Physicochemical Characterization of the Oral Biotherapeutic Drug IMUNOR®
Abstract
1. Introduction
2. Results
2.1. Leukocytes
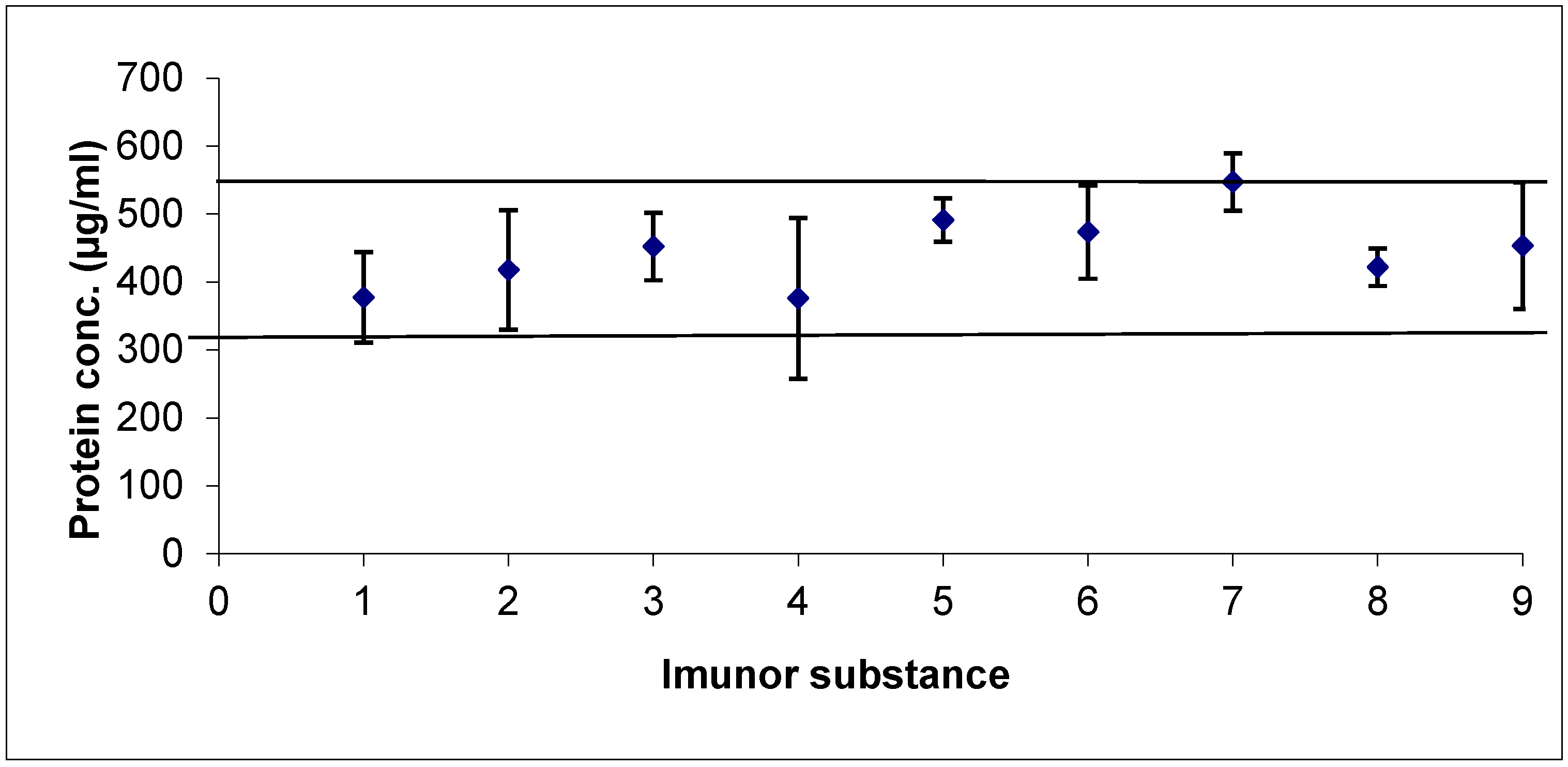
2.2. Protein Quantification
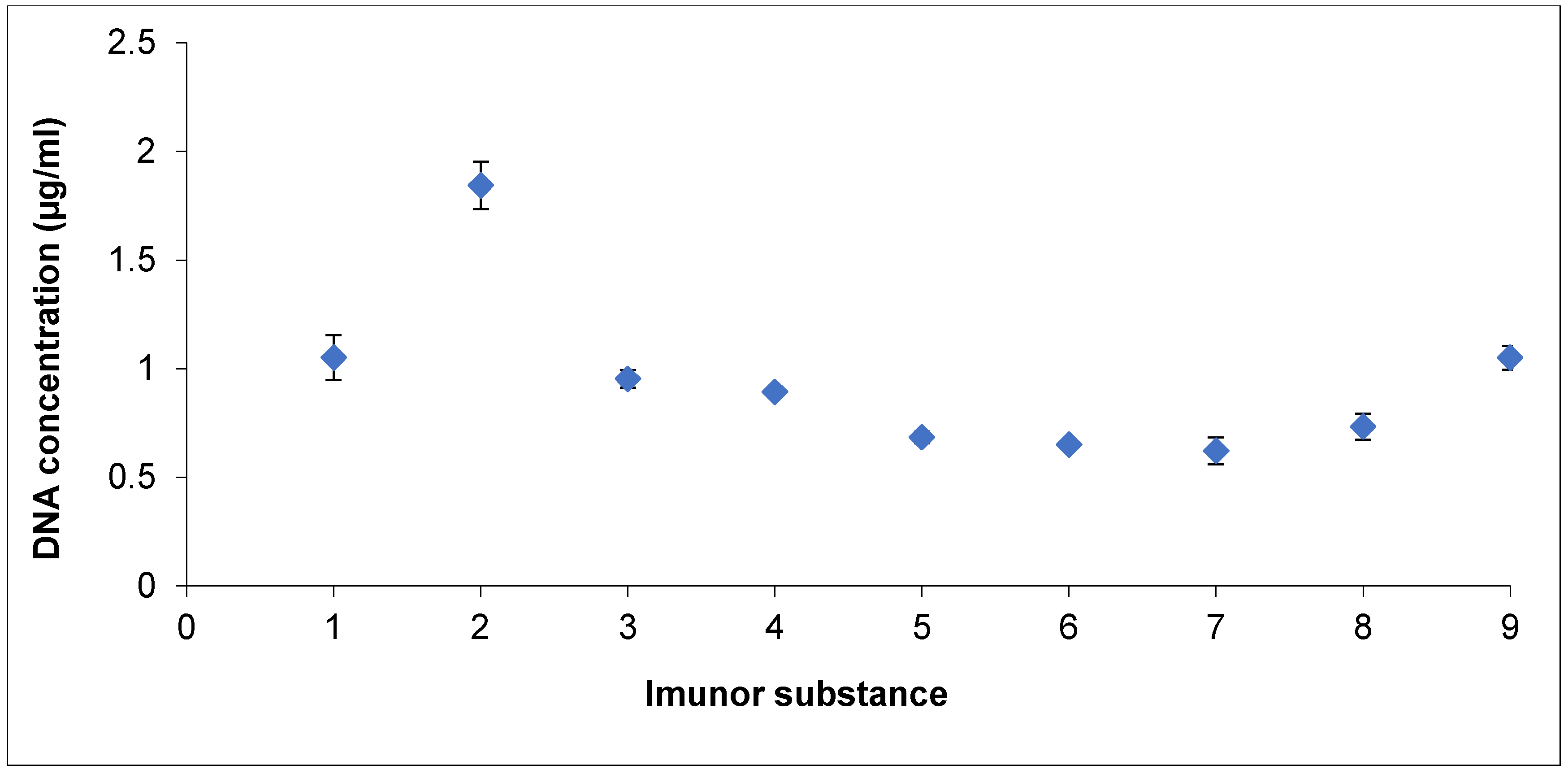
2.3. DNA Quantification
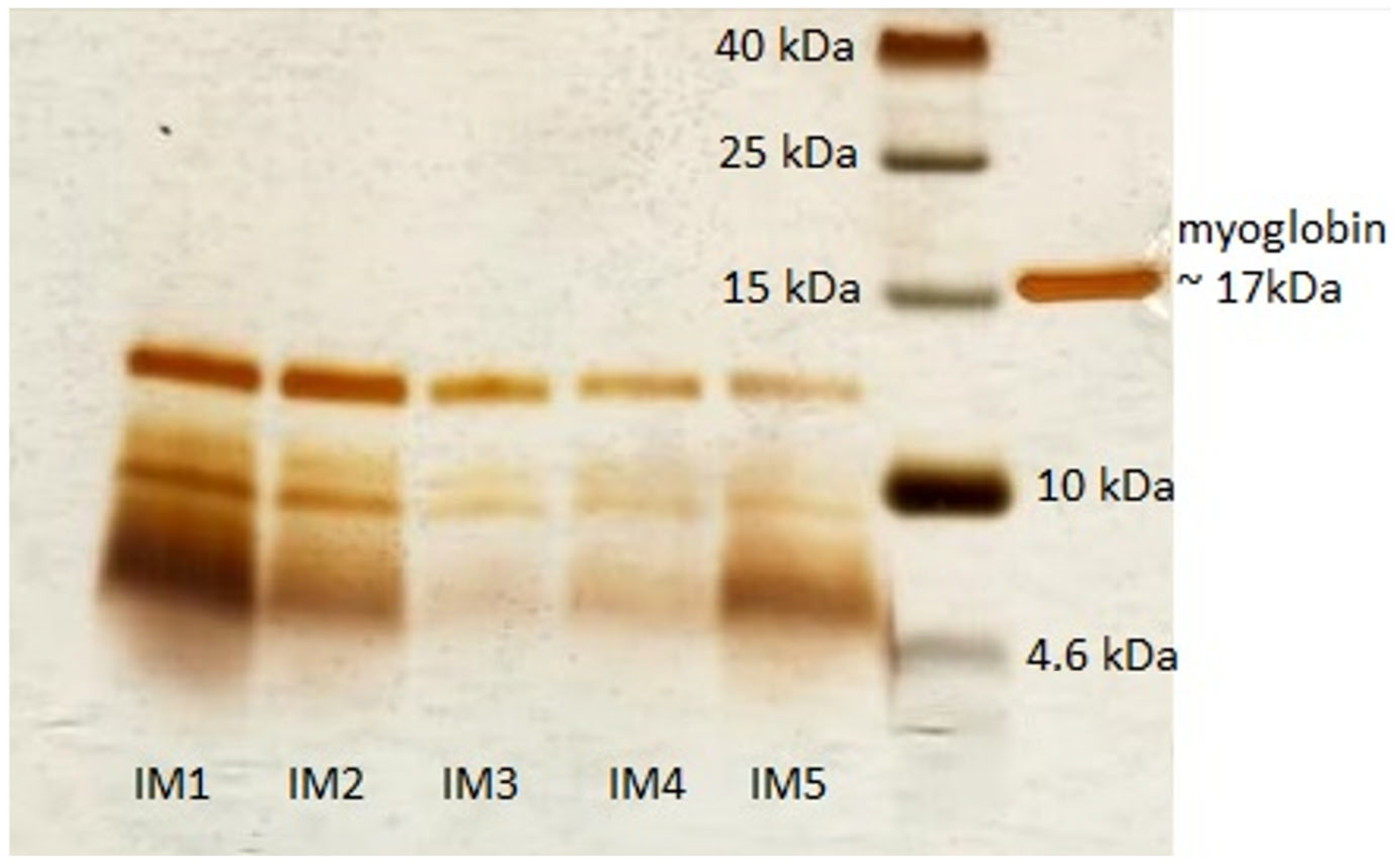
2.4. SDS-PAGE Profile
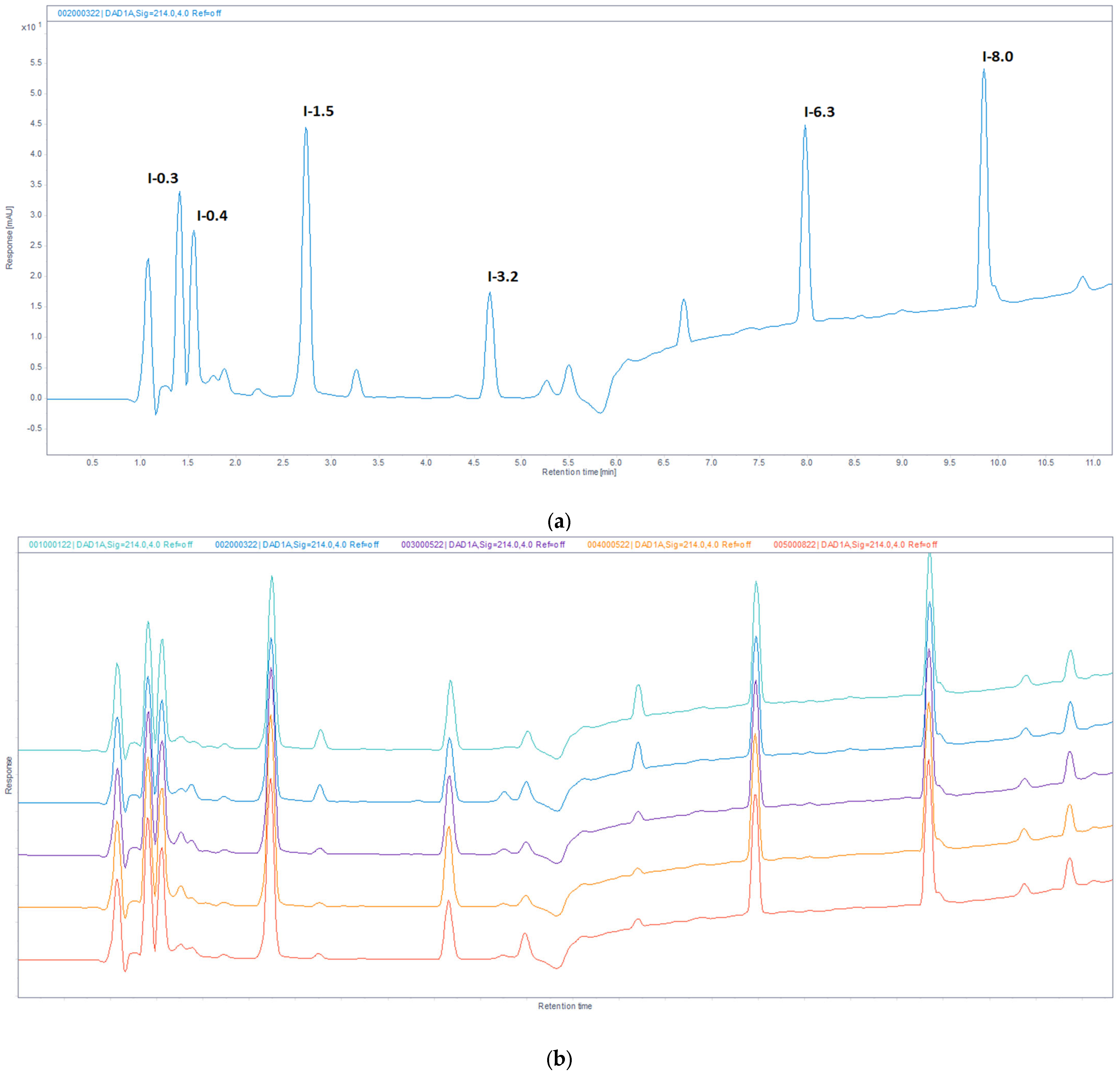
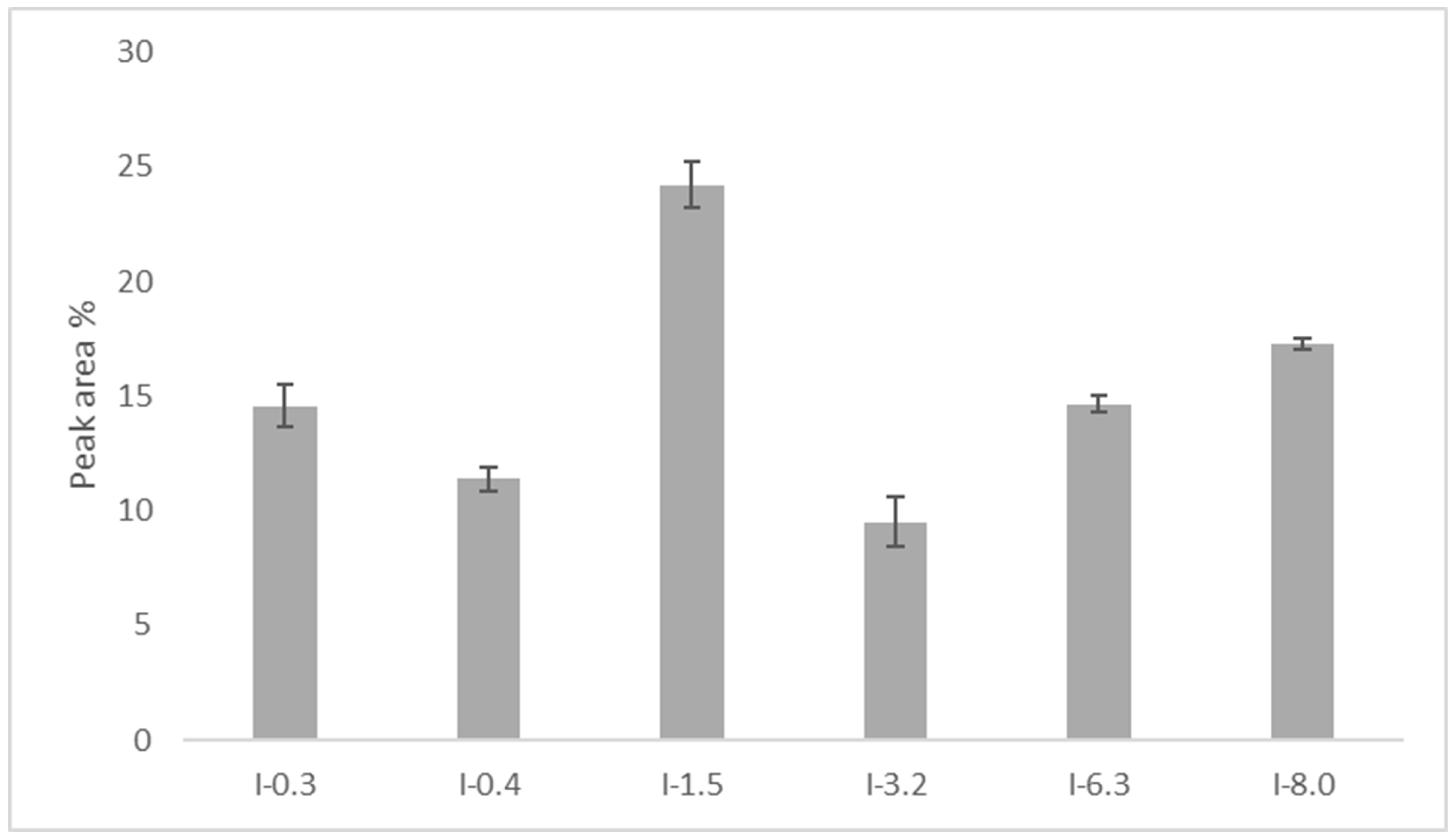
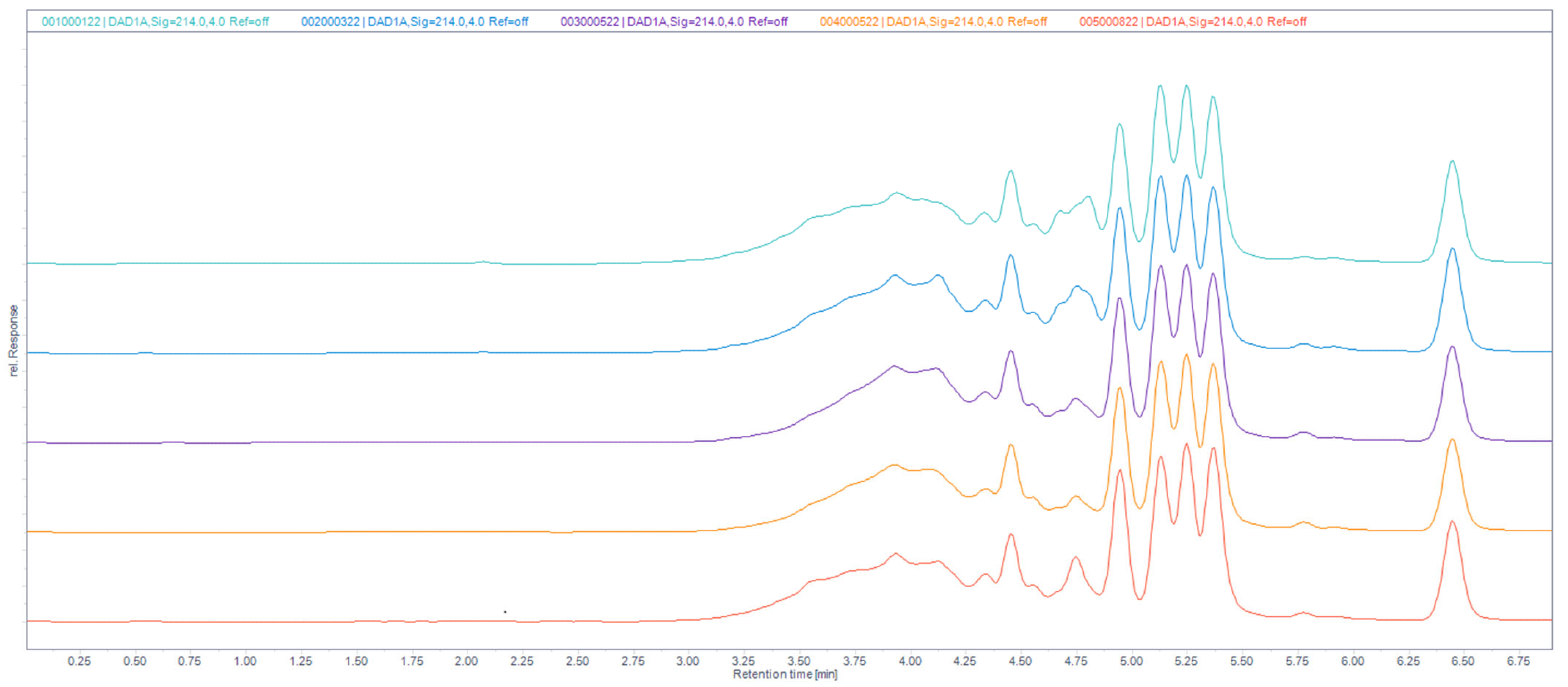
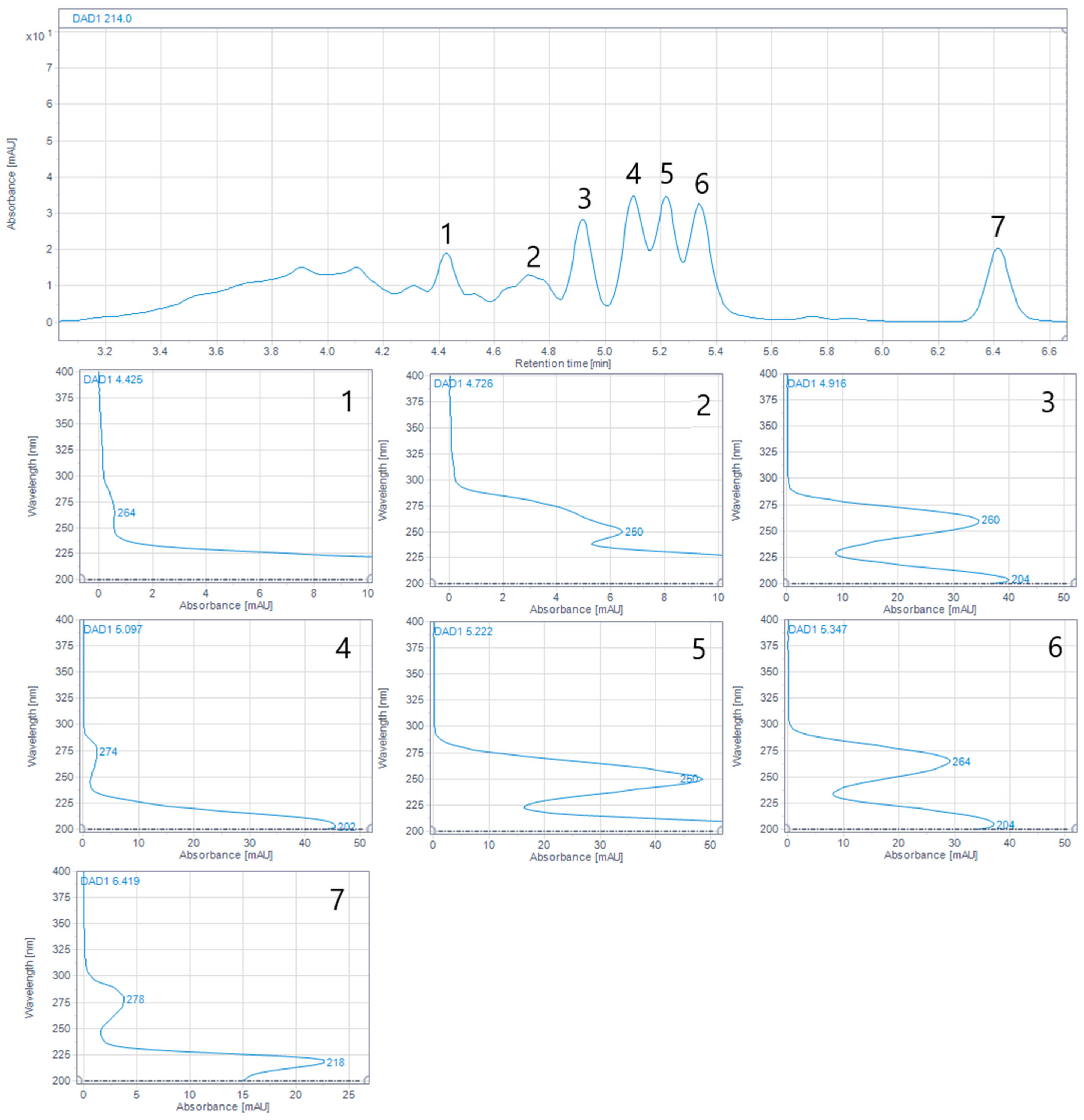
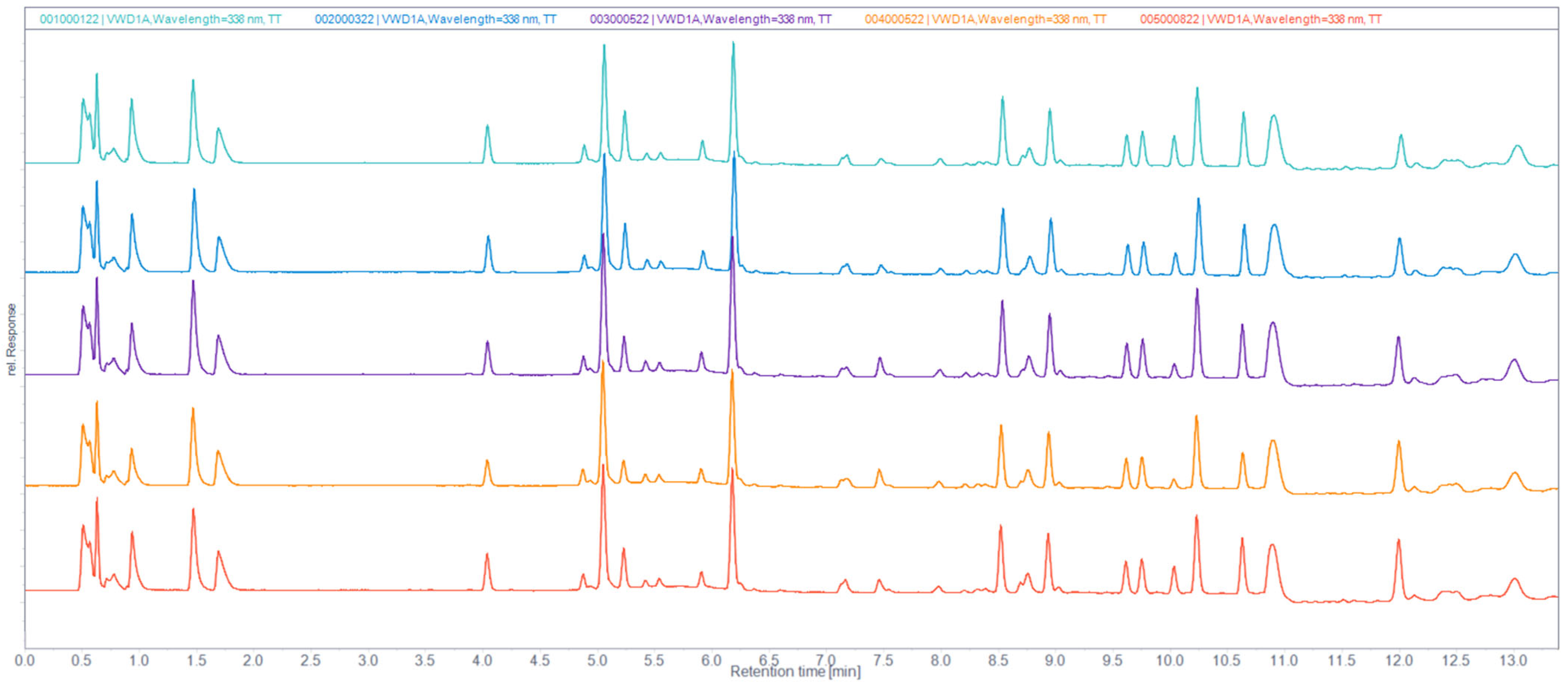
2.5. Reversed-Phase (RP) UHPLC
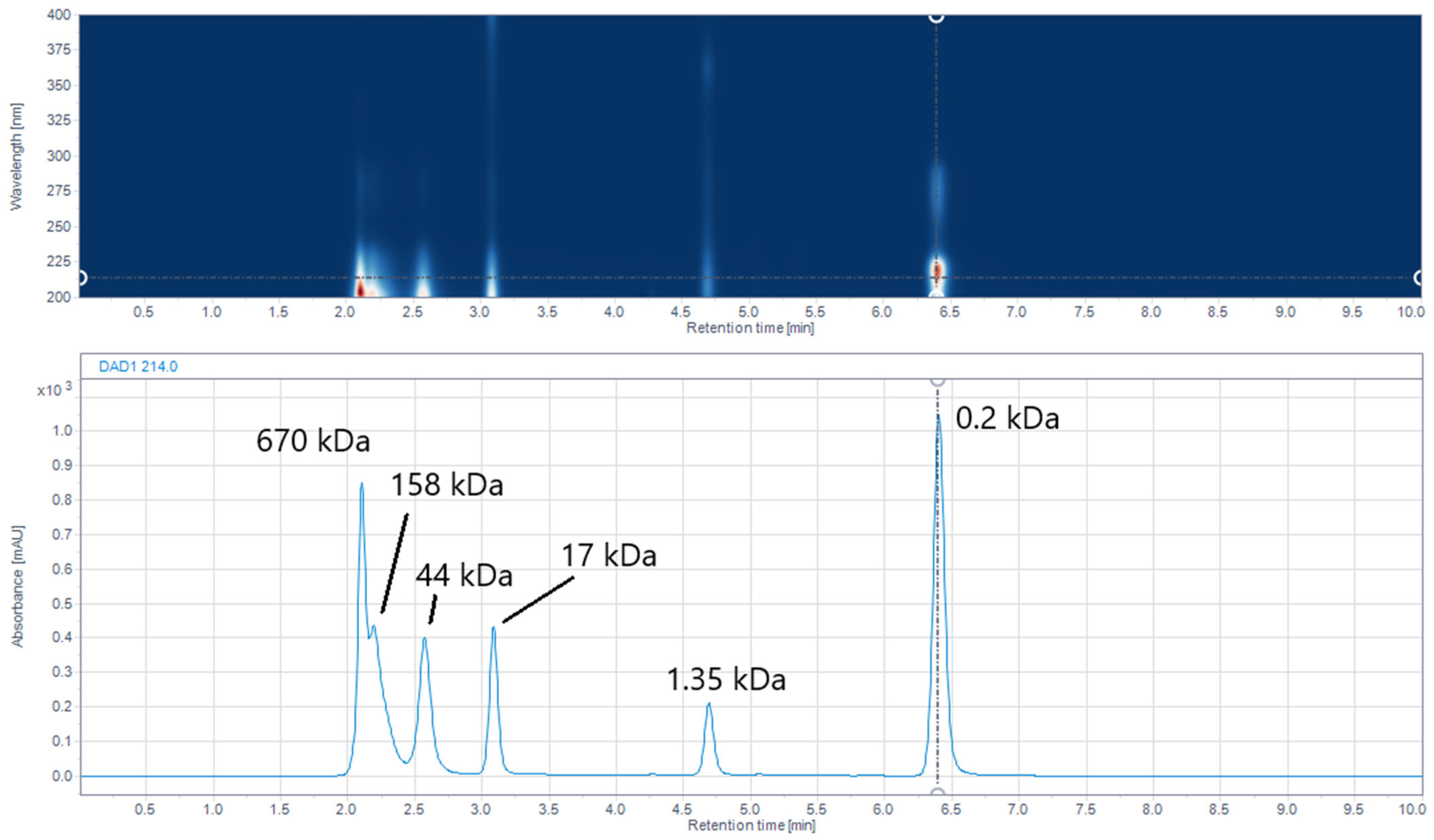
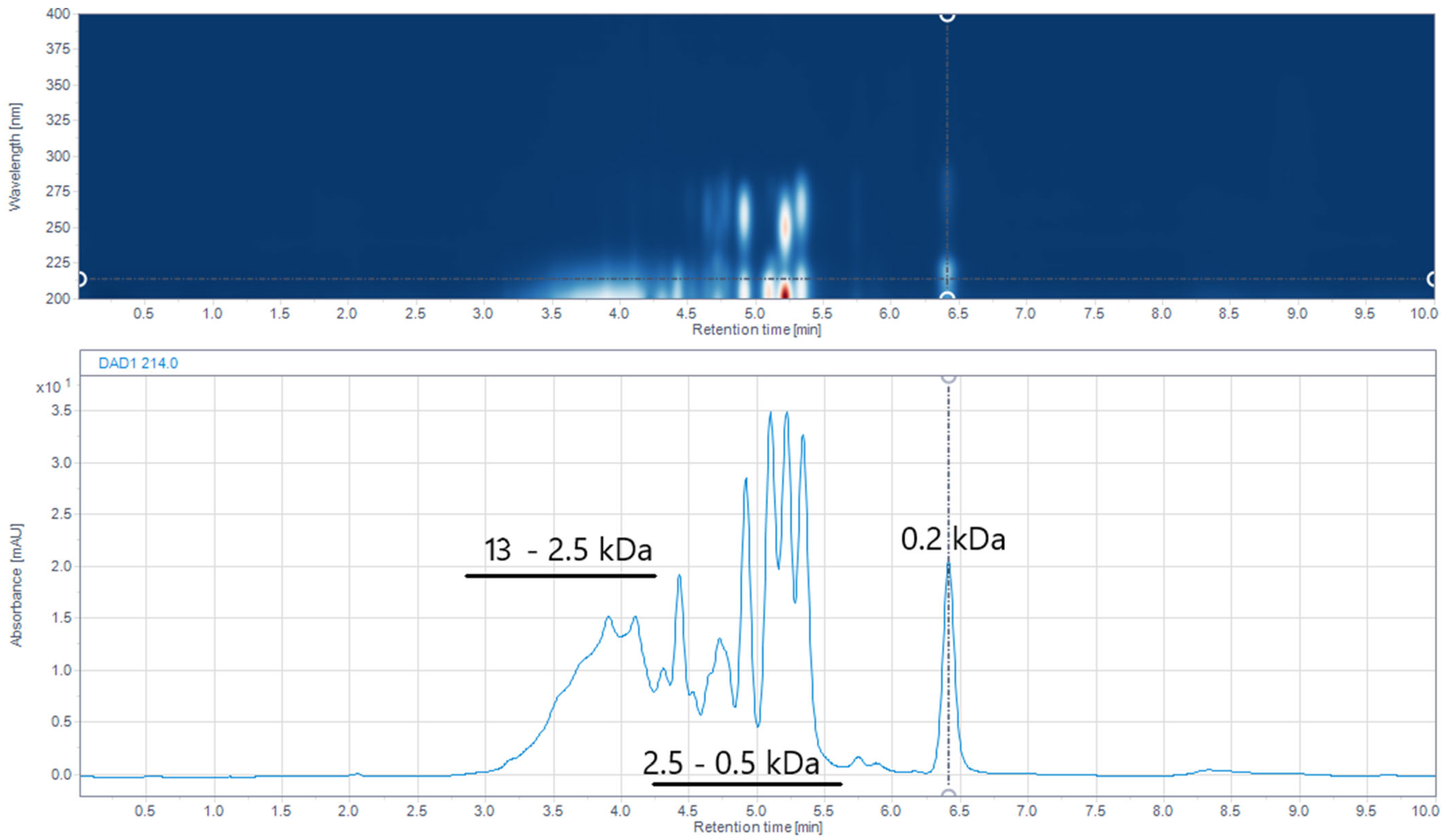
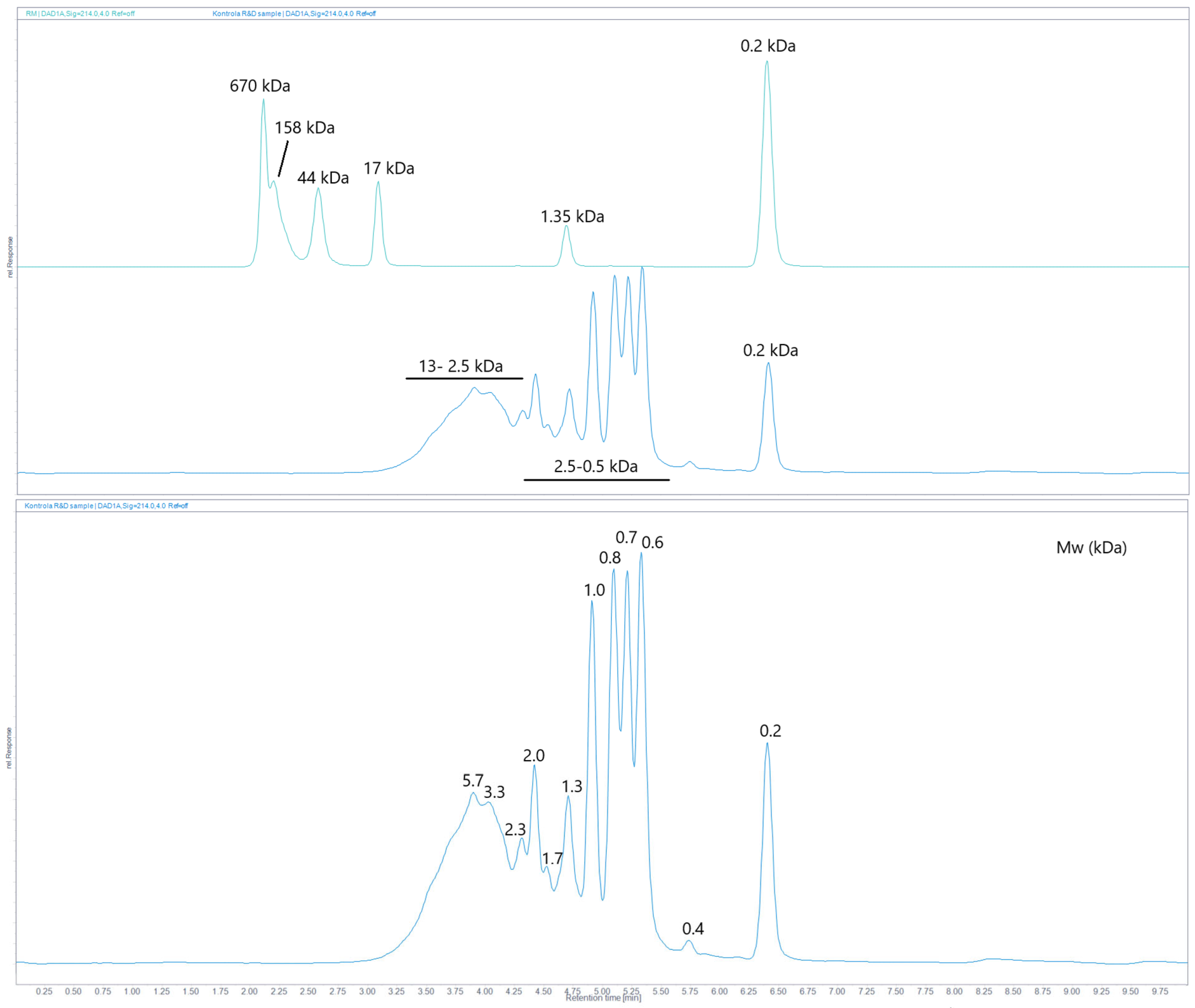
2.6. Size-Exclusion (SE) UHPLC
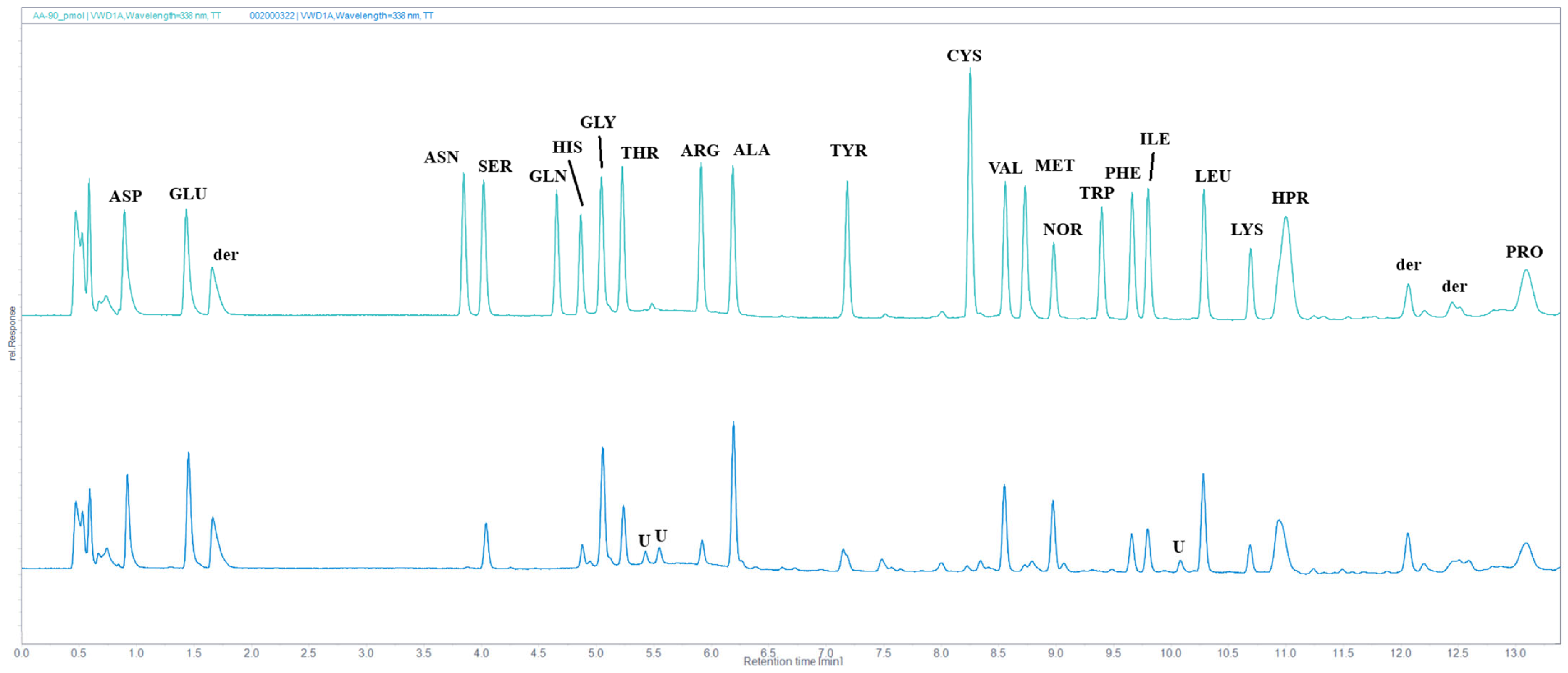
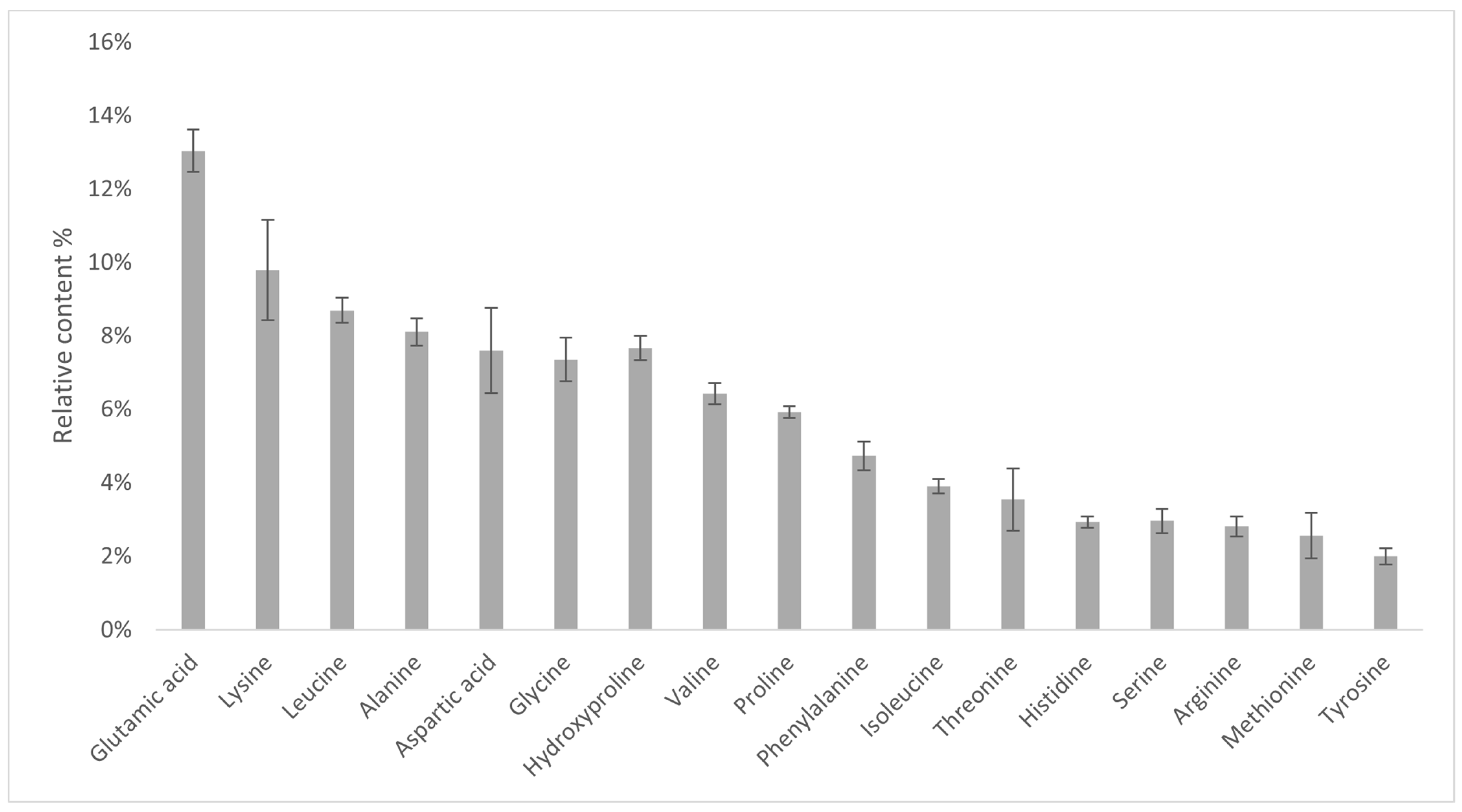
2.7. Amino Acid Analysis
3. Discussion
4. Materials and Methods
4.1. Analytical Samples
4.2. Porcine Leucocyte Quantification
4.3. Protein Quantification
4.4. DNA or RNA Quantification
4.5. Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.6. Reversed-Phase (RP) UHPLC
4.7. Size-Exclusion (SE) HPLC
4.8. Amino Acid Analysis
4.9. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hofer, M.; Vacek, A.; Lojek, A.; Holá, J.; Štreitová, D. Ultrafiltered Pig Leukocyte Extract (IMUNOR®) Decreases Nitric Oxide Formation and Hematopoiesis-Stimulating Cytokine Production in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. Int. Immunopharmacol. 2007, 7, 1369–1374. [Google Scholar] [CrossRef]
- Kirkpatrick, C.H. Transfer Factors: Identification of Conserved Sequences in Transfer Factor Molecules. Mol. Med. 2000, 6, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Berrón-Pérez, R.; Chávez-Sánchez, R.; Estrada-García, I.; Espinosa-Padilla, S.; Serrano-Miranda, E.; Ondarza-Aguilera, R.; Pérez-Tapia, M.; Olvera, B.P.; Cano, L.; Pacheco, P.U.; et al. Indications, Usage, and Dosage of the Transfer Factor. Rev. Alerg. México 2007, 54, 134–139. [Google Scholar]
- Pérez-Tapia, S.M.; Pavón, L.; Vallejo-Castillo, L.; López-Morales, C.A.; Mellado-Sánchez, G.; Nieto-Patlán, A.; Velasco-Velázquez, M.A.; Medina-Rivero, E.; Estrada-Parra, S. Transferon® en el 2020. Rev. Bio Cienc. 2020, 7, 10. [Google Scholar] [CrossRef]
- Medina-Rivero, E.; Merchand-Reyes, G.; Pavón, L.; Vázquez-Leyva, S.; Pérez-Sánchez, G.; Salinas-Jazmín, N.; Estrada-Parra, S.; Velasco-Velázquez, M.; Pérez-Tapia, S.M. Batch-to-Batch Reproducibility of TransferonTM. J. Pharm. Biomed. Anal. 2014, 88, 289–294. [Google Scholar] [CrossRef]
- Salinas-Jazmín, N.; Estrada-Parra, S.; Becerril-García, M.A.; Limón-Flores, A.Y.; Vázquez-Leyva, S.; Medina-Rivero, E.; Pavón, L.; Velasco-Velázquez, M.A.; Pérez-Tapia, S.M. Herpes Murine Model as a Biological Assay to Test Dialyzable Leukocyte Extracts Activity. J. Immunol. Res. 2015, 2015, 146305. [Google Scholar] [CrossRef] [PubMed]
- Medina-Rivero, E.; Vallejo-Castillo, L.; Vázquez-Leyva, S.; Pérez-Sánchez, G.; Favari, L.; Velasco-Velázquez, M.; Estrada-Parra, S.; Pavón, L.; Pérez-Tapia, S.M. Physicochemical Characteristics of TransferonTM Batches. BioMed Res. Int. 2016, 2016, 7935181. [Google Scholar] [CrossRef] [PubMed]
- Zajícová, A.; Javorková, E.; Trošan, P.; Chudíčková, M.; Krulová, M.; Holáň, V. A Low-Molecular-Weight Dialysable Leukocyte Extract Selectively Enhances Development of CD4+RORγt+ T Cells and IL-17 Production. Folia Biol. 2014, 60, 253–260. [Google Scholar] [CrossRef]
- Cardoso, F.M.; Tomkova, M.; Petrovajova, D.; Bubanova, M.; Ragac, O.; Hornakova, T. New and Cost Effective Cell-Based Assay for Dialyzed Leukocyte Extract (DLE)-Induced Jurkat Cells Proliferation under Azathioprine Treatment. J. Pharm. Biomed. Anal. 2017, 138, 100–108. [Google Scholar] [CrossRef]
- Carballo-Uicab, G.; Linares-Trejo, J.E.; Mellado-Sánchez, G.; López-Morales, C.A.; Velasco-Velázquez, M.; Pavón, L.; Estrada-Parra, S.; Pérez-Tapia, S.M.; Medina-Rivero, E. Validation of a Cell Proliferation Assay to Assess the Potency of a Dialyzable Leukocyte Extract Intended for Batch Release. Molecules 2019, 24, 3426. [Google Scholar] [CrossRef]
- Vallejo-Castillo, L.; Favari, L.; Vázquez-Leyva, S.; Mellado-Sánchez, G.; Macías-Palacios, Z.; López-Juárez, L.E.; Valencia-Flores, L.; Medina-Rivero, E.; Chacón-Salinas, R.; Pavón, L.; et al. Sequencing Analysis and Identification of the Primary Peptide Component of the Dialyzable Leukocyte Extract “Transferon Oral”: The Starting Point to Understand Its Mechanism of Action. Front. Pharmacol. 2020, 11, 569039. [Google Scholar] [CrossRef] [PubMed]
- Zuniga-Navarrete, F.; Zavala-Meneses, S.G.; Zelnik, V.; Kopacek, J.; Skultety, L. Initial Proteomic Characterization of IMMODIN, Commercially Available Dialysable Leukocytes Extract. Chem. Pap. 2021, 75, 1959–1968. [Google Scholar] [CrossRef]
- Merchand-Reyes, G.; Pavón, L.; Pérez-Sánchez, G.; Vázquez-Leyva, S.; Salinas-Jazmín, N.; Velasco-Velázquez, M.; Medina-Rivero, E.; Pérez-Tapia, S.M. Swine Dialyzable Spleen Extract as Antiviral Prophylaxis. J. Med. Food 2015, 18, 1239–1246. [Google Scholar] [CrossRef]
- Oliveira, C.R.; Vieira, R.P.; de Oliveira Ferreira, A.; de Souza Schmidt Gonçalves, A.E.; Polonini, H. Immunoregulatory Effects of Imuno TF® (Transfer Factors) on Th1/Th2/Th17/Treg Cytokines. bioRxiv 2020. [Google Scholar] [CrossRef]
- Polonini, H.; Gonçalves, A.E.D.S.S.; Dijkers, E.; Ferreira, A.D.O. Characterization and Safety Profile of Transfer Factors Peptides, a Nutritional Supplement for Immune System Regulation. Biomolecules 2021, 11, 665. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.A.F.; Krímskaya, S.E.S.; Guerra, R.T.; Padilla, C.R. A Review of IMMUNEPOTENT CRP, A Modifier of Biological Response: Efficacy and Current Practice. Biomed. J. Sci. Tech. Res. 2019, 14, 001–004. [Google Scholar] [CrossRef]
- Hromas, J.; Vacek, A.; Hofer, M.; Lukšíková, E.; Svoboda, J.; Schneiderová, H. Hemopoiesis-Stimulating Effects and Enhanced Survival of Irradiated Mice after Peroral or Intraperitoneal Administration of Ultrafiltered Pig Leukocyte Extract (UPLE, IMUNOR®). Immunopharmacol. Immunotoxicol. 2002, 24, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Arnaudov, A. Immunotherapy with Dialyzable Leukocyte Extracts Containing Transfer Factor. In Immunotherapy—Myths, Reality, Ideas, Future; Metodiev, K., Ed.; InTech: London, UK, 2017; ISBN 978-953-51-3105-2. [Google Scholar]
- Bílková, A.; Souček, R.; Vokrouhlický, L. Transfer Faktor VUFB Lyofilizovany Roztok k Peroralní Aplikaci: Souhrnné Výsledky Klinickeho Hodnoceni. OKF VÚFB Praha 1993, 286. [Google Scholar]
- Gutová, V.; Hanzlíková, J. IMUNOR v Lecbe Deti s Recidivujicimi Respiracnimi Infekty. Klin. Imunol. Alergol. 2004, 14. [Google Scholar]
- Bystroň, J.; Petrů, V.; Kopecká, K.; Richterová, I. Effects of the dialyzed pig leukocyte extract—IMUNOR on the clinical condition and basic laboratory parameters of immunity in patients with recurring or chronic infections. Alergie 2007, 9, 61–66. [Google Scholar]
- Pružinec, P.; Košturiak, R.; Kossárová, K.; Hochmuth, L.; Benedik, R. The role of IMUNOR® in the management of patients with recurrent infections—The results of a multicenter observational study evaluating its clinical efficacy and safety in normal clinical practice. Alergie 2018, 20, 245–254. [Google Scholar]
- Bystroň, J.; Petrů, V. The Effect of Oral Transfer Factor Administration on the Quality of Life of Children with Recurrent Respiratory Infections and Their Parents. Alergie 2023, 25, 49–55. [Google Scholar]
- Arnaudov, A.; Kostova, Z. Dialysable Leukocyte Extracts in Immunotherapy. Biotechnol. Biotechnol. Equip. 2015, 29, 1017–1023. [Google Scholar] [CrossRef]
- European Medicines Agency. Q 6 B Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products CPMP/ICH/365/96; European Medicines Agency: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Friendship, R.M.; Lumsden, J.H.; McMillan, I.; Wilson, M.R. Hematology and Biochemistry Reference Values for Ontario Swine. Can. J. Comp. Med. 1984, 48, 390–393. [Google Scholar] [PubMed]
- Sipos, W.; Duvigneau, C.J.; Hartl, R.T.; Schwendenwein, I. Exploratory Reference Intervals on Hematology and Cellular Immune System of Multiparous Large White Sows. Vet. Immunol. Immunopathol. 2011, 141, 307–311. [Google Scholar] [CrossRef][Green Version]
- Patterson, J.; Mura, C. Rapid Colorimetric Assays to Qualitatively Distinguish RNA and DNA in Biomolecular Samples. J. Vis. Exp. JoVE 2013, 72, 50225. [Google Scholar] [CrossRef]
- Hernández-Esquivel, M.A.; Pérez-Torres, A.; Romero-Romero, L.; Reyes-Matute, A.; Loaiza, B.; Mellado-Sánchez, G.; Pavón, L.; Medina-Rivero, E.; Pestell, R.G.; Pérez-Tapia, S.M.; et al. The Dialyzable Leukocyte Extract TransferonTM Inhibits Tumor Growth and Brain Metastasis in a Murine Model of Prostate Cancer. Biomed. Pharmacother. 2018, 101, 938–944. [Google Scholar] [CrossRef]
- European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, France, 2022.
- Summerfield, A.; Guzylack-Piriou, L.; Schaub, A.; Carrasco, C.P.; Tâche, V.; Charley, B.; McCullough, K.C. Porcine Peripheral Blood Dendritic Cells and Natural Interferon-Producing Cells. Immunology 2003, 110, 440–449. [Google Scholar] [CrossRef]
- Lu, Y. Analysis of Amino Acids Derived Online Using an Agilent AdvanceBio AAA Column. In Pharmaceutical and Food Testing; Application Note; Agilent Technologies, Inc.: Santa Clara, CA, USA, 2017. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mucksová, J.; Borošová, G.; Blazsek, M.; Kalina, J.; Minaříková, L.; Svobodová, Z. Physicochemical Characterization of the Oral Biotherapeutic Drug IMUNOR®. Pharmaceuticals 2024, 17, 1114. https://doi.org/10.3390/ph17091114
Mucksová J, Borošová G, Blazsek M, Kalina J, Minaříková L, Svobodová Z. Physicochemical Characterization of the Oral Biotherapeutic Drug IMUNOR®. Pharmaceuticals. 2024; 17(9):1114. https://doi.org/10.3390/ph17091114
Chicago/Turabian StyleMucksová, Jitka, Gabriela Borošová, Miloš Blazsek, Jiří Kalina, Lucie Minaříková, and Zdeňka Svobodová. 2024. "Physicochemical Characterization of the Oral Biotherapeutic Drug IMUNOR®" Pharmaceuticals 17, no. 9: 1114. https://doi.org/10.3390/ph17091114
APA StyleMucksová, J., Borošová, G., Blazsek, M., Kalina, J., Minaříková, L., & Svobodová, Z. (2024). Physicochemical Characterization of the Oral Biotherapeutic Drug IMUNOR®. Pharmaceuticals, 17(9), 1114. https://doi.org/10.3390/ph17091114




