Abstract
Taraxaci herba, as a traditional Chinese medicine, is the name of the Taraxacum genus in the Asteraceae family. Documented in the Tang Herbal Medicine (Tang Dynasty, AD 657–659), its medicinal properties cover a wide range of applications such as acute mastitis, lung abscess, conjunctival congestion, sore throat, damp-heat jaundice, and vision improvement. In the Chinese Pharmacopoeia (Edition 2020), more than 40 kinds of China-patented drugs containing Taraxaci herba were recorded. This review explores the evolving scientific understanding of Taraxaci herba, covering facets of ethnopharmacology, botany, phytochemistry, pharmacology, artificial cultivation, and quality control. In particular, the chemical constituents and pharmacological research are reviewed. Taraxaci herba has been certified as a traditional medicine plant, and its flavonoids, phenolic acids, and terpenoids have been identified and separated, which include Chicoric acid, taraxasterol, Taraxasteryl acetate, Chlorogenic acid, isorhamnetin, and luteolin; they are responsible for anti-inflammatory, antioxidant, antibacterial, anti-tumor, and anti-cancer activities. These findings validate the traditional uses of Taraxaci herba and lay the groundwork for further scientific exploration. The sources used in this study include Web of Science, Pubmed, the CNKI site, classic monographs, the Chinese Pharmacopoeia, the Chinese Medicine Dictionary, and doctoral and master’s theses.
1. Introduction
Taraxaci herba is the name of the Taraxacum genus in the Asteraceae family, which include T. mongolicum, T. sinicum Kitag, and several plants of the same genus, and the whole grass is used for medicine. Dandelions are the English common name of Taraxaci herba. Taraxaci herba is widely distributed in the north temperate zone, including the northeast, north, northwest, central, east, and southwest provinces of China [1,2], and it is also widely distributed in Korea, Mongolia, and Russia, mainly in hillside grasslands, roadsides, fields, and river beaches at middle and low altitudes [3,4].
According to the Chinese Pharmacopoeia (Edition 2020) [5], the medicinal effects of Taraxaci herba involve clearing heat and eliminating toxins, hematoma, inflammation, diuresis, and jaundice. Taraxaci herba can be employed to treat acute mastitis, lung abscess, conjunctival congestion, sore throat, damp-heat jaundice, and so forth. The dosage of decoction is 10–15 g, and the maximum dosage is 60 g. In addition, the edible part of Taraxaci herba is as high as 84%. People in East Asia have the habit of eating Dandelion pickled products, Dandelion porridge, and Dandelion soup since ancient times [6,7].
Taraxaci herba is composed of a variety of complex chemical components. The main ones that determine its biological activity are flavonoids, terpenoids, phenolic acids, and polysaccharides. Pharmacological experiments have confirmed that it has anti-inflammatory, antioxidant, antibacterial, anti-cancer, and anti-tumor effects [8,9,10,11]. It has become an urgent matter to clarify the effective components and pharmacological effects of Taraxaci herba and find out the factors that affect the accumulation of effective components in Taraxaci herba so as to improve the yield and quality of Taraxaci herba. In this review, the chemical composition, biological activity, growth conditions, and quality control of Taraxaci herba were integrated and analyzed, with the aim of providing a reference for the establishment of a planting system and quality control system of Taraxaci herba and the development of new products.
2. Materials and Methods
Relevant literature was obtained from scientific databases such as PubMed (https://pubmed.ncbi.nlm.nih.gov, accessed on 6 June 2024), SciFinder, Web of Science, and CNKI (https://www.cnki.net, accessed on 6 June 2024). The keywords used to search were “Taraxaci herba”, “Phytochemistry”, “Pharmacology”, “Dandelion”, and “Quality control”. TCMSP (https://old.tcmsp-e.com/tcmsp.php, accessed on 8 June 2024), Pubchem, and Web of Huayuan (https://www.chemsrc.com, accessed on 8 June 2024) were used to find the chemical composition of Taraxaci herba. And the chemical structures were accurately depicted using the ChemDraw 23 software. The review also included results from the Flora of China (https://www.iplant.cn/frps, accessed on 10 June 2024), China Plant Science Data Center (https://www.plantplus.cn/cn, accessed on 11 June 2024), Plants of the World Online (https://powo.science.kew.org, accessed on 20 June 2024), classic monographs, Chinese Pharmacopoeia, and doctoral and master’s theses.
3. Botanical Features and Distribution
The original plant of Taraxaci herba belongs to the genus Taraxacum, which mainly grows in temperate and subtropical regions of the northern hemisphere. In China, Taraxacum plants are widely distributed in the northeast, north, northwest, central, east, and southwest provinces [1,2]. Taraxacum plants contain Taraxasterol, stigmasterol, sitosterol, choline, organic acids, and inulin, so they have very high medicinal and nutritional values [12,13].
With reference to China Dictionary of Chinese Herbal Medicine Resources, Flora of China (https://www.iplant.cn/frps, accessed on 1 July 2024), China Plant Science Data Center (https://www.plantplus.cn/cn, accessed on 2 July 2024), and other references [1,2,14,15,16,17], 84 species of Taraxacum distributed in China have been identified, as shown in Table 1. And the distribution map of the Taraxacum genus is presented in Figure 1.

Table 1.
A total of 84 species of the genus Taraxacum in China.
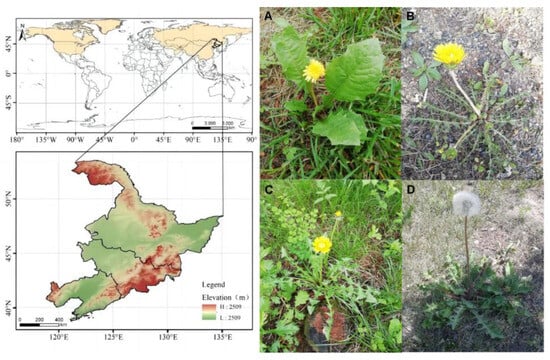
Figure 1.
Distribution of Taraxaci herba (the yellow shading represents the distribution of Taraxaci herba; white is the area where Taraxaci herba almost does not exist, and the bottom half of the image is the typical height legend of Taraxaci herba): (A–C) Growth period of Taraxaci herba; (D) Seed maturity of Taraxaci herba.
In China, the most important medicinal species of Taraxacum is T. mongolicum. T. mongolicum is a perennial herb with cylindrical and curved roots, 3–7 cm long, dark brown, and stout. Leaves obovate-lanceolate, obovate-lanceolate, or oblong-lanceolate, 4–20 cm long and 1–5 cm wide, with blunt or acute apex and sometimes wavy teeth or pinnately parted edges, with 3–5 lobes on each side, triangular or triangular-lanceolate, usually toothed; Scapes 1 to several, equal to or slightly longer than leaves, 10–25 cm high; The capitulum is about 30–40 mm in diameter; Involucre bell-shaped, 12–14 mm long, pale green, involucre 2–3 layers [18]. The tongue-shaped flower is yellow, with a tongue length of about 8 mm and a width of about 1.5 mm. The back of the edge flower tongue has purple-red stripes, and the anther and stigma are dark green. The flowering period is 4–9 months [19]. T. sinicum is also one of the medicinal varieties of Taraxacum. Scapes 1 to several, 5–20 cm high, longer than leaves; The capitulum is about 20–25 mm in diameter; The involucre is small, 8–12 mm long, light green, and the involucre has three layers; Tongue-shaped flowers are yellow and thin white. The tongue is about 8 mm long and 1–1.5 mm wide, and the flowering period is 6–8 months. Achenes obovate-lanceolate, light brown, about 3–4 mm long; The crest is white and 5–6 mm long [18,20]. In addition, T. scariosum, T. platycarpum, T. officinale, and other plants of Taraxacum are often used as medicinal varieties.
4. Traditional Uses
Taraxaci herba is called Pugongying (Dandelion) in China’s classical medical works. Taraxaci herba has a long medicinal history and was recorded as a medicinal plant for the first time in Liu Juanzi Gui Yifang (Jin Dynasty, AD 442–499), and it was recorded that Taraxaci herba soup can cure “Carbuncle of breast”. Tang Bencao (Tang Dynasty, AD 657–659) introduced the medicinal value of Taraxaci herba systematically to the government for the first time. Bencao Yanyi (Song Dynasty, AD 1116) and Bencao Yanyi Buyi (Yuan Dynasty, AD 1347) systematically introduced Taraxaci herba from the unique Four natures of drugs, Five flavors and Channel tropism of traditional Chinese medicine. In addition, Bencao Gangmu (Ming Dynasty, AD 1578–1596), Diannan Bencao (Ming Dynasty, AD 1436), and Gangmu Shiyi (Qing Dynasty, AD 1765–1864) also supplemented the medicinal functions of Taraxaci herba. In addition, the medical classics of ethnic minorities in China, such as Tibetan Medicine in China, Qingzang Yaojian, Mengzhi Yaozhi, Dianyao Lu, Miao Yiyao, Laoai, Tujia Yao, and Dedai Yao, also contain relevant records about Taraxaci herba as a medicinal plant.
Taraxaci herba is widely used in traditional Chinese medicine prescriptions, such as Yingtengtang, Zhirubianyongfang, Lixiaotang, Pugongsan, and Kaiyaguchiqifang, which all take Taraxaci herba as the main ingredient, and have been widely used in clinics. At present, there have been reports regarding the combination of Taraxaci herba with other traditional Chinese medicines in the treatment of mastitis [21,22], colitis [23], acute tonsillitis [24], and intestinal obstruction [25]. This article collects a large number of traditional Chinese medicine formulas about Taraxaci herba, as shown in Table 2.

Table 2.
Traditional Prescription Drugs Related to Taraxaci herba in China.
5. Phytochemistry
Taraxaci herba possesses distinctive biological characteristics and excellent biological activity, and its complex chemical composition determines its unique biological activity. The main chemical components of Taraxaci herba include flavonoids, phenolic acids, polysaccharides, terpenoids, volatile oils, and alkaloids, among which flavonoids, phenolic acids, and terpenoids are the main active components of Taraxaci herba.
5.1. Flavonoid
Flavonoids are a type of natural product that widely exists in the plant kingdom and generally take C6-C3-C6 as the basic carbon chain skeleton. Flavonoid metabolites generally possess the ability of defense, allelopathy, inhibition of harmful bacteria, and antioxidation [38]. The grass, roots, stems, leaves, and flowers of Taraxaci herba all contain flavonoids, and the aboveground parts are the most abundant. The content of flavonoids in Taraxaci herba is approximately 1.35%, of which luteolin (1) is the highest, followed by luteolin-7-O-β-D-glucoside [39,40]. Yun Ling separated 9 kinds of flavonoids from the ethanol crude extract of Taraxaci herba, which were as follows luteolin (1), diosmetin [41], apigenin (11), hesperetin 7-O-β-D-glucuronide (19), rutin [41], isoquercitrin (17), quercetin-3-O-β-D-galacoside, quercetin (2), luteolin-7-O-β-D-glucoside [39,42,43]. Yao Wei [44] also separated luteolin (1), luteolin-7-O-β-D-glucoside [39], and quercetin (2) from the crude ethanol extract of Taraxaci herba by chromatography. Rong Wang et al. [45] extracted hesperetin 7-O-β-D-glucocuronide (19) and hesperetin 5-O-glucoside (20) from Taraxaci herba for the first time by optimizing the water extraction method. Milovanovic Stoja extracted the flavonoids (208.6–564.5 μg QE/g) and phenolic (5.5–12.1 mg GAE/g) active components from dandelion seeds by supercritical fluid extraction technology [46].
Flavonoids, due to their remarkable functions such as liver protection, bacteria inhibition, and oxidation resistance, are widely utilized in the health care products, medicine, and cosmetics industries. Hence, we have summarized the previous research reports regarding flavonoids in Taraxaci herba, as presented in Table 3 and Figure 2.

Table 3.
Main Flavonoids in Taraxaci herba.
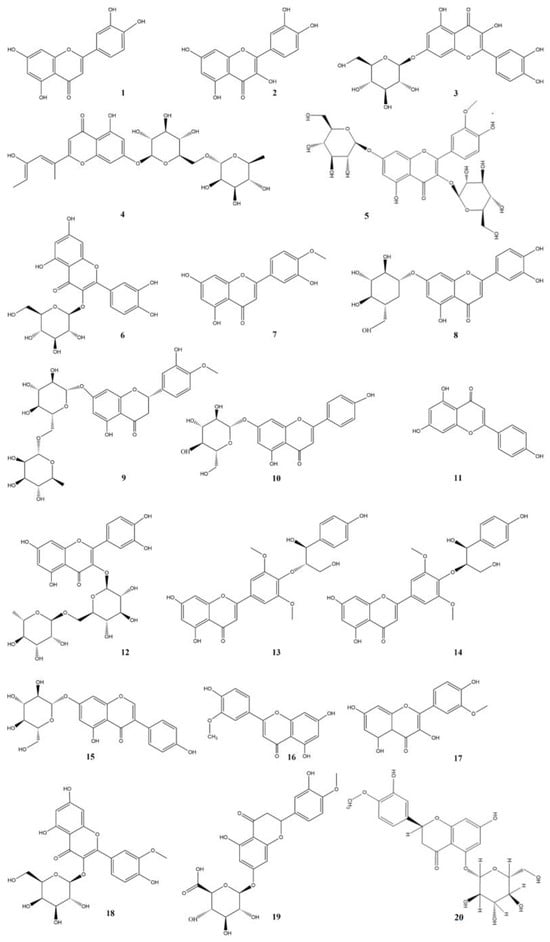
Figure 2.
Structures of flavonoids isolated from the Taraxaci herba.
5.2. Terpenoids and Steroids
Terpenoids are the products of advanced plant evolution and are the general names of isoprene polymers and their derivatives, which are usually expressed by the general formula of carbon skeleton (C5H8)n [52]. Triterpenoids and sesquiterpenes are the main terpenoids in Taraxaci herba, which possess anti-inflammatory and anti-tumor activities [53].
The triterpenoids in Taraxaci herba are mainly pentacyclic triterpenoids. Yun Ling [54] initially discovered two triterpenoids from the crude ethanol extract of Taraxaci herba, namely psi-taraxasterol acetate (1) and psi-taraxasterol (4) palmitate. Taraxasterol [41] is a triterpene compound isolated and identified from the pollen, roots, and leaves of Taraxaci herba, and it is one of the main active components of Taraxaci herba with anti-inflammatory and anti-tumor effects. Schütz et al. isolated taraxasterol [41], psi-taraxasterol (4), and arnidiol [41] from Taraxaci herba, and the content in the root of Taraxaci herba was significantly higher than that in other parts [9]. Sesquiterpenes in Taraxaci herba are mainly sesquiterpene lactones (19), and sesquiterpenes are the main components of the bitter source of Taraxaci herba [55]. Zhiyun Meng et al. identified the content of monomeric sterols in Taraxaci herba. The results showed that the content of β-sitosterol (11) was the highest, followed by stigmasterol (13) and campesterol (15) [56].
In order to present the terpenoids in Taraxaci herba more intuitively, we choose to present them in tabular form, as shown in Table 4 and Figure 3.

Table 4.
Major Terpenoids and Steroids in Taraxaci herba.
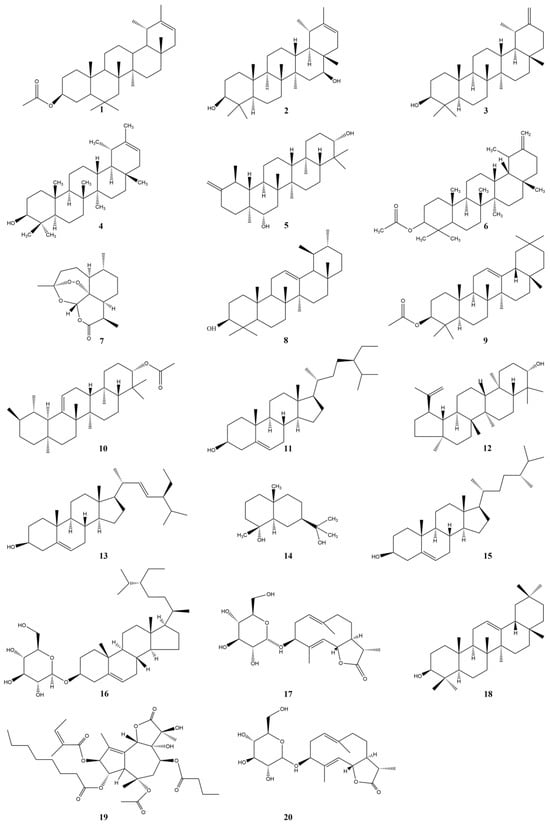
Figure 3.
Structures of Terpenoids and Steroids isolated from the Taraxaci herba.
5.3. Phenolic Acid
Phenolic acid compounds are one of the important secondary metabolites in Taraxaci herba, which are a kind of compounds formed by combining one or more aromatic rings with one or more hydroxyl groups, and have certain anti-inflammatory, antibacterial, anti-cancer, and antioxidant activities [64].
Shuyun Shi has successfully extracted phenolic acids such as gallic acid (11), ferulic acid [41], chlorogenic acid (1), caftaric acid [41], and caffeic acid [41] from Taraxaci herba [61,65,66,67]. Deqian Peng et al. isolated three phenolic acids from Taraxaci herba roots for the first time, namely ethyl (p-Hydroxyphenyl) acetate, methyl caffeate acid (13), and vanillin (17) [68]. Kenny O et al. isolated and identified three kinds of phenolic acids with antibacterial activity from the methanol crude extract of the root of Taraxaci herba, including vanillin (17), coniferaldehyde (18), and p-methoxyphenylglyoxylic acid (19) [57].
Currently, the phenolic acids isolated and identified from Taraxaci herba are presented in Table 5 and Figure 4.

Table 5.
Phenolic acids in Taraxaci herba.
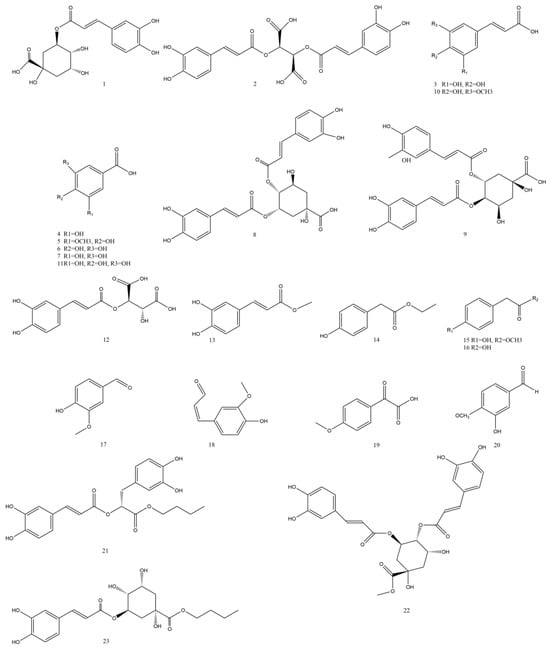
Figure 4.
Structures of phenolic acids isolated from the Taraxaci herba.
5.4. Others
Taraxaci herba is abundant in polysaccharides, pigments, alkaloids, volatile oils, and other chemical components [71,72,73]. Hongkun Xue extracted polysaccharides by an ultrasonic-assisted hot water extraction method. The highest polysaccharide yield was (12.08 ± 0.14)%, and the IC50 values for scavenging DPPH and OH free radicals were 0.71 mg/mL and 0.75 mg/mL, respectively [74]. The polysaccharide in Taraxaci herba accounts for 30~50% of its dry weight, and the polysaccharide content in Taraxaci herba root is 83.31%, which is significantly higher than that in leaves and flowers, and the inulin content accounts for 45% of the root [75]. Taraxaci herba contains certain fatty acids, such as myristic acid, linoleic acid, palmitic acid, and linolenic acid, as well as various amino acids, proteins, and minerals [76,77].
6. Pharmacology
6.1. Anti-Inflammatory and Liver Protection
In recent years, the anti-inflammatory effect and mechanism of pure compounds and crude extracts of Taraxaci herba plants have been studied through cell and animal models, which have proven that the anti-inflammatory effect of Taraxaci herba plants is remarkable. Taraxasterol (TS) is one of the main active components in Taraxaci herba, which plays an anti-inflammatory role, and its content is the highest in the roots [78,79]. Xuemei Zhang et al. utilized the lipopolysaccharide (LPS) induced inflammatory model of peritoneal macrophages in mice to assess the anti-inflammatory effect of TS. The experimental results indicate that TS can inhibit the production of inflammatory factors PGE2, TNF-α, IL-1, and IL-6 in inflammatory tissue cells and prevent NF-κB from translocation from cytoplasm to nucleus, thereby exerting an anti-inflammatory role [80]. San Zhihao perfused the mouse mammary duct with LPS, thus establishing a mouse mammary inflammatory injury model to evaluate the anti-inflammatory activity of TS [81]. The results demonstrated that the production of inflammatory factors TNF-α, IL-1β, and IL-6 in breast tissue was inhibited, the activation of NF-κB signaling pathway was blocked, and MPO activity decreased, suggesting that TS has obvious anti-inflammatory activity. Feng Zheng et al. used LPS to stimulate human umbilical vein endothelial cells (HUVECs) to establish a vascular inflammation model and explored the anti-inflammatory effect and mechanism of TS. The experimental results show that the anti-inflammatory mechanism of TS inhibits the production of inflammatory factors TNF-α, IL-8, IL-1β, and NO and suppresses the activation of NF-κB and the expression of VCAM-1 and ICAM-1 [82]. By observing the effect of TS on hepatocytes of mice with LPS-induced hepatitis, Yifang Yi et al. discovered that TS regulates the JAK2/STAT3 and JNK signaling pathways by up-regulating the expression of the SOCS3 gene, thus inhibiting the inflammatory reaction of mouse hepatocytes [83]. Bingjie Ge et al. used acetaminophen (APAP) to stimulate AML12 cells to establish a mouse liver injury model in order to clarify the protective mechanism of TS on liver injury. The results showed that TS could alleviate the pathological changes of the AML12 cells, promote the expression of the Nrf2/HO-1 pathway, inhibit JNK phosphorylation, reduce the ratio of Bax/Bcl-2 and the expression of caspase-3, demonstrating unique anti-inflammatory and liver-protecting activities [84].
Taraxaci herba polysaccharides (TPS), as another potential anti-inflammatory component in Taraxaci herba, have been increasingly reported in relation to their anti-inflammatory mechanism. Park, through the influence of TPS on LPS-stimulated RAW 264.7 cells, explores the anti-inflammatory mechanism of TPS. Studies have shown that TPS can effectively inhibit the inflammatory reaction mediated by NFκB by regulating the PI3K/Akt pathway and stimulate the antioxidant potential mediated by Nrf2 [85]. Chaoyong Xiao explores the anti-inflammatory mechanism of TPS through the LPS-induced RAW264.7 cell model. The experiment showed that TPS significantly inhibited the mRNA expression of inflammatory factors COX-2, TNF-α, IL-6, and IL-1β in the cell model, which provided the basis for its anti-inflammatory activity [86]. Taking the LPS-induced enteritis model as the research object, Zhu Li et al. explored the protective and therapeutic effects of TPS on acute enteritis. The results show that TDS can not only promote the repair of the intestinal barrier in the enteritis model but also down-regulate the expression of inflammatory factors (TNF-α, IL-6, IL-1β) and up-regulate the expression of the anti-inflammatory factor IL-22, thereby protecting acute intestinal inflammation [75]. In addition, Han Seok Hee found that the ethanol extract of Taraxaci herba significantly inhibited the production of NO and inflammatory factors (Cox-2, TNF-α, IL-6, IL-1β), thus effectively alleviating intestinal inflammation [87]. To sum up, TS and TDS in Taraxaci herba are the main active components that play an anti-inflammatory role, and they can reduce the inflammatory damage of the host by inhibiting the secretion of inflammatory factors. The above modern pharmacological and animal experimental studies provide a theoretical basis for dandelion as a traditional anti-inflammatory medicine plant, and the anti-inflammatory mechanism of Taraxaci herba should be further explored for the development and utilization of new drugs. Jun Qi et al. have achieved remarkable results in treating acute mastitis with Taraxaci herba in the clinic. Among the 58 patients in the treatment group, 57 of them were treated with Taraxaci herba, and the redness, swelling, fever, and pain were relieved or disappeared, while the body temperature was significantly decreased or returned to normal, but one case was ineffective [21]. Nan Qiao used Taraxaci herba combined with minimally invasive catheter drainage to treat 89 patients with acute mastitis, and a control group was set up. Compared with other groups, the combined treatment group has the advantages of less trauma, quicker recovery, and no impact on breastfeeding [22]. Doctor Lijing An treated patients with acute mastitis using Taraxaci herba combined with cefradine, and an experimental group and a control group were respectively set up. The experimental group was the combined medication group, while the control group was given cefradine intravenously. After treatment, the levels of inflammatory factors IL-6, PCT, ICAM-1 and TNF-α in both groups groups decreased significantly, and the experimental group was superior to the control group [88].
6.2. Anti-Oxidation
Flavonoids are the main antioxidant products of Taraxaci herba, which have an obvious anti-aging effect [89]. Mingyue Pan extracted flavonoids from Taraxaci herba by means of ethyl acetate and dichloromethane and tested their antioxidant activity. The results showed that Taraxaci herba flavone (TF) had a strong scavenging ability for hydroxyl radical (OH), superoxide anion (O2), and 1,1-diphenyl-2-picrylhydrazyl (DPPH), and its main component was isorhamnetin [90]. The flavonoids extracted from the flowers of Taraxaci herba have a scavenging ability for superoxide anion (O2) and hydroxyl radical (OH) and cooperate with α-tocopherol to scavenge DPPH to play an antioxidant role [91]. In addition, the antioxidant activity of TF is related to the up-regulation of the mRNA levels of Nrf2 and SOD1, which leads to the increase of SOD and GSH and the decrease of MDA [92].
Not only does TF play an antioxidant role, the polysaccharides in Taraxaci herba also have obvious antioxidant activity. By extracting TDS with water and analyzing its antioxidant activity, Yunfeng et al. found that TDS can effectively neutralize DPPH, superoxide anion (O2), and hydroxyl radical (OH), suggesting that it has obvious antioxidant activity [93]. Zhou studied the antioxidant mechanism of TDS through animal model experiments. The results show that TDS can exert antioxidant activity by reducing the level of malondialdehyde (MDA) and increasing the activities of superoxide dismutase and glutathione peroxidase [94]. TDS activates a series of antioxidant genes via Nrf2 and then up-regulates the activity of antioxidant protease to play an antioxidant role [95,96]. To sum up, Taraxaci herba can reduce the oxidative damage of cells by scavenging free radicals and improving the activity of antioxidant enzymes, which provides convenience for developing Taraxaci herba as a new antioxidant.
6.3. Anti-Bacterial Activity
Taraxaci herba has a broad anti-bacterial spectrum and obvious anti-bacterial activity. Through the anti-bacterial activity verification of the ethanol extract of Taraxaci herba, Demin verified that it has obvious antibacterial activity against staphylococcus aureus and escherichia coli [97]. Qiao and Sun respectively tested the antibacterial ability of the ethanol and ethyl acetate extracts from the flowers of Taraxaci herba and determined the MIC value. The results showed that the anti-bacterial activity of the ethyl acetate extract against Pseudomonas aeruginosa and Bacillus subtilis (the MIC values were 125 μg/mL and 62.5 μg/mL, respectively) was significantly stronger than that of the ethanol extract [98]. Katy tested the antibacterial activity of the ethyl acetate extract from the leaves of Taraxaci herba. The results showed that the extract of Taraxaci herba leaves had relatively strong antibacterial activity against staphylococcus aureus, with a MIC of 200 μg/mL, and the MIC of escherichia coli and streptococcus pneumoniae was 400 μg/mL [99]. Xuefeng found that the phenolic extract from the Taraxaci herba flower can rupture the cell membrane of staphylococcus aureus, cause the leakage of adenosine triphosphate and Na-K-ATPase in the cell, and lead to bacterial death. Its main active components are Caffeic acid and Luteolin-7-O-glucoside [100].
In addition, Taraxaci herba also possesses certain antiviral and antifungal pharmacological activities. Yinku et al. found that the ethanol extract of Taraxaci herba could destroy the cell wall and cell membrane of candida albicans, resulting in the leakage of nucleic acid macromolecules, destroying cell metabolism and ultimately leading to the death of candida albicans [101]. At the concentration range of 25~100 μg/mL, the combined extract of Taraxaci herba ethanol and ethyl acetate significantly blocked the protein synthesis and DNA replication of the hepatitis B virus, and the antiviral effect was remarkable [102]. The aqueous extract of Taraxaci herba has potential anti-human immunodeficiency virus (HIV) activity, and its mechanism may be the inhibition of HIV-1 replication and reverse transcriptase activity [103]. In addition, Taraxaci herba also has an anti-hepatitis C virus (HCV) effect [104]. That is to say, the Taraxaci herba extract exerts anti-bacterial activity by destroying cell walls and cell membranes and interferes with the replication of virus DNA and protein, thus exerting antiviral activity. The drug concentration shows a positive correlation with both the rate of inhibition of bacterial growth and the rate of inhibition of biofilm formation. We present the pharmacological effects of Taraxaci herba in the form of pictures. For details, please refer to Figure 5.
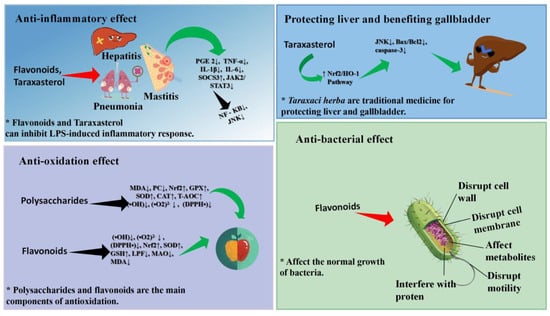
Figure 5.
The Pharmacological properties of Taraxaci herba.
6.4. Anti-Tumor and Anti-Cancer
Cancer is the most prevalent type of malignant tumor and one of the main causes of human mortality. In recent years, its incidence rate has been on the rise. Based on clinical practice and modern pharmacological research, Taraxaci herba has remarkable anti-tumor effects, mainly in breast cancer, liver cancer, stomach cancer, bladder cancer, and so on [105,106].
6.4.1. Breast Cancer
Breast cancer is a heterogeneous disease with significant individual differences. It ranks first among the most common cancers in women and has a high mortality rate [107,108]. Sophia conducted a study on the anti-cancer mechanism of Taraxaci herba for the first time. The aqueous extract of Taraxaci herba leaves inhibited the proliferation and differentiation of breast cancer cells (MCF-7) in an ERK-dependent manner, which exhibited a certain anti-cancer effect [109]. By observing the apoptosis of TNBC cell line under the influence of the ethanol extract of Taraxaci herba, Li et al. studied the anti-cancer mechanism of Taraxaci herba. The experimental results indicated that the ethanol extract of Taraxaci herba significantly reduced the activity of MDA-MB-231 cells, triggered G2/M phase arrest, increased caspase-3 and PARP protein, and promoted the apoptosis of cancer cells [110]. Shan combined network pharmacology and multiomics technology to study the mechanism of action of the ethanol extract of Taraxaci herba on breast cancer. The results showed that the anti-cancer mechanism of the ethanol extract of Taraxaci herba might be down-regulating the expression of CHKA, inhibiting the PI3K/AKT pathway and its downstream targets SREBP and FADS2 to interfere with the metabolism of glycerophospholipid and unsaturated fatty acids [111]. According to Xin-Xin et al., the experimental results show that the ethanol extract of Taraxaci herba can inhibit the proliferation, migration, and invasion of TNBC cells in the TAMs microenvironment by inhibiting the IL-10/STAT3/PD-L1 signaling pathway [112].
Hu Niu et al. extracted TDS by ultrasonic extraction and constructed a tumor model of breast cancer MCF-7 cells transplanted subcutaneously in nude mice to study the anti-tumor activity of TDS in vivo. The experimental data showed that TDS could significantly increase the expression of Pro-apoptosis proteins (p53, Bax) and significantly decrease the expression of anti-apoptosis protein Bcl-2 in MCF-7 cells, which was positively correlated with the dose [113]. Yumin observed the effects of different concentrations of Taraxaci herba flavonoids (TF) on the proliferation and apoptosis of MCF-7 cells by culturing MCF-7 cells in vitro. The results showed that TF promoted the apoptosis of MCF-7 cells by down-regulating the expression of Bcl-2 mRNA and up-regulating the expression of P53 and Bax mRNA [114]. Hamed et al. observed the proliferation and metastasis activities of MCF-7 and MDA-MB231 breast cancer cells under the combined action of TF and all-trans-retinoic acid (ATRA). The results showed that the expressions of MMP-9 and IL-1β in the treated two cell lines decreased significantly, while the expressions of p53 and KAI1 increased, and TF had obvious anti-tumor activity [115]. In addition, the triterpenoid compound TS in Taraxaci herba also has obvious Anti-tumor activity, and its mechanism may be that it inhibits the migration and invasion of MDA-MB-231 cells through ERK/Slug axis [116].
6.4.2. Prostate Cancer
Prostate cancer (PCa) accounts for 13.5% of the incidence of male malignant tumors, ranking second. More than 80% of the patients are men over 65 years old, and the incidence rate of urban men is 3.7 times that of rural men [117].
Christopher Nguyen et al., by observing the apoptosis of the PCa cell line induced by water extract of Taraxaci herba root, discovered that the Taraxaci herba root extract induced PCa cell apoptosis in a dose- and time-dependent manner and also had good tolerance, but its mechanism was not clarified [118]. Therefore, Jingqiu et al., through the modeling experiment of xenotransplantation of the PCa cell line, clarified the mechanism of TS inhibiting the proliferation of prostate cancer cells. The results showed that TS inhibited the proliferation of prostate cancer cells by inhibiting the activation of the PI3K/AKT signaling pathway and the expression of FGFR2 [119]. Morteza, through pharmacological experiments, found that the expression of uPA-uPAR, MMP-9, and MMP-2 in PCa cells treated with TS decreased significantly, while the expression of TIMP-2 and TIMP-1 increased significantly, which confirmed that TS had a therapeutic effect on prostate cancer [120].
6.4.3. Liver Cancer
Hepatocellular carcinoma (HCC) is a malignant tumor of the digestive tract with high morbidity and mortality worldwide, and its treatment and prognosis are difficult [121]. Ren investigated the mechanism of TDS in the treatment of liver cancer (HCC) through cell and animal experiments. The experimental results indicated that TDS significantly increased the spleen index, regulated T cell activation, and inhibited the growth of cancer cells, and the expression levels of P-PI3K, P-AKT, and P-mTOR genes in HCC cells were significantly reduced [122,123].
As one of the components with anti-cancer activity in Taraxaci herba, TS also has a remarkable inhibitory effect on liver cancer cells. Feng studied the anti-cancer mechanism of TS through the H22 hepatoma mouse cell model. The results showed that TS could significantly inhibit the proliferation of HCC cells, induce their apoptosis in vitro, and inhibit the growth of cancer cells and the expression of Ki67 in H22 hepatoma mice. The mechanism might be related to the regulation of Apoptosis-related proteins and the IL-6/STAT3 pathway [124]. Tianhao found that the apoptosis of mouse subcutaneous transplanted liver cancer cells induced by TS might be caused by increasing the level of Hint1 to regulate Bax and down-regulating the expressions of Bcl2 and cyclinD1 [41].
6.4.4. Gastric Cancer
Gastric cancer (GC) is the fifth most common cancer and the fourth most common cause of cancer death worldwide, and it is a disease with high molecular and phenotypic heterogeneity [125].
Hui conducted in vitro experiments to test the cytotoxicity of the water extract of Taraxaci herba on the human gastric cancer cell line (SGC-7901). The results revealed that the aqueous extract of Taraxaci herba could promote the apoptosis of gastric cancer cells, reduce the migration ability of gastric cancer cells, and reduce the expression of pro-proliferation and anti-apoptosis genes (Erk, survivin and Bcl2) [126]. Long-chain noncoding RNA (lncRNA) can impact the proliferation of tumor cells. Through the study of targeting lncRNA-CCAT1 with the aqueous extract of Taraxaci herba root, Zhu discovered that CCAT1 in GC cells was significantly down-regulated, and it inhibited the proliferation and migration of GC cells [127]. Wei C established a subcutaneous xenotransplantation model of gastric cancer by subcutaneously injecting MKN-28 cells in nude mice and then observed the growth of gastric cancer cells after TS administration. The results showed that TS inhibited the growth of gastric cancer by down-regulating the expression of EGFR and AKT1 in cancer cells and inhibiting the EGFR/AKT1 signaling pathway [128]. Yang, through cell experiments, it was found that TS inhibited the proliferation of gastric cancer cells and promoted the apoptosis of gastric cancer cells by inhibiting glycolysis mediated by GPD2 [129].
6.4.5. Others Cancer
Colorectal cancer (CRC) is the third most common cancer in the world, as recognized by the WHO [130]. Tao C observed that the mortality of CRC patients with high expression of the RNF31 protein was much higher than that of patients with low expression of RNF31. Taraxasterol acetate could promote the degradation of RNF31 and then inhibit the mutation of the P35 gene, thus playing an anti-cancer role [131,132]. Le, by testing the effect of Taraxaci herba flavone (TF) on cancer cells in the Lewis mouse lung cancer model, it was found that under the influence of 200 μg/mL TF, the levels of CD4+, CD8+ and CD4+/CD8+ in cancer cells increased, the levels of IL-2, IL-3, IFN-γ, and TNF-α increased, and the expression of Ki67 decreased significantly, thus inhibiting the proliferation of lung cancer cells and improving the host’s immunity [133]. In addition, Taraxaci herba has an inhibitory effect on glioma, leukemia, pancreatic cancer, melanoma, and esophageal cancer in vitro and in vivo, or it can be used as a radiosensitizer to assist in the treatment of cancer [134,135,136]. The anti-cancer and anti-tumor mechanisms of Taraxaci herba need to be further explored through experimental research and clinical practice in order to develop them into potential natural anti-cancer drugs. We present the anti-cancer mechanism of Taraxaci herba in the form of pictures. For details, please refer to Figure 6.
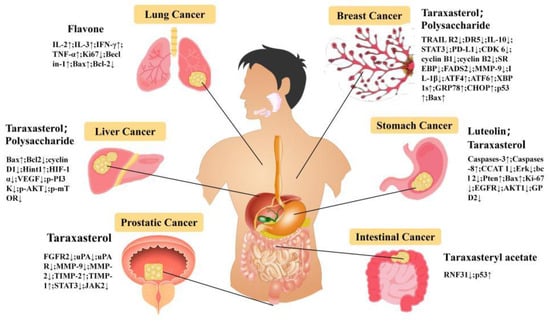
Figure 6.
The Anti-Cancer of Taraxaci herba.
6.5. Other Pharmacological Effects
Taraxaci herba not only has certain anti-inflammatory, anti-oxidation, and anti-cancer effects but also has the functions of improving immunity [137,138,139], lowering blood sugar and blood fat [140,141,142,143,144], protecting liver and gallbladder [145,146], diuresis, anticoagulation [147,148] and depression [149].
7. Artificial Cultivations
7.1. Sowing and Harvesting
Taraxaci herba can be sown from April to September every year, which has strong adaptability and can survive in most soils. The seeds of Taraxaci herba have no dormancy characteristics, and their vigor decreases rapidly after harvesting. It is best to choose new seeds for sowing, which can generally emerge in 7–15 days, and weeds need to be removed as soon as possible.
The Pharmacopoeia of China (2020 edition) stipulates that Taraxaci herba should be harvested at the beginning of flowering from spring to autumn. In order to ensure multiple harvesting, only the aerial parts of Taraxaci herba are collected the first few times. The aerial parts of artificially cultivated Taraxaci herba can usually be harvested after growing for 2–3 months in the first year and can be used as medicine after removing impurities, washing, and drying in the sun. Traditional methods of drying or refrigerating medicinal materials have damaged the bioavailability of bioactive components in medicinal materials to some extent. In the future, the emerging nano-encapsulation technology will be used to improve the bioavailability of bioactive components [150]. However, wild Taraxaci herba usually needs to grow for about 6 months due to insufficient nutritional conditions [151]. The underground part of medicinal Taraxaci herba is usually harvested in autumn. Shiyu Li and Minghao Shen determined the content of active ingredients in Taraxaci herba roots collected in different periods [152]. The content of flavonoids and saponins in Taraxaci herba roots collected at the end of August was the highest (12.78 mg/g, 0.78 mg/g), the choline content in Taraxaci herba roots collected at the end of July reached the peak of 5.06 mg/g, and the antibacterial activity of Taraxaci herba collected from August to September was the most obvious. Ruiyi et al. The content of active ingredients in Taraxaci herba roots at different harvest times was determined [153]. The results showed that the content of flavonoids in Taraxaci herba roots reached the peak of 10.61 mg/g at the beginning of September, the content of total polysaccharides reached the peak of 131.98 mg/g at the end of September, and the content of phenolic acids (caftaric acid, chlorogenic acid, caffeic acid, and cichoric acid) reached the highest of 0.98% in mid-September.
7.2. Cultivation
Taraxaci herba, as a commonly used bulk medicine, should not only ensure the supply of raw materials for pharmaceutical enterprises but also ensure that its medicinal value reaches the standard. Wild species are unsustainable, so it is particularly important to cultivate Taraxaci herba artificially. There are many factors that affect the growth of Taraxaci herba and the accumulation of its active components, including light intensity, fertilizer dosage, soil environment, harvesting and storage, etc. Therefore, the previous studies are summarized, as shown in Table 6.

Table 6.
Environmental factors that influence Taraxaci herba.
8. Quality Control and Toxicology
8.1. Quality Control
Due to the imperfection of market supervision and the identification system of traditional Chinese medicine, there have always been problems with variety misidentification and adulteration, but as a drug, the quality standard must comply with relevant laws and regulations. At present, the China Pharmacopoeia (2020 edition) [5] regulates the quality of Taraxaci herba from three aspects: Character identification, microscopic identification, and TLC. The moisture content of Taraxaci herba is no more than 13.0%, the total ash content is no more than 20.0%, and the leaching solution content should not be less than 18.0%. Cichoric acid in Taraxaci herba was determined to be ≥0.45% by HPLC, and cichoric acid in processed Taraxaci herba pieces was ≥0.30%. The European Pharmacopoeia (11.0 edition) regulates the quality of Taraxaci herba from three aspects: Character identification, microscopic identification, and TLC. The loss on drying of Taraxaci herba is not more than 12.0%, the total ash content is not more than 10.0%, the proportion of ash insoluble in hydrochloric acid should not exceed 3%, and the leaching solution content should not be less than 20.0% [163]. However, it may not be enough to evaluate the quality system of Taraxaci herba only by HPLC. With the progress of science and technology, people can jointly use other methods to determine the substance content of Taraxaci herba and improve the quality control system of Taraxaci herba.
Chemical fingerprint, the fingerprint of traditional Chinese medicine, is a comprehensive and quantifiable identification method based on the systematic study of chemical components of traditional Chinese medicine, which is used to evaluate the authenticity, stability, consistency, and effectiveness of traditional Chinese medicine [164,165]. Aipeng L established a new chemical fingerprint of Taraxaci herba, corrected nine common peaks, and determined the contents of chlorogenic acid, cichoric acid, caffeic acid, caftaric acid, and Isochlorogenic acid A and luteolin can be used as markers to evaluate the quality of Taraxaci herba [166]. Bo H, the fingerprints of 15 batches of Taraxaci herba from different habitats were determined, 23 common peaks were identified, and the contents of 11 chemical components (chlorogenic acid, chicoric acid, caffeic acid, quercetin, apigenin, rutin, diosmetin, luteolin-7-O-β-D-glucoside, kaempferol, genkwanin, 7-Hydroxycoumarine) were identified [167]. Meng Ran et al. calibrated six common peaks by fingerprint combined with multi-component content determination and chemical pattern recognition and determined that caftaric acid, chlorogenic acid, caffeic acid, and cichoric acid can be used as material indexes for quality evaluation of Taraxaci herba [168]. Twenty-two phenolic compounds in Taraxaci herba were determined by HPLC-DAD-MS/MS, and cichoric acid, caffeic acid, and luteolin were identified as quality markers, which further improved the quality evaluation system of Taraxaci herba [169]. Wu Zhe, through the comparative analysis of chemical fingerprint and high-performance liquid chromatography, caftaric acid, chlorogenic acid, caffeic acid, and cichoric acid were selected as quality evaluation markers of Taraxaci herba [64]. With the continuous development of technology, the quality control methods of medicinal materials are also constantly innovating, and the innovation of quality control methods and technologies of Taraxaci herba is becoming more and more important.
8.2. Toxicology
To date, little literature has reported the toxicity of Taraxaci herba. People’s Republic of China Pharmacopoeia (2020 edition) [5] stipulates that the dosage of dried dandelion is 10–15 g, and no adverse reactions have been reported in normal dosage. Up to now, only 3 cases of adverse reactions caused by clinical use of dandelion have been reported, all of which exceeded the normal dose, respectively 20 g, 30 g, and 30 g [170,171,172]. Toxicological safety evaluation is essential for plant application and new drug development. Therefore, further research on toxicity is needed to provide a more reliable basis for medication use.
9. Conclusions and Future Perspectives
In this paper, the latest review of Taraxaci herba was made through textual research of herbal medicine, traditional medicine, phytochemistry, pharmacology, and artificial cultivation. According to historical documents, Taraxaci herba is a plant medicine that can be used as a whole herb, and it has been used for a long time to treat acute inflammation, colds and fever, bacterial infections, and so on. In China, Taraxaci herba is often used in combination with other natural medicines, and a large number of medicinal prescriptions for Taraxaci herba are recorded.
There are a total of 84 species of Taraxacum in China, and 27 of them can be used for medicinal purposes, among which T. mongolicum, T. sinicum, T. officinale, T. scariosum and T. platycarpum are the most common. Therefore, the research on the chemical components of Taraxacum plants mainly focuses on the above five species, and there is a lack of research on other species of Taraxacum. The research on the extraction of active components of dandelions lags, and the application of advanced technologies, such as ultrasonic-assisted extraction, microwave-assisted extraction, or supercritical fluid extraction technology, is relatively lacking.
The roots, stems, leaves, and flowers of Taraxaci herba are all rich in flavonoids, but the content of flavonoids in different parts is obviously different (flowers > leaves > roots), which are mainly concentrated in the aboveground parts. Terpenoids in Taraxacum plants are mainly triterpenoids and sesquiterpenes, among which pentacyclic triterpenoid taraxasterol is one of the main active components in Taraxacum plants, and sesquiterpenes are the main source of the bitter taste of Taraxacum.
Current phytochemical and pharmacological studies show that Taraxaci herba has significant anti-inflammatory, antioxidant, anti-tumor, and antibacterial effects. Pharmacological research provides a scientific theoretical basis for the routine application of Taraxaci herba in classical medicine, especially in the treatment of inflammation. Taraxaci herba has a remarkable therapeutic effect on mastitis in clinical practice in China, mainly achieved through external application, oral administration, a combination with other natural medicines, and integrated Chinese and Western medicine approaches. Previous pharmacological experiments have confirmed that dandelion has a certain inhibitory effect on the growth and reproduction of cancer cells such as in breast cancer, liver cancer, lung cancer, and gastric cancer. It is basically non-toxic, has few adverse reactions, and can be developed as a potential natural anti-cancer drug or an adjunct for cancer treatment. Taraxaci herba is usually used in combination with other natural plants and animal drugs, but there is relatively little research on the synergistic mechanism. Therefore, it seems to be a new way to explore the synergistic mechanism of drugs between specific natural animals, plants, and Taraxaci herba. Furthermore, the processing and application methods of dandelion are single, inevitably causing problems such as the loss of active ingredients and low bioavailability. Innovative nano-encapsulation technology and sustained-release dosage forms can effectively improve bioavailability and therapeutic targeting, which is worthy of further research. Taraxaci herba plants are widely distributed, and the accumulation of metabolites in Taraxaci herba plants is quite different due to different harvesting times and environmental differences between different producing areas. Taraxaci herba has broad commercial potential. Its rich medicinal value provides a new research and development direction for the pharmaceutical industry, which is expected to develop a series of efficient and low-toxic natural medicine products. In addition, Taraxaci herba also has huge development space in the fields of food and health care products. For example, it can be made into dandelion tea and dandelion health care products. In the cosmetics industry, it can be used as a natural antioxidant and anti-inflammatory ingredient and applied to the development of skin care products. In the medical market, drugs made from its extracts may occupy a certain market share. With people’s preference for natural ingredients, the market demand for natural medicines and health care products based on Taraxaci herba is expected to continue to grow. At the same time, its application value in ecological restoration and environmental governance may also attract investment and development from related enterprises. We hope that the above information will help people better understand the traditional medicinal plant Taraxaci herba, establish a perfect yield and quality control system, and provide convenience for the development and application of Taraxaci herba in the future.
Author Contributions
J.W.: Conceptualization, Writing—original draft, Writing—review and editing. J.S.: Writing—review and editing. M.L.: Writing—review and editing. X.Z.: Writing—review and editing. L.K.: Writing—review and editing. L.M.: Writing—review and editing. S.J.: Writing—review and editing. X.L.: Conceptualization, Writing—review and editing, Supervision, Project administration. W.M.: Conceptualization, Writing—review and editing, Supervision, Project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Project, Research and Demonstration of Collection, Screening, and Breeding Technology of Ginseng and other Genuine Medicinal Materials, grant number 2021YFD1600901, Study on Growth and Quality Optimization of Artificial Cultivation of Bupleurum chinense, grant number LBH-Z23276 and Heilongjiang Touyan Innovation Team Program, grant number [2019] No. 5. And the APC was funded by 2021YFD1600901.
Data Availability Statement
No data were used for the research described in the article.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Glossary
| TS | Taraxasterol | TDS | Taraxaci herba polysaccharides |
| TF | Taraxaci herba flavone | Bcl2 | B-cell lymphoma-2 |
| STAT | Signal transducer and activator of transcription | JAK | Janus kinase |
| JNK | c-Jun N-terminal kinase | SOCS3 | Cytokine signaling pathway inhibitor 3 |
| APAP | Acetaminophen | HO-1 | Heme Oxygenase-1 |
| Nrf2 | Nuclearrespiratoty factor 2 | PGE2 | Prostaglandin E2 |
| Hint1 | Histidine triad nucleotide-binding protein 1 | IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 | NF-κB | Nuclear factor-κB |
| MAPK | Mitogen-activated protein kinase | TIMP | Tissue inhibitor of matrix metalloproteinases |
| Cox-2 | Cyclooxygenase-2 | TNF-α | Tumor necrosis factor alpha |
| IL-1β | Interleukin—1β | MPO | Myeloperoxidase |
| SOD | Superoxide dismutase | GSH | Glutathione |
| MDA | Malondialdehyde | MAO | Monoamine oxidase |
| LPF | Leukocytosis promoting factor | ROS | Reactive oxygen species |
| MMP | Matrix metallopeptidase | CAT | Catalase |
| MIC | Minimum inhibitory concentration | TNBC | Triple negative breast cancer |
| IL-10 | Interleukin-10 | ERK | Extracellular signal-regulated kinase |
| MAPK | Activated protein kinase | BAX | Bcl2 Associated x protein |
References
- Ge, X.; Lin, Y.; Zhai, D. Preliminary arrangement of Taraxacum plants in China. Plant Res. 1998, 4, 1–21. [Google Scholar]
- Gong, Z.; Zhang, W.; Liu, C.; He, B.; Tan, R. Resources of Taraxacum in China. Wild Plant Resour. China 2001, 3, 9–14+15. [Google Scholar]
- Shanghai Scientific and Technical Publishers. Dictionary of Traditional Chinese Medicine; Jiangsu New Medical College: Shanghai, China, 2020. [Google Scholar]
- Li, L.; Liu, Q.; Liu, T.; Cui, X.; Ning, W. Expression of putative luteolin biosynthesis genes and WRKY transcription factors in Taraxacum antungense kitag. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 145, 1–17. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020. [Google Scholar]
- Sergiu, P.; Sonia, A.; Gheorghe, G.; Beniamin, P. The Evaluation of Dandelion (Taraxacum officinale) Properties as a Valuable Food Ingredient. Rom. Biotechnol. Lett. 2016, 21, 11569–11575. [Google Scholar]
- Hu, C. Taraxacum: Phytochemistry and health benefits. Chin. Herb. Med. 2018, 10, 353–361. [Google Scholar] [CrossRef]
- Laura, G.; Stefano, E.; Bruna, D.F.; Virginia, L.; Giuliano, B. Common dandelion: A review of its botanical, phytochemical and pharmacological profiles. Phytochem. Rev. 2019, 18, 1115–1132. [Google Scholar] [CrossRef]
- Schütz, K.; Carle, R.; Schieber, A. Taraxacum—A review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef]
- Martinez, M.; Poirrier, P.; Chamy, R.; Prüfer, D.; Schulze-Gronover, C.; Jorquera, L.; Ruiz, G. Taraxacum officinale and related species-An ethnopharmacological review and its potential as a commercial medicinal plant. J. Ethnopharmacol. 2015, 169, 244–262. [Google Scholar] [CrossRef]
- Hao, F.; Deng, X.; Yu, X.; Wen, W.; Yan, W.; Zhao, X.; Wang, X.; Bai, C.; Lu, H. Taraxacum: A Review of Ethnopharmacology, Phytochemistry and Pharmacological Activity. Am. J. Chin. Med. 2024, 52, 183–215. [Google Scholar] [CrossRef]
- Murtaza, I.; Laila, O.; Drabu, I.; Ahmad, A.; Charifi, W.; Popescu, S.M.; Mansoor, S. Nutritional Profiling, Phytochemical Composition and Antidiabetic Potential of Taraxacum officinale, an Underutilized Herb. Molecules 2022, 27, 5380. [Google Scholar] [CrossRef]
- Ruiz-Juarez, D.; Melo-Ruiz, V.E.; Gutierrez-Rojas, M.; Sanchez-Herrera, K.; Cuamatzi-Tapia, O. Nutrient value in dandelion flower (taraxacum officinale). Ann. Nutr. Metab. 2020, 76, 102. [Google Scholar]
- Chen, G.; Li, R. Investigation on the Current Situation of Medicinal Plant Resources in Shandong Province. Chin. Herb. Med. 2023, 46, 2155–2159. [Google Scholar] [CrossRef]
- Ning, W.; Wu, J.; Zhao, T.; Zhao, X.; Li, T. Karyotype study on seven species of Taraxacum mongolicum in Northeast China. China J. Tradit. Chin. Med. 2012, 37, 771–776. [Google Scholar]
- Qiao, Y.; Wang, Y.; Cao, Y.; He, J. Cluster analysis of karyotype similarity coefficient of 13 species of Taraxacum. Acta Agrestia Sin. 2020, 28, 285–290. [Google Scholar]
- Li, Z.; Zhang, T.; Yang, M.; Sun, L. Investigation and Analysis of Medicinal Plant Resources in Shandong Province. Chin. Herb. Med. 2024, 2, 313–318. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, S.; Liu, J.; Guo, Y. Leaf structure changes and ecological adaptability of three compositae plants at different altitudes in Qinghai-Tibet Plateau. Acta Ecol. Sin. 2016, 36, 1559–1570. [Google Scholar]
- Wu, J.; Liu, Q.; Haitao, C.; Wei, N.; Wei, C. Evaluation and identification of morphological characters suitable for delimitation of Taraxacum species distributed in northeastern China. Food Sci. Nutr. 2022, 10, 2999–3008. [Google Scholar] [CrossRef]
- Li, H.; Zhao, X.; Jia, Q.; Li, T.; Ning, W. Achene morphology cluster analysis of Taraxacum F. H. Wigg. from northeast China and molecule systematics evidence determined by SRAP. Yao xue xue bao = Acta Pharm. Sin. 2012, 47, 1063–1069. [Google Scholar]
- Qi, J.; Jia, Q.; Guo, X.; Fan, X. Clinical application of dandelion external application in acute mastitis. Guizhou Med. J. 2014, 38, 360–361. [Google Scholar]
- Qiao, N.; Ding, X.; Ni, S. Clinical observation on treatment of acute lactation mastitis complicated with abscess formation with combination of traditional Chinese and western medicine. China J. Tradit. Chin. Med. Pharm. 2020, 35, 1580–1582. [Google Scholar]
- Yuan, Y.; Weichen, H. Clinical observation on the treatment of ulcerative colitis with Gegen Qinlian decoction and dandelion enema. Prog. Mod. Biomed. 2007, 9, 1336–1337. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, L.; Chen, S. Clinical observation on treatment of acute suppurative tonsillitis with compound dandelion decoction. J. Anhui Tradit. Chin. Med. Coll. 2010, 29, 9–12. [Google Scholar]
- Wang, C.; Xu, M. Clinical observation on the treatment of 38 cases of intestinal obstruction in the elderly with twelve dandelion syrup. Hebei J. Tradit. Chin. Med. 2013, 35, 1146–1147. [Google Scholar]
- Li, S. Grass chapter. In Bencao Gangmu; Plant Part; 1596; Volume 16. [Google Scholar]
- Chen, S. Strange prescription middle volume. In Secret Record of Surgery; 1694; Volume 15. [Google Scholar]
- Inner Mongolia Autonomous Region Revolutionary Committee Health Bureau. Selected Materials of New Medical Treatment of Chinese Herbal Medicine; Inner Mongolia Autonomous Region Revolutionary Committee Health Bureau: Inner Mongolia, China, 1971; pp. 162–164.
- Lan, M. Herbal Medicine in Southern Yunnan; 1436; Volume 1. [Google Scholar]
- Ge, H.; Tao, H.; Yang, Y. Breast prescription. In Meishi Prescription; 820; Volume 5. [Google Scholar]
- Xie, Z.; Fan, C.; Zhu, Z. Compilation of Chinese Herbal Medicine in China; People’s Health Publishing House: Beijing, China, 1994; pp. 898–899. [Google Scholar]
- Ministry of Health, L.D.o.N.M.R. Commonly Used Chinese Herbal Medicines in Nanjing; Jiangsu Revolutionary Committee Publishing Bureau: Nanjing, China, 1969; pp. 464–465. [Google Scholar]
- Chen, S. Strange prescription rolled up. In Secret Record of Surgery; 1694; Volume 14. [Google Scholar]
- China Anhui Provincial Revolutionary Committee Health Bureau. Anhui Chinese Herbal Medicine; Anhui People’s Publishing House: Hefei, China, 1975; pp. 49–51.
- He, Z. Summary of prescription addition and subtraction changes. In Heshijishenglun; 1672; Volume 7. [Google Scholar]
- China Qingdao Chinese Herbal Medicine Manual Compilation Group. Qingdao Handbook of Chinese Herbal Medicine; People’s Health Publishing House: Qingdao, China, 1975; pp. 255–257. [Google Scholar]
- China Jilin Provincial Institute of Traditional Chinese Medicine. Flora of Changbai Mountain; Jilin People’s Publishing House: Jilin, China, 1982; pp. 1259–1260. [Google Scholar]
- Hasnat, H.; Shompa, S.A.; Islam, M.M.; Alam, S.; Richi, F.T.; Emon, N.U.; Ashrafi, S.; Ahmed, N.U.; Chowdhury, M.N.R.; Fatema, N.; et al. Flavonoids: A treasure house of prospective pharmacological potentials. Heliyon 2024, 10, e27533. [Google Scholar] [CrossRef]
- Choi, J.; Yoon, K.D.; Kim, J. Chemical constituents from Taraxacum officinale and their α-glucosidase inhibitory activities. Bioorganic Med. Chem. Lett. 2018, 28, 476–481. [Google Scholar] [CrossRef]
- Xie, S.; Yang, X.; Ding, Z.; Li, M. Chemical constituents and pharmacological effects of Taraxacum mongolicum. Nat. Prod. Res. Dev. 2012, 24, 141–151. [Google Scholar] [CrossRef]
- Bao, T.; Ke, Y.; Wang, Y.; Wang, W.; Li, Y.; Wang, Y.; Kui, X.; Zhou, Q.; Zhou, H.; Zhang, C.; et al. Taraxasterol suppresses the growth of human liver cancer by upregulating Hint1 expression. J. Mol. Med. 2018, 96, 661–672. [Google Scholar] [CrossRef]
- Ling, Y.; Zhou, Y.; Chen, S. Studies on chemical constituents of Taraxacum mongolicum in alkali land. China J. Chin. Mater. Medica 1998, 4, 40–64. [Google Scholar]
- Ling, Y.; Bo, Y. Studies on chemical constituents of Taraxacum mongolicum. J. Nav. Gen. Hosp. 1998, 2, 167–169. [Google Scholar]
- Wei, Y.; Wenyan, L.; Changxin, Z. Studies on chemical constituents of Taraxacum mongolicum. China J. Chin. Mater. Medica 2007, 10, 926–929. [Google Scholar]
- Rong, W.; Weihua, L.; Cao, F.; Xinxin, Z.; Chao, L.; Qing, H. Extraction and identification of new flavonoid compounds in dandelion Taraxacum mongolicum Hand.-Mazz. with evaluation of antioxidant activities. Sci. Rep. 2023, 13, 2166. [Google Scholar] [CrossRef]
- Stoja, M.; Agnieszka, G.; Łukasz, Ś.; Agnieszka, D.; Katarzyna, T.; Marcin, K. Dandelion seeds as a new and valuable source of bioactive extracts obtained using the supercritical fluid extraction technique. Sustain. Chem. Pharm. 2022, 29, 100796. [Google Scholar]
- Wang, Y.; Li, Y.; Yang, N. Research progress on chemical constituents and pharmacological effects of Taraxacum plants. Spec. Res. 2017, 39, 67–75. [Google Scholar] [CrossRef]
- Songbao, Z.; Chao, L.; Wei, G.; Rongli, Q.; Jianguo, C.; Lingfeng, P.; Lijie, M.; Yangfang, G.; Rong, T. Metabolomics analysis of dandelions from different geographical regions in China. Phytochem. Anal. 2021, 32, 899–906. [Google Scholar] [CrossRef]
- Xiaoyan, L.; Feng, L.; Yu, Z.; Xue, L.; Xian, Z. Research progress on anti-tumor mechanism of effective components in dandelion. Chin. Herb. Med. 2023, 54, 3391–3400. [Google Scholar]
- Wu, X.; Du, S.; Chen, H. Research and application of Taraxacum mongolicum. J. Pharm. Pract. 2002, 4, 246–248. [Google Scholar]
- Yang, Y.; Li, T.; Wang, X. Ultrasonic Assisted Extraction- Gas Chromatography-mass Spectrometry Analysis of Chemical Components of Dandelion Root. Chem. Eng. Trans. (CET J.) 2018, 66. [Google Scholar] [CrossRef]
- Albert, B.; Manuel, R.-C. Terpenoid biosynthesis in prokaryotes. Adv. Biochem. Eng./Biotechnol. 2015, 148, 3–18. [Google Scholar] [CrossRef]
- D, M. Triterpene alcohols from Taraxacum pollen. C R Acad. Sci. Ser. D 1969. [Google Scholar]
- Ling, Y.; Xu, Y.; Zhang, J. Studies on triterpenoids from Taraxacum mongolicum. Chin. Herb. Med. 1998, 4, 224–225. [Google Scholar]
- Anne, B.; Maik, B.; Alberto, M.; Giovanni, A.; Wolfgang, M. Broad tuning of the human bitter taste receptor hTAS2R46 to various sesquiterpene lactones, clerodane and labdane diterpenoids, strychnine, and denatonium. J. Agric. Food Chem. 2007, 55, 6236–6243. [Google Scholar] [CrossRef]
- Ming, Z.; Xu, S. Chemistry and Pharmacology of Taraxacum mongolicum. J. Shenyang Pharm. Univ. 1997, 2, 62–68. [Google Scholar]
- Kenny, O.; Brunton, N.P.; Walsh, D.; Hewage, C.M.; McLoughlin, P.; Smyth, T.J. Characterisation of antimicrobial extracts from dandelion root (Taraxacum officinale) using LC-SPE-NMR. Phytother. Res. PTR 2015, 29, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Triebwasser-Freese, D.; Reichelt, M.; Heiling, S.; Paetz, C.; Chandran, J.N.; Bartram, S.; Schneider, B.; Gershenzon, J.; Erb, M. Identification, quantification, spatiotemporal distribution and genetic variation of major latex secondary metabolites in the common dandelion (Taraxacum officinale agg.). Phytochemistry 2015, 115, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, J. The effects of Taraxacum officinale extracts (TOE) supplementation on physical fatigue in mice. Afr. J. Tradit. Complement. Altern. Med. AJTCAM 2011, 8, 128–133. [Google Scholar] [CrossRef]
- Liu, T.; Liao, J.; Shi, M.; Li, L.; Liu, Q.; Cui, X.; Ning, W.; Kai, G. A Jasmonate-Responsive bHLH Transcription Factor TaMYC2 Positively Regulates Triterpenes Biosynthesis in Taraxacum antungense Kitag. Plant Sci. Int. J. Exp. Plant Biol. 2022, 326, 111506. [Google Scholar] [CrossRef]
- Shuyun, S.; Changxin, Z.; Yan, X.; Qiaofeng, T. Studies on chemical constituents of Taraxacum mongolicum. China J. Chin. Mater. Medica 2008, 10, 1147–1157. [Google Scholar]
- Sharifi-Rad, M.; Roberts, T.H.; Matthews, K.R.; Bezerra, C.F.; Morais-Braga, M.F.B.; Coutinho, H.D.M.; Sharopov, F.; Salehi, B.; Yousaf, Z.; Sharifi-Rad, M.; et al. Ethnobotany of the genus Taraxacum-Phytochemicals and antimicrobial activity. Phytother. Res. 2018, 32, 2131–2145. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, B.; Kuang, H. Studies on Chemical Constituents of Taraxacum mongolicum Root in Northeast China. Chin. Pat. Med. 2023, 45, 1887–1891. [Google Scholar]
- Wu, Z.; Yang, Y.; Li, Z.; Lu, X.; Wang, X. Development of dandelion (Taraxacum spp.) quality evaluation technology based on phenolic acids. Folia Hortic. 2022, 34, 187–209. [Google Scholar] [CrossRef]
- Chen, H.; Qiao, H.; Sun, T. Study on antitumor activity of Taraxacum mongolicum flower extract in vitro. Chin. Remedies Clin. 2014, 14, 1179–1181+1309. [Google Scholar]
- Shi, S.; Zhou, H. Studies on chemical constituents of Taraxacum tubulosa. China J. Tradit. Chin. Med. 2009, 34, 1002–1004. [Google Scholar]
- Shi, S.; Zhang, C.; Xu, Y.; Tiao, Q.; Bai, H.; Lu, F.; Lin, W.; Li, X.; Qu, J. Studies on chemical constituents from herbs of Taraxacum mongolicum. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Medica 2008, 33, 1147–1157. [Google Scholar]
- Peng, D.; Gao, J.; Guo, X.; Wang, J. Studies on the chemical constituents of Taraxacum mongolicum roots. Chin. Pat. Med. 2014, 36, 1462–1466. [Google Scholar]
- Ren, H.; Zhang, W.; Zhang, Y.; Zhang, Z. Research progress on functional components and biological activities of Taraxacum mongolicum. Food Drug 2022, 24, 193–201. [Google Scholar] [CrossRef]
- Liu, X.; Guan, J.; Zhang, G.; Wang, J. Effect of Taraxasterol on Proliferation of Human Tongue Cancer CAL-27 Cells and Its Mechanism. Trace Elem. Health Res. 2020, 37, 39–41. [Google Scholar]
- Yang, C.; Yan, Q.; Tao, J.; Xia, B. Analysis of volatile oil from Taraxacum mongolicum and its anti-inflammatory and anti-tumor activities. China J. Tradit. Chin. Med. Pharm. 2018, 33, 3106–3111. [Google Scholar]
- Shouxun, Z.; Bingqian, H. Chemical constituents and pharmacological effects of Taraxacum mongolicum. In Wild Plant Resources in China; 2001; pp. 1–3. [Google Scholar]
- Jin, Y.; Zhu, G. Study on extraction technology of total alkaloids from Taraxacum mongolicum by orthogonal test. Jiangsu Agric. Sci. 2009, 3, 329–330. [Google Scholar]
- Xue, H.; Nima, L.; Wang, S.; Tan, J. Ultrasound assisted hot water extraction of polysaccharides from Taraxacum mongolicum: Optimization, purification, structure characterization, and antioxidant activity. J. Food Sci. 2024, 89, 2827–2842. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Y.; Zou, J.; Zhang, X.; Zhai, B.; Guo, D.; Sun, J.; Luan, F. Extraction, purification, structural features, biological activities, modifications, and applications from Taraxacum mongolicum polysaccharides: A review. Int. J. Biol. Macromol. 2024, 259, 129193. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Yasmeen, S.; Ali, Z.; Khan, M.A.; Choudhary, M.I.; Akhtar, F.; Miana, G.A.; Zahid, M. Taraxacin, a new guaianolide from Taraxacum wallichii. J. Nat. Prod. 2000, 63, 1010–1011. [Google Scholar] [CrossRef]
- Ling, Y.; Bo, Y. Analysis of Trace Elements in Dandelion. Trace Elem. Health Res. 1998, 2, 54–56. [Google Scholar]
- Che, L. Protective Effect of Dandelion Sterol on Ulcerative Colitis and Its Mechanism. Ph.D. Thesis, Zhengzhou University, Zhengzhou, China, 2020. [Google Scholar]
- Zhang, Y.; Bin Shaari, R.; Nawi, M.A.B.A.; Bin Hassan, A.; Cui, C. Pharmacological Action and Research Progress of Taraxasterol. Curr. Pharm. Biotechnol. 2024, 25, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiong, H.; Liu, L. Effects of taraxasterol on inflammatory responses in lipopolysaccharide-induced RAW 264.7 macrophages. J. Ethnopharmacol. 2012, 141, 206–211. [Google Scholar] [CrossRef] [PubMed]
- San, Z. Study on Anti-Inflammatory Effect and Regulatory Mechanism of Taraxasterol on LPS-Induced Mastitis. Ph.D. Thesis, Jilin University, Jilin, China, 2014. [Google Scholar]
- Zheng, F.; Dong, X.; Meng, X. Anti-Inflammatory Effects of Taraxasterol on LPS-Stimulated Human Umbilical Vein Endothelial Cells. Inflammation 2018, 41, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Li, H.; Tao, T.; Xu, B. Study on the protective effect of Taraxasterol on acute severe hepatitis based on JAK2/STAT3 and JNK signaling pathway. Pharmacol. Clin. Chin. Mater. Medica 2023, 39, 57–61. [Google Scholar] [CrossRef]
- Bingjie, G.; Rui, S.; Wei, W.; Kexin, Y.; Yifan, Y.; Lin, K.; Minghong, Y.; Xinman, L.; Xuemei, Z. Protection of taraxasterol against acetaminophen-induced liver injury elucidated through network pharmacology and in vitro and in vivo experiments. Phytomedicine 2023, 116, 154872. [Google Scholar] [CrossRef]
- Park, C.M.; Cho, C.W.; Song, Y.S. TOP 1 and 2, polysaccharides from Taraxacum officinale, inhibit NFκB-mediated inflammation and accelerate Nrf2-induced antioxidative potential through the modulation of PI3K-Akt signaling pathway in RAW 264.7 cells. Food Chem. Toxicol. 2014, 66, 56–64. [Google Scholar] [CrossRef]
- Chaoyong, X.; Yu, Z.; Yuliang, W. Extraction, Purification and in vitro Anti-inflammatory Activity Analysis of Total Polysaccharides from Taraxacum mongolicum. Chin. J. Exp. Tradit. Med. Formulae 2016, 22, 25–28. [Google Scholar] [CrossRef]
- Hee, H.S.; HakDong, L.; Sanghyun, L.; Young, L.A. Taraxacum coreanum Nakai extract attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier dysfunction in Caco-2 cells. J. Ethnopharmacol. 2023, 319, 117105. [Google Scholar] [CrossRef]
- An, L. Clinical study on dandelion granules combined with cefradine in the treatment of acute mastitis. Drugs Clin. 2021, 36, 796–798. [Google Scholar]
- Sui, H.; Wang, Y.; Luan, H. Antioxidant effect of Taraxacum mongolicum total flavonoids extract on brain tissue of aging model mice. Chin. Pat. Med. 2009, 31, 1289–1290. [Google Scholar]
- Pang, M.; Li, T.; Chen, W.; Long, S. Study on antioxidant active components and mechanism of Taraxacum mongolicum based on HPLC and network pharmacology. J. Jilin Univ. 2023, 61, 437–442. [Google Scholar] [CrossRef]
- Hu, C.; Kitts, D.D. Dandelion (Taraxacum officinale) flower extract suppresses both reactive oxygen species and nitric oxide and prevents lipid oxidation in vitro. Phytomedicine Int. J. Phytother. Phytopharm. 2005, 12, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, F. Antioxidant activity participates in the protective effect of Taraxacum mongolicum on experimental gastric ulcer in mice. World Chin. Med. 2018, 13, 993–996. [Google Scholar]
- Shu, Y.; Lu, J.; Chen, X. Study on extraction and antioxidant activity of dandelion polysaccharide. Mod. Agric. Sci. Technol. 2022, 186–189+193. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, Z.; Hao, Y.; An, P.; Luo, J.; Luo, Y. Dandelion Polysaccharides Ameliorate High-Fat-Diet-Induced Atherosclerosis in Mice through Antioxidant and Anti-Inflammatory Capabilities. Nutrients 2023, 15, 4120. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Shi, P.; Li, P.; Fu, Y.; Tan, G.; Zhou, J.; Zeng, J.; Huang, P. Modulation of Acute Intestinal Inflammation by Dandelion Polysaccharides: An In-Depth Analysis of Antioxidative, Anti-Inflammatory Effects and Gut Microbiota Regulation. Int. J. Mol. Sci. 2024, 25, 1429. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, J.-L.; Bai, Z.; Du, J.; Shi, Y.; Wang, Y.; Wang, Y.; Liu, Y.; Yu, Z.; Li, M.-Y. Polysaccharide from dandelion enriched nutritional composition, antioxidant capacity, and inhibited bioaccumulation and inflammation in Channa asiatica under hexavalent chromium exposure. Int. J. Biol. Macromol. 2022, 201, 557–568. [Google Scholar] [CrossRef]
- Gao, D. Analysis of nutritional components of Taraxacum mongolicum and its antibacterial activity. Pharmacogn. J. 2010, 2, 502–505. [Google Scholar]
- Han, Q.; Sun, T. Antibacterial activity of ethanol extract and fractions obtained from Taraxacum mongolicum flower. Res. J. Pharmacogn. 2014, 1, 35–39. [Google Scholar]
- Katy, D.; Luis, E.; Alejandro, M.; Leonardo, P.; Rolando, C. Isolation and Identification of Compounds from Bioactive Extracts of Taraxacum officinale Weber ex F. H. Wigg. (Dandelion) as a Potential Source of Antibacterial Agents. Evid.-Based Complement. Altern. Med. eCAM 2018, 2018, 2706417. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Xie, P. Screening and unveiling antibacterial mechanism of dandelion phenolic extracts against Staphylococcus aureus by inhibiting intracellular Na+-K+ ATPase based on molecular docking and molecular dynamics simulation. J. Biomol. Struct. Dyn. 2024, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Duan, H.; Zhang, P.; Han, H.; Gao, F.; Li, Y.; Xu, Z. Extraction and isolation of the active ingredients of dandelion and its antifungal activity against Candida albicans. Mol. Med. Rep. 2020, 21, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Guo, R.; Wang, Y. Taraxacum mongolicum extract exhibits a protective effect on hepatocytes and an antiviral effect against hepatitis B virus in animal and human cells. Mol. Med. Rep. 2014, 9, 1381–1387. [Google Scholar] [CrossRef]
- Han, H.; He, W.; Wang, W.; Gao, B. Inhibitory effect of aqueous dandelion extract on HIV-1 replication and reverse transcriptase activity. BMC Complement. Altern. Med. 2011, 11, 112. [Google Scholar] [CrossRef]
- Tu, G. Chemical constituents, pharmacological effects and clinical application of Taraxacum mongolicum. Strait Pharm. J. 2012, 24, 33–35. [Google Scholar]
- Qi, X.; Gao, P.; Qiao, T. Research progress on anti-tumor effect of Taraxacum mongolicum. China Cancer 2015, 24, 53–56. [Google Scholar]
- Sun, Y.; Liu, X. Understanding of breast cancer in traditional Chinese medicine and principles of postoperative treatment. Chin. Arch. Tradit. Chin. Med. 2005, 180–182. [Google Scholar] [CrossRef]
- Veronesi, U.; Boyle, P.; Goldhirsch, A.; Orecchia, R.; Viale, G. Breast cancer. Lancet 2005, 365, 1727–1741. [Google Scholar] [CrossRef]
- Qu, J.; Ke, F.; Liu, Z.; Yang, X.; Li, X.; Xu, H.; Li, Q.; Bi, K. Uncovering the mechanisms of dandelion against triple-negative breast cancer using a combined network pharmacology, molecular pharmacology and metabolomics approach. Phytomedicine 2022, 99, 153986. [Google Scholar] [CrossRef] [PubMed]
- Sigstedt, S.C.; Hooten, C.J.; Callewaert, M.C.; Jenkins, A.R.; Romero, A.E.; Pullin, M.J.; Kornienko, A.; Lowrey, T.K.; Slambrouck, S.V.; Steelant, W.F. Evaluation of aqueous extracts of Taraxacum officinale on growth and invasion of breast and prostate cancer cells. Int. J. Oncol. 2008, 32, 1085–1090. [Google Scholar] [CrossRef]
- Li, X.; He, X.; Zhou, Y.; Zhao, H.; Zheng, W.; Jiang, S.; Zhou, Q.; Li, P.; Han, S. Taraxacum mongolicum extract induced endoplasmic reticulum stress associated-apoptosis in triple-negative breast cancer cells. J. Ethnopharmacol. 2017, 206, 55–64. [Google Scholar] [CrossRef]
- Wang, S.; Hao, H.; Jiao, Y.; Fu, J.; Guo, Z.; Guo, Y.; Yuan, Y.; Li, P.; Han, S. Dandelion extract inhibits triple-negative breast cancer cell proliferation by interfering with glycerophospholipids and unsaturated fatty acids metabolism. Front. Pharmacol. 2022, 13, 942996. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Jiao, Y.; Hao, H.; Xue, D.; Bai, C.; Han, S. Taraxacum mongolicum extract inhibited malignant phenotype of triple-negative breast cancer cells in tumor-associated macrophages microenvironment through suppressing IL-10/STAT3/PD-L1 signaling pathways. J. Ethnopharmacol. 2021, 274, 113978. [Google Scholar] [CrossRef]
- Niu, H.; Fan, J.; Wang, G.; Wang, J.; Chu, Y.; Yang, Q.; Wang, L.; Tian, B. Anti-tumor effect of polysaccharides isolated from Taraxacum mongolicum Hand-Mazz on MCF-7 human breast cancer cells. Trop. J. Pharm. Res. 2017, 16, 83. [Google Scholar] [CrossRef]
- Sun, Y. Taraxacum flavonoids Also Have Inhibitory Effect on Human Breast Cancer Cells. Master’s Thesis, Qinghai University, Xining, China, 2020. [Google Scholar]
- Hamed, R.; Reza, A.M.; Farhad, J.; CT, C.C.; Vahideh, A.; Ali, N. Combined dandelion extract and all-trans retinoic acid induces cytotoxicity in human breast cancer cells. Sci. Rep. 2023, 13, 15074. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, Y.; Che, L.; Min, L.; Huang, D.; Zhang, Y.; Li, C.; Li, Z. Suppression of migration and invasion by taraxerol in the triple-negative breast cancer cell line MDA-MB-231 via the ERK/Slug axis. PLoS ONE 2023, 18, e0291693. [Google Scholar] [CrossRef]
- He, J.; Cao, W.; Lin, N. Guidelines for screening, early diagnosis and early treatment of prostate cancer in China (2022, Beijing). China Cancer 2022, 31, 1–30. [Google Scholar]
- Nguyen, C.; Mehaidli, A.; Baskaran, K.; Grewal, S.; Pupulin, A.; Ruvinov, I.; Scaria, B.; Parashar, K.; Vegh, C.; Pandey, S. Dandelion Root and Lemongrass Extracts Induce Apoptosis, Enhance Chemotherapeutic Efficacy, and Reduce Tumour Xenograft Growth In Vivo in Prostate Cancer. Evid.-Based Complement. Altern. Med. eCAM 2019. [Google Scholar] [CrossRef]
- Yang, J.; Xin, C.; Yin, G.; Li, J. Taraxasterol suppresses the proliferation and tumor growth of androgen-independent prostate cancer cells through the FGFR2-PI3K/AKT signaling pathway. Sci. Rep. 2023, 13, 13072. [Google Scholar] [CrossRef] [PubMed]
- Morteza, M.; Mona, P.; Gouvarchin, G.H.E.; Jalali, K.B. Anti-metastatic effect of taraxasterol on prostate cancer cell lines. Res. Pharm. Sci. 2023, 18, 439–448. [Google Scholar] [CrossRef]
- Wu, M.; Tang, Z.; Liu, T. Guidelines for standardized pathological diagnosis of primary liver cancer (2015 edition). J. Clin. Hepatol. 2015, 31, 833–839. [Google Scholar]
- Ren, F.; Li, J.; Yuan, X.; Wang, Y.; Wu, K.; Kang, L.; Luo, Y.; Zhang, H.; Yuan, Z. Dandelion polysaccharides exert anticancer effect on Hepatocellular carcinoma by inhibiting PI3K/AKT/mTOR pathway and enhancing immune response. J. Funct. Foods 2019, 55, 263–274. [Google Scholar] [CrossRef]
- Ren, F.; Wu, K.; Yang, Y.; Yang, Y.; Wang, Y.; Li, J. Dandelion Polysaccharide Exerts Anti-Angiogenesis Effect on Hepatocellular Carcinoma by Regulating VEGF/HIF-1α Expression. Front. Pharmacol. 2020, 11, 460. [Google Scholar] [CrossRef]
- Ren, F.; Zhang, Y.; Qin, Y.; Shang, J.; Wang, Y.; Wei, P.; Guo, J.; Jia, H.; Zhao, T. Taraxasterol prompted the anti-tumor effect in mice burden hepatocellular carcinoma by regulating T lymphocytes. Cell Death Discov. 2022, 8, 264. [Google Scholar] [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Hui, H.; Zhen, C.G.; Kang, Z.S.; Ru, X.R.; Liang, W.C. In vitro anti-tumor activity in SGC-7901 human gastric cancer cells treated with dandelion extract. Trop. J. Pharm. Res. 2018, 17, 65. [Google Scholar]
- Zhu, H.; Zhao, H.; Zhang, L.; Xu, J.; Zhu, C.; Zhao, H.; Lv, G. Dandelion root extract suppressed gastric cancer cells proliferation and migration through targeting lncRNA-CCAT1. Biomed. Pharmacother. 2017, 93, 1010–1017. [Google Scholar] [CrossRef]
- Chen, W.; Li, J.; Chen, L.; Fan, H.; Zhang, J.; Zhu, J. Network pharmacology-based identification of the antitumor effects of taraxasterol in gastric cancer. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420933107. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Guo, M.; Yang, H. Taraxasterol suppresses cell proliferation and boosts cell apoptosis via inhibiting GPD2-mediated glycolysis in gastric cancer. Cytotechnology 2021, 73, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Zheng, R.; Zhang, S. Analysis of Incidence and Death of Malignant Tumors in China in 2015. China Cancer 2019, 28, 1–11. [Google Scholar]
- Tao, T.; Yang, J.; Liu, Z.; Chen, Y.; Zeng, C. Taraxasterol acetate targets RNF31 to inhibit RNF31/p53 axis-driven cell proliferation in colorectal cancer. Cell Death Discov. 2021, 7, 66. [Google Scholar] [CrossRef]
- Tang, C. Study on the Mechanism of Dandelion Sterol Regulating the Occurrence and Development of Colon Cancer through Activating Autophagy and Mediating RNF31/p53 Signal Axis. Ph.D. Thesis, Nanchang University, Nanchang, China, 2022. [Google Scholar]
- Kang, L.; Mang, M.; Song, Y.; Fang, X.; Zhang, J.; Zhang, Y.; Miao, J. Total flavonoids of Taraxacum mongolicum inhibit non-small cell lung cancer by regulating immune function. J. Ethnopharmacol. 2021, 281, 114514. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Fu, X. Study on Ant-itumor and Anti-mutagenic effects of dandelion polysaccharide in vitro. Lishizhen Med. Mater. Medica Res. 2009, 20, 2470–2471. [Google Scholar]
- Li, Y.; Deng, Y.; Zhang, X.; Wang, T. Dandelion Seed Extract Affects Tumor Progression and Enhances the Sensitivity of Cisplatin in Esophageal Squamous Cell Carcinoma. Front. Pharmacol. 2022, 13, 897465. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, R.; He, Y.; Mao, G.; Kong, Z. Taraxasterol enhanced bladder cancer cells radiosensitivity via inhibiting the COX-2/PGE2/JAK2/STAT3/MMP pathway. Int. J. Radiat. Biol. 2024, 100, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, W.; He, Y. Effect of Taraxacum mongolicum polysaccharide on immune organs in mice. Prog. Vet. Med. 2008, 10–12. [Google Scholar] [CrossRef]
- Natchanok, T.; Subramanian, P.; ChangSheng, L.; Nan, M.; Marimuthu, P.N.; SangGuan, Y. Polysaccharide extracted from Taraxacum platycarpum root exerts immunomodulatory activity via MAPK and NF-κB pathways in RAW264.7 cells. J. Ethnopharmacol. 2021, 281, 114519. [Google Scholar] [CrossRef]
- Gu, W.; Sun, M.; Wang, L.; Chen, Z. Effects of four common Chinese medicines on immune function and intestinal flora in immunosuppressed mice. China Anim. Husb. Vet. Med. 2019, 46, 147–156. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, W.; Chen, G. Study on decolorization and deproteinization of dandelion polysaccharide and its hypoglycemic activity. Food Res. Dev. 2020, 41, 24–28. [Google Scholar]
- Li, J.; Luo, J.; Chai, Y.; Guo, Y.; Tianzhi, Y.; Bao, Y. Hypoglycemic effect of Taraxacum officinale root extract and its synergism with Radix Astragali extract. Food Sci. Nutr. 2021, 9, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Shuang, Y.; Guangyao, L.; Congshu, D.; Haozhen, C. Effect of Taraxacum mongolicum extract on lowering blood sugar in type 2 diabetic rats. Food Mach. 2020, 36, 138–142. [Google Scholar] [CrossRef]
- Carrasco, B.G.; Dacosta, R.F.; Dávalos, A.; Ordovás, J.M.; Casado, A.R. In vitro Hypolipidemic and Antioxidant Effects of Leaf and Root Extracts of Taraxacum officinale. Med. Sci. 2015, 3, 38–54. [Google Scholar] [CrossRef]
- Sui, H.; Zhao, X.; Qi, S. Effect of dandelion on monoamine oxidase and monoamine neurotransmitters in brain tissue of aging model mice. Chin. Pat. Med. 2007, 08, 1223–1225. [Google Scholar]
- Mbarka, H.; Dalel, B.; Lazhar, Z. Hepatoprotective effect of Taraxacum officinale leaf extract on sodium dichromate-induced liver injury in rats. Environ. Toxicol. 2016, 31, 339–349. [Google Scholar] [CrossRef]
- Mahboubi, M.; Mahboubi, M. Hepatoprotection by dandelion (Taraxacum officinale) and mechanisms. Asian Pac. J. Trop. Biomed. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Jifeng, L.; Nenling, Z.; Mengqi, L. A new inositol triester from Taraxacum mongolicum. Nat. Prod. Res. 2014, 28, 420–423. [Google Scholar] [CrossRef]
- Chen, M.; Wu, J.; Shi, S.; Chen, Y.; Wang, H.; Fan, H.; Wang, S. Structure analysis of a heteropolysaccharide from Taraxacum mongolicum Hand.-Mazz. and anticomplementary activity of its sulfated derivatives. Carbohydr. Polym. 2016, 152, 241–252. [Google Scholar] [CrossRef]
- Berté, T.E.; Dalmagro, A.P.; Zimath, P.L.; Gonçalves, A.E.; Meyre-Silva, C.; Bürger, C.; Weber, C.J.; Santos, D.A.d.; Cechinel-Filho, V.; Souza, M.M.d. Taraxerol as a possible therapeutic agent on memory impairments and Alzheimer’s disease: Effects against scopolamine and streptozotocin-induced cognitive dysfunctions. Steroids 2018, 132, 5–11. [Google Scholar] [CrossRef]
- Lemos, J.d.A.; Oliveira, A.E.M.; Araujo, R.S.; Townsend, D.M.; Ferreira, L.A.M.; de Barros, A.L.B. Recent progress in micro and nano-encapsulation of bioactive derivatives of the Brazilian genus Pterodon. Biomed Pharmacother 2021, 143, 112137. [Google Scholar] [CrossRef]
- Li, Z.; Wu, C.; Xu, B. Investigation on the Collection, Primary Processing, Storage and Packaging of Taraxacum mongolicum. J. Basic Chin. Med. 2021, 27, 498–501. [Google Scholar] [CrossRef]
- Li, S.; Sun, M. Content changes of effective components in dandelion at different harvest times. J. Jilin Agric. Univ. 2013, 35, 438–441. [Google Scholar] [CrossRef]
- Hu, R.; Wang, J.; Yu, H. Study on the Effect of Harvest Time on Chemical Constituents in Dandelion Roots Based on Characteristic Atlas. J. Jilin Agric. Univ. 2023. [Google Scholar] [CrossRef]
- Zhang, Z.; Meng, Q.; Wang, Y.; Han, L. Research progress on pharmacological substance basis of Taraxacum mongolicum. Chin. Arch. Tradit. Chin. Med. 2022, 40, 148–152. [Google Scholar] [CrossRef]
- Xue, Z. Effects of Shading on Growth and Accumulation of Main Active Components of Taraxacum mongolicum. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2023. [Google Scholar]
- Xu, Y.; Xie, X.; Guo, Y. Effects of illumination on photosynthetic efficiency and main flavonoids content of Taraxacum mongolicum. Hubei Agric. Sci. 2021. [Google Scholar] [CrossRef]
- Zhao, L. Effects of Light Intensity, Nitrogen Level and Storage Temperature on Quality and Physiological Metabolism of Taraxacum mongolicum. Master’s Thesis, Qingdao Agricultural University, Qingdao, China, 2008. [Google Scholar]
- Su, Y. Study on the Influence of Storage Temperature and Cooking Methods on the Quality of Dandelion. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2016. [Google Scholar]
- Zhao, L.; Yang, Y.; Lin, D. Effect of storage temperature on postharvest physiology and quality of Taraxacum mongolicum. J. N. China Agric. 2008, 3, 143–145. [Google Scholar]
- Qin, Y. Analysis of Some Functional Components and Nutritional Components of Dry and Wet Dandelion. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2014. [Google Scholar]
- Zhu, Y.; Gu, W.; Qiu, R.; Tang, J. Effects of salt stress on growth characteristics, accumulation of effective components and ion absorption and distribution of medicinal dandelion. Subtrop. Plant Sci. 2022, 2, 81–91. [Google Scholar]
- Jia, M. Effects of Saline-Alkali Stress on Growth and Quality of Taraxacum mongolicum. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2023. [Google Scholar]
- European Pharmacopoeia Committee. European Pharmacopoeia (11.0 Edition) European Directorate for the Quality of Medicines and HealthCare; EU: Brussels, Belgium, 2023. [Google Scholar]
- Yan, H.; Zhou, C. Application progress and prospect of fingerprints (characteristics) of traditional Chinese medicine in China Pharmacopoeia (2010 edition~2020 edition). J. South. Med. Univ. 2022, 42, 150–155. [Google Scholar]
- Li, Q.; Du, S.; Zhang, Z.; Lü, C. Technical progress and future development prospect of fingerprint of traditional Chinese Medicine. Chin. Herb. Med. 2013, 44, 3095–3104. [Google Scholar]
- Liu, A.; Guo, L.; Xue, Z. Study on quality control of Taraxacum mongolicum based on fingerprint and multi-component content determination. China J. Chin. Mater. Medica 2018, 43, 3715–3721. [Google Scholar] [CrossRef]
- Hong, B.; Liu, R.; Hou, Y. UPLC Fingerprint of Taraxacum mongolicum and Determination of 10 Components. Chin. J. Pharm. Anal. 2023, 43, 1858–1865. [Google Scholar] [CrossRef]
- Ran, M.; Wu, Z.; Feng, W. Quality evaluation of Taraxacum mongolicum based on HPLC fingerprint combined with chemical pattern recognition and multi-component quantification. Chin. Herb. Med. 2022, 53, 7887–7896. [Google Scholar]
- Liu, F.; Yang, J.; Chen, X.; Yu, T.; Ni, H.; Feng, L.; Li, P.; Li, H. Chemometrics integrated with in silico pharmacology to reveal antioxidative and anti-inflammatory markers of dandelion for its quality control. Chin. Med. 2022, 17, 125. [Google Scholar] [CrossRef]
- Niu, K.; Wang, J. A case of allergic reaction caused by decoction. Chin. J. Inf. Tradit. Chin. Med. 2009, 16, 110. [Google Scholar]
- Hu, Y. Acute allergic reaction caused by compound houttuynia cordata syrup: A case report. Chin. J. Clin. Ration. Drug Use 2014, 7, 2. [Google Scholar] [CrossRef]
- Wang, Y. A case report of allergic reaction caused by taking Paishi decoction. Bull. Tradit. Chin. Med. 1988, 11, 52. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).