Analgesic Effect of Human Placenta Hydrolysate on CFA-Induced Inflammatory Pain in Mice
Abstract
1. Introduction
2. Results
2.1. Antinociceptive Effect of HPH in CFA-Induced Cold, Mechanical Allodynia
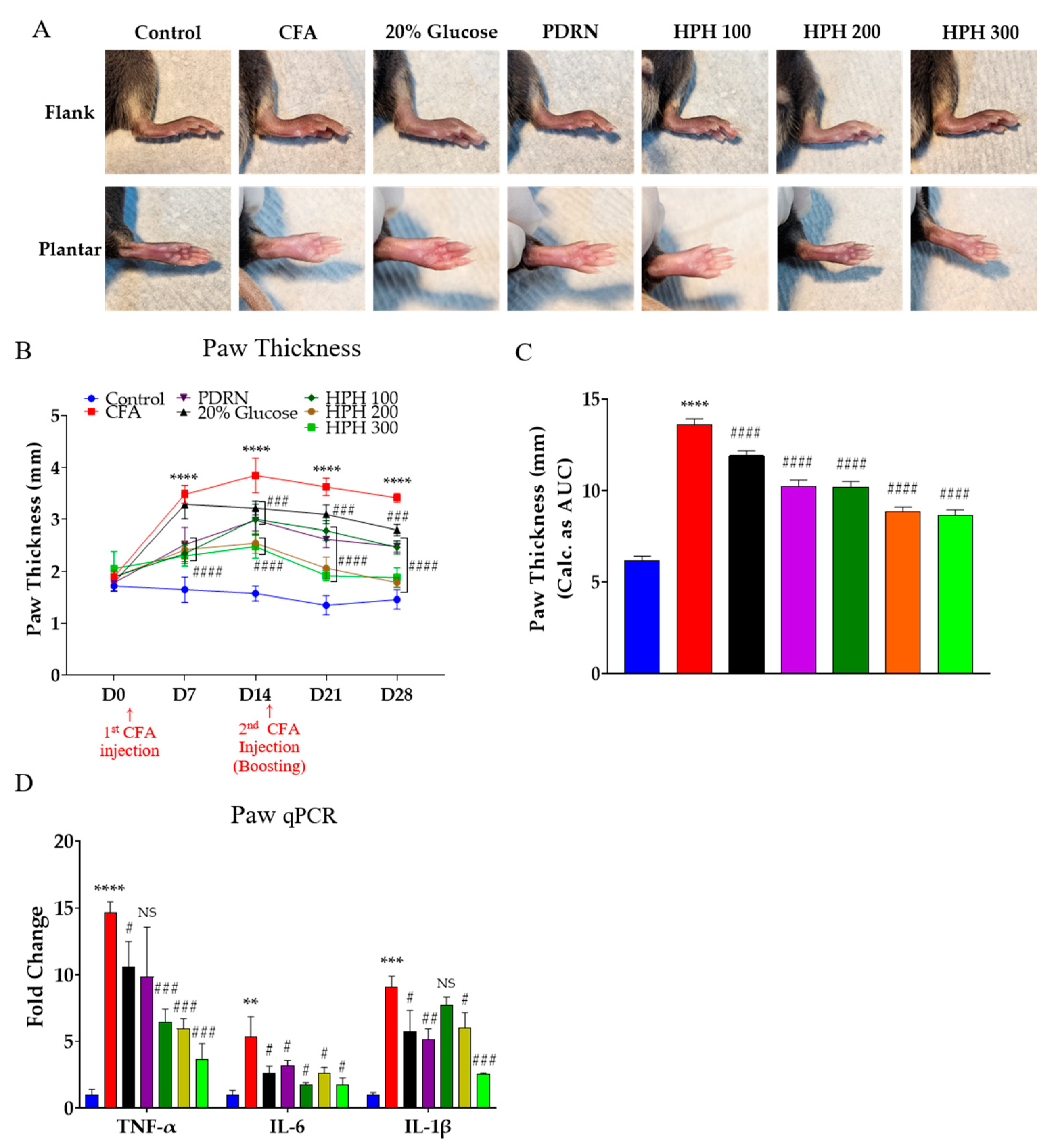
2.2. HPH Decreases Paw Thickness and Pro-Inflammatory Cytokines Increased by CFA Injection
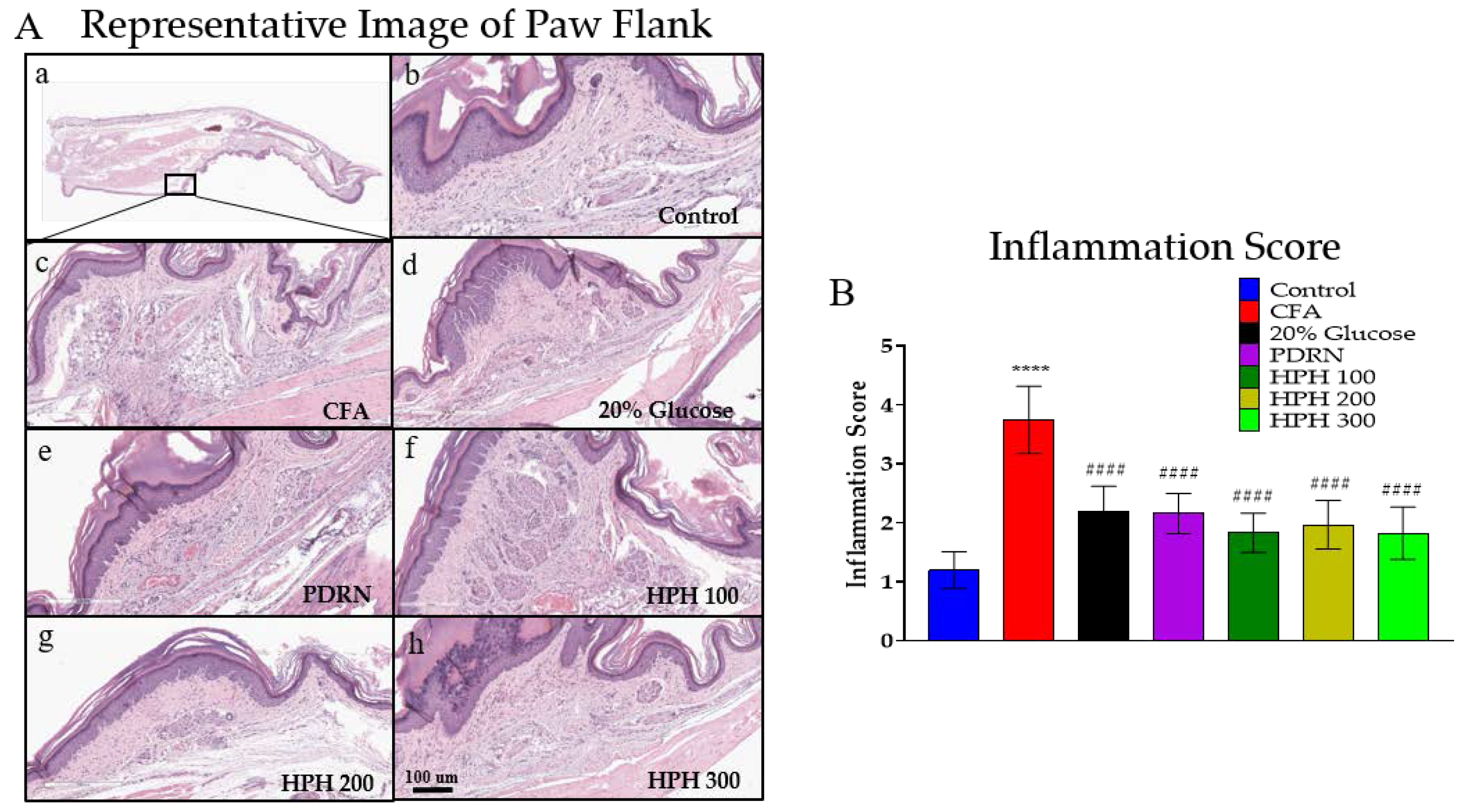
2.3. Ameliorating Effect of HPH on CFA-Induced Histological Damage
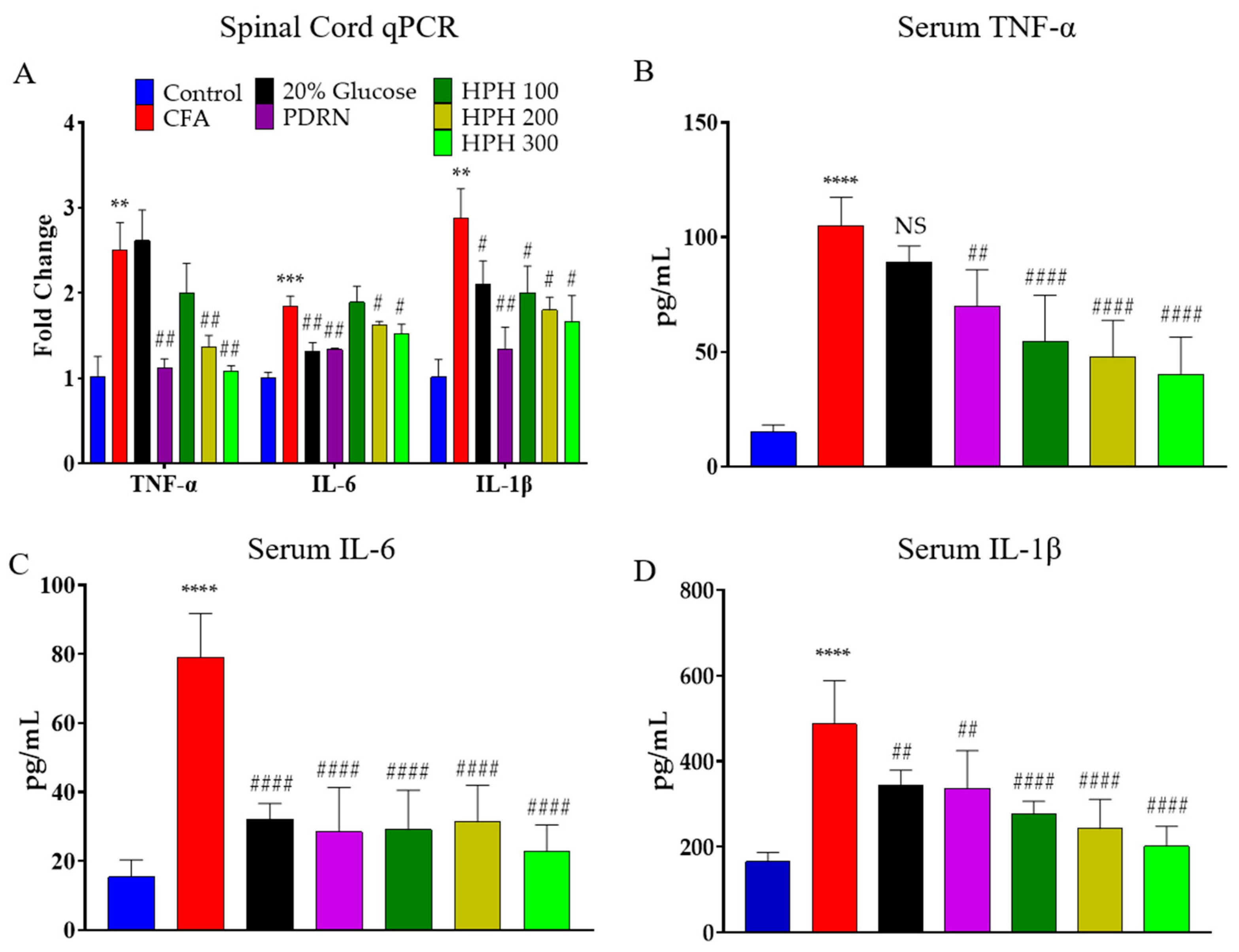
2.4. mRNA Expression of Pro-Inflammatory Cytokine Using qPCR in Spinal Cord and Levels of Cytokine of Serum
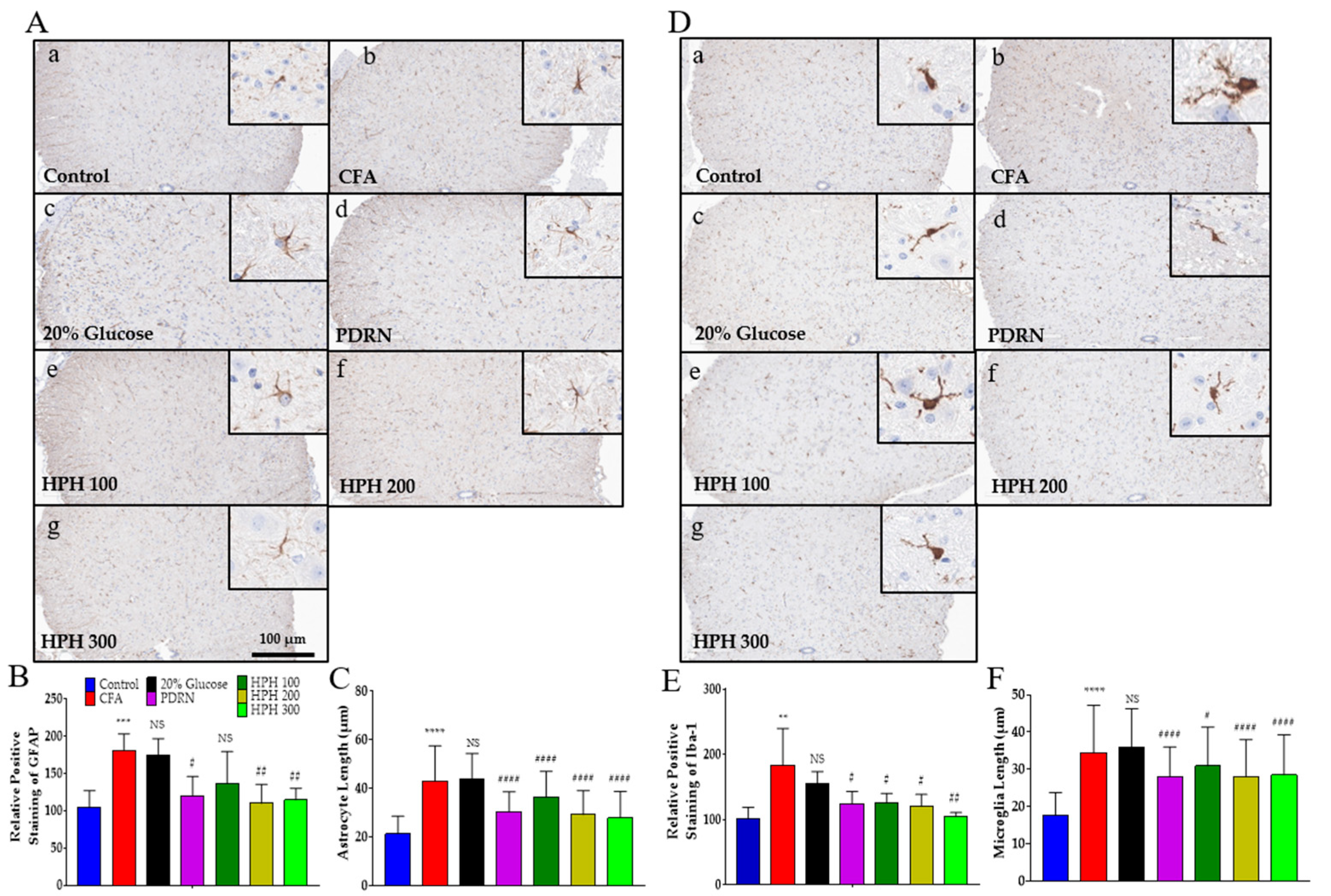
2.5. Glial Cell Activation
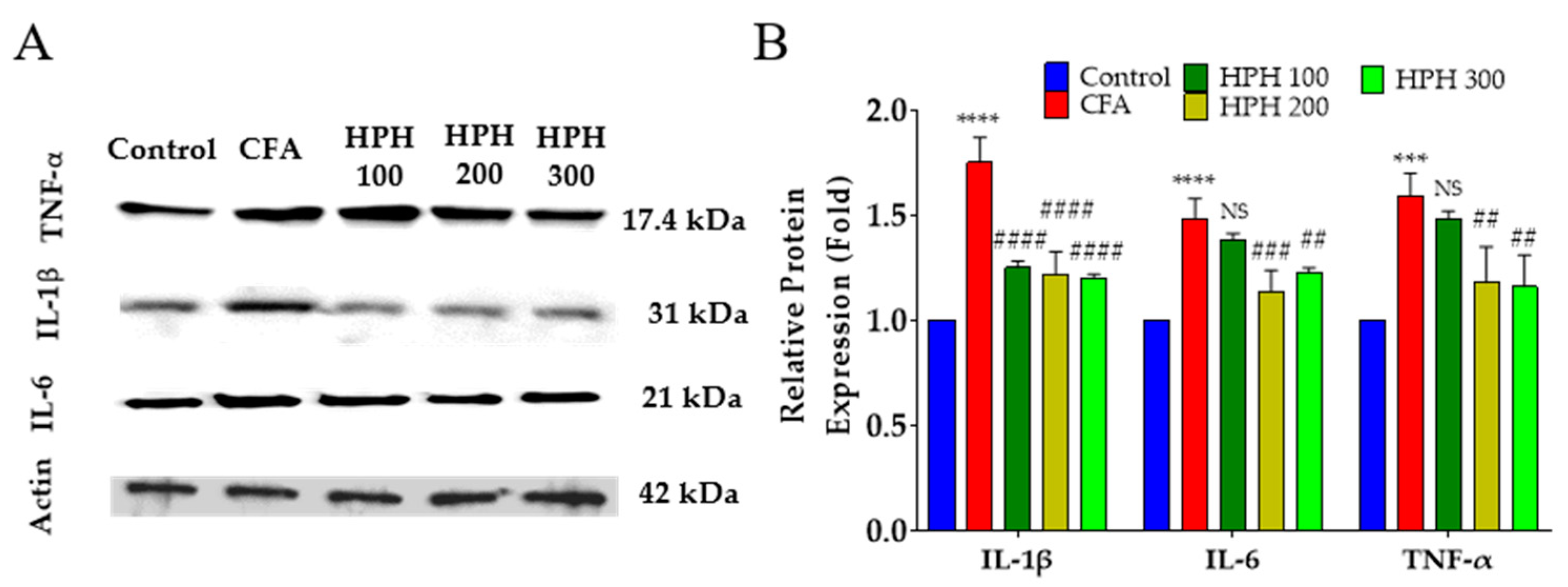
2.6. Protein Expression of Pro-Inflammatory Cytokines Using Western Blot in Spinal Cord
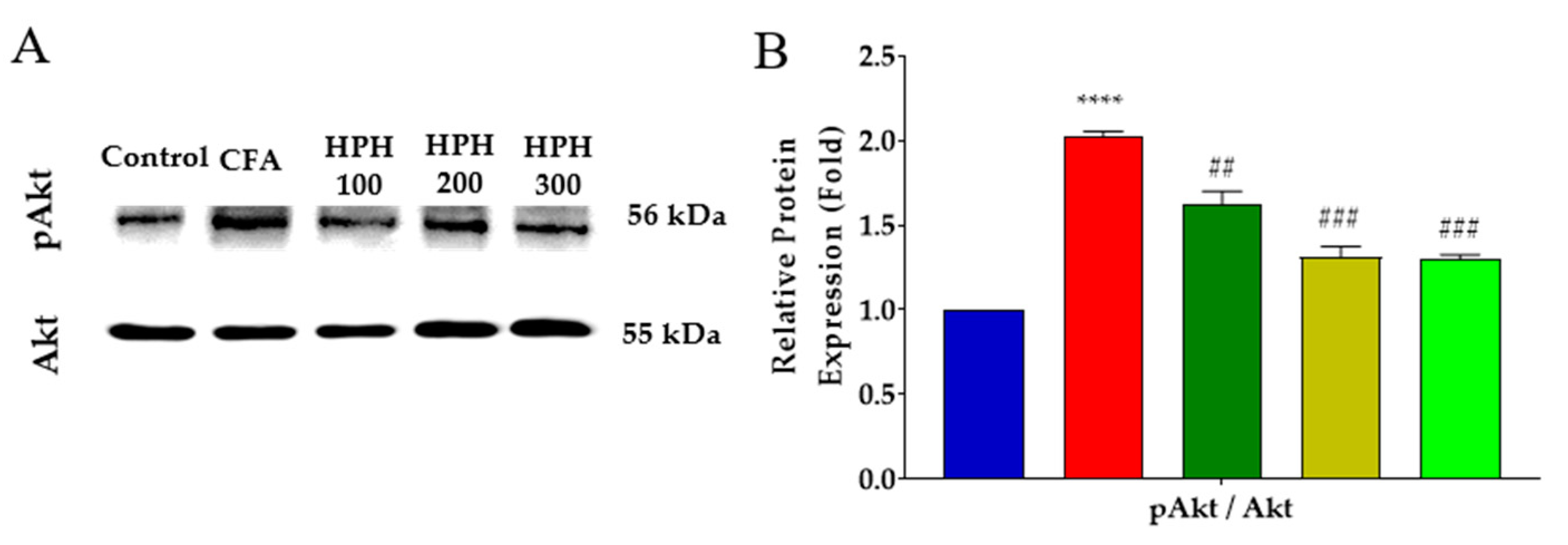
2.7. Decreased Expression of Phosphorylated Akt in the Spinal Cord after HPH Administration
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Establishment of an Inflammatory Pain Model Using CFA Injection
4.4. Behavioral Assessment
4.5. Gene Expression Using Gene-Specific Primers
4.6. Western Blot
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Hematoxylin and Eosin Staining and Inflammation Scoring
4.9. Immunohistochemistry Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patapoutian, A.; Tate, S.; Woolf, C.J. Transient receptor potential channels: Targeting pain at the source. Nat. Rev. Drug Discov. 2009, 8, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C.; Lindenlaub, T.; Teuteberg, P.; Schäfers, M.; Hartung, T.; Toyka, K.V. Anti-TNF-neutralizing antibodies reduce pain-related behavior in two different mouse models of painful mononeuropathy. Brain Res. 2001, 913, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Fongang, A.L.M.; Nguemfo, E.L.; Nangue, Y.D.; Zangueu, C.B.; Fouokeng, Y.; Azebaze, A.G.B.; Llorent-Martínez, E.J.; Fernández-de Córdova, M.L.; Dongmo, A.B.; Vierling, W. Antinociceptive and anti-inflammatory effects of the methanolic stem bark extract of Antrocaryon klaineanum Pierre (Anacardiaceae) in mice and rat. J. Ethnopharmacol. 2017, 203, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Milligan, E.; Zapata, V.; Schoeniger, D.; Chacur, M.; Green, P.; Poole, S.; Martin, D.; Maier, S.; Watkins, L. An initial investigation of spinal mechanisms underlying pain enhancement induced by fractalkine, a neuronally released chemokine. Eur. J. Neurosci. 2005, 22, 2775–2782. [Google Scholar] [CrossRef]
- Cunha, F.Q.; Poole, S.; Lorenzetti, B.B.; Ferreira, S.H. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br. J. Pharmacol. 1992, 107, 660–664. [Google Scholar] [CrossRef]
- Ferreira, S.H.; Lorenzetti, B.B.; Bristow, A.F.; Poole, S. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature 1988, 334, 698–700. [Google Scholar] [CrossRef]
- Verri, W.A., Jr.; Cunha, T.M.; Parada, C.A.; Poole, S.; Cunha, F.Q.; Ferreira, S.H. Hypernociceptive role of cytokines and chemokines: Targets for analgesic drug development? Pharmacol. Ther. 2006, 112, 116–138. [Google Scholar] [CrossRef]
- Uçeyler, N.; Sommer, C. Cytokine regulation in animal models of neuropathic pain and in human diseases. Neurosci. Lett. 2008, 437, 194–198. [Google Scholar] [CrossRef]
- Üçeyler, N.; Sommer, C. Cytokine-induced pain: Basic science and clinical implications. Rev. Analg. 2007, 9, 87–103. [Google Scholar] [CrossRef]
- Liu, L.R.; Liu, J.C.; Bao, J.S.; Bai, Q.Q.; Wang, G.Q. Interaction of Microglia and Astrocytes in the Neurovascular Unit. Front. Immunol. 2020, 11, 1024. [Google Scholar] [CrossRef]
- Erta, M.; Quintana, A.; Hidalgo, J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012, 8, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Gajtkó, A.; Bakk, E.; Hegedűs, K.; Ducza, L.; Holló, K. IL-1β Induced Cytokine Expression by Spinal Astrocytes Can Play a Role in the Maintenance of Chronic Inflammatory Pain. Front. Physiol. 2020, 11, 543331. [Google Scholar] [CrossRef]
- Ji, R.-R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- Donnelly, C.R.; Andriessen, A.S.; Chen, G.; Wang, K.; Jiang, C.; Maixner, W.; Ji, R.-R. Central nervous system targets: Glial cell mechanisms in chronic pain. Neurotherapeutics 2020, 17, 846–860. [Google Scholar] [CrossRef]
- Grace, P.M.; Hutchinson, M.R.; Maier, S.F.; Watkins, L.R. Pathological pain and the neuroimmune interface. Nat. Rev. Immunol. 2014, 14, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Yekkirala, A.S.; Roberson, D.P.; Bean, B.P.; Woolf, C.J. Breaking barriers to novel analgesic drug development. Nat. Rev. Drug Discov. 2017, 16, 545–564. [Google Scholar] [CrossRef]
- Mochizuki, H.; Kada, T. Antimutagenic action of mammalian placental extracts on mutations induced in Escherichia coli by UV radiation, γ-rays and N-methyl-N′-nitro-N-nitrosoguanidine. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1982, 95, 457–474. [Google Scholar] [CrossRef]
- Banerjee, K.; Bishayee, A.; Chatterjee, M. Anti-inflammatory effect of human placental extract: A biochemical mechanistic approach. Riv. Eur. Sci. Med. Farmacol. = Eur. Rev. Med. Pharmacol. Sci. = Rev. Eur. Sci. Med. Pharmacol. 1992, 14, 361–366. [Google Scholar]
- Liu, K.-X.; Kato, Y.; Kaku, T.-i.; Sugiyama, Y. Human placental extract stimulates liver regeneration in rats. Biol. Pharm. Bull. 1998, 21, 44–49. [Google Scholar] [CrossRef]
- Jung, J.; Lee, H.-J.; Lee, J.M.; Na, K.-H.; Hwang, S.-G.; Kim, G.J. Placenta extract promote liver regeneration in CCl4-injured liver rat model. Int. Immunopharmacol. 2011, 11, 976–984. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, T.H.; Lee, W.C.; Kim, S.H.; Lee, S.Y.; Lee, S.M. Anti-inflammatory and analgesic effects of human placenta extract. Nat. Prod. Res. 2011, 25, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Sur, T.K.; Biswas, T.K.; Ali, L.; Mukherjee, B. Anti-inflammatory and anti-platelet aggregation activity of human placental extract. Acta Pharmacol. Sin. 2003, 24, 187–192. [Google Scholar]
- Kwon, T.R.; Oh, C.T.; Choi, E.J.; Park, H.M.; Han, H.J.; Ji, H.J.; Kim, B.J. Human placental extract exerts hair growth-promoting effects through the GSK-3β signaling pathway in human dermal papilla cells. Int. J. Mol. Med. 2015, 36, 1088–1096. [Google Scholar] [CrossRef]
- Shibasaki, T.; Odagiri, E.; Shizume, K.; Ling, N. Corticotropin-releasing factor-like activity in human placental extracts. J. Clin. Endocrinol. Metab. 1982, 55, 384–386. [Google Scholar] [CrossRef]
- Cho, T.H.; Park, K.M. Complex regional pain syndrome type 1 relieved by acupuncture point injections with placental extract. J. Acupunct. Meridian Stud. 2014, 7, 155–158. [Google Scholar] [CrossRef][Green Version]
- Cho, T.; Park, K. Use of acupuncture point injection with placental extract for treatment of complex regional pain syndrome. J. Pain Relief 2016, 5, 246. [Google Scholar] [CrossRef]
- Park, K.M.; Cho, T.H. Therapeutic effect of acupuncture point injection with placental extract in knee osteoarthritis. J. Integr. Med. 2017, 15, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Ramon, G. Sur l’augmentation anormale de l’antitoxine chez les chevaux producteurs de serum antidiphterique. Bull. Soc. Centr. Med. Vet. 1925, 101, 227–234. [Google Scholar]
- Ramon, G. Procedes pour acroitre la production des antitoxins. Ann. Inst. Pasteur. 1926, 40, 1–10. [Google Scholar]
- Casals, J.; Freund, J. Sensitization and antibody formation in monkeys injected with tubercle bacilli in paraffin oil. J. Immunol. 1939, 36, 399–404. [Google Scholar] [CrossRef]
- Allison, A.C.; Byars, N.E. Immunological adjuvants: Desirable properties and side-effects. Mol. Immunol. 1991, 28, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, M.T.; Galli, C.; Guizzardi, S. Polydeoxyribonucleotide Regulation of Inflammation. Adv. Wound Care 2020, 9, 576–589. [Google Scholar] [CrossRef]
- Lee, W.Y.; Park, K.D.; Park, Y. The effect of polydeoxyribonucleotide on the treatment of radiating leg pain due to cystic mass lesion in inner aspect of right sciatic foramen: A CARE compliant case report. Medicine 2018, 97, e12794. [Google Scholar] [CrossRef] [PubMed]
- Lyftogt, J. Subcutaneous prolotherapy for achilles tendinopathy: The best solution? Australas. Musculoskelet. Med. 2007, 12, 107–109. [Google Scholar]
- Li, T.-Y.; Chen, S.-R.; Shen, Y.-P.; Chang, C.-Y.; Su, Y.-C.; Chen, L.-C.; Wu, Y.-T. Long-term outcome after perineural injection with 5% dextrose for carpal tunnel syndrome: A retrospective follow-up study. Rheumatology 2021, 60, 881–887. [Google Scholar] [CrossRef]
- Freire, M.O.; Van Dyke, T.E. Natural resolution of inflammation. Periodontology 2000 2013, 63, 149–164. [Google Scholar] [CrossRef]
- Chandrasoma, P.; Taylor, C.R. Chapter 3. The Acute Inflammatory Response. In Concise Pathology; The McGraw-Hill Companies: New York, NY, USA, 1998. [Google Scholar]
- Ji, R.-R.; Kohno, T.; Moore, K.A.; Woolf, C.J. Central sensitization and LTP: Do pain and memory share similar mechanisms? Trends Neurosci. 2003, 26, 696–705. [Google Scholar] [CrossRef]
- Scholz, J.; Woolf, C.J. The neuropathic pain triad: Neurons, immune cells and glia. Nat. Neurosci. 2007, 10, 1361–1368. [Google Scholar] [CrossRef]
- Chen, S.-P.; Zhou, Y.-Q.; Liu, D.-Q.; Zhang, W.; Manyande, A.; Guan, X.-H.; Tian, Y.-K.; Ye, D.-W.; Mohamed Omar, D. PI3K/Akt pathway: A potential therapeutic target for chronic pain. Curr. Pharm. Des. 2017, 23, 1860–1868. [Google Scholar] [CrossRef]
- Li, D.; Chen, H.; Luo, X.-H.; Sun, Y.; Xia, W.; Xiong, Y.-C. CX3CR1-mediated Akt1 activation contributes to the paclitaxel-induced painful peripheral neuropathy in rats. Neurochem. Res. 2016, 41, 1305–1314. [Google Scholar] [CrossRef]
- Shin, E.H.; Kim, M.; Hada, B.; Oh, C.T.; Jang, M.J.; Kim, J.Y.; Han, H.J.; Kim, D.H.; Choi, B.H.; Kim, B.S. Effects of human placenta extract (Laennec) on ligament healing in a rodent model. Biol. Pharm. Bull. 2019, 42, 1988–1995. [Google Scholar] [CrossRef] [PubMed]
- Pratt, J.P.; Kaucher, M.; Richards, A.J.; Williams, H.H.; Macy, I.C. Composition of the Human Placenta: I. Proximate Composition. Am. J. Obstet. Gynecol. 1946, 52, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef] [PubMed]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef]
- Squadrito, F.; Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Minutoli, L.; Altavilla, D. Pharmacological activity and clinical use of PDRN. Front. Pharmacol. 2017, 8, 224. [Google Scholar] [CrossRef]
- Jeong, W.; Yang, C.E.; Roh, T.S.; Kim, J.H.; Lee, J.H.; Lee, W.J. Scar prevention and enhanced wound healing induced by polydeoxyribonucleotide in a rat incisional wound-healing model. Int. J. Mol. Sci. 2017, 18, 1698. [Google Scholar] [CrossRef]
- Chung, K.I.; Kim, H.K.; Kim, W.S.; Bae, T.H. The effects of polydeoxyribonucleotide on the survival of random pattern skin flaps in rats. Arch. Plast. Surg. 2013, 40, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Park, G.; Lee, J.; Bae, H. The effect of polydeoxyribonucleotide on chronic non-healing wound of an amputee: A case report. Ann. Rehabil. Med. 2018, 42, 630–633. [Google Scholar] [CrossRef]
- Guizzardi, S.; Galli, C.; Govoni, P.; Boratto, R.; Cattarini, G.; Martini, D.; Belletti, S.; Scandroglio, R. Polydeoxyribonucleotide (PDRN) promotes human osteoblast proliferation: A new proposal for bone tissue repair. Life Sci. 2003, 73, 1973–1983. [Google Scholar] [CrossRef]
- Han, J.H.; Jung, J.; Hwang, L.; Ko, I.G.; Nam, O.H.; Kim, M.S.; Lee, J.W.; Choi, B.J.; Lee, D.W. Anti-inflammatory effect of polydeoxyribonucleotide on zoledronic acid-pretreated and lipopolysaccharide-stimulated RAW 264.7 cells. Exp. Ther. Med. 2018, 16, 400–405. [Google Scholar] [CrossRef]
- Baek, A.; Kim, M.; Kim, S.H.; Cho, S.-R.; Kim, H.J. Anti-inflammatory effect of DNA polymeric molecules in a cell model of osteoarthritis. Inflammation 2018, 41, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Jo, S.Y.; Lee, M.H.; Won, C.H.; Lee, M.W.; Choi, J.H.; Chang, S.E. The effect of MCP-1/CCR2 on the proliferation and senescence of epidermal constituent cells in solar lentigo. Int. J. Mol. Sci. 2016, 17, 948. [Google Scholar] [CrossRef]
- Edgar, S.; Hopley, B.; Genovese, L.; Sibilla, S.; Laight, D.; Shute, J. Effects of collagen-derived bioactive peptides and natural antioxidant compounds on proliferation and matrix protein synthesis by cultured normal human dermal fibroblasts. Sci. Rep. 2018, 8, 10474. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef]
- Ma, W.; Chabot, J.-G.; Quirion, R. A role for adrenomedullin as a pain-related peptide in the rat. Proc. Natl. Acad. Sci. USA 2006, 103, 16027–16032. [Google Scholar] [CrossRef]
- Buel, G.R.; Dang, H.Q.; Asara, J.M.; Blenis, J.; Mutvei, A.P. Prolonged deprivation of arginine or leucine induces PI3K/Akt-dependent reactivation of mTORC1. J. Biol. Chem. 2022, 298, 102030. [Google Scholar] [CrossRef]
- Guo, L.; Liang, Z.; Zheng, C.; Liu, B.; Yin, Q.; Cao, Y.; Yao, J. Leucine Affects α-Amylase Synthesis through PI3K/Akt-mTOR Signaling Pathways in Pancreatic Acinar Cells of Dairy Calves. J. Agric. Food Chem. 2018, 66, 5149–5156. [Google Scholar] [CrossRef] [PubMed]
- Songjang, W.; Nensat, C.; Tohtong, R.; Suthiphongchai, T.; Phimsen, S.; Rattanasinganchan, P.; Metheenukul, P.; Kasekarn, W.; Jiraviriyakul, A. Porcine placenta extract induced Akt, ERK, and JNK signaling to heighten the osteogenic activity of human osteoblasts. J. Appl. Pharm. Sci. 2022, 12, 018–025. [Google Scholar] [CrossRef]
- Aioi, A.; Muromoto, R.; Kashiwakura, J.-i.; Mogami, S.; Nishikawa, M.; Ogawa, S.; Matsuda, T. Porcine placenta extract upregulates ceramide synthase 3 expression via the PPARδ/ILK/Akt/mTOR/STAT3 pathway. bioRxiv 2022. [Google Scholar] [CrossRef]
- Boddeke, E.W. Involvement of chemokines in pain. Eur. J. Pharmacol. 2001, 429, 115–119. [Google Scholar] [CrossRef]
- Raghavendra, V.; DeLeo, J.A. The role of astrocytes and microglia in persistent pain. Adv. Mol. Cell Biol. 2003, 31, 951–966. [Google Scholar]
- Raghavendra, V.; Tanga, F.Y.; DeLeo, J.A. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur. J. Neurosci. 2004, 20, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zeng, H.; Wang, Q.; Yu, Q.; Wu, J.; Feng, Y.; Deng, P.; Zhang, H. Glycyrrhizin ameliorates inflammatory pain by inhibiting microglial activation-mediated inflammatory response via blockage of the HMGB1-TLR4-NF-kB pathway. Exp. Cell Res. 2018, 369, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.D.; Christensen, A.D.; Tewari, D.; McMahon, S.B.; Hamilton, J.A. Immune Cytokines and Their Receptors in Inflammatory Pain. Trends Immunol. 2018, 39, 240–255. [Google Scholar] [CrossRef]
- Catterall, W.A.; Goldin, A.L.; Waxman, S.G. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Rev. 2005, 57, 397–409. [Google Scholar] [CrossRef]
- Clark, A.K.; Gruber-Schoffnegger, D.; Drdla-Schutting, R.; Gerhold, K.J.; Malcangio, M.; Sandkühler, J. Selective activation of microglia facilitates synaptic strength. J. Neurosci. 2015, 35, 4552–4570. [Google Scholar] [CrossRef]
- Kronschläger, M.; Drdla-Schutting, R.; Gassner, M.; Honsek, S.; Teuchmann, H.; Sandkühler, J. Gliogenic LTP spreads widely in nociceptive pathways. Science 2016, 354, 1144–1148. [Google Scholar] [CrossRef]
- Gruber-Schoffnegger, D.; Drdla-Schutting, R.; Hönigsperger, C.; Wunderbaldinger, G.; Gassner, M.; Sandkühler, J. Induction of thermal hyperalgesia and synaptic long-term potentiation in the spinal cord lamina I by TNF-α and IL-1β is mediated by glial cells. J. Neurosci. 2013, 33, 6540–6551. [Google Scholar] [CrossRef]
- Beloeil, H.; Ababneh, Z.; Chung, R.; Zurakowski, D.; Mulkern, R.V.; Berde, C.B. Effects of Bupivacaine and Tetrodotoxin on Carrageenan-induced Hind Paw Inflammation in Rats (Part 1) Hyperalgesia, Edema, and Systemic Cytokines. J. Am. Soc. Anesthesiol. 2006, 105, 128–138. [Google Scholar] [CrossRef]
- Sweitzer, S.; Colburn, R.; Rutkowski, M.; DeLeo, J. Acute peripheral inflammation induces moderate glial activation and spinal IL-1β expression that correlates with pain behavior in the rat. Brain Res. 1999, 829, 209–221. [Google Scholar] [CrossRef]
- Fiorentino, P.M.; Tallents, R.H.; Miller, J.N.H.; Brouxhon, S.M.; O’Banion, M.K.; Puzas, J.E.; Kyrkanides, S. Spinal interleukin-1β in a mouse model of arthritis and joint pain. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2008, 58, 3100–3109. [Google Scholar] [CrossRef] [PubMed]
- Gauldie, J.; Richards, C.; Harnish, D.; Lansdorp, P.; Baumann, H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc. Natl. Acad. Sci. USA 1987, 84, 7251–7255. [Google Scholar] [CrossRef] [PubMed]
- Fann, M.-J.; Patterson, P.H. Neuropoietic cytokines and activin A differentially regulate the phenotype of cultured sympathetic neurons. Proc. Natl. Acad. Sci. USA 1994, 91, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Hama, T.; Kushima, Y.; Miyamoto, M.; Kubota, M.; Takei, N.; Hatanaka, H. Interleukin-6 improves the survival of mesencephalic catecholaminergic and septal cholinergic neurons from postnatal, two-week-old rats in cultures. Neuroscience 1991, 40, 445–452. [Google Scholar] [CrossRef]
- Fattori, E.; Lazzaro, D.; Musiani, P.; Modesti, A.; Alonzi, T.; Ciliberto, G. IL-6 expression in neurons of transgenic mice causes reactive astrocytosis and increase in ramified microglial cells but no neuronal damage. Eur. J. Neurosci. 1995, 7, 2441–2449. [Google Scholar] [CrossRef]
- Chiang, C.-S.; Stalder, A.; Samimi, A.; Campbell, I.L. Reactive gliosis as a consequence of interleukin-6 expression in the brain: Studies in transgenic mice. Dev. Neurosci. 1994, 16, 212–221. [Google Scholar] [CrossRef]
- Campbell, I.L.; Abraham, C.R.; Masliah, E.; Kemper, P.; Inglis, J.D.; Oldstone, M.; Mucke, L. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc. Natl. Acad. Sci. USA 1993, 90, 10061–10065. [Google Scholar] [CrossRef]
- Nakafuku, M.; Satoh, T.; Kaziro, Y. Differentiation factors, including nerve growth factor, fibroblast growth factor, and interleukin-6, induce an accumulation of an active Ras. GTP complex in rat pheochromocytoma PC12 cells. J. Biol. Chem. 1992, 267, 19448–19454. [Google Scholar] [CrossRef]
- Oka, T.; Oka, K.; Hosoi, M.; Hori, T. Intracerebroventricular injection of interleukin-6 induces thermal hyperalgesia in rats. Brain Res. 1995, 692, 123–128. [Google Scholar] [CrossRef]
- DeLEO, J.A.; Colburn, R.W.; Nichols, M.; Malhotra, A. Interleukin-6-mediated hyperalgesia/allodynia and increased spinal IL-6 expression in a rat mononeuropathy model. J. Interferon Cytokine Res. 1996, 16, 695–700. [Google Scholar] [CrossRef]
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Vila, M.; Jackson-Lewis, V.; Guégan, C.; Teismann, P.; Choi, D.-K.; Tieu, K.; Przedborski, S. The role of glial cells in Parkinson’s disease. Curr. Opin. Neurol. 2001, 14, 483–489. [Google Scholar] [CrossRef]
- Watkins, L.R.; Maier, S.F. Glia: A novel drug discovery target for clinical pain. Nat. Rev. Drug Discov. 2003, 2, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, T.; Chen, X.; Li, L.; Feng, M.; Zhang, Y.; Wan, L.; Zhang, C.; Yao, W. Microglia induce the transformation of A1/A2 reactive astrocytes via the CXCR7/PI3K/Akt pathway in chronic post-surgical pain. J. Neuroinflamm. 2020, 17, 211. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.Q.; Mantyh, P.W.; Carlson, E.J.; Gillespie, A.-M.; Epstein, C.J.; Basbaum, A.I. Primary afferent tachykinins are required to experience moderate to intense pain. Nature 1998, 392, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Alhadeff, A.L.; Su, Z.; Hernandez, E.; Klima, M.L.; Phillips, S.Z.; Holland, R.A.; Guo, C.; Hantman, A.W.; De Jonghe, B.C.; Betley, J.N. A neural circuit for the suppression of pain by a competing need state. Cell 2018, 173, 140–152.E15. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ji, H.; Ko, S.-G.; Kim, W. Ji017 attenuates oxaliplatin-induced cold allodynia via spinal trpv1 and astrocytes inhibition in mice. Int. J. Mol. Sci. 2021, 22, 8811. [Google Scholar] [CrossRef]
- Lee, J.H.; Min, D.; Lee, D.; Kim, W. Zingiber officinale roscoe rhizomes attenuate oxaliplatin-induced neuropathic pain in mice. Molecules 2021, 26, 548. [Google Scholar] [CrossRef]
- Dixon, W.J. Efficient analysis of experimental observations. Annu. Rev. Pharmacol. Toxicol. 1980, 20, 441–462. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.; Chung, J.; Yaksh, T. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Hussein, S.Z.; Mohd Yusoff, K.; Makpol, S.; Mohd Yusof, Y.A. Gelam honey attenuates carrageenan-induced rat paw inflammation via NF-κB pathway. PLoS ONE 2013, 8, e72365. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.-T.; Jo, H.; Jeon, S.-H.; Jeong, K.; Im, M.; Kim, J.-W.; Jung, J.-P.; Jung, H.C.; Lee, J.h.; Kim, W. Analgesic Effect of Human Placenta Hydrolysate on CFA-Induced Inflammatory Pain in Mice. Pharmaceuticals 2024, 17, 1179. https://doi.org/10.3390/ph17091179
Park K-T, Jo H, Jeon S-H, Jeong K, Im M, Kim J-W, Jung J-P, Jung HC, Lee Jh, Kim W. Analgesic Effect of Human Placenta Hydrolysate on CFA-Induced Inflammatory Pain in Mice. Pharmaceuticals. 2024; 17(9):1179. https://doi.org/10.3390/ph17091179
Chicago/Turabian StylePark, Keun-Tae, Heejoon Jo, So-Hyun Jeon, Kyeongsoo Jeong, Minju Im, Jae-Won Kim, Jong-Pil Jung, Hoe Chang Jung, Jae hun Lee, and Woojin Kim. 2024. "Analgesic Effect of Human Placenta Hydrolysate on CFA-Induced Inflammatory Pain in Mice" Pharmaceuticals 17, no. 9: 1179. https://doi.org/10.3390/ph17091179
APA StylePark, K.-T., Jo, H., Jeon, S.-H., Jeong, K., Im, M., Kim, J.-W., Jung, J.-P., Jung, H. C., Lee, J. h., & Kim, W. (2024). Analgesic Effect of Human Placenta Hydrolysate on CFA-Induced Inflammatory Pain in Mice. Pharmaceuticals, 17(9), 1179. https://doi.org/10.3390/ph17091179





