Multitarget-Directed Ligands Hitting Serotonin Receptors: A Medicinal Chemistry Survey
Abstract
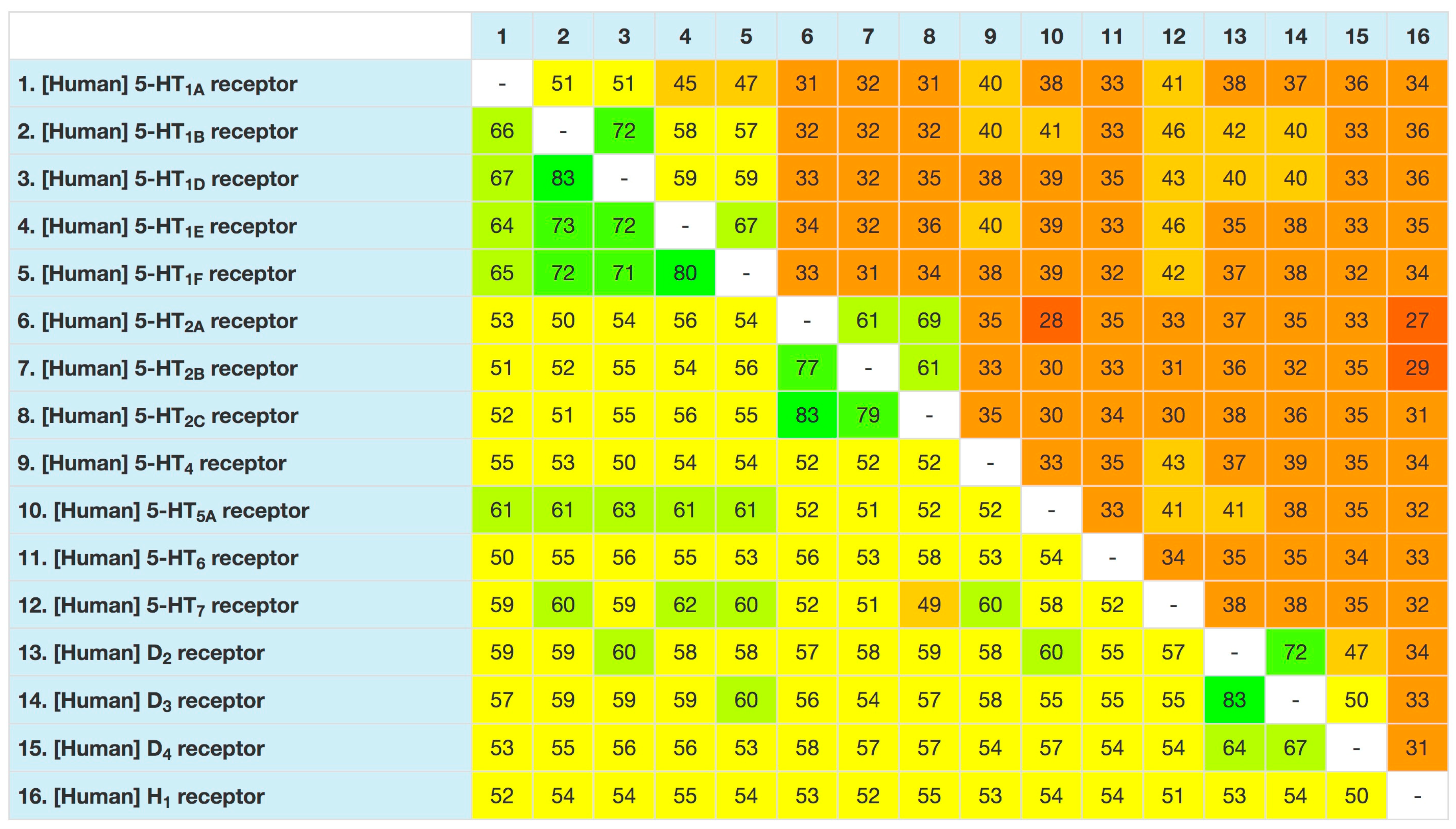
1. Introduction
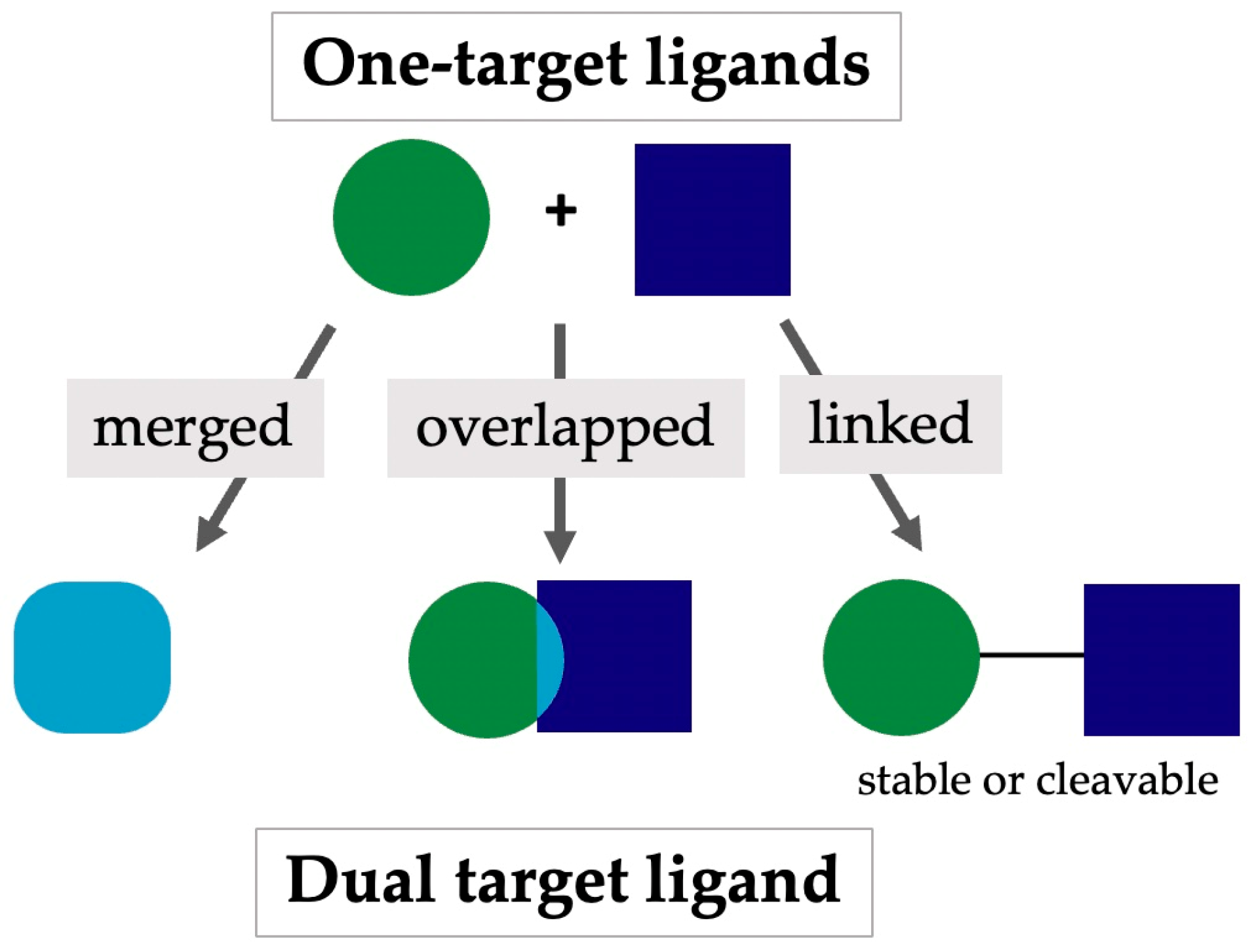
2. Multitarget-Directed Ligands Encompassing Serotonergic Activity
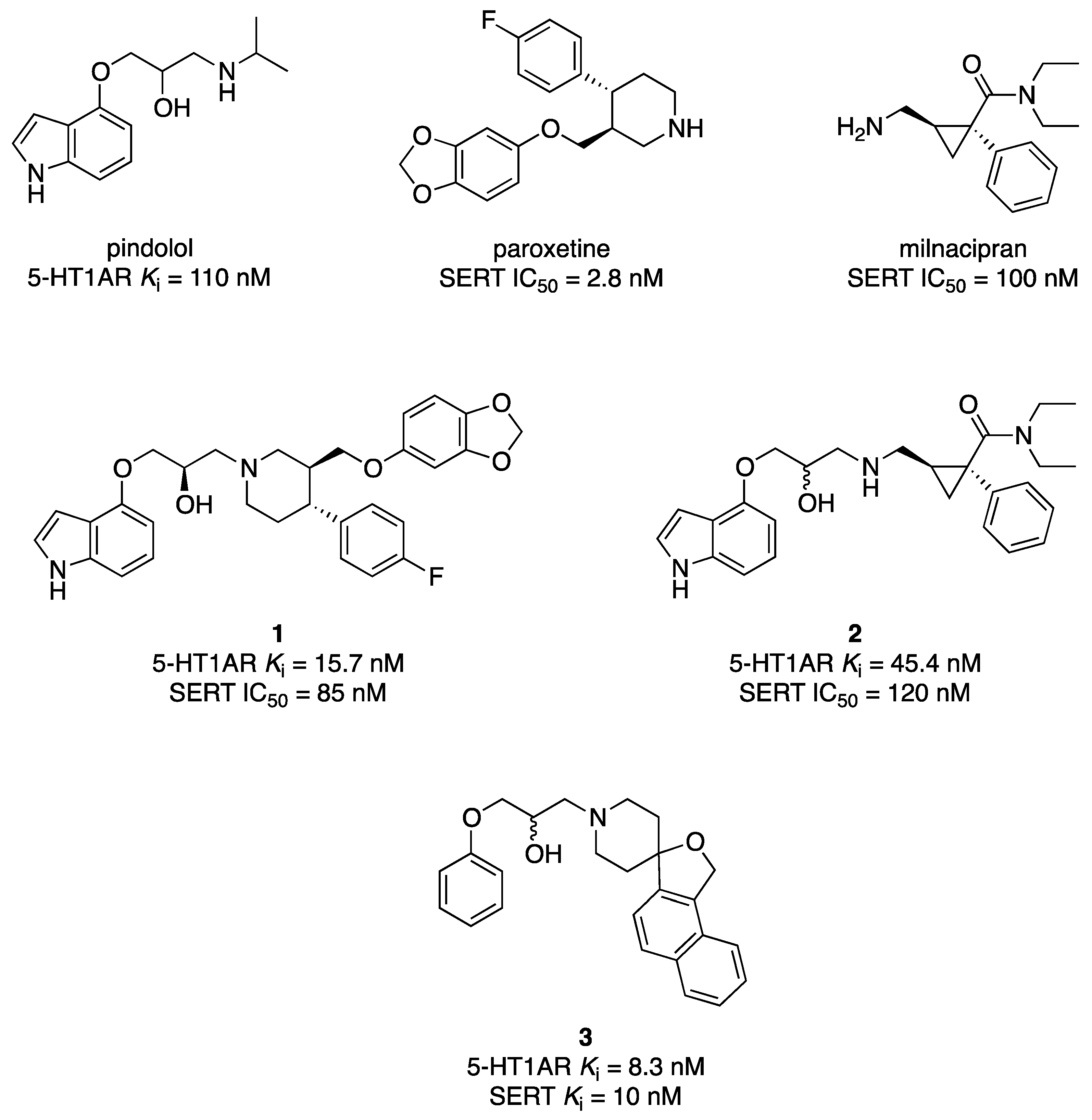
2.1. Multitarget-Directed Ligands Targeting Serotonin Receptors and Serotonin Transporter
2.2. Multitarget-Directed Ligands Targeting Serotonin Receptors and Norepinephrine Transporter
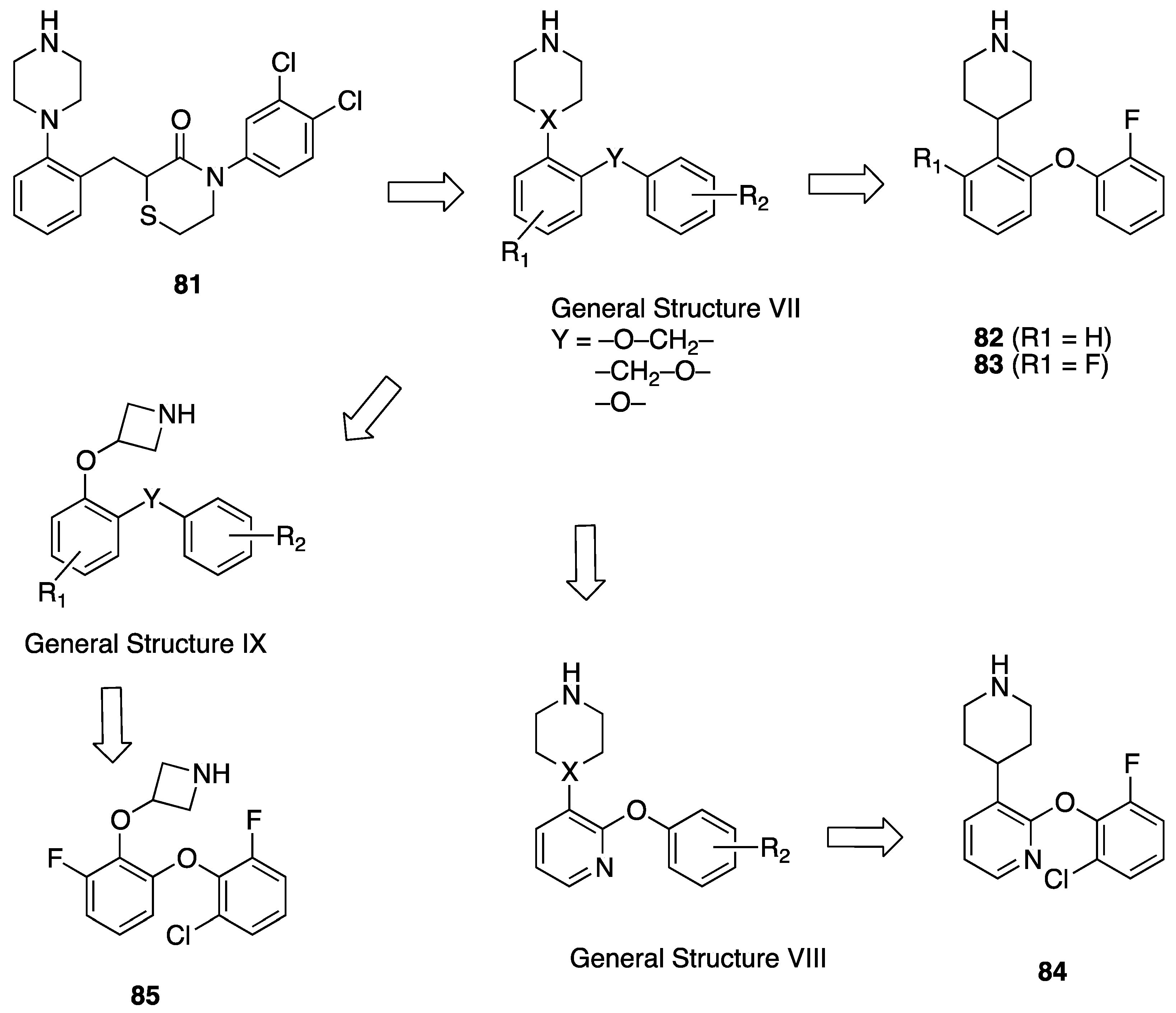
2.3. Multitarget-Directed Ligands Targeting Serotonin Receptors and Sigma Receptors
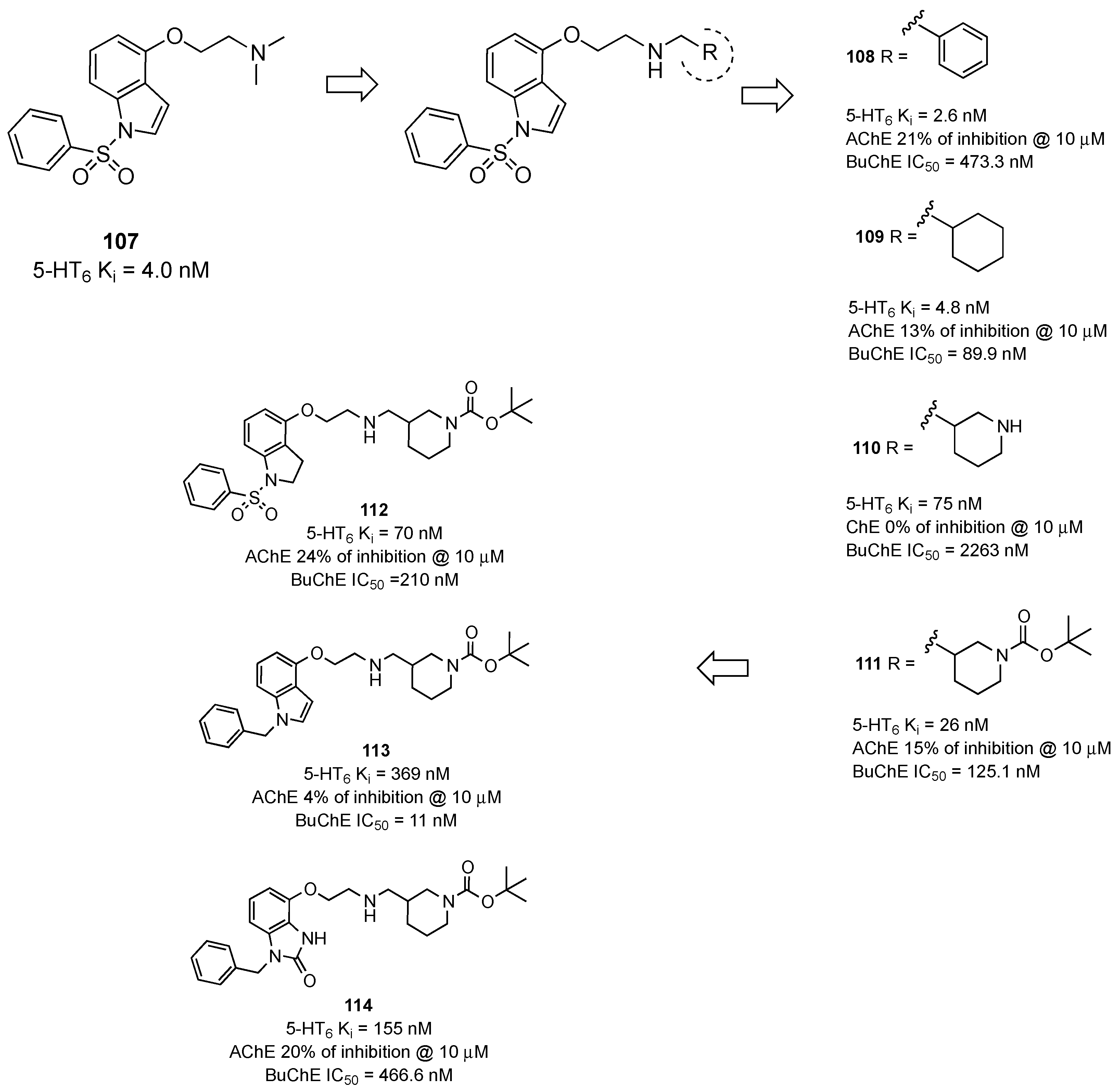
2.4. Multitarget-Directed Ligands Targeting Serotonin Receptors and Acetyl Cholinesterase/Butirryl Cholinesterase
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, J.A.; Uhlik, M.T.; Moxham, C.M.; Tomandl, D.; Sall, D.J. Modern phenotypic drug discovery is a viable, neoclassic pharma strategy. J. Med. Chem. 2012, 55, 4527–4538. [Google Scholar] [CrossRef] [PubMed]
- Croston, G.E. The utility of target-based discovery. Expert Opin. Drug Discov. 2017, 12, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Anighoro, A.; Bajorath, J.; Rastelli, G. Polypharmacology: Challenges and opportunities in drug discovery. J. Med. Chem. 2014, 57, 7874–7887. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Liu, F.; Li, S.; Shi, D. Rational multitargeted drug design strategy from the perspective of a medicinal chemist. J. Med. Chem. 2021, 64, 10581–10605. [Google Scholar] [CrossRef]
- Korcsmáros, T.; Szalay, M.S.; Böde, C.; Kovács, I.A.; Csermely, P. How to design multi-target drugs. Expert Opin. Drug Discov. 2007, 2, 799–808. [Google Scholar] [CrossRef]
- Barnes, N.M.; Ahern, G.P.; Becamel, C.; Bockaert, J.; Camilleri, M.; Chaumont-Dubel, S.; Claeysen, S.; Cunningham, K.A.; Fone, K.C.; Gershon, M.; et al. International Union of Basic and Clinical Pharmacology. CX. Classification of Receptors for 5-hydroxytryptamine; Pharmacology and Function. Pharmacol. Rev. 2021, 73, 310–520. [Google Scholar]
- Kristensen, A.S.; Andersen, J.; Jørgensen, T.N.; Sørensen, L.; Eriksen, J.; Loland, C.J.; Strømgaard, K.; Gether, U. SLC6 neurotransmitter transporters: Structure, function, and regulation. Pharmacol. Rev. 2011, 63, 585–640. [Google Scholar] [CrossRef]
- Masand, P.S.; Gupta, S. Selective serotonin-reuptake inhibitors: An update. Harv. Rev. Psychiatry. 1999, 7, 69–84. [Google Scholar] [CrossRef]
- Meltzer, H.Y. Clinical studies on the mechanism of action of clozapine: The dopamine-serotonin hypothesis of schizophrenia. Psychopharmacology 1989, 99, S18–S27. [Google Scholar] [CrossRef]
- Kondej, M.; Stępnicki, P.; Kaczor, A.A. Multi-Target Approach for Drug Discovery against Schizophrenia. Int. J. Mol. Sci. 2018, 19, 3105. [Google Scholar] [CrossRef]
- Jones, C.A.; Johnston, L.C.; Jackson, M.J.; Smith, L.A.; van Scharrenburg, G.; Rose, S.; Jenner, P.G.; McCreary, A.C. An in vivo pharmacological evaluation of pardoprunox (SLV308)-a novel combined dopamine D(2)/D(3) receptor partial agonist and 5-HT(1A) receptor agonist with efficacy in experimental models of Parkinson’s disease. Eur. Neuropsychopharmacol. 2010, 20, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Lacivita, E.; Niso, M.; Mastromarino, M.; Garcia Silva, A.; Resch, C.; Zeug, A.; Loza, M.I.; Castro, M.; Ponimaskin, E.; Leopoldo, M. Knowledge-Based Design of Long-Chain Arylpiperazine Derivatives Targeting Multiple Serotonin Receptors as Potential Candidates for Treatment of Autism Spectrum Disorder. ACS Chem. Neurosci. 2021, 12, 1313–1327. [Google Scholar] [CrossRef] [PubMed]
- Bojarski, A.J. Pharmacophore models for metabotropic 5-HT receptor ligands. Curr. Top. Med. Chem. 2006, 6, 2005–2026. [Google Scholar] [CrossRef] [PubMed]
- Chidester, C.G.; Lin, C.H.; Lahti, R.A.; Haadsma-Svensson, S.R.; Smith, M.W. Comparison of 5-HT1A and dopamine D2 pharmacophores. X-ray structures and affinities of conformationally constrained ligands. J. Med. Chem. 1993, 36, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Giorgioni, G.; Bonifazi, A.; Botticelli, L.; Cifani, C.; Matteucci, F.; Micioni Di Bonaventura, E.; Micioni Di Bonaventura, M.V.; Giannella, M.; Piergentili, A.; Piergentili, A.; et al. Advances in drug design and therapeutic potential of selective or multitarget 5-HT1A receptor ligands. Med. Res. Rev. 2024, in press. [Google Scholar] [CrossRef]
- Ye, N.; Song, Z.; Zhang, A. Dual ligands targeting dopamine D2 and serotonin 5-HT1A receptors as new antipsychotical or anti-Parkinsonian agents. Curr. Med. Chem. 2014, 21, 437–457. [Google Scholar] [CrossRef]
- Isberg, V.; Mordalski, S.; Munk, C.; Rataj, K.; Harpsoe, K.; Hauser, A.S.; Vroling, B.; Bojarski, A.J.; Vriend, G.; Gloriam, D.E. GPCRdb: An information system for G protein-coupled receptors. Nucleic Acids Res. 2016, 44, D356–D364. [Google Scholar] [CrossRef]
- Munk, C.; Isberg, V.; Mordalski, S.; Harpsøe, K.; Rataj, K.; Hauser, A.S.; Kolb, P.; Bojarski, A.J.; Vriend, G.; Gloriam, D.E. GPCRdb: The G protein-coupled receptor database—An introduction. Br. J. Pharmacol. 2012, 173, 2195–2207. [Google Scholar] [CrossRef]
- Artigas, F.; Bortolozzi, A.; Celada, P. Can we increase speed and efficacy of antidepressant treatments? Part I: General aspects and monoamine-based strategies. Eur. Neuropsychopharmacol. 2018, 28, 445–456. [Google Scholar] [CrossRef]
- Artigas, F.; Romero, L.; de Montigny, C.; Blier, P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci. 1996, 19, 378–383. [Google Scholar] [CrossRef]
- Hjorth, S.; Westlin, D.; Bengtsson, H.J. WAY100635-induced augmentation of the 5-HT-elevating action of citalopram: Relative importance of the dose of the 5-HT1A (auto)receptor blocker versus that of the 5-HT reuptake inhibitor. Neuropharmacology 1997, 36, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Blier, P.; Bergeron, R.; de Montigny, C. Selective activation of postsynaptic 5-HT1A receptors induces rapid antidepressant response. Neuropsychopharmacology 1997, 16, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Pauwels, P.J.; Pallard-Sigogneau, I.; Fourrier, C.; Chopin, P.; Palmier, C.; Colovray, V.; Halazy, S. Design and synthesis of new potent, silent 5-HT1A antagonists by covalent coupling of aminopropanol derivatives with selective serotonin reuptake inhibitors. Bioorg. Med. Chem. Lett. 1998, 8, 3423–3428. [Google Scholar] [CrossRef] [PubMed]
- van Niel, M.B.; Beer, M.S.; Castro, J.L.; Cheng, S.K.; Evans, D.C.; Heald, A.; Hitzel, L.; Hunt, P.; Mortishire-Smith, R.; O’Connor, D.; et al. Parallel synthesis of 3-aryloxy-2-propanolamines and evaluation as dual affinity 5-HT(1A) and 5-HT re-uptake ligands. Bioorg. Med. Chem. Lett. 1999, 9, 3243–3248. [Google Scholar] [CrossRef] [PubMed]
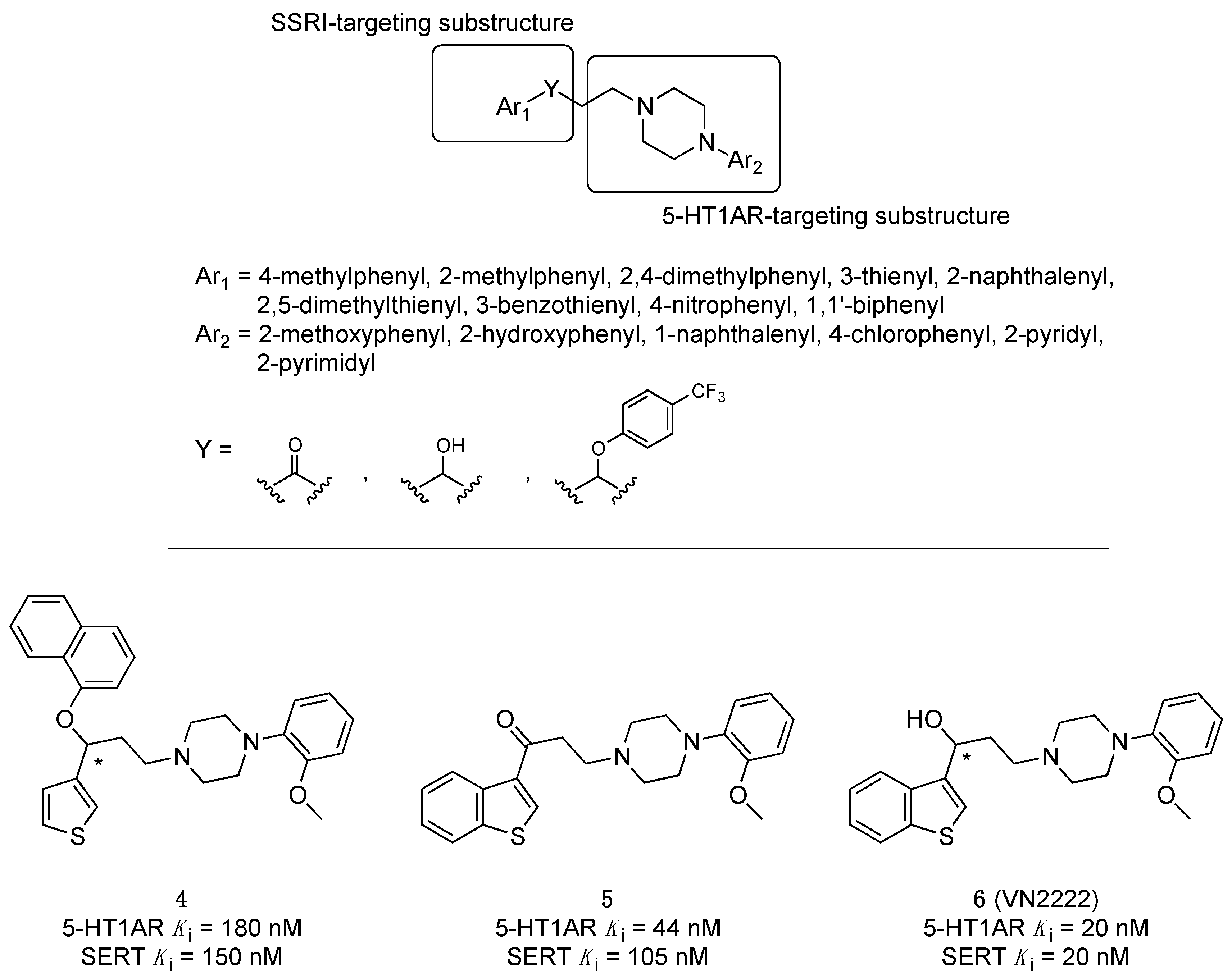
- Oficialdegui, A.M.; Martinez, J.; Pérez, S.; Heras, B.; Irurzun, M.; Palop, J.A.; Tordera, R.; Lasheras, B.; del Río, J.; Monge, A. Design, synthesis and biological evaluation of new 3-[(4-aryl)piperazin-1-yl]-1-arylpropane derivatives as potential antidepressants with a dual mode of action: Serotonin reuptake inhibition and 5-HT1A receptor antagonism. Farmaco 2000, 55, 345–353. [Google Scholar]
- Martínez-Esparza, J.; Oficialdegui, A.M.; Pérez-Silanes, S.; Heras, B.; Orús, L.; Palop, J.A.; Lasheras, B.; Roca, J.; Mourelle, M.; Bosch, A.; et al. New 1-aryl-3-(4-arylpiperazin-1-yl)propane derivatives, with dual action at 5-HT1A serotonin receptors and serotonin transporter, as a new class of antidepressants. J. Med. Chem. 2001, 44, 418–428. [Google Scholar] [CrossRef]
- Tordera, R.M.; Monge, A.; Del Río, J.; Lasheras, B. Antidepressant-like activity of VN2222, a serotonin reuptake inhibitor with high affinity at 5-HT1A receptors. Eur. J. Pharmacol. 2002, 442, 63–71. [Google Scholar] [CrossRef]
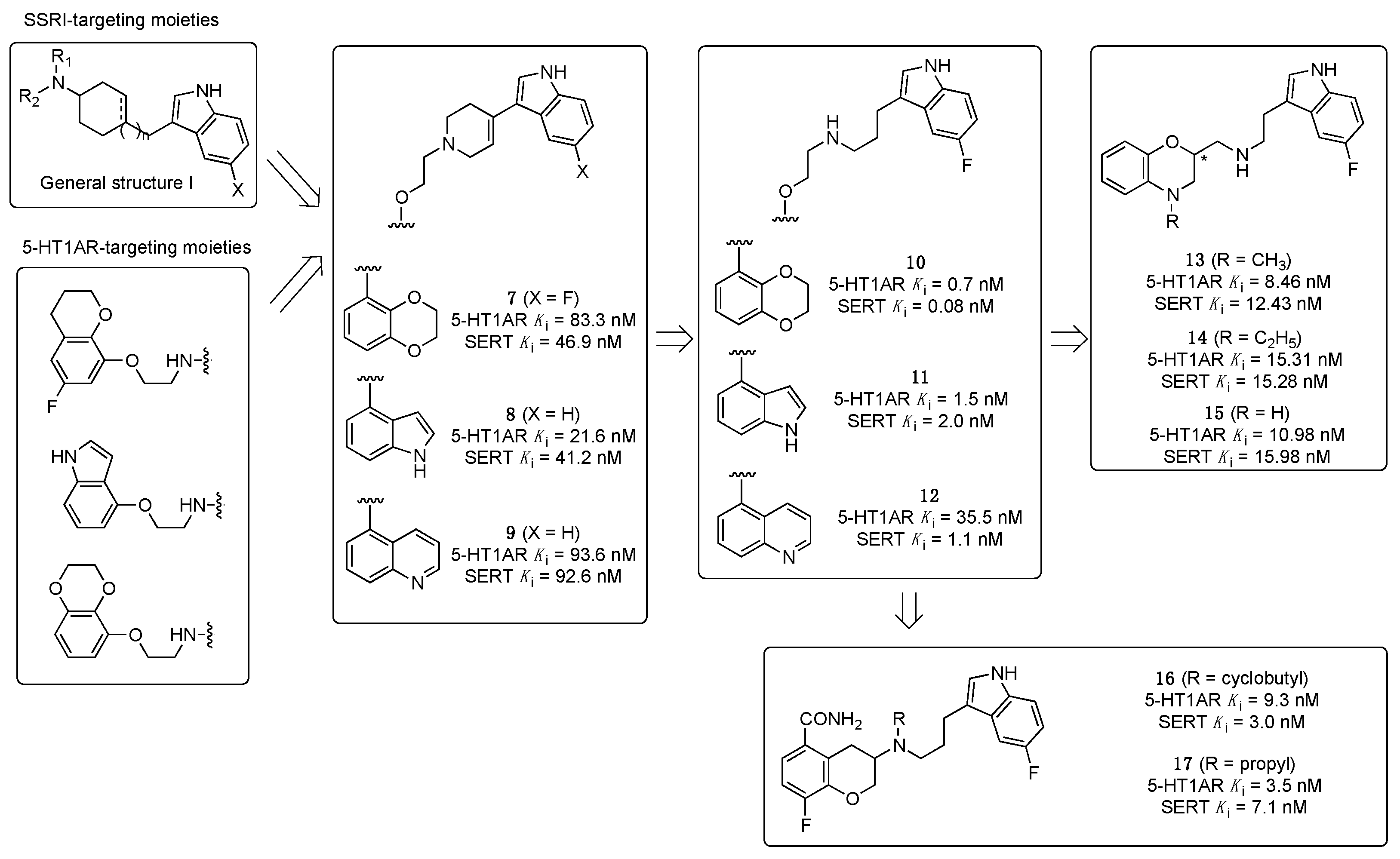
- Meagher, K.L.; Mewshaw, R.E.; Evrard, D.A.; Zhou, P.; Smith, D.L.; Scerni, R.; Spangler, T.; Abulhawa, S.; Shi, X.; Schechter, L.E.; et al. Studies towards the next generation of antidepressants. Part 1: Indolylcyclohexylamines as potent serotonin reuptake inhibitors. Bioorg. Med. Chem. Lett. 2001, 11, 1885–1888. [Google Scholar] [CrossRef]
- Mewshaw, R.E.; Zhou, D.; Zhou, P.; Shi, X.; Hornby, G.; Spangler, T.; Scerni, R.; Smith, D.; Schechter, L.E.; Andree, T.H. Studies toward the discovery of the next generation of antidepressants. 3. Dual 5-HT1A and serotonin transporter affinity within a class of N-aryloxyethylindolylalkylamines. J. Med. Chem. 2004, 47, 3823–3842. [Google Scholar] [CrossRef]
- Zhou, D.; Harrison, B.L.; Shah, U.; Andree, T.H.; Hornby, G.A.; Scerni, R.; Schechter, L.E.; Smith, D.L.; Sullivan, K.M.; Mewshaw, R.E. Studies toward the discovery of the next generation of antidepressants. Part 5: 3,4-Dihydro-2H-benzo[1,4]oxazine derivatives with dual 5-HT1A receptor and serotonin transporter affinity. Bioorg. Med. Chem. Lett. 2006, 16, 1338–1341. [Google Scholar]
- Hatzenbuhler, N.T.; Evrard, D.A.; Harrison, B.L.; Huryn, D.; Inghrim, J.; Kraml, C.; Mattes, J.F.; Mewshaw, R.E.; Zhou, D.; Hornby, G.; et al. Synthesis and biological evaluation of novel compounds within a class of 3-aminochroman derivatives with dual 5-HT1A receptor and serotonin transporter affinity. J. Med. Chem. 2006, 49, 4785–4789. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Kohn, T.J.; Honigschmidt, N.A.; Rocco, V.P.; Spinazze, P.G.; Hemrick-Luecke, S.K.; Thompson, L.K.; Evans, D.C.; Rasmussen, K.; Koger, D.; et al. Advances toward new antidepressants beyond SSRIs: 1-aryloxy-3-piperidinylpropan-2-ols with dual 5-HT1A receptor antagonism/SSRI activities. Part 5. Bioorg. Med. Chem. Lett. 2006, 16, 2347–2351. [Google Scholar] [CrossRef]
- Sahli, Z.T.; Banerjee, P.; Tarazi, F.I. The Preclinical and Clinical Effects of Vilazodone for the Treatment of Major Depressive Disorder. Expert Opin. Drug Discov. 2016, 11, 515–523. [Google Scholar] [CrossRef]
- Heinrich, T.; Böttcher, H.; Bartoszyk, G.D.; Greiner, H.E.; Seyfried, C.A.; Van Amsterdam, C. Indolebutylamines as selective 5-HT(1A) agonists. J. Med. Chem. 2004, 47, 4677–4683. [Google Scholar] [CrossRef] [PubMed]
- Herold, F.; Chodkowski, A.; Izbicki, Ł.; Król, M.; Kleps, J.; Turło, J.; Nowak, G.; Stachowicz, K.; Dybała, M.; Siwek, A. Novel 4-aryl-pyrido[1,2-c]pyrimidines with dual SSRI and 5-HT1A activity, part 1. Eur. J. Med. Chem. 2009, 44, 1710–1717. [Google Scholar] [PubMed]
- Herold, F.; Chodkowski, A.; Izbicki, Ł.; Turło, J.; Dawidowski, M.; Kleps, J.; Nowak, G.; Stachowicz, K.; Dybała, M.; Siwek, A.; et al. Novel 4-aryl-pyrido[1,2-c]pyrimidines with dual SSRI and 5-HT(1A) activity. part 3. Eur. J. Med. Chem. 2011, 46, 142–149. [Google Scholar] [PubMed]
- Vaatstra, W.J.; Deiman-Van Aalst, W.M.; Eigeman, L. Du 24565, a quipazine derivative, a potent selective serotonin uptake inhibitor. Eur. J. Pharmacol. 1981, 70, 195–202. [Google Scholar] [CrossRef]
- Perrone, R.; Berardi, F.; Colabufo, N.A.; Lacivita, E.; Larizza, C.; Leopoldo, M.; Tortorella, V. Design and synthesis of long-chain arylpiperazines with mixed affinity for serotonin transporter (SERT) and 5-HT(1A) receptor. J. Pharm. Pharmacol. 2005, 57, 1319–1327. [Google Scholar] [CrossRef]
- Gomółka, A.; Ciesielska, A.; Wróbel, M.Z.; Chodkowski, A.; Kleps, J.; Dawidowski, M.; Siwek, A.; Wolak, M.; Stachowicz, K.; Sławińska, A.; et al. Novel 4-aryl-pyrido[1,2-c]pyrimidines with dual SSRI and 5-HT(1A) activity. Part 5. Eur. J. Med. Chem. 2015, 98, 221–236. [Google Scholar]
- Zhou, D.; Stack, G.P.; Lo, J.; Failli, A.A.; Evrard, D.A.; Harrison, B.L.; Hatzenbuhler, N.T.; Tran, M.; Croce, S.; Yi, S.; et al. Synthesis, potency, and in vivo evaluation of 2-piperazin-1-ylquinoline analogues as dual serotonin reuptake inhibitors and serotonin 5-HT1A receptor antagonists. J. Med. Chem. 2009, 52, 4955–4959. [Google Scholar] [CrossRef]
- Shalom, G.; Gur, E.; Van de Kar, L.D.; Newman, M.E. Repeated administration of the 5-HT(1B) receptor antagonist SB-224289 blocks the desensitisation of 5-HT(1B) autoreceptors induced by fluoxetine in rat frontal cortex. Naunyn Schmiedebergs Arch. Pharmacol. 2004, 370, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Gobert, A.; Dekeyne, A.; Millan, M.J. The ability of WAY100,635 to potentiate the neurochemical and functional actions of fluoxetine is enhanced by co-administration of SB224,289, but not BRL15572. Neuropharmacology. 2000, 39, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, P.J.; Bromidge, S.M.; Duxon, M.S.; Gaster, L.M.; Hadley, M.S.; Hammond, B.; Johnson, C.N.; Middlemiss, D.N.; North, S.E.; Price, G.W.; et al. 3,4-Dihydro-2H-benzoxazinones are 5-HT(1A) receptor antagonists with potent 5-HT reuptake inhibitory activity. Bioorg. Med. Chem. Lett. 2005, 15, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Serafinowska, H.T.; Blaney, F.E.; Lovell, P.J.; Merlo, G.G.; Scott, C.M.; Smith, P.W.; Starr, K.R.; Watson, J.M. Novel 5-HT(1A/1B/1D) receptors antagonists with potent 5-HT reuptake inhibitory activity. Bioorg. Med. Chem. Lett. 2008, 18, 5581–5585. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J. Multi-target strategies for the improved treatment of depressive states: Conceptual foundations and neuronal substrates, drug discovery and therapeutic application. Pharmacol. Ther. 2006, 110, 135–370. [Google Scholar] [PubMed]
- Eliás, O.; Agai-Csongor, E.; Domány, G.; Keserű, G.M.; Gere, A.; Kiss, B.; Hellinger, E.; Vastag, M.; Gyertyán, I. Design of novel multiple-acting ligands towards SERT and 5-HT2C receptors. Bioorg. Med. Chem. Lett. 2014, 24, 2118–2122. [Google Scholar] [CrossRef]
- Bonaventure, P.; Kelly, L.; Aluisio, L.; Shelton, J.; Lord, B.; Galici, R.; Miller, K.; Atack, J.; Lovenberg, T.W.; Dugovic, C. Selective blockade of 5-hydroxytryptamine (5-HT)7 receptors enhances 5-HT transmission, antidepressant-like behavior, and rapid eye movement sleep suppression induced by citalopram in rodents. J. Pharmacol. Exp. Ther. 2007, 321, 690–698. [Google Scholar] [CrossRef]
- Wesołowska, A.; Tatarczyńska, E.; Nikiforuk, A.; Chojnacka-Wójcik, E. Enhancement of the anti-immobility action of antidepressants by a selective 5-HT7 receptor antagonist in the forced swimming test in mice. Eur. J. Pharmacol. 2007, 555, 43–47. [Google Scholar] [CrossRef]
- Hedlund, P.B.; Leopoldo, M.; Caccia, S.; Sarkisyan, G.; Fracasso, C.; Martelli, G.; Lacivita, E.; Berardi, F.; Perrone, R. LP-211 is a brain penetrant selective agonist for the serotonin 5-HT7 receptor. Neurosci. Lett. 2010, 481, 12–16. [Google Scholar] [CrossRef][Green Version]
- Gu, Z.S.; Zhou, A.N.; Xiao, Y.; Zhang, Q.W.; Li, J.Q. Synthesis and antidepressant-like activity of novel aralkyl piperazine derivatives targeting SSRI/5-HT1A/5-HT7. Eur. J. Med. Chem. 2018, 144, 701–715. [Google Scholar] [CrossRef]
- Wang, W.T.; Qian, H.; Wu, J.W.; Chen, X.W.; Li, J.Q. Synthesis and antidepressant-like activity of novel alkoxy-piperidine derivatives targeting SSRI/5-HT1A/5-HT7. Bioorg. Med. Chem. Lett. 2019, 29, 126769. [Google Scholar] [CrossRef] [PubMed]
- Jensen, N.H.; Rodriguiz, R.M.; Caron, M.G.; Wetsel, W.C.; Rothman, R.B.; Roth, B.L. N-desalkylquetiapine, a potent norepinephrine reuptake inhibitor and partial 5-HT1A agonist, as a putative mediator of quetiapine’s antidepressant activity. Neuropsychopharmacology 2008, 33, 2303–2312. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.L.; Xu, W.; Campbell, B.M.; Dounay, A.B.; Barta, N.; Boroski, S.; Denny, L.; Evans, L.; Stratman, N.; Probert, A. Discovery and pharmacological characterization of aryl piperazine and piperidine ethers as dual acting norepinephrine reuptake inhibitors and 5-HT1A partial agonists. Bioorg. Med. Chem. Lett. 2009, 19, 6604–6607. [Google Scholar] [CrossRef]
- Dounay, A.B.; Barta, N.S.; Campbell, B.M.; Coleman, C.; Collantes, E.M.; Denny, L.; Dutta, S.; Gray, D.L.; Hou, D.; Iyer, R.; et al. Design, synthesis, and pharmacological evaluation of phenoxy pyridyl derivatives as dual norepinephrine reuptake inhibitors and 5-HT1A partial agonists. Bioorg. Med. Chem. Lett. 2010, 20, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, M.; Campbell, B.M.; Dounay, A.B.; Gray, D.L.; Xie, L.; O’Donnell, C.J.; Stratman, N.C.; Zoski, K.; Drummond, E.; Bora, G.; et al. Design, synthesis, and pharmacological evaluation of azetedine and pyrrolidine derivatives as dual norepinephrine reuptake inhibitors and 5-HT(1A) partial agonists. Bioorg. Med. Chem. Lett. 2011, 21, 865–868. [Google Scholar] [CrossRef]
- Schmidt, H.R.; Kruse, A.C. The Molecular Function of σ Receptors: Past, Present, and Future. Trends Pharmacol. Sci. 2019, 40, 636–654. [Google Scholar] [CrossRef] [PubMed]
- Piechal, A.; Jakimiuk, A.; Mirowska-Guzel, D. Sigma receptors and neurological disorders. Pharmacol. Rep. 2021, 73, 1582–1594. [Google Scholar] [CrossRef]
- Zeng, C.; Riad, A.; Mach, R.H. The Biological Function of Sigma-2 Receptor/TMEM97 and Its Utility in PET Imaging Studies in Cancer. Cancers 2020, 12, 1877. [Google Scholar] [CrossRef]
- Lizama, B.N.; Kahle, J.; Catalano, S.M.; Caggiano, A.O.; Grundman, M.; Hamby, M.E. Sigma-2 Receptors-From Basic Biology to Therapeutic Target: A Focus on Age-Related Degenerative Diseases. Int. J. Mol. Sci. 2023, 24, 6251. [Google Scholar] [CrossRef]
- Glennon, R.A. Pharmacophore identification for sigma-1 (sigma1) receptor binding: Application of the “deconstruction-reconstruction-elaboration” approach. Mini Rev. Med. Chem. 2005, 5, 927–940. [Google Scholar] [CrossRef]
- Perrone, R.; Berardi, F.; Colabufo, N.A.; Leopoldo, M.; Abate, C.; Tortorella, V. N-aryl- or N-alkylpiperazine derivatives: The role of N-substituent on s1, s2, 5-HT1A and D2 receptor affinity. Med. Chem. Res. 2000, 10, 201–207. [Google Scholar]
- Berardi, F.; Ferorelli, S.; Colabufo, N.A.; Leopoldo, M.; Perrone, R.; Tortorella, V. A multireceptorial binding reinvestigation on an extended class of sigma ligands: N-[omega-(indan-1-yl and tetralin-1-yl)alkyl] derivatives of 3,3-dimethylpiperidine reveal high affinities towards sigma1 and EBP sites. Bioorg. Med. Chem. 2001, 9, 1325–1335. [Google Scholar] [PubMed]
- Costantino, L.; Gandolfi, F.; Sorbi, C.; Franchini, S.; Prezzavento, O.; Vittorio, F.; Ronsisvalle, G.; Leonardi, A.; Poggesi, E.; Brasili, L. Synthesis and structure-activity relationships of 1-aralkyl-4-benzylpiperidine and 1-aralkyl-4-benzylpiperazine derivatives as potent sigma ligands. J. Med. Chem. 2005, 48, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Tottori, K.; Miwa, T.; Uwahodo, Y.; Yamada, S.; Nakai, M.; Oshiro, Y.; Kikuchi, T.; Altar, C.A. Antidepressant-like responses to the combined sigma and 5-HT1A receptor agonist OPC-14523. Neuropharmacology 2001, 41, 976–988. [Google Scholar] [CrossRef]
- Ago, Y.; Hasebe, S.; Hiramatsu, N.; Hashimoto, H.; Takuma, K.; Matsuda, T. Psychopharmacology of combined activation of the serotonin1A and σ1 receptors. Eur. J. Pharmacol. 2017, 809, 172–177. [Google Scholar] [CrossRef]
- Perregaard, J.; Moltzen, E.K.; Meier, E.; Sánchez, C. Sigma ligands with subnanomolar affinity and preference for the sigma 2 binding site. 1. 3-(omega-aminoalkyl)-1H-indoles. J. Med. Chem. 1995, 38, 1998–2008. [Google Scholar] [CrossRef]
- Porter, M.R.; Xiao, H.; Wang, J.; Smith, S.B.; Topczewski, J.J. 3-Amino-chromanes and Tetrahydroquinolines as Selective 5-HT2B, 5-HT7, or σ1 Receptor Ligands. ACS Med. Chem. Lett. 2019, 10, 1436–1442. [Google Scholar] [CrossRef]
- Abatematteo, F.S.; Niso, M.; Contino, M.; Leopoldo, M.; Abate, C. Multi-Target Directed Ligands (MTDLs) Binding the σ1 Receptor as Promising Therapeutics: State of the Art and Perspectives. Int. J. Mol. Sci. 2021, 22, 6359. [Google Scholar] [CrossRef]
- Eremin, D.V.; Kondaurova, E.M.; Rodnyy, A.Y.; Molobekova, C.A.; Kudlay, D.A.; Naumenko, V.S. Serotonin receptors as a potential target in the treatment of Alzheimer’s Disease. Biochem. (Mosc.) 2023, 88, 2023–2042. [Google Scholar] [CrossRef]
- Riemer, C.; Borroni, E.; Levet-Trafit, B.; Martin, J.R.; Poli, S.; Porter, R.H.; Bös, M. Influence of the 5-HT6 receptor on acetylcholine release in the cortex: Pharmacological characterization of 4-(2-bromo-6-pyrrolidin-1-ylpyridine-4-sulfonyl)phenylamine, a potent and selective 5-HT6 receptor antagonist. J. Med. Chem. 2003, 46, 1273–1276. [Google Scholar] [CrossRef]
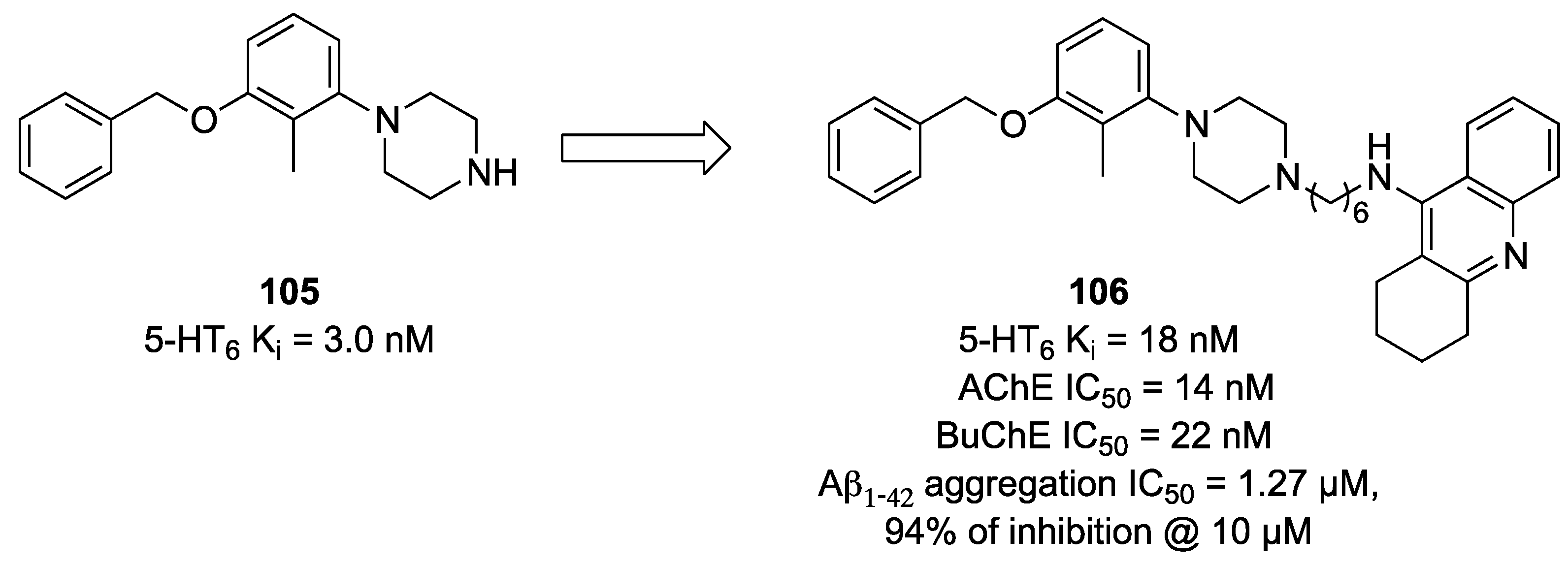
- Więckowska, A.; Kołaczkowski, M.; Bucki, A.; Godyń, J.; Marcinkowska, M.; Więckowski, K.; Zaręba, P.; Siwek, A.; Kazek, G.; Głuch-Lutwin, M.; et al. Novel multi-target-directed ligands for Alzheimer’s disease: Combining cholinesterase inhibitors and 5-HT6 receptor antagonists. Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2016, 124, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Więckowska, A.; Wichur, T.; Godyń, J.; Bucki, A.; Marcinkowska, M.; Siwek, A.; Więckowski, K.; Zaręba, P.; Knez, D.; Głuch-Lutwin, M.; et al. Novel Multitarget-Directed Ligands Aiming at Symptoms and Causes of Alzheimer’s Disease. ACS Chem. Neurosci. 2018, 9, 1195–1214. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowska, M.; Bucki, A.; Panek, D.; Siwek, A.; Fajkis, N.; Bednarski, M.; Zygmunt, M.; Godyń, J.; Del Rio Valdivieso, A.; Kotańska, M.; et al. Anti-Alzheimer’s multitarget-directed ligands with serotonin 5-HT6 antagonist, butyrylcholinesterase inhibitory, and antioxidant activity. Arch. Pharm. 2019, 352, e1900041. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yan, Y.; Bernotas, R.; Harrison, B.L.; Huryn, D.; Robichaud, A.J.; Zhang, G.M.; Smith, D.L.; Schechter, L.E. 4-(2-Aminoethoxy)-N-(phenylsulfonyl)indoles as novel 5-HT6 receptor ligands. Bioorg. Med. Chem. Lett. 2005, 15, 1393–1396. [Google Scholar] [CrossRef] [PubMed]
- Wichur, T.; Godyń, J.; Góral, I.; Latacz, G.; Bucki, A.; Siwek, A.; Głuch-Lutwin, M.; Mordyl, B.; Śniecikowska, J.; Walczak, M.; et al. Development and crystallography-aided SAR studies of multifunctional BuChE inhibitors and 5-HT6R antagonists with β-amyloid anti-aggregation properties. Eur. J. Med. Chem. 2021, 225, 113792. [Google Scholar] [CrossRef]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.S.; Mamczarz, J.; Holloway, H.W.; Giordano, T.; et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef]
- Więckowski, K.; Szałaj, N.; Gryzło, B.; Wichur, T.; Góral, I.; Sługocka, E.; Sniecikowska, J.; Latacz, G.; Siwek, A.; Godyń, J.; et al. Serotonin 5-HT6 Receptor Ligands and Butyrylcholinesterase Inhibitors Displaying Antioxidant Activity-Design, Synthesis and Biological Evaluation of Multifunctional Agents against Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 9443. [Google Scholar] [CrossRef]
- Wichur, T.; Pasieka, A.; Godyń, J.; Panek, D.; Góral, I.; Latacz, G.; Honkisz-Orzechowska, E.; Bucki, A.; Siwek, A.; Głuch-Lutwin, M.; et al. Discovery of 1-(phenylsulfonyl)-1H-indole-based multifunctional ligands targeting cholinesterases and 5-HT6 receptor with an-ti-aggregation properties against amyloid-beta and tau. Eur. J. Med. Chem. 2021, 225, 113783. [Google Scholar] [CrossRef]
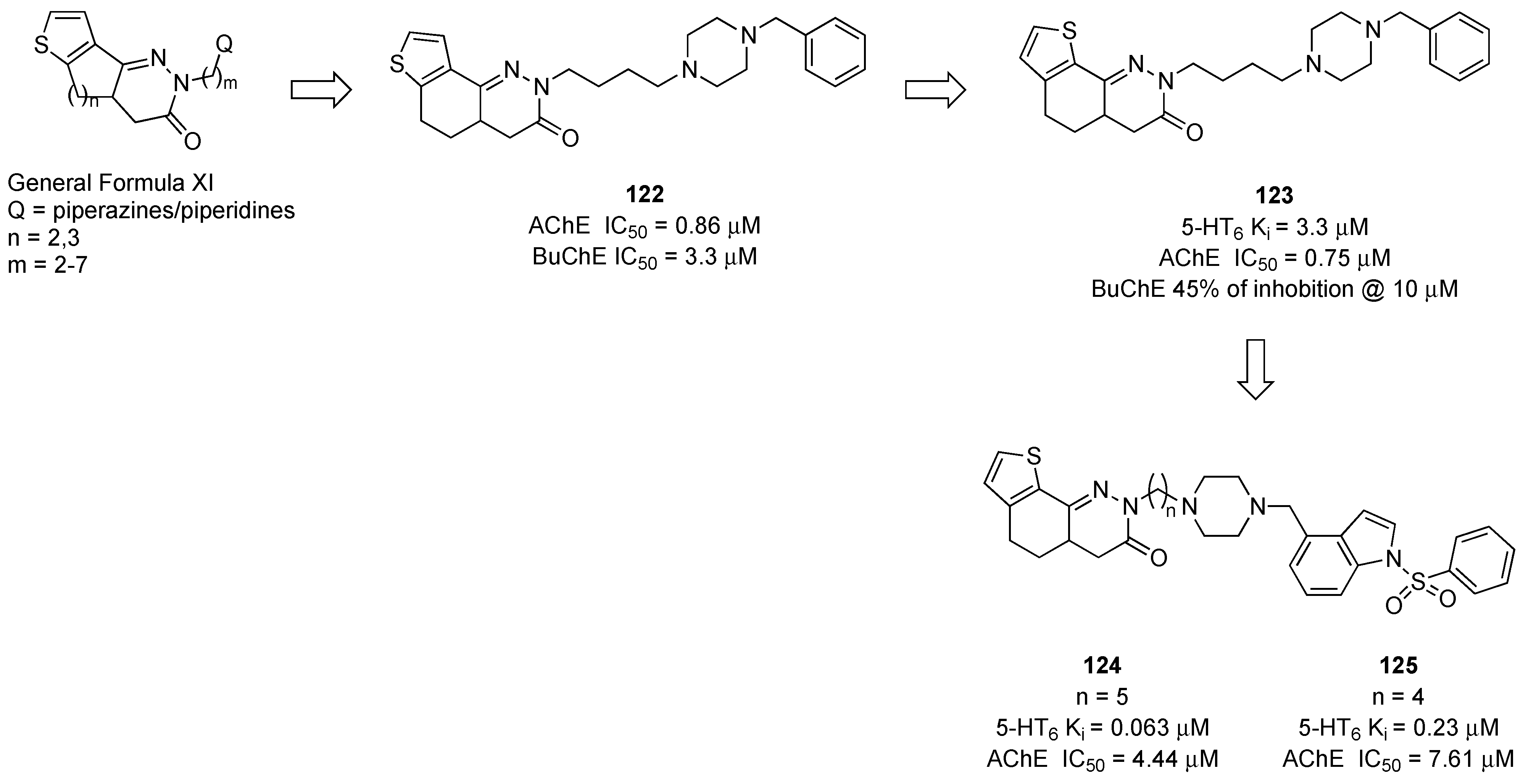
- Asproni, B.; Catto, M.; Loriga, G.; Murineddu, G.; Corona, P.; Purgatorio, R.; Cichero, E.; Fossa, P.; Scarano, N.; Martínez, A.L.; et al. Novel thienocycloalkylpyridazinones as useful scaffolds for acetylcholinesterase inhibition and serotonin 5-HT6 receptor interaction. Bioorg. Med. Chem. 2023, 84, 117256. [Google Scholar] [CrossRef]
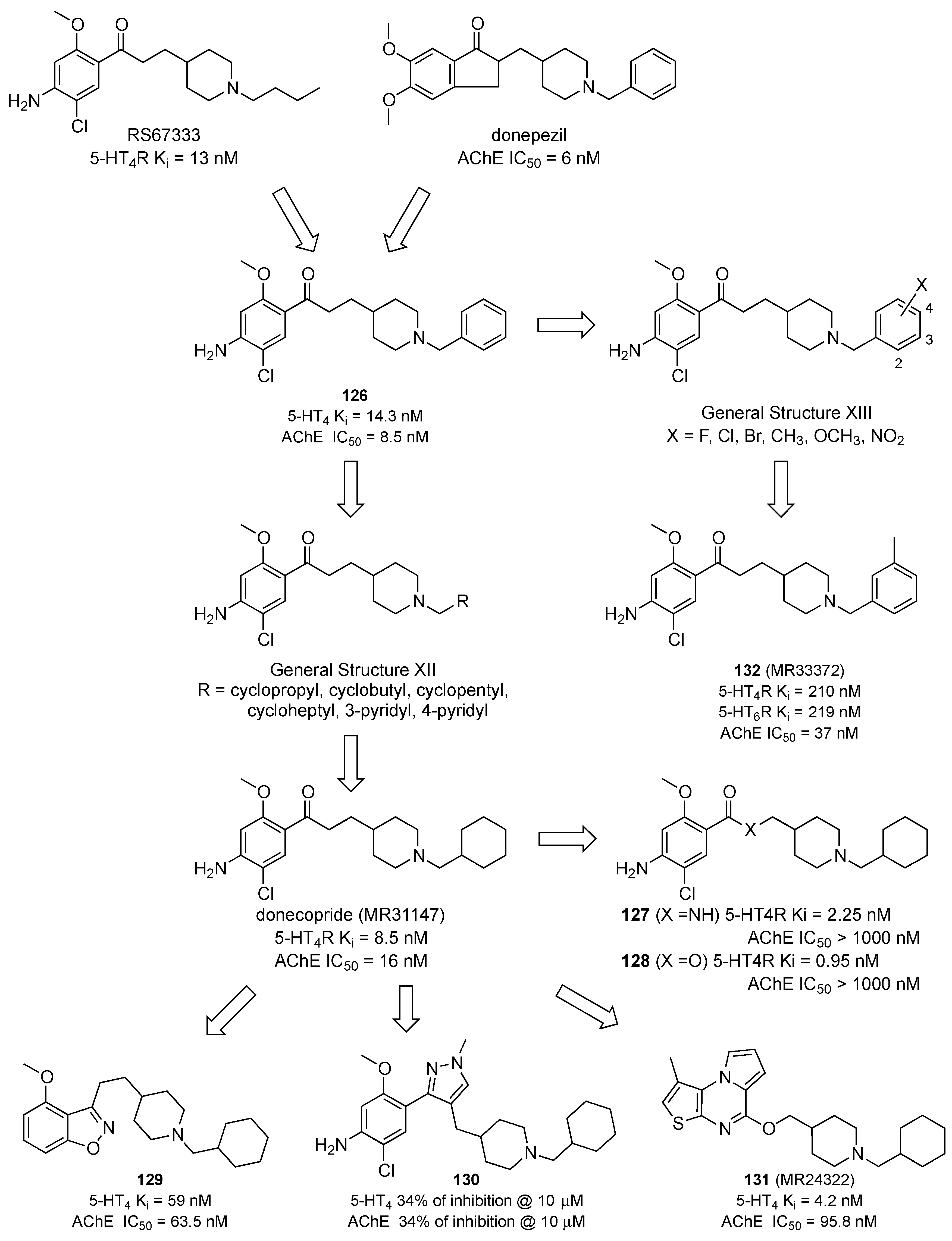
- Freret, T.; Bouet, V.; Quiedeville, A.; Nee, G.; Dallemagne, P.; Rochais, C.; Boulouard, M. Synergistic effect of acetylcholinesterase inhibition (donepezil) and 5-HT(4) receptor activation (RS67333) on object recognition in mice. Behav. Brain Res. 2012, 230, 304–308. [Google Scholar] [CrossRef]
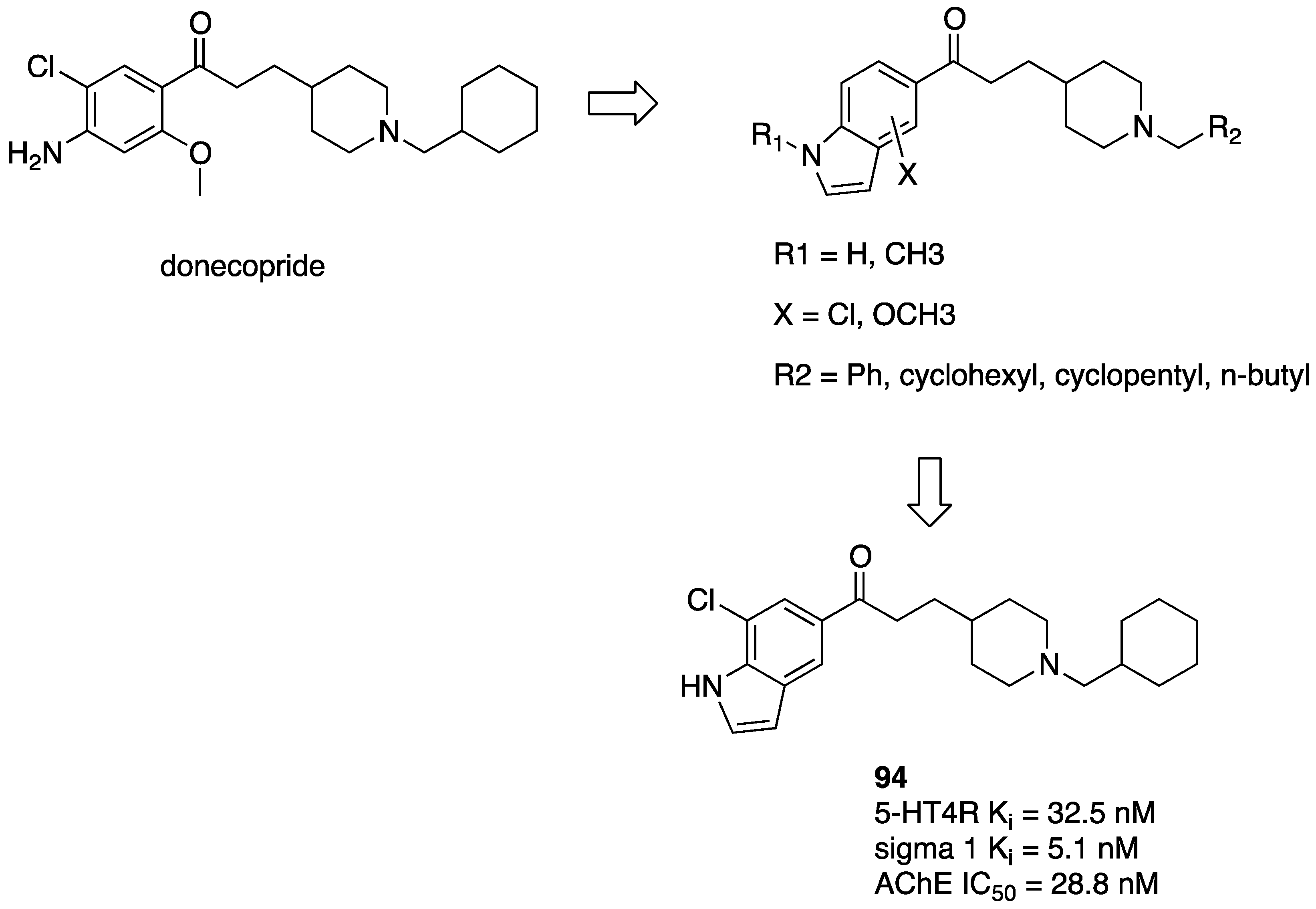
- Lecoutey, C.; Hedou, D.; Freret, T.; Giannoni, P.; Gaven, F.; Since, M.; Bouet, V.; Ballandonne, C.; Corvaisier, S.; Malzert Fréon, A.; et al. Design of donecopride, a dual serotonin subtype 4 receptor agonist/acetylcholinesterase inhibitor with potential interest for Alzheimer’s disease treatment. Proc. Natl. Acad. Sci. USA 2014, 111, E3825–E3830. [Google Scholar] [CrossRef] [PubMed]
- Lecoutey, C.; Rochais, C.; Genest, D.; Butt-Gueulle, S.; Ballandonne, C.; Corvaisier, S.; Dulin, F.; Lepailleur, A.; Sopkova-de Oliveira Santos, J.; Dallemagne, P. Synthesis of dual AChE/5-HT4 receptor multi-target directed ligands. MedChemCom 2012, 3, 627. [Google Scholar] [CrossRef]
- Hatat, B.; Yahiaoui, S.; Lecoutey, C.; Davis, A.; Freret, T.; Boulouard, M.; Claeysen, S.; Rochais, C.; Dallemagne, P. A Novel in vivo anti-amnesic agent, specially designed to express both acetylcholinesterase (AChE) inhibitory, serotonergic subtype 4 receptor (5-HT4R) agonist and serotonergic subtype 6 receptor (5-HT6R) inverse agonist activities, with a potential interest against Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 148. [Google Scholar]
- Pahwa, M.; Sleem, A.; Elsayed, O.H.; Good, M.E.; El-Mallakh, R.S. New antipsychotic medications in the last decade. Curr. Psychiatry Rep. 2021, 23, 87. [Google Scholar] [CrossRef] [PubMed]
- Saarinen, M.; Mantas, I.; Flais, I.; Ågren, R.; Sahlholm, K.; Millan, M.J.; Svenningsson, P. TAAR1 dependent and independent actions of the potential antipsychotic and dual TAAR1/5-HT1A receptor agonist SEP-383856. Neuropsychopharmacology 2022, 47, 2319–2329. [Google Scholar] [CrossRef]
- Rehan, S.T.; Siddiqui, A.H.; Khan, Z.; Imran, L.; Syed, A.A.; Tahir, M.J.; Jassani, Z.; Singh, M.; Asghar, M.S.; Ahmed, A. Samidorphan/olanzapine combination therapy for schizophrenia: Efficacy, tolerance and adverse outcomes of regimen, evidence-based review of clinical trials. Ann. Med. Surg. 2022, 79, 104115. [Google Scholar] [CrossRef]
- Burnat, G.; Brański, P.; Solich, J.; Kolasa, M.; Chruścicka, B.; Dziedzicka-Wasylewska, M.; Pilc, A. The functional cooperation of 5-HT1A and mGlu4R in HEK-293 cell line. Pharmacol. Rep. 2020, 72, 1358–1369. [Google Scholar] [CrossRef]
- Yang, W.; Gong, X.; Sun, H.; Wu, C.; Suo, J.; Ji, J.; Jiang, X.; Shen, J.; He, Y.; Aisa, H.A. Discovery of a CB2 and 5-HT1A receptor dual agonist for the treatment of depression and anxiety. Eur. J. Med. Chem. 2024, 265, 116048. [Google Scholar] [CrossRef]
- Pathak, C.; Kabra, U.D. A comprehensive review of multi-target directed ligands in treating Alzheimer’s disease. Bioorg. Chem. 2024, 144, 107152. [Google Scholar] [CrossRef]
- Rochais, C.; Lecoutey, C.; Hamidouche, K.; Giannoni, P.; Gaven, F.; Cem, E.; Mignani, S.; Baranger, K.; Freret, T.; Bockaert, J.; et al. Donecopride, a Swiss army knife with potential against Alzheimer’s disease. Br. J. Pharmacol. 2020, 177, 1988–2005. [Google Scholar] [CrossRef]
- Stuivenga, M.; Giltay, E.J.; Cools, O.; Roosens, L.; Neels, H.; Sabbe, B. Evaluation of vilazodone for the treatment of depressive and anxiety disorders. Expert Opin. Pharmacother. 2019, 20, 251–260. [Google Scholar] [CrossRef] [PubMed]
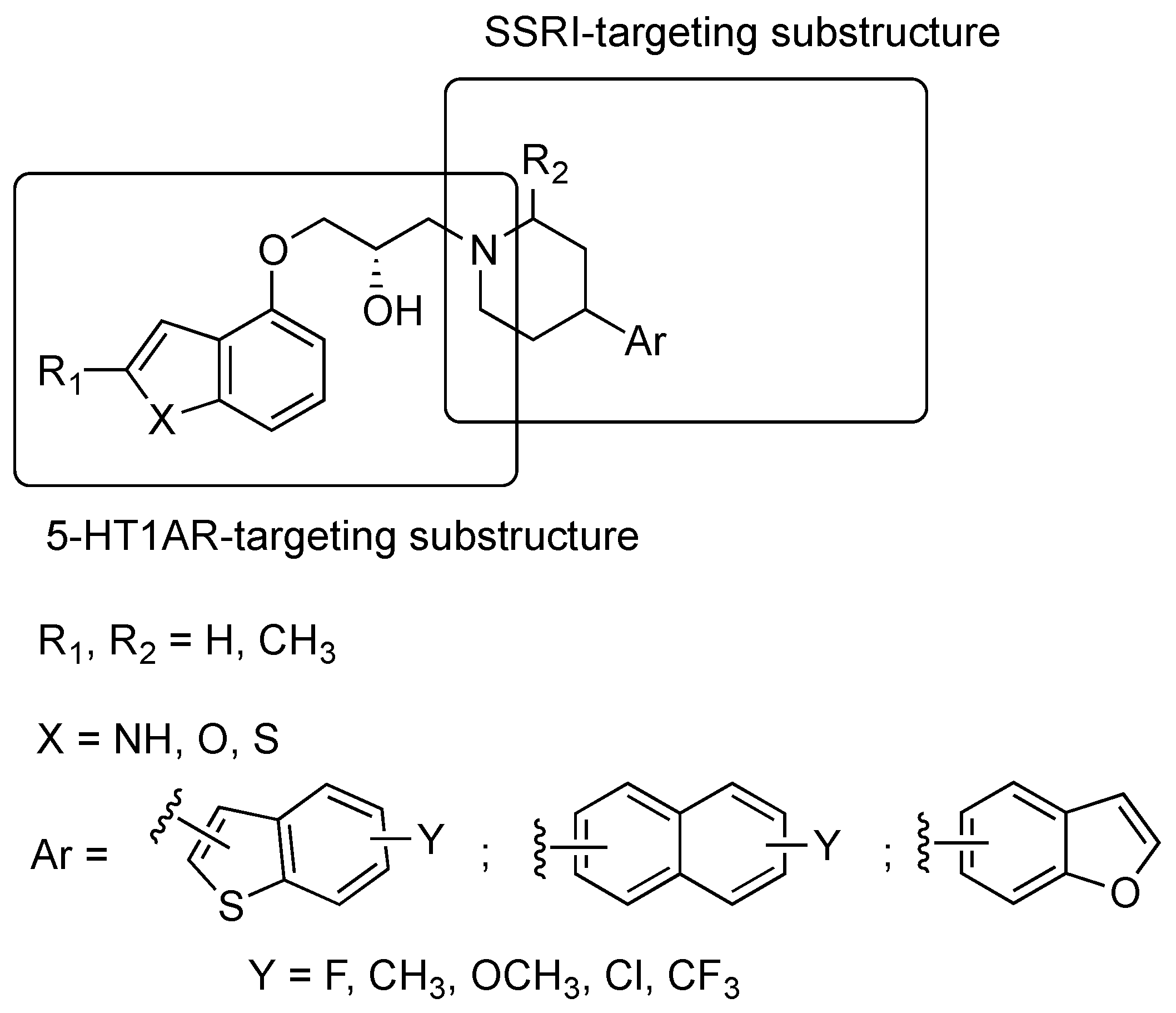
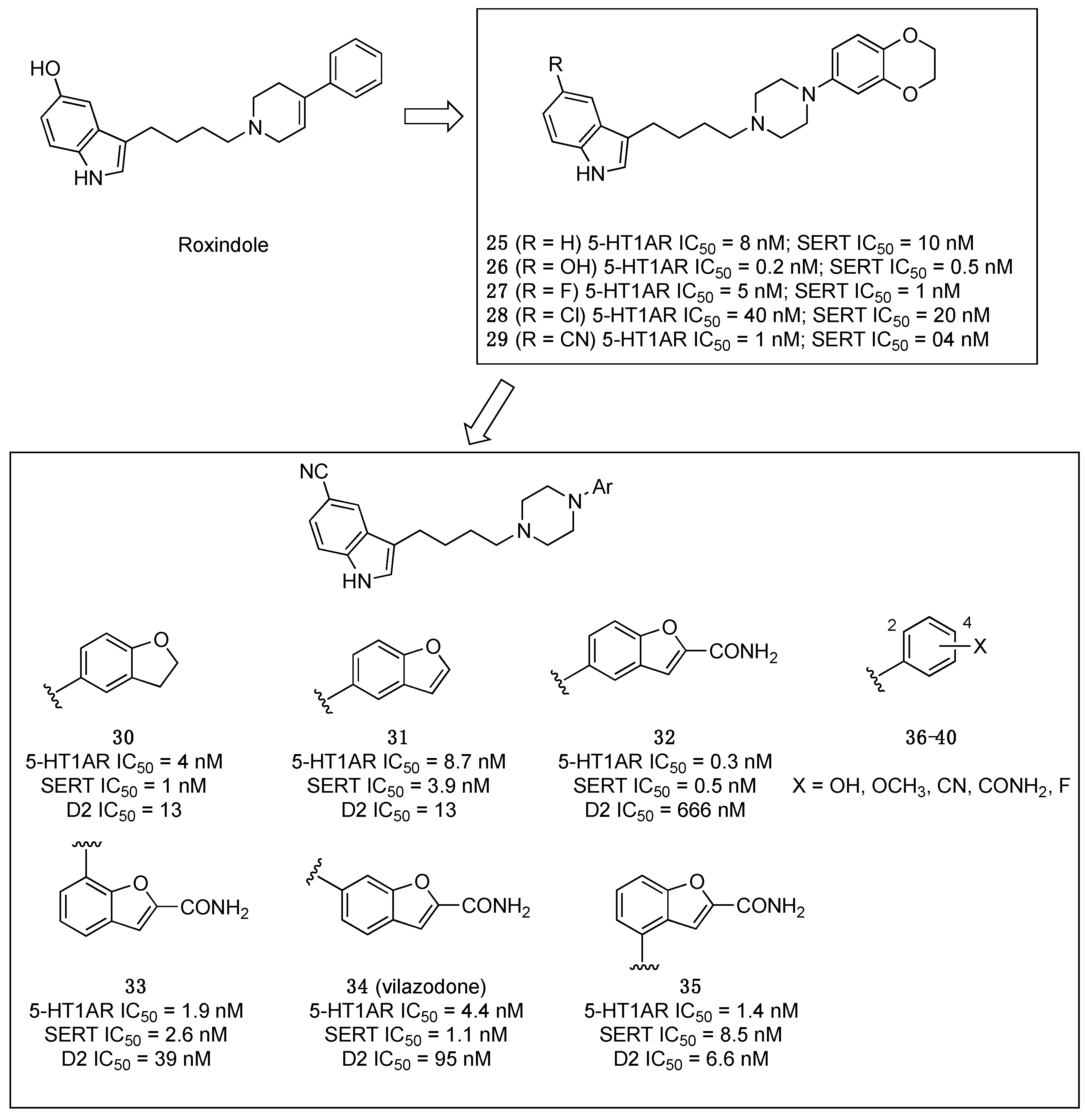

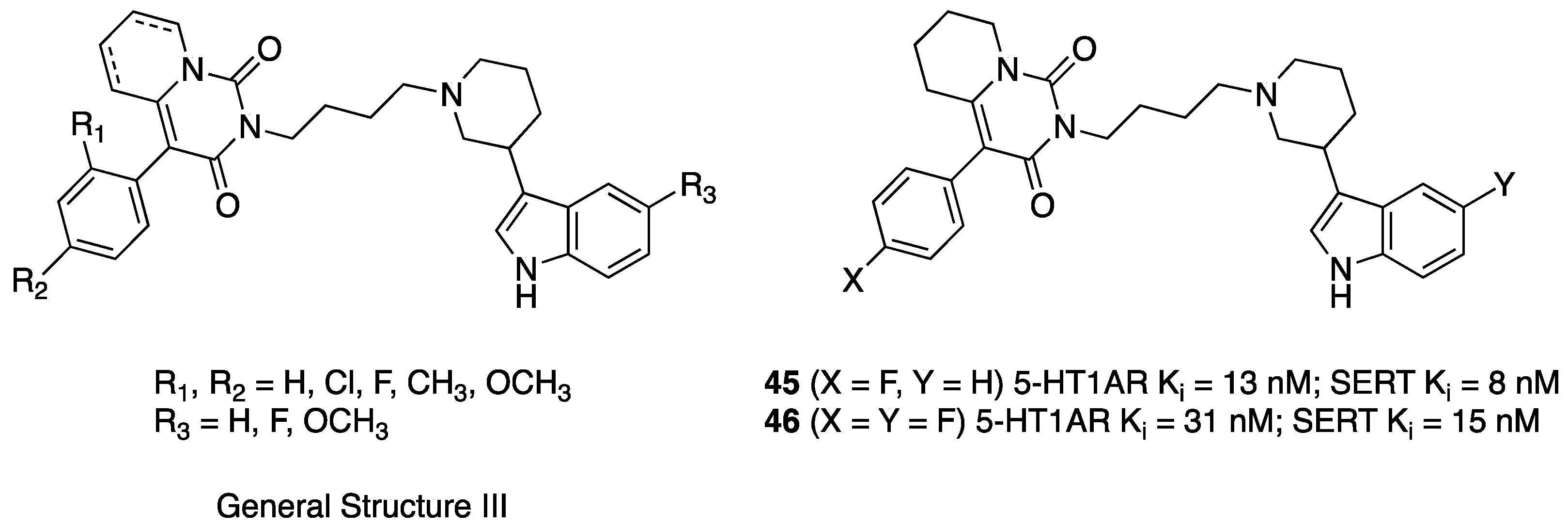
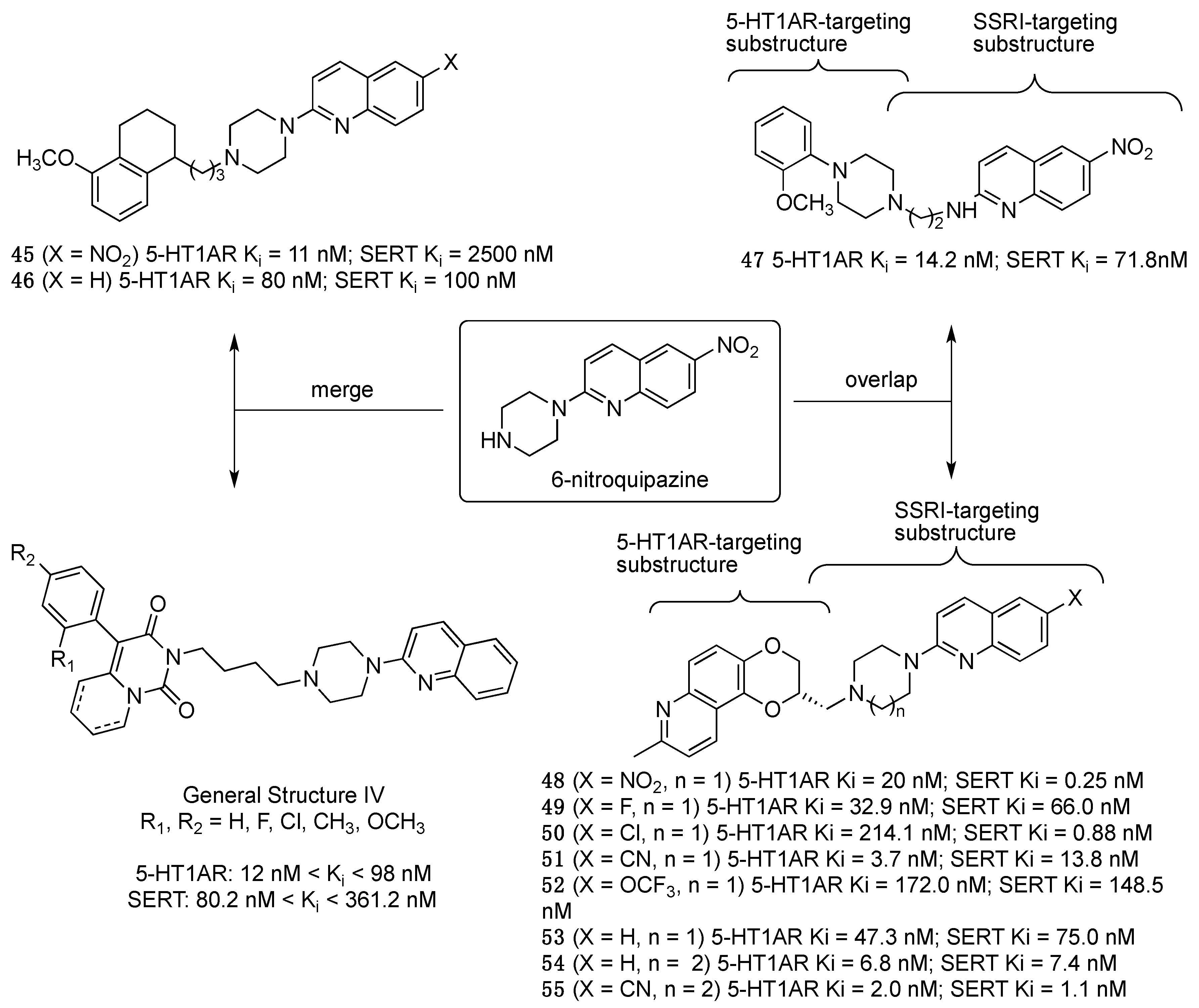


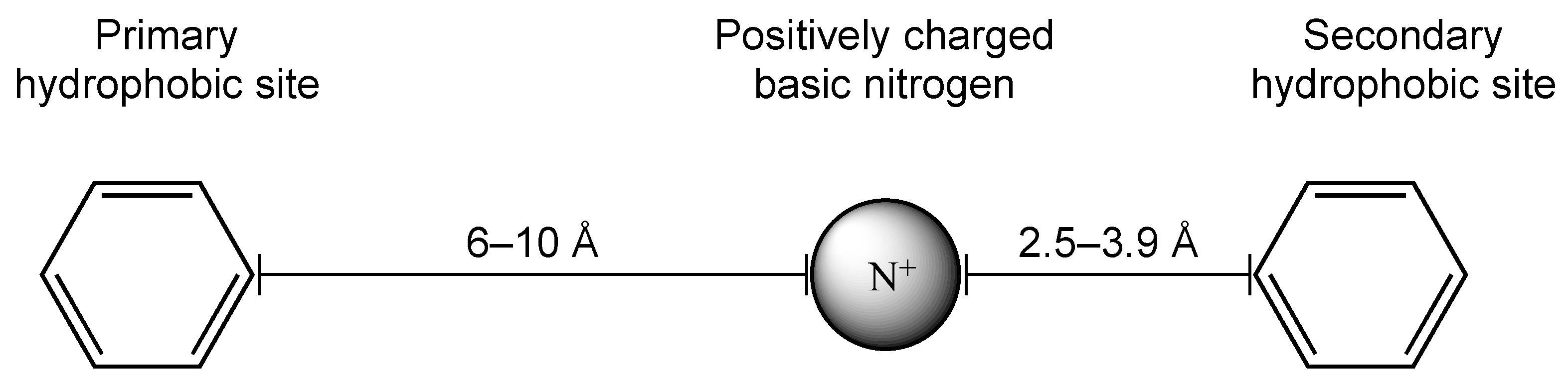

| Name | Structural Formula | 5-HT1AR Ki [nM] | 5-HT2AR Ki [nM] | 5-HT7R Ki [nM] | D2R Ki [nM] | Sigma 1 | Sigma 2 |
|---|---|---|---|---|---|---|---|
| Chlorpromazine | 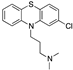 | 3115 | 3.2 | 21 | 4.8 | -- | -- |
| Haloperidol | 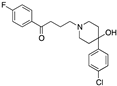 | 1202 | 46 | 378 | 3.7 | 48.7 | |
| Trifluperazine | 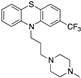 | 12,022 | 8.8 | 288.4 | 3.8 | 350 b | -- |
| Pimozide | 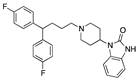 | 650 | 19 | 0.5 | 0.33 | 508 b | -- |
| Risperidone | 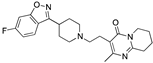 | 427 | 0.64 | 3.5 | 4.9 | 950 | -- |
| Olanzapine | 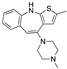 | 2063 | 9.2 | 157 | 21 | 5000 | -- |
| Aripiprazole | 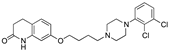 | 5.6 | 15.0 | 10.0 | 3.3 | -- | -- |
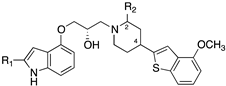 | |||||||
|---|---|---|---|---|---|---|---|
| Compd | R1 | R2 | Piperidine Chirality | 5-HT1AR Ki [nM] | SERT Ki [nM] | % Displacement of the Specific Radioligand Ex Vivo | |
| 5-HT1AR 1 | SERT 2 | ||||||
| 18 | H | H | N.A. 3 | 1.89 | 12.63 | 96 | 0 |
| 19 | CH3 | H | N.A. | 4.83 | 51.16 | N.D. 4 | N.D. |
| 20 | H | CH3 | 2R,4R | 2.76 | 14.71 | 72 | 30 |
| 21 | H | CH3 | 2S,4S | 14.45 | 13.59 | 60 | 12 |
| 22 | H | CH3 | 2S,4R | 3.64 | 0.27 | 87 | 89 |
| 23 | H | CH3 | 2R,4S | 8.47 | 1.15 | 67 | 92 |
| 24 | CH3 | CH3 | 2S,4R | 14.35 | 0.24 | 100 | 74 |
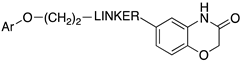 | |||||
|---|---|---|---|---|---|
| Compd | Ar | LINKER | 5-HT1AR pKi | IA 1 | SERT pKi |
| 58 | 4-indolyl | 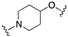 | 8.4 | 0.2 | 8.3 |
| 59 | 1-naphthyl | 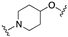 | 8.6 | 0.4 | 7.0 |
| 60 | 1-isoquinolinyl | 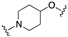 | 8.1 | 0.8 | 7.3 |
| 61 | 4-quinolinyl | 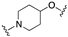 | 7.0 | N.D.2 | 7.0 |
| 62 | 8-quinolinyl | 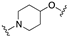 | 8.2 | 0.5 | 7.4 |
| 63 | 5-quinolinyl | 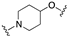 | 7.9 | 0.1 | 7.5 |
| 64 | 5-quinolinyl |  | 8.8 | 0.3 | 7.1 |
| 65 | 5-quinolinyl | 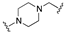 | 8.0 | 0.2 | 7.5 |
| 66 | 5-quinolinyl | 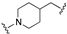 | 8.9 | 0.2 | 8.2 |
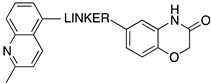 | |||||
|---|---|---|---|---|---|
| Compd | LINKER | 5-HT1AR pKi | 5-HT1BR pKi | 5-HT1DR pKi | SERT pKi |
| 67 (SB-6499115) | 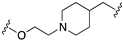 | 8.6 | 8.0 | 8.8 | 8.1 |
| 68 | 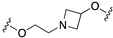 | 7.3 | 7.3 | 8.1 | 7.2 |
| 69 | 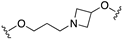 | 7.1 | 6.5 | 6.8 | N.D. |
| 70 | 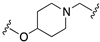 | <5.0 | <5.6 | 5.0 | 6.7 |
| 71 | 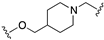 | <5.8 | <5.0 | 5.9 | 7.5 |
| 72 |  | 6.5 | 7.6 | 8.2 | 7.0 |
| 73 | 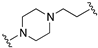 | 9.6 | 9.3 | 9.7 | 8.4 |
| 74 |  | 8.6 | 7.3 | 8.0 | 7.6 |
| 75 | 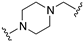 | 6.2 | <5.0 | 6.9 | 6.1 |
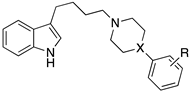 | ||||||
|---|---|---|---|---|---|---|
| Compd | X | R | Sigma 1 | Sigma 2 | 5-HT1AR Ki [nM] | 5-HT2AR Ki [nM] |
| 86 | N | 2-OCH3 | 14 | 21 | 17 | 150 |
| 87 | CH | 2-OCH3 | 4.5 | 3.3 | 56 | 83 |
| 88 | N | 4-F | 5.6 | 1.3 | 37 | 14 |
| 89 | CH | H | 1.5 | 0.48 | 110 | 25 |
| 90 | CH | 4-F | 1.4 | 4.0 | 27 | 34 |
| Receptor Affinity (pKi) | 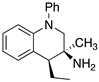 (±)-91 | 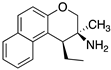 (±)-92 | 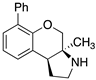 (±)-93 |
|---|---|---|---|
| 5-HT1AR | 7.13 | 6.5 | 6.7 |
| 5-HT2BR | 6.1 | 7.7 | 6.8 |
| 5-HT7R | 6.0 | 6.18 | 7.63 |
| Sigma 1 | 6.5 | 6.0 | 7.4 |
| Sigma 2 | 6.2 | 5.7 | 6.9 |
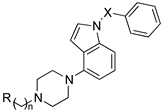 | ||||||
|---|---|---|---|---|---|---|
| Compd | R | N | X | 5-HT6R Ki [nM] | AChE IC50 [nM] | BChE IC50 [nM] |
| 96 | 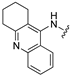 | 2 | SO2 | 10 | 43.1 | 16.8 |
| 97 | 6 | SO2 | 2.0 | 12.9 | 8.2 | |
| 98 | 2 | CH2 | 36 | 26 | 5 | |
| 99 | 6 | CH2 | 94 | 13 | 15 | |
| 100 | 8 | SO2 | 130 | 1.3 | 12.4 | |
| 101 | 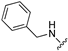 | 6 | SO2 | 2.0 | (37%) | 2384 |
| 102 | 6 | CH2 | 510 | 8217 | 1562 | |
| 103 | 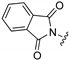 | 2 | CH2 | 17 | (<10%) | 6820 |
| 104 | 3 | CH2 | 149.8 | (<10%) | 3440 | |
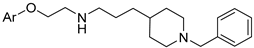 | ||||
|---|---|---|---|---|
| Compd | Ar | 5-HT6 Ki [nM] | AChE IC50 [nM] | BChE IC50 [nM] |
| 115 | 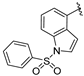 | 22 | 930 | 16 |
| 116 | 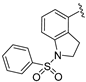 | 598 | 821 | 487 |
| 117 | 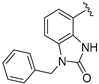 | 480 | 544 | 613 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghafir El Idrissi, I.; Santo, A.; Lacivita, E.; Leopoldo, M. Multitarget-Directed Ligands Hitting Serotonin Receptors: A Medicinal Chemistry Survey. Pharmaceuticals 2024, 17, 1238. https://doi.org/10.3390/ph17091238
Ghafir El Idrissi I, Santo A, Lacivita E, Leopoldo M. Multitarget-Directed Ligands Hitting Serotonin Receptors: A Medicinal Chemistry Survey. Pharmaceuticals. 2024; 17(9):1238. https://doi.org/10.3390/ph17091238
Chicago/Turabian StyleGhafir El Idrissi, Imane, Angela Santo, Enza Lacivita, and Marcello Leopoldo. 2024. "Multitarget-Directed Ligands Hitting Serotonin Receptors: A Medicinal Chemistry Survey" Pharmaceuticals 17, no. 9: 1238. https://doi.org/10.3390/ph17091238
APA StyleGhafir El Idrissi, I., Santo, A., Lacivita, E., & Leopoldo, M. (2024). Multitarget-Directed Ligands Hitting Serotonin Receptors: A Medicinal Chemistry Survey. Pharmaceuticals, 17(9), 1238. https://doi.org/10.3390/ph17091238