Characterization of Antineoplastic Agents Inducing Taste and Smell Disorders Using the FAERS Database
Abstract
1. Introduction
2. Results
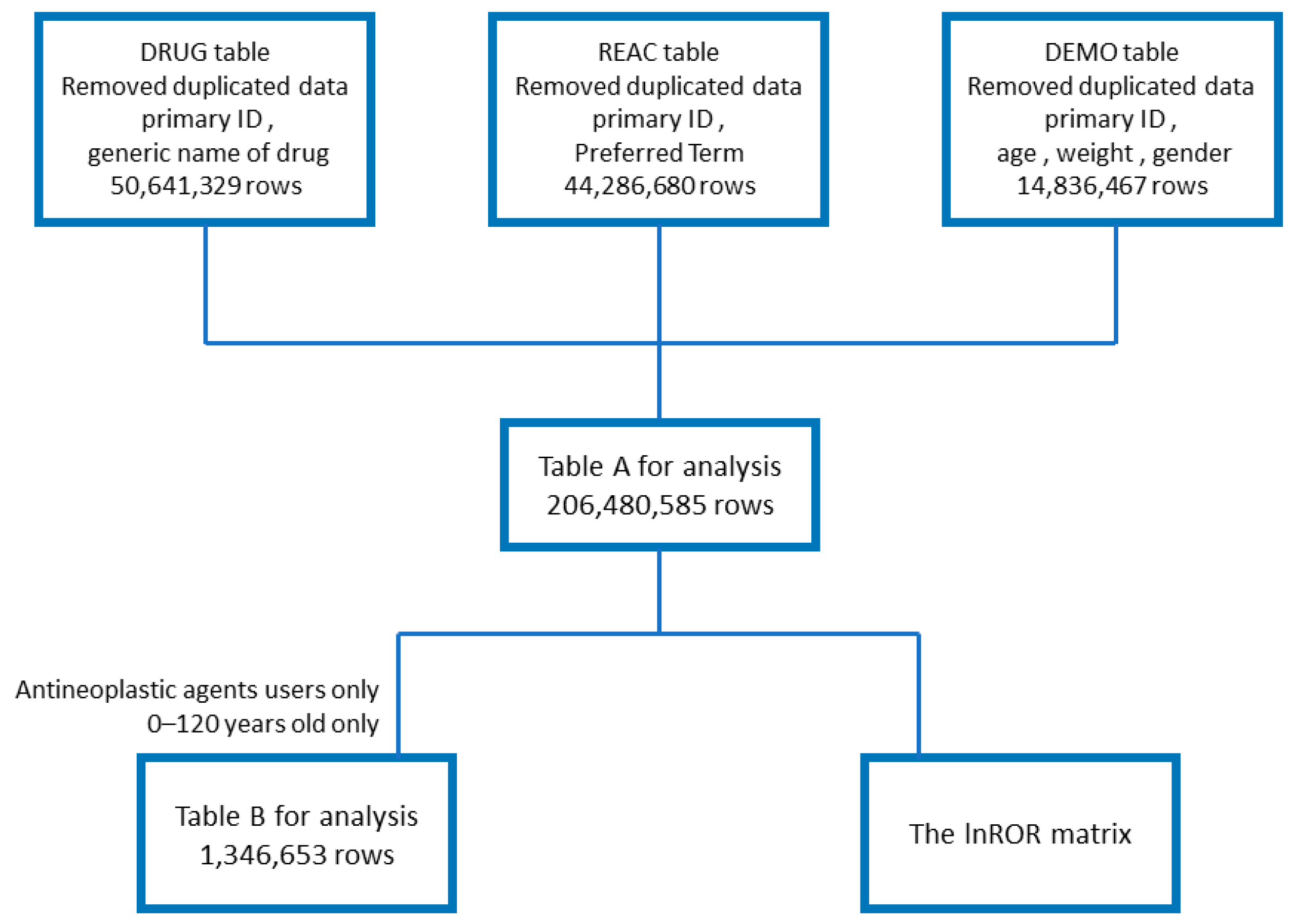
2.1. Creation of Data Tables
2.2. Number of Reports of Taste and Smell Disorders
2.3. Relationships of Patient Age and Gender with Taste and Smell Disorders Induced by Antineoplastic Agents
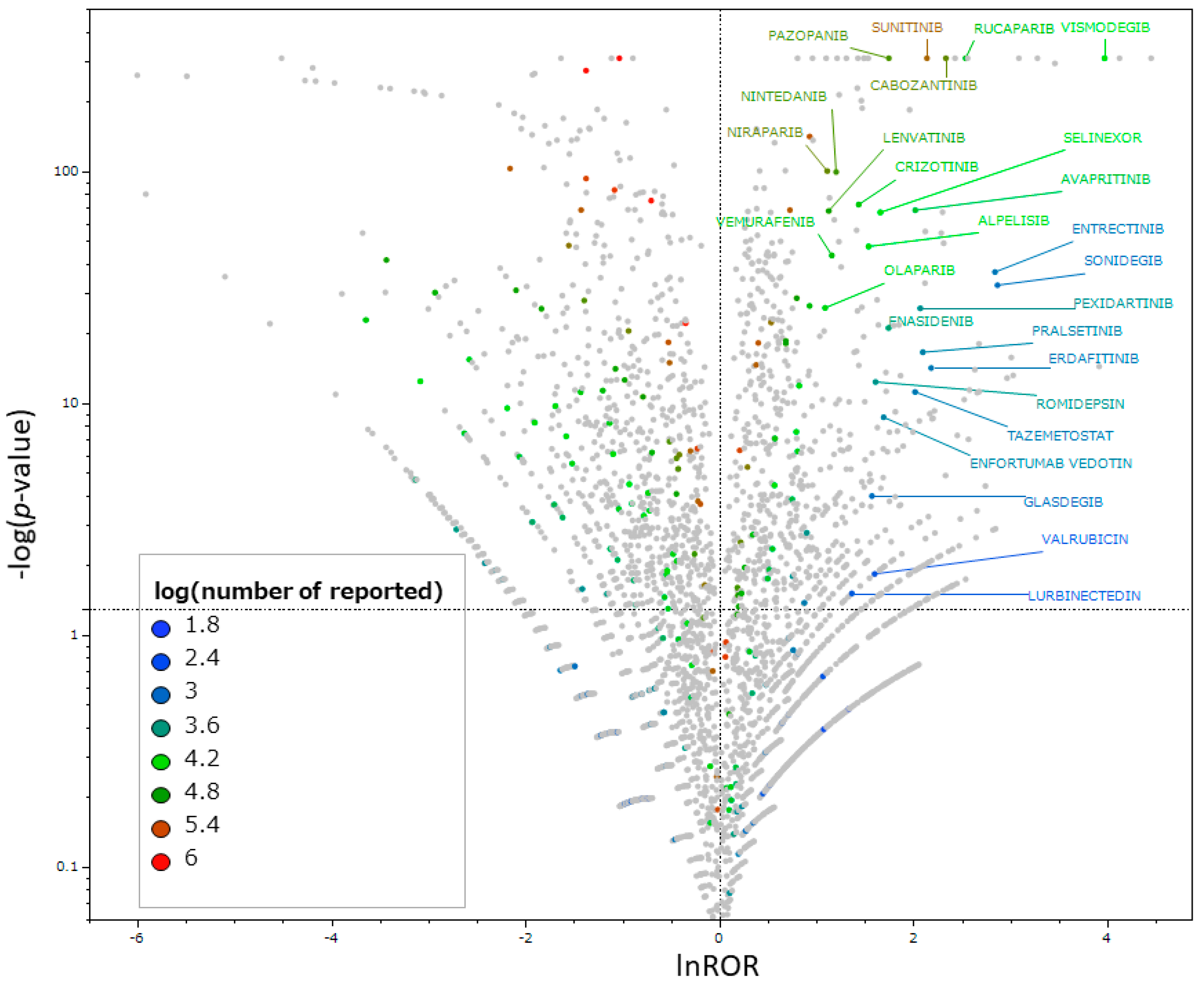
2.4. Identification of Antineoplastic Agents That Induce Taste and Smell Disorders
2.5. Relationships between the Class of Antineoplastic Agents and Taste and Smell Disorders
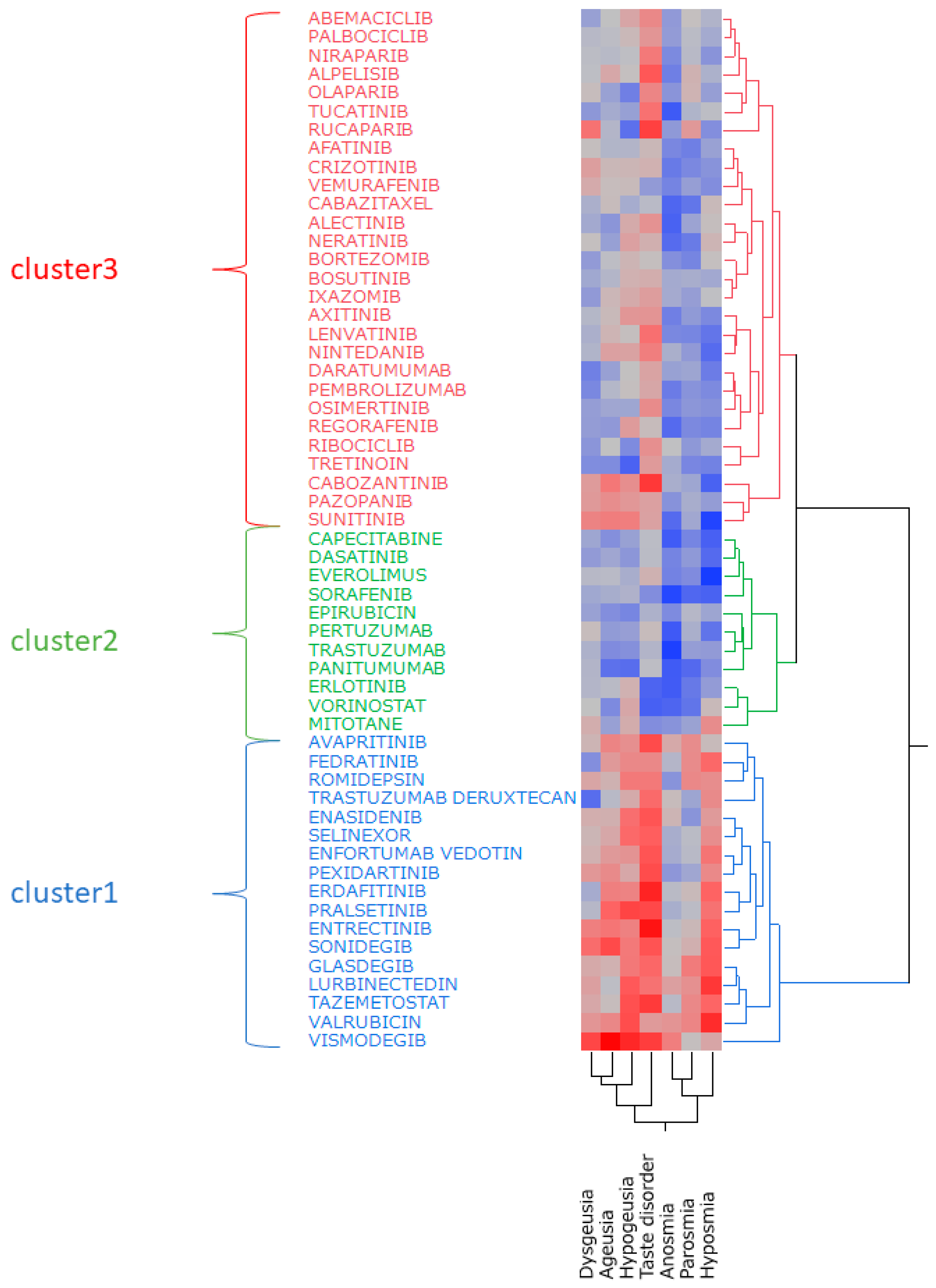
2.6. Hierarchical Cluster Analysis
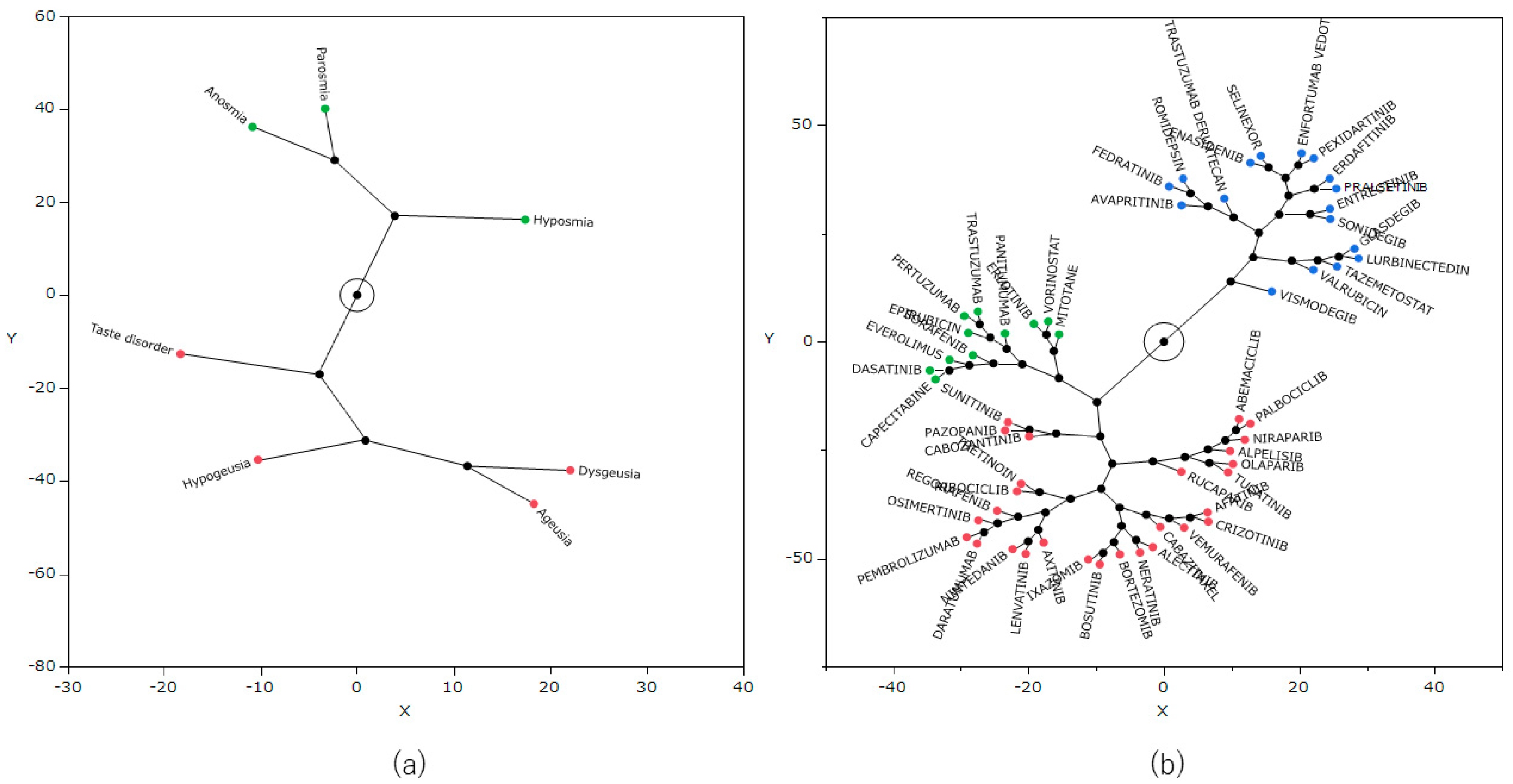
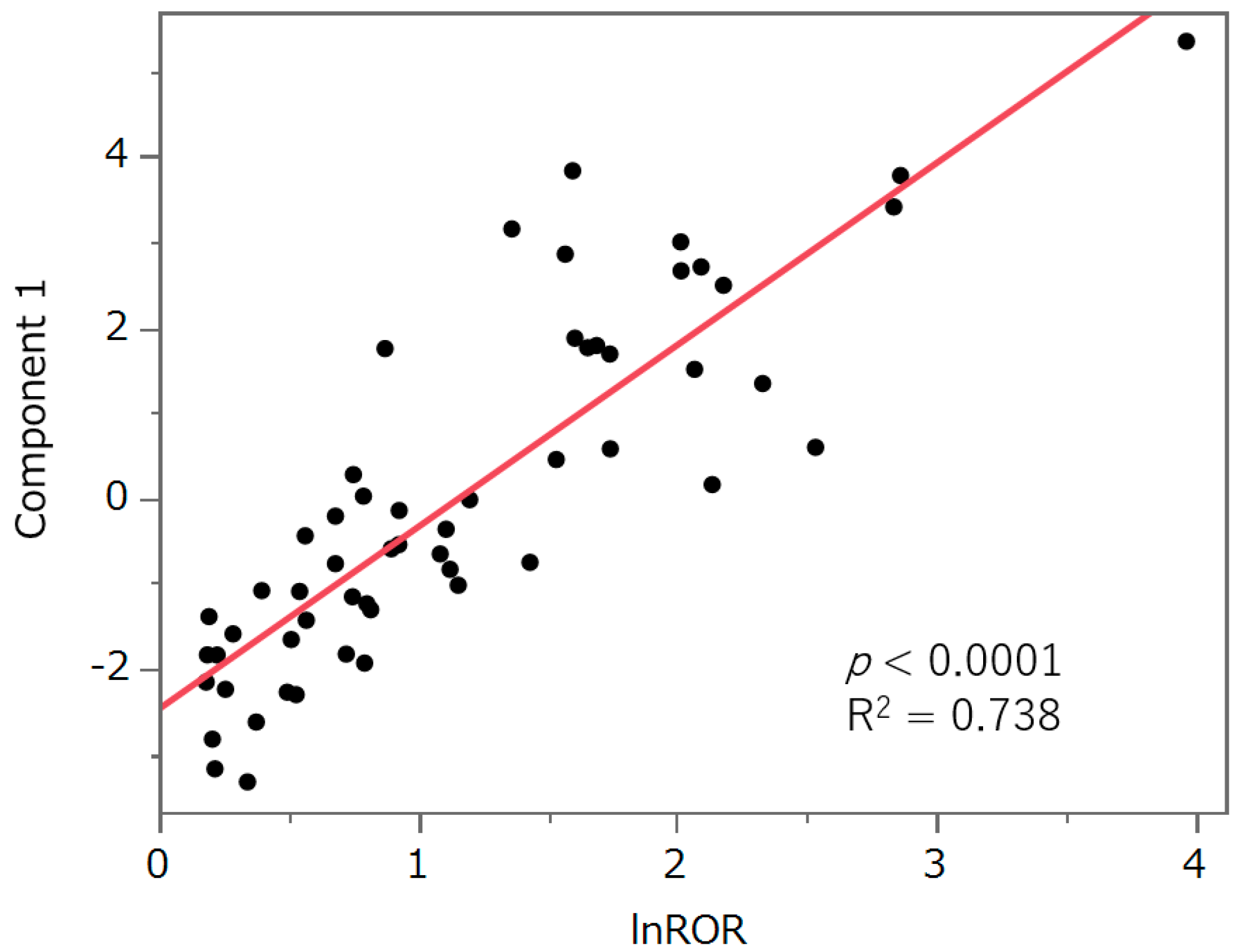
2.7. Principal Component Analysis
3. Discussion
3.1. Number of Reports of Taste and Smell Disorders
3.2. Characteristics of Patients with Taste and Smell Disorders and Antineoplastic Agents
3.3. Relationships of Antineoplastic Agents with Taste and Smell Disorders
3.4. Relationship between the Class of Antineoplastic Agents and Taste and Smell Disorders
3.5. Hierarchical Cluster Analysis
3.6. Principal Component Analysis
3.7. Limitations
4. Materials and Methods
4.1. FAERS Database
4.2. Adverse Event Terms and Drugs for Analysis
4.3. Number of Reports of Taste and Smell Disorders
4.4. Relationships of Taste and Smell Disorders with Age and Gender Among Patients Using Antineoplastic Agents
4.5. Relationships between Antineoplastic Agents and Taste and Smell Disorders
4.6. Relationships of the Class of Antineoplastic Agents with Taste and Smell Disorders
4.7. Creation of Data Tables for Principal Component and Cluster Analyses
4.8. Hierarchical Cluster Analysis
4.9. Principal Component Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cohen, J.; Wakefield, C.E.; Laing, D.G. Smell and taste disorders resulting from cancer and chemotherapy. Curr. Pharm. Des. 2016, 22, 2253–2263. [Google Scholar] [CrossRef]
- Hutton, J.L.; Baracos, V.E.; Wismer, W.V. Chemosensory dysfunction is a primary factor in the evolution of declining nutritional status and quality of life in patients with advanced cancer. J. Pain Symptom Manag. 2007, 33, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Wickham, R.S.; Rehwaldt, M.; Kefer, C.; Shott, S.; Abbas, K.; Glynn-Tucker, E.; Potter, C.; Blendowski, C. Taste changes experienced by patients receiving chemotherapy. Oncol. Nurs. Forum 1999, 26, 697–706. [Google Scholar] [PubMed]
- Comeau, T.B.; Epstein, J.B.; Migas, C. Taste and smell dysfunction in patients receiving chemotherapy: A review of current knowledge. Support Care Cancer 2001, 9, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Brisbois, T.D.; de Kock, I.H.; Watanabe, S.M.; Baracos, V.E.; Wismer, W.V. Characterization of chemosensory alterations in advanced cancer reveals specific chemosensory phenotypes impacting dietary intake and quality of life. J. Pain Symptom Manag. 2011, 41, 673–683. [Google Scholar] [CrossRef]
- Tuccori, M.; Lapi, F.; Testi, A.; Ruggiero, E.; Moretti, U.; Vannacci, A.; Bonaiuti, R.; Antonioli, L.; Fornai, M.; Giustarini, G.; et al. Drug-induced taste and smell alterations: A case/non-case evaluation of an italian database of spontaneous adverse drug reaction reporting. Drug Saf. 2011, 34, 849–859. [Google Scholar] [CrossRef]
- Gamper, E.M.; Zabernigg, A.; Wintner, L.M.; Giesinger, J.M.; Oberguggenberger, A.; Kemmler, G.; Sperner-Unterweger, B.; Holzner, B. Coming to your senses: Detecting taste and smell alterations in chemotherapy patients. A systematic review. J. Pain Symptom Manag. 2012, 44, 880–895. [Google Scholar] [CrossRef]
- Henkin, R.I. Drug-induced taste and smell disorders. Incidence, mechanisms and management related primarily to treatment of sensory receptor dysfunction. Drug Saf. 1994, 11, 318–377. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Zervakis, J. Taste and smell perception in the elderly: Effect of medications and disease. Adv. Food Nutr. Res. 2002, 44, 247–346. [Google Scholar] [CrossRef]
- Debbaneh, P.; McKinnon, L.; Haidari, M.; Liang, J. Drug-induced olfactory and gustatory dysfunction: Analysis of FDA adverse events reporting system. Auris Nasus Larynx 2023, 50, 558–564. [Google Scholar] [CrossRef]
- Deems, D.A.; Doty, R.L.; Settle, R.G.; Moore-Gillon, V.; Shaman, P.; Mester, A.F.; Kimmelman, C.P.; Brightman, V.J.; Snow, J.B., Jr. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch. Otolaryngol. Head Neck Surg. 1991, 117, 519–528. [Google Scholar] [CrossRef]
- Goodspeed, R.B.; Gent, J.F.; Catalanotto, F.A. Chemosensory dysfunction. Clinical evaluation results from a taste and smell clinic. Postgrad. Med. 1987, 81, 251–257, 260. [Google Scholar] [CrossRef] [PubMed]
- Buttiron Webber, T.; Briata, I.M.; DeCensi, A.; Cevasco, I.; Paleari, L. Taste and smell disorders in cancer treatment: Results from an integrative rapid systematic review. Int. J. Mol. Sci. 2023, 24, 2538. [Google Scholar] [CrossRef]
- Malta, C.E.N.; de Lima Martins, J.O.; Carlos, A.C.A.M.; Freitas, M.O.; Magalhães, I.A.; de Vasconcelos, H.C.A.; de Lima Silva-Fernandes, I.J.; de Barros Silva, P.G. Risk factors for dysgeusia during chemotherapy for solid tumors: A retrospective cross-sectional study. Support Care Cancer 2022, 30, 313–325. [Google Scholar] [CrossRef]
- Amézaga, J.; Alfaro, B.; Ríos, Y.; Larraioz, A.; Ugartemendia, G.; Urruticoechea, A.; Tueros, I. Assessing taste and smell alterations in cancer patients undergoing chemotherapy according to treatment. Support Care Cancer 2018, 26, 4077–4086. [Google Scholar] [CrossRef]
- Zabernigg, A.; Gamper, E.M.; Giesinger, J.M.; Rumpold, G.; Kemmler, G.; Gattringer, K.; Sperner-Unterweger, B.; Holzner, B. Taste alterations in cancer patients receiving chemotherapy: A neglected side effect? Oncologist 2010, 15, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Eravcı, F.C.; Uçar, G.; Özcan, K.M.; Çolak, M.; Ergün, Y.; Açıkgöz, Y.; Ikincioğulları, A.; Uncu, D.; Dere, H.H. The effect of chemotherapy on olfactory function and mucociliary clearance. Support Care Cancer 2021, 29, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Pugnaloni, S.; Vignini, A.; Borroni, F.; Sabbatinelli, J.; Alia, S.; Fabri, M.; Taus, M.; Mazzanti, L.; Berardi, R. Modifications of taste sensitivity in cancer patients: A method for the evaluations of dysgeusia. Support Care Cancer 2020, 28, 1173–1181. [Google Scholar] [CrossRef]
- Riga, M.; Chelis, L.; Papazi, T.; Danielides, V.; Katotomichelakis, M.; Kakolyris, S. Hyposmia: An underestimated and frequent adverse effect of chemotherapy. Support Care Cancer 2015, 23, 3053–3058. [Google Scholar] [CrossRef]
- Ikeda, M.; Ikui, A.; Komiyama, A.; Kobayashi, D.; Tanaka, M. Causative factors of taste disorders in the elderly, and therapeutic effects of zinc. J. Laryngol. Otol. 2008, 122, 155–160. [Google Scholar] [CrossRef]
- Search for Ethical Drug Information on the Website of the Pharmaceuticals and Medical Devices Agency (PMDA). Available online: https://www.pmda.go.jp/PmdaSearch/iyakuSearch/ (accessed on 26 June 2024).
- Search for drug information on the website of the FDA. Available online: https://dailymed.nlm.nih.gov/dailymed/ (accessed on 10 August 2024).
- van der Werf, A.; Rovithi, M.; Langius, J.A.E.; de van der Schueren, M.A.E.; Verheul, H.M.W. Insight in taste alterations during treatment with protein kinase inhibitors. Eur. J. Cancer 2017, 86, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, P.; Xiao, Q.; Chen, L.; Li, S.; Jian, J.M.; Zhong, Y.B. Sunitinib malate inhibits intestinal tumor development in male ApcMin/+ mice by down-regulating inflammation-related factors with suppressing β-cateinin/c-Myc pathway and re-balancing Bcl-6 and Caspase-3. Int. Immunopharmacol. 2021, 90, 107128. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, H.; Wang, N.; Zhao, C.; Zhang, H.; Deng, F.; Wu, N.; He, Y.; Chen, X.; Zhang, J.; et al. Modulation of β-catenin signaling by the inhibitors of MAP kinase, tyrosine kinase, and PI3-kinase pathways. Int. J. Med. Sci. 2013, 10, 1888–1898. [Google Scholar] [CrossRef] [PubMed]
- Barlow, L.A. Progress and renewal in gustation: New insights into taste bud development. Development 2015, 142, 3620–3629. [Google Scholar] [CrossRef]
- Miura, H.; Kusakabe, Y.; Sugiyama, C.; Kawamatsu, M.; Ninomiya, Y.; Motoyama, J.; Hino, A. Shh and Ptc are associated with taste bud maintenance in the adult mouse. Mech. Dev. 2001, 106, 143–145. [Google Scholar] [CrossRef]
- Hummel, T.; Whitcroft, K.L.; Rueter, G.; Haehner, A. Intranasal vitamin A is beneficial in post-infectious olfactory loss. Eur. Arch. Otorhinolaryngol. 2017, 274, 2819–2825. [Google Scholar] [CrossRef]
- Everitt, B.S.; Landau, S.; Leese, M.; Stahl, D. Cluster Analysis, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-0-470-74991-3. [Google Scholar]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Maeda, R. JADER from pharmacovigilance point of view. Jpn. J. Pharmacoepidemiol. Yakuzai Ekigaku 2014, 19, 51–56. [Google Scholar] [CrossRef]
- Noguchi, Y.; Tachi, T.; Teramachi, H. Detection algorithms and attentive points of safety signal using spontaneous reporting systems as a clinical data source. Brief. Bioinform. 2021, 22, bbab347. [Google Scholar] [CrossRef]
- Pariente, A.; Gregoire, F.; Fourrier-Reglat, A.; Haramburu, F.; Moore, N. Impact of safety alerts on measures of disproportionality in spontaneous reporting databases: The notoriety bias. Drug Saf. 2007, 30, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Hartnell, N.R.; Wilson, J.P. Replication of the Weber effect using postmarketing adverse event reports voluntarily submitted to the United States Food and Drug Administration. Pharmacotherapy 2004, 24, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Hochberg, A.M.; Pearson, R.K.; Hauben, M. An experimental investigation of masking in the US FDA adverse event reporting system database. Drug Saf. 2010, 33, 1117–1133. [Google Scholar] [CrossRef]
- Pariente, A.; Avillach, P.; Salvo, F.; Thiessard, F.; Miremont-Salamé, G.; Fourrier-Reglat, A.; Haramburu, F.; Bégaud, B.; Moore, N.; Association Française des Centres Régionaux de Pharmacovigilance (CRPV). Effect of competition bias in safety signal generation: Analysis of a research database of spontaneous reports in France. Drug Saf. 2012, 35, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Poleksic, A.; Xie, L. Database of adverse events associated with drugs and drug combinations. Sci. Rep. 2019, 9, 20025. [Google Scholar] [CrossRef]
- FDA Adverse Event Reporting System (FAERS). Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-adverse-event-reporting-system-faers (accessed on 26 June 2024).
- Lumini, A.; Nanni, L. Convolutional neural networks for ATC classification. Curr. Pharm. Des. 2018, 24, 4007–4012. [Google Scholar] [CrossRef]
- MedDRA Japanese Maintenance Organization. Available online: https://www.meddra.org/ (accessed on 26 June 2024).
- Watanabe, H.; Matsushita, Y.; Watanabe, A.; Maeda, T.; Nukui, K.; Ogawa, Y.; Sawa, J.; Maeda, H. Early detection of important safety information. Recent methods for signal detection. Jpn. J. Biomet. 2004, 25, 37–60. [Google Scholar] [CrossRef]
- Ohyama, K.; Sugiura, M. Evaluation of the association between topical prostaglandin F2α analogs and asthma using the JADER database: Comparison with β-blockers. Yakugaku Zasshi 2018, 138, 559–564. [Google Scholar] [CrossRef]
- Greenland, S.; Schwartzbaum, J.A.; Finkle, W.D. Problems due to small samples and sparse data in conditional logistic regression analysis. Am. J. Epidemiol. 2000, 151, 531–539. [Google Scholar] [CrossRef]
- Gravel, C.A.; Douros, A. Considerations on the use of different comparators in pharmacovigilance: A methodological review. Br. J. Clin. Pharmacol. 2023, 89, 2671–2676. [Google Scholar] [CrossRef]
- Hosoya, R.; Uesawa, Y.; Ishii-Nozawa, R.; Kagaya, H. Analysis of factors associated with hiccups based on the Japanese Adverse Drug Event Report database. PLoS ONE 2017, 12, e0172057. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, A.; Uesawa, Y. Analysis of corticosteroid-induced glaucoma using the Japanese adverse drug event reporting database. Pharmaceuticals 2023, 16, 948. [Google Scholar] [CrossRef] [PubMed]
- Okunaka, M.; Kano, D.; Matsui, R.; Kawasaki, T.; Uesawa, Y. Comprehensive analysis of chemotherapeutic agents that induce infectious neutropenia. Pharmaceuticals 2021, 14, 681. [Google Scholar] [CrossRef] [PubMed]
- Kan, Y.; Nagai, J.; Uesawa, Y. Evaluation of antibiotic-induced taste and smell disorders using the FDA adverse event reporting system database. Sci. Rep. 2021, 11, 9625. [Google Scholar] [CrossRef]
- Nakao, Y.; Asada, M.; Uesawa, Y. Comprehensive study of drug-induced pruritus based on adverse drug reaction report database. Pharmaceuticals 2023, 16, 1500. [Google Scholar] [CrossRef]
- Chen, J.J.; Wang, S.J.; Tsai, C.A.; Lin, C.J. Selection of differentially expressed genes in microarray data analysis. Pharmacogenom. J. 2007, 7, 212–220. [Google Scholar] [CrossRef]
- Van Puijenbroek, E.P.; Bate, A.; Leufkens, H.G.M.; Lindquist, M.; Orre, R.; Egberts, A.C. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol. Drug. Saf. 2002, 11, 3–10. [Google Scholar] [CrossRef]

| Number of Reports | Proportion (%) | |
|---|---|---|
| Taste or smell disorders | 19,342 | 100 |
| Taste disorders | 18,764 | 97.0 |
| Taste disorders only | 17,872 | 92.4 |
| Smell disorders | 1470 | 7.6 |
| Smell disorders only | 578 | 3.0 |
| Both taste and smell disorders | 892 | 4.6 |
| Gender | Taste and Smell Disorders | Non-Taste and Smell Disorders | p (Fisher’s Exact Test) | ROR | 95% CI |
|---|---|---|---|---|---|
| Male | 5391 | 565,885 | 0.569 | 0.990 | 0.955–1.025 |
| Female | 7164 | 744,229 | |||
| Age | Taste and Smell Disorders | Non-Taste and Smell Disorders | p (Fisher’s Exact Test) | ROR | 95% CI |
| ≥70 | 4561 | 387,134 | <0.0001 | 1.381 | 1.332–1.432 |
| <70 | 8077 | 946,863 |
| Medicinal Classification by the ATC Classification | Antineoplastic Agents Likely to Induce Taste and Smell Disorders *# | Number of All Drugs by Drug Class # | Antineoplastic Agents Likely to Induce Taste and Smell Disorders/Number of All Drugs by Drug Class (%) |
|---|---|---|---|
| Alkylating agents (L01A) | 1 | 26 | 3.85 |
| Antimetabolites (L01B) | 1 | 19 | 5.26 |
| Plant alkaloids and other natural products (L01C) | 1 | 17 | 5.88 |
| Cytotoxic antibiotics and related substances (L01D) | 2 | 16 | 12.5 |
| Protein kinase inhibitors (L01E) | 29 | 73 | 39.73 |
| Monoclonal antibodies and antibody drug conjugates (L01F) | 7 | 45 | 15.56 |
| Other antineoplastic agents (L01X) | 16 | 65 | 24.62 |
| Medicinal Classification by ATC Classification | ROR | 95% CI |
|---|---|---|
| Alkylating agents | 0.439 | 0.410–0.470 |
| Antimetabolites | 0.491 | 0.474–0.509 |
| Plant alkaloids and other natural products | 0.710 | 0.677–0.743 |
| Cytotoxic antibiotics and related substances | 0.391 | 0.356–0.430 |
| Protein kinase inhibitors | 2.545 | 2.498–2.592 |
| Monoclonal antibodies and antibody drug conjugates | 0.729 | 0.703–0.756 |
| Other antineoplastic agents | 1.570 | 1.532–1.609 |
| Cluster 1 | Cluster 2 | Cluster 3 |
|---|---|---|
| Avapritinib | Capecitabine | Abemaciclib |
| Enasidenib | Dasatinib | Afatinib |
| Enfortumab vedotin | Epirubicin | Alectinib |
| Entrectinib | Erlotinib | Alpelisib |
| Erdafitinib | Everolimus | Axitinib |
| Fedratinib | Mitotane | Bortezomib |
| Glasdegib | Panitumumab | Bosutinib |
| Lurbinectedin | Pertuzumab | Cabazitaxel |
| Pexidartinib | Sorafenib | Cabozantinib |
| Pralsetinib | Trastuzumab | Crizotinib |
| Romidepsin | Vorinostat | Daratumumab |
| Selinexor | Ixazomib | |
| Sonidegib | Lenvatinib | |
| Tazemetostat | Neratinib | |
| Trastuzumab deruxtecan | Nintedanib | |
| Valrubicin | Niraparib | |
| Vismodegib | Olaparib | |
| Osimertinib | ||
| Palbociclib | ||
| Pazopanib | ||
| Pembrolizumab | ||
| Regorafenib | ||
| Ribociclib | ||
| Rucaparib | ||
| Sunitinib | ||
| Tretinoin | ||
| Tucatinib | ||
| Vemurafenib |
| PT Name | PT Code | Number of Records |
|---|---|---|
| Dysgeusia | 10013911 | 220,220 |
| Ageusia | 10001480 | 70,503 |
| Taste disorder | 10082490 | 34,849 |
| Anosmia | 10002653 | 30,875 |
| Parosmia | 10034018 | 22,325 |
| Hypogeusia | 10020989 | 6926 |
| Hyposmia | 10050515 | 3547 |
| Hallucination, olfactory | 10019072 | 1371 |
| Olfactory nerve disorder | 10056388 | 320 |
| Hypergeusia | 10069147 | 184 |
| Hallucination, gustatory | 10019071 | 157 |
| Gustometry abnormal | 10064480 | 11 |
| Olfactory test abnormal | 10062927 | 9 |
| Olfactory dysfunction | 10086567 | 0 |
| Taste and Smell Disorders | Non-Taste and Smell Disorders | |
|---|---|---|
| Reports with the suspected drug | a | b |
| All other reports | c | d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamazaki, R.; Uesawa, Y. Characterization of Antineoplastic Agents Inducing Taste and Smell Disorders Using the FAERS Database. Pharmaceuticals 2024, 17, 1116. https://doi.org/10.3390/ph17091116
Hamazaki R, Uesawa Y. Characterization of Antineoplastic Agents Inducing Taste and Smell Disorders Using the FAERS Database. Pharmaceuticals. 2024; 17(9):1116. https://doi.org/10.3390/ph17091116
Chicago/Turabian StyleHamazaki, Risa, and Yoshihiro Uesawa. 2024. "Characterization of Antineoplastic Agents Inducing Taste and Smell Disorders Using the FAERS Database" Pharmaceuticals 17, no. 9: 1116. https://doi.org/10.3390/ph17091116
APA StyleHamazaki, R., & Uesawa, Y. (2024). Characterization of Antineoplastic Agents Inducing Taste and Smell Disorders Using the FAERS Database. Pharmaceuticals, 17(9), 1116. https://doi.org/10.3390/ph17091116







