, J.; Gao, Z.; Liu, C.; Lu, Y.; Wang, D.; Li, H.; et al. Modification of lily polysaccharide by selenylation and the immune-enhancing activity. Carbohydr. Polym. 2016, 142, 73–81. [Google Scholar] [CrossRef]
- Jiang, R.; Kuang, W.Y.; Wu, S.H. Isolation and composition of immunoreactive polysaccharides from lily. J. Fourth Milit. Med. Univ. 1998, 2, 188. [Google Scholar]
- Kwon, O.K.; Lee, M.Y.; Yuk, J.E.; Oh, S.R.; Chin, Y.W.; Lee, H.K.; Ahn, K.S. Anti-inflammatory effects of methanol extracts of the root of Lilium lancifolium on LPS-stimulated Raw264.7 cells. J. Ethnopharmacol. 2010, 130, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, J.; Qian, J.; Wu, G.; Ma, Z. Emodin alleviates CCl4-induced liver fibrosis by suppressing epithelial-mesenchymal transition and transforming growth factor-β1 in rats. Mol. Med. Rep. 2018, 18, 3262–3270. [Google Scholar] [CrossRef] [PubMed]
- He, C.L.; Yang, X.H.; Huang, H.; Yang, Q.Z. Anti-weary Pharmacological Action of Lily Polysaccharide. J. Hunan Normal Univ. (Med. Sci.) 2009, 6, 9–11+15. [Google Scholar]
- Shao, X.H.; Lu, L.H.; Xu, D.S.; Zhang, J. The Study of the Two Different Bulbus Lilii on the Anti-anoxia Effect. J. Shandong Univ. TCM 2000, 5, 387–388. [Google Scholar] [CrossRef]
- Li, W.M.; Meng, X.S.; Yu, T.F.; Gao, Y. Studies on the Pharmacological effects of Lilium. J. Chin. Med. Mater. 1990, 6, 31–35. [Google Scholar] [CrossRef]
- Hu, M.M.; Cai, B.C.; Zhang, Z.J.; Zhang, H.F. Pharmacodynamics Research of Lilium brownii Polysaccharide. Tradit. Chin. Drug Res. Clin. Pharmacol. 2007, 2, 107–109. [Google Scholar] [CrossRef]
- Peng, Y.R.; Qian, D.W.; Ding, Y.; Luo, Y.H. Study on Pharmacological Effects of Extracts Different Position from Lilium lancifolium. Res. Pract. Chin. Med. 2006, 1, 31–32. [Google Scholar] [CrossRef]
- Munafo, J.P., Jr.; Gianfagna, T.J. Antifungal activity and fungal metabolism of steroidal glycosides of Easter lily (Lilium longiflorum Thunb.) by the plant pathogenic fungus, Botrytis cinerea. J. Agric. Food Chem. 2011, 59, 5945–5954. [Google Scholar] [CrossRef]
- Jin, H.; Jiang, M.; Liu, Z.G.; Gao, Y.; Wang, J.G. The extraction and antibacterial activity of Lily polysaccharide. Food Sci. Technol. 2015, 40, 167–169. [Google Scholar] [CrossRef]
- Niu, L.X.; Jin, L.; Zhang, Y.L.; Guo, Q.J.; Li, H.J. An evaluation on the antibacterial effects of the bulb extract from three Lilium species. Guihaia 2008, 6, 842–846. [Google Scholar]
- Shimomura, H.; Sashida, Y.; Mimaki, Y. Steroidal saponins, pardarinoside A–G from the bulbs of Lilium pardarinum. Phytochemistry 1989, 28, 3163–3170. [Google Scholar] [CrossRef]
- Obmann, A.; Tsendayush, D.; Thalhammer, T.; Zehl, M.; Vo, T.P.N.; Purevsuren, S.; Natsagdorj, D.; Narantuya, S.; Kletter, C.; Glasl, S. Extracts from the Mongolian traditional medicinal plants Dianthus versicolor Fisch. and Lilium pumilum Delile stimulate bile flow in an isolated perfused rat liver model. J. Ethnopharmacol. 2010, 131, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Jovtchev, G.; Gateva, S.; Stankov, A. Lilium compounds kaempferol and jatropham can modulate cytotoxic and genotoxic effects of radiomimetic zeocin in plants and human lymphocytes in vitro. Environ. Toxicol. 2016, 31, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.S.; Gong, J.; Shen, M.J. Effects of Xiangxi Longshan Lilium on melanin content and tyrosinase activity of melanoma cells in Benz16 mice. J. Hunan Univ. Chin. Med. 2017, 37, 145–148. [Google Scholar]
- Peng, L.L.; Liu, C.S.; Cheng, X.; Chen, Y.; Yang, J.J.; Li, Y.L. Effects of the main active ingredients from Baihe (Bulbus Lilii) on melanin content and tyrosinase activity in mice B-16 cells. J. Hunan Univ. Chin. Med. 2023, 43, 605–611. [Google Scholar]
- Esposito, D.; Munafo, J.P., Jr.; Lucibello, T.; Baldeon, M.; Komarnytsky, S.; Gianfagna, T.J. Steroidal glycosides from the bulbs of Easter lily (Lilium longiflorum Thunb.) promote dermal fibroblast migration in vitro. J. Ethnopharmacol. 2013, 148, 433–440. [Google Scholar] [CrossRef]
- Zhou, Z.-L.; Lin, S.-Q.; Yang, H.-Y.; Zhang, H.-L.; Xia, J.-M. Antiviral Constituents from the Bulbs of Lilium lancifolium. Asian J. Chem. 2014, 26, 7616–7618. [Google Scholar] [CrossRef]
- Zhou, Z.L.; Feng, Z.C.; Fu, C.Y.; Zhang, H.L.; Xia, J.M. Steroidal and phenolic glycosides from the bulbs of Lilium pumilum DC and their potential Na+/K+ ATPase inhibitory activity. Molecules 2012, 17, 10494–10502. [Google Scholar] [CrossRef]
- Cheng, H.; Li, Y. Clinical trial on the modified Baihe-Gujin Decoction combined with routine chemotherapy for the elderly patients with primary treated pulmonary tuberculosis and lung-kidney yin deficiency. Int. J. Tradit. Chin. Med. 2020, 6, 842–846. [Google Scholar]
- Fitzgerald, K.T. Lily toxicity in the cat. Top. Companion. Anim. Med. 2010, 25, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.D.; Clarke, C.F. Angel trumpet lily poisoning in five adolescents: Clinical findings and management. J. Paediatr. Child Health 1999, 35, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.L.; Xiao, W.L.; Wang, Y.M.; Cheng, W.N. One case of anaphylactic shock caused by lilium consumption. People′s Mil. Surgenon 2012, 55, 703. [Google Scholar]
- Song, Y.; Li, L.X.; Zhao, K.W.; Zhang, Y.H.; Zhang, M. Discussion on the medicinal consumption of lilium and the relationship between medicine and food. J. Basic Chin. Med. 2023, 29, 276–279. [Google Scholar] [CrossRef]
- Poisoning by Colchicin. Hospital (Lond 1886) 1890, 9, 75.
- Wang, X.M.; Xu, K.Y.; Wu, H.R.; Wei, X.X.; Zhang, H.Y.; Li, K. Clinical application and dosage of lily bulb. J. Chang. Univ. Chin. Med. 2020, 36, 637–639. [Google Scholar] [CrossRef]
- Ji, J.; Jin, W.; Liu, S.J.; Jiao, Z.; Li, X. Probiotics, prebiotics, and postbiotics in health and disease. MedComm (2020) 2023, 4, e420. [Google Scholar] [CrossRef]
- Mo, Q.Y.; Xiao, D.Q.; Zhu, Y.W.; Yang, L.; Wang, W.Z. Research Progress in the Mechanism of Degradation of Plant Polysacchairdes by Intestinal Microorganisms. China Anim. Husb. Vet. Med. 2023, 50, 4058–4069. [Google Scholar] [CrossRef]
- Sun, Y.; Ho, C.T.; Zhang, Y.; Hong, M.; Zhang, X. Plant polysaccharides utilized by gut microbiota: New players in ameliorating cognitive impairment. J. Tradit. Complement. Med. 2023, 13, 128–134. [Google Scholar] [CrossRef]
- Fang, Y.C.; Huang, H.C.; Chen, H.H.; Juan, H.F. TCMGeneDIT: A database for associated traditional Chinese medicine, gene and disease information using text mining. BMC Complement. Altern. Med. 2008, 8, 58. [Google Scholar] [CrossRef]
- van Hasselt, J.G.; van der Graaf, P.H. Towards integrative systems pharmacology models in oncology drug development. Drug Discov. Today Technol. 2015, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Shi, H.; Zang, Z.; Meng, Q.; Cheng, Y.; Liang, L.; Zhai, Y.; Yin, G.; Sun, L.; Ma, K. Research progress on classical traditional Chinese medicine formula Baihe Zhimu (Lilium lancifolium bulb and Anemarrhena asphodeloides rhizome) decoction in the treatment of depression. Heliyon 2024, 10, e25171. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.N.; Chen, C.; Wang, X.; Feng, Y.; Song, Y.M. Study of Baihe Dihuang Decoction. J. Chang. Univ. Chin. Med. 2019, 35, 987–990. [Google Scholar] [CrossRef]
- Liu, W.Q.; Wu, S.Y. Research Progress of Baihe Dihuang Decoction in the Treatment of Psychiatric Diseases. Clin. J. Tradit. Chin. Med. 2019, 31, 1816–1819. [Google Scholar] [CrossRef]
- Peng, X.J.; Yang, X.J.; Chen, Y.B.; Lu, L.; Xu, H.Y.; Xu, H.Y.; Ding, T.; Liu, F. Action mechanism of Baihe Dihuang decoction on depression based onintegrative pharmacology of traditional Chinese medicine. Chin. J. Chin. Mater. Medica 2018, 43, 1338–1344. [Google Scholar] [CrossRef]
- Zhao, C.J.; Su, J.L.; Zheng, J. Effect of Baihe Dihuang decoction on chemotherapy and PG-SGA level of non-small cell lung cancer PG-SGA. J. Hebei TCM Pharmacol. 2021, 36, 26–29. [Google Scholar] [CrossRef]
- Huang, S.X.; Wu, S.X.; Zhang, P.; Luo, J.P.; Wang, M.; Guo, Y.B.; Li, H.; Zhang, L.; Qiang, Z. The potential targets and mechanisms of modified Baihe dihuang decoction applied in post-stroke depression. China Pharm. 2023, 34, 2483–2489. [Google Scholar]
- Feng, X.; Liu, Y.Q.; Liu, B.; Wang, D.F.; Li, L.; Zhu, L.; Liu, H.F.; Zhang, C.J.; Yang, W.P. Study on metabonomics of Baihe Dihuang decoction in depression rats. Acta Chin. Med. Pharmacol. 2023, 51, 36–45. [Google Scholar] [CrossRef]
- Yang, W.; Chen, F.; Cheng, L.; Li, F.; Deng, L.; Wang, L.J.; Zhao, H.N. Clinical efficacy of Baihe Dihuang decoction combined with acupoint acupuncture in the treatment of uremic hemodialysis sleep disorder. Inn. Mong. J. Tradit. Chin. Med. 2023, 42, 44–45. [Google Scholar] [CrossRef]
- Pan, J. Protective Effect and Mechanism of Lily Bulb and Rehmannia Decoction Medicated Serum on CORT-Induced Nerve Cell Injury; Shandong University of Chinese Medicine: Jinan, China, 2023. [Google Scholar]
- Duan, S.P. Baihe Dihuang decoction combined with droperidoxine in the treatment of peri-menopausal depression. Pract. Clin. J. Integr. Tradit. Chin. West. Med. 2020, 20, 84–86. [Google Scholar] [CrossRef]
- Guan, G.X.; Li, S.R.; Tian, F.L.; Zhang, L.Y. Clinical observation of Baihe Dihuang decoction combined with Trazodone Hydrochloride in the treatment of Insomnia related to Depression of Yin deficiency. China Prac. Med. 2020, 15, 165–167. [Google Scholar] [CrossRef]
- Chen, Z.; Ding, L. Clinical observation on treating 40 cases of depression with the Chaihu plus Longgu Muli decoction and lily anemarrhena decoction. Clin. J. Chin. Med. 2012, 4, 38–39. [Google Scholar]
- Hu, G.G. Summary of Clinical Application of Baihe Zhimu decoction. Guangming J. Chin. Med. 2020, 35, 1604–1606. [Google Scholar] [CrossRef]
- Lv, B.N.; Zhao, X.R.; Zhang, X.J.; Liu, Y.Q. Analysis of Therapeutic effect of Tongdu Tiaoshen Acupuncture combined with Baihe Zhimu decoction in the treatment of Post-Stroke Depression. J. Sichuan Tradit. Chin. Med. 2023, 41, 200–203. [Google Scholar]
- Zhang, C.F.; Cheng, D.J.; Feng, Y.J.; Zhao, L.Q.; Wang, J.B. Effects of Morita Therapy Combined with Baihe Zhimu Decoction on Serum BDNF and DOPAC Quality of Life, Daily Life Ability and Efficacy in Patients with First-episode Depression. Chin. Arch. Tradit. Chin. Med. 2021, 39, 73–76. [Google Scholar] [CrossRef]
- Zhang, H.W.; Xing, H.G. Clinical effect of Baihe Zhimu decoction combined with acupoint acupuncture in the treatment of senile post-stroke depression. Chin. J. Gerontol. 2020, 40, 5001–5004. [Google Scholar] [CrossRef]
- Xu, H.Y.; Liu, Y.H.; Wang, S.; Hou, M.N.; Wang, Q.; Peng, X.J. Molecular Mechanism of Baihe Zhimu Tang against Anxiety and Insomnia Based on Network Pharmacology. West. J. Tradit. Chin. Med. 2024, 37, 48–53. [Google Scholar] [CrossRef]
- Xiao, W.; Zhan, T.; Yi, X.F.; Xiao, L.; Wen, H.H. Baihe Gujin Decoction Combined with Fermented Cordyceps Powder in the Treatment of Lung-kidney Yin Deficiency Lung Cancer. Chin. Med. Mod. Distance Educ. China 2023, 21, 87–89. [Google Scholar] [CrossRef]
- Zhao, S.H.; Wang, W.H.; Liang, Y.C.; Zhang, K.X.; Chen, K.; Wang, H.L.; Wang, X.Q. Research Progress of Baihe Gujin Decoction in the Treatment of Lung Cancer. Cancer Manag. Res. 2024, 16, 347–359. [Google Scholar] [CrossRef]
- Liu, Q.L.; Hou, S.J.; Lin, Y.Q. Efficacy observation of modified Baihe Gujin decoction and needle-embedding at acupoint on chronic obstructive pulmonary disease. Shanxin J. TCM 2023, 39, 19–21. [Google Scholar] [CrossRef]
- Xie, F.L.; Huang, W.D.; Meng, X.S.; Li, X.S.; Li, J.P. Effect of Baihe Gujin decoction combined with anti-tuberculosis drugs on clinical efficacy and T lymphocyte subsets in patients with pulmonary tuberculosis. Mod. Med. Health Res. 2023, 7, 89–92. [Google Scholar] [CrossRef]
- Zhong, Y.D.; Fan, S.H.; Wen, W.P. Modified Baihe Gujin decoction in the treatment of 46 cases of smear positive pulmonary tuberculosis of yin deficiency and fire exuberance type. Hunan J. Tradit. Chin. Med. 2021, 37, 35–37. [Google Scholar] [CrossRef]
- Nie, G.R. Clinical efficacy of Baihe Gujin decoction combined with compound andrographis paniculata in the treatment of bronchiectasis of yin deficiency and lung heat. Chin. J. Clin. Ration. Drug Use 2020, 13, 131–133. [Google Scholar] [CrossRef]
- Chen, X.L.; Tang, Y.Y.; Chen, L. Observation on 30 cases of ventilator-associated pneumonia of Lung and Kidney Yin deficiency treated with Baihe Gujin decoction. Zhejiang J. Tradit. Chin. Med. 2020, 55, 800–801. [Google Scholar] [CrossRef]
- Jiang, W.Z.; Chi, J.Y. Observation on the efficacy of Baihe Gujin decoction in the treatment of diabetes mellitus complicated with pulmonary tuberculosis. Electron. J. Clin. Med. Lit. 2020, 7, 144–146. [Google Scholar] [CrossRef]
- Wang, L.; Yang, S.H. Clinical study on Baihe Gujin decoction and Zhisou powder in treating lung cancer cough (lung yin deficiency syndrome). China Med. Pharm. 2020, 10, 43–45+69. [Google Scholar]
- Chen, H.P.; Huang, W.L.; Yan, J.; He, J.B. Study on Hemostatic Activity and Mechanism of Baihe Gujin Decoction. J. Emerg. Tradit. Chin. Med. 2020, 29, 653–656. [Google Scholar] [CrossRef]
- Lin, R.F. Effect of Ganwei Baihe Decoction on Autophagy of Gastric Mucosa in Rats with Stress Ulcer; Hunan University of Chinese Medicine: Changsha, China, 2023. [Google Scholar]
- Yan, A.; Luo, Z.H.; Xu, Y.; Yu, B. Efficacy observation of Ganwei Baihe decoction and vonoprazan fumarate tablets on reflux esophagitis. Shanxi J. TCM 2023, 39, 11–13. [Google Scholar] [CrossRef]
- Guo, R.H. Clinical Observation of Baihe Runjing Decoction Combined with Artificial Tears in the Treatment of Dry Eyes with Lung Yin deficiency Syndrome; Hunan University of Chinese Medicine: Changsha, China, 2023. [Google Scholar]
- Fu, X.Z.; Mu, Y.; Zhao, J.X.; Zhang, K.T.; Zhang, Y.F.; Liu, J.T.; Zhao, Y. Experience of Baihe Danshen Prescription in the treatment of diabetes and its complications. China J. Tradit. Chin. Med. Pharm. 2022, 37, 3253–3255. [Google Scholar]
- Xue, L.H.; Song, H.Y.; Gao, Q.; Li, S.; Zhao, C.L.; Bai, Y.; Zhang, B.N.; Qi, Y.J. Protective Effect and Its Mechanism Analysis of Baihe Wuyao Decoction on Treatment of Type 1 Diabetes Mellitus and Associated Hepatic Injury. Sci. Technol. Food Ind. 2022, 43, 376–383. [Google Scholar] [CrossRef]
- Chen, Y.; Li, R.; Hu, N.; Yu, C.; Song, H.; Li, Y.; Dai, Y.; Guo, Z.; Li, M.; Zheng, Y.; et al. Baihe Wuyao decoction ameliorates CCl(4)-induced chronic liver injury and liver fibrosis in mice through blocking TGF-β1/Smad2/3 signaling, anti-inflammation and anti-oxidation effects. J. Ethnopharmacol. 2020, 263, 113227. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.F. Clinical observation on 33 cases of Lung Adenocarcinoma treated by Western Medicine combined with Baihe Adenocarcinoma decoction. Chin. J. Ethnomedicine Ethnopharmacy 2020, 29, 102–104. [Google Scholar]
- Xie, Y.; Zai, G.T. Baihe Dihuang Pingkang decoction in the treatment of hyperthyroidism. Chin. J Clin. Res. 2021, 34, 236–239. [Google Scholar] [CrossRef]
- Zhao, H.Q.; Wang, Y.H.; Meng, P.; Han, Y.S.; Qiu, X.Y.; Tang, R.S.; Yang, A.N.; Wang, K.M. Effects of Baihe Shugan Anshen decoction and its disassembled prescriptions on HPA axis and monoamine neurotransmitters in rats with anxiety depression. Chin. Remedies Clin. 2021, 37, 160–166. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Li, Z.; Lin, M.Y.; Li, Z.Y.; Chen, N.H.; Liu, F.; Li, L. Effect of Baihe Jizi decoction on antidepressant activity in CUMS rats through BDNF/TrkB-mediated PI3K/Akt/mTOR signal pathway. Chin. Tradit. Pat. Med. 2021, 43, 778–782. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Ji, F. Effect of Baihe Shuxin decoction on Serum CK, CK-MB and LDH levels in patients with angina Pectoris after PCI. Mod. Med. Health Res. 2020, 4, 76–77. [Google Scholar]
- Gu, H.F.; Wang, X.Q.; Huang, C.H.; Yang, B.X.; Ge, F.H. Observation on the efficacy of Shuanghua Lily tablet in the treatment of 40 cases of radiation oral mucositis. Med. J. Commun. 2014, 28, 673–674+677. [Google Scholar]
- Rasoulinezhad, S.; Yekta, N.H.; Fallah, E. Promising pain-relieving activity of an ancient Persian remedy (mixture of white Lily in sesame oil) in patients with chronic low back pain. J. Family Med. Prim. Care 2019, 8, 634–639. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-F.; An, Z.-Y.; Yuan, L.-Q.; Wang, T.; Jin, W.-L. Lilium brownii/Baihe as Nutraceuticals: Insights into Its Composition and Therapeutic Properties. Pharmaceuticals 2024, 17, 1242. https://doi.org/10.3390/ph17091242
Wang Y-F, An Z-Y, Yuan L-Q, Wang T, Jin W-L. Lilium brownii/Baihe as Nutraceuticals: Insights into Its Composition and Therapeutic Properties. Pharmaceuticals. 2024; 17(9):1242. https://doi.org/10.3390/ph17091242
Chicago/Turabian StyleWang, Yong-Fei, Zi-Yi An, Le-Qi Yuan, Ting Wang, and Wei-Lin Jin. 2024. "Lilium brownii/Baihe as Nutraceuticals: Insights into Its Composition and Therapeutic Properties" Pharmaceuticals 17, no. 9: 1242. https://doi.org/10.3390/ph17091242
APA StyleWang, Y.-F., An, Z.-Y., Yuan, L.-Q., Wang, T., & Jin, W.-L. (2024). Lilium brownii/Baihe as Nutraceuticals: Insights into Its Composition and Therapeutic Properties. Pharmaceuticals, 17(9), 1242. https://doi.org/10.3390/ph17091242








