Methanolic Extract of Rhizophora mangle (Rhizophoraceae) Leaves: Phytochemical Characterization and Anthelmintic Evaluation against Schistosoma mansoni
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of MELRm Extract
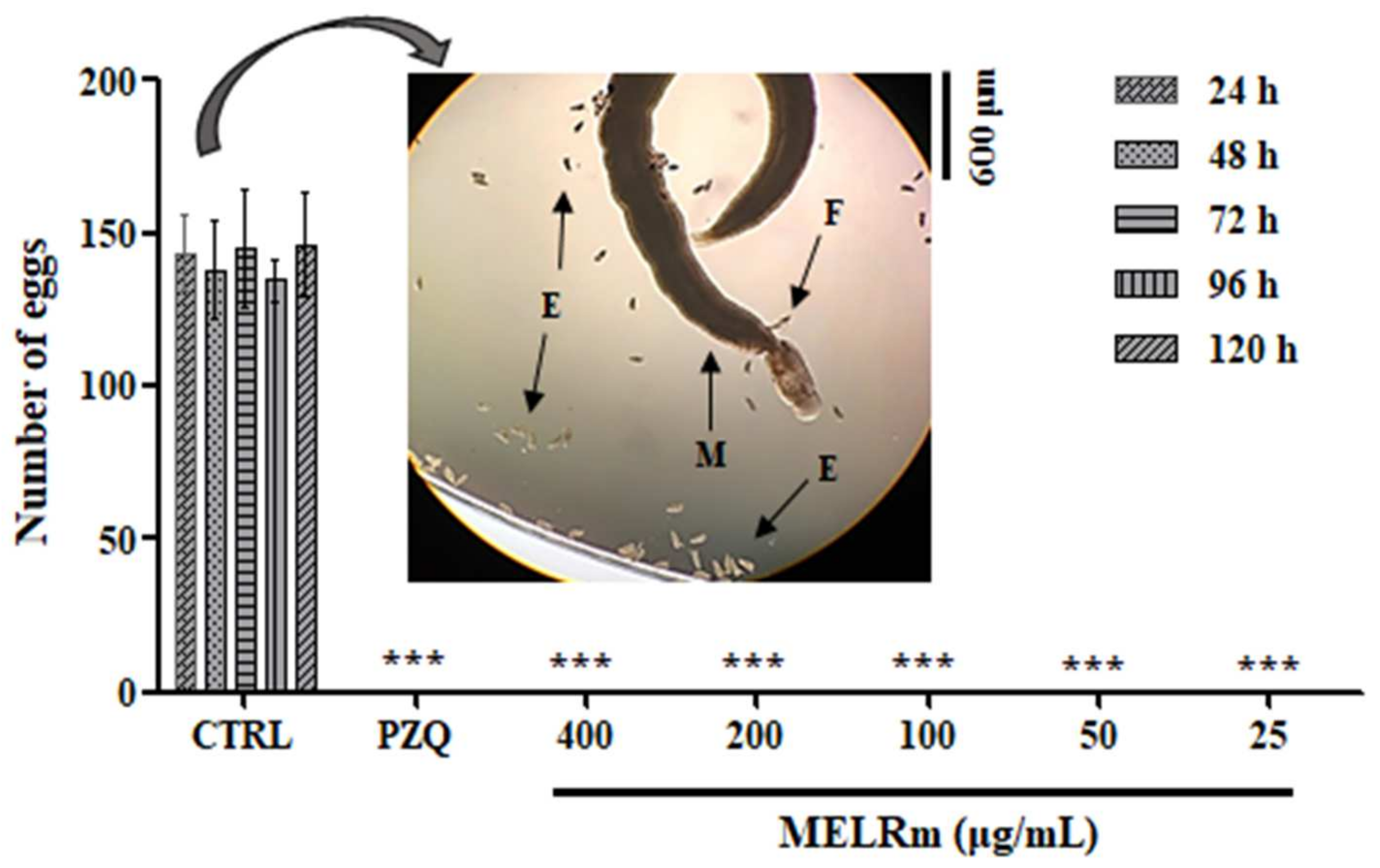
2.2. MELRm Induced Mortality, Altered Motility, and Caused Detachment of Adult S. mansoni Worms
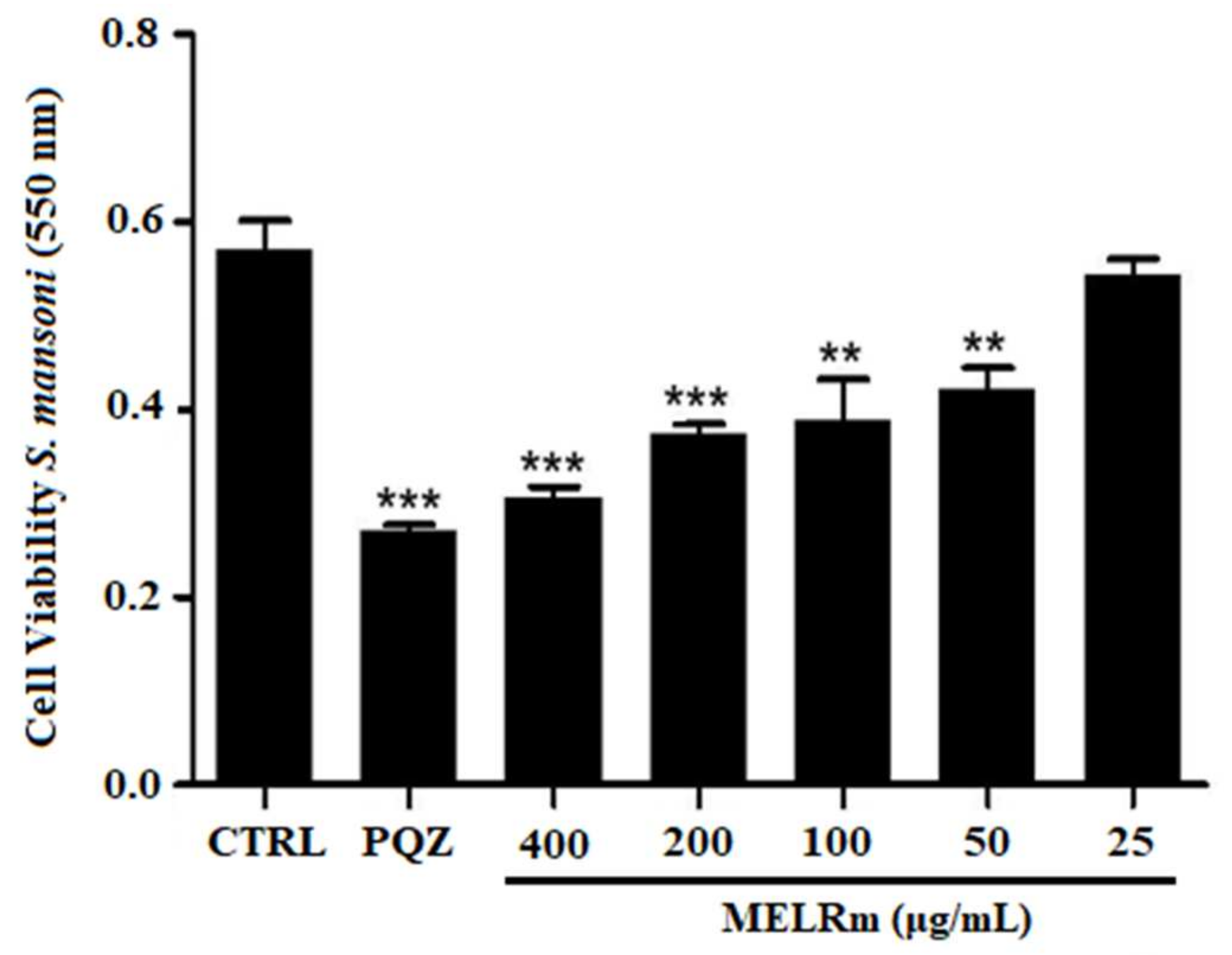
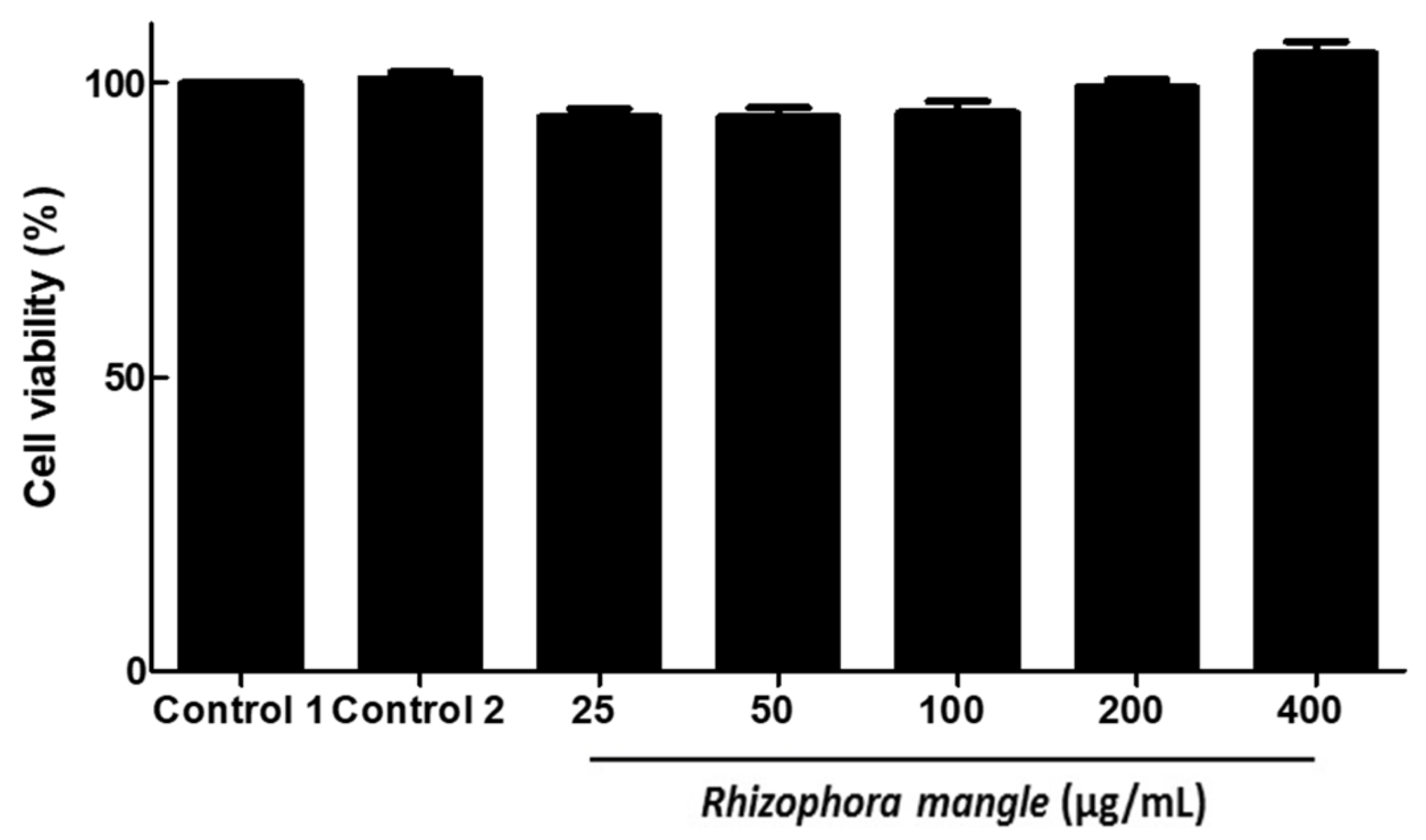
2.3. MELRm Induced Cell Death in Adult S. mansoni Worms but Showed No Cytotoxicity towards RAW 264.7 Cells
3. Discussion
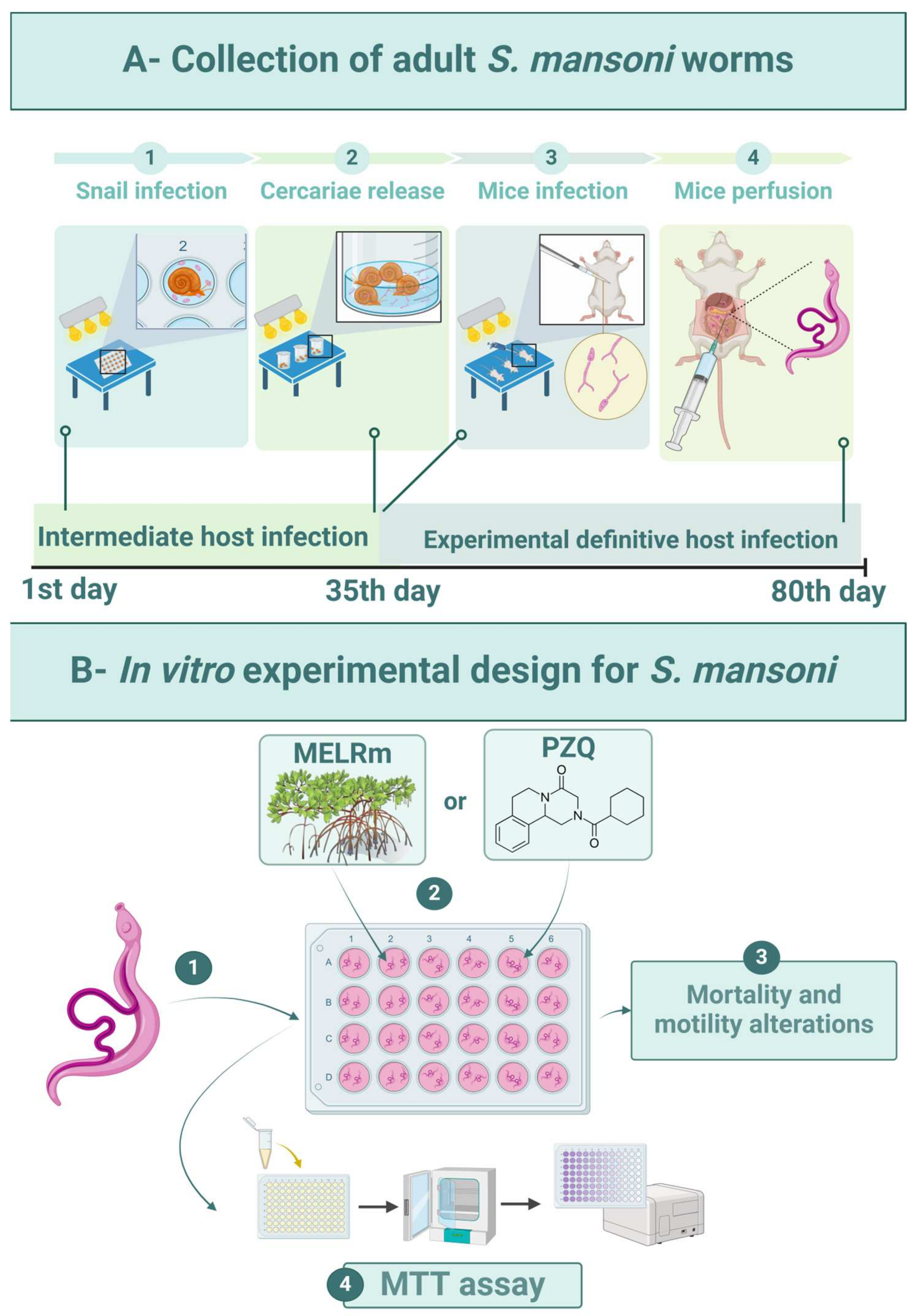
4. Materials and Methods
4.1. Plant Material
4.2. Drugs and Reagents
4.3. Extract Preparation
Phytochemical Characterization
4.4. Animals and Ethical Considerations
4.5. Recovery of Adult Worm Couples
4.6. In Vitro Susceptibility against to S. mansoni
4.6.1. Criteria of Schistosomicide Evaluation
Mortality and Changes in Motility
Cell Viability Assay of Couples of S. mansoni Worms
4.7. Cytotoxic Evaluation of MELRm
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva da Paz, W.; Dos Santos Reis, E.; Leal, I.B.; Barbosa, Y.M.; de Araújo, K.C.G.; de Jesus, A.R.; de Souza, C.D.F.; Dos Santos, A.D.; Bezerra-Santos, M. Basic and associated causes of schistosomiasis-related mortality in Brazil: A population-based study and a 20-year time series of a disease still neglected. J. Glob. Health 2021, 11, 04061. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 21 June 2024).
- Nogueira, R.A.; Lira, M.G.S.; Licá, I.C.L.; Frazão, G.C.C.G.; Dos Santos, V.A.F.; Filho, A.C.C.M.; Rodrigues, J.G.M.; Miranda, G.S.; Carvalho, R.C.; Nascimento, F.R.F. Praziquantel: An update on the mechanism of its action against schistosomiasis and new therapeutic perspectives. Mol. Biochem. Parasito 2022, 252, 111531. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.C.S.; da Silva, I.E.P.; de Araújo, H.D.A.; Barbosa, C.S. Malacological, socio-environmental evaluation, and evidence of local transmission and maintenance of schistosomiasis in an urban area of Northeast Brazil. Acta Trop. 2024, 252, 07145. [Google Scholar] [CrossRef]
- Ernould, J.C.; Ba, K.; Sellin, B. Increase of intestinal schistosomiasis after praziquantel treatment in a Schistosoma haematobium and Schistosoma mansoni mixed focus. Acta Trop. 1999, 73, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Pica-Mattoccia, L.; Doenhoff, M.J.; Valle, C.; Basso, A.; Troiani, A.R.; Liberti, P.; Festucci, A.; Guidi, A.; Cioli, D. Genetic analysis of decreased praziquantel sensitivity in a laboratory strain of Schistosoma mansoni. Acta Trop. 2009, 111, 82–85. [Google Scholar] [CrossRef]
- Wu, M.J.; Xu, B.; Guo, Y.W. Unusual Secondary Metabolites from the Mangrove Ecosystems: Structures, Bioactivities, Chemical, and Bio-Syntheses. Mar. Drugs 2022, 20, 535. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, Q.; Xu, J.; Li, M.Y.; Pan, J.Y.; Yang, M.H. Natural products from true mangrove flora: Source, chemistry and bioactivities. Nat. Prod. Rep. 2008, 25, 955. [Google Scholar] [CrossRef]
- Ramasubburayan, R.; Prakash, S.; Pitchiah, S.; Dhanraj, G. Antifouling activity and biodegradable potential of the bioactive metabolites isolated from mangrove Avicennia officinalis L. Nat. Prod. Res. 2024, 38, 1680–1686. [Google Scholar] [CrossRef]
- Ramalingam, V.; Rajaram, R. Enhanced antimicrobial, antioxidant and anticancer activity of Rhizophora apiculata: An experimental report. 3 Biotech 2018, 8, 200. [Google Scholar] [CrossRef]
- He, Z.; Feng, X.; Chen, Q.; Li, L.; Li, S.; Han, K.; Guo, Z.; Wang, J.; Liu, M.; Shi, C.; et al. Evolution of coastal forests based on a full set of mangrove genomes. Nat. Ecol. Evol. 2022, 6, 738–749. [Google Scholar] [CrossRef]
- Pan American Health Organization. Available online: https://www.paho.org/en/topics/schistosomiasis (accessed on 19 June 2024).
- Lima, K.O.O.; Tognella, M.M.P.; Cunha, S.R.; Andrade, H.A. Growth models of Rhizophora mangle L. seedlings in tropical southwestern Atlantic. Estuar. Coast. Shelf Sci. 2018, 207, 154–163. [Google Scholar] [CrossRef]
- de-Faria, F.M.; Almeida, A.C.; Luiz-Ferreira, A.; Takayama, C.; Dunder, R.J.; da Silva, M.A.; Salvador, M.J.; Abdelnur, P.V.; Eberlin, M.N.; Vilegas, W.; et al. Antioxidant Action of Mangrove Polyphenols against Gastric Damage Induced by Absolute Ethanol and Ischemia-Reperfusion in the Rat. Sci. World J. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- de Souza Mesquita, L.M.; Caria, C.R.E.P.; Santos, P.S.; Ruy, C.C.; da Silva Lima, N.; Moreira, D.K.T.; da Rocha, C.Q.; Murador, D.C.; de Rosso, V.V.; Gambero, A.; et al. Modulatory Effect of Polyphenolic Compounds from the Mangrove Tree Rhizophora mangle L. on Non-Alcoholic Fatty Liver Disease and Insulin Resistance in High-Fat Diet Obese Mice. Molecules 2018, 23, 2114. [Google Scholar] [CrossRef] [PubMed]
- de Souza Mesquita, L.M.; Rodrigues, C.F.B.; da Rocha, C.Q.; Bianchim, M.S.; Rodrigues, C.M.; de Oliveira, V.M.; Gaeta, H.H.; Belchor, M.N.; Toyama, M.H.; Vilegas, W. LC–ESI–IT-MS/MS and MALDI-TOF Approach: Identification of Natural Polymers from Rhizophora mangle Barks and Determination of Their Analgesic and Anti-inflammatory Properties. Nat. Prod. Bioprospect. 2019, 9, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.J.X.; Leal, L.B.; Sá, J.G.A.; Sabino, L.R.A.; Cavalcanti, I.M.F.S.D.; Silva, L.A.; Santana, E.S.; Fernandes, F.H.P.; C Filho, I.J.; Brandão, W.F.M.; et al. A preliminary study of cutaneous wound healing on the upper eyelid in a small Brazilian population using Rhizophora mangle-based cream. Anais da Academia Brasileira de Ciências 2024, 96, e20231143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Lin, Y.M.; Zhou, H.C.; Wei, S.D.; Chen, J.H. Condensed Tannins from Mangrove Species Kandelia candel and Rhizophora mangle and Their Antioxidant Activity. Molecules 2010, 15, 420–431. [Google Scholar] [CrossRef]
- Cruz, S.M.; Marroquín, N.; Alvarez, L.E.; Chang, D.E.; Cáceres, A. Evaluation of Mangrove (Rhizophora mangle L.) products as coloring, antimicrobial and antioxidant agents. Int. J. Phytocosmet. Nat. Ingred. 2015, 2, 12. [Google Scholar] [CrossRef]
- Alarcon-Aguilara, F.J.; Roman-Ramos, R.; Perez-Gutierrez, S.; Aguilar-Contreras, A.; Contreras-Weber, C.C.; Flores-Saenz, J.L. Study of the anti-hyperglycemic effect of plants used as antidiabetics. J. Ethnopharmacol. 1998, 61, 101–110. [Google Scholar] [CrossRef]
- Sánchez Perera, L.M.; Varcalcel, L.; Escobar, A.; Noa, M. Polyphenol and Phytosterol Composition in an Antibacterial Extract from Rhizophora mangle L. Bark. J. Herb. Pharmacother. 2008, 7, 107–128. [Google Scholar] [CrossRef]
- Baker, S.; Bisht, N.; Bhat, P.; Karthik, R.N.; Prasad, A.; Prasad, H.; Prasad, M.N.N. Phytogenic synthesis of nanoparticles from Rhizophora mangle and their bactericidal potential with DNA damage activity. Nano-Struct. Nano-Objects 2017, 10, 112–115. [Google Scholar] [CrossRef]
- Rodrigues Neto, A.A.; Gomes Júnior, P.P.; Silva, M.C.; Lima, C.S.A.; Yara, R.; Guimarães, E.B.; Santana, E.S.; Silva, L.A.D.; Lira, E.J.R.V.; Vieira, J.R.C. Evaluation of embryotoxic and embryostatic effects of the aqueous extract of Rhizophora mangle and tannic acid on eggs and larvae of Aedes aegypti. Anais da Academia Brasileira de Ciências 2018, 90, 2141–2148. [Google Scholar] [CrossRef]
- Mendes, R.J.A.; Pereira Filho, A.A.; Nogueira, A.J.L.; Araújo, K.R.F.; França, C.R.C.; Carvalho, I.B.; Silva, N.M.L.D.; Azevedo, A.S.; Rosa, I.G. Evaluation of molluscicidal activity of three mangrove species (Avicennia schaueriana, Laguncularia racemosa and Rhizophora mangle) and their effects on the bioactivity of Biomphalaria glabrata Say, 1818. Rev. Inst. Med. Trop. Sao Paulo 2018, 60, e7. [Google Scholar] [CrossRef]
- Silva, L.M.N.; França, W.W.M.; Santos, V.H.B.; Souza, R.A.F.; Silva, A.M.; Diniz, E.G.M.; Aguiar, T.W.A.; Rocha, J.V.R.; Souza, M.A.A.; Nascimento, W.R.C.; et al. Plumbagin: A Promising In Vivo Antiparasitic Candidate against Schistosoma mansoni and In Silico Pharmacokinetic Properties (ADMET). Biomedicines 2023, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Simoben, C.V.; Ntie-Kang, F.; Akone, S.H.; Sippl, W. Compounds from African Medicinal Plants with Activities Against Selected Parasitic Diseases: Schistosomiasis, Trypanosomiasis and Leishmaniasis. Nat. Prod. Bioprospect 2018, 8, 151–169. [Google Scholar] [CrossRef]
- Duarte Galhardo de Albuquerque, R.D.; Mahomoodally, M.F.; Lobine, D.; Suroowan, S.; Rengasamy, K.R. Botanical Products in the Treatment and Control of Schistosomiasis: Recent Studies and Distribution of Active Plant Resources According to Affected Regions. Biology 2020, 9, 223. [Google Scholar] [CrossRef]
- Patra, J.K.; Thatoi, H.N. Metabolic diversity and bioactivity screening of mangrove plants: A review. Acta Physiol. Plant 2011, 33, 1051–1061. [Google Scholar] [CrossRef]
- Costa, F.N.; da Silva, M.D.; Borges, R.M.; Leitão, G.G. Isolation of phenolics from Rhizophora mangle by combined counter-current chromatography and gel-filtration. Nat. Prod. Commun. 2014, 9, 1729–1731. [Google Scholar] [CrossRef] [PubMed]
- Nabeelah Bibi, S.; Fawzi, M.M.; Gokhan, Z.; Rajesh, J.; Nadeem, N.; RR, R.K.; RDDG, A.; Pandian, S.K. Ethnopharmacology, Phytochemistry, and Global Distribution of Mangroves—A Comprehensive Review. Mar. Drugs 2019, 17, 231. [Google Scholar] [CrossRef] [PubMed]
- Willian, N.; Syukri, S.; Zulhadjri, Z.; Arief, S. Marine plant mediated green synthesis of silver nanoparticles using mangrove Rhizophora stylosa: Effect of variable process and their antibacterial activity. F1000Research 2022, 10, 768. [Google Scholar] [CrossRef]
- Kandil, F.; Grace, M.; Seigler, D.; Cheeseman, J. Polyphenolics in Rhizophora mangle L. leaves and their changes during leaf development and senescence. Trees 2004, 18, 5. [Google Scholar] [CrossRef]
- da Silva Pontes, A.L.; Monteiro Leal, C.; Pereira Lucas, M.; Caamaño Muiño da Silva, G.; Braga Alves Peixoto, J.V.; Barbosa Succar, J.; Ribeiro Flores, V.; Neves Direito, I.C.; Ribeiro da Silva, A.J.; de Oliveira Chaves, F.; et al. Dereplication Tools for Rhizophora mangle Extracts from Different Mangrove Areas and their Potential Against Staphylococcus aureus. Chem. Biodivers. 2024, 21, e202400687. [Google Scholar] [CrossRef]
- Sánchez, J.; Melchor, G.; Martínez, G.; Escobar, A.; Faure, R. Antioxidant activity of Rhizophora mangle bark. Fitoterapia 2006, 77, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Kalasuba, K.; Miranti, M.; Rahayuningsih, S.R.; Safriansyah, W.; Syamsuri, R.R.P.; Farabi, K.; Oktavia, D.; Alhasnawi, A.N.; Doni, F. Red Mangrove (Rhizophora stylosa Griff.)—A Review of Its Botany, Phytochemistry, Pharmacological Activities, and Prospects. Plants 2023, 12, 2196. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Escandón-Rivera, S.M.; Torres-Valle, G.M.; Quijano, L. Phytochemical composition and chronic hypoglycemic effect of Rhizophora mangle cortex on STZ-NA-induced diabetic rats. Rev. Bras. Farmacogn. 2017, 27, 744–750. [Google Scholar] [CrossRef]
- Sormin, R.B.D.; Nendissa, D.M.; Mailoa, M.N.; Rieuwpassa, F.; Wenno, M.R. Antibacterial activity of Rhizophora apiculata extract originated from Inner Ambon Bay against selected pathogen bacteria. IOP Conf. Ser. Earth Environ. Sci. 2021, 797, 012017. [Google Scholar] [CrossRef]
- Kathiresan, K. A review of studies on Pichavaram mangrove, southeast India. Hydrobiologia 2000, 430, 185–205. [Google Scholar] [CrossRef]
- Tobar-Delgado, E.; Mejía-España, D.; Osorio-Mora, O.; Serna-Cock, L. Rutin: Family Farming Products’ Extraction Sources, Industrial Applications and Current Trends in Biological Activity Protection. Molecules 2023, 28, 5864. [Google Scholar] [CrossRef] [PubMed]
- El-Ashmawy, I.M.; Al-Wabel, N.A.; Bayad, A.E. Achillea fragrantissima, rich in flavonoids and tannins, potentiates the activity of diminazine aceturate against Trypanosoma evansi in rats. Asian Pac. J. Trop. Med. 2016, 9, 228–234. [Google Scholar] [CrossRef]
- Hamad, R.S. Rutin, a Flavonoid Compound Derived from Garlic, as a Potential Immunomodulatory and Anti-Inflammatory Agent against Murine Schistosomiasis mansoni. Nutrients 2023, 15, 1206. [Google Scholar] [CrossRef]
- Tasdemir, D.; Kaiser, M.; Brun, R.; Yardley, V.; Schmidt, T.J.; Tosun, F.; Rüedi, P. Antitrypanosomal and Antileishmanial Activities of Flavonoids and Their Analogues: In Vitro, In Vivo, Structure-Activity Relationship, and Quantitative Structure-Activity Relationship Studies. Antimicrob. Agents Chemother. 2006, 50, 1352–1364. [Google Scholar] [CrossRef]
- Cunha, F.; Tintino, S.R.; Figueredo, F.; Barros, L.; Duarte, A.E.; Vega Gomez, M.C.; Coronel, C.C.; Rolón, M.; Leite, N.; Sobral-Souza, C.E.; et al. HPLC-DAD phenolic profile, cytotoxic and anti-kinetoplastidae activity of Melissa officinalis. Pharm. Biol. 2016, 54, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Silva, F.; Inacio, J.D.; Canto-Cavalheiro, M.M.; Almeida-Amaral, E.E. Reactive Oxygen Species Production by Quercetin Causes the Death of Leishmania amazonensis Intracellular Amastigotes. J. Nat. Prod. 2013, 76, 1505–1508. [Google Scholar] [CrossRef]
- Silva, E.R.; Brogi, S.; Lucon-Júnior, J.F.; Campiani, G.; Gemma, S.; Maquiaveli, C. Dietary polyphenols rutin, taxifolin and quercetin related compounds target Leishmania amazonensis arginase. Food Funct. 2019, 10, 3172–3180. [Google Scholar] [CrossRef] [PubMed]
- Alemán Gainza, Y.; Sánchez Perera, L.; Tania, P.; Rodriguez Perdomo, Y.; Olivares, J.L.; Rodríguez, J.G. Actividad larvicida de extractos de Rhizophora mangle l. contra estrongílidos gastrointestinales de ovinos larvicidal activity of extracts from Rhizophora mangle l. against gastrointestinal strongylid of sheep. Rev. Salud Anim. 2011, 33, 111–115. [Google Scholar]
- de Souza Silva, M.S.; Dos Santos, M.L.M.F.; da Silva, A.M.; França, W.W.M.; Araújo, S.B.; da Silva, R.L.; do Nascimento, W.R.C.; da Silva Santos, N.P.; da Cruz Filho, I.J.; de Azevedo Albuquerque, M.C.P.; et al. Sanguinarine: An alkaloid with promising in vitro and in vivo antiparasitic activity against different developmental stages of Schistosoma mansoni and in silico pharmacokinetic properties (ADMET). Parasitol. Res. 2024, 123, 2. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Hussain, K.; Abbas, R.; Abbas, A.; Samiullah, K.; Ahmed, T.; Siddique, F.; Mohsin, M.; Rehman, A.; Rahman, A.; Waqas, M. Anticoccidial potential of Ageratum conyzoides and its effect on Blood parameters of experimentally infected Broiler Chickens. J. Hell. Vet. Med. Soc. 2021, 72, 3085–3090. [Google Scholar] [CrossRef]
- Mohsin, M.; Aleem, M.T.; Goraya, M.U.; Aguilar-Marcelino, L.; Abbas, R.Z.; Abbas, A. Editorial: Natural products and pseudo-natural products against veterinary disease-causing microorganisms. Front. Vet. Sci. 2024, 11, 1429587. [Google Scholar] [CrossRef]
- Thao, N.; Linh, K.; Quan, N.; Trung, V.; Binh, P.; Nguyen, C.; Nam, N.; Van Thanh, N. Cytotoxic metabolites from the leaves of the mangrove Rhizophora apiculata. Phytochem. Lett. 2022, 47, 51–55. [Google Scholar] [CrossRef]
- Santana, E.; Leal, L.; da Silva, L.; Barbosa, I.; Melo, C.; Santos, D.; Vieira, J. Association of Rhizophora mangle and ascorbic acid in hydrogels: Evaluation of cytotoxic and immunomodulatory effects. Braz. J. Pharm. Sci. 2023, 59, e20179. [Google Scholar] [CrossRef]
- Sá, J.; Brandão, W.; Santana, M.; da Silva, L.; Santana, E.; Silva, E.; de Carvalho, E.; Vieira, J. Avaliação da citotoxicidade e caracterização do perfil fitoquímico de Rhizophora mangle l. Do mangue brasileiro. In Ciências da Saúde e suas Descobertas Científicas; Seven Editora: São José dos Pinhais, Brazil, 2023. [Google Scholar] [CrossRef]
- Mena-Rejon, G.; Caamal-Fuentes, E.; Cantillo-Ciau, Z.; Cedillo-Rivera, R.; Flores-Guido, J.; Moo-Puc, R. In vitro cytotoxic activity of nine plants used in Mayan traditional medicine. J. Ethnopharmacol. 2009, 121, 462–465. [Google Scholar] [CrossRef]
- Youssef, A.M.M.; Maaty, D.A.M.; Al-Saraireh, Y.M. Phytochemistry and Anticancer Effects of Mangrove (Rhizophora mucronata Lam.) Leaves and Stems Extract against Different Cancer Cell Lines. Pharmaceuticals 2022, 16, 4. [Google Scholar] [CrossRef]
- Nerys, L.; Jacob, I.; Silva, P.; da Silva, A.; Macário, A.; Rocha, W.; Pereira, D.; Abreu, A.; Silva, R.; Filho, I.; et al. Photoprotective, biological activities and chemical composition of the non-toxic hydroalcoholic extract of Clarisia racemosa with cosmetic and pharmaceutical applications. Ind. Crops Prod. 2022, 180, 114762. [Google Scholar] [CrossRef]
- Olivier, L.; Stirewalt, M.A. An efficient method for exposure of mice to cercariae of Schistosoma mansoni. J. Parasitol. 1952, 38, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Smithers, S.R.; Terry, R.J. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology 1965, 55, 695–700. [Google Scholar] [CrossRef]
- Araújo, H.D.A.; Aires, A.L.; Soares, C.L.R.; Brito, T.G.S.; Nascimento, W.M.; Martins, M.C.B.; Silva, T.G.; Brayner, F.A.; Alves, L.C.; Silva, N.H.; et al. Usnic acid potassium salt from Cladonia substellata (Lichen): Synthesis, cytotoxicity and in vitro anthelmintic activity and ultrastructural analysis against adult worms of Schistosoma mansoni. Acta Trop. 2019, 192, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Araújo, H.D.A.; Silva, N.H.; Albuquerque, M.C.P.A.; Aires, A.L.; Lima, V.L.M. Potassium usnate, a water-soluble usnic acid salt, shows enhanced activity against Schistosoma mansoni in vitro. Exp. Parasitol. 2020, 208, 107779. [Google Scholar] [CrossRef]
- Aires, A.L.; Ximenes, E.C.; Silva, R.A.; Barbosa, V.X.; Góes, A.J.; Peixoto, C.A.; Souza, V.M.; Albuquerque, M.C. Ultrastructural analysis of β-lapachone-induced surface membrane damage in male adult Schistosoma mansoni BH strain worms. Exp. Parasitol. 2014, 142, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari Nejad, A.S.; Fotouhi, F.; Mehrbod, P.; Keshavarz, M.; Alikhani, M.Y.; Ghaemi, A. Oncolytic effects of Hitchner B1 strain of newcastle disease virus against cervical cancer cell proliferation is mediated by the increased expression of cytochrome C, autophagy and apoptotic pathways. Microb. Pathog. 2020, 147, 104438. [Google Scholar] [CrossRef] [PubMed]
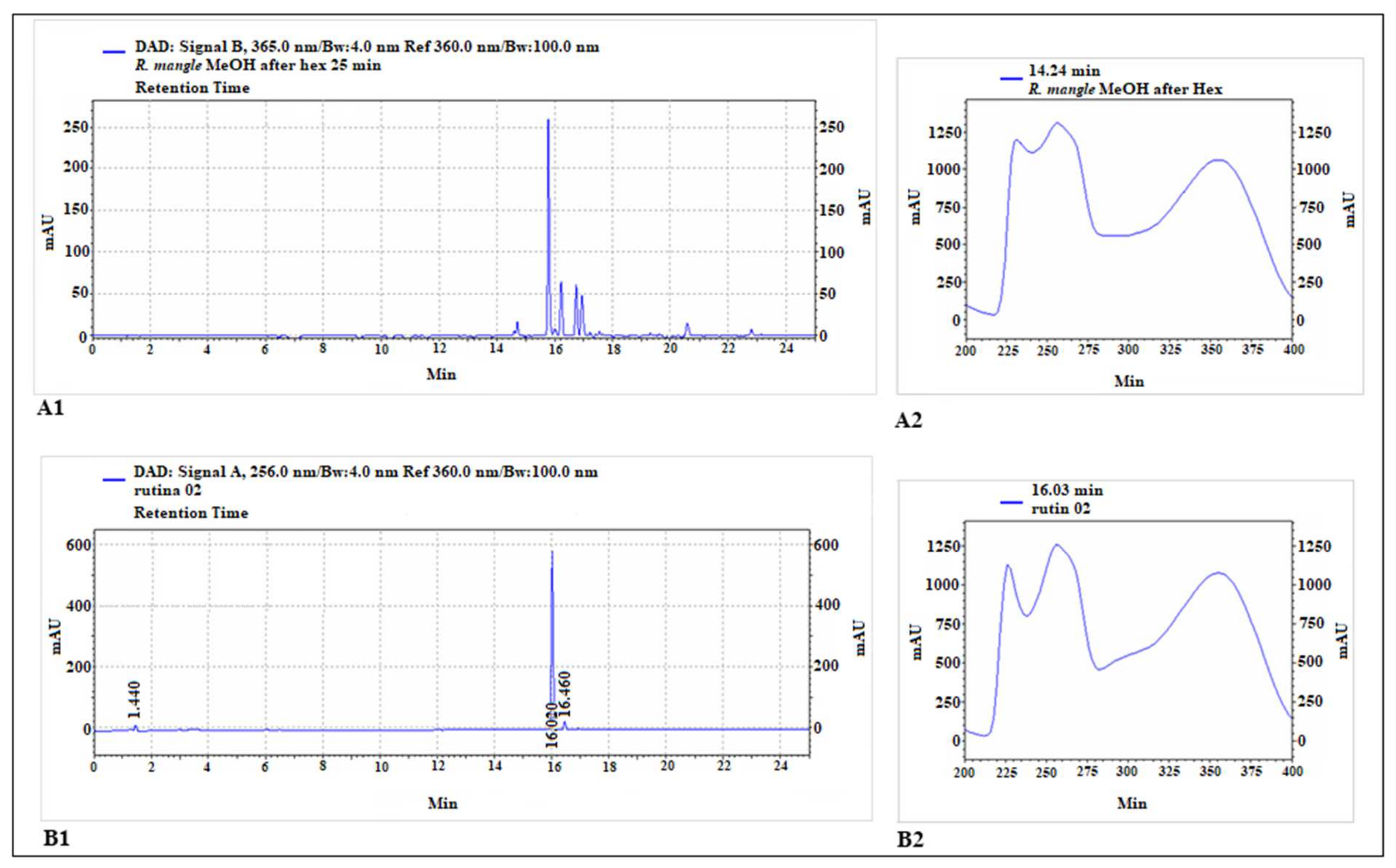

| Peak | Compound | Class | Rt, min * |
|---|---|---|---|
| 1 | p-Coumaric acid | Phenolic acid | 14.7 |
| 2 | Rutin | Flavonoid | 15.8 |
| 3 | Elargic acid | Polyphenol | 16.2 |
| 4 | Quercetin | Flavonoid | 16.6 |
| 5 | Apigenin | Flavonoid | 17.1 |
| 6 | Geranium | Tannin | 20.6 |
| Groups Score | Percentage of Worms (%) in Each Motility Score after Incubation | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | 120 h | ||||||||||||||||
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | |
| Control 1 | 16 ± 0.0 (100%) | 16 ± 0.0 (100%) | 16 ± 0.0 (100%) | 16 ± 0.0 (100%) | 16 ± 0.0 (100%) | |||||||||||||||
| Control 2 | 16 ± 0.0 (100%) | 16 ± 0.0 (100%) | 16 ± 0.0 (100%) | 16 ± 0.0 (100%) | 16 ± 0.0 (100%) | |||||||||||||||
| PZQ | ||||||||||||||||||||
| 10 µM | 16 ± 0.0 (100%) | 16 ± 0.0 (100%) | 16 ± 0.0 (100%) | 16 ± 0.0 (100%) | 16 ± 0.0 (100%) | |||||||||||||||
| MELRm μg/mL | ||||||||||||||||||||
| 400 | 13 ± 1.5 (81.25%) | 3 ± 1.0 (18.75%) | 16 ± 0.0 (100%) | 9 ± 0.5 (56.25%) | 7 ± 1.0 (43.75) | 11 ± 2.0 (68.75%) | 5 ± 1.0 (31.25%) | 12 ± 0.5 (75%) | 4 ± 0.0 (25%) | |||||||||||
| 200 | 6 ± 1.5 (37.5%) | 10 ± 2.0 (62.5%) | 4 ± 0.0 (25%) | 12 ± 0.0 (75%) | 16 ± 0.0 (100%) | 9 ± 1.5 (56.25%) | 7 ± 1.0 (43.75%) | 10 ± 0.0 (62.5%) | 6 ± 0.0 (37.5%) | |||||||||||
| 100 | 4 ± 2.0 (25%) | 12 ± 1.5 (75%) | 12 ± 1.0 (75%) | 4 ± 0.5 (25%) | 16 ± 0.0 (100%) | 5 ± 1.0 (43.75%) | 11 ± 1.5 (56.25%) | 8 ± 0.0 (50%) | 8 ± 0.0 (50%) | |||||||||||
| 50 | 16 ± 0.0 (100%) | 8 ± 0.0 (50%) | 8 ± 0.0 (50%) | 8 ± 0.0 (50%) | 8 ± 0.0 (50%) | 14 ± 3.0 (87.5%) | 2 ± 0.0 (12.5%) | 16 ± 0.0 (100%) | ||||||||||||
| 25 | 16 ± 0.0 (100%) | 16 ± 0.0 (100%) | 16 ± 0.0 (100%) | 5 ± 1.0 (43.75%) | 11 ± 1.0 (56.25%) | 16 ± 0.0 (100%) | ||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
França, W.W.M.; Filho, S.D.M.; Cavalcante, L.A.O.; Gomes, M.A.A.S.; Gonçalves, M.T.V.; Diniz, E.G.M.; Nascimento, W.R.C.; Neto, R.G.L.; Albuquerque, M.C.P.A.; Filho, I.J.C.; et al. Methanolic Extract of Rhizophora mangle (Rhizophoraceae) Leaves: Phytochemical Characterization and Anthelmintic Evaluation against Schistosoma mansoni. Pharmaceuticals 2024, 17, 1178. https://doi.org/10.3390/ph17091178
França WWM, Filho SDM, Cavalcante LAO, Gomes MAAS, Gonçalves MTV, Diniz EGM, Nascimento WRC, Neto RGL, Albuquerque MCPA, Filho IJC, et al. Methanolic Extract of Rhizophora mangle (Rhizophoraceae) Leaves: Phytochemical Characterization and Anthelmintic Evaluation against Schistosoma mansoni. Pharmaceuticals. 2024; 17(9):1178. https://doi.org/10.3390/ph17091178
Chicago/Turabian StyleFrança, Wilza W. M., Sérgio D. Magalhães Filho, Lucas A. O. Cavalcante, Mary A. A. S. Gomes, Maria T. V. Gonçalves, Emily G. M. Diniz, Wheverton R. C. Nascimento, Reginaldo G. Lima Neto, Mônica C. P. A. Albuquerque, Iranildo J. Cruz Filho, and et al. 2024. "Methanolic Extract of Rhizophora mangle (Rhizophoraceae) Leaves: Phytochemical Characterization and Anthelmintic Evaluation against Schistosoma mansoni" Pharmaceuticals 17, no. 9: 1178. https://doi.org/10.3390/ph17091178
APA StyleFrança, W. W. M., Filho, S. D. M., Cavalcante, L. A. O., Gomes, M. A. A. S., Gonçalves, M. T. V., Diniz, E. G. M., Nascimento, W. R. C., Neto, R. G. L., Albuquerque, M. C. P. A., Filho, I. J. C., Araújo, H. D. A., Aires, A. L., & Vieira, J. R. C. (2024). Methanolic Extract of Rhizophora mangle (Rhizophoraceae) Leaves: Phytochemical Characterization and Anthelmintic Evaluation against Schistosoma mansoni. Pharmaceuticals, 17(9), 1178. https://doi.org/10.3390/ph17091178









