Neuroregeneration Improved by Sodium-D,L-Beta-Hydroxybutyrate in Primary Neuronal Cultures
Abstract
1. Introduction
2. Results
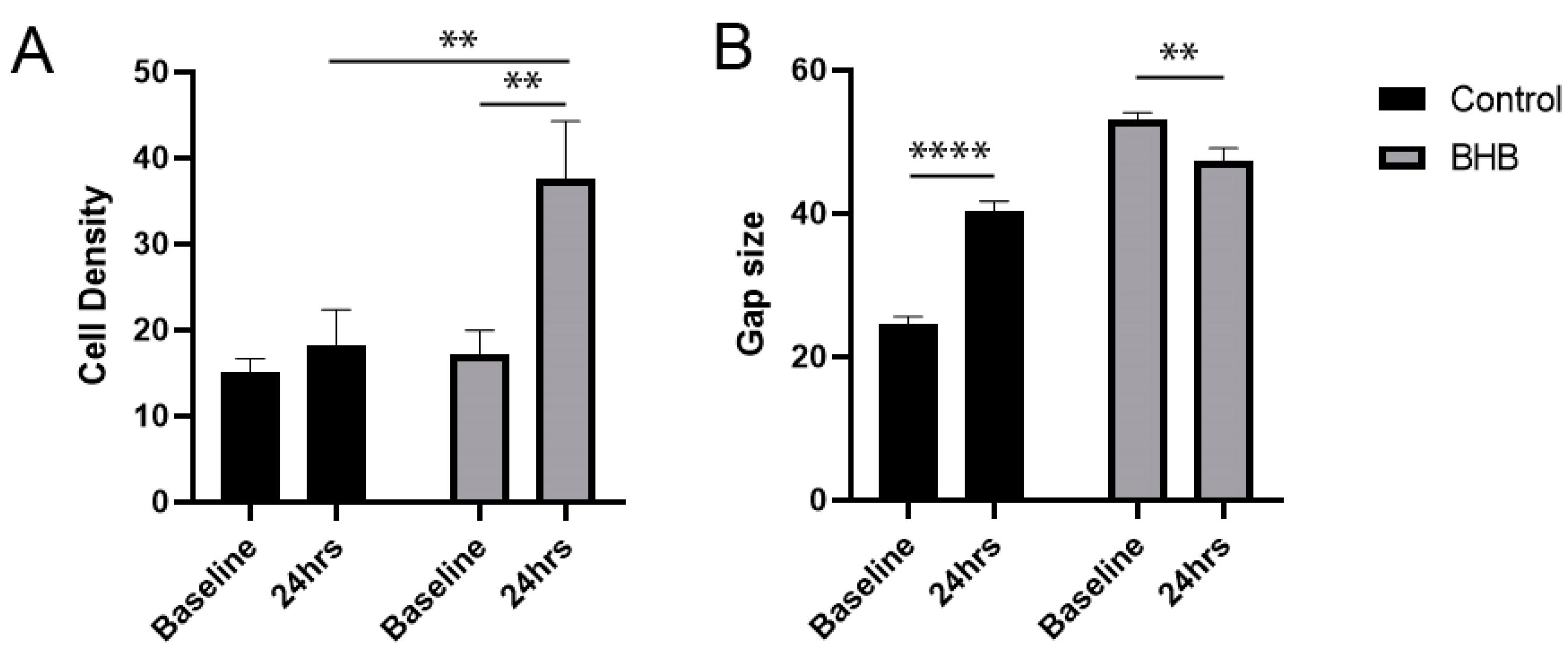
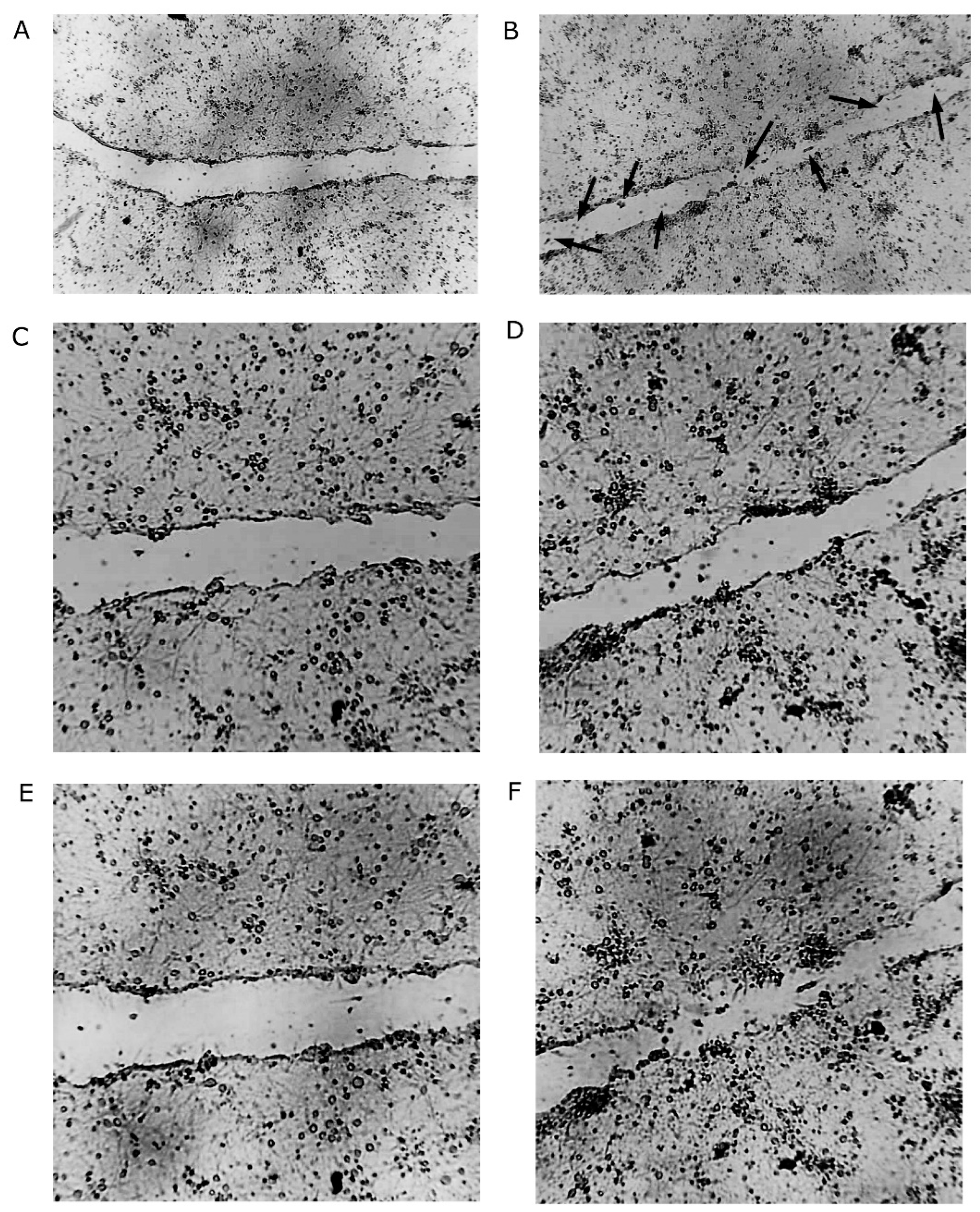
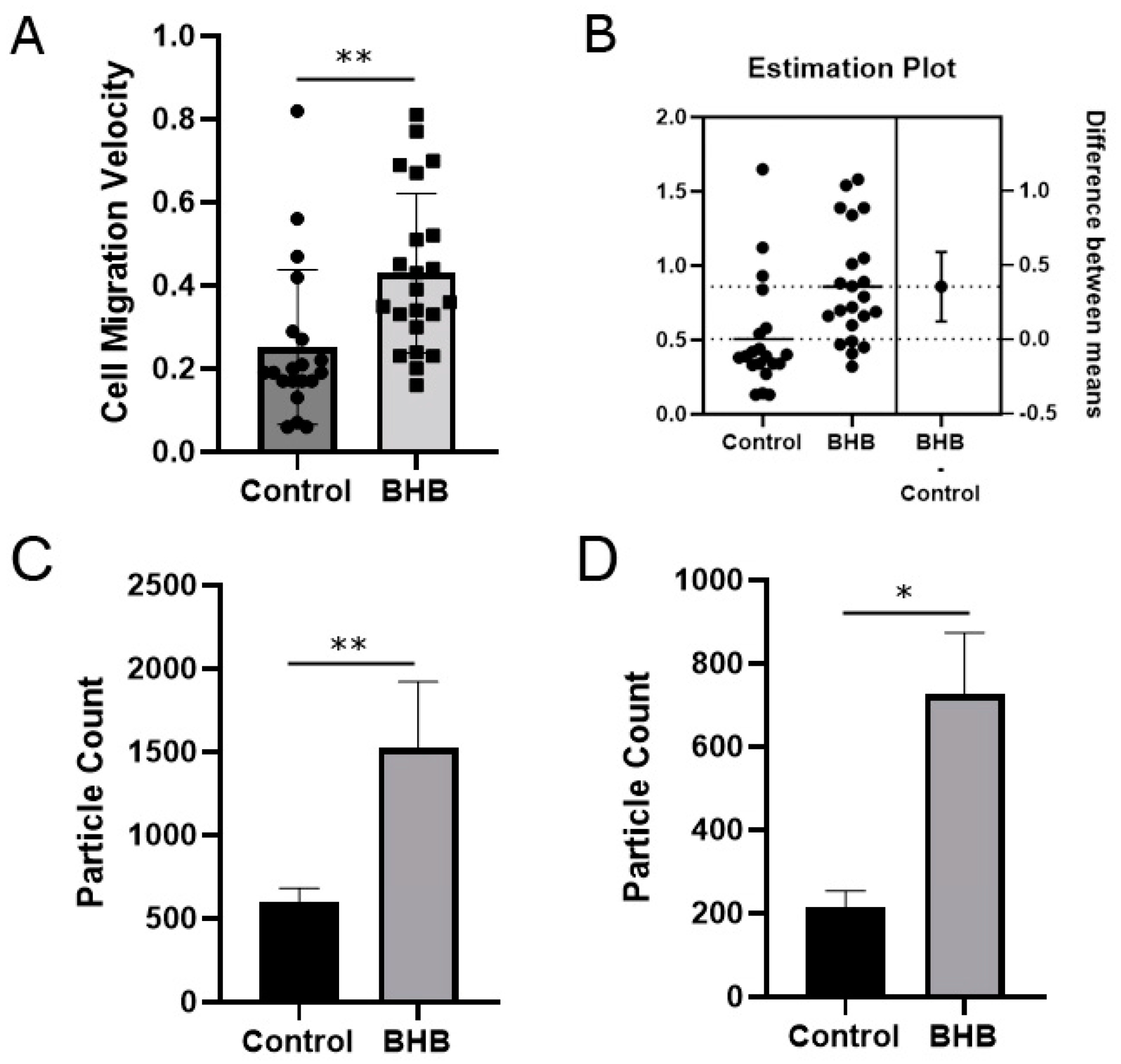
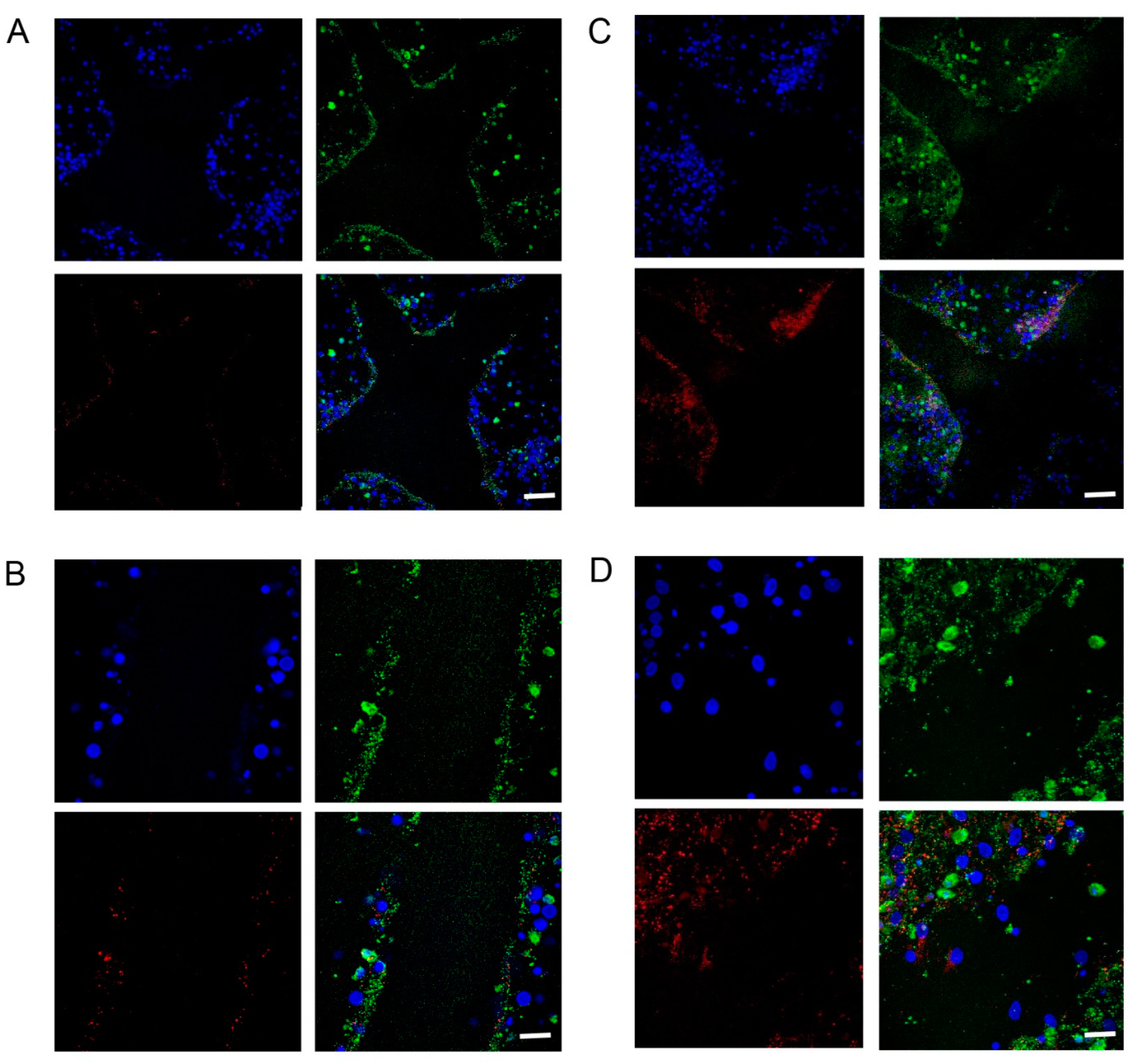
2.1. Increased Density of Cell Nuclei and Enhanced Cell Migration with BHB Treatment
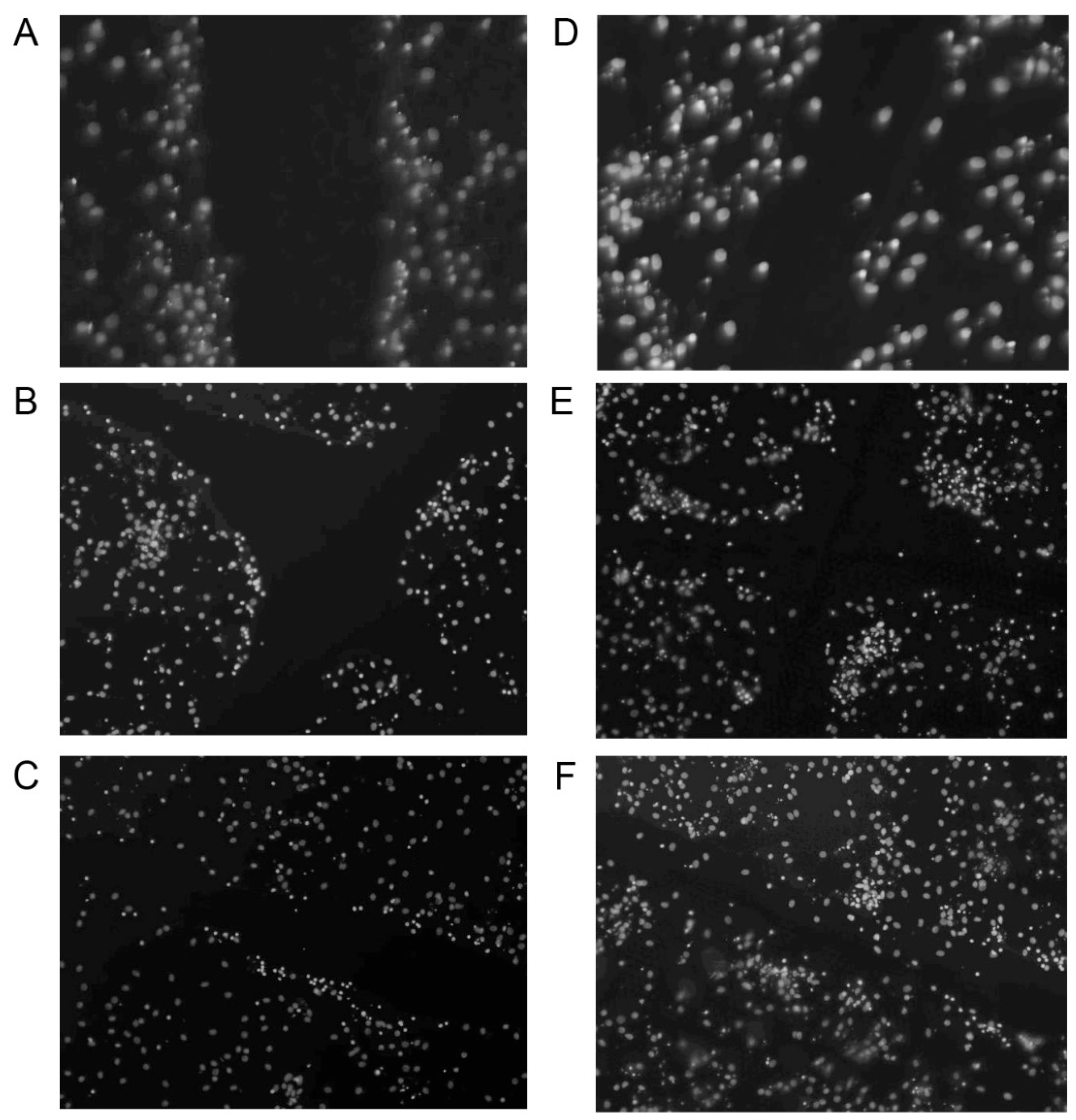
2.2. Increased Synapsin and Tubulin Staining in BHB-Treated Cultures
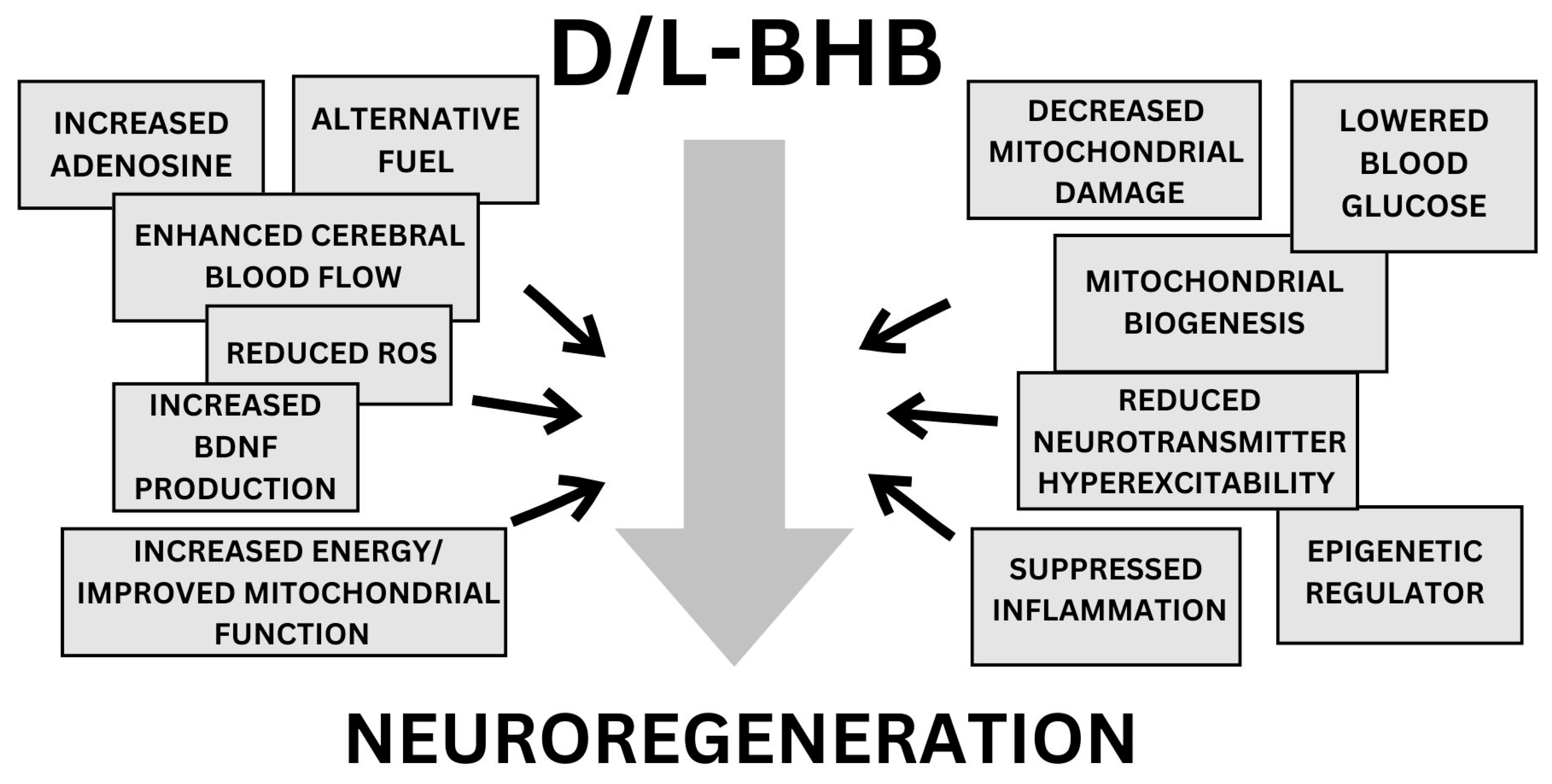
3. Discussion
3.1. Ketones as Alternative Fuel
3.2. Enhanced Cerebral Blood Flow
3.3. Increased BDNF Production
3.4. Improved Mitochondrial Function
3.5. Suppressed Inflammation
3.6. Reduced ROS and Decreased Neurotransmitter Hyperexcitability
3.7. Epigenetic Regulator
3.8. Lower Blood Glucose
3.9. Increased Adenosine
4. Materials and Methods
4.1. Immunocytochemistry
4.2. Imaging
4.3. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29, Erratum in Int. J. Stroke 2022, 17, 478. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Anderson, D.B.; Chen, L.; Feng, S.; Zhou, H. Global, regional and national burden of traumatic brain injury and spinal cord injury, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e075049. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lawrence, A.B.; Orman, J.A.; Miller, T.R.; Spicer, R.S.; Hendrie, D. Cost of traumatic brain injuries in the United States and the return on helmet investments. In Neurotrauma and Critical Care of the Brain, 2nd ed.; Jallo, J., Loftus, C.M., Eds.; Thieme Medical Publishers, Inc.: New York, NY, USA, 2018; Chapter 32. [Google Scholar]
- Yoshino, A.; Hovda, D.A.; Kawamata, T.; Katayama, Y.; Becker, D.P. Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: Evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991, 561, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Giza, C.C.; Prins, M.L. Is being plastic fantastic? Mechanisms of altered plasticity after developmental traumatic brain injury. Dev. Neurosci. 2006, 28, 364–379. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trojan, S.; Langmeier, M.; Maresová, D.; Pokorný, J. Dehydrogenase activity in the blood and brain after adaptation to intermittent hypoxia. Sb. Lek. 2000, 101, 11–16. [Google Scholar] [PubMed]
- Tieu, P.; Lee, M.H.; Dhawan, T.; Nguyen, H.H.; Afraz, S.; Chung, J.Z.; Khan, S.; Yusuf, I.; Liu, S.S. Cell-free DNA as a potential biomarker in stroke: A comprehensive review of observational studies. J. Transl. Genet. Genom. 2020, 4, 133–143. [Google Scholar] [CrossRef]
- Blázquez, E.; Hurtado-Carneiro, V.; LeBaut-Ayuso, Y.; Velázquez, E.; García-García, L.; Gómez-Oliver, F.; Ruiz-Albusac, J.M.; Ávila, J.; RPozo, M.Á. Significance of Brain Glucose Hypometabolism, Altered Insulin Signal Transduction, and Insulin Resistance in Several Neurological Diseases. Front. Endocrinol. 2022, 13, 873301. [Google Scholar] [CrossRef]
- Griffin, W.S.; Sheng, J.G.; Royston, M.C.; Gentleman, S.M.; McKenzie, J.E.; Graham, D.I.; Roberts, G.; Mrak, R. Glial-Neuronal Interactions in Alzheimer’s Disease: The Potential Role of a “Cytokine Cycle” in Disease Progression. Brain Pathol. 1998, 8, 65–72. [Google Scholar] [CrossRef]
- Heni, M.; Kullmann, S.; Ahlqvist, E.; Wagner, R.; Machicao, F.; Staiger, H.; Häring, H.-U.; Almgren, P.; Groop, L.C.; Small, D.M.; et al. Interaction Between the Obesity-Risk Gene FTO and the Dopamine D2 Receptor Gene ANKK1/TaqIA on Insulin Sensitivity. Diabetologia 2016, 59, 2622–2631. [Google Scholar] [CrossRef]
- de Ceballos, M.L.; Köfalvi, A. Boosting Brain Glucose Metabolism to Fight Neurodegeneration? Oncotarget 2017, 8, 14273–14274. [Google Scholar] [CrossRef]
- Blass, J.P. Brain Metabolism and Brain Disease: Is Metabolic Deficiency the Proximate Cause of Alzheimer Dementia? J. Neurosci. Res. 2001, 66, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Grimm, A.; Friedland, K.; Eckert, A. Mitochondrial Dysfunction: The Missing Link Between Aging and Sporadic Alzheimer’s Disease. Biogerontology 2016, 17, 281–296. [Google Scholar] [CrossRef]
- Petrou, M.; Dwamena, B.A.; Foerster, B.R.; MacEachern, M.P.; Bohnen, N.I.; Müller, M.L.; Albin, R.L.; Frey, K.A.; Mlis, M.P.M. Amyloid Deposition in Parkinson’s Disease and Cognitive Impairment: A Systematic Review. Mov. Disord. Off. J. Mov. Disord. Soc. 2015, 30, 928–935. [Google Scholar] [CrossRef] [PubMed]
- de la Monte, S.M. Insulin Resistance and Neurodegeneration: Progress Towards the Development of New Therapeutics for Alzheimer’s Disease. Drugs 2017, 77, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, S.; Heni, M.; Hallschmid, M.; Fritsche, A.; Preissl, H.; Häring, H.-U. Brain Insulin Resistance at the Crossroads of Metabolic and Cognitive Disorders in Humans. Physiol. Rev. 2016, 96, 1169–1209. [Google Scholar] [CrossRef]
- Blázquez, E.; Velázquez, E.; Hurtado-Carneiro, V.; Ruiz-Albusac, J.M. Insulin in the Brain: Its Pathophysiological Implications for States Related with Central Insulin Resistance, Type 2 Diabetes and Alzheimer’s Disease. Front. Endocrinol. 2014, 5, 161. [Google Scholar] [CrossRef]
- Zilberter, Y.; Zilberter, M. The Vicious Circle of Hypometabolism in Neurodegenerative Diseases: Ways and Mechanisms of Metabolic Correction. J. Neurosci. Res. 2017, 95, 2217–2235. [Google Scholar] [CrossRef]
- Gambardella, I.; Ascione, R.; D’agostino, D.P.; Ari, C.; Worku, B.; Tranbaugh, R.F.; Ivascu, N.; Villena-Vargas, J.; Girardi, L.N. Systematic Review Neuroprotection of ketosis in acute injury of the mammalian central nervous system: A meta-analysis. J. Neurochem. 2021, 158, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Jensen, N.J.; Nilsson, M.; Ingerslev, J.S.; Olsen, D.A.; Fenger, M.; Svart, M.; Møller, N.; Zander, M.; Miskowiak, K.W.; Rungby, J. Effects of β-hydroxybutyrate on cognition in patients with type 2 diabetes. Eur. J. Endocrinol. 2020, 182, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Ari, C.; Kovács, Z.; Juhasz, G.; Murdun, C.; Goldhagen, C.R.; Koutnik, A.P.; Poff, A.M.; Kesl, S.L.; D’Agostino, D.P. Exogenous Ketone Supplements Reduce Anxiety-Related Behavior in Sprague-Dawley and Wistar Albino Glaxo/Rijswijk Rats. Front. Mol. Neurosci. 2016, 9, 137, Erratum in Front. Mol. Neurosci. 2017, 10, 36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Henderson, S.T. Ketone bodies as a therapeutic for Alzheimer’s disease. Neurotherapeutics 2008, 5, 470–480. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Appelberg, K.S.; Hovda, D.A.; Prins, M.L. The effects of a ketogenic diet on behavioral outcome after controlled cortical impact injury in the juvenile and adult rat. J. Neurotrauma 2009, 26, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Grigolon, R.B.; Gerchman, F.; Schöffel, A.C.; Hawken, E.R.; Gill, H.; Vazquez, G.H.; Mansur, R.B.; McIntyre, R.S.; Brietzke, E. Mental, emotional, and behavioral effects of ketogenic diet for non-epileptic neuropsychiatric conditions. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2020, 102, 109947. [Google Scholar] [CrossRef]
- Herbert, M.R.; Buckley, J.A. Autism and dietary therapy: Case report and review of the literature. J. Child Neurol. 2013, 28, 975–982. [Google Scholar] [CrossRef]
- Krikorian, R.; Shidler, M.D.; Dangelo, K.; Couch, S.C.; Benoit, S.C.; Clegg, D.J. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol. Aging 2012, 33, 425.e19–425.e27. [Google Scholar] [CrossRef]
- Maalouf, M.; Rho, J.M.; Mattson, M.P. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res. Rev. 2009, 59, 293–315. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Sun, X.; Eroku, B.O.; Tsipis, C.P.; Puchowicz, M.A.; LaManna, J.C. Diet-induced ketosis improves cognitive performance in aged rats. Adv. Exp. Med. Biol. 2010, 662, 71–75. [Google Scholar] [PubMed]
- Hallböök, T.; Köhler, S.; Rosén, I.; Lundgren, J. Effects of ketogenic diet on epileptiform activity in children with therapy resistant epilepsy. Epilepsy Res. 2007, 77, 134–140. [Google Scholar] [CrossRef] [PubMed]
- IJff, D.M.; Postulart, D.; Lambrechts DA, J.E.; Majoie MH, J.M.; de Kinderen RJ, A.; Hendriksen JG, M.; Evers SM, A.A.; Aldenkamp, A.P. Cognitive and behavioral impact of the ketogenic diet in children and adolescents with refractory epilepsy: A randomized controlled trial. Epilepsy Behav. 2016, 60, 153–157. [Google Scholar] [CrossRef]
- Kasprowska-Liśkiewicz, D.; Liśkiewicz, A.D.; Nowacka-Chmielewska, M.M.; Nowicka, J.; Małecki, A.; Barski, J.J. The ketogenic diet affects the social behavior of young male rats. Physiol. Behav. 2017, 179, 168–177. [Google Scholar] [CrossRef]
- Kinsman, S.L.; Vining, E.P.; Quaskey, S.A.; Mellits, D.; Freeman, J.M. Efficacy of the ketogenic diet for intractable seizure disorders: Review of 58 cases. Epilepsia 1992, 33, 1132–1136. [Google Scholar] [CrossRef]
- Nordli, D.R., Jr.; Kuroda, M.M.; Carroll, J.; Koenigsberger, D.Y.; Hirsch, L.J.; Bruner, H.J.; Seidel, W.T.; De Vivo, D.C. Experience with the ketogenic diet in infants. Pediatrics 2001, 108, 129–133. [Google Scholar] [CrossRef]
- Pulsifer, M.B.; Gordon, J.M.; Brandt, J.; Vining, E.P.; Freeman, J.M. Effects of ketogenic diet on development and behavior: Preliminary report of a prospective study. Dev. Med. Child Neurol. 2001, 43, 301–306. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, D.P.; Pilla, R.; Held, H.E.; Landon, C.S.; Puchowicz, M.; Brunengraber, H.; Ari, C.; Arnold, P.; Dean, J.B. Therapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R829–R836. [Google Scholar] [CrossRef] [PubMed]
- Ari, C.; Kovács, Z.; Murdun, C.; Koutnik, A.P.; Goldhagen, C.R.; Rogers, C.; Diamond, D.; D’agostino, D.P. Nutritional ketosis delays the onset of isoflurane induced anesthesia. BMC Anesthesiol. 2018, 18, 85. [Google Scholar] [CrossRef]
- Ari, C.; Koutnik, A.P.; DeBlasi, J.; Landon, C.; Rogers, C.Q.; Vallas, J.; Bharwani, S.; Puchowicz, M.; Bederman, I.; Diamond, D.M.; et al. Delaying latency to hyperbaric oxygen-induced CNS oxygen toxicity seizures by combinations of exogenous ketone supplements. Physiol. Rep. 2019, 7, e13961. [Google Scholar] [CrossRef]
- Kovács, Z.; D’agostino, D.P.; Diamond, D.; Kindy, M.S.; Rogers, C.; Ari, C. Therapeutic Potential of Exogenous Ketone Supplement Induced Ketosis in the Treatment of Psychiatric Disorders: Review of Current Literature. Front. Psychiatry 2019, 10, 363. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Z.; Brunner, B.; Ari, C. Beneficial Effects of Exogenous Ketogenic Supplements on Aging Processes and Age-Related Neurodegenerative Diseases. Nutrients 2021, 13, 2197. [Google Scholar] [CrossRef]
- Yin, J.; Han, P.; Tang, Z.; Liu, Q.; Shi, J. Sirtuin 3 mediates neuroprotection of ketones against ischemic stroke. J. Cereb. Blood Flow Metab. 2015, 35, 1783–1789. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, B.T.; Jiang, H.; Moulson, A.J.; Wu, X.L.; Wang, W.C.; Liu, J.; Plunet, W.T.; Tetzlaff, W. Neuroprotective effects of a ketogenic diet in combination with exogenous ketone salts following acute spinal cord injury. Neural. Regen. Res. 2020, 15, 1912–1919. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- White, H.; Venkatesh, B.; Jones, M.; Kruger, P.S.; Walsham, J.; Fuentes, H. Inducing ketogenesis via an enteral formulation in patients with acute brain injury: A phase II study. Neurol. Res. 2020, 42, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Edwards Maria, G.P.; Furuholmen-Jenssen, T.; Søegaard, E.G.I.; Thapa, S.; Andersen, J. Exploring diet-induced ketosis with exogenous ketone supplementation as a potential intervention in Post-Traumatic Stress Disorder: A feasibility study. Front. Nutr. 2024. [Google Scholar] [CrossRef]
- Fioretto, P.; Trevisan, R.; Velussi, M.; Cernigoi, A.; De Riva, C.; Bressan, M.; Doria, A.; Pauletto, N.; Angeli, P.; De Donà, C.; et al. Glomerular filtration rate is increased in man by the infusion of both D,L-3-hydroxybutyric acid and sodium D,L-3-hydroxybutyrate. J. Clin. Endocrinol. Metab. 1987, 65, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Van Hove, J.L.; Grünewald, S.; Jaeken, J.; Demaerel, P.; Declercq, P.E.; Bourdoux, P.; Niezen-Koning, K.; Deanfeld, J.E.; Leonard, J.V. D,L-3-hydroxybutyrate treatment of multiple acyl-CoA dehydrogenase deficiency (MADD). Lancet 2003, 361, 1433–1435. [Google Scholar] [CrossRef] [PubMed]
- Kackley, M.L.; Short, J.A.; Hyde, P.N.; LaFountain, R.A.; Buga, A.; Miller, V.J.; Dickerson, R.M.; Sapper, T.N.; Barnhart, E.C.; Krishnan, D.; et al. A Pre-Workout Supplement of Ketone Salts, Caffeine, and Amino Acids Improves High-Intensity Exercise Performance in Keto-Naïve and Keto-Adapted Individuals. J. Am. Coll. Nutr. 2020, 39, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, B.C.; Des Rosiers, C.; Brunengraber, H. Metabolism of S-3-hydroxybutyrate in the perfused rat liver. Arch Biochem Biophys. 1987, 259, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Webber, R.J.; Edmond, J. Utilization of L(+) 3 hydroxybutyrate, D(−) 3 hydroxybutyrate, acetoacetate and glucose for respiration and lipid synthesis in the 18 day old rat. J. Biol. Chem. 1977, 252, 5222–5226. [Google Scholar] [CrossRef]
- Desrochers, S.; Dubreuil, P.; Brunet, J.; Jetté, M.; David, F.; Landau, B.R.; Brunengraber, H. Metabolism of (R,S)-1,3-butanediol acetoacetate esters, potential parenteral and enteral nutrients in conscious pigs. Am. J. Physiol. 1995, 268 Pt 1, E660–E667. [Google Scholar] [CrossRef] [PubMed]
- Cuenoud, B.; Hartweg, M.; Godin, J.-P.; Croteau, E.; Maltais, M.; Castellano, C.-A.; Carpentier, A.C.; Cunnane, S.C. Metabolism of Exogenous D-Beta-Hydroxybutyrate, an Energy Substrate Avidly Consumed by the Heart and Kidney. Front. Nutr. 2020, 7, 13. [Google Scholar] [CrossRef]
- Stubbs, B.J.; Cox, P.J.; Evans, R.D.; Santer, P.; Miller, J.J.; Faull, O.K.; Magor-Elliott, S.; Hiyama, S.; Stirling, M.; Clarke, K. On the Metabolism of Exogenous Ketones in Humans. Front. Physiol. 2017, 8, 848. [Google Scholar] [CrossRef]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Rijt, W.J.; Van Hove, J.L.K.; Vaz, F.M.; Havinga, R.; Allersma, D.P.; Zijp, T.R.; Bedoyan, J.K.; Heiner-Fokkema, M.R.; Reijngoud, D.J.; Geraghty, M.T.; et al. Enantiomer-specific pharmacokinetics of D,L-3-hydroxybutyrate: Implications for the treatment of multiple acyl-CoA dehydrogenase deficiency. J. Inherit. Metab. Dis. 2021, 44, 926–938. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scafidi, S.; Jernberg, J.; Fiskum, G.; McKenna, M.C. Metabolism of Exogenous [2,4-13C]β-Hydroxybutyrate following Traumatic Brain Injury in 21-22-Day-Old Rats: An Ex Vivo NMR Study. Metabolites 2022, 12, 710. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Newman, J.C.; Verdin, E. Beta-hydroxybutyrate: Much more than a metabolite. Diabetes Res. Clin. Pract. 2014, 106, 173–181. [Google Scholar] [CrossRef]
- Zhang, H.; Uchimura, K.; Kadomatsu, K. Brain keratan sulfate and glial scar formation. Ann. N. Y. Acad. Sci. 2006, 1086, 81–90. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, D.; Weng, Y.; Delaney, K.; Tang, Z.; Yan, C.; Qi, S.; Peng, C.; Cole, P.A.; Roeder, R.G.; et al. The regulatory enzymes and protein substrates for the lysine β-hydroxybutyrylation pathway. Sci. Adv. 2021, 7, eabe2771. [Google Scholar] [CrossRef] [PubMed]
- Svart, M.; Gormsen, L.C.; Hansen, J.; Zeidler, D.; Gejl, M.; Vang, K.; Aanerud, J.; Moeller, N. Regional cerebral effects of ketone body infusion with 3-hydroxybutyrate in humans: Reduced glucose uptake, unchanged oxygen consumption and increased blood flow by positron emission tomography. A randomized, controlled trial. PLoS ONE 2018, 13, e0190556. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bernini, A.; Masoodi, M.; Solari, D.; Miroz, J.P.; Carteron, L.; Christinat, N.; Morelli, P.; Beaumont, M.; Abed-Maillard, S.; Hartweg, M.; et al. Modulation of cerebral ketone metabolism following traumatic brain injury in humans. J. Cereb. Blood Flow Metab. 2020, 40, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Laffel, L. Ketone bodies: A review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab. Res. Rev. 1999, 15, 412–426. [Google Scholar] [CrossRef]
- Volek, J.S.; Phinney, S.D.; Forsythe, C.E.; Quann, E.E.; Wood, R.J.; Puglisi, M.J.; Kraemer, W.J.; Bibus, D.M.; Fernandez, M.L.; Feinman, R.D. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids 2009, 44, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Rauch, E.; Ari, C.; Kovács, Z. Dose-Dependent Beneficial Effect of Ketone Supplement-Evoked Ketosis on Anxiety Level in Female WAG/Rij Rats: Sometimes Less Is More. Nutrients 2023, 15, 4412. [Google Scholar] [CrossRef]
- Ferrari, A.; Filoni, J.; Di Dedda, C.; Piemonti, L.; Monti, P. Ketone bodies rescue T cell impairments induced by low glucose availability. Eur. J. Nutr. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Craft, S.; Newcomer, J.; Kanne, S.; Dagogo-Jack, S.; Cryer, P.; Sheline, Y.; Luby, J.; Dagogo-Jack, A.; Alderson, A. Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiol. Aging 1996, 17, 123–130. [Google Scholar] [CrossRef]
- Evans, M.; Egan, B. Intermittent Running and Cognitive Performance after Ketone Ester Ingestion. Med. Sci. Sports. Exerc. 2018, 50, 2330–2338. [Google Scholar] [CrossRef] [PubMed]
- Mujica-Parodi, L.R.; Amgalan, A.; Sultan, S.F.; Antal, B.; Sun, X.; Skiena, S.; Lithen, A.; Adra, N.; Ratai, E.M.; Weistuch, C.; et al. Diet modulates brain network stability, a biomarker for brain aging, in young adults. Proc. Natl. Acad. Sci. USA 2020, 117, 6170–6177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walsh, J.J.; Neudorf, H.; Little, J.P. 14-Day Ketone Supplementation Lowers Glucose and Improves Vascular Function in Obesity: A Randomized Crossover Trial. J. Clin. Endocrinol. Metab. 2021, 106, e1738–e1754. [Google Scholar] [CrossRef]
- Linde, R.; Hasselbalch, S.G.; Topp, S.; Paulson, O.B.; Madsen, P.L.J. Global cerebral blood flow and metabolism during acute hyperketonemia in the awake and anesthetized rat. Cereb. Blood Flow Metab. 2006, 26, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife 2016, 5, e15092. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Duan, W.; Mattson, M.P. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. 2002, 82, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Brownlow, M.L.; Jung, S.H.; Moore, R.J.; Bechmann, N.; Jankord, R. Nutritional Ketosis Affects Metabolism and Behavior in Sprague-Dawley Rats in Both Control and Chronic Stress Environments. Front. Mol. Neurosci. 2017, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Mohorko, N.; Černelič-Bizjak, M.; Poklar-Vatovec, T.; Grom, G.; Kenig, S.; Petelin, A.; Jenko-Pražnikar, Z. Weight loss, improved physical performance, cognitive function, eating behavior, and metabolic profile in a 12-week ketogenic diet in obese adults. Nutr. Res. 2018, 62, 64–77. [Google Scholar] [CrossRef]
- Walsh, J.J.; Myette-Côté, É.; Little, J.P. The Effect of Exogenous Ketone Monoester Ingestion on Plasma BDNF during an Oral Glucose Tolerance Test. Front. Physiol. 2020, 11, 1094. [Google Scholar] [CrossRef]
- Verweij, B.H.; Muizelaar, J.P.; Vinas, F.C.; Peterson, P.L.; Xiong, Y.; Lee, C.P. Impaired cerebral mitochondrial function after traumatic brain injury in humans. J. Neurosurg. 2000, 93, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Cahill, G.F., Jr. Fuel metabolism in starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.D.; Sokoloff, L. Circulation and energy metabolism of the brain. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects; Siegel, G.J., Agranoff, B.W., Albers, R.W., Risher, S.K., Uhler, M.D., Eds.; Lippincott: Philadelphia, PA, USA, 1999; pp. 637–670. [Google Scholar]
- Sokoloff, L.; Mangold, R.; Wechsler, R.L.; Kenney, C.; Kety, S.S. The effect of mental arithmetic on cerebral circulation and metabolism. J. Clin. Investig. 1955, 34, 1101–1108. [Google Scholar] [CrossRef]
- Sato, K.; Kashiwaya, Y.; Keon, C.A.; Tsuchiya, N.; King, M.T.; Radda, G.K.; Chance, B.; Clarke, K.; Veech, R.L. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995, 9, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Prata, C.; Sega, F.V.D.; Piperno, R.; Hrelia, S. Traumatic Brain Injury and NADPH Oxidase: A Deep Relationship. Oxid. Med. Cell. Longev. 2015, 2015, 370312. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, S.G.; Kim, S.H.; Kim, J.H.; Choi, E.; Cho, W.; Rim, J.H.; Hwang, I.; Lee, C.J.; Lee, M.; et al. Sglt2 Inhibition Modulates Nlrp3 Inflammasome Activity Via Ketones and Insulin in Diabetes with Cardiovascular Disease. Nat. Commun. 2020, 11, 2127. [Google Scholar] [CrossRef]
- Hinzman, J.M.; Thomas, T.C.; Burmeister, J.J.; Quintero, J.E.; Huettl, P.; Pomerleau, F.; Gerhardt, G.A.; Lifshitz, J. Diffuse brain injury elevates tonic glutamate levels and potassium-evoked glutamate release in discrete brain regions at two days post-injury: An enzyme-based microelectrode array study. J. Neurotrauma 2010, 27, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.C.; Verdin, E. β-Hydroxybutyrate: A Signaling Metabolite. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Erecińska, M.; Nelson, D.; Daikhin, Y.; Yudkoff, M. Regulation of GABA level in rat brain synaptosomes: Fluxes through enzymes of the GABA shunt and effects of glutamate, calcium, and ketone bodies. J. Neurochem. 1996, 67, 2325–2334. [Google Scholar] [CrossRef]
- McNally, M.A.; Hartman, A.L. Ketone bodies in epilepsy. J. Neurochem. 2012, 121, 28–35. [Google Scholar] [CrossRef]
- Achanta, L.B.; Rae, C.D. β-Hydroxybutyrate in the brain: One molecule, multiple mechanisms. Neurochem. Res. 2017, 42, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Wang, J.; Ghena, N.; Zhao, Q.; Perone, I.; King, T.M.; Veech, R.L.; Gorospe, M.; Wan, R.; Mattson, M.P. SIRT3 haploinsufficiency aggravates loss of GABAergic interneurons and neuronal network hyperexcitability in an Alzheimer’s disease model. J. Neurosci. 2020, 40, 694–709. [Google Scholar] [CrossRef]
- Sharma, A.K.; Rani, E.; Waheed, A.; Rajput, S.K. Pharmacoresistant epilepsy: A current update on non- conventional pharmacological and non-pharmacological interventions. J. Epilepsy Res. 2015, 5, 1–8. [Google Scholar] [CrossRef]
- Yudkoff, M.; Daikhin, Y.; Melo, T.M.; Nissim, I.; Sonnewald, U.; Nissim, I. The ketogenic diet and brain metabolism of amino acids: Relationship to the anticonvulsant effect. Annu. Rev. Nutr. 2007, 27, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.S.; Kim, Y.S.; Choi, W.S. Neuroprotective effects of the ketogenic diet. Epilepsia 2008, 49, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Juge, N.; Gray, J.A.; Omote, H.; Miyaji, T.; Inoue, T.; Hara, C.; Uneyama, H.; Edwards, R.H.; Nicoll, R.A.; Moriyama, Y. Metabolic control of vesicular glutamate transport and release. Neuron 2010, 68, 99–112. [Google Scholar] [CrossRef]
- Simeone, T.A.; Simeone, K.A.; Rho, J.M. Ketone bodies as anti-seizure agents. Neurochem. Res. 2017, 42, 2011–2018. [Google Scholar] [CrossRef]
- Yamamoto, I.; Absalom, N.; Carland, J.E.; Doddareddy, M.R.; Gavande, N.; Johnston, G.A.R.; Hanrahan, J.R.; Chebib, M. Differentiating enantioselective actions of GABOB: A possible role for threonine 244 in the binding site of GABAC ρ1 receptors. ACS Chem. Neurosci. 2012, 3, 665–673. [Google Scholar] [CrossRef]
- Bough, K.J.; Wetherington, J.; Hassel, B.; Pare, J.F.; Gawryluk, J.W.; Greene, J.G.; Shaw, R.; Smith, Y.; Geiger, J.D.; Dingledine, R.J. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann. Neurol. 2006, 60, 223–235. [Google Scholar] [CrossRef]
- Ahola-Erkkila, S.; Carroll, C.J.; Peltola-Mjösund, K.; Tulkki, V.; Mattila, I.; Seppänen-Laakso, T.; Orešič, M.; Tyynismaa, H.; Suomalainen, A. Ketogenic diet slows down mitochondrial myopathy progression in mice. Hum. Mol. Genet. 2010, 19, 1974–1984. [Google Scholar] [CrossRef]
- Gershuni, V.M.; Yan, S.L.; Medici, V. Nutritional Ketosis for Weight Management and Reversal of Metabolic Syndrome. Curr. Nutr. Rep. 2018, 7, 97–106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Cao, R.; Niu, J.; Yang, S.; Ma, H.; Zhao, S.; Li, H. Molecular basis for hierarchical histone de-β-hydroxybutyrylation by SIRT3. Cell Discov. 2019, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Salim, A.; Hadjizacharia, P.; Dubose, J.; Brown, C.; Inaba, K.; Chan, L.S.; Margulies, D. Persistent Hyperglycemia in Severe Traumatic Brain Injury: An Independent Predictor of Outcome. Am. Surg. 2009, 75, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Ari, C.; Murdun, C.; Koutnik, A.P.; Goldhagen, C.R.; Rogers, C.; Park, C.; Bharwani, S.; Diamond, D.M.; Kindy, M.S.; D’Agostino, D.P.; et al. Exogenous Ketones Lower Blood Glucose Level in Rested and Exercised Rodent Models. Nutrients 2019, 11, 2330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fredholm, B.B. Adenosine and neuroprotection. Int. Rev. Neurobiol. 1997, 40, 259–280. [Google Scholar] [PubMed]
- Boison, D. Adenosine kinase, epilepsy and stroke: Mechanisms and therapies. Trends Pharmacol. Sci. 2006, 27, 652–658. [Google Scholar] [CrossRef]
- Masino, S.A.; Geiger, J.D. Are purines mediators of the anticonvulsant/neuroprotective effects of ketogenic diets? Trends Neurosci. 2008, 31, 273–278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wilder, R.M. The effects of ketonemia on the course of epilepsy. Mayo Clin. Proc. 1921, 2, 307–308. [Google Scholar]
- Gasior, M.; Rogawski, M.A.; Hartman, A.L. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav. Pharmacol. 2006, 17, 431–439. [Google Scholar] [CrossRef]
- Prins, M.L.; Fujima, L.; Hovda, D. Age-dependent reduction of cortical contusion volume by ketones after traumatic brain injury. J. Neurosci. Res. 2005, 82, 413–420. [Google Scholar] [CrossRef]
- Yamada, K.A.; Rensing, N.; Thio, L.L. Ketogenic diet reduces hypoglycemia-induced neuronal death in young rats. Neurosci. Lett. 2005, 385, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ren, G.; Lusardi, T.; Wilz, A.; Lan, J.Q.; Iwasato, T.; Itohara, S.; Simon, R.P.; Boison, D. Adenosine kinase is a target for the prediction and prevention of epileptogenesis. J. Clin. Investig. 2008, 118, 571–582. [Google Scholar] [CrossRef]
- Maurer, G.D.; Brucker, D.P.; Bähr, O.; Harter, P.N.; Hattingen, E.; Walenta, S.; Mueller-Klieser, W.; Steinbach, J.P.; Rieger, J. Differential utilization of ketone bodies by neurons and glioma cell lines: A rationale for ketogenic diet as experimental glioma therapy. BMC Cancer 2011, 11, 315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huebner, E.A.; Strittmatter, S.M. Axon regeneration in the peripheral and central nervous systems. Results Probl. Cell Differ. 2009, 48, 339–351. [Google Scholar] [PubMed]
- Abe, N.; Borson, S.H.; Gambello, M.J.; Wang, F.; Cavalli, V. Mammalian target of rapamycin (mTOR) activation increases axonal growth capacity of injured peripheral nerves. J. Biol. Chem. 2010, 285, 28034–28043. [Google Scholar] [CrossRef]
- Christie, K.J.; Webber, C.A.; Martinez, J.A.; Singh, B.; Zochodne, D.W. PTEN inhibition to facilitate intrinsic regenerative outgrowth of adult peripheral axons. J. Neurosci. 2010, 30, 9306–9315. [Google Scholar] [CrossRef]
- McDaniel, S.S.; Rensing, N.R.; Thio, L.L.; Yamada, K.A.; Wong, M. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia 2011, 52, e7–e11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hwang, C.Y.; Choe, W.; Yoon, K.S.; Ha, J.; Kim, S.S.; Yeo, E.J.; Kang, I. Molecular Mechanisms for Ketone Body Metabolism, Signaling Functions, and Therapeutic Potential in Cancer. Nutrients 2022, 14, 4932. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koshinaga, M.; Whittemore, S.R. The temporal and spatial activation of microglia in fiber tracts undergoing anterograde and retrograde degeneration following spinal cord lesion. J. Neurotrauma 1995, 12, 209–222. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Moon, L.D.; Popat, R.J.; King, V.R.; Bennett, G.S.; Patel, P.N.; Fawcett, J.W.; McMahon, S.B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 2002, 416, 636–640. [Google Scholar] [CrossRef]
- Fouad, K.; Schnell, L.; Bunge, M.B.; Schwab, M.E.; Liebscher, T.; Pearse, D.D. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J. Neurosci. 2005, 25, 1169–1178. [Google Scholar] [CrossRef]
- Alilain, W.J.; Horn, K.P.; Hu, H.; Dick, T.E.; Silver, J. Functional regeneration of respiratory pathways after spinal cord injury. Nature 2011, 475, 196–200. [Google Scholar] [CrossRef]
- Wittenberg, G.F. Experience, cortical remapping, and recovery in brain disease. Neurobiol. Dis. 2010, 37, 252–258. [Google Scholar] [CrossRef]
- Dancause, N.; Barbay, S.; Frost, S.B.; Zoubina, E.V.; Plautz, E.J.; Mahnken, J.D.; Nudo, R.J. Effects of small ischemic lesions in the primary motor cortex on neurophysiological organization in ventral premotor cortex. J. Neurophysiol. 2006, 96, 3506–3511. [Google Scholar] [CrossRef] [PubMed]
- Higley, M.J.; Contreras, D. Balanced excitation and inhibition determine spike timing during frequency adaptation. J. Neurosci. 2006, 26, 448–457. [Google Scholar] [CrossRef]
- Okun, M.; Lampl, I. Instantaneous correlation of excitation and inhibition during ongoing and sensory-evoked activities. Nat. Neurosci. 2008, 11, 535–537. [Google Scholar] [CrossRef]
- Loy, K.; Bareyre, F.M. Rehabilitation following spinal cord injury: How animal models can help our understanding of exercise-induced neuroplasticity. Neural Regen. Res. 2019, 14, 405–412. [Google Scholar] [PubMed]
- Asboth, L.; Friedli, L.; Beauparlant, J.; Martinez-Gonzalez, C.; Anil, S.; Rey, E.; Baud, L.; Pidpruzhnykova, G.; Anderson, M.A.; Shkorbatova, P.; et al. Cortico-reticulo-spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nat. Neurosci. 2018, 21, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, A.; Duan, H.; Zhang, S.; Hao, P.; Ye, K.; Sun, Y.E.; Li, X. NT3-chitosan elicits robust endogenous neurogenesis to enable functional recovery after spinal cord injury. Proc. Natl. Acad. Sci. USA 2015, 112, 13354–13359. [Google Scholar] [CrossRef]
- Rao, J.S.; Zhao, C.; Zhang, A.; Duan, H.; Hao, P.; Wei, R.H.; Shang, J.; Zhao, W.; Liu, Z.; Yu, J.; et al. NT3-chitosan enables de novo regeneration and functional recovery in monkeys after spinal cord injury. Proc. Natl. Acad. Sci. USA 2018, 115, E5595–E5604. [Google Scholar] [CrossRef]
- Rosenzweig, E.S.; Brock, J.H.; Lu, P.; Kumamaru, H.; Salegio, E.A.; Kadoya, K.; Weber, J.L.; Liang, J.J.; Moseanko, R.; Hawbecker, S.; et al. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat. Med. 2018, 24, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Marosi, K.; Kim, S.W.; Moehl, K.; Scheibye-Knudsen, M.; Cheng, A.; Cutler, R.; Camandola, S.; Mattson, M.P. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J. Neurochem. 2016, 139, 769–781. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holmes, D.J.; Kristan, D.M. Comparative and alternative approaches and novel animal models for aging research. Age 2008, 30, 63–73. [Google Scholar] [CrossRef]
- Hodge, R.D.; Bakken, T.E.; Miller, J.A.; Smith, K.A.; Barkan, E.R.; Graybuck, L.T.; Close, J.L.; Long, B.; Johansen, N.; Penn, O.; et al. Conserved cell types with divergent features in human versus mouse cortex. Nature 2019, 573, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, M.; Rey, F.; Fantini, V.; Pansarasa, O.; Di Giulio, A.; Carelli, S.; Cereda, C. From neuronal differentiation of iPSCs to 3D neuro-organoids: Modelling and therapy of neurodegenerative diseases. Int. J. Mol. Sci. 2018, 19, 3972. [Google Scholar] [CrossRef]
- Zimmermann, J.A.; Schaffer, D.V. Engineering biomaterials to control the neural differentiation of stem cells. Brain Res. Bull. 2019, 150, 50–60. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ari, C.; D’Agostino, D.P.; Cha, B.J. Neuroregeneration Improved by Sodium-D,L-Beta-Hydroxybutyrate in Primary Neuronal Cultures. Pharmaceuticals 2024, 17, 1160. https://doi.org/10.3390/ph17091160
Ari C, D’Agostino DP, Cha BJ. Neuroregeneration Improved by Sodium-D,L-Beta-Hydroxybutyrate in Primary Neuronal Cultures. Pharmaceuticals. 2024; 17(9):1160. https://doi.org/10.3390/ph17091160
Chicago/Turabian StyleAri, Csilla, Dominic P. D’Agostino, and Byeong J. Cha. 2024. "Neuroregeneration Improved by Sodium-D,L-Beta-Hydroxybutyrate in Primary Neuronal Cultures" Pharmaceuticals 17, no. 9: 1160. https://doi.org/10.3390/ph17091160
APA StyleAri, C., D’Agostino, D. P., & Cha, B. J. (2024). Neuroregeneration Improved by Sodium-D,L-Beta-Hydroxybutyrate in Primary Neuronal Cultures. Pharmaceuticals, 17(9), 1160. https://doi.org/10.3390/ph17091160








