Efficient Improvement of Eugenol Water Solubility by Spray Drying Encapsulation in Soluplus® and Lutrol F 127
Abstract
1. Introduction
2. Results and Discussion
2.1. Solubilization Capacity and Stability of the Emulsions for Spray Drying
2.2. Spray-Dried Powders
2.2.1. Morphology of the Particles
2.2.2. Encapsulation Efficiency
2.2.3. FTIR Analysis
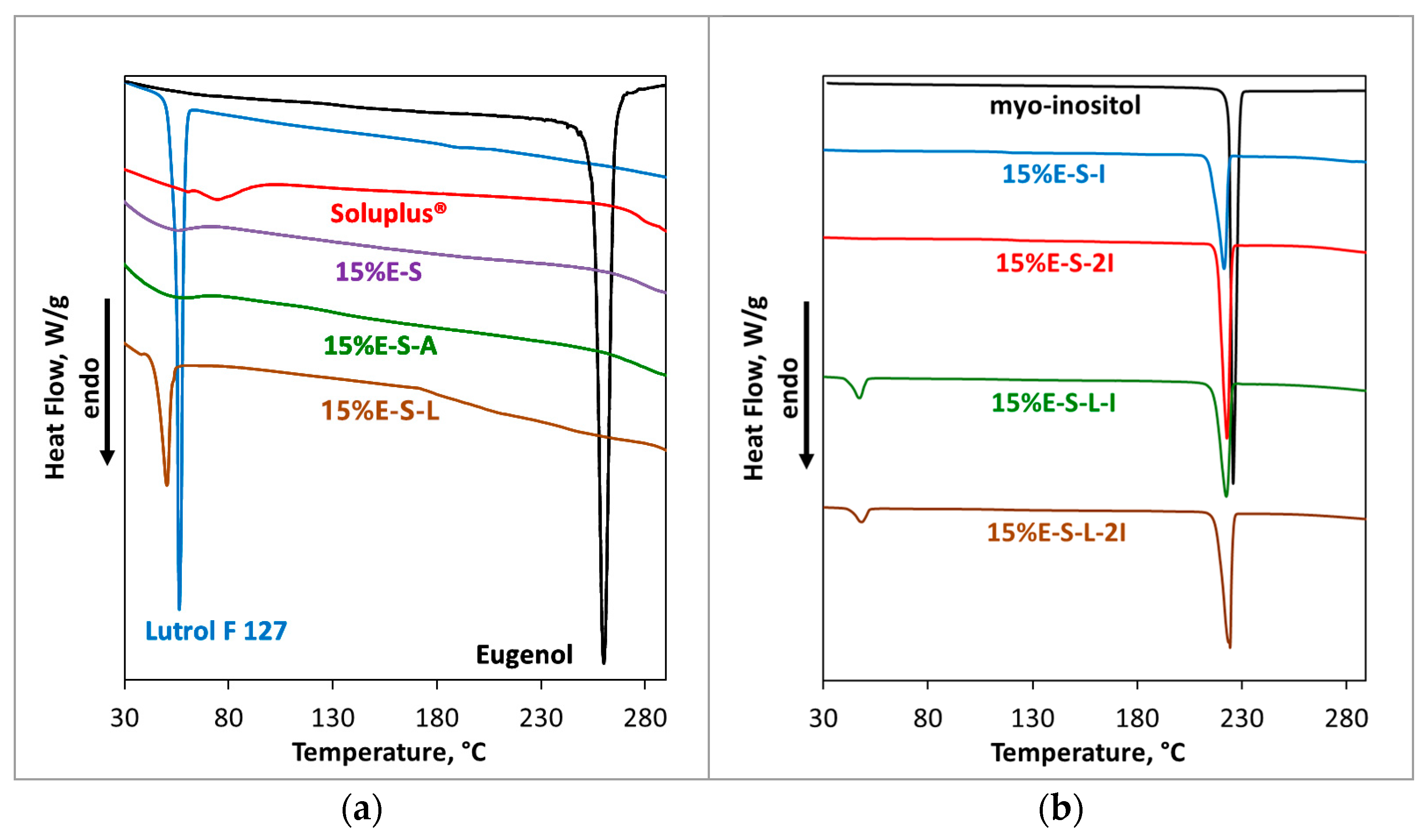
2.2.4. DSC Analysis
2.2.5. Particle Size in a Water Solution
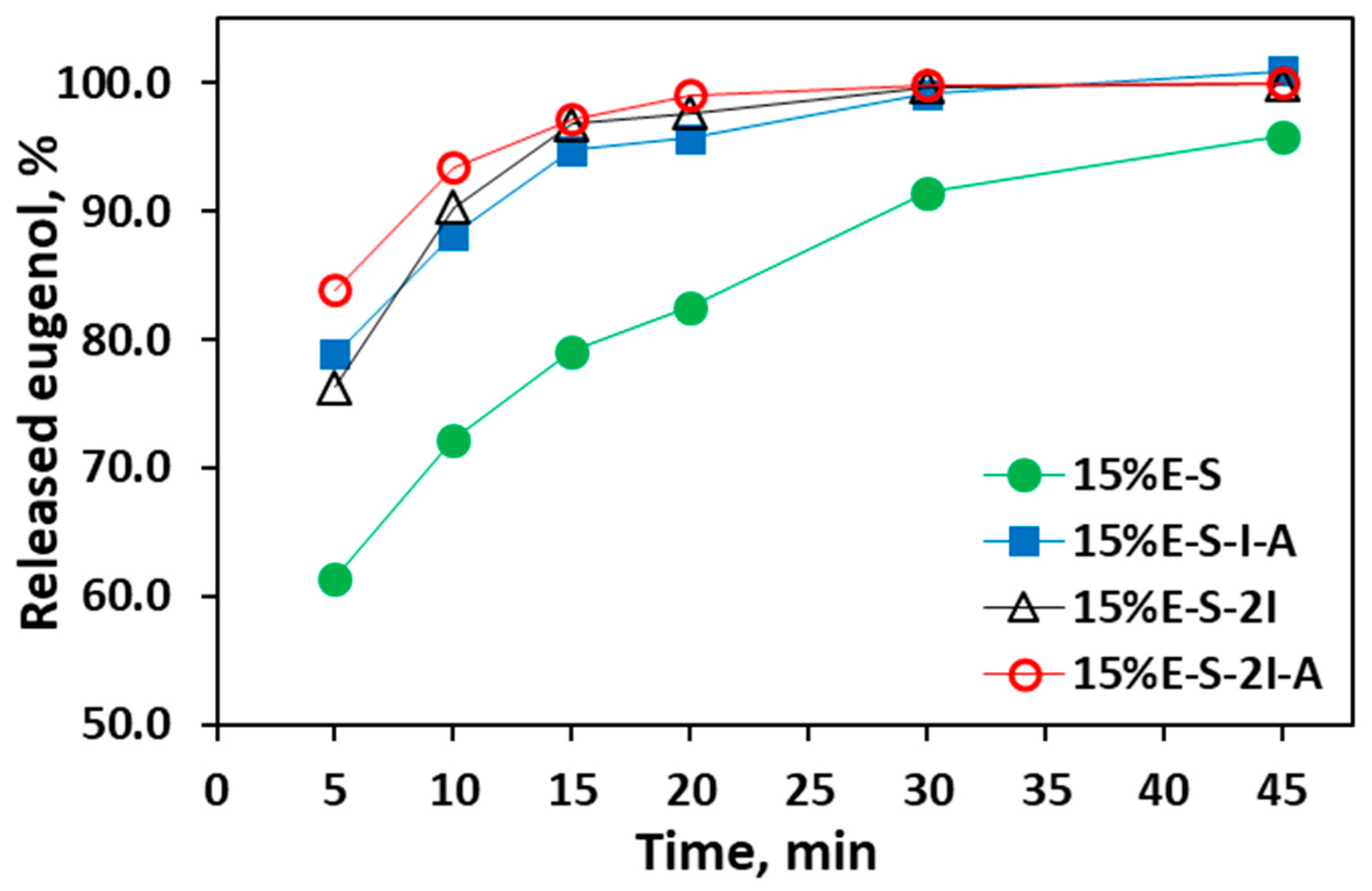
2.2.6. Dissolving Properties of the Spray-Dried Powders
3. Materials and Methods
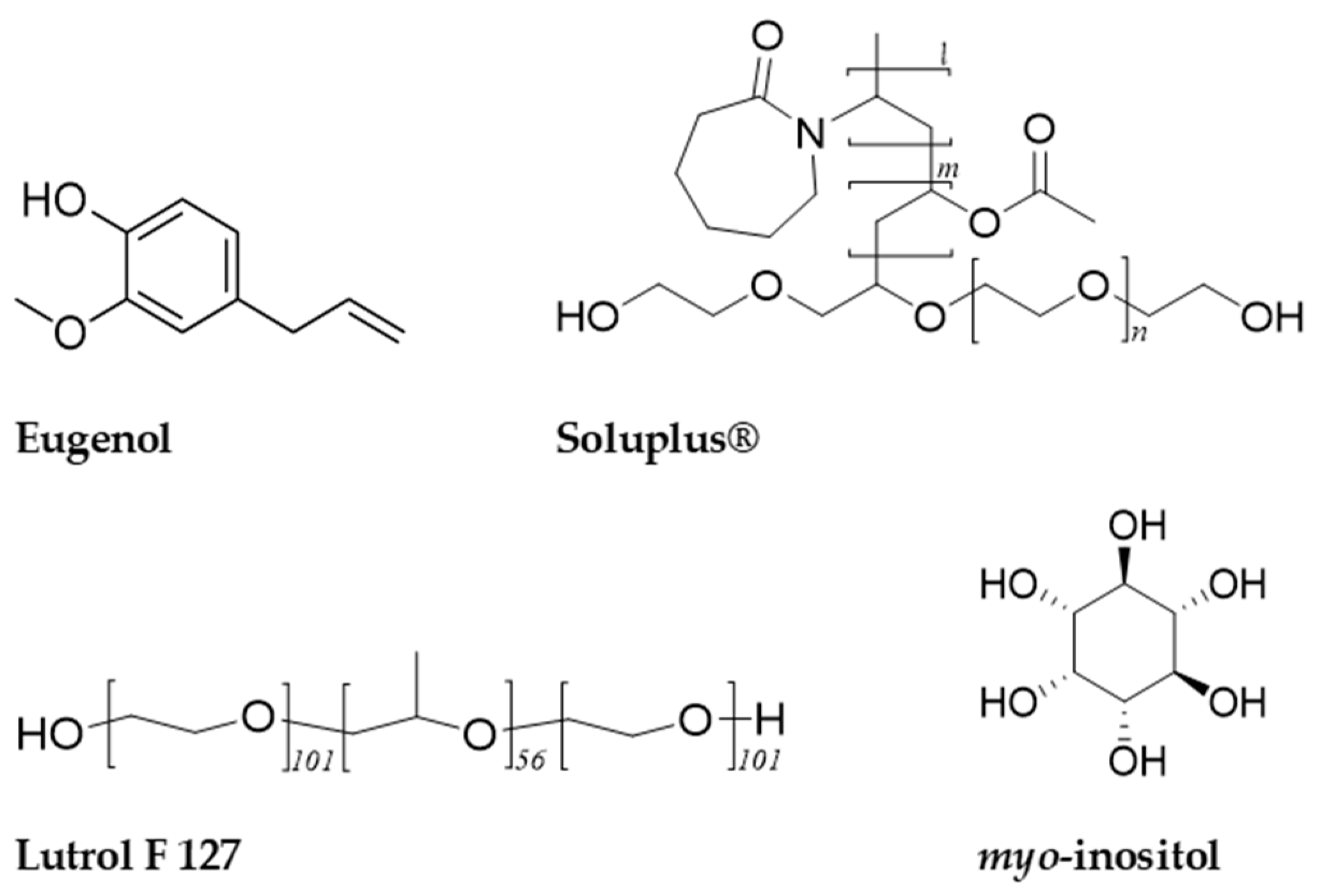
3.1. Materials and Reagents
3.2. Preparation of the Solutions for Spray Drying (Summarized in Supplementary Materials, Table S2, and in Table S3 the Excipients Are Expressed as Fractions Considering That the Sum of All Excipients Is 1.000 g)
3.2.1. Formulations Only with Soluplus®—5%E-S, 10%E-S, and 15%E-S
3.2.2. Formulations Containing Lutrol F 127 and 10%/15% Eugenol with Respect to the Total Mass of the Polymers—10%E-S-L and 15%E-S-L and 15%E-S-L-I, 15%E-S-L-2I, 15%E-S-L-I-A, and 15%E-S-L-2I-A
3.2.3. Formulations containing myo-inositol and/or Aerosil® 200—15%E-S-A, 15%E-S-L-A, 15%E-S-I, 15%E-S-2I, 15%E-S-L-I, 15%E-S-L-2I, 15%E-S-I-A, 15%E-S-2I-A, 15%E-S-L-I-A, and 15%E-S-L-2I-A:
3.3. Spray Drying Conditions
3.4. Scanning Electron Microscopy (SEM) and Particle Size
3.5. Assay of the Encapsulated Eugenol (Encapsulation Efficiency and Entrapment Efficiency)
3.6. Fourier-Transform Infrared (FTIR) Spectroscopy
3.7. Differential Scanning Calorimetry (DSC)
3.8. Particle Size in Water Solution
3.9. Loss on Drying (Water Content)
3.10. Dissolution of Eugenol from the Spray-Dried Powders
3.11. 1H NMR Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cortés Rojas, D.F.; de Souza, C.R.; Oliveira, W.P. Clove (Syzygium aromaticum): A precious spice. Asian Pac. J. Trop. Biomed. 2014, 4, 90–96. [Google Scholar] [CrossRef]
- Ulanowska, M.; Olas, B. Biological Properties and Prospects for the Application of Eugenol—A Review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef]
- Lee, M.H.; Yeon, K.-Y.; Park, C.-K.; Li, H.-Y.; Fang, Z.; Kim, M.S.; Choi, S.-Y.; Lee, S.J.; Lee, S.; Park, K.; et al. Eugenol inhibits calcium currents in dental afferent neurons. J. Dent. Res. 2005, 84, 848–851. [Google Scholar] [CrossRef]
- Park, C.-K.; Li, H.Y.; Yeon, K.-Y.; Jung, S.J.; Choi, S.-Y.; Lee, S.J.; Lee, S.; Park, K.; Kim, J.S.; Oh, S.B. Eugenol inhibits sodium currents in dental afferent neurons. J. Dent. Res. 2006, 85, 900–904. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant activity of eugenol: A structure-activity relationship study. J. Med. Food. 2011, 14, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Nagababu, E.; Rifkind, J.M.; Boindala, S.; Nakka, L. Assessment of antioxidant activity of eugenol in vitro and in vivo. Methods Mol. Biol. 2010, 610, 165–180. [Google Scholar] [CrossRef]
- Karapinar, M.; Aktuğ, Ş.E. Inhibition of foodborne pathogens by thymol, eugenol, menthol and anethole. Int. J. Food Microbiol. 1987, 4, 161–166. [Google Scholar] [CrossRef]
- Didry, N.; Dubreuil, L.; Pinkas, M. Activity of thymol, carvacrol, cinnamaldehyde and eugenol on oral bacteria. Pharm. Acta Helv. 1994, 69, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.M.; de Lima, E.O.; de Souza, E.L.; de Diniz, M.F.F.M.; Trajano, V.N.; de Medeiros, I.A. Inhibitory effect of beta-pinene, alpha-pinene and eugenol on the growth of potential infectious endocarditis causing Gram-positive bacteria. Rev. Bras. Cienc. Farm. 2007, 43, 121–126. [Google Scholar] [CrossRef]
- Tippayatum, P.; Chonhenchob, V. Antibacterial activities of thymol, eugenol and nisin against some food spoilage bacteria. Kasetsart J. (Nat. Sci.) 2007, 41, 319–323. [Google Scholar]
- Moon, S.-E.; Kim, H.-Y.; Cha, J.-D. Synergistic effect between clove oil and its major compounds and antibiotics against oral bacteria. Arch. Oral Biol. 2011, 56, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Bae, Y.-M.; Jung, K.-S.; Heu, S.; Lee, S.-Y. Antimicrobial activity of natural antimicrobial substances against spoilage bacteria isolated from fresh produce. Food Control 2013, 32, 665–672. [Google Scholar] [CrossRef]
- Takahashi, H.; Nakamura, A.; Fujino, N.; Sawaguchi, Y.; Sato, M.; Kuda, T.; Kimura, B. Evaluation of the antibacterial activity of allyl isothiocyanate, clove oil, eugenol and carvacrol against spoilage lactic acid bacteria. LWT Food Sci. Technol. 2021, 145, 111263. [Google Scholar] [CrossRef]
- de Almeida, A.L.; Caleffi-Ferracioli, K.R.; de L Scodro, R.B.; Baldin, V.P.; Montaholi, D.C.; Spricigo, L.F.; Nakamura-Vasconcelos, S.S.; Hegeto, L.A.; Sampiron, E.G.; Costacurta, G.F.; et al. Eugenol and derivatives activity against Mycobacterium tuberculosis, nontuberculous mycobacteria and other bacteria. Future Microbiol. 2019, 14, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Abrishami, A.; Manjarrés, V.; Gutmann, J.L. Historical Perspectives on the Evolution of Root Canal Sealer/Cements. J. Hist. Dent. 2022, 70, 107–118. [Google Scholar]
- Camps, J.; Pommel, L.; Bukiet, F.; About, I. Influence of the powder/liquid ratio on the properties of zinc oxide-eugenol-based root canal sealers. Dent. Mater. 2004, 20, 915–923. [Google Scholar] [CrossRef]
- Budnavi, S. (Ed.) The Merck Index, 11th ed.; Merck & Co Inc.: Rahway, NJ, USA, 1989. [Google Scholar]
- BASF. Soluplus® Technical Information (03_90801e-06). Available online: https://pharma.basf.com/products/soluplus (accessed on 10 January 2024).
- Jin, I.S.; Jo, M.J.; Park, C.W.; Chung, Y.B.; Kim, J.S.; Shin, D.H. Physicochemical, Pharmacokinetic, and Toxicity Evaluation of Soluplus® Polymeric Micelles Encapsulating Fenbendazole. Pharmaceutics 2020, 12, 1000. [Google Scholar] [CrossRef]
- Attia, M.S.; Elshahat, A.; Hamdy, A.; Fathi, A.M.; Emad-Eldin, M.; Ghazy, F.-E.S.; Chopra, H.; Ibrahim, T.M. Soluplus® as a solubilizing excipient for poorly water-soluble drugs: Recent advances in formulation strategies and pharmaceutical product features. J. Drug. Deliv. Sci. Technol. 2023, 84, 104519. [Google Scholar] [CrossRef]
- Linn, M.; Collnot, E.-M.; Djuric, D.; Hempel, K.; Fabian, E.; Kolter, K.; Lehr, C.-M. Soluplus® as an effective absorption enhancer of poorly soluble drugs in vitro and in vivo. Eur. J. Pharm. Sci. 2012, 45, 336–343. [Google Scholar] [CrossRef]
- Alopaeus, J.F.; Hagesæther, E.; Tho, I. Micellisation Mechanism and Behaviour of Soluplus®–Furosemide Micelles: Preformulation Studies of an Oral Nanocarrier-Based System. Pharmaceuticals 2019, 12, 15. [Google Scholar] [CrossRef]
- Pignatello, R.; Corsaro, R.; Bonaccorso, A.; Zingale, E.; Carbone, C.; Musumeci, T. Soluplus® polymeric nanomicelles improve solubility of BCS-class II drugs. Drug. Deliv. Transl. Res. 2022, 12, 1991–2006. [Google Scholar] [CrossRef]
- BASF. Lutrol L and Lutrol F-Grades, Technical Information (03_100102e-06). Available online: https://pharma.basf.com/products/ (accessed on 28 September 2023).
- Broadhead, J.; Edmond Rouan, S.K.; Rhodes, C.T. The Spray Drying of Pharmaceuticals. Drug Dev. Ind. Pharm. 1992, 18, 1169–1206. [Google Scholar] [CrossRef]
- Vehringa, R.; Foss, W.R.; Lechuga-Ballesteros, D. Particle formation in spray drying. Aerosol. Sci. 2007, 38, 728–746. [Google Scholar] [CrossRef]
- Tran, P.; Pyo, Y.C.; Kim, D.H.; Lee, S.E.; Kim, J.K.; Park, J.S. Overview of the Manufacturing Methods of Solid Dispersion Technology for Improving the Solubility of Poorly Water-Soluble Drugs and Application to Anticancer Drugs. Pharmaceutics 2019, 11, 132. [Google Scholar] [CrossRef]
- Ziaeea, A.; Albadarina, A.B.; Padrela, L.; Femmer, T.; O’Reilly, E.; Walker, G. Spray drying of pharmaceuticals and biopharmaceuticals: Critical parameters and experimental process optimization approaches. Eur. J. Pharm. Sci. 2019, 127, 300–318. [Google Scholar] [CrossRef]
- Vehring, R. Pharmaceutical particle engineering via spray drying. Pharm. Res. 2008, 25, 999–1021. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Klojdová, I.; Milota, T.; Smetanová, J.; Stathopoulos, C. Encapsulation: A Strategy to Deliver Therapeutics and Bioactive Compounds? Pharmaceuticals 2023, 16, 362. [Google Scholar] [CrossRef]
- Bajaj, T.; Das Gupta, G.; Singh, C. Spray dried mebendazole–loaded Soluplus-based polymeric micelles for improved biopharmaceutical attributes: In vitro and in vivo studies. Colloid Polym. Sci. 2024, 302, 1067–1080. [Google Scholar] [CrossRef]
- Anwer, M.K.; Ahmed, M.M.; Alshetaili, A.; Almutairy, B.K.; Alalaiwe, A.; Fatima, F.; Ansari, M.N.; Iqbal, M. Preparation of spray dried amorphous solid dispersion of diosmin in soluplus with improved hepato-renoprotective activity: In vitro anti-oxidant and in-vivo safety studies. J. Drug Deliv. Technol. 2020, 60, 102101. [Google Scholar] [CrossRef]
- Kamal, M.M.; Salawi, A.; Lam, M.; Nokhodchi, A.; Abu-Fayyad, A.; El Sayed, K.A.; Nazzal, S. Development and characterization of curcumin-loaded solid self-emulsifying drug delivery system (SEDDS) by spray drying using Soluplus® as solid carrier. Powder Technol. 2020, 369, 137–145. [Google Scholar] [CrossRef]
- Peng, S.; Zou, L.; Liu, W.; Gan, L.; Liu, W.; Liang, R.; Liu, C.; Niu, J.; Cao, Y.; Liu, Z.; et al. Storage Stability and Antibacterial Activity of Eugenol Nanoliposomes Prepared by an Ethanol Injection–Dynamic High-Pressure Microfluidization Method. JFP 2015, 78, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, B.; Si, H.Y.; Lin, L.; Chen, L. Release characteristics and antibacterial activity of solid state eugenol/betacyclodextrin inclusion complex. J. Incl. Phenom. Macrocycl. Chem. 2011, 71, 207–213. [Google Scholar] [CrossRef]
- Hill, L.E.; Gomes, C.; Taylor, T.M. Characterization of beta-cyclodextrin inclusion complexes containing essential oils. (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT-Food Sci. Technol. 2013, 51, 86–93. [Google Scholar] [CrossRef]
- Celebioglu, A.; Yildiz, Z.I.; Uyar, T. Fabrication of Electrospun Eugenol/Cyclodextrin Inclusion Complex Nanofibrous Webs for Enhanced Antioxidant Property, Water Solubility, and High Temperature Stability. J. Agric. Food Chem. 2018, 66, 457–466. [Google Scholar] [CrossRef]
- Talón, E.; Lampi, A.-M.; Vargas, M.; Chiralt, A.; Jouppila, K.; González-Martínez, C. Encapsulation of eugenol by spray-drying using whey protein isolate or lecithin: Release kinetics, antioxidant and antimicrobial properties. Food Chem. 2019, 295, 588–598. [Google Scholar] [CrossRef]
- Bittencourta, P.R.S.; dos Santos da Veigab, R.; da Silva-Buzanellob, R.A.; Scremina, F.R.; Becker-Algerib, T.A.; Corsob, M.P.; de Moraes Floresa, É.L.; Cananb, C. Optimization of eugenol-loaded microcapsules obtained by spray drying using a simplex-centroid mixture design. Quim. Nova 2022, 45, 152–158. [Google Scholar] [CrossRef]
- Sahlan, M.; Lestari, S.F.; Indrawati, T.; Pratami, D.K.; Wijarnako, A.; Hermansyah, H.; Lischer, K.; Rabbani, A.N. Microencapsulation of clove oil using spray dry with casein encapsulator and activity test towards Streptococcus mutans. AIP Conf. Proc. 2019, 2193, 030006. [Google Scholar] [CrossRef]
- Woranuch, S.; Yoksan, R. Eugenol-loaded chitosan nanoparticles: I. Thermal stability improvement of eugenol through encapsulation. Carbohydr. Polym. 2013, 96, 578–585. [Google Scholar] [CrossRef]
- Paulo, F.; Santos, L. Double emulsion solvent evaporation approach as a novel eugenol delivery system—Optimization by response surface methodology. Ind. Crops Prod. 2018, 126, 287–301. [Google Scholar] [CrossRef]
- Veneranda, M.; Hu, Q.; Wang, T.; Luo, Y.; Castro, K.; Madariaga, J.M. Formation and characterization of zein-caseinate-pectin complex nanoparticles for encapsulation of eugenol. LWT-Food Sci. Technol. 2018, 89, 596–603. [Google Scholar] [CrossRef]
- Shao, Y.; Wu, C.; Wu, T.; Li, Y.; Chen, S.; Yuan, C.; Hu, Y. Eugenol-chitosan nanoemulsions by ultrasound-mediated emulsification: Formulation, characterization and antimicrobial activity. Carbohydr. Polym. 2018, 193, 144–152. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H. Myo-inositol for insulin resistance, metabolic syndrome, polycystic ovary syndrome and gestational diabetes. Open Heart 2022, 9, e001989. [Google Scholar] [CrossRef] [PubMed]
- Croze, M.L.; Soulage, C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef]
- York, P. Application of powder failure testing equipment in assessing effect of glidants on flowability of cohesive pharmaceutical powders. J. Pharm. Sci. 1975, 64, 1216–1221. [Google Scholar] [CrossRef]
- Sofroniou, C.; Baglioni, M.; Mamusa, M.; Resta, C.; Doutch, J.; Smets, J.; Baglioni, P. Self-Assembly of Soluplus in Aqueous Solutions: Characterization and Prospectives on Perfume Encapsulation. ACS Appl. Mater. Interfaces 2022, 14, 14791–14804. [Google Scholar] [CrossRef] [PubMed]


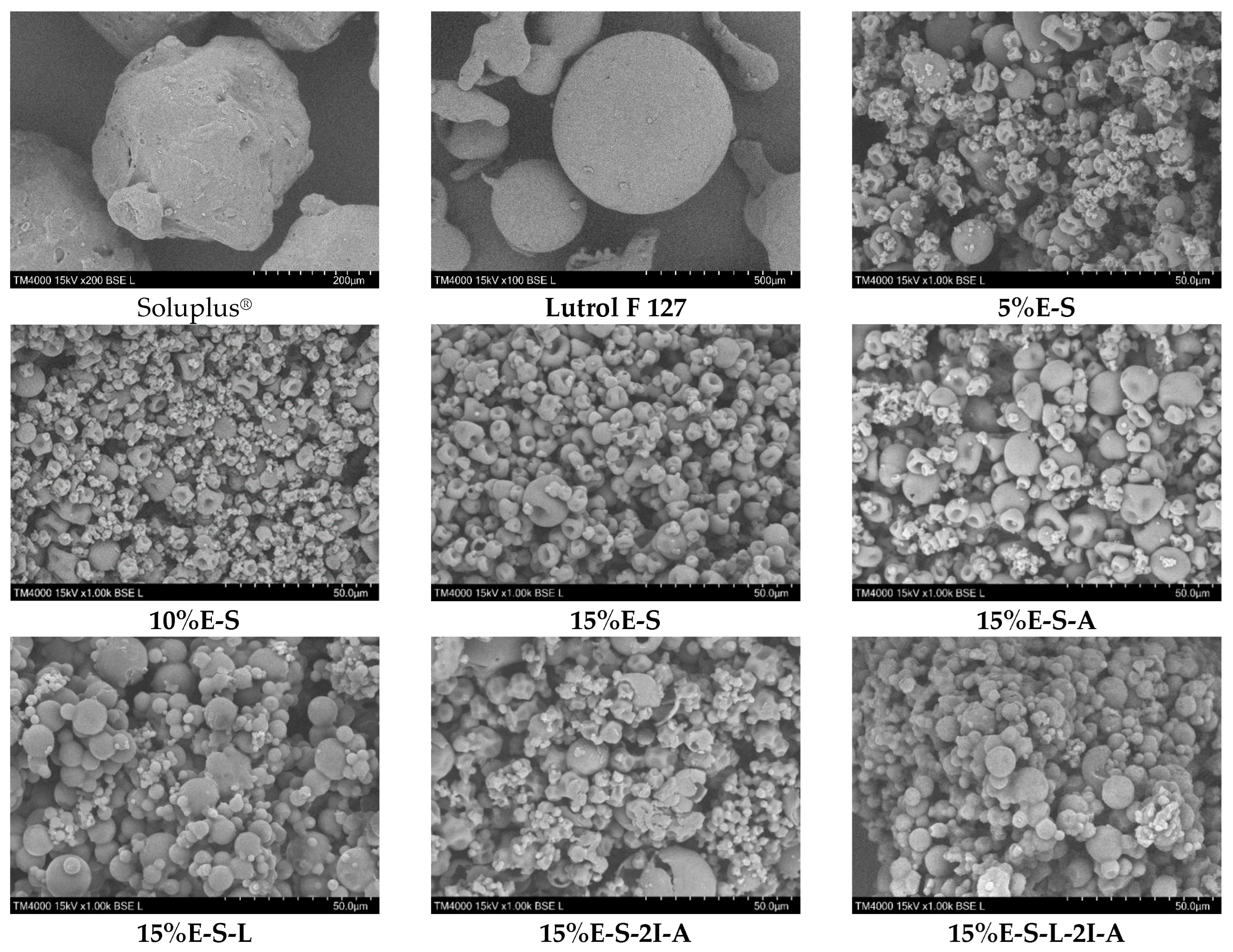

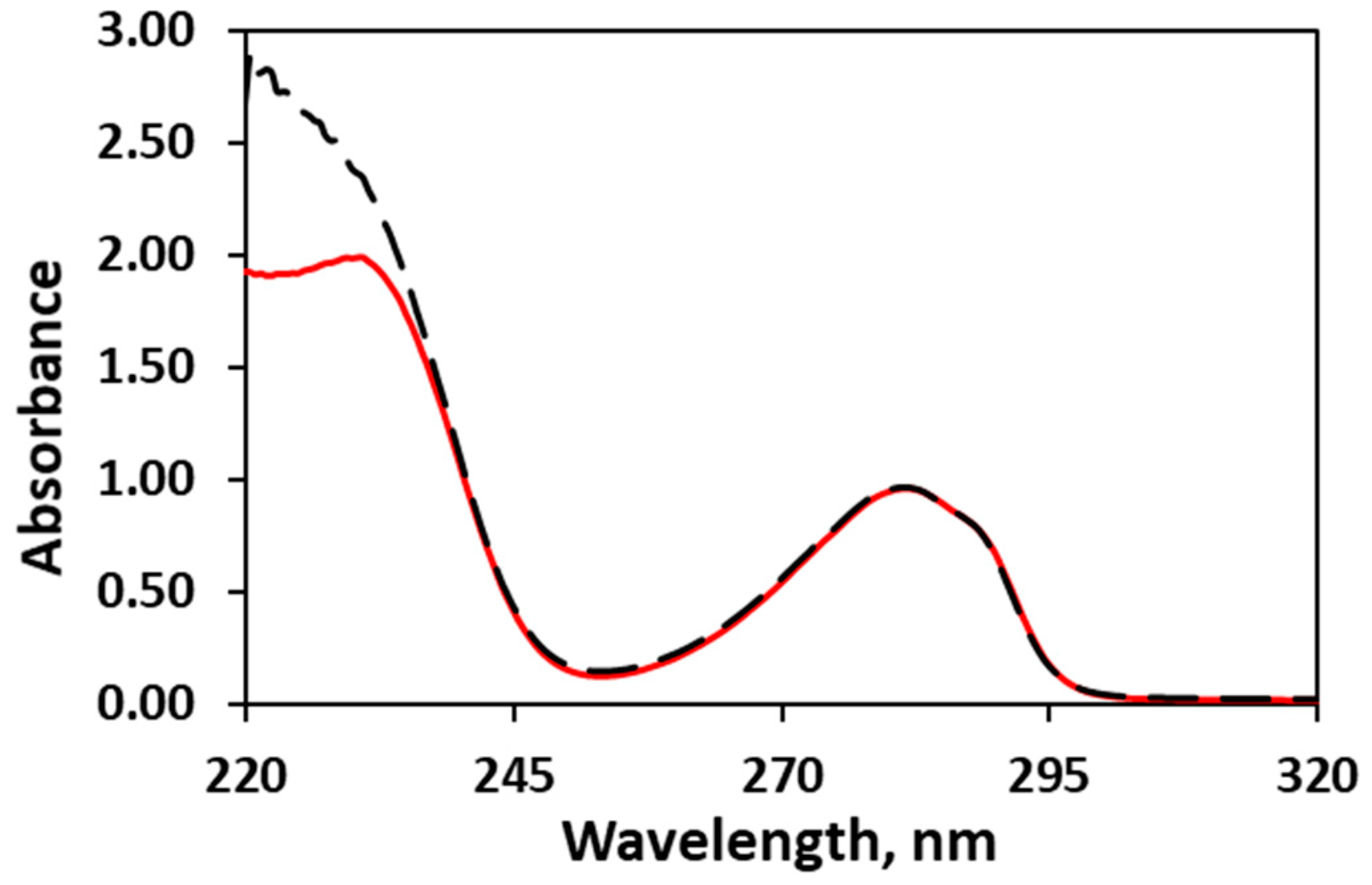
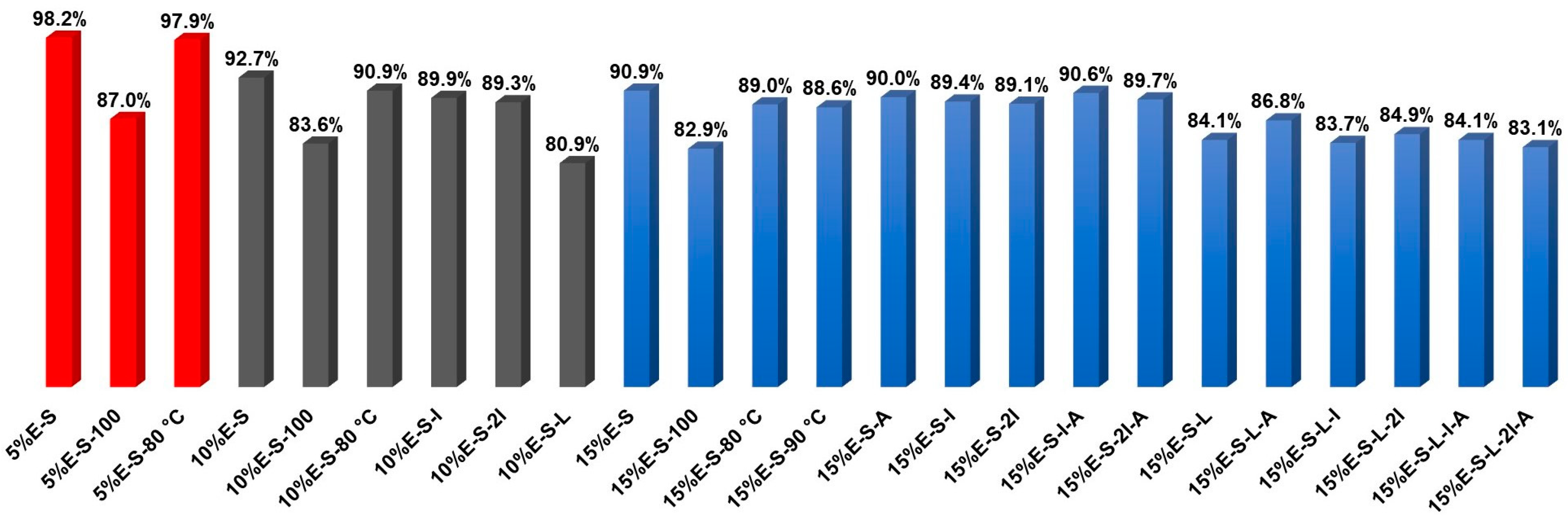

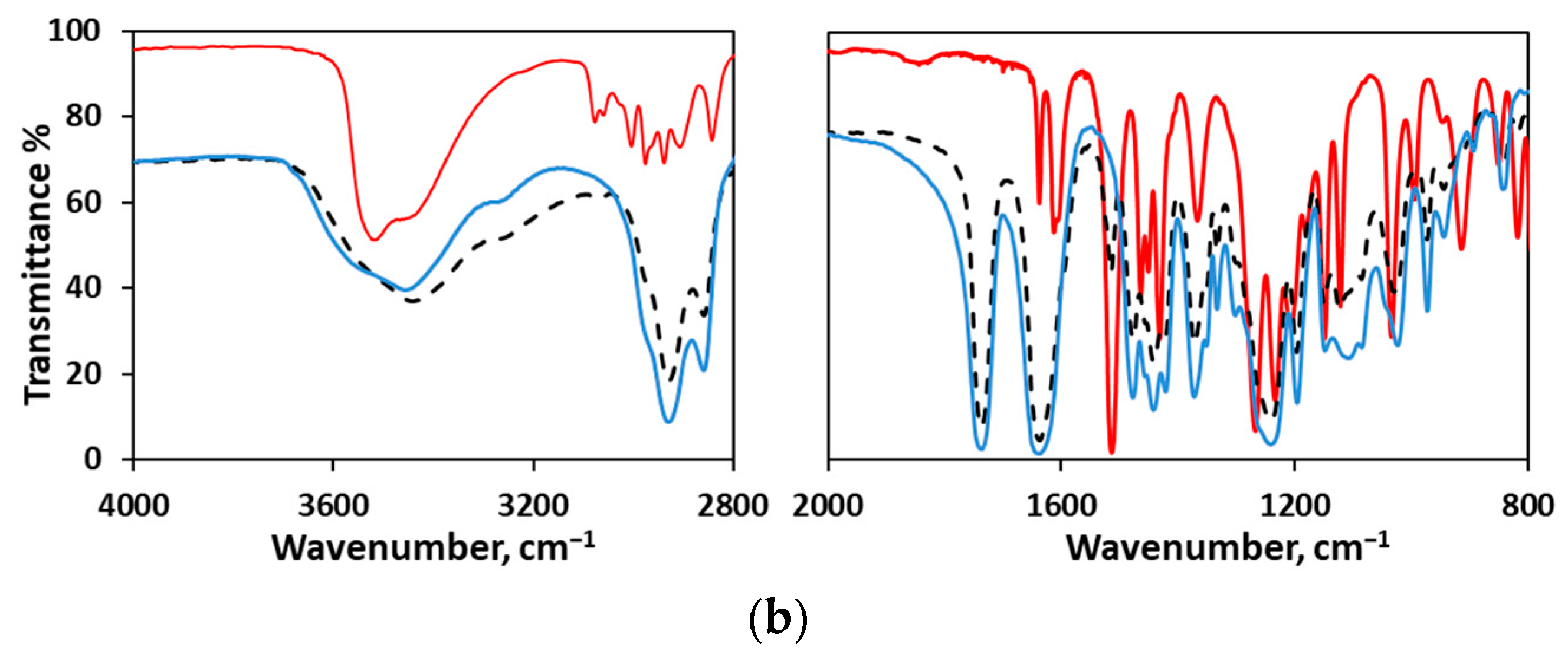
| Formulation | Solids/H2O (g/g) | Inlet/Outlet Air Temperature (°C) 1 | Encapsulated Eugenol (mg/g) 2 | Theoretical Eugenol (mg/g) 3 | Encapsulation Efficiency (%) |
|---|---|---|---|---|---|
| 5% Eugenol with respect to the mass of the polymer | |||||
| 5%E-S | 4/40 | 70/30 | 49.5 | 50.4 | 98.2 ± 1.9 |
| 5%E-S-100 | 4/100 | 70/30 | 44.0 | 50.5 | 87.0 ± 1.3 |
| 5%E-S-80 °C | 4/40 | 80/42 | 49.9 | 50.9 | 97.9 ± 1.8 |
| 10% Eugenol with respect to the mass of the polymer(s) | |||||
| 10%E-S | 4/40 | 70/30 | 93.1 | 100.4 | 92.7 ± 1.4 |
| 10%E-S-80 °C | 4/40 | 80/41 | 91.1 | 100.2 | 90.9 ± 2.5 |
| 10%E-S-100 | 4/100 | 80/41 | 84.1 | 100.7 | 83.6 ± 2.4 |
| 10%E-S-I | 8/80 | 80/41 | 90.0 | 100.2 | 89.9 ± 2.8 |
| 10%E-S-2I | 12/80 | 80/41 | 89.5 | 100.3 | 89.3 ± 1.9 |
| 10%E-S-L | 4/40 | 80/41 | 81.0 | 100.1 | 80.9 ± 1.5 |
| 15% Eugenol with respect to the mass of the polymer(s) | |||||
| 15%E-S | 4/40 | 70/30 | 136.7 | 150.4 | 90.9 ± 2.1 4 |
| 15%E-S-100 | 4/100 | 70/30 | 124.6 | 150.4 | 82.9 ± 1.1 |
| 15%E-S-80 °C | 4/40 | 80/41 | 134.0 | 150.6 | 89.0 ± 2.4 |
| 15%E-S-90 °C | 4/40 | 90/45 | 133.4 | 150.5 | 88.6 ± 1.8 |
| 15%E-S-A | 4.1/40 | 80/41 | 135.4 | 150.4 | 90.0 ± 0.9 |
| 15%E-S-I | 8/80 | 80/41 | 134.5 | 150.5 | 89.4 ± 1.1 |
| 15%E-S-2I | 12/80 | 80/41 | 134.1 | 150.5 | 89.1 ± 1.6 |
| 15%E-S-I-A | 8.1/80 | 80/41 | 136.2 | 150.3 | 90.6 ± 0.8 |
| 15%E-S-2I-A | 12.1/80 | 80/41 | 134.8 | 150.4 | 89.7 ± 1.5 |
| 15%E-S-L | 4/40 | 70/30 | 126.7 | 150.8 | 84.1 ± 1.8 |
| 15%E-S-L-A | 4.1/40 | 70/30 | 130.7 | 150.5 | 86.8 ± 1.6 |
| 15%E-S-L-I | 8/80 | 70/30 | 125.8 | 150.3 | 83.7 ± 1.0 |
| 15%E-S-L-2I | 12/80 | 70/30 | 125.8 | 150.5 | 84.9 ± 1.8 |
| 15%E-S-L-I-A | 8.1/80 | 70/30 | 126.4 | 150.4 | 84.1 ± 1.6 |
| 15%E-S-L-2I-A | 12.1/80 | 70/30 | 124.8 | 150.2 | 83.1 ± 1.5 |
| Formulation | DH ± SD | PDI ± SD |
|---|---|---|
| 5%E-S | 66.4 ± 2.1 | 0.082 ± 0.075 |
| 10%E-S | 68.7 ± 2.2 | 0.075 ± 0.082 |
| 15%E-S | 64.5 ± 1.8 | 0.080 ± 0.057 |
| 15%E-S-A | 63.1 ± 2.1 | 0.156 ± 0.101 |
| 15%E-S-I | 69.7 ± 2.4 | 0.161 ± 0.107 |
| 15%E-S-2I | 66.1 ± 2.8 | 0.202 ± 0.093 |
| 15%E-S-I-A | 81.6 ± 3.9 | 0.163 ± 0.054 |
| 15%E-S-2I-A | 74.4 ± 1.7 | 0.114 ± 0.064 |
| 15%E-S-L | 69.2 ± 2.7 | 0.128 ± 0.128 |
| 15%E-S-L-A | 75.2 ± 6.1 | 0.247 ± 0.107 |
| 15%E-S-L-I | 71.0 ± 7.1 | 0.195 ± 0.128 |
| 15%E-S-L-I-A | 82.8 ± 6.3 | 0.299 ± 0.084 |
| 15%E-S-L-2I | 75.3 ± 1.8 | 0.101 ± 0.080 |
| 15%E-S-L-2I-A | 77.8 ± 5.7 | 0.197 ± 0.165 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koleva, I.Z.; Tzachev, C.T. Efficient Improvement of Eugenol Water Solubility by Spray Drying Encapsulation in Soluplus® and Lutrol F 127. Pharmaceuticals 2024, 17, 1156. https://doi.org/10.3390/ph17091156
Koleva IZ, Tzachev CT. Efficient Improvement of Eugenol Water Solubility by Spray Drying Encapsulation in Soluplus® and Lutrol F 127. Pharmaceuticals. 2024; 17(9):1156. https://doi.org/10.3390/ph17091156
Chicago/Turabian StyleKoleva, Iskra Z., and Christo T. Tzachev. 2024. "Efficient Improvement of Eugenol Water Solubility by Spray Drying Encapsulation in Soluplus® and Lutrol F 127" Pharmaceuticals 17, no. 9: 1156. https://doi.org/10.3390/ph17091156
APA StyleKoleva, I. Z., & Tzachev, C. T. (2024). Efficient Improvement of Eugenol Water Solubility by Spray Drying Encapsulation in Soluplus® and Lutrol F 127. Pharmaceuticals, 17(9), 1156. https://doi.org/10.3390/ph17091156







