High-Altitude Medicinal Plants as Promising Source of Phytochemical Antioxidants to Combat Lifestyle-Associated Oxidative Stress-Induced Disorders
Abstract
1. Introduction
1.1. Oxidative Stress: Source, Mechanism and Lifestyle-Related Diseases
1.1.1. Source of Oxidative Stress
1.1.2. Mechanism of ROS Production
1.1.3. Lifestyle-Associated Oxidative Stress-Induced Disorders
Cardiovascular Diseases
- Atherosclerosis
- 2.
- Hypertension
- 3.
- Myocardial Infarction
Neurodegenerative Diseases
- Alzheimer’s Disease (AD)
- 2.
- Parkinson’s Disease (PD)
Cancer
- DNA Damage and Mutation
- 2.
- Tumour Angiogenesis
Metabolic Disorders
- Insulin Resistance
- 2.
- Obesity
1.2. Antioxidant Defence Systems
2. Phytochemicals as Antioxidants
2.1. Carotenoids
2.2. Ascorbic Acid (AsA)
2.3. Tocopherols and Tocotrienols
2.4. Polyphenols
2.5. Polysterols
| Phytochemical Class | Sub-Class | Representative Compounds | Chemical Formulae | PubChem ID | High Altitude Plant Source | Preventive Activity Against | Reference |
|---|---|---|---|---|---|---|---|
| Carotenoids | Carotenes | Alpha-carotene | C40H56 | 6419725 | Gentiana algida Pall., Rhododendron ferrugineum L., Ranunculus glacialis L., Saxifraga oppositifolia L., Primula hirsuta All. | Cardiovascular diseases, type 2 diabetes, cancer, skin and eye diseases, ageing, inflammation | [96,97] |
| Beta-carotene | C40H56 | 5280489 | |||||
| Lycopene | C40H56 | 446925 | |||||
| Phytoene | C40H64 | 5280784 | |||||
| Phytofluene | C40H62 | 6436722 | |||||
| Xanthophylls | Lutein | C40H56O2 | 5281243 | ||||
| Canthaxanthin | C40H52O2 | 5281227 | |||||
| Antheraxanthin | C40H56O3 | 5281223 | |||||
| Zeaxanthin | C40H56O2 | 5280899 | |||||
| β-cryptoxanthin | C40H56O | 5281235 | |||||
| Astaxanthin | C40H52O4 | 5281224 | |||||
| Fucoxanthin | C42H58O6 | 5281239 | |||||
| Rubixanthin | C40H56O | 5281252 | |||||
| Violaxanthin | C40H56O4 | 448438 | |||||
| Vitamins | Ascorbic Acid | C6H8O6 | 54670067 | Vaccinium macrocarpon Aiton. (Mountain cranberry), Sorbus aucuparia Poir., Sorbus scopulina Greene, Juniperus recurva Buch. -Ham. ex D. Don. | Age-related muscular degeneration, cataract, cardiovascular diseases, immunosuppression | [98,99] | |
| Tocopherols | Alpha-tocopherol | C29H50O2 | 14985 | Cardiovascular diseases, cancer, obesity, diabetes | |||
| Beta-tocopherol | C28H48O2 | 6857447 | |||||
| Gama-tocopherol | C28H48O2 | 92729 | |||||
| Delta-tocopherol | C27H46O2 | 92094 | |||||
| Tocotrienols | Alpha-tocotrienol | C29H44O2 | 5282347 | ||||
| Polyphenols | Flavonoids | Quercetin | C15H10O7 | 5280343 | Rhodiola rosea L., Vaccinium vitis-idaea L., Dipsacus fullonum L., Dipsacus sylvestris Huds., Juniperus recurva Buch. -Ham. ex D. Don. | Obesity, neurodegenerative diseases, type 2 diabetes, and cardiovascular diseases | [100,101] |
| Kaempferol | C15H10O6 | 5280863 | |||||
| Fisetin | C15H10O6 | 5281614 | |||||
| Isorhamnetin | C16H12O7 | 5281654 | |||||
| Myricetin | C15H10O8 | 5281672 | |||||
| Luteolin | C15H10O6 | 5280445 | |||||
| Apigenin | C15H10O5 | 5280443 | |||||
| Sinensetin | C20H20O7 | 145659 | |||||
| Isosinensetin | C20H20O7 | 632135 | |||||
| Nobiletin | C21H22O8 | 72344 | |||||
| Tangeretin | C20H20O7 | 68077 | |||||
| Galangin | C15H10O5 | 5281616 | |||||
| Chrysin | C15H10O4 | 5281607 | |||||
| Baicalin | C21H18O11 | 64982 | |||||
| Catechin | C15H14O6 | 9064 | |||||
| Epicatechin | C15H14O6 | 72276 | |||||
| Epicatechin gallate | C22H18O10 | 107905 | |||||
| Gallocatechin | C15H14O7 | 65084 | |||||
| Epigallocatechin | C15H14O7 | 72277 | |||||
| Epigallocatechin gallate | C22H18O11 | 65064 | |||||
| Daidzein | C15H10O4 | 5281708 | |||||
| Genistein | C15H10O5 | 5280961 | |||||
| Daidzin | C21H20O9 | 107971 | |||||
| Naringenin | C15H12O5 | 439246 | |||||
| Naringin | C27H32O14 | 442428 | |||||
| Hesperidin | C28H34O15 | 10621 | |||||
| Hesperetin | C16H14O6 | 72281 | |||||
| Eriodicytol | C15H12O6 | 11095 | |||||
| Pelargonidin | C15H11O5⁺ | 440832 | |||||
| Cyanidin | C15H11O6⁺ | 128861 | |||||
| Delphinidin | C15H11ClO7 | 68245 | |||||
| Peonidin | C16H13O6⁺ | 441773 | |||||
| Petunidin | C16H13O7⁺ | 441774 | |||||
| Malvidin | C17H15O7⁺ | 159287 | |||||
| Stilbenes | Resveratrol | C14H12O3 | 445154 | ||||
| Pinosylvin | C14H12O2 | 5280457 | |||||
| Piceatannol | C14H12O4 | 667639 | |||||
| Pterostilbene | C16H16O3 | 5281727 | |||||
| Rhapontigenin | C15H14O4 | 5320954 | |||||
| Isorhapontigenin | C15H14O4 | 5318650 | |||||
| Phenolic acids | Salicylic acid | C7H6O3 | 338 | ||||
| Hydroxybenzoic acid | C7H6O3 | 135 | |||||
| Protocatechuic acid | C7H6O4 | 72 | |||||
| Gallic acid | C7H6O5 | 370 | |||||
| Syringic acid | C9H10O5 | 10742 | |||||
| Vanillic acid | C8H8O4 | 8468 | |||||
| Gentisic acid | C7H6O4 | 3469 | |||||
| Coumaric acid | C9H6O2 | 323 | |||||
| Phytosterols | Campesterol | C28H48O | 173183 | Rhodiola spp., Dipsacus spp., Juniperus spp. | Elevated cholesterol level, inflammation, oxidative stress, immunosuppression. | [102,103] | |
| Sitosterol | C29H50O | 222284 | |||||
| Stigmasterol | C29H48O | 5280794 | |||||
| Campestanol | C28H50O | 119394 | |||||
| Stigmastanol | C29H52O | 241572 | |||||
3. Role of Phytochemical Antioxidants in Mitigating Major Lifestyle-Associated Oxidative Stress-Induced Health Disorders
3.1. Cardiovascular Diseases
| Phytochemical | Plant | Chemical Structure | Treatment | Mechanism of Action | Reference |
|---|---|---|---|---|---|
| Allicin | Allium humile Kunth |  | Hypertension | Inhibits the formation of LPO and MDA | [108,115] |
| Berberine | Berberis aristata DC. | 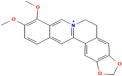 | Hypertension | Reduces O2 and H2O2 levels | [116] |
| Delphinidin-3-glucoside | Vaccinium myrtillus L. | 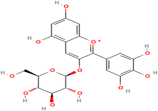 | Coronary heart disease, ischemia-reperfusion injury | Inhibits caspase-3, bax, and ap-JNK expression | [117,118] |
| Gastrodin | Gastrodia elata Blume. | 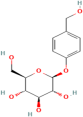 | Heart failure | Regulates AMPK, Akt, mTOR, and Bcl-2 | [119] |
| Gypenoside | Gynostemma pentaphyllum Thunb. | 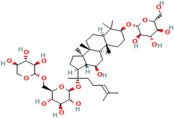 | Acute myocardial infarction | Regulates the PI3K/Akt/mTOR signalling pathway | [120,121] |
| Matrine | Sophora flavescens Aiton. | 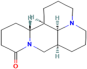 | Arrhythmia | Increases production of SOD | [122,123,124] |
| Orientin | Millettia nitida Benth. | 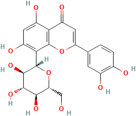 | Coronary heart disease, atherosclerosis | Reduces ROS | [125,126,127] |
| Paeonol | Paeonia suffruticosa Andrews |  | Arrhythmia, coronary heart disease | Inhibits free radical reaction | [122,128] |
| Polysaccharides | Astragalus propinquus Schischk. | Coronary heart disease, acute myocardial infarction | Inhibits the expression of NOX | [129] | |
| Quercetin | Dendrobium nobile Lindl. | 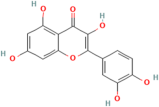 | Acute myocardial infarction, ischemia Reperfusion | Reduce ROS | [130] |
| Tanshinone II-A | Salvia miltiorrhiza Bunge. | 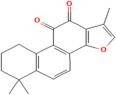 | Coronary heart disease, acute myocardial infarction | Regulates Nrf2/ARE/HO-1 and TGF-beta1/signal transduction | [131,132] |
| Tetramethylpyrazine | Ligusticum chuanxiong | 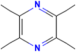 | Heart failure, coronary heart disease | Increases the activity of SOD, CAT and GSH-Px | [133,134] |
3.2. Neurodegenerative Disorders
| Phytochemicals | Plant | Structure | Mode of Action | Reference |
|---|---|---|---|---|
| 1,8-Cineole | Salvia officinalis L. | 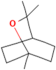 | Selectively suppresses NF- κB and activation of pro-inflammatory gene expression and cytokine production, enhances neurogenesis | [152] |
| Asiatic acid | Centella asiatica (L.) urban | 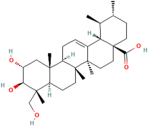 | Inhibits pro-inflammatory cytokines and inflammatory pathway and promotes neurogenesis | [153,154] |
| Asiaticoside | Centella asiatica (L.) urban | 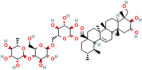 | Inhibits pro-inflammatory cytokines | [155,156] |
| Bacoside A | Bacopa monniera (L.) Pennel | 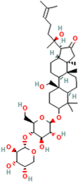 | Reduces oxidative stress-induced neuronal damage, enhances cholinergic neurotransmission, improves cognitive function, inhibits pro-inflammatory cytokines, inhibits amyloid-beta (Aβ) peptide aggregation, and promotes synaptic remodelling | [157,158] |
| Baohuoside I | Centella asiatica (L.) urban | 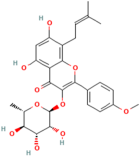 | Promotes the antioxidant activity of essential enzyme such as SOD, CAT and GSH-Px. | [159] |
| Betulic acid | Centella asiatica (L.) urban | 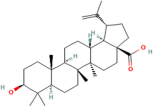 | Inhibiting pro-inflammatory cytokines and signalling pathways and promotes neurotrophic factor BDNF expression contributing to overall brain health | [160] |
| Borneol | Salvia officinalis L. | 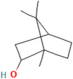 | Exhibits antioxidant properties and suppresses pro-inflammatory cytokine production | [161] |
| Brahmic acid | Centella asiatica (L.) urban |  | Promotes neurogenesis; modulates neurotransmitter levels, including acetylcholine, serotonin, and dopamine; and reduces the production of pro-inflammatory cytokines | [155] |
| Camphor | Salvia officinalis L. | 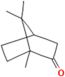 | Exhibits antioxidant properties and suppresses NF-κB activation and pro-inflammatory cytokine production | [162] |
| Caryophyllene | Salvia officinalis L. | 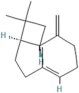 | Demonstrates anti-inflammatory activity, modulates neurotransmitter systems and enhances neurogenesis | [163] |
| Herpestine | Bacopa monniera (L.) Pennel |  | Enhances neuronal synthesis, increases kinase activity, and restores synaptic activity and nerve impulse transmission | [164] |
| Linalool | Salvia officinalis L. | 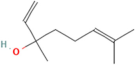 | Scavenges free radicals, suppresses NF-κB activation and pro-inflammatory cytokine production, modulates neurotransmitter systems and enhances neurogenesis | [152] |
| Luteolin | Picrorhiza scrophulariiflora Pennell. |  | Reduces neuroinflammation, promotes expression of brain-derived neurotrophic factor (BNDF) and modulates neurotransmitter systems, such as dopamine and serotonin | [165] |
| Madecassic acid | Centella asiatica (L.) urban | 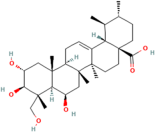 | Inhibits pro-inflammatory cytokines and signalling pathways and promotes neurotrophic factors’ BDNF expression | [160,166] |
| Picroside II | Picrorhiza scrophulariiflora Pennell. | 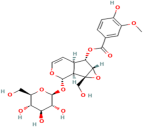 | Inhibits neuronal apoptosis | [167] |
3.3. Metabolic Disorders: Diabetes and Obesity
| Phytochemical | Plant | Chemical Structure | Mode of Action | Reference |
|---|---|---|---|---|
| Anthocyanin | Aristotelia chilensis (Molina) Stuntz | 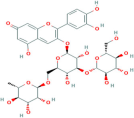 | Inhibits synthesis of the pro-inflammatory cytokines, TNF-α and IL-6, further reducing inflammation associated with diabetes and obesity, and modulates the NF-κB signalling pathway, leading to decreased expression of inflammatory mediators | [184] |
| Ascorbic acid | Rosehips produced by Rosa pendulina L. | 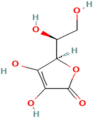 | Enhances insulin sensitivity, facilitating the uptake of glucose into cells; reduces risk of hyperglycaemia; and modulates lipid metabolism by reducing lipid peroxidation and inhibiting fatty acid synthesis, which prevents dyslipidemia | [185,186] |
| Caffeine | Ilex guayusa Loes. | 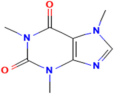 | Stimulates lipolysis and thermogenesis, caffeine may help reduce circulating levels of LDL cholesterol and triglycerides, thereby preventing the development of atherosclerotic plaques | [187] |
| Niazirin | Moringa oleifera Lam. |  | Helps regulate lipid metabolism, reducing the level of triglyceride and LDL cholesterol while increasing the production of HDL cholesterol; modulates lipid metabolism and helps prevent the formation of atherosclerotic plaques; and maintains vascular health in diabetic individuals. | [188,189] |
| Proanthocyanidins | Vitis vinifera L. |  | Promotes endothelial NO production, leading to vasodilation and improved blood flow; inhibits endothelial cell apoptosis and preserve vascular homeostasis; prevents formation of atherosclerotic plaques; and maintains cardiovascular health | [190] |
| Phenolic acids (Protocatechuic acid) and saponins | Androsace umbellata (Lour.) Merr. |  | Promotes the production of serum antioxidant enzymes, upregulates the expression of hepatic antioxidant genes, and inhibits the NF-κB signalling pathway, leading to the decreased expression of inflammatory mediators | [191,192] |
4. High-Altitude Medicinal Plants: Bulk Producers of Antioxidants
4.1. Environmental Factors Influencing Antioxidant Production in High-Altitude Medicinal Plants
4.1.1. Solar Radiation Intensity and Ultraviolet (UV) Exposure
4.1.2. Temperature Fluctuations
4.1.3. Low Oxygen Levels (Hypoxia)
4.1.4. Water Scarcity and Drought Stress
4.1.5. Soil Composition and Nutrient Availability
4.1.6. Altitude-Dependent Factors
4.2. High-Altitude Plants and Their Antioxidant Potential
4.2.1. Saussurea lappa (Decne.) C. B. Clarke
4.2.2. Arnebia benthamii (Wall. ex G. Don) I. M. Johnst.
4.2.3. Pinus nigra Aiton, Hort. Kew. [W. Aiton]
4.2.4. Cedrus deodara (Roxb. ex D. Don) G. Don
4.2.5. Podophyllum hexandrum Royle
4.2.6. Valeriana jatamansi D. Don
4.2.7. Berberis aristata DC.
4.2.8. Pedicularis longiflora Rudolph
4.2.9. Aconitum heterophyllum Wall. ex Royle
4.3. Underutilization of High-Altitude Medicinal Plants
| S. No. | Plant Name | Plant Family | Altitude (m above m.s.l.) | Parts Used | Principle Bioactive Compound | Pharmacological Activity | Reference |
|---|---|---|---|---|---|---|---|
| Allium humile Kunth | Amaryllidaceae | 3200–4500 | Whole plant | Allicin | Antioxidant | [232] |
| Allium semenovii Regel. | 2000–3000 | Whole plant | Alliin | Antioxidant | [233] | |
| Allium stoliczki Regel | 3200–3700 | Bulbs | S-Allyl-L-cysteine sulfoxide | Antioxidant, Cardiovascular health benefits | [234] | |
| Pistacia integerrima L. | Anacardiaceae | 800–2200 | Fruits | Gallic acid, Quercetin | Antioxidant, Anti-inflammatory | [235] |
| Angelica glauca Edgew. | Apiaceae | 2000–3800 | Roots | Angelicin, Umbelliferone | Antioxidant, Hepatoprotective | [236] |
| Bupleurum falcatum L | 2130–3500 | Roots | Saikosaponins | Anti-inflammatory, Hepatoprotective | [237] | |
| Chaerophyllum aromaticum L. | 2800–3200 | Roots | Coumarin, Umbelliferone | Antioxidant, Anti-inflammatory | [238] | |
| Ferula jaeschkeana Vatke | 2600–3000 | Rhizomes | Ferutinin, Ferulenol | Antioxidant | [239] | |
| Heracleum candicans L. | 1800–4000 | Leaves, Stem Roots | Bergapten, Psoralen | Antioxidant, Anti-inflammatory | [240] | |
| Pleurospermum brunonis Benth. ex C.B Clarke | 3000–4000 | Leaves | Psoralen, Isopsoralen | Antioxidant, Anti-inflammatory | [241] | |
| Selinum vaginatum C.B. Clarke | 2700–3800 | Roots Bhutkeshi | Selinidin, Selinidiol | Antioxidant, Anti-inflammatory | [242] | |
| Arisaema flavum (Forsk.) Schott. | Araceae | 2000–3400 | Rhizome | Arisarumol | Antioxidant, Anti-inflammatory | [243] |
| Hedera nepalensis C. Koch | Araliaceae | 1500–3000 | Leaves, Stems | Hederacoside C, Hederagenin | Antioxidant, Anti-inflammatory | [244] |
| Achillea millefolium L. | Asteraceae | 3200–3700 | Leaves, Flowers | Apigenin, Luteolin | Antioxidant, Anti-inflammatory | [245] |
| Artemisia absinthium L. | 2000–3660 | Whole plant | Absinthin, Anabsinthin | Antioxidant | [246] | |
| Artemisia macrocephala Jacq. ex Bess | 3400–5500 | Aerial parts | Artemisinin, Dihydroartemisinin | Antioxidant, Anticancer | [247] | |
| Carduus nutans L. | 2600–3000 | Leaves, Roots | Silymarin | Hepatoprotective, Antioxidant | [248] | |
| Cichorium intybus L. | 2600–3000 | Leaves, Roots | Inulin, Lactucin | Hepatoprotective, Hypoglycemic | [249] | |
| Erigeron acris L. | 2600–3400 | Roots | Quercetin, Kaempferol | Anti-inflammatory, Antioxidant | [250] | |
| Inula cappa DC. | 2600–3500 | Roots | Alantolactone, Isoalantolactone | Antioxidant, Anti-inflammatory | [251] | |
| Inula racemosa Hook. f. | 2000–3100 | Roots | Alantolactone, Isoalantolactone | Antioxidant, Anti-inflammatory | [252] | |
| Jurinea dolomiaea Boiss. | 3000–4000 | Roots | Jurineol, Jurineol acetate | Antioxidant, Anti-inflammatory | [253] | |
| Jurinea macrocephala DC. | 3000–4000 | Roots Leaves | Jurineol, Jurineol acetate | Antioxidant, Anti-inflammatory | [254] | |
| Saussurea albescens Hook. f. et. Thomson | 2000–3600 | Leaves | Costunolide, Eupatilin | Antioxidant, Anti-inflammatory | [255] | |
| Saussurea costus (Falc.) Lipsch. | 2600–4000 | Roots | Costunolide, Dehydrocostus lactone | Antioxidant, Anti-inflammatory | [256] | |
| Saussurea gossypiphora D. Don | 4500–5300 | Flowers | Saussureamine | Antioxidant, Anti-inflammatory | [257] | |
| Scorzonera virgata DC. | 2700–4200 | Leaves | Inulin, Scorzodioside B | Hepatoprotective, Hypoglycemic | [258] | |
| Waldhemia glabra (Decne.) Regel. | 4000–5000 | Aerial parts | Waldhemiol, Waldhemidin | Antioxidant, Anti-inflammatory | [259] | |
| Waldhemia tomentosa (Decne.) Regel. | 3800–4500 | Whole plant | Waldhemiol, Waldhemidin | Antioxidant, Anti-inflammatory | [260] | |
| Impatiens sulcata Wall. | Balsaminaceae | 2000–3900 | Whole plant | Lawsone | Antioxidant, Anti-inflammatory | [261] |
| Berberis lycium Royle | Berberidaceae | 1200–3000 | Roots, stems | Berberine, Palmatine | Antioxidant, Antidiabetic | [262] |
| Betula utilis D. Don | Betulaceae | 2900–4000 | Bark | Betulin, Betulinic acid | Antioxidant, Anti-inflammatory | [263] |
| Biebersteinia odora Steph. ex Fish | Biebersteiniaceae | 4200–5030 | Rootstocks | Coumarin, Umbelliferone | Antioxidant, Anti-inflammatory | [264] |
| Arnebia benthamii (Wall. ex G. Don.) Johnston | Boraginaceae | 3000–3900 | Roots | Alkannin, Shikonin | Antioxidant, Anti-inflammatory | [265] |
| Cynoglossum wallichii G. Don | 2600–3700 | Leaves | Shikonin, Deoxyshikonin | Antioxidant, Anti-inflammatory | [266] | |
| Cynoglossum zeylanicum Thunb. ex Lehm. Brand. | 2600–3350 | Roots | Shikonin, Deoxyshikonin | Antioxidant, Anti-inflammatory | [266] | |
| Myosotis silvatica Ehrh. ex Hoffm. | 3200–4200 | Whole plant | Tannins, Flavonoids | Antioxidant, Anti-inflammatory | [267] | |
| Onosma hispida Wall. ex G. Don | 2000–3400 | Roots, Leaves | Alkannin, Shikonin | Antioxidant, Anti-inflammatory | [268] | |
| Arabidopsis mollissma (C. May.) N. Busch | Brassicaceae | 3800–4300 | Leaves | Sinapine, Sinapic acid | Antioxidant, Anti-inflammatory | [269] |
| Arabis nova Vill. | 3500–3900 | Fruits | Glucosinolates | Antioxidant, Anticancer | [270] | |
| Brassica rapa L. ssp. | 3200–4500 | Whole plant | Glucosinolates | Antioxidant, Anticancer | [271] | |
| Descurainia sophia (L.) Webb. ex Prantl | 2600–3500 | Whole plant | Linalool, Thymoquinone | Antioxidant, Anti-inflammatory | [272] | |
| Lepidium latifolium L. | 2500–4300 | Aerial parts | Glucosinolates | Antioxidant | [273] | |
| Nasturtium officinale W.T. Ait. Hort. | 2600–3500 | Whole plant | Glucosinolates | Antioxidant | [274] | |
| Sisymbrium orientale L. | 2600–3600 | Seeds | Glucosinolates | Antioxidant | [275] | |
| Sarcococca saligna (D. Don) Muell.-Arg. | Buxaceae | 1500–2300 | Leaves, Stem | Sarcococcin | Antioxidant, Anti-inflammatory | [276] |
| Codonopsis clematidea (Schrenk) C.B. Clarke | Campanulaceae | 3000–3800 | Flowers | Codonopsin, Codonopsidic acid | Antioxidant, Immunomodulatory | [277] |
| Codonopsis ovata Benth. | 2700–3200 | Whole plant | Codonopsin, Codonopsidic acid | Antioxidant, Immunomodulatory | [278] | |
| Cyananthus lobatus Wall. ex Benth | 3000–4000 | Leaves, flowers | Cyanolobatolide | Antioxidant, Anti-inflammatory | [279] | |
| Capparis himalayensis Jafri | Capparaceae | 2800–3300 | Leaves | Flavonoids, Glucosinolates | Antioxidant | [280] |
| Lonicera hypoleuca Decne. | Caprifoliaceae | 2900–3100 | Stem | Chlorogenic acid, Luteolin | Antioxidant, Anti-inflammatory | [281] |
| Lonicera quinquelocularis Hardw. | 2600–3500 | Stems, Leaves, Fruit | Chlorogenic acid, Luteolin | Antioxidant, Anti-inflammatory | [282] | |
| Viburnum cotinifolium D. Don | 2300–2600 | Fruits | Iridoids, Flavonoids | Antioxidant, Anti-inflammatory | [283] | |
| Viburnum grandiflorum Buch-Ham. ex D. Don | 2800–4300 | Fruits, seeds | Iridoids, Flavonoids | Antioxidant, Anti-inflammatory | [283] | |
| Cerastium cerastoides (L.) Britt. | Caryophyllaceae | 2000–4000 | Whole plant | Tannins, Flavonoids | Antioxidant, Anti-inflammatory | [86] |
| Myosoton aquaticum (L.) Moench | 2000–2800 | Leaves, Stem | Tannins, Flavonoids | Antioxidant, Anti-inflammatory | [284] | |
| Silene vulgaris (Moench) Garcke | 2740–3450 | Leaves, Twigs | Tannins, Flavonoids | Antioxidant, Anti-inflammatory | [285] | |
| Stellaria media (L.) Vill. | 2600–3000 | Leaves | Tannins, Flavonoids | Antioxidant, Anti-inflammatory | [286] | |
| Chenopodium album L. | Chenopodiaceae | 350–4300 | Leaves, Seeds | Saponins, Flavonoids | Antioxidant, Anti-inflammatory | [287] |
| Chenopodium foliosum Wall. | 2000–4000 | Fruits | Saponins, Flavonoids | Antioxidant, Anti-inflammatory | [288] | |
| Convolvulus arvensis L. | Convolvulaceae | 3000–4000 | Flower buds | Alkaloids, Flavonoids | Antioxidant, Neuroprotective | [289] |
| Corylus jacquemontii Decne. | Corylaceae | 2000–3300 | Seeds | Catechins, Quercetin | Antioxidant, Anti-inflammatory | [290] |
| Rosularia alpestris (Kar. and Kir.) Boriss. | Crassulaceae | 3000–4300 | Whole plant | Phenolic compounds, Flavonoids | Antioxidant, Anti-inflammatory | [102] |
| Juniperus communis L. | Cupressaceae | 3000–4200 | Needles | Monoterpenes, Flavonoids | Antioxidant | [291] |
| Juniperus indica Bertol. | 3500–4500 | Wood | Monoterpenes, Flavonoids | Antioxidant | [292] | |
| Cuscuta reflexa Roxb. | Cuscutaceae | 800–2500 | Whole plant | Flavonoids, Alkaloids | Antioxidant, Hepatoprotective | [293] |
| Datisca cannabina L. | Datiscaceae | 2800–3200 | Leaves, Roots | Tannins, Flavonoids | Antioxidant, Anti-inflammatory | [294] |
| Dioscorea deltoidea Wall. ex Kunth | Dioscoreaceae | 2000–2800 | Tuber | Diosgenin, Dioscin | Antioxidant, Anti-inflammatory | [15] |
| Elaeagnus conferta Roxb. | Elaeagnaceae | 1500–2200 | Fruits | Triterpenoids, Flavonoids | Antioxidant, Anti-inflammatory | [295] |
| Hippophae rhamnoides L. | 2600–3500 | Fruits, Stem | Flavonoids, Vitamin C | Antioxidant, Immunomodulatory | [296] | |
| Hippophae salicifolia D. Don | 2800–3500 | Fruits | Flavonoids, Vitamin C | Antioxidant, Immunomodulatory | [297] | |
| Cassiope fastigiata (Wall.) D. Don | Ericaceae | 3800–4600 | Leaves | Polyphenols, Flavonoids | Antioxidant, Anti-inflammatory | [298] |
| Rhododendron anthopogon D. Don | 3200–4500 | Leaves, Flowers | Rhododendrin, Ursolic acid | Antioxidant, Anti-inflammatory | [299] | |
| Rhododendron arboretum Sm. | 2000–4000 | Leaves, Flowers | Arbutin, Quercetin | Antioxidant, Anti-inflammatory | [300] | |
| Rhododendron campanulatum D. Don | 3000–4300 | Leaves | Arbutin, Quercetin | Antioxidant, Anti-inflammatory | [301] | |
| Gentiana kurroo Royle | Gentianaceae | 1800–4200 | Roots | Gentisin, Swertiamarin | Antioxidant, Hepatoprotective | [302] |
| Gentiana leucomelaena Maxim. ex Kusn. | 2500–5000 | Whole plant | Gentisin, Swertiamarin | Antioxidant, Hepatoprotective | [303] | |
| Gentiana moorcroftiana | 2700–5000 | Leaves | Gentisin, Swertiamarin | Antioxidant, Hepatoprotective | [304] | |
| Gentiana tianshanica Rupr. | 3900–3900 | Whole plant | Gentisin, Swertiamarin | Antioxidant, Hepatoprotective | [305] | |
| Gentiana tubiflora (G. Don) Grirseb. | 4000–5300 | Whole plant | Gentisin, Swertiamarin | Antioxidant, Hepatoprotective | [306] | |
| Gentianopsis detonsa (Rottb.) Ma | 2700–4200 | Whole plant | Gentisin, Swertiamarin | Antioxidant, Hepatoprotective | [303] | |
| Gentianopsis paludosa (Hook.) Ma | 3000–4000 | Whole plant | Gentisin, Swertiamarin | Antioxidant, Hepatoprotective | [307] | |
| Swertia chirayita (Roxb. ex Fleming) Karst. | 1500–3000 | Whole plant | Amarogentin, Swertiamarin | Antioxidant, Hepatoprotective | [308] | |
| Geranium pratense L. | Geraniaceae | 2680–3900 | Whole plant | Geraniin, Tannins | Antioxidant, Anti-inflammatory | [309] |
| Geranium wallichianum D. Don ex Sweet | 2600–3980 | Whole plant | Geraniin, Tannins | Antioxidant, Anti-inflammatory | [310] | |
| Juglans regia L. | Juglandaceae | 1000–3300 | Leaves, seeds | Juglone, Quercetin | Antioxidant, Anti-inflammatory | [311] |
| Lamium album L. | Lamiaceae | 1500–2400 | Roots, Rhizomes | Rosmarinic acid, Flavonoids | Antioxidant, Anti-inflammatory | [312] |
| Origanum vulgare L | 1800–3600 | Leaves, Stems | Carvacrol, Thymol | Antioxidant | [313] | |
| Phlomis bracteosa Royle ex Benth. | 3200–4400 | Whole plant | Ursolic acid | Antioxidant, Anti-inflammatory | [314] | |
| Salvia nubicola Wall. ex Sweet | 2000–2700 | Roots, Leaves | Salvianolic acid, Rosmarinic acid | Antioxidant, Anti-inflammatory | [315] | |
| Astragalus bicuspis Fischer | Leguminosae | 3100–3500 | Whole plant | Astragaloside IV | Antioxidant, Immunomodulatory | [316] |
| Astragalus candolleanus Royle | 3000–4000 | Roots | Astragaloside IV | Antioxidant, Immunomodulatory | [317] | |
| Astragalus grahamianus Royle ex Benth. | 3000–3500 | Whole plant | Astragaloside IV | Antioxidant, Immunomodulatory | [318] | |
| Astragalus himalayanus Klotzsch | 3200–4400 | Flowers Seeds | Astragaloside IV | Antioxidant, Immunomodulatory | [319] | |
| Astragalus strobiliferus Royle | 3000–4000 | Roots | Astragaloside IV | Antioxidant, Immunomodulatory | [320] | |
| Astragalus zanskarensis Benth. ex Bunge | 3200–4600 | Roots | Astragaloside IV | Antioxidant, Immunomodulatory | [321] | |
| Cicer microphyllum Benth. | 3200–4600 | Aerial parts, | Flavonoids, Saponins | Antioxidant, Anti-inflammatory | [322] | |
| Desmodium elegans DC. | 2000–4000 | Leaves | Flavonoids, Alkaloids | Anti-inflammatory | [323] | |
| Lotus corniculatus L. | 2500–3400 | Whole plant | Rutin, Quercetin | Antioxidant, Anti-inflammatory | [324] | |
| Medicago falcata L. | 2700–3500 | Aerial parts | Isoflavones, Saponins | Antioxidant, Anti-inflammatory | [325] | |
| Trifolium pratense L. | 2600–3800 | Whole plant | Formononetin, Biochanin A | Antioxidant | [326] | |
| Trifolium repens L. | 2600–3200 | Whole plant | Trifoside, Genistein | Antioxidant, Anti-inflammatory | [327] | |
| Trigonella emodi Benth. | 2600–3800 | Whole plant | Trigonelline, Diosgenin | Antioxidant, Antidiabetic, Hypolipidemic | [328] | |
| Vicia sativa L. | 2600–3000 | Whole plant | Vicine, Convicine | Antioxidant, Antidiabetic | [329] | |
| Eremurus himalaicus Baker | Liliaceae | 3200–4500 | Fruits | Steroidal saponins | Anti-inflammatory, Immunomodulatory | [330] |
| Viscum album L. | Loranthaceae | 2000–3000 | Bark | Viscotoxins, Lectins | Antioxidant, Immunomodulatory | [331] |
| Malva neglecta Wallr. | Malvaceae | 2600–4500 | Whole plant | Mucilage | Antioxidant, Anti-inflammatory | [332] |
| Malva verticillata L. | 2500–3800 | Seeds | Mucilage | Antioxidant, Anti-inflammatory | [333] | |
| Morus serrata Roxb. | Moraceae | 2000–2300 | Leaves, Fruits | Morin, Resveratrol | Antioxidant, Anti-inflammatory | [334] |
| Morina coulteriana Royle | Morinaceae | 3000–3700 | Flowers | Morin | Antioxidant, Anti-inflammatory | [335] |
| Morina longifolia Wall. ex DC. | 3000–4300 | Roots, Flowers | Morin | Antioxidant, Anti-inflammatory | [336] | |
| Jasminum officinale L. | Oleaceae | 1800–4000 | Leaves Stems | Jasmonic acid, Quercetin | Antioxidant, Anti-inflammatory | [337] |
| Epilobium angustifolium L. | Onagraceae | 3000–4700 | Roots | Oenothein B, Quercetin | Antioxidant, Anti-inflammatory | [338] |
| Oenothera glazioviana Micheli | 2000–2700 | Whole plant | Linoleic acid, Gamma-linolenic acid | Antioxidant, Anti-inflammatory | [339] | |
| Dactylorhiza hatagirea D. Don | Orchidaceae | 3000–3800 | Rhizome | Phenanthrenes | Antioxidant, Anti-inflammatory | [340] |
| Meconopsis aculeata Royle | Papaveraceae | 2400–4200 | Whole plant | Alkaloids, Flavonoids | Antioxidant, Anti-inflammatory | [341] |
| Parnassia nubicola Hook. f. | Parnassiaceae | 1900–3400 | Roots | Parnassiol | Antioxidant, Hepatoprotective, Anti-inflammatory | [342] |
| Cedrus deodara (Royle ex D. Don) | Pinaceae | 1600–3000 | Wood | Deodarone, Cedrol | Antioxidant | [343] |
| Pinus gerardiana Wall. ex Lambert. | 2500–3000 | Fruits/Kernels | Pinene, Pinenes | Antioxidant, Anti-inflammatory | [344] | |
| Pinus nigra Aiton, Hort. Kew. [W. Aiton] | 1300–2200 | Fruits/Kernels | Pinene, limonene borneol | Antioxidant, Anti-inflammatory | [345] | |
| Plantago depressa Willd. | Plantaginaceae | 2000–4500 | Whole plant | Glycosides, Flavonoids | Antioxidant, Anti-inflammatory | [346] |
| Plantago major L. | 2000–2800 | Leaves, Roots, | Aucubin, Ursolic acid | Antioxidant, Anti-inflammatory | [347] | |
| Bistorta vaccinifolia (Wall. ex Meisn.) Greene | Polygonaceae | 3000–4600 | Whole plant | Tannins, Flavonoids | Antioxidant, Anti-inflammatory | [348] |
| Koenigia delicatula (Meisn.) H. Hara | 3000–4500 | Stems | Tannins, Flavonoids | Antioxidant, Anti-inflammatory | [349] | |
| Oxyria digyna Hill | 2600–5300 | Whole plant | Oxycoumarins | Antioxidant, Anti-inflammatory | [350] | |
| Polygonum alpinum Allioni. | 1500–2400 | Stems, Leaves | Rutin, Quercetin | Antioxidant, Anti-inflammatory | [351] | |
| Polygonum aviculare L. | 2000–4200 | Flower buds | Polyphenols, Flavonoids | Antioxidant, Anti-inflammatory | [352] | |
| Polygonum plebejum R.Br. | 1000–4000 | Whole plant | Polyphenols, Flavonoids | Antioxidant, Anti-inflammatory | [353] | |
| Polygonum pubescens Blume | 1500–3700 | Roots | Polyphenols, Flavonoids | Antioxidant, Anti-inflammatory | [354] | |
| Polygonum tortuosum D. Don | 3600–4900 | Young peduncle | Polyphenols, Flavonoids | Antioxidant, Anti-inflammatory | [352] | |
| Rheum australe D. Don | 3300–5200 | Roots | Anthraquinones, Tannins | Antioxidant | [355] | |
| Rheum spiciforme Royle | 4000–5000 | Peduncle | Anthraquinones, Tannins | Antioxidant | [356] | |
| Rumex acetosa L. | 1500–4000 | Leaves | Anthraquinones, Tannins | Antioxidant | [357] | |
| Rumex hestatus D. Don | 1500–3700 | Leaves, Stem | Anthraquinones, Tannins | Antioxidant | [358] | |
| Rumex nepalensis Spreng. | 1200–4000 | Roots | Anthraquinones, Tannins | Antioxidant | [359] | |
| Aconitum heterophyllum Wall. ex Royle | Ranunculaceae | 3200–4500 | Roots | Aconitine, Pseudoaconitine | Antioxidant, Anti-inflammatory | [360] |
| Aconitum rotundifolium Kar. and Kir. | 3500–4800 | Stem | Aconitine, Pseudoaconitine | Antioxidant, Anti-inflammatory | [361] | |
| Aconitum violaceum Jacq. ex Stapf | 3200–4400 | Roots | Aconitine, Pseudoaconitine | Antioxidant, Anti-inflammatory | [362] | |
| Aconitum heterophyllum Wall. ex Royle. | 2000–4000 | Roots | Aconitine, atisine, heteratisine, hetisine | Antioxidant, Anti-inflammatory | [363] | |
| Anemone rivularis Buch. Ham. ex DC. | 2400–3300 | Leaves, Roots | Saponins, Tannins | Antioxidant, Anti-inflammatory | [364] | |
| Aquilegia fragrans Benth. | 2900–3500 | Whole plant | Alkaloids, Flavonoids | Antioxidant, Anti-inflammatory | [365] | |
| Aquilegia moorcroftiana Wall. ex Royle | 3300–3700 | Twigs | Alkaloids, Flavonoids | Antioxidant, Anti-inflammatory | [366] | |
| Caltha palustris L. | 3020–3500 | Leaves, Roots | Protoanemonin | Antioxidant, Anti-inflammatory | [367] | |
| Clematis grata Wall. | 2000–2600 | Leaves | Clematichinenoside | Antioxidant, Anti-inflammatory | [368] | |
| Clematis ladakhiana C. Grey-Wilson | 3200–3900 | Roots Shoots | Clematichinenoside | Antioxidant, Anti-inflammatory | [369] | |
| Clematis orientalis L. | 3400–5200 | Whole plant | Clematichinenoside | Antioxidant, Anti-inflammatory | [370] | |
| Crataegus songarica K. Koch | 1500–2000 | Fruits, Leaves | Flavonoids, Triterpenes | Antioxidant, Cardioprotective | [371] | |
| Fragaria nubicola Lindl. | 2500–3900 | Fruit, Roots | Anthocyanins, Ellagic acid | Antioxidant | [372] | |
| Geum elatum Wall. ex G. Don | 3500–4500 | Roots | Tannins, Flavonoids | Antioxidant, Anti-inflammatory | [373] | |
| Potentilla atrisanguinea Lodd. var. argyrophylla (Wall. ex Lehm.) Griers. and Long | 3000–4500 | Roots | Tannins, Flavonoids | Antioxidant, Anti-inflammatory | [374] | |
| Potentilla eriocarpa Wall. ex Lehm. | 3000–5000 | Whole plant | Tannins, Flavonoids | Antioxidant, Anti-inflammatory | [375] | |
| Potentilla fulgens Wall. | 2000–3200 | Roots | Tannins, Flavonoids | Antioxidant, Anti-inflammatory | [376] | |
| Potentilla nubicola Lindl. ex Lacaita | 2900–4000 | Fruits | Tannins, Flavonoids | Antioxidant, Anti-inflammatory | [377] | |
| Prinsepia utilis Royle | 1800–3000 | Seeds, Roots | Triterpenes, Flavonoids | Antioxidant, Hepatoprotectiv | [378] | |
| Pyracantha crenulata (D. Don) Roemer | 1000–2600 | Fruits | Flavonoids, Triterpenes | Antioxidant, Anti-inflammatory | [379] | |
| Pyrus lanata D. Don. | 2700–3400 | Fruits | Triterpenes, Flavonoids | Antioxidant, Hepatoprotective | [380] | |
| Rosa brunonii Lindl. | 2100–4500 | Flowers | Anthocyanins, Flavonoids | Antioxidant, Anti-inflammatory | [381] | |
| Rosa webbiana Wall. ex Royle | 3000–3800 | Fruits, Stem, Flowers | Anthocyanins, Flavonoids | Antioxidant, Anti-inflammatory | [382] | |
| Rubus ellipticus Sm. | 1800–2600 | Fruits | Anthocyanins, Ellagic acid | Antioxidant | [383] | |
| Rubus niveus Thunb. | 2000–2800 | Fruits | Anthocyanins, Ellagic acid | Antioxidant | [384] | |
| Spiraea canescens D. Don | 2600–4000 | Stem | Tannins, Flavonoids | Antioxidant, Anti-inflammatory | [385] | |
| Rubia cordifolia L. | Rubiaceae | 1800–3000 | Leaves, Stem, Roots | Anthraquinones, Tannins | Antioxidant, Anti-inflammatory | [386] |
| Euphrasia flabellate Pennell | Scrophulariaceae | 3000–4000 | Whole plant | Iridoid glycosides, Flavonoids | Antioxidant, Anti-inflammatory | [387] |
| Euphrasia paucifolia Wettst. | 3000–4300 | Leaves | Iridoid glycosides, Flavonoids | Antioxidant, Anti-inflammatory | [388] | |
| Picrorhiza kurroa Royle ex Benth. | 3000–4000 | Roots | Picroside I, Picroside II | Antioxidant, Hepatoprotective | [389] | |
| Scrophularia calycina Benth. | 3000–4000 | Whole plant | Iridoid glycosides, Flavonoids | Antioxidant, Anti-inflammatory | [390] | |
| Scrophularia decomposita Royle ex Benth. | 3000–4200 | Leaves | Iridoid glycosides, Flavonoids | Antioxidant, Anti-inflammatory | [391] | |
| Urtica dioica Jacq. ex Wedd. | Urticaceae | 2000–3000 | Leaves | Acetylcholine, Histamine | Anti-inflammatory | [392] |
5. Challenges of Using High-Altitude Phytochemicals in Medicine
5.1. Challenges in Extraction and Utilization
5.1.1. Harsh Environmental Conditions
5.1.2. Low Biomass and Slow Growth
5.1.3. Species Rarity and Endemism
5.1.4. Seasonal Variability
5.1.5. Complex Chemistry
5.1.6. Extraction Efficiency
5.1.7. Cultural and Traditional Knowledge
5.2. Regulatory Challenges
6. Future Prospects
6.1. Dietary Phytochemicals as Antioxidants
6.2. Novel Delivery Systems for Sustained Release
7. Methodology
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Valle, N.R.-D.; Huang, P. Redox Regulation of Cell Survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- de Almeida, A.J.P.O.; de Oliveira, J.C.P.L.; da Silva Pontes, L.V.; de Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and Its Implications in Aging Pathways. Oxid. Med. Cell. Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef] [PubMed]
- Fujii, J.; Homma, T.; Osaki, T. Superoxide Radicals in the Execution of Cell Death. Antioxidants 2022, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Forcados, G.E.; Muhammad, A.; Oladipo, O.O.; Makama, S.; Meseko, C.A. Metabolic Implications of Oxidative Stress and Inflammatory Process in SARS-CoV-2 Pathogenesis: Therapeutic Potential of Natural Antioxidants. Front. Cell. Infect. Microbiol. 2021, 11, 654813. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef] [PubMed]
- Mudau, F.N.; Chimonyo, V.G.P.; Modi, A.T.; Mabhaudhi, T. Neglected and Underutilised Crops: A Systematic Review of Their Potential as Food and Herbal Medicinal Crops in South Africa. Front. Pharmacol. 2022, 12, 809866. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH Oxidases: An Overview from Structure to Innate Immunity-Associated Pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, N.; Vaziri, N.D.; Dafoe, D.C.; Ichii, H. The Role of Oxidative Stress in Pancreatic β Cell Dysfunction in Diabetes. Int. J. Mol. Sci. 2021, 22, 1509. [Google Scholar] [CrossRef] [PubMed]
- Muscolo, A.; Mariateresa, O.; Giulio, T.; Mariateresa, R. Oxidative Stress: The Role of Antioxidant Phytochemicals in the Prevention and Treatment of Diseases. Int. J. Mol. Sci. 2024, 25, 3264. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, C.; Qian, H. Pharmaceutical Potential of High-Altitude Plants for Fatigue-Related Disorders: A Review. Plants 2022, 11, 2004. [Google Scholar] [CrossRef]
- Semwal, P.; Painuli, S.; Cruz-Martins, N. Dioscorea Deltoidea Wall. Ex Griseb: A Review of Traditional Uses, Bioactive Compounds and Biological Activities. Food Biosci. 2021, 41, 100969. [Google Scholar] [CrossRef]
- Llauradó Maury, G.; Méndez Rodríguez, D.; Hendrix, S.; Escalona Arranz, J.C.; Fung Boix, Y.; Pacheco, A.O.; García Díaz, J.; Morris-Quevedo, H.J.; Ferrer Dubois, A.; Aleman, E.I.; et al. Antioxidants in Plants: A Valorization Potential Emphasizing the Need for the Conservation of Plant Biodiversity in Cuba. Antioxidants 2020, 9, 1048. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial Dysfunction and Oxidative Stress in Metabolic Disorders—A Step towards Mitochondria Based Therapeutic Strategies. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The Impact of Oxidative Stress in Human Pathology: Focus on Gastrointestinal Disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as Novel Anti-Inflammatory Drugs. Future Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide Dismutases: Dual Roles in Controlling ROS Damage and Regulating ROS Signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Carraro, E.; Schilirò, T.; Biorci, F.; Romanazzi, V.; Degan, R.; Buonocore, D.; Verri, M.; Dossena, M.; Bonetta, S.; Gilli, G. Physical Activity, Lifestyle Factors and Oxidative Stress in Middle Age Healthy Subjects. Int. J. Environ. Res. Public Health 2018, 15, 1152. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative Stress and Neurodegeneration: Where Are We Now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative Stress and Metabolic Disorders: Pathogenesis and Therapeutic Strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Dubois-deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef]
- Griendling, K.K.; Camargo, L.L.; Rios, F.J.; Alves-Lopes, R.; Montezano, A.C.; Touyz, R.M. Oxidative Stress and Hypertension. Circ. Res. 2021, 128, 993–1020. [Google Scholar] [CrossRef]
- Murphy, E.; Liu, J.C. Mitochondrial Calcium and Reactive Oxygen Species in Cardiovascular Disease. Cardiovasc. Res. 2023, 119, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- DeTure, M.A.; Dickson, D.W. The Neuropathological Diagnosis of Alzheimer’s Disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Li, X.; Li, X.; Liu, Q.; Cheng, Y. Oxidative Stress in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Mol. Neurosci. 2018, 11, 236. [Google Scholar] [CrossRef]
- Davalli, P.; Marverti, G.; Lauriola, A.; D’Arca, D. Targeting Oxidatively Induced DNA Damage Response in Cancer: Opportunities for Novel Cancer Therapies. Oxid. Med. Cell. Longev. 2018, 2018, 2389523. [Google Scholar] [CrossRef] [PubMed]
- Klaunig, J.E. Oxidative Stress and Cancer. Curr. Pharm. Des. 2019, 24, 4771–4778. [Google Scholar] [CrossRef] [PubMed]
- Tangvarasittichai, S. Oxidative Stress, Insulin Resistance, Dyslipidemia and Type 2 Diabetes Mellitus. World J. Diabetes 2015, 6, 456. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 01162. [Google Scholar] [CrossRef] [PubMed]
- Dias, T.R.; Martin-Hidalgo, D.; Silva, B.M.; Oliveira, P.F.; Alves, M.G. Endogenous and Exogenous Antioxidants As a Tool to Ameliorate Male Infertility Induced by Reactive Oxygen Species. Antioxid. Redox Signal. 2020, 33, 767–785. [Google Scholar] [CrossRef]
- Moussa, Z.; Judeh, Z.M.A.; Ahmed, S.A. Nonenzymatic Exogenous and Endogenous Antioxidants. In Free Radical Medicine and Biology; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Wadhwani, A. Antioxidant Enzymes and Human Health. In Antioxidant Enzyme; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Irato, P.; Santovito, G. Enzymatic and Non-Enzymatic Molecules with Antioxidant Function. Antioxidants 2021, 10, 579. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The Role of Glutathione Reductase and Related Enzymes on Cellular Redox Homoeostasis Network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Cox, G.M.; Harrison, T.S.; McDade, H.C.; Taborda, C.P.; Heinrich, G.; Casadevall, A.; Perfect, J.R. Superoxide Dismutase Influences the Virulence of Cryptococcus neoformans by Affecting Growth within Macrophages. Infect. Immun. 2003, 71, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Oxidative Stress and Antioxidants: Distress or Eustress? Arch. Biochem. Biophys. 2016, 595, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Bohn, T. Exogenous Antioxidants—Double-Edged Swords in Cellular Redox State: Health Beneficial Effects at Physiologic Doses versus Deleterious Effects at High Doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of Antioxidant Potential of Plants and Its Relevance to Therapeutic Applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The Role and Place of Medicinal Plants in the Strategies for Disease Prevention. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 210–229. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Guleria, S.; Ghosh, D.; Dogra, V.; Kumar, S. Managing Reactive Oxygen Species—Some Learnings from High Altitude Extremophytes. Environ. Exp. Bot. 2021, 189, 104525. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Lee, M.T.; Lin, W.C.; Yu, B.; Lee, T.T. Antioxidant Capacity of Phytochemicals and Their Potential Effects on Oxidative Status in Animals—A Review. Asian-Australas. J. Anim. Sci. 2016, 30, 299–308. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Kiani, A.; Santhiravel, S.; Holman, B.W.B.; Lauridsen, C.; Dunshea, F.R. The Importance of Dietary Antioxidants on Oxidative Stress, Meat and Milk Production, and Their Preservative Aspects in Farm Animals: Antioxidant Action, Animal Health, and Product Quality—Invited Review. Animals 2022, 12, 3279. [Google Scholar] [CrossRef]
- Kumar, A.; P, N.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; K, S.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef] [PubMed]
- González-Peña, M.A.; Ortega-Regules, A.E.; Anaya de Parrodi, C.; Lozada-Ramírez, J.D. Chemistry, Occurrence, Properties, Applications, and Encapsulation of Carotenoids—A Review. Plants 2023, 12, 313. [Google Scholar] [CrossRef] [PubMed]
- Carazo, A.; Macáková, K.; Matoušová, K.; Krčmová, L.K.; Protti, M.; Mladěnka, P. Vitamin A Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity. Nutrients 2021, 13, 1703. [Google Scholar] [CrossRef] [PubMed]
- Yabuzaki, J. Carotenoids Database: Structures, chemical fingerprints and distribution among organisms. Database J. Biol. Databases Curation 2017, 2017, bax004. [Google Scholar] [CrossRef] [PubMed]
- Merhan, O. The Biochemistry and Antioxidant Properties of Carotenoids. In Carotenoids; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Sindhu, E.R.; Kavya, A.K.; Binitha, P.P. Role of Carotenoids in Preventing Oxidative Stress—Induced Cancer. In Handbook of Oxidative Stress in Cancer: Therapeutic Aspects; Springer: Singapore, 2022; pp. 351–363. [Google Scholar] [CrossRef]
- Khoo, H.-E.; Prasad, K.N.; Kong, K.-W.; Jiang, Y.; Ismail, A. Carotenoids and Their Isomers: Color Pigments in Fruits and Vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The Role of Carotenoids in the Prevention of Human Pathologies. Biomed. Pharmacother. 2004, 58, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Ramel, F.; Birtic, S.; Cuiné, S.; Triantaphylidès, C.; Ravanat, J.-L.; Havaux, M. Chemical Quenching of Singlet Oxygen by Carotenoids in Plants. Plant Physiol. 2012, 158, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-A.; Hayden, M.M.; Bannerman, S.; Jansen, J.; Crowe-White, K.M. Anti-Apoptotic Effects of Carotenoids in Neurodegeneration. Molecules 2020, 25, 3453. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E. Carotenoids: How Effective Are They to Prevent Age-Related Diseases? Molecules 2019, 24, 1801. [Google Scholar] [CrossRef]
- Maria, A.G.; Graziano, R.; Nicolantonio, D. Carotenoids: Potential Allies of Cardiovascular Health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar] [CrossRef]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration—Neurodegenerative Disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic Acid-A Potential Oxidant Scavenger and Its Role in Plant Development and Abiotic Stress Tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef] [PubMed]
- Gallie, D.R. L-Ascorbic Acid: A Multifunctional Molecule Supporting Plant Growth and Development. Scientifica 2013, 2013, 795964. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in Plants: Biosynthesis and Physiological Role in Environmental Stress Tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Carr, A.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef] [PubMed]
- Morelli, M.B.; Gambardella, J.; Castellanos, V.; Trimarco, V.; Santulli, G. Vitamin C and Cardiovascular Disease: An Update. Antioxidants 2020, 9, 1227. [Google Scholar] [CrossRef] [PubMed]
- Chambial, S.; Dwivedi, S.; Shukla, K.K.; John, P.J.; Sharma, P. Vitamin C in Disease Prevention and Cure: An Overview. Indian J. Clin. Biochem. 2013, 28, 314–328. [Google Scholar] [CrossRef]
- Jiang, Q. Natural Forms of Vitamin E: Metabolism, Antioxidant, and Anti-Inflammatory Activities and Their Role in Disease Prevention and Therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef]
- Almagro, L.; Sabater-Jara, A.B.; Belchí-Navarro, S.; Pedreño, M.Á. Recent Trends in the Biotechnological Production of Tocopherols Using in Vitro Cultures. Phytochem. Rev. 2021, 20, 1193–1207. [Google Scholar] [CrossRef]
- Pritam, P.; Deka, R.; Bhardwaj, A.; Srivastava, R.; Kumar, D.; Jha, A.K.; Jha, N.K.; Villa, C.; Jha, S.K. Antioxidants in Alzheimer’s Disease: Current Therapeutic Significance and Future Prospects. Biology 2022, 11, 212. [Google Scholar] [CrossRef]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and Tocotrienols—Bioactive Dietary Compounds; What Is Certain, What Is Doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The Role of Vitamin e in Human Health and Some Diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157-65. [Google Scholar] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Chen, W.; Lu, S.; Zhang, H.; Yin, L. Metabolic Engineering of Shikimic Acid Biosynthesis Pathway for the Production of Shikimic Acid and Its Branched Products in Microorganisms: Advances and Prospects. Molecules 2022, 27, 4779. [Google Scholar] [CrossRef]
- Kanner, J. Food Polyphenols as Preventive Medicine. Antioxidants 2023, 12, 2103. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Hassanein, E.H.M.; Althagafy, H.S.; Baraka, M.A.; Abd-alhameed, E.K.; Ibrahim, I.M.; Abd El-Maksoud, M.S.; Mohamed, N.M.; Ross, S.A. The Promising Antioxidant Effects of Lignans: Nrf2 Activation Comes into View. Naunyn Schmiedebergs Arch. Pharmacol. 2024. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and Molecular Mechanisms of Antioxidants: Experimental Approaches and Model Systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Reinisalo, M.; Kårlund, A.; Koskela, A.; Kaarniranta, K.; Karjalainen, R.O. Polyphenol Stilbenes: Molecular Mechanisms of Defence against Oxidative Stress and Aging-Related Diseases. Oxid. Med. Cell. Longev. 2015, 2015, 340520. [Google Scholar] [CrossRef]
- Pinyaev, S.I.; Kuzmenko, T.P.; Revina, N.V.; Parchaykina, M.V.; Pronin, A.S.; Syusin, I.V.; Novozhilova, O.S.; Revin, V.V.; Chudaikina, E.V.; Revina, E.S. Influence of Resveratrol on Oxidation Processes and Lipid Phase Characteristics in Damaged Somatic Nerves. BioMed Res. Int. 2019, 2019, 2381907. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Liu, M.; Portincasa, P.; Wang, D.Q.-H. Recent Advances in the Critical Role of the Sterol Efflux Transporters ABCG5/G8 in Health and Disease. In Lipid Transfer in Lipoprotein Metabolism and Cardiovascular Disease. Advances in Experimental Medicine and Biology; Spinger: Berlin/Heidelberg, Germany, 2020; pp. 105–136. [Google Scholar] [CrossRef]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.-H.; El Omari, N.; Sheikh, R.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Health Benefits and Pharmacological Properties of Stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef]
- Arivarasu, L. In-Vitro Antioxidant Potential of Beta-Sitosterol: A Preface. Cureus 2023, 15, e45617. [Google Scholar] [CrossRef]
- Li, X.; Xin, Y.; Mo, Y.; Marozik, P.; He, T.; Guo, H. The Bioavailability and Biological Activities of Phytosterols as Modulators of Cholesterol Metabolism. Molecules 2022, 27, 523. [Google Scholar] [CrossRef]
- Hashim, A.M.; Alharbi, B.M.; Abdulmajeed, A.M.; Elkelish, A.; Hozzein, W.N.; Hassan, H.M. Oxidative Stress Responses of Some Endemic Plants to High Altitudes by Intensifying Antioxidants and Secondary Metabolites Content. Plants 2020, 9, 869. [Google Scholar] [CrossRef] [PubMed]
- Crupi, P.; Faienza, M.F.; Naeem, M.Y.; Corbo, F.; Clodoveo, M.L.; Muraglia, M. Overview of the Potential Beneficial Effects of Carotenoids on Consumer Health and Well-Being. Antioxidants 2023, 12, 1069. [Google Scholar] [CrossRef]
- Mannino, G.; Di Stefano, V.; Lauria, A.; Pitonzo, R.; Gentile, C. Vaccinium macrocarpon (Cranberry)-Based Dietary Supplements: Variation in Mass Uniformity, Proanthocyanidin Dosage and Anthocyanin Profile Demonstrates Quality Control Standard Needed. Nutrients 2020, 12, 992. [Google Scholar] [CrossRef] [PubMed]
- Fagbohun, O.F.; Gillies, C.R.; Murphy, K.P.J.; Rupasinghe, H.P.V. Role of Antioxidant Vitamins and Other Micronutrients on Regulations of Specific Genes and Signaling Pathways in the Prevention and Treatment of Cancer. Int. J. Mol. Sci. 2023, 24, 6092. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 370438. [Google Scholar] [CrossRef]
- Chiang, H.-M.; Chen, H.-C.; Wu, C.-S.; Wu, P.-Y.; Wen, K.-C. Rhodiola Plants: Chemistry and Biological Activity. J. Food Drug Anal. 2015, 23, 359–369. [Google Scholar] [CrossRef]
- Vezza, T.; Canet, F.; de Marañón, A.M.; Bañuls, C.; Rocha, M.; Víctor, V.M. Phytosterols: Nutritional Health Players in the Management of Obesity and Its Related Disorders. Antioxidants 2020, 9, 1266. [Google Scholar] [CrossRef]
- Radomska-Leśniewska, D.M.; Skopiński, P.; Bałan, B.J.; Białoszewska, A.; Jóźwiak, J.; Rokicki, D.; Skopińska-Różewska, E.; Borecka, A.; Hevelke, A. Review Paper Angiomodulatory Properties of Rhodiola spp. and other Natural Antioxidants. Cent. Eur. J. Immunol. 2015, 2, 249–262. [Google Scholar] [CrossRef]
- Panth, N.; Paudel, K.R.; Parajuli, K. Reactive Oxygen Species: A Key Hallmark of Cardiovascular Disease. Adv. Med. 2016, 2016, 9152732. [Google Scholar] [CrossRef]
- Münzel, T.; Camici, G.G.; Maack, C.; Bonetti, N.R.; Fuster, V.; Kovacic, J.C. Impact of Oxidative Stress on the Heart and Vasculature. J. Am. Coll. Cardiol. 2017, 70, 212–229. [Google Scholar] [CrossRef]
- Padovan, J.C.; Dourado, T.M.H.; Pimenta, G.F.; Bruder-Nascimento, T.; Tirapelli, C.R. Reactive Oxygen Species Are Central Mediators of Vascular Dysfunction and Hypertension Induced by Ethanol Consumption. Antioxidants 2023, 12, 1813. [Google Scholar] [CrossRef] [PubMed]
- Leventopoulos, G.; Koros, R.; Travlos, C.; Perperis, A.; Chronopoulos, P.; Tsoni, E.; Koufou, E.-E.; Papageorgiou, A.; Apostolos, A.; Kaouris, P.; et al. Mechanisms of Atrial Fibrillation: How Our Knowledge Affects Clinical Practice. Life 2023, 13, 1260. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhang, T.; Zhang, W.; Zhao, Z.; Sun, J. Natural Drugs as a Treatment Strategy for Cardiovascular Disease through the Regulation of Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 5430407. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A Review of Anti-Cancer Properties and Therapeutic Activity in Head and Neck Squamous Cell Carcinoma. Mol. Cancer 2011, 10, 12. [Google Scholar] [CrossRef]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic Effects of Green Tea Polyphenol (—)-Epigallocatechin-3-Gallate (EGCG) in Relation to Molecular Pathways Controlling Inflammation, Oxidative Stress, and Apoptosis. Int. J. Mol. Sci. 2022, 24, 340. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxid. Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B.; Kitts, D.D. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Dobhal, Y.; Parcha, V.; Dhasmana, D.C. Characterization of New Cardioprotective Principle Isolated from Methanolic Extract of Allium humile Leaves from Himalayan Region. Bangladesh J. Pharmacol. 2016, 11, 383. [Google Scholar] [CrossRef]
- Giuseppe, D.; Angela, D.; Davide, R.; Pamela, M. Effects of a Combination of Berberis aristata, Silybum marianum and Monacolin on Lipid Profile in Subjects at Low Cardiovascular Risk; A Double-Blind, Randomized, Placebo-Controlled Trial. Int. J. Mol. Sci. 2017, 18, 343. [Google Scholar] [CrossRef]
- Habanova, M.; Saraiva, J.A.; Haban, M.; Schwarzova, M.; Chlebo, P.; Predna, L.; Gažo, J.; Wyka, J. Intake of Bilberries (Vaccinium myrtillus L.) Reduced Risk Factors for Cardiovascular Disease by Inducing Favorable Changes in Lipoprotein Profiles. Nutr. Res. 2016, 36, 1415–1422. [Google Scholar] [CrossRef]
- Karcheva-Bahchevanska, D.; Nikolova, M.; Iliev, I. Inhibitory Potential of Different Bilberry (Vaccinium myrtillus L.) Extracts on Human Salivary α-Amylase. Molecules 2023, 28, 5820. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Jia, B.; Sun, J.; Lin, J.; Lu, B.; Duan, J.; Li, C.; Wang, Q.; Zhang, X.; Tan, M.; et al. Gastrodia elata Blume: A Review of Its Mechanisms and Functions on Cardiovascular Systems. Fitoterapia 2023, 167, 105511. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, H.-Y.; He, D.; Rao, C.-M.; Xu, B. Cardioprotective Effect of Gynostemma pentaphyllum against Streptozotocin Induced Cardiac Toxicity in Rats via Alteration of AMPK/Nrf2/HO-1 Pathway. J. Oleo Sci. 2022, 71, ess21281. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.; Zhao, F.; Fang, M.; Pu, F.; Kong, L.; Liu, J. Gynostemma pentaphyllum for Dyslipidemia: A Systematic Review of Randomized Controlled Trials. Front. Pharmacol. 2022, 13, 917521. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Yu, Z.; Li, X.; Zhang, X.; Wang, S.; Yang, S.; Hu, L.; Liu, L. Paeonol for the Treatment of Atherosclerotic Cardiovascular Disease: A Pharmacological and Mechanistic Overview. Front. Cardiovasc. Med. 2021, 8, 690116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, L.; Sun, X.; Yang, Q.; Wan, L.; Guo, C. Matrine: A Promising Natural Product with Various Pharmacological Activities. Front. Pharmacol. 2020, 11, 588. [Google Scholar] [CrossRef]
- Jung, Y.A.; Wan, X.; Yan, H.; Row, K.H. Determination of Matrine and Oxymatrine in Sophora flavescens Ait. via High Performance Liquid Chromatography. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 2752–2761. [Google Scholar] [CrossRef]
- Janda, K.; Wojtkowska, K.; Jakubczyk, K.; Antoniewicz, J.; Skonieczna-Żydecka, K. Passiflora incarnata in Neuropsychiatric Disorders—A Systematic Review. Nutrients 2020, 12, 3894. [Google Scholar] [CrossRef]
- Achika, J.I.; Yusuf, A.J.; Ayo, R.G.; Liman, D.U. Flavonoids from Nigerian Indigenous Medicinal Plants and Their Bioactivities: A Review. Phytomed. Plus 2023, 3, 100405. [Google Scholar] [CrossRef]
- Lam, K.Y.; Ling, A.P.K.; Koh, R.Y.; Wong, Y.P.; Say, Y.H. A Review on Medicinal Properties of Orientin. Adv. Pharmacol. Sci. 2016, 2016, 4104595. [Google Scholar] [CrossRef]
- Ding, L.; Liu, Z.; Zhao, F.; Bai, G.; Chen, L.; Yao, X.; Qiu, F. Isolation and Identification of the Metabolites of Paeonol in Human Urine. Xenobiotica 2012, 42, 1206–1212. [Google Scholar] [CrossRef]
- Zhang, M.-X.; Huang, X.-Y.; Song, Y.; Xu, W.-L.; Li, Y.-L.; Li, C. Astragalus propinquus Schischkin and Salvia miltiorrhiza Bunge Promote Angiogenesis to Treat Myocardial Ischemia via Ang-1/Tie-2/FAK Pathway. Front. Pharmacol. 2023, 13, 1103557. [Google Scholar] [CrossRef]
- Fan, C.; Sun, X.; Wang, X.; Yu, H. Therapeutic Potential of the Chemical Composition of Dendrobium nobile Lindl. Front. Pharmacol. 2023, 14, 1163830. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Fu, L.; Nile, S.H.; Zhang, J.; Kai, G. Salvia miltiorrhiza in Treating Cardiovascular Diseases: A Review on Its Pharmacological and Clinical Applications. Front. Pharmacol. 2019, 10, 753. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Gao, W.; Huang, L. Tanshinones, Critical Pharmacological Components in Salvia miltiorrhiza. Front. Pharmacol. 2019, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Long, Y.; Yu, S.; Shi, A.; Wan, J.; Wen, J.; Li, X.; Liu, S.; Zhang, Y.; Li, N.; et al. Research Advances in Cardio-Cerebrovascular Diseases of Ligusticum Chuanxiong Hort. Front. Pharmacol. 2022, 12, 832673. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Q.; Zhou, S.; Xu, S.; Yao, K. Tetramethylpyrazine: A Review on Its Mechanisms and Functions. Biomed. Pharmacother. 2022, 150, 113005. [Google Scholar] [CrossRef]
- Jellinger, K.A. Basic Mechanisms of Neurodegeneration: A Critical Update. J. Cell. Mol. Med. 2010, 10, 457–487. [Google Scholar] [CrossRef]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative Stress in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative Diseases and Oxidative Stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic Life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Alavi Naini, S.M.; Soussi-Yanicostas, N. Tau Hyperphosphorylation and Oxidative Stress, a Critical Vicious Circle in Neurodegenerative Tauopathies? Oxid. Med. Cell. Longev. 2015, 2015, 151979. [Google Scholar] [CrossRef] [PubMed]
- Csala, M.; Kardon, T.; Legeza, B.; Lizák, B.; Mandl, J.; Margittai, É.; Puskás, F.; Száraz, P.; Szelényi, P.; Bánhegyi, G. On the Role of 4-Hydroxynonenal in Health and Disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2015, 1852, 826–838. [Google Scholar] [CrossRef]
- Brand-Yavin, A.; Yavin, E. Brain Oxidative Stress from a Phospholipid Perspective. In Handbook of Neurochemistry and Molecular Neurobiology; Springer: Boston, MA, USA, 2009; pp. 603–630. [Google Scholar] [CrossRef]
- Piccialli, I.; Tedeschi, V.; Caputo, L.; D’Errico, S.; Ciccone, R.; De Feo, V.; Secondo, A.; Pannaccione, A. Exploring the Therapeutic Potential of Phytochemicals in Alzheimer’s Disease: Focus on Polyphenols and Monoterpenes. Front. Pharmacol. 2022, 13, 876614. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Palanivelu, K. The Effect of Curcumin (Turmeric) on Alzheimer′s Disease: An Overview. Ann. Indian Acad. Neurol. 2008, 11, 13. [Google Scholar] [CrossRef]
- Rahman, M.H.; Akter, R.; Bhattacharya, T.; Abdel-Daim, M.M.; Alkahtani, S.; Arafah, M.W.; Al-Johani, N.S.; Alhoshani, N.M.; Alkeraishan, N.; Alhenaky, A.; et al. Resveratrol and Neuroprotection: Impact and Its Therapeutic Potential in Alzheimer’s Disease. Front. Pharmacol. 2020, 11, 619024. [Google Scholar] [CrossRef]
- Minocha, T.; Birla, H.; Obaid, A.A.; Rai, V.; Sushma, P.; Shivamallu, C.; Moustafa, M.; Al-Shehri, M.; Al-Emam, A.; Tikhonova, M.A.; et al. Flavonoids as Promising Neuroprotectants and Their Therapeutic Potential against Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2022, 2022, 6038996. [Google Scholar] [CrossRef]
- Kujawska, M.; Jodynis-Liebert, J. Polyphenols in Parkinson’s Disease: A Systematic Review of In Vivo Studies. Nutrients 2018, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Klomparens, E.; Ding, Y. The Neuroprotective Mechanisms and Effects of Sulforaphane. Brain Circ. 2019, 5, 74. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, B.-L.; Xiang, Y.; Tian, D.-Y.; Zhu, C.; Li, W.-W.; Liu, Y.-H.; Bu, X.-L.; Shen, L.-L.; Jin, W.-S.; et al. Capsaicin Consumption Reduces Brain Amyloid-Beta Generation and Attenuates Alzheimer’s Disease-Type Pathology and Cognitive Deficits in APP/PS1 Mice. Transl. Psychiatry 2020, 10, 230. [Google Scholar] [CrossRef]
- Magalingam, K.B.; Radhakrishnan, A.K.; Haleagrahara, N. Protective Mechanisms of Flavonoids in Parkinson’s Disease. Oxid. Med. Cell. Longev. 2015, 2015, 314560. [Google Scholar] [CrossRef]
- Chandran, R.; Abrahamse, H. Identifying Plant-Based Natural Medicine against Oxidative Stress and Neurodegenerative Disorders. Oxid. Med. Cell. Longev. 2020, 2020, 8648742. [Google Scholar] [CrossRef] [PubMed]
- Uță, G.; Manolescu, D.Ș.; Avram, S. Therapeutic Properties of Several Chemical Compounds of Salvia officinalis L. in Alzheimer’s Disease. Mini-Rev. Med. Chem. 2021, 21, 1421–1430. [Google Scholar] [CrossRef]
- Ariani, A.; Ghofar, I.; Khotimah, H.; Nurdiana, N.; Rahayu, M. Asiatic Acid in Centella asiatica Extract towards Morphological Development in an Intermittent Hypoxia Intrauterine Embryo Model and Molecular Prediction Pathway of Insulin-like Growth Factor-1 (IGF-1) Receptor Signalling. Open Vet. J. 2023, 13, 629. [Google Scholar] [CrossRef]
- Mushtaq, Z.; Imran, M.; Hussain, M.; Saeed, F.; Imran, A.; Umar, M.; Abdelgawad, M.A.; El-Ghorab, A.H.; Ahmed, A.; Alsagaby, S.A.; et al. Asiatic Acid: A Review on Its Polypharmacological Properties and Therapeutic Potential against Various Maladies. Int. J. Food Prop. 2023, 26, 1244–1263. [Google Scholar] [CrossRef]
- Wong, J.H.; Barron, A.M.; Abdullah, J.M. Mitoprotective Effects of Centella asiatica (L.) Urb.: Anti-Inflammatory and Neuroprotective Opportunities in Neurodegenerative Disease. Front. Pharmacol. 2021, 12, 687935. [Google Scholar] [CrossRef]
- Bandopadhyay, S.; Mandal, S.; Ghorai, M.; Jha, N.K.; Kumar, M.; Radha; Ghosh, A.; Proćków, J.; Pérez de la Lastra, J.M.; Dey, A. Therapeutic Properties and Pharmacological Activities of Asiaticoside and Madecassoside: A Review. J. Cell. Mol. Med. 2023, 27, 593–608. [Google Scholar] [CrossRef]
- Fatima, U.; Roy, S.; Ahmad, S.; Al-Keridis, L.A.; Alshammari, N.; Adnan, M.; Islam, A.; Hassan, M.I. Investigating Neuroprotective Roles of Bacopa Monnieri Extracts: Mechanistic Insights and Therapeutic Implications. Biomed. Pharmacother. 2022, 153, 113469. [Google Scholar] [CrossRef] [PubMed]
- Gubbannavar, J.; Chandola, H.; Harisha, C.; Khanpara, K.; Shukla, V. A Comparative Pharmacognostical and Preliminary Physico-Chemical Analysis of Stem and Leaf of Bacopa monnieri (L.) Pennel and Bacopa Floribunda (R.BR.) Wettst. AYU 2013, 34, 95. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.E.; Alcazar Magana, A.; Lak, P.; Wright, K.M.; Quinn, J.; Stevens, J.F.; Maier, C.S.; Soumyanath, A. Centella asiatica: Phytochemistry and Mechanisms of Neuroprotection and Cognitive Enhancement. Phytochem. Rev. 2018, 17, 161–194. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E. Centella Asiatica (L.) Urban: From Traditional Medicine to Modern Medicine with Neuroprotective Potential. Evid.-Based Complement. Altern. Med. 2012, 2012, 946259. [Google Scholar] [CrossRef] [PubMed]
- Ehrnhöfer-Ressler, M.M.; Fricke, K.; Pignitter, M.; Walker, J.M.; Walker, J.; Rychlik, M.; Somoza, V. Identification of 1,8-Cineole, Borneol, Camphor, and Thujone as Anti-Inflammatory Compounds in a Salvia officinalis L. Infusion Using Human Gingival Fibroblasts. J. Agric. Food Chem. 2013, 61, 3451–3459. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological Properties of Salvia officinalis and Its Components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Tundis, R.; Leporini, M.; Bonesi, M.; Rovito, S.; Passalacqua, N.G. Salvia officinalis L. from Italy: A Comparative Chemical and Biological Study of Its Essential Oil in the Mediterranean Context. Molecules 2020, 25, 5826. [Google Scholar] [CrossRef]
- Shoukat, S.; Zia, M.A.; Uzair, M.; Attia, K.A.; Abushady, A.M.; Fiaz, S.; Ali, S.; Yang, S.H.; Ali, G.M. Bacopa monnieri: A Promising Herbal Approach for Neurodegenerative Disease Treatment Supported by in Silico and in Vitro Research. Heliyon 2023, 9, e21161. [Google Scholar] [CrossRef]
- Wu, P.; Chang, C.; Zhu, G.; Zhai, L.; Zhang, X.; Huan, Q.; Gao, Z.; Deng, H.; Liang, Y.; Xiao, H. Network Pharmacology Study of Bioactive Components and Molecular Mechanisms of the Glycoside Fraction from Picrorhiza scrophulariiflora Against Experimental Colitis. Drug Des. Dev. Ther. 2023, 17, 1531–1546. [Google Scholar] [CrossRef]
- He, Z.; Hu, Y.; Niu, Z.; Zhong, K.; Liu, T.; Yang, M.; Ji, L.; Hu, W. A Review of Pharmacokinetic and Pharmacological Properties of Asiaticoside, a Major Active Constituent of Centella asiatica (L.) Urb. J. Ethnopharmacol. 2023, 302, 115865. [Google Scholar] [CrossRef]
- Agnihotri, V.; Walia, M.; Pathania, V.; Singh, B.; Kant, K. Evaluation of Antioxidant Activity of Picrorhiza kurroa (Leaves) Extracts. Indian J. Pharm. Sci. 2013, 75, 324. [Google Scholar] [CrossRef]
- Burchardt, P.; Żurawski, J.; Zuchowski, B.; Kubacki, T.; Murawa, D.; Wiktorowicz, K.; Wysocki, H. State of the Art Paper Low-Density Lipoprotein, Its Susceptibility to Oxidation and the Role of Lipoprotein-Associated Phospholipase A2 and Carboxyl Ester Lipase Lipases in Atherosclerotic Plaque Formation. Arch. Med. Sci. 2013, 1, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A.; Loscalzo, J. Oxidative Mechanisms and Atherothrombotic Cardiovascular Disease. Drug Discov. Today Ther. Strateg. 2008, 5, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Savini, I.; Catani, M.; Evangelista, D.; Gasperi, V.; Avigliano, L. Obesity-Associated Oxidative Stress: Strategies Finalized to Improve Redox State. Int. J. Mol. Sci. 2013, 14, 10497–10538. [Google Scholar] [CrossRef] [PubMed]
- Blagov, A.; Nedosugova, L.; Kirichenko, T.; Sukhorukov, V.; Melnichenko, A.; Orekhov, A. Mitochondrial Dysfunction as a Factor of Energy Metabolism Disorders in Type 2 Diabetes Mellitus. Front. Biosci. Sch. 2024, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Mabhida, S.E.; Ziqubu, K.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Hanser, S.; Basson, A.K.; Pheiffer, C.; Kengne, A.P. Pancreatic β-Cell Dysfunction in Type 2 Diabetes: Implications of Inflammation and Oxidative Stress. World J. Diabetes 2023, 14, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Holvoet, P. Stress in Obesity and Associated Metabolic and Cardiovascular Disorders. Scientifica 2012, 2012, 205027. [Google Scholar] [CrossRef] [PubMed]
- Moutia, M.; El Azhary, K.; Elouaddari, A.; Al Jahid, A.; Jamal Eddine, J.; Seghrouchni, F.; Habti, N.; Badou, A. Capparis spinosa L. Promotes Anti-Inflammatory Response in Vitro through the Control of Cytokine Gene Expression in Human Peripheral Blood Mononuclear Cells. BMC Immunol. 2016, 17, 26. [Google Scholar] [CrossRef]
- Rocha, D.H.A.; Pinto, D.C.G.A.; Silva, A.M.S. Macroalgae Specialized Metabolites: Evidence for Their Anti-Inflammatory Health Benefits. Mar. Drugs 2022, 20, 789. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Cione, E.; La Torre, C.; Cannataro, R.; Caroleo, M.C.; Plastina, P.; Gallelli, L. Quercetin, Epigallocatechin Gallate, Curcumin, and Resveratrol: From Dietary Sources to Human MicroRNA Modulation. Molecules 2019, 25, 63. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Shoorei, H.; Bahroudi, Z.; Hussen, B.M.; Talebi, S.F.; Taheri, M.; Ayatollahi, S.A. Nrf2-Related Therapeutic Effects of Curcumin in Different Disorders. Biomolecules 2022, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, B.I.; Ruiz-Ramos, M.; Pedraza-Chaverri, J.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Effect of Resveratrol on Markers of Oxidative Stress and Sirtuin 1 in Elderly Adults with Type 2 Diabetes. Int. J. Mol. Sci. 2023, 24, 7422. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Gao, Z.; Zhang, Q.; Gu, C. The Mechanism of Berberine Alleviating Metabolic Disorder Based on Gut Microbiome. Front. Cell. Infect. Microbiol. 2022, 12, 854885. [Google Scholar] [CrossRef]
- Capece, U.; Moffa, S.; Improta, I.; Di Giuseppe, G.; Nista, E.C.; Cefalo, C.M.A.; Cinti, F.; Pontecorvi, A.; Gasbarrini, A.; Giaccari, A.; et al. Alpha-Lipoic Acid and Glucose Metabolism: A Comprehensive Update on Biochemical and Therapeutic Features. Nutrients 2022, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Tao, G.; Yang, L.; Wu, X.; Liu, J.; Dagher, F.; Ou, S.; Song, Y.; Huang, J. Dietary Phytochemical and Metabolic Disease Prevention: Focus on Plant Proteins. Front. Nutr. 2023, 10, 1089487. [Google Scholar] [CrossRef] [PubMed]
- Martchenko, A.; Papaelias, A.; Bolz, S.-S. Physiologic Effects of the Maqui Berry (Aristotelia chilensis): A Focus on Metabolic Homeostasis. Food Funct. 2024, 15, 4724–4740. [Google Scholar] [CrossRef] [PubMed]
- Kunc, N.; Hudina, M.; Osterc, G.; Bavcon, J.; Ravnjak, B.; Mikulič-Petkovšek, M. Phenolic Compounds of Rose Hips of Some Rosa Species and Their Hybrids Native Grown in the South-West of Slovenia during a Two-Year Period (2020–2021). Foods 2023, 12, 1952. [Google Scholar] [CrossRef]
- Oprica, L.; Bucsa, C.; Zamfirache, M.M. Ascorbic Acid Content of Rose Hip Fruit Depending on Altitude. Iran. J. Public Health 2015, 44, 138–139. [Google Scholar]
- Gan, R.-Y.; Zhang, D.; Wang, M.; Corke, H. Health Benefits of Bioactive Compounds from the Genus Ilex, a Source of Traditional Caffeinated Beverages. Nutrients 2018, 10, 1682. [Google Scholar] [CrossRef] [PubMed]
- Louisa, M.; Patintingan, C.G.H.; Wardhani, B.W.K. Moringa Oleifera Lam. in Cardiometabolic Disorders: A Systematic Review of Recent Studies and Possible Mechanism of Actions. Front. Pharmacol. 2022, 13, 792794. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Bao, Y.; Shen, X.; Zengin, G.; Lyu, Y.; Xiao, J.; Weng, Z. Niazirin from Moringa oleifera Lam. Attenuates High Glucose-Induced Oxidative Stress through PKCζ/Nox4 Pathway. Phytomedicine 2021, 86, 153066. [Google Scholar] [CrossRef] [PubMed]
- Akaberi, M.; Hosseinzadeh, H. Grapes (Vitis vinifera) as a Potential Candidate for the Therapy of the Metabolic Syndrome. Phytother. Res. 2016, 30, 540–556. [Google Scholar] [CrossRef] [PubMed]
- Graf, B.L.; Raskin, I.; Cefalu, W.T.; Ribnicky, D.M. Plant-Derived Therapeutics for the Treatment of Metabolic Syndrome. Curr. Opin. Investig. Drugs 2010, 11, 1107–1115. [Google Scholar] [PubMed]
- Wang, Y.; Zhang, D.; Ye, W.; Yin, Z.; Fung, K.-P.; Zhao, S.; Yao, X. Triterpenoid Saponins from Androsace umbellata and Their Anti-Proliferative Activities in Human Hepatoma Cells. Planta Med. 2008, 74, 1280–1284. [Google Scholar] [CrossRef]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Askew, E.W. Work at High Altitude and Oxidative Stress: Antioxidant Nutrients. Toxicology 2002, 180, 107–119. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Siyar, S.; Sami, S.; Majeed, A. Heavy Metal Stress in Plants: Effects on Nutrients and Water Uptake. In Cellular and Molecular Phytotoxicity of Heavy Metals; Springer: Berlin/Heidelberg, Germany, 2020; pp. 89–98. [Google Scholar] [CrossRef]
- Petrovska, B. Historical Review of Medicinal Plants’ Usage. Pharmacogn. Rev. 2012, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, P.; Ahlawat, P.; Singh, K.; Singh, R. Chemical Constituents, Pharmacological Activities, and Uses of Common Ayurvedic Medicinal Plants: A Future Source of New Drugs. Adv. Tradit. Med. 2021, 23, 673–714. [Google Scholar] [CrossRef]
- Butola, J.S.; Samant, S.S. Saussurea Species in Indian Himalayan Region: Diversity, Distribution and Indigenous Uses. Int. J. Plant Biol. 2010, 1, e9. [Google Scholar] [CrossRef]
- Pandey, M.M.; Rastogi, S.; Rawat, A.K.S. Saussurea costus: Botanical, Chemical and Pharmacological Review of an Ayurvedic Medicinal Plant. J. Ethnopharmacol. 2007, 110, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Wen, C.; Wang, D.; Shao, H.; Liu, C.; He, C.; Olatunji, O.J. Cardiorenal Protective Effect of Costunolide against Doxorubicin-Induced Toxicity in Rats by Modulating Oxidative Stress, Inflammation and Apoptosis. Molecules 2022, 27, 2122. [Google Scholar] [CrossRef]
- Mlejnek, P.; Dolezel, P.; Kriegova, E.; Pastvova, N. N-Acetylcysteine Can Induce Massive Oxidative Stress, Resulting in Cell Death with Apoptotic Features in Human Leukemia Cells. Int. J. Mol. Sci. 2021, 22, 12635. [Google Scholar] [CrossRef] [PubMed]
- Elshaer, S.E.; Hamad, G.M.; Hafez, E.E.; Baghdadi, H.H.; El-Demerdash, F.M.; Simal-Gandara, J. Root Extracts of Saussurea costus as Prospective Detoxifying Food Additive against Sodium Nitrite Toxicity in Male Rats. Food Chem. Toxicol. 2022, 166, 113225. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Bhardwaj, P.; Pandey, S.S.; Kumar, S. Arnebia euchroma, a Plant Species of Cold Desert in the Himalayas, Harbors Beneficial Cultivable Endophytes in Roots and Leaves. Front. Microbiol. 2021, 12, 696667. [Google Scholar] [CrossRef] [PubMed]
- Parray, J.A.; Kamili, A.N.; Jan, S.; Mir, M.Y.; Shameem, N.; Ganai, B.A.; Abd_Allah, E.F.; Hashem, A.; Alqarawi, A.A. Manipulation of Plant Growth Regulators on Phytochemical Constituents and DNA Protection Potential of the Medicinal Plant Arnebia benthamii. BioMed Res. Int. 2018, 2018, 6870139. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Shashni, S.; Kumar, P.; Pant, D.; Singh, A.; Verma, R.K. Phytochemical Constituents, Distributions and Traditional Usages of Arnebia euchroma: A Review. J. Ethnopharmacol. 2021, 271, 113896. [Google Scholar] [CrossRef]
- Su, L.; Yan, G.; Guan, B.; Xu, W.; Hao, Y.; Wang, Y.; Zhang, Y.; Liu, L. Shikonin Derivatives Protect Immune Organs from Damage and Promote Immune Responses In Vivo in Tumour-Bearing Mice. Phytother. Res. 2012, 26, 26–33. [Google Scholar] [CrossRef]
- Ganie, S.A.; Dar, T.A.; Hamid, R.; Zargar, O.; Abeer, S.U.; Masood, A.; Amin, S.; Zargar, M.A. In Vitro Antioxidant and Cytotoxic Activities of Arnebia benthamii (Wall Ex. G. Don): A Critically Endangered Medicinal Plant of Kashmir Valley. Oxid. Med. Cell. Longev. 2014, 2014, 792574. [Google Scholar] [CrossRef]
- Shameem, N.; Kamili, A.N.; Parray, J.A.; Hamid, R.; Bandh, S.A. Antimicrobial and Antioxidant Activity of Methanol Extracts of Arnebia benthamii (Wall Ex. G. Don) Johnston—A Critically Endangered Medicinal Plant of North Western Himalaya. J. Anal. Sci. Technol. 2015, 6, 36. [Google Scholar] [CrossRef]
- Papp, N.; Purger, D.; Czigle, S.; Czégényi, D.; Stranczinger, S.; Tóth, M.; Dénes, T.; Kocsis, M.; Takácsi-Nagy, A.; Filep, R. The Importance of Pine Species in the Ethnomedicine of Transylvania (Romania). Plants 2022, 11, 2331. [Google Scholar] [CrossRef]
- Gülçin, İ.; Büyükokuroǧlu, M.E.; Oktay, M.; Küfrevioǧlu, Ö.İ. Antioxidant and Analgesic Activities of Turpentine of Pinus nigra Arn. subsp. pallsiana (Lamb.) Holmboe. J. Ethnopharmacol. 2003, 86, 51–58. [Google Scholar] [CrossRef]
- Kurti, F.; Giorgi, A.; Beretta, G.; Mustafa, B.; Gelmini, F.; Testa, C.; Angioletti, S.; Giupponi, L.; Zilio, E.; Pentimalli, D.; et al. Chemical Composition, Antioxidant and Antimicrobial Activities of Essential Oils of Different Pinus Species from Kosovo. J. Essent. Oil Res. 2019, 31, 263–275. [Google Scholar] [CrossRef]
- Bisht, A.; Jain, S.; Misra, A.; Dwivedi, J.; Paliwal, S.; Sharma, S. Cedrus deodara (Roxb. Ex D.Don) G.Don: A Review of Traditional Use, Phytochemical Composition and Pharmacology. J. Ethnopharmacol. 2021, 279, 114361. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, K.; Silva, A.S.; Atanassova, M.; Sharma, R.; Nepovimova, E.; Musilek, K.; Sharma, R.; Alghuthaymi, M.A.; Dhanjal, D.S.; Nicoletti, M.; et al. Conifers Phytochemicals: A Valuable Forest with Therapeutic Potential. Molecules 2021, 26, 3005. [Google Scholar] [CrossRef]
- Samant, S.S.; Pant, S. Diversity, Distribution Pattern and Conservation Status of the Plants Used in Liver Diseases/Ailments in Indian Himalayan Region. J. Mt. Sci. 2006, 3, 28–47. [Google Scholar] [CrossRef]
- Anand, U.; Biswas, P.; Kumar, V.; Ray, D.; Ray, P.; Loake, V.I.P.; Kandimalla, R.; Chaudhary, A.; Singh, B.; Routhu, N.K.; et al. Podophyllum hexandrum and Its Active Constituents: Novel Radioprotectants. Biomed. Pharmacother. 2022, 146, 112555. [Google Scholar] [CrossRef]
- Cornara, L.; Ambu, G.; Trombetta, D.; Denaro, M.; Alloisio, S.; Frigerio, J.; Labra, M.; Ghimire, G.; Valussi, M.; Smeriglio, A. Comparative and Functional Screening of Three Species Traditionally Used as Antidepressants: Valeriana officinalis L., Valeriana jatamansi Jones Ex Roxb. and Nardostachys jatamansi (D. Don) DC. Plants 2020, 9, 994. [Google Scholar] [CrossRef] [PubMed]
- Raina, A.; Negi, K. Essential Oil Composition of Valeriana jatamansi Jones from Himalayan Regions of India. Indian J. Pharm. Sci. 2015, 77, 218. [Google Scholar] [CrossRef] [PubMed]
- Thusoo, S.; Gupta, S.; Sudan, R.; Kour, J.; Bhagat, S.; Hussain, R.; Bhagat, M. Antioxidant Activity of Essential Oil and Extracts of Valeriana jatamansi Roots. BioMed Res. Int. 2014, 2014, 614187. [Google Scholar] [CrossRef] [PubMed]
- Keet, J.-H.; Cindi, D.D.; du Preez, P.J. Assessing the Invasiveness of Berberis aristata and B. julianae (Berberidaceae) in South Africa: Management Options and Legal Recommendations. S. Afr. J. Bot. 2016, 105, 288–298. [Google Scholar] [CrossRef]
- Singh, J.; Kakkar, P. Antihyperglycemic and Antioxidant Effect of Berberis aristata Root Extract and Its Role in Regulating Carbohydrate Metabolism in Diabetic Rats. J. Ethnopharmacol. 2009, 123, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Frezza, C.; Venditti, A.; Toniolo, C.; De Vita, D.; Serafini, I.; Ciccòla, A.; Franceschin, M.; Ventrone, A.; Tomassini, L.; Foddai, S.; et al. Pedicularis L. Genus: Systematics, Botany, Phytochemistry, Chemotaxonomy, Ethnopharmacology, and Other. Plants 2019, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Yatoo, M.I.; Dimri, U.; Gopalakrishnan, A.; Karthik, K.; Gopi, M.; Khandia, R.; Saminathan, M.; Saxena, A.; Alagawany, M.; Farag, M.R.; et al. Beneficial Health Applications and Medicinal Values of Pedicularis Plants: A Review. Biomed. Pharmacother. 2017, 95, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Jeelani, S.M.; Siddique, M.A.A.; Rani, S. Variations of Morphology, Ecology and Chromosomes of Aconitum heterophyllum Wall., an Endangered Alpine Medicinal Plant in Himalayas. Caryologia 2015, 68, 294–305. [Google Scholar] [CrossRef][Green Version]
- Konda, V.G.R. Antioxidant and Nephroprotective Activities of Aconitum heterophyllum Root in Glycerol Induced Acute Renal Failure in Rats. J. Clin. Diagn. Res. 2016, 10, FF01. [Google Scholar] [CrossRef]
- Nengroo, Z.R.; Ganie, A.S.; Azeem, M. Aconitum heterophylum from Kashmir: Evaluation of Fatty Acid Profile, Antibacterial, Antioxidant Activities and Functional Group Analysis. Carbohydr. Polym. Technol. Appl. 2021, 2, 100105. [Google Scholar] [CrossRef]
- Manzoor, M.; Ahmad, M.; Zafar, M.; Gillani, S.W.; Shaheen, H.; Pieroni, A.; Al-Ghamdi, A.A.; Elshikh, M.S.; Saqib, S.; Makhkamov, T.; et al. The Local Medicinal Plant Knowledge in Kashmir Western Himalaya: A Way to Foster Ecological Transition via Community-Centred Health Seeking Strategies. J. Ethnobiol. Ethnomed. 2023, 19, 56. [Google Scholar] [CrossRef] [PubMed]
- Jahan, F.; Alvi, S.S.; Islam, M.H. Berberis aristata and Its Secondary Metabolites: Insights into Nutraceutical and Therapeutical Applications. Pharmacol. Res. Mod. Chin. Med. 2022, 5, 100184. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, Y.; Yue, J.; Wang, Z.; Zou, H.; Ji, X.; Zhang, S.; Liu, Z. Prediction of Potential Suitable Areas and Priority Protection for Cupressus gigantea on the Tibetan Plateau. Plants 2024, 13, 896. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Sarker, D.; Kar, P.; Gupta, P.; Sen, A. Indigenous Knowledge of Plants in Local Healthcare Management Practices by Tribal People of Malda District, India. J. Intercult. Ethnopharmacol. 2014, 3, 179. [Google Scholar] [CrossRef]
- Ravindra, J.; UG, Y.; Pandyanda Nanjappa, D.; Kalladka, K.; Dhakal, R.; Chakraborty, A.; Chakraborty, G. Allicin Extracted from Allium sativum Shows Potent Anti-Cancer and Antioxidant Properties in Zebrafish. Biomed. Pharmacother. 2023, 169, 115854. [Google Scholar] [CrossRef] [PubMed]
- Barbu, I.A.; Ciorîță, A.; Carpa, R.; Moț, A.C.; Butiuc-Keul, A.; Pârvu, M. Phytochemical Characterization and Antimicrobial Activity of Several Allium Extracts. Molecules 2023, 28, 3980. [Google Scholar] [CrossRef] [PubMed]
- Yudhistira, B.; Punthi, F.; Lin, J.; Sulaimana, A.S.; Chang, C.; Hsieh, C. S-Allyl Cysteine in Garlic (Allium sativum): Formation, Biofunction, and Resistance to Food Processing for Value-added Product Development. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2665–2687. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, M.; Zafar, R.; Rahman, N.U. Isolation and Identification of Phenolic Antioxidants from Pistacia integerrima Gall and Their Anticholine Esterase Activities. Heliyon 2018, 4, e01007. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.-L.; Liu, C.-F.; Huang, C.-L.; Huang, T.-C. Hepatoprotective Effect of Angelica archangelica in Chronically Ethanol-Treated Mice. Pharmacology 2003, 68, 70–73. [Google Scholar] [CrossRef]
- Park, W.H.; Kang, S.; Piao, Y.; Pak, C.J.; Oh, M.S.; Kim, J.; Kang, M.S.; Pak, Y.K. Ethanol Extract of Bupleurum falcatum and Saikosaponins Inhibit Neuroinflammation via Inhibition of NF-ΚB. J. Ethnopharmacol. 2015, 174, 37–44. [Google Scholar] [CrossRef]
- Witaicenis, A.; Seito, L.N.; da Silveira Chagas, A.; de Almeida, L.D.; Luchini, A.C.; Rodrigues-Orsi, P.; Cestari, S.H.; Di Stasi, L.C. Antioxidant and Intestinal Anti-Inflammatory Effects of Plant-Derived Coumarin Derivatives. Phytomedicine 2014, 21, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, U.; Nawchoo, I.A.; Ahmad, M. Phytochemical Evaluation of Various Solvent Extracts of the Leaves, Fruits and Shoots of Ferula jaeschkeana Vatke. Herb. Med. Open Access 2016, 2, 2472-0151. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Dinparast, L.; Zengin, G. The Genus Heracleum: A Comprehensive Review on Its Phytochemistry, Pharmacology, and Ethnobotanical Values as a Useful Herb. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1018–1039. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Song, X.; Tan, L.; Guo, C.; Wang, M.; Liu, H.; Cao, Z.; Li, Y.; Peng, C. A Review of the Pharmacological Properties of Psoralen. Front. Pharmacol. 2020, 11, 571535. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.P.; Dixit, P.; Singh, L.; Verma, P.C.; Saxena, G. Status of Selinum spp. L. a Himalayan Medicinal Plant in India: A Review of Its Pharmacology, Phytochemistry and Traditional Uses. Curr. Pharm. Biotechnol. 2019, 19, 1122–1134. [Google Scholar] [CrossRef]
- Nisa, S.; Bibi, Y.; Masood, S.; Ali, A.; Alam, S.; Sabir, M.; Qayyum, A.; Ahmed, W.; Alharthi, S.; Santali, E.Y.; et al. Isolation, Characterization and Anticancer Activity of Two Bioactive Compounds from Arisaema flavum (Forssk.) Schott. Molecules 2022, 27, 7932. [Google Scholar] [CrossRef]
- Akhtar, M.; Shaukat, A.; Zahoor, A.; Chen, Y.; Wang, Y.; Yang, M.; Umar, T.; Guo, M.; Deng, G. Anti-Inflammatory Effects of Hederacoside-C on Staphylococcus aureus Induced Inflammation via TLRs and Their Downstream Signal Pathway in Vivo and in Vitro. Microb. Pathog. 2019, 137, 103767. [Google Scholar] [CrossRef]
- Farasati Far, B.; Behzad, G.; Khalili, H. Achillea Millefolium: Mechanism of Action, Pharmacokinetic, Clinical Drug-Drug Interactions and Tolerability. Heliyon 2023, 9, e22841. [Google Scholar] [CrossRef]
- Mohammed, H.A. Phytochemical Analysis, Antioxidant Potential, and Cytotoxicity Evaluation of Traditionally Used Artemisia absinthium L. (Wormwood) Growing in the Central Region of Saudi Arabia. Plants 2022, 11, 1028. [Google Scholar] [CrossRef]
- Li, Z.; Li, Q.; Wu, J.; Wang, M.; Yu, J. Artemisinin and Its Derivatives as a Repurposing Anticancer Agent: What Else Do We Need to Do? Molecules 2016, 21, 1331. [Google Scholar] [CrossRef]
- Kozyra, M.; Kukula-Koch, W.; Szymański, M. Phenolic Composition of Inflorescences of Carduus nutans L. Chem. Biodivers. 2022, 19, e202100827. [Google Scholar] [CrossRef] [PubMed]
- shaikh, T.; Rub, R.A.; Sasikumar, S. Antimicrobial Screening of Cichorium intybus Seed Extracts. Arab. J. Chem. 2016, 9, S1569–S1573. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.; Asaf, S.; Lubna; Asif, S.; Kim, K.-M. Bioactivity and Therapeutic Potential of Kaempferol and Quercetin: New Insights for Plant and Human Health. Plants 2022, 11, 2623. [Google Scholar] [CrossRef] [PubMed]
- Kalola, J.; Shah, R.; Patel, A.; Lahiri, S.K.; Shah, M.B. Anti-Inflammatory and Immunomodulatory Activities of Inula cappa Roots (Compositae). J. Complement. Integr. Med. 2017, 14, 20160083. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, P.; Murugan, M. Antimutagenic and Antiapoptotic Effects of Aqueous Root Extract of Inula racemosa Hook. f. on 4-NQO-Induced Genetic Damage in Mice. ISRN Pharmacol. 2013, 2013, 768359. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.A.; Khan, M.R.; Naz, K.; Khan, M.A. Antioxidant Potential, DNA Protection, and HPLC-DAD Analysis of Neglected Medicinal Jurinea dolomiaea Roots. BioMed Res. Int. 2014, 2014, 726241. [Google Scholar] [CrossRef][Green Version]
- Kumar, A.; Agnihotri, V.K. Phytochemical Studies of Jurinea macrocephala Roots from Western Himalaya. Nat. Prod. Res. 2020, 34, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-M.; Choi, S.-I.; Kim, G.-H. Anti-Oxidant Activity of Saussurea lappa C.B. Clarke Roots. Prev. Nutr. Food Sci. 2012, 17, 306–309. [Google Scholar] [CrossRef]
- Rathore, S.; Debnath, P.; Kumar, R. Kuth Saussurea costus (Falc.) Lipsch.: A Critically Endangered Medicinal Plant from Himalaya. J. Appl. Res. Med. Aromat. Plants 2021, 20, 100277. [Google Scholar] [CrossRef]
- Mishra, A.P.; Saklani, S.; Parcha, V.; Nigam, M.; Coutinho, H.D.M. Antibacterial Activity and Phytochemical Characterisation of Saussurea gossypiphora D. Don. Arch. Microbiol. 2021, 203, 5055–5065. [Google Scholar] [CrossRef]
- Jaghthmi, O.; Zeid, I. Hypoglycemic and Hepatoprotective Effect of Rhizophora mucronata and Avicennia marina against Streptozotocin-Induced Diabetes in Male Rats. J. Adv. Vet. Anim. Res. 2020, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- Manzo, A.; Musso, L.; Panseri, S.; Iriti, M.; Dallavalle, S.; Catalano, E.; Scarì, G.; Giorgi, A. Screening of the Chemical Composition and Bioactivity of Waldheimia glabra (Decne.) Regel Essential Oil. J. Sci. Food Agric. 2016, 96, 3195–3201. [Google Scholar] [CrossRef] [PubMed]
- Hemeg, H.A.; Moussa, I.M.; Ibrahim, S.; Dawoud, T.M.; Alhaji, J.H.; Mubarak, A.S.; Kabli, S.A.; Alsubki, R.A.; Tawfik, A.M.; Marouf, S.A. Antimicrobial Effect of Different Herbal Plant Extracts against Different Microbial Population. Saudi J. Biol. Sci. 2020, 27, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, R.; Sati, N.; Sati, O.P. Antioxidant, Antibacterial and Antifungal Activity of Impatiens sulcata Wallich in Roxb. Extracts. Int. J. Life Sci. Sci. Res. 2016, 2, 671–677. [Google Scholar] [CrossRef]
- Gulfraz, M.; Mehmood, S.; Ahmad, A.; Fatima, N.; Praveen, Z.; Williamson, E.M. Comparison of the Antidiabetic Activity of Berberis lyceum Root Extract and Berberine in Alloxan-induced Diabetic Rats. Phytother. Res. 2008, 22, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Costa, J.F.; Meira, C.S.; das Neves, M.V.G.; Dos Reis, B.P.Z.C.; Soares, M.B.P. Anti-Inflammatory Activities of Betulinic Acid: A Review. Front. Pharmacol. 2022, 13, 883857. [Google Scholar] [CrossRef] [PubMed]
- Monsef-Esfahani, H.R.; Amini, M.; Goodarzi, N.; Saiedmohammadi, F.; Hajiaghaee, R.; Faramarzi, M.A.; Tofighi, Z.; Ghahremani, M.H. Coumarin Compounds of Biebersteinia multifida Roots Show Potential Anxiolytic Effects in Mice. DARU J. Pharm. Sci. 2013, 21, 51. [Google Scholar] [CrossRef] [PubMed]
- Parray, J.A.; Hamid, R.; Kamili, A.N.; Shameem, N.; Jan, S.; Ganai, B.A. Biological Efficacy and Radical Scavenging Potential of Shikonin in Arnebia benthamii (Wall Ex. G Don) Johnston. Ind. Crops Prod. 2015, 74, 434–439. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, A.; Nayik, G.A.; Cooper, R.; Bhardwaj, G.; Sohal, H.S.; Mutreja, V.; Kaur, R.; Areche, F.O.; AlOudat, M.; et al. Review of Shikonin and Derivatives: Isolation, Chemistry, Biosynthesis, Pharmacology and Toxicology. Front. Pharmacol. 2022, 13, 905755. [Google Scholar] [CrossRef]
- Chouhan, H.S.; Singh, S.K. Phytochemical Analysis, Antioxidant and Anti-Inflammatory Activities of Phyllanthus simplex. J. Ethnopharmacol. 2011, 137, 1337–1344. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, R.; Kishore, K. Onosma L.: A Review of Phytochemistry and Ethnopharmacology. Pharmacogn. Rev. 2013, 7, 140. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; d’Erme, M.; Trovato, M.; Mancini, P.; Piacentini, L.; Casale, A.; Wessjohann, L.; Gazzino, R.; Costantino, P.; et al. Anti-Inflammatory Activity of A Polyphenolic Extract from Arabidopsis thaliana in In Vitro and In Vivo Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 708. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J. The Glucosinolates: A Sulphur Glucoside Family of Mustard Anti-Tumour and Antimicrobial Phytochemicals of Potential Therapeutic Application. Biomedicines 2019, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Han, S.; Kim, H.; Won, S.Y.; Park, H.W.; Choi, H.; Choi, M.; Lee, M.Y.; Ha, I.J.; Lee, S.-G. Anticancer Effects of High Glucosinolate Synthesis Lines of Brassica rapa on Colorectal Cancer Cells. Antioxidants 2022, 11, 2463. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.-C.; Kuo, C.-Y.; Lee, Y.-H.; Wu, Y.-K.; Yang, M.-C.; Tzeng, I.-S.; Lan, C.-C. Therapeutic Effects and Mechanisms of Actions of Descurainia sophia. Int. J. Med. Sci. 2020, 17, 2163–2170. [Google Scholar] [CrossRef] [PubMed]
- Kaur, T.; Hussain, K.; Koul, S.; Vishwakarma, R.; Vyas, D. Evaluation of Nutritional and Antioxidant Status of Lepidium latifolium Linn.: A Novel Phytofood from Ladakh. PLoS ONE 2013, 8, e69112. [Google Scholar] [CrossRef] [PubMed]
- Bahramikia, S.; Yazdanparast, R. Antioxidant Efficacy of Nasturtium officinale Extracts Using Various In Vitro Assay Systems. J. Acupunct. Meridian Stud. 2010, 3, 283–290. [Google Scholar] [CrossRef]
- Đulović, A.; Popović, M.; Burčul, F.; Čikeš Čulić, V.; Marijan, S.; Ruščić, M.; Anđelković, N.; Blažević, I. Glucosinolates of Sisymbrium officinale and S. orientale. Molecules 2022, 27, 8431. [Google Scholar] [CrossRef] [PubMed]
- Farrukh, M.; Saleem, U.; Ahmad, B.; Chauhdary, Z.; Alsharif, I.; Manan, M.; Qasim, M.; Alhasani, R.H.; Shah, G.M.; Shah, M.A. Sarcococca saligna Hydroalcoholic Extract Ameliorates Arthritis in Complete Freund’s Adjuvant-Induced Arthritic Rats via Modulation of Inflammatory Biomarkers and Suppression of Oxidative Stress Markers. ACS Omega 2022, 7, 13164–13177. [Google Scholar] [CrossRef]
- Jan, H.A.; Hussain, W.; Bussmann, R.W.; Paniagua-Zambrana, N.Y. Codonopsis clematidea (Schrenk Ex Fisch. & C.A. Mey.) C.B. Clarke Campanulaceae. In Ethnobotany of the Himalayas. Ethnobotany of Mountain Regions; Springer: Cham, Switzerland, 2021; pp. 591–593. [Google Scholar] [CrossRef]
- He, J.-Y.; Ma, N.; Zhu, S.; Komatsu, K.; Li, Z.-Y.; Fu, W.-M. The Genus Codonopsis (Campanulaceae): A Review of Phytochemistry, Bioactivity and Quality Control. J. Nat. Med. 2015, 69, 1–21. [Google Scholar] [CrossRef]
- Huang, S.K.-H.; Bueno, P.R.P.; Garcia, P.J.B.; Lee, M.-J.; De Castro-Cruz, K.A.; Leron, R.B.; Tsai, P.-W. Antioxidant, Anti-Inflammatory and Antiproliferative Effects of Osmanthus fragrans (Thunb.) Lour. Flower Extracts. Plants 2023, 12, 3168. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Marcotullio, M.C.; Menichini, F.; Statti, G.A.; Vannutelli, L.; Burini, G.; Menichini, F.; Curini, M. The Influence of Collection Zone on Glucosinolates, Polyphenols and Flavonoids Contents and Biological Profiles of Capparis sicula ssp. Sicula. Food Sci. Technol. Int. 2011, 17, 87–97. [Google Scholar] [CrossRef]
- Hsu, H.-F.; Hsiao, P.-C.; Kuo, T.-C.; Chiang, S.-T.; Chen, S.-L.; Chiou, S.-J.; Ling, X.-H.; Liang, M.-T.; Cheng, W.-Y.; Houng, J.-Y. Antioxidant and Anti-Inflammatory Activities of Lonicera japonica Thunb. Var. Sempervillosa hayata Flower Bud Extracts Prepared by Water, Ethanol and Supercritical Fluid Extraction Techniques. Ind. Crops Prod. 2016, 89, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, Z.; Jiang, C.; Wang, X.; Huang, L. Exploiting Genes and Functional Diversity of Chlorogenic Acid and Luteolin Biosyntheses in Lonicera japonica and Their Substitutes. Gene 2014, 534, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Vergara, C.V.; Kitic, D.; Kostic, M.; Armstrong, L.; Shinwari, Z.K.; Khalil, A.T.; Brdar-Jokanović, M.; Ljevnaić-Mašić, B.; et al. Genus Viburnum: Therapeutic Potentialities and Agro-Food-Pharma Applications. Oxid. Med. Cell. Longev. 2021, 2021, 3095514. [Google Scholar] [CrossRef] [PubMed]
- Belahcene, S.; Kebsa, W.; Akingbade, T.V.; Umar, H.I.; Omoboyowa, D.A.; Alshihri, A.A.; Abo Mansour, A.; Alhasaniah, A.H.; Oraig, M.A.; Bakkour, Y.; et al. Chemical Composition Antioxidant and Anti-Inflammatory Activities of Myrtus communis L. Leaf Extract: Forecasting ADMET Profiling and Anti-Inflammatory Targets Using Molecular Docking Tools. Molecules 2024, 29, 849. [Google Scholar] [CrossRef] [PubMed]
- Boukhira, S.; El Mansouri, L.; Bouarfa, M.; Ouhammou, A.; Achour, S.; Khadhr, M.; Bousta, D. Phytochemical Screening, Anti-Inflammatory and Analgesic Activities Of Formulation Cream of Silene vulgaris. Res. J. Med. Plant 2016, 10, 150–158. [Google Scholar] [CrossRef][Green Version]
- Oladeji, O.S.; Oyebamiji, A.K. Stellaria media (L.) Vill.-A Plant with Immense Therapeutic Potentials: Phytochemistry and Pharmacology. Heliyon 2020, 6, e04150. [Google Scholar] [CrossRef] [PubMed]
- Poonia, A.; Upadhayay, A. Chenopodium album Linn: Review of Nutritive Value and Biological Properties. J. Food Sci. Technol. 2015, 52, 3977–3985. [Google Scholar] [CrossRef]
- Ajayi, A.; Tanayen, J.; Magomere, A.; Ezeonwumelu, J. Antinociceptive and Anti-Inflammatory Effects of Aqueous Extract of Chenopodium opulifolium Schrad Leaves. J. Intercult. Ethnopharmacol. 2017, 6, 14. [Google Scholar] [CrossRef]
- Salamatullah, A.M. Convolvulus Arvensis: Antioxidant, Antibacterial, and Antifungal Properties of Chemically Profiled Essential Oils: An Approach against Nosocomial Infections. Life 2022, 12, 2138. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, P.; Koundal, R.; Agnihotri, V.K. Antioxidant Properties and UPLC-MS/MS Profiling of Phenolics in Jacquemont’s Hazelnut Kernels (Corylus Jacquemontii) and Its Byproducts from Western Himalaya. J. Food Sci. Technol. 2016, 53, 3522–3531. [Google Scholar] [CrossRef] [PubMed]
- Bais, S.; Gill, N.S.; Rana, N.; Shandil, S. A Phytopharmacological Review on a Medicinal Plant: Juniperus communis. Int. Sch. Res. Notices 2014, 2014, 634723. [Google Scholar] [CrossRef] [PubMed]
- Miceli, N.; Trovato, A.; Dugo, P.; Cacciola, F.; Donato, P.; Marino, A.; Bellinghieri, V.; La Barbera, T.M.; Güvenç, A.; Taviano, M.F. Comparative Analysis of Flavonoid Profile, Antioxidant and Antimicrobial Activity of the Berries of Juniperus communis L. Var. Communis and Juniperus communis L. Var. Saxatilis Pall. from Turkey. J. Agric. Food Chem. 2009, 57, 6570–6577. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Alhodieb, F.S.; Barkat, M.A.; Hassan, M.Z.; Barkat, H.A.; Ali, R.; Alam, P.; Alam, O. Antitumor and Hepatoprotective Effect of Cuscuta reflexa Roxb. in a Murine Model of Colon Cancer. J. Ethnopharmacol. 2022, 282, 114597. [Google Scholar] [CrossRef] [PubMed]
- Deveoglu, O.; Cakmakcı, E.; Taskopru, T.; Torgan, E.; Karadag, R. Identification by RP-HPLC-DAD, FTIR, TGA and FESEM-EDAX of natural pigments prepared from Datisca cannabina L. Dye. Pigment. 2012, 94, 437–442. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Chang, Z.-Q.; Oh, B.-C.; Park, S.-C.; Shin, S.-R.; Kim, N.-W. Antioxidant Activity, Anti-Inflammatory Activity, and Whitening Effects of Extracts of Elaeagnus multiflora Thunb. J. Med. Food 2007, 10, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Gong, X.; Li, X.; Wang, C.; Li, M. Advanced Research on the Antioxidant Activity and Mechanism of Polyphenols from Hippophae Species—A Review. Molecules 2020, 25, 917. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.K.; Basistha, B.C.; Sen, A.; Middha, S.K. Antioxidant Profiling of Hippophae salicifolia Growing in Sacred Forests of Sikkim, India. Funct. Plant Biol. 2011, 38, 697. [Google Scholar] [CrossRef]
- Rosero, S.; Del Pozo, F.; Simbaña, W.; Álvarez, M.; Quinteros, M.F.; Carrillo, W.; Morales, D. Polyphenols and Flavonoids Composition, Anti-Inflammatory and Antioxidant Properties of Andean Baccharis macrantha Extracts. Plants 2022, 11, 1555. [Google Scholar] [CrossRef]
- Baral, B.; Shrestha Vaidya, G.; Laxmi Maharjan, B.; Teixeira Da Silva, J.A. Phytochemical And Antimicrobial Characterization Of Rhododendron anthopogon From High Nepalese Himalaya. Bot. Lith. 2015, 20, 142–152. [Google Scholar] [CrossRef][Green Version]
- Ahmad, A.; Wali, A.F.; Rehman, M.U.; Khan, A.; Raish, M.; Kazi, M.; Alnemer, O.; Rao, P.G.M. Therapeutic Potential of Rhododendron arboreum Polysaccharides in an Animal Model of Lipopolysaccharide-Inflicted Oxidative Stress and Systemic Inflammation. Molecules 2020, 25, 6045. [Google Scholar] [CrossRef]
- Zong, L.; Zhang, J.; Dai, L.; Liu, J.; Yang, Y.; Xie, J.; Luo, X. The Anti-Inflammatory Properties of Rhododendron molle Leaf Extract in LPS-Induced RAW264.7. Chem. Biodivers. 2020, 17, e2000477. [Google Scholar] [CrossRef]
- Ghazanfar, K.; Mubashir, K.; Dar, S.A.; Nazir, T.; Hameed, I.; Ganai, B.A.; Akbar, S.; Masood, A. Gentiana kurroo Royle Attenuates the Metabolic Aberrations in Diabetic Rats; Swertiamarin, Swertisin and Lupeol Being the Possible Bioactive Principles. J. Complement. Integr. Med. 2017, 14. [Google Scholar] [CrossRef]
- Jaishree, V.; Badami, S. Antioxidant and Hepatoprotective Effect of Swertiamarin from Enicostemma axillare against D-Galactosamine Induced Acute Liver Damage in Rats. J. Ethnopharmacol. 2010, 130, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Mihailović, V.; Mihailović, M.; Uskoković, A.; Arambašić, J.; Mišić, D.; Stanković, V.; Katanić, J.; Mladenović, M.; Solujić, S.; Matić, S. Hepatoprotective Effects of Gentiana asclepiadea L. Extracts against Carbon Tetrachloride Induced Liver Injury in Rats. Food Chem. Toxicol. 2013, 52, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Pasdaran, A.; Naychov, Z.; Batovska, D.; Kerr, P.; Favre, A.; Dimitrov, V.; Aneva, I.; Hamedi, A.; Kozuharova, E. Some European Gentiana Species Are Used Traditionally to Cure Wounds: Bioactivity and Conservation Issues. Diversity 2023, 15, 467. [Google Scholar] [CrossRef]
- Mirzaee, F.; Hosseini, A.; Jouybari, H.B.; Davoodi, A.; Azadbakht, M. Medicinal, Biological and Phytochemical Properties of Gentiana Species. J. Tradit. Complement. Med. 2017, 7, 400–408. [Google Scholar] [CrossRef]
- Nagalekshmi, R.; Menon, A.; Chandrasekharan, D.K.; Nair, C.K.K. Hepatoprotective Activity of Andrographis paniculata and Swertia chirayita. Food Chem. Toxicol. 2011, 49, 3367–3373. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Van Staden, J. A Review of Swertia chirayita (Gentianaceae) as a Traditional Medicinal Plant. Front. Pharmacol. 2016, 6, 308. [Google Scholar] [CrossRef]
- Velázquez-González, C.; Cariño-Cortés, R.; Gayosso de Lucio, J.A.; Ortiz, M.I.; De la O Arciniega, M.; Altamirano-Báez, D.A.; Ángeles, L.J.; Bautista-Ávila, M. Antinociceptive and Anti-Inflammatory Activities of Geranium bellum and Its Isolated Compounds. BMC Complement. Altern. Med. 2014, 14, 506. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, Q.; Haider, S.I.; Asif, A.; Rasheed, R.; Gul, S.; Arshad, S. Geranium wallichianum D. Don Ex Sweet Ameliorates Rheumatoid Arthritis by Curtailing the Expression of COX-II and Inflammatory Cytokines as Well as by Alleviating the Oxidative Stress. Dose-Response 2022, 20, 155932582211126. [Google Scholar] [CrossRef] [PubMed]
- Vieira, V.; Pereira, C.; Pires, T.C.S.P.; Calhelha, R.C.; Alves, M.J.; Ferreira, O.; Barros, L.; Ferreira, I.C.F.R. Phenolic Profile, Antioxidant and Antibacterial Properties of Juglans regia L. (Walnut) Leaves from the Northeast of Portugal. Ind. Crops Prod. 2019, 134, 347–355. [Google Scholar] [CrossRef]
- Czerwińska, M.; Świerczewska, A.; Granica, S. Bioactive Constituents of Lamium album L. as Inhibitors of Cytokine Secretion in Human Neutrophils. Molecules 2018, 23, 2770. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Burton, D.; Parra, F.; López, J.; Muñoz, P.; Escobar, H.; Parra, C. Antioxidant and Antibacterial Capacities of Origanum vulgare L. Essential Oil from the Arid Andean Region of Chile and Its Chemical Characterization by GC-MS. Metabolites 2020, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wu, F.; Tang, Z.; Yang, X.; Liu, Y.; Wang, F.; Chen, B. Anti-Inflammatory and Antioxidant Activity of Ursolic Acid: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2023, 14, 1256946. [Google Scholar] [CrossRef]
- Brindisi, M.; Bouzidi, C.; Frattaruolo, L.; Loizzo, M.R.; Cappello, M.S.; Dugay, A.; Deguin, B.; Lauria, G.; Cappello, A.R.; Tundis, R. New Insights into the Antioxidant and Anti-Inflammatory Effects of Italian Salvia officinalis Leaf and Flower Extracts in Lipopolysaccharide and Tumor-Mediated Inflammation Models. Antioxidants 2021, 10, 311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, C.; Gao, L.; Du, G.; Qin, X. Astragaloside IV Derived from Astragalus Membranaceus: A Research Review on the Pharmacological Effects. Adv. Pharmacol. 2020, 87, 89–112. [Google Scholar] [CrossRef]
- Stępnik, K.; Kukula-Koch, W.; Plazinski, W.; Gawel, K.; Gaweł-Bęben, K.; Khurelbat, D.; Boguszewska-Czubara, A. Significance of Astragaloside IV from the Roots of Astragalus mongholicus as an Acetylcholinesterase Inhibitor—From the Computational and Biomimetic Analyses to the In Vitro and In Vivo Studies of Safety. Int. J. Mol. Sci. 2023, 24, 9152. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, B.; Liang, D.; Quan, X.; Gu, R.; Meng, Z.; Gan, H.; Wu, Z.; Sun, Y.; Liu, S.; et al. Pharmacological Effects of Astragaloside IV: A Review. Molecules 2023, 28, 6118. [Google Scholar] [CrossRef]
- Gong, G.; Yu, H.; Zheng, Y.; Qi, B.; He, H.; Yin, T.; Dong, T.T.X.; Tsim, K.W.K. Astragaloside IV, a Saponin from Astragalus membranaceus Var. Mongholicus, Induces Expressions of Heme Recycle Proteins via Signaling of Nrf2/ARE in Cultured Macrophages. J. Ethnopharmacol. 2021, 265, 113389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-D.; Chen, H.; Zhang, C.; Liu, R.-H.; Li, H.-L.; Chen, H.-Z. Astragaloside IV from Astragalus membranaceus Shows Cardioprotection during Myocardial Ischemia in Vivo and in Vitro. Planta Med. 2006, 72, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hou, X.; Xu, R.; Liu, C.; Tu, M. Research Review on the Pharmacological Effects of Astragaloside. Fundam. Clin. Pharmacol. 2017, 31, 17–36. [Google Scholar] [CrossRef]
- Agour, A.; Mssillou, I.; Es-safi, I.; Conte, R.; Mechchate, H.; Slighoua, M.; Amrati, F.E.-Z.; Parvez, M.K.; Numan, O.; Bari, A.; et al. The Antioxidant, Analgesic, Anti-Inflammatory, and Wound Healing Activities of Haplophyllum tuberculatum (Forsskal) A. Juss Aqueous and Ethanolic Extract. Life 2022, 12, 1553. [Google Scholar] [CrossRef]
- Zhi, K.-K.; Yang, Z.-D.; Shi, D.-F.; Yao, X.-J.; Wang, M.-G. Desmodeleganine, a New Alkaloid from the Leaves of Desmodium elegans as a Potential Monoamine Oxidase Inhibitor. Fitoterapia 2014, 98, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Baali, N.; Mezrag, A.; Bouheroum, M.; Benayache, F.; Benayache, S.; Souad, A. Anti-Inflammatory and Antioxidant Effects of Lotus corniculatus on Paracetamol-Induced Hepatitis in Rats. Antiinflamm. Antiallergy Agents Med. Chem. 2020, 19, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Quiñones-Muñoz, T.A.; Villanueva-Rodríguez, S.J.; Torruco-Uco, J.G. Nutraceutical Properties of Medicago sativa L., Agave Spp., Zea Mays L. and Avena Sativa L.: A Review of Metabolites and Mechanisms. Metabolites 2022, 12, 806. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Bai, Y.-H.; Wang, S.-T.; Zhu, Z.-M.; Zhang, Y.-W. Research on Antioxidant Effects and Estrogenic Effect of Formononetin from Trifolium pratense (Red Clover). Phytomedicine 2009, 16, 314–319. [Google Scholar] [CrossRef]
- Ahmad, S.; Zeb, A. Phytochemical Profile and Pharmacological Properties of Trifolium repens. J. Basic. Clin. Physiol. Pharmacol. 2021, 32, 20200015. [Google Scholar] [CrossRef]
- Zhou, J.; Chan, L.; Zhou, S. Trigonelline: A Plant Alkaloid with Therapeutic Potential for Diabetes and Central Nervous System Disease. Curr. Med. Chem. 2012, 19, 3523–3531. [Google Scholar] [CrossRef]
- Liu, L.-F.; Li, W.-H.; Li, M.-Y.; Wu, X.-Z.; Yang, F.; Xu, J.-N.; Yuan, C.-S. Chemical Constituents from Common Vetch ( Vicia sativa L.) and Their Antioxidant and Cytotoxic Activities. Nat. Prod. Res. 2020, 34, 3205–3211. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.R.S.; Araújo-Filho, H.G.; Monteiro, B.S.; Shanmugam, S.; de Souza Araújo, A.A.; da Silva Almeida, J.R.; Thangaraj, P.; Júnior, L.J.Q.; Quintans, J.D. Anti-Inflammatory and Modulatory Effects of Steroidal Saponins and Sapogenins on Cytokines: A Review of Pre-Clinical Research. Phytomedicine 2022, 96, 153842. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Melo, M.N.; Oliveira, A.P.; Wiecikowski, A.F.; Carvalho, R.S.; de Lima Castro, J.; de Oliveira, F.A.G.; Pereira, H.M.G.; da Veiga, V.F.; Capella, M.M.A.; Rocha, L.; et al. Phenolic Compounds from Viscum album Tinctures Enhanced Antitumor Activity in Melanoma Murine Cancer Cells. Saudi Pharm. J. 2018, 26, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Akkol, E.K.; Karpuz, B.; Türkcanoğlu, G.; Coşgunçelebi, F.G.; Taştan, H.; Aschner, M.; Khatkar, A.; Sobarzo-Sánchez, E. The Phytochemical Profile and Biological Activity of Malva neglecta Wallr. in Surgically Induced Endometriosis Model in Rats. Molecules 2022, 27, 7869. [Google Scholar] [CrossRef] [PubMed]
- Altyar, A.E.; Munir, A.; Ishtiaq, S.; Rizwan, M.; Abbas, K.; Kensara, O.; Elhady, S.S.; Rizg, W.Y.; Youssef, F.S.; Ashour, M.L. Malva parviflora Leaves and Fruits Mucilage as Natural Sources of Anti-Inflammatory, Antitussive and Gastro-Protective Agents: A Comparative Study Using Rat Models and Gas Chromatography. Pharmaceuticals 2022, 15, 427. [Google Scholar] [CrossRef] [PubMed]
- Suriyaprom, S.; Srisai, P.; Intachaisri, V.; Kaewkod, T.; Pekkoh, J.; Desvaux, M.; Tragoolpua, Y. Antioxidant and Anti-Inflammatory Activity on LPS-Stimulated RAW 264.7 Macrophage Cells of White Mulberry (Morus alba L.) Leaf Extracts. Molecules 2023, 28, 4395. [Google Scholar] [CrossRef] [PubMed]
- Alhakmani, F.; Kumar, S.; Khan, S.A. Estimation of Total Phenolic Content, in-Vitro Antioxidant and Anti—Inflammatory Activity of Flowers of Moringa oleifera. Asian Pac. J. Trop. Biomed. 2013, 3, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.S.; Nautiyal, M.C.; Tava, A.; Cecotti, R. Essential Oil Composition of Morina longifolia Wall. Ex DC. from the Himalayan Region. J. Essent. Oil Res. 2012, 24, 461–463. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; EL-Hefnawy, H.M.; Osman, S.M.; El-Raey, M.A.; Mokhtar, F.A.; Ibrahim, H.A. Antioxidant, Anti-Inflammatory and Cytotoxic Activities of Jasminum multiflorum (Burm. F.) Andrews Leaves towards MCF-7 Breast Cancer and HCT 116 Colorectal Cell Lines and Identification of Bioactive Metabolites. Anticancer. Agents Med. Chem. 2021, 21, 2572–2582. [Google Scholar] [CrossRef]
- Kiss, A.K.; Bazylko, A.; Filipek, A.; Granica, S.; Jaszewska, E.; Kiarszys, U.; Kośmider, A.; Piwowarski, J. Oenothein B’s Contribution to the Anti-Inflammatory and Antioxidant Activity of Epilobium sp. Phytomedicine 2011, 18, 557–560. [Google Scholar] [CrossRef]
- Timoszuk, M.; Bielawska, K.; Skrzydlewska, E. Evening Primrose (Oenothera biennis) Biological Activity Dependent on Chemical Composition. Antioxidants 2018, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, V.; Seth, C.A.; Sourirajan, A.; El-Shazly, M.; Dev, K. A Comprehensive Review on the Phytochemistry, Pharmacological Properties, and in Vitro Propagation of an Endemic Medicinal Orchid, Dactylorhiza hatagirea. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 2621–2635. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Kaloo, Z.A.; Ganai, B.A.; Ganaie, H.A.; Singh, S. Phytochemical Screening of Meconopsis aculeata Royle an Important Medicinal Plant of Kashmir Himalaya: A Perspective. Res. J. Phytochem. 2016, 10, 1–9. [Google Scholar] [CrossRef]
- Jaydeokar, A.V.; Bandawane, D.D.; Bibave, K.H.; Patil, T.V. Hepatoprotective Potential of Cassia auriculata Roots on Ethanol and Antitubercular Drug-Induced Hepatotoxicity in Experimental Models. Pharm. Biol. 2014, 52, 344–355. [Google Scholar] [CrossRef]
- Charalambous, D.; Eliades, N.-G.H.; Christoforou, M.; Kakouri, E.; Kanakis, C.; Tarantilis, P.A.; Pantelidou, M. Chemical Characterization, Antioxidant and Antimicrobial Properties of Different Types of Tissue of Cedrus brevifolia Henry Extracts. Molecules 2022, 27, 2717. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, K.; Sharma, R.; Cruz-Martins, N.; Valko, M.; Upadhyay, N.K.; Kuča, K.; Bhardwaj, P. Studies of Phytochemicals, Antioxidant, and Antibacterial Activities of Pinus gerardiana and Pinus roxburghii Seed Extracts. BioMed Res. Int. 2022, 2022, 5938610. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Zhakipbekov, K.; Turgumbayeva, A.; Issayeva, R.; Kipchakbayeva, A.; Kadyrbayeva, G.; Tleubayeva, M.; Akhayeva, T.; Tastambek, K.; Sainova, G.; Serikbayeva, E.; et al. Antimicrobial and Other Biomedical Properties of Extracts from Plantago major, Plantaginaceae. Pharmaceuticals 2023, 16, 1092. [Google Scholar] [CrossRef]
- Samuelsen, A.B. The Traditional Uses, Chemical Constituents and Biological Activities of Plantago major L. A Review. J. Ethnopharmacol. 2000, 71, 1–21. [Google Scholar] [CrossRef]
- Pawłowska, K.A.; Hałasa, R.; Dudek, M.K.; Majdan, M.; Jankowska, K.; Granica, S. Antibacterial and Anti-Inflammatory Activity of Bistort (Bistorta officinalis) Aqueous Extract and Its Major Components. Justification of the Usage of the Medicinal Plant Material as a Traditional Topical Agent. J. Ethnopharmacol. 2020, 260, 113077. [Google Scholar] [CrossRef]
- Manivannan, V.; Johnson, M. Total Phenolic, Tannin, Triterpenoid, Flavonoid and Sterol Contents, Anti-Diabetic, Anti-Inflammatory and Cytotoxic Activities of Tectaria paradoxa (Fee.) Sledge. Toxicol. Rep. 2020, 7, 1465–1468. [Google Scholar] [CrossRef]
- Nakamura, T.; Kodama, N.; Arai, Y.; Kumamoto, T.; Higuchi, Y.; Chaichantipyuth, C.; Ishikawa, T.; Ueno, K.; Yano, S. Inhibitory Effect of Oxycoumarins Isolated from the Thai Medicinal Plant Clausena guillauminii on the Inflammation Mediators, INOS, TNF-α, and COX-2 Expression in Mouse Macrophage RAW 264.7. J. Nat. Med. 2009, 63, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, D.; Roza, J.M. Assessment of Anti-Inflammatory and Antioxidant Activity of Quercetin—Rutin Blend (SophorOxTM)—An Invitro Cell Based Assay. J. Complement. Integr. Med. 2022, 19, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Granica, S.; Czerwińska, M.E.; Żyżyńska-Granica, B.; Kiss, A.K. Antioxidant and Anti-Inflammatory Flavonol Glucuronides from Polygonum aviculare L. Fitoterapia 2013, 91, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-J.; Zhang, Y.-J.; Yang, C.-R. Antioxidant Phenolic Compounds from Rhizomes of Polygonum paleaceum. J. Ethnopharmacol. 2005, 96, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Pukhrambam, P.D.; Devi, K.K.; Maibam, C.; Mutum, R.D.; Devi, M.L.; Das, S. Phenolics and Flavonoids from Polygonum posumbu and Comparision of Flavonoid Compounds Content in Different Tissues (Leaves, Stems and Roots). Fitoterapia 2024, 174, 105864. [Google Scholar] [CrossRef] [PubMed]
- Pandith, S.A.; Dar, R.A.; Lattoo, S.K.; Shah, M.A.; Reshi, Z.A. Rheum. australe, an Endangered High-Value Medicinal Herb of North Western Himalayas: A Review of Its Botany, Ethnomedical Uses, Phytochemistry and Pharmacology. Phytochem. Rev. 2018, 17, 573–609. [Google Scholar] [CrossRef] [PubMed]
- Zhumashova, G.; Kukula-Koch, W.; Koch, W.; Baj, T.; Sayakova, G.; Shukirbekova, A.; Głowniak, K.; Sakipova, Z. Phytochemical and Antioxidant Studies on a Rare Rheum. cordatum Losinsk. Species from Kazakhstan. Oxid. Med. Cell. Longev. 2019, 2019, 5465463. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, T.; Akhter, S.; Sultan, P.; Hassan, Q.P. Critical Review on Rumex dentatus L. a Strong Pharmacophore and the Future Medicine: Pharmacology, Phytochemical Analysis and Traditional Uses. Heliyon 2023, 9, e14159. [Google Scholar] [CrossRef]
- Ahmad, S.; Ullah, F.; Ayaz, M.; Sadiq, A.; Imran, M. Antioxidant and Anticholinesterase Investigations of Rumex hastatus D. Don: Potential Effectiveness in Oxidative Stress and Neurological Disorders. Biol. Res. 2015, 48, 20. [Google Scholar] [CrossRef]
- Demirezer, L.Ö.; Kuruüzüm-Uz, A.; Bergere, I.; Schiewe, H.-J.; Zeeck, A. The Structures of Antioxidant and Cytotoxic Agents from Natural Source: Anthraquinones and Tannins from Roots of Rumex patientia. Phytochemistry 2001, 58, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Wani, T.A.; Kaloo, Z.A.; Dangroo, N.A. Aconitum heterophyllum Wall. Ex Royle: A Critically Endangered Medicinal Herb with Rich Potential for Use in Medicine. J. Integr. Med. 2022, 20, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Siyiti, M.; Zhang, J.; Yao, M.; Zhao, F. Anti-inflammatory and Anti-rheumatic Activities in Vitro of Alkaloids Separated from Aconitum soongoricum Stapf. Exp. Ther. Med. 2021, 21, 493. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Bibi, Y. Medicinal Uses and Conservation Status of Aconitum violaceum. J. Plant Environ. 2020, 2, 19–23. [Google Scholar] [CrossRef]
- Wani, Z.A.; Pant, S. Aconitum heterophyllum Wall. Ex Royle: An Endemic, Highly Medicinal and Critically Endangered Plant Species of Northwestern Himalaya in Peril. Curr. Tradit. Med. 2021, 7, 2–7. [Google Scholar] [CrossRef]
- Anh Minh, C.T.; Khoi, N.M.; Thuong, P.T.; Hwang, I.H.; Kim, D.W.; Na, M. A New Saponin and Other Constituents from Anemone rivularis Buch.-Ham. Biochem. Syst. Ecol. 2012, 44, 270–274. [Google Scholar] [CrossRef]
- Shrivastava, A.K.; Keshari, M.; Neupane, M.; Chaudhary, S.; Dhakal, P.K.; Shrestha, L.; Palikhey, A.; Yadav, C.K.; Lamichhane, G.; Shekh, M.U.; et al. Evaluation of Antioxidant and Anti-Inflammatory Activities, and Metabolite Profiling of Selected Medicinal Plants of Nepal. J. Trop. Med. 2023, 2023, 6641018. [Google Scholar] [CrossRef] [PubMed]
- Jan, H.; Usman, H.; Shah, M.; Zaman, G.; Mushtaq, S.; Drouet, S.; Hano, C.; Abbasi, B.H. Phytochemical Analysis and Versatile in Vitro Evaluation of Antimicrobial, Cytotoxic and Enzyme Inhibition Potential of Different Extracts of Traditionally Used Aquilegia pubiflora Wall. Ex Royle. BMC Complement. Med. Ther. 2021, 21, 165. [Google Scholar] [CrossRef] [PubMed]
- Mubashir, S.; Dar, M.Y.; Lone, B.A.; Zargar, M.I.; Shah, W.A. Anthelmintic, Antimicrobial, Antioxidant and Cytotoxic Activity of Caltha palustris Var. Alba Kashmir, India. Chin. J. Nat. Med. 2014, 12, 567–572. [Google Scholar] [CrossRef]
- Mostafa, M.; Appidi, J.R.; Yakubu, M.T.; Afolayan, A.J. Anti-Inflammatory, Antinociceptive and Antipyretic Properties of the Aqueous Extract of Clematis brachiata Leaf in Male Rats. Pharm. Biol. 2010, 48, 682–689. [Google Scholar] [CrossRef]
- Yesilada, E.; Küpeli, E. Clematis vitalba L. Aerial Part Exhibits Potent Anti-Inflammatory, Antinociceptive and Antipyretic Effects. J. Ethnopharmacol. 2007, 110, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Hasan, U.H.; Alamgeer; Shahzad, M.; Jahan, S.; Niazi, Z.R.; Bukhari, I.A.; Assiri, A.M.; Riaz, H. Inhibitory Effects of Clematis orientalis Aqueous Ethanol Extract and Fractions on Inflammatory Markers in Complete Freund’s Adjuvant-Induced Arthritis in Sprague—Dawley Rats. Inflammopharmacology 2019, 27, 781–797. [Google Scholar] [CrossRef]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef] [PubMed]
- Bahukhandi, A.; Barola, A.; Sekar, K.C. Antioxidant Activity and Polyphenolics of Fragaria nubicola: A Wild Edible Fruit Species of Himalaya. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 761–767. [Google Scholar] [CrossRef]
- Orlova, A.; Kysil, E.; Tsvetkova, E.; Meshalkina, D.; Whaley, A.; Whaley, A.O.; Laub, A.; Francioso, A.; Babich, O.; Wessjohann, L.A.; et al. Phytochemical Characterization of Water Avens (Geum rivale L.) Extracts: Structure Assignment and Biological Activity of the Major Phenolic Constituents. Plants 2022, 11, 2859. [Google Scholar] [CrossRef] [PubMed]
- Tangpu, V.; Deori, K.; Yadav, A. Evaluation of Safety and Protective Effects of Potentilla fulgens Root Extract in Experimentally Induced Diarrhoea in Mice. J. Intercult. Ethnopharmacol. 2014, 3, 103. [Google Scholar] [CrossRef] [PubMed]
- Wölfle, U.; Hoffmann, J.; Haarhaus, B.; Rao Mittapalli, V.; Schempp, C.M. Anti-Inflammatory and Vasoconstrictive Properties of Potentilla erecta—A Traditional Medicinal Plant from the Northern Hemisphere. J. Ethnopharmacol. 2017, 204, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Radhika, M.; Ghoshal, N.; Chatterjee, A. Comparison of Effectiveness in Antitumor Activity between Flavonoids and Polyphenols of the Methanolic Extract of Roots of Potentilla fulgens in Breast Cancer Cells. J. Complement. Integr. Med. 2012, 9. [Google Scholar] [CrossRef] [PubMed]
- Mashaal, K.; Shabbir, A.; Shahzad, M.; Mobashar, A.; Akhtar, T.; Fatima, T.; Riaz, B.; Alharbi, R.; Fatima, A.; Alanezi, A.A.; et al. Amelioration of Rheumatoid Arthritis by Fragaria nubicola (Wild Strawberry) via Attenuation of Inflammatory Mediators in Sprague Dawley Rats. Medicina 2023, 59, 1917. [Google Scholar] [CrossRef] [PubMed]
- Bagale, R.; Acharya, S.; Gupta, A.; Chaudhary, P.; Chaudhary, G.P.; Pandey, J. Antibacterial and Antioxidant Activities of Prinsepia utilis Royle Leaf and Seed Extracts. J. Trop. Med. 2022, 2022, 3898939. [Google Scholar] [CrossRef]
- Tewari, D.; Bawari, S.; Sah, A.N.; Sharma, H.; Joshi, B.C.; Gupta, P.; Sharma, V.K. Himalayan Pyracantha crenulata (D.Don) M.Roem. Leaf and Fruit Extracts Alleviate Algesia through COX-2 and Mu-Opioid Receptor Mediated Pathways. J. Ethnopharmacol. 2024, 318, 117004. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, N.; Gogoi, A.; Neog, B.; Baruah, D.; Singh, K. Evaluation of Antioxidant and Hepatoprotective Activity of Fruit Rind Extract of Garcinia dulcis (Roxburgh) Kurz. Pharmacogn. Res. 2017, 9, 266. [Google Scholar] [CrossRef]
- Ishaque, M.; BiBi, Y.; Valant-Vetschera, K.M.; Schinnerl, J.; Bacher, M. Fruits of Rosa brunonii—A Source of Antioxidant Phenolic Compounds. Nat. Prod. Commun. 2017, 12, 1934578X1701201. [Google Scholar] [CrossRef]
- Abla, M.; Cai, Y.; Gao, L.; Wu, J.; Yang, L. Changes in the Antioxidant and Anti-Inflammatory Activities of Rosa rugosa ‘Mohong’ during Fermentation. Heliyon 2024, 10, e25982. [Google Scholar] [CrossRef] [PubMed]
- Dhatwalia, J.; Kumari, A.; Chauhan, A.; Mansi, K.; Thakur, S.; Saini, R.V.; Guleria, I.; Lal, S.; Kumar, A.; Batoo, K.M.; et al. Rubus ellipticus Sm. Fruit Extract Mediated Zinc Oxide Nanoparticles: A Green Approach for Dye Degradation and Biomedical Applications. Materials 2022, 15, 3470. [Google Scholar] [CrossRef] [PubMed]
- Krauze-Baranowska, M.; Majdan, M.; Hałasa, R.; Głód, D.; Kula, M.; Fecka, I.; Orzeł, A. The Antimicrobial Activity of Fruits from Some Cultivar Varieties of Rubus idaeus and Rubus occidentalis. Food Funct. 2014, 5, 2536–2541. [Google Scholar] [CrossRef] [PubMed]
- Kostikova, V.A.; Petrova, N.V. Phytoconstituents and Bioactivity of Plants of the Genus Spiraea L. (Rosaceae): A Review. Int. J. Mol. Sci. 2021, 22, 11163. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, B.; Prabhakara, S.; Mohan, T.; Shabeer, D.; Bhandare, B.; Nalini, M.; Sharmila, P.; Meghana, D.; Reddy, B.; Hanumantha Rao, H.; et al. Characterization of Rubia cordifolia L. Root Extract and Its Evaluation of Cardioprotective Effect in Wistar Rat Model. Indian J. Pharmacol. 2018, 50, 12. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, A.; Mncwangi, N.; Vermaak, I. Anti-Inflammatory Iridoids of Botanical Origin. Curr. Med. Chem. 2012, 19, 2104–2127. [Google Scholar] [CrossRef]
- Zhu, W.; Pang, M.; Dong, L.; Huang, X.; Wang, S.; Zhou, L. Anti-Inflammatory and Immunomodulatory Effects of Iridoid Glycosides from Paederia scandens (LOUR.) MERRILL (Rubiaceae) on Uric Acid Nephropathy Rats. Life Sci. 2012, 91, 369–376. [Google Scholar] [CrossRef]
- Ganeshkumar, Y.; Ramarao, A.; Veeresham, C. Picroside I and Picroside II from Tissue Cultures of Picrorhiza kurroa. Pharmacognosy Res. 2017, 9, 53. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Shangguan, Z.-S.; Chen, C.; Zhang, H.-J.; Lin, Y. Anti-Inflammatory Effects of Guggulsterone on Murine Macrophage by Inhibiting LPS-Induced Inflammatory Cytokines in NF-κB Signaling Pathway. Drug Des. Devel Ther. 2016, 10, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, F.; Russo, R.; Sanna, C.; Celaj, O.; Caredda, A.; Corona, A.; Tramontano, E.; Fiorentino, A.; Esposito, F.; D’Abrosca, B. Chemical Characterization and Anti-HIV-1 Activity Assessment of Iridoids and Flavonols from Scrophularia trifoliata. Molecules 2021, 26, 4777. [Google Scholar] [CrossRef] [PubMed]
- Taheri, Y.; Quispe, C.; Herrera-Bravo, J.; Sharifi-Rad, J.; Ezzat, S.M.; Merghany, R.M.; Shaheen, S.; Azmi, L.; Prakash Mishra, A.; Sener, B.; et al. Urtica dioica-Derived Phytochemicals for Pharmacological and Therapeutic Applications. Evid.-Based Complement. Altern. Med. 2022, 2022, 4024331. [Google Scholar] [CrossRef] [PubMed]
- Raina, R.; Gautam, K. Conservation and Utilization of High-Altitude Threatened Medicinal Plants. In Conservation and Utilization of Threatened Medicinal Plants; Springer International Publishing: Cham, Switzerland, 2020; pp. 369–387. [Google Scholar] [CrossRef]
- Giordano, D. Bioactive Molecules from Extreme Environments. Mar. Drugs 2020, 18, 640. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Amelot, M.E. High Altitude Plants, Chemistry of Acclimation and Adaptation. Stud. Nat. Prod. Chem. 2008, 34, 883–982. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Wang, J.; Yu, M.; Zhang, R.; Mi, Y.; Xu, J.; Jiang, R.; Gao, J. Elevation Influences Belowground Biomass Proportion in Forests by Affecting Climatic Factors, Soil Nutrients and Key Leaf Traits. Plants 2024, 13, 674. [Google Scholar] [CrossRef] [PubMed]
- Sekar, K.C.; Thapliyal, N.; Pandey, A.; Joshi, B.; Mukherjee, S.; Bhojak, P.; Bisht, M.; Bhatt, D.; Singh, S.; Bahukhandi, A. Plant Species Diversity and Density Patterns along Altitude Gradient Covering High-Altitude Alpine Regions of West Himalaya, India. Geol. Ecol. Landsc. 2023, 1–15. [Google Scholar] [CrossRef]
- Rathore, N.; Thakur, D.; Chawla, A. Seasonal Variations Coupled with Elevation Gradient Drives Significant Changes in Eco-Physiological and Biogeochemical Traits of a High Altitude Evergreen Broadleaf Shrub, Rhododendron anthopogon. Plant Physiol. Biochem. 2018, 132, 708–719. [Google Scholar] [CrossRef]
- Arjona-García, C.; Blancas, J.; Beltrán-Rodríguez, L.; López Binnqüist, C.; Colín Bahena, H.; Moreno-Calles, A.I.; Sierra-Huelsz, J.A.; López-Medellín, X. How Does Urbanization Affect Perceptions and Traditional Knowledge of Medicinal Plants? J. Ethnobiol. Ethnomed. 2021, 17, 48. [Google Scholar] [CrossRef]
- Roufogalis, B.D. Challenges in Integrating Herbal Medicine in Healthcare Systems. Focus. Altern. Complement. Ther. 2015, 20, 34–35. [Google Scholar] [CrossRef]
- Ekor, M. The Growing Use of Herbal Medicines: Issues Relating to Adverse Reactions and Challenges in Monitoring Safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S. Current Status of Herbal Product: Regulatory Overview. J. Pharm. Bioallied Sci. 2015, 7, 293. [Google Scholar] [CrossRef]
- Smith, A.; Jogalekar, S.; Gibson, A. Regulation of Natural Health Products in Canada. J. Ethnopharmacol. 2014, 158, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Harnett, J.; McIntyre, E.; Adams, J.; Addison, T.; Bannerman, H.; Egelton, L.; Ma, J.; Zabakly, L.; Steel, A. Prevalence and Characteristics of Australians’ Complementary Medicine Product Use, and Concurrent Use with Prescription and Over-the-Counter Medications—A Cross Sectional Study. Nutrients 2023, 15, 327. [Google Scholar] [CrossRef]
- Sahoo, N.; Manchikanti, P. Herbal Drug Regulation and Commercialization: An Indian Industry Perspective. J. Altern. Complement. Med. 2013, 19, 957–963. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Lavecchia, T.; Rea, G.; Antonacci, A.; Giardi, M.T. Healthy and Adverse Effects of Plant-Derived Functional Metabolites: The Need of Revealing Their Content and Bioactivity in a Complex Food Matrix. Crit. Rev. Food Sci. Nutr. 2013, 53, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects—A Review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Gligorijević, N.; Stanić-Vučinić, D.; Radomirović, M.; Stojadinović, M.; Khulal, U.; Nedić, O.; Ćirković Veličković, T. Role of Resveratrol in Prevention and Control of Cardiovascular Disorders and Cardiovascular Complications Related to COVID-19 Disease: Mode of Action and Approaches Explored to Increase Its Bioavailability. Molecules 2021, 26, 2834. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhu, K.; Liu, Z.; Huang, J. Prooxidant Effects of Epigallocatechin-3-Gallate in Health Benefits and Potential Adverse Effect. Oxid. Med. Cell. Longev. 2020, 2020, 9723686. [Google Scholar] [CrossRef]
- Chauhan, D.; Yadav, P.K.; Sultana, N.; Agarwal, A.; Verma, S.; Chourasia, M.K.; Gayen, J.R. Advancements in Nanotechnology for the Delivery of Phytochemicals. J. Integr. Med. 2024, 385–398. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, S.; Wang, J.; Chen, Q. A Review on Polymer and Lipid-Based Nanocarriers and Its Application to Nano-Pharmaceutical and Food-Based Systems. Front. Nutr. 2021, 8, 783831. [Google Scholar] [CrossRef]
- El-Hefny, M.; Mohamed, A.A.; Abdelkhalek, A.; Salem, M.Z.M. Productivity and Phytochemicals of Asclepias curassavica in Response to Compost and Silver Nanoparticles Application: HPLC Analysis and Antibacterial Activity of Extracts. Plants 2023, 12, 2274. [Google Scholar] [CrossRef]
- Ashaolu, T.J. Nanoemulsions for Health, Food, and Cosmetics: A Review. Environ. Chem. Lett. 2021, 19, 3381–3395. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.; Gil-Izquierdo, Á.; García-Viguera, C.; Domínguez-Perles, R. Nanoparticles and Controlled Delivery for Bioactive Compounds: Outlining Challenges for New “Smart-Foods” for Health. Foods 2018, 7, 72. [Google Scholar] [CrossRef]
- Parveen, S.; Gupta, P.; Kumar, S.; Banerjee, M. Lipid Polymer Hybrid Nanoparticles as Potent Vehicles for Drug Delivery in Cancer Therapeutics. Med. Drug Discov. 2023, 20, 100165. [Google Scholar] [CrossRef]
- Marisa Ribeiro, A.; Estevinho, B.N.; Rocha, F. Microencapsulation of Polyphenols—The Specific Case of the Microencapsulation of Sambucus nigra L. Extracts—A Review. Trends Food Sci. Technol. 2020, 105, 454–467. [Google Scholar] [CrossRef]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
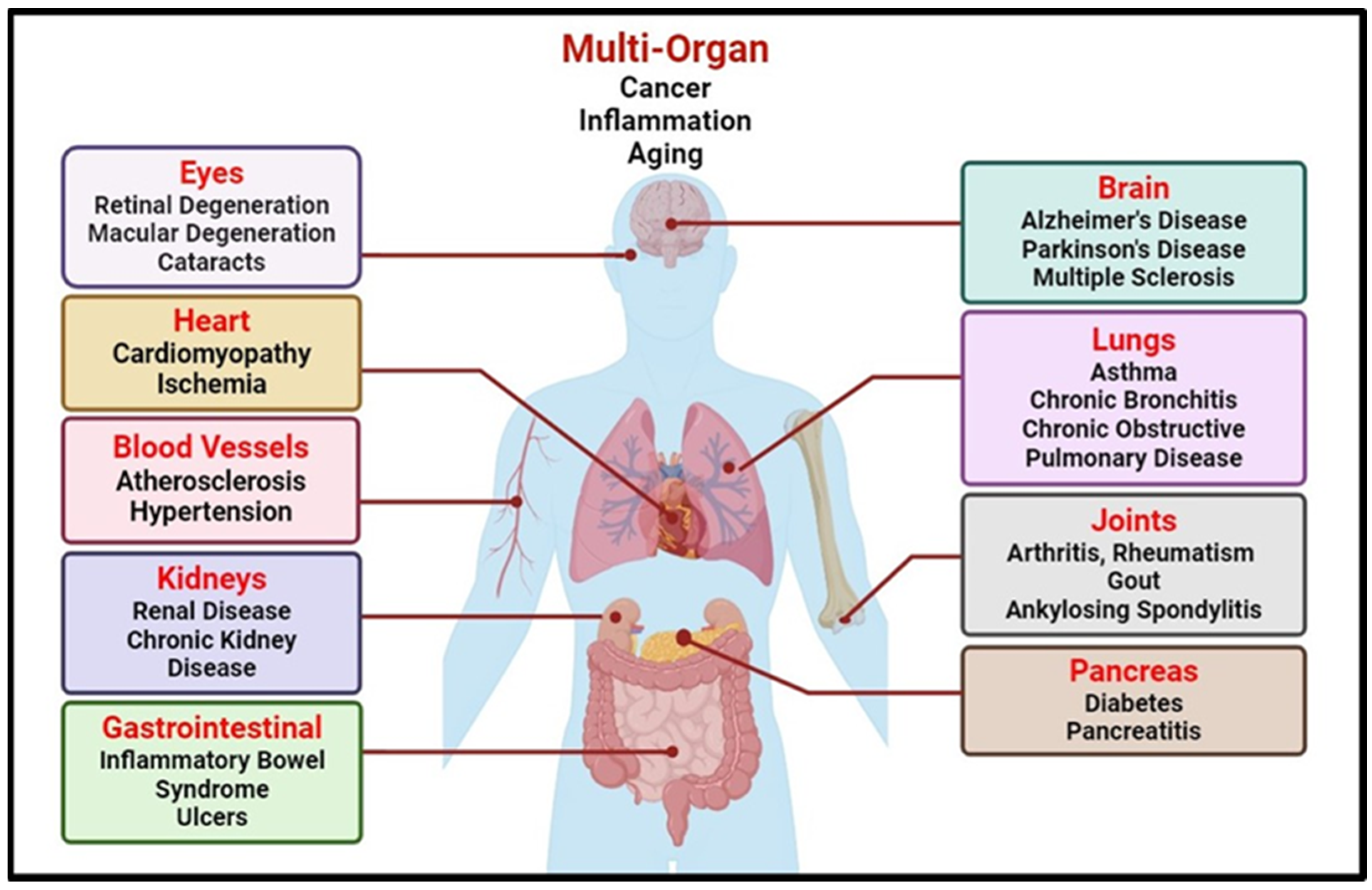
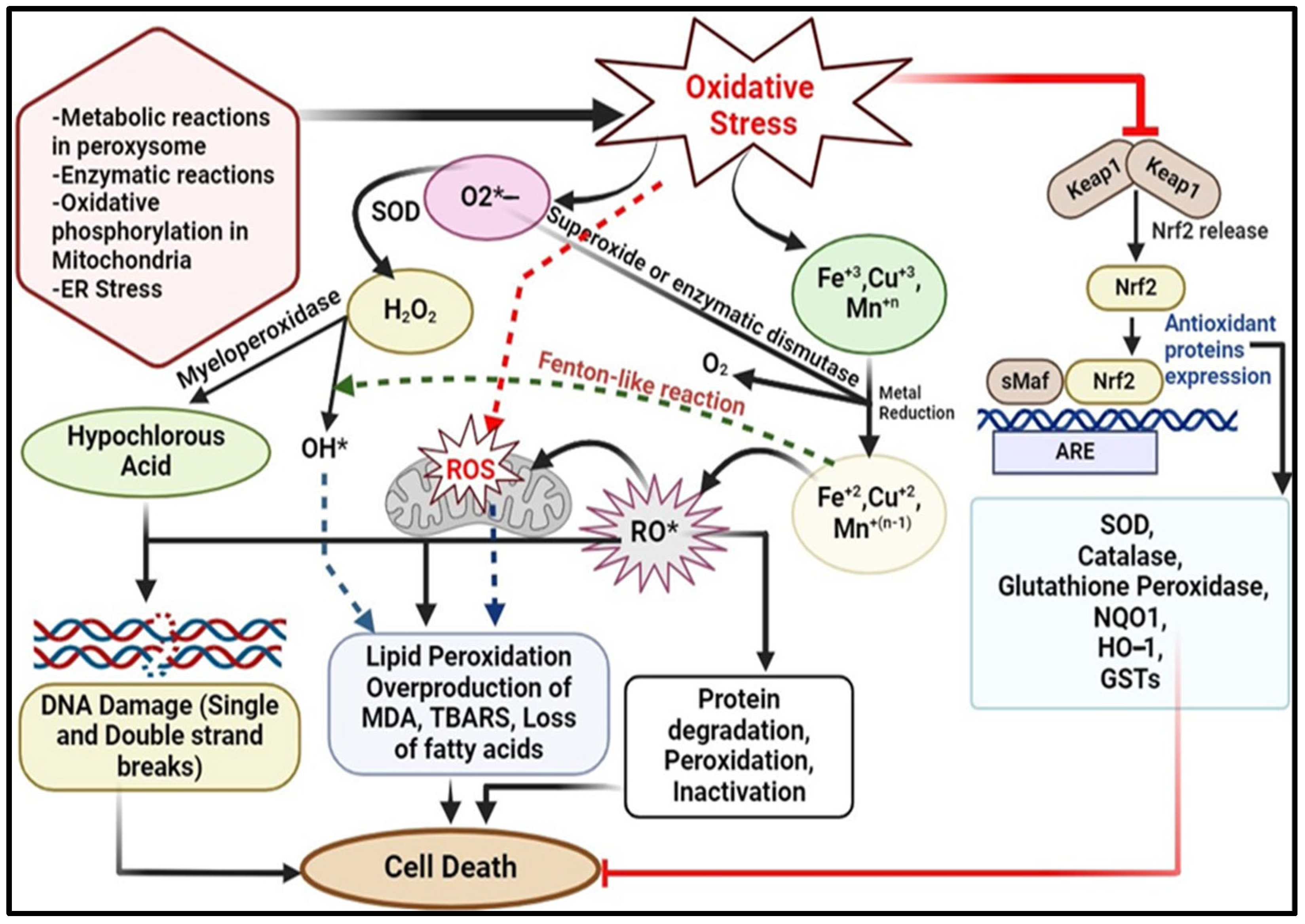



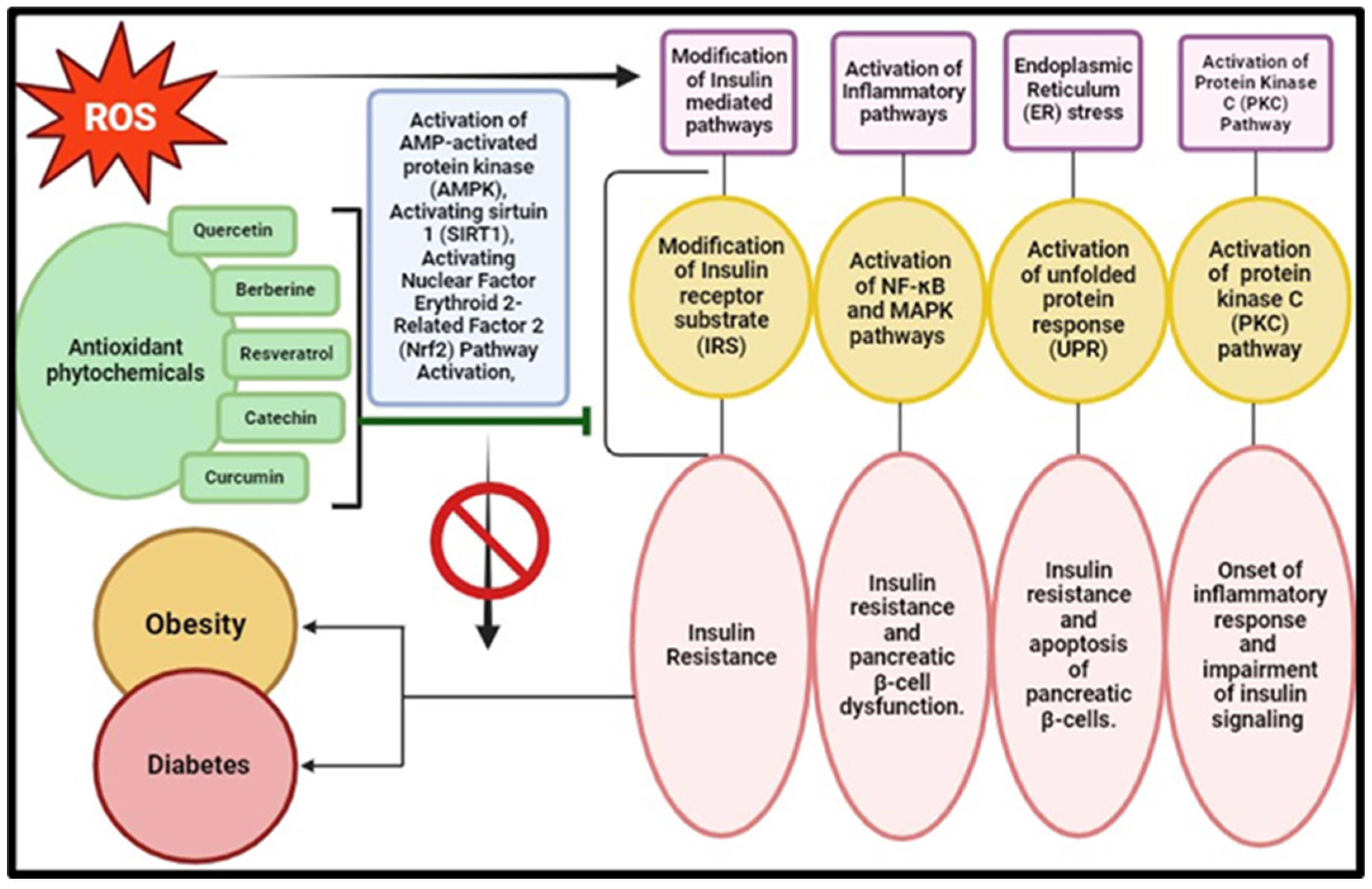
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashraf, M.V.; Khan, S.; Misri, S.; Gaira, K.S.; Rawat, S.; Rawat, B.; Khan, M.A.H.; Shah, A.A.; Asgher, M.; Ahmad, S. High-Altitude Medicinal Plants as Promising Source of Phytochemical Antioxidants to Combat Lifestyle-Associated Oxidative Stress-Induced Disorders. Pharmaceuticals 2024, 17, 975. https://doi.org/10.3390/ph17080975
Ashraf MV, Khan S, Misri S, Gaira KS, Rawat S, Rawat B, Khan MAH, Shah AA, Asgher M, Ahmad S. High-Altitude Medicinal Plants as Promising Source of Phytochemical Antioxidants to Combat Lifestyle-Associated Oxidative Stress-Induced Disorders. Pharmaceuticals. 2024; 17(8):975. https://doi.org/10.3390/ph17080975
Chicago/Turabian StyleAshraf, Mohammad Vikas, Sajid Khan, Surya Misri, Kailash S. Gaira, Sandeep Rawat, Balwant Rawat, M. A. Hannan Khan, Ali Asghar Shah, Mohd Asgher, and Shoeb Ahmad. 2024. "High-Altitude Medicinal Plants as Promising Source of Phytochemical Antioxidants to Combat Lifestyle-Associated Oxidative Stress-Induced Disorders" Pharmaceuticals 17, no. 8: 975. https://doi.org/10.3390/ph17080975
APA StyleAshraf, M. V., Khan, S., Misri, S., Gaira, K. S., Rawat, S., Rawat, B., Khan, M. A. H., Shah, A. A., Asgher, M., & Ahmad, S. (2024). High-Altitude Medicinal Plants as Promising Source of Phytochemical Antioxidants to Combat Lifestyle-Associated Oxidative Stress-Induced Disorders. Pharmaceuticals, 17(8), 975. https://doi.org/10.3390/ph17080975









