Evaluating the Anti-Osteoporotic Potential of Mediterranean Medicinal Plants: A Review of Current Evidence
Abstract
1. Introduction
2. Methodology
3. Bone Anatomy and Osteoporotic Fractures
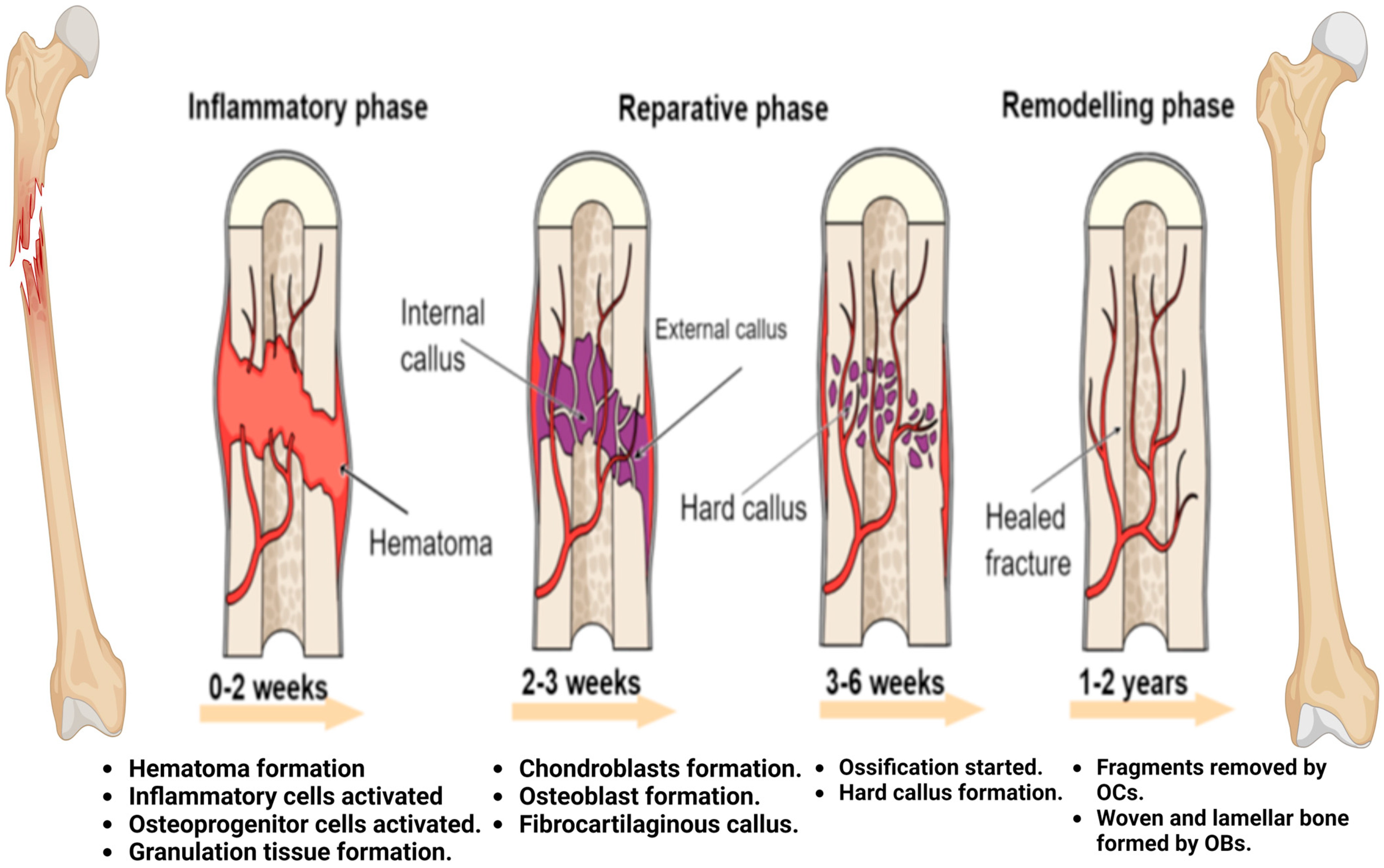
4. Fracture Healing Process
4.1. Inflammatory Phase
4.2. Callus Formation
4.3. Bone Remodeling
5. Current and Commercial Therapies in Osteoporosis
| Drugs (Anabolic Agents and Anti-Resorptive Agents) | Targets | References |
|---|---|---|
| Bisphosphonates (anti-resorptive agents): Alendronate and risedronate (short-term doses). Ibandronate and risedronate (long-term doses). | Bisphosphonate and nitrogen complex that inhibits guanosine triphosphatases (GTPases) by inhibiting mevalonate pathway components to prevent osteoclast survival. Complex of bisphosphonate without nitrogen that binds with the mineral stage to stimulate osteoclast apoptosis. | [79] |
| Denosumab (anti-resorptive effects) | Inhibits bone resorption by binding to the receptor activator of nuclear factor-κβ ligand to reduce activation of osteoclasts and bone resorption (antibody against RANKL). | [73] |
| Teriparatide and abaloparatide (anabolic agent) | Increasing bone formation rather than decreasing resorption. | [75] |
| Menopausal hormone therapy (MHT) or estrogen therapy (anabolic and anti-resorptive agent) | Stimulating osteoblasts and inhibiting osteoclasts. | [80] |
| Odanacatib (anti-resorptive agent) | Inhibit Cathepsin K (an osteoclastic enzyme that degrades collagens). | [15] |
| Saracatinib (anti-resorptive agent) | Inhibit c-src kinase (an enzyme involved in osteoclast activation). | [10] |
| BHQ 880 (anabolic activity) | Antibody against Dickkopf-1 (inhibitor of the Wnt/β-catenin pathway). | [10] |
| AMG 785 (anabolic activity) | Antibody against Sclerostin (inhibitor of the Wnt/β-catenin pathway). | [10] |
| Selective estrogen receptor modulators (SERMs): raloxifene (anti-resorptive agent) | Osteoclast inhibition. | [81] |
| Parathyroid hormone PTH (anabolic agent) | PTH binds with osteoprogenitor cells, PTHrP, and Indian Hedgehog to chondrocytes formation. PTH binds with Wnt signaling to stimulate osteoblast formation | [79] |
| Sclerostin blocking (anabolic agent) | Osteoblast formation and increased bone strength. | [82] |
6. A Historical Overview of Medicinal Plants and Osteoporosis
| Scientific Name | Family | Place of Origin | Active Component | Outcomes | References |
|---|---|---|---|---|---|
| Acanthopanax senticosus | Araliaceae | Russia and East Asia | Syringin and eleutheroside E | Increase bone mineral density (BMD). Decrease the production of osteoclasts by inhibiting the NF-kB and MAPK signaling pathways. | [98] |
| Erythrina variegate | Fabaceae | India and Southeast Asia | N. D | Increase mechanical properties and decrease bone loss. Histomorphometry analysis showed increases in the area and thickness of the trabecular region. | [99] |
| Actaea racemosa | Ranunculaceae | Eastern North America | Isopropanol and rhizomes | Inhibit osteoclastogenesis by inhibiting the NF-κB signaling pathway. Inhibit the production of TNF-a by suppressing lipopolysaccharide. | [100] |
| Asparagus racemosus | Asparagaceae | India | Carbohydrates, flavonoids, steroids, organic acids, and saponin glycosides. | Improvements in biomechanical, biochemical, and histological properties in experiments using ovariectomized (OVX) rats. | [101] |
| Epimedium | Berberidaceae | Eastern Asia | Flavonoids, lignins, diadzein, icariin, and genistein. | Improvements in bone mineral density (BMD), pain, and impact rate. Improvements in biomarkers, especially alkaline phosphatase (ALP). | [102] |
| Allium cepa | Amaryllidaceae | Iran Pakistan | Flavonoids (quercetin), and phenolic acids | Prevents bone resorption. Inhibits osteoclastogenesis. Maintains calcium in bone. Increases secretion of IL-3 and IL-4. Decreases secretion of IL-6 and TNF-α. | [103] |
| Cronus mas L. | Cornaceae | Iran | Flavonoids (quercetin), and kaempferol. | Enhances osteoblastic bone formation-related genes such as RUNX2 and ALP. Suppresses bone-resorption-related genes such as Ctsk, Acp5, and Nfatc1. | [104] |
| Fructus Malvae Verticillatae | Malvaceae | Eastern Asia. | Polysaccharides and flavonoids. | Anti-resorptive agent (inhibits osteoclasts by suppressing RANKL). Inhibits all markers that relate to the proliferation and differentiation of osteoclast. | [105] |
| Cissus quadrangularis | Vitaceae | India | Vitamin C, triterpenes, and flavonoids (quercetin). | Improves fracture healing by enhancing mineral absorption and metabolism. | [106] |
7. Mediterranean Medicinal Plants with Anti-Osteoporosis Effects
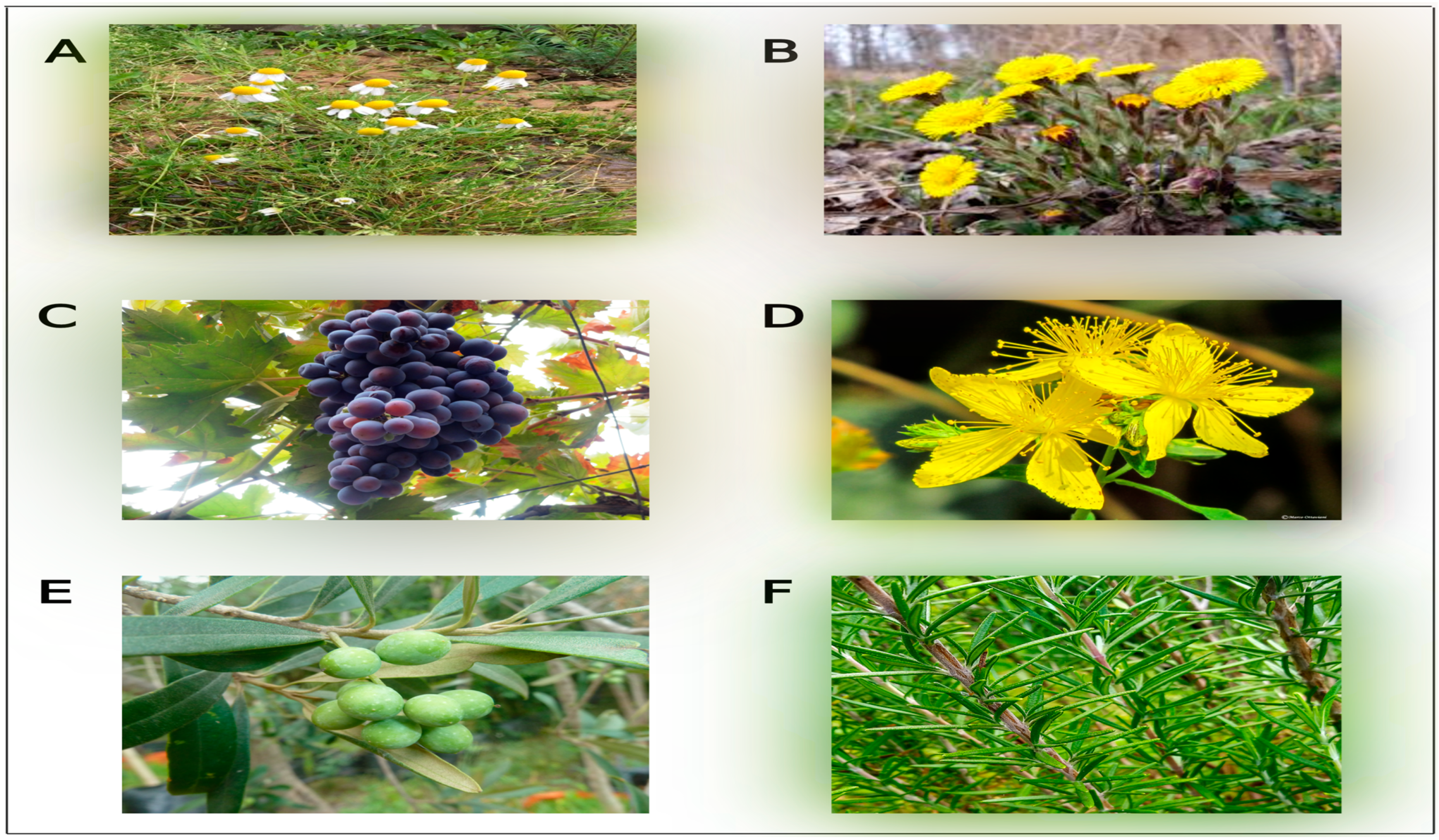
7.1. Ammi majus
7.2. Brassica oleracea
7.3. Ceratonia siliqua L.
7.4. Foeniculum vulgare
7.5. Licorice
7.6. Salvia officinalis
7.7. Silybum marianum
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Reznikov, N.; Shahar, R.; Weiner, S. Bone hierarchical structure in three dimensions. Acta Biomater. 2014, 10, 3815–3826. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, P.; Jagadeesan, R.; Sekaran, S.; Dhanasekaran, A.; Vimalraj, S. Flavonoids: Classification, Function, and Molecular Mechanisms Involved in Bone Remodelling. Front. Endocrinol. 2021, 12, 779638. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.; Body, J.J.; Aapro, M.; Hadji, P.; Herrstedt, J.; ESMO Guidelines Working Group. Bone health in cancer patients: ESMO clinical practice guidelines. Ann. Oncol. 2014, 25, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, H.K.; Shrestha, S.; Rokka, K.; Shakya, R. Vitamin D, Calcium, Parathyroid Hormone, and Sex Steroids in Bone Health and Effects of Aging. J. Osteoporos. 2020, 2020, 9324505. [Google Scholar] [CrossRef] [PubMed]
- Karpouzos, A.; Diamantis, E.; Farmaki, P.; Savvanis, S.; Troupis, T. Nutritional Aspects of Bone Health and Fracture Healing. J. Osteoporos. 2017, 2017, 4218472. [Google Scholar] [CrossRef]
- Pelletier, S.; Chapurlat, R. Optimizing bone health in chronic kidney disease. Maturitas 2010, 65, 325–333. [Google Scholar] [CrossRef]
- Fardellone, P.; Salawati, E.; Le Monnier, L.; Goëb, V. Bone loss, osteoporosis, and fractures in patients with rheumatoid arthritis: A review. J. Clin. Med. 2020, 9, 3361. [Google Scholar] [CrossRef]
- Sheng, B.; Li, X.; Nussler, A.K.; Zhu, S. The relationship between healthy lifestyles and bone health: A narrative review. Medicine 2021, 100, E24684. [Google Scholar] [CrossRef]
- Bigham-Sadegh, A.; Oryan, A. Basic concepts regarding fracture healing and the current options and future directions in managing bone fractures. Int. Wound J. 2015, 12, 238–247. [Google Scholar] [CrossRef]
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef]
- Fu, C.; Shi, R. Osteoclast biology in bone resorption: A review. STEMedicine 2020, 1, e57. [Google Scholar] [CrossRef]
- Compston, J.; McClung, M.; Leslie, W. Osteoporosis seminar 2019. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Iantomasi, T.; Romagnoli, C.; Palmini, G.; Donati, S.; Falsetti, I.; Miglietta, F.; Aurilia, C.; Marini, F.; Giusti, F.; Brandi, M.L. Oxidative Stress and Inflammation in Osteoporosis: Molecular Mechanisms Involved and the Relationship with microRNAs. Int. J. Mol. Sci. 2023, 24, 3772. [Google Scholar] [CrossRef] [PubMed]
- Ayub, N.; Faraj, M.; Ghatan, S.; Reijers, J.A.A.; Napoli, N.; Oei, L. The treatment gap in osteoporosis. J. Clin. Med. 2021, 10, 3002. [Google Scholar] [CrossRef]
- Boggild, M.K.; Gajic-Veljanoski, O.; McDonald-Blumer, H.; Ridout, R.; Tile, L.; Josse, R.; Cheung, A.M. Odanacatib for the treatment of osteoporosis. Expert Opin. Pharmacother. 2015, 16, 1717–1726. [Google Scholar] [CrossRef]
- Eguia, A.; Bagan, L.; Cardona, F. Review and update on drugs related to the development of osteonecrosis of the jaw. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e71–e83. [Google Scholar] [CrossRef]
- Pan, T.-Y.; Chang, C.-C.; Chen, H.-T.; Tsou, H.-K.; Lin, Y.-C.; Hsu, C.-H. Effectiveness of Teriparatide for Spine Fusion in Osteoporotic Patient: A Systematic Review and Meta-Analysis of Comparative Studies. World Neurosurg. 2023, 179, 8–17. [Google Scholar] [CrossRef]
- Shirwaikar, A.; Khan, S.; Kamariya, Y.H.; Patel, B.D.; Gajera, F.P. Medicinal Plants for the Management of Post Menopausal Osteoporosis: A Review. Open Bone J. 2010, 2, 1–13. [Google Scholar] [CrossRef]
- Baertl, S.; Metsemakers, W.J.; Morgenstern, M.; Alt, V.; Richards, R.G.; Moriarty, T.F.; Young, K. Fracture-related infection. Bone Jt. Res. 2021, 10, 351–353. [Google Scholar] [CrossRef]
- Nuwaha, F.; Musinguzi, G. Use of alternative medicine for hypertension in Buikwe and Mukono districts of Uganda: A cross sectional study. BMC Complement. Altern. Med. 2013, 13, 2–7. [Google Scholar] [CrossRef]
- Rabito, M.J.; Kaye, A.D. Complementary and alternative medicine and cardiovascular disease: An evidence-based review. Evid.-Based Complement. Altern. Med. 2013, 2013, 672097. [Google Scholar] [CrossRef] [PubMed]
- Al-garawyi, A.M.A.; Hussein, T.A.; Jassim, M.M.A. Inhibition of viral infection by using of natural herbal remedies as alternative treatment. Syst. Rev. Pharm. 2020, 11, 416–419. [Google Scholar] [CrossRef]
- Martín Ortega, A.M.; Segura Campos, M.R. Bioactive Compounds as Therapeutic Alternatives. In Bioactive Compounds; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Moghadam, E.T.; Yazdanian, M.; Tahmasebi, E.; Tebyanian, H.; Ranjbar, R.; Yazdanian, A.; Seifalian, A.; Tafazoli, A. Current herbal medicine as an alternative treatment in dentistry: In vitro, in vivo and clinical studies. Eur. J. Pharmacol. 2020, 889, 173665. [Google Scholar] [CrossRef]
- Dehyab, A.S.; Bakar, M.F.A.; AlOmar, M.K.; Sabran, S.F. A review of medicinal plant of Middle East and North Africa (MENA) region as source in tuberculosis drug discovery. Saudi J. Biol. Sci. 2020, 27, 2457–2478. [Google Scholar] [CrossRef]
- Mesraoua, B.; Kissani, N.; Deleu, D.; Elsheikh, L.; Ali, M.; Melikyan, G.; Al Hail, H.; Wiebe, S.; Asadi-Pooya, A.A. Complementary and alternative medicine (CAM) for epilepsy treatment in the Middle East and North Africa (MENA) region. Epilepsy Res. 2021, 170, 106538. [Google Scholar] [CrossRef]
- González-Tejero, M.R.; Casares-Porcel, M.; Sánchez-Rojas, C.P.; Ramiro-Gutiérrez, J.M.; Molero-Mesa, J.; Pieroni, A.; Giusti, M.E.; Censorii, E.; de Pasquale, C.; Della, A.; et al. Medicinal plants in the Mediterranean area: Synthesis of the results of the project Rubia. J. Ethnopharmacol. 2008, 116, 341–357. [Google Scholar] [CrossRef]
- Mssillou, I.; Bakour, M.; Slighoua, M.; Laaroussi, H.; Saghrouchni, H.; Ez-Zahra Amrati, F.; Lyoussi, B.; Derwich, E. Investigation on wound healing effect of Mediterranean medicinal plants and some related phenolic compounds: A review. J. Ethnopharmacol. 2022, 298, 115663. [Google Scholar] [CrossRef]
- Carrubba, A.; Scalenghe, R. The scent of Mare Nostrum: Medicinal and aromatic plants in Mediterranean soils. J. Sci. Food Agric. 2012, 92, 1150–1170. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Amromanoh, O. Bone Organic-Inorganic Phase Ratio Is a Fundamental Determinant of Bone Material Quality. Appl. Bionics Biomech. 2021, 2021, 4928396. [Google Scholar] [CrossRef]
- Ralston, S.H. Bone structure and metabolism. Medicine 2017, 45, 560–564. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, S.; Chen, W.; Hu, Y.; Geng, Z.; Su, J. Bone/cartilage targeted hydrogel: Strategies and applications. Bioact. Mater. 2023, 23, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Hart, N.H.; Newton, R.U.; Tan, J.; Rantalainen, T.; Chivers, P.; Siafarikas, A.; Nimphius, S. Biological basis of bone strength: Anatomy, physiology and measurement. J. Musculoskelet. Neuronal Interact. 2020, 20, 347–371. [Google Scholar] [PubMed]
- Oftadeh, R.; Perez-Viloria, M.; Villa-Camacho, J.C.; Vaziri, A.; Nazarian, A. Biomechanics and Mechanobiology of Trabecular Bone: A Review. J. Biomech. Eng. 2015, 137, 010802. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hernandez, M.D.L.L.; Rangel-Moreno, J.; Garcia-Castaneda, M.; Kenney, H.M.; Paine, A.; Thullen, M.; Anandarajah, A.P.; Schwarz, E.M.; Dirksen, R.T.; Ritchlin, C.T. Dendritic cell-specific transmembrane protein is required for synovitis and bone resorption in inflammatory arthritis. Front. Immunol. 2022, 13, 1026574. [Google Scholar] [CrossRef] [PubMed]
- Marcadet, L.; Bouredji, Z.; Argaw, A.; Frenette, J. The Roles of RANK/RANKL/OPG in Cardiac, Skeletal, and Smooth Muscles in Health and Disease. Front. Cell Dev. Biol. 2022, 10, 903657. [Google Scholar] [CrossRef]
- Fujii, T.; Murata, K.; Mun, S.H.; Bae, S.; Lee, Y.J.; Pannellini, T.; Kang, K.; Oliver, D.; Park-Min, K.H.; Ivashkiv, L.B. MEF2C regulates osteoclastogenesis and pathologic bone resorption via c-FOS. Bone Res. 2021, 9, 4. [Google Scholar] [CrossRef]
- Tan, E.M.; Li, L.; Indran, I.R.; Chew, N.; Yong, E.L. TRAF6 Mediates Suppression of Osteoclastogenesis and Prevention of Ovariectomy-Induced Bone Loss by a Novel Prenylflavonoid. J. Bone Miner. Res. 2017, 32, 846–860. [Google Scholar] [CrossRef]
- Mun, S.H.; Park, P.S.U.; Park-Min, K.H. The M-CSF receptor in osteoclasts and beyond. Exp. Mol. Med. 2020, 52, 1239–1254. [Google Scholar] [CrossRef]
- Chu, A.; Zirngibl, R.A.; Manolson, M.F. The V-ATPase a3 subunit: Structure, function and therapeutic potential of an essential biomolecule in osteoclastic bone resorption. Int. J. Mol. Sci. 2021, 22, 6934. [Google Scholar] [CrossRef]
- Kang, S.; Kang, Y.K.; Lee, J.A.; Kim, D.H.; Lim, J.S. A case of autosomal dominant osteopetrosis type 2 with a CLCN7 gene mutation. JCRPE J. Clin. Res. Pediatr. Endocrinol. 2019, 11, 439–443. [Google Scholar] [CrossRef]
- Dai, R.; Wu, Z.; Chu, H.Y.; Lu, J.; Lyu, A.; Liu, J.; Zhang, G. Cathepsin K: The Action in and Beyond Bone. Front. Cell Dev. Biol. 2020, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Whole Aspect of Runx2 Functions in Skeletal Development. Int. J. Mol. Sci. 2022, 23, 5776. [Google Scholar] [CrossRef]
- Liu, Q.; Li, M.; Wang, S.; Xiao, Z.; Xiong, Y.; Wang, G. Recent Advances of Osterix Transcription Factor in Osteoblast Differentiation and Bone Formation. Front. Cell Dev. Biol. 2020, 8, 601224. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhu, K.; Lai, Y.; Zhao, Z.; Fan, J.; Im, H.-J.; Chen, D.; Xiao, G. ATF4 Promotes β-Catenin Expression and Osteoblastic Differentiation of Bone Marrow Mesenchymal Stem Cells. Int. J. Biol. Sci. 2013, 9, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, E.; Eggers, B.; Heim, N.; Kramer, F.J.; Nokhbehsaim, M.; Götz, W. Bevacizumab and sunitinib mediate osteogenic and pro-inflammatory molecular changes in primary human alveolar osteoblasts in vitro. Odontology 2022, 110, 634–647. [Google Scholar] [CrossRef]
- Komori, T. Functions of osteocalcin in bone, pancreas, testis, and muscle. Int. J. Mol. Sci. 2020, 21, 7513. [Google Scholar] [CrossRef]
- Whyte, M.P.; Mumm, S.; Baker, J.C.; Zhang, F.; Sedighi, H.; Duan, S.; Cundy, T. LRP6 High Bone Mass Characterized in Two Generations Harboring a Unique Mutation of Low-Density Lipoprotein Receptor-Related Protein 6. JBMR Plus 2023, 7, e10717. [Google Scholar] [CrossRef]
- Lewiecki, E.M. Role of sclerostin in bone and cartilage and its potential as a therapeutic target in bone diseases. Ther. Adv. Musculoskelet. Dis. 2014, 6, 48–57. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, W.; Masson, A.; Li, Y.P. Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis. In Cell Discovery; Springer: New York, NY, USA, 2024; Volume 10, ISBN 4142102400. [Google Scholar]
- Mitchell, S.A.T.; Majuta, L.A.; Mantyh, P.W. New Insights in Understanding and Treating Bone Fracture Pain. Curr. Osteoporos. Rep. 2018, 16, 325–332. [Google Scholar] [CrossRef]
- Sabet, F.A.; Raeisi Najafi, A.; Hamed, E.; Jasiuk, I. Modelling of bone fracture and strength at different length scales: A review. Interface Focus 2016, 6, 20–30. [Google Scholar] [CrossRef]
- Singh, S.; Foster, R.; Khan, K.M. Accident or osteoporosis? Survey of community follow-up after low-trauma fracture. Can. Fam. Physician 2011, 57, 128–133. [Google Scholar]
- Bouvard, B.; Annweiler, C.; Legrand, E. Osteoporosis in older adults. Jt. Bone Spine 2021, 88, 105135. [Google Scholar] [CrossRef]
- Oryan, A.; Monazzah, S.; Bigham-Sadegh, A. Bone injury and fracture healing biology. Biomed. Environ. Sci. 2015, 28, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Lorentzon, M.; Johansson, H.; Harvey, N.C.; Liu, E.; Vandenput, L.; McCloskey, E.V.; Kanis, J.A. Osteoporosis and fractures in women: The burden of disease. Climacteric 2022, 25, 4–10. [Google Scholar] [CrossRef]
- Winston, K.R. Cranial Bone: Anatomy and Healing. In Plastic Neurosurgery; Springer International Publishing: Cham, Switzerland, 2023; pp. 191–219. [Google Scholar] [CrossRef]
- Gorter, E.A.; Reinders, C.R.; Krijnen, P.; Appelman-Dijkstra, N.M.; Schipper, I.B. The effect of osteoporosis and its treatment on fracture healing a systematic review of animal and clinical studies. Bone Rep. 2021, 15, 101117. [Google Scholar] [CrossRef]
- Schmidt-Bleek, K.; Schell, H.; Schulz, N.; Hoff, P.; Perka, C.; Buttgereit, F.; Volk, H.D.; Lienau, J.; Duda, G.N. Inflammatory phase of bone healing initiates the regenerative healing cascade. Cell Tissue Res. 2012, 347, 567–573. [Google Scholar] [CrossRef]
- Ghiasi, M.S.; Chen, J.; Vaziri, A.; Rodriguez, E.K.; Nazarian, A. Bone fracture healing in mechanobiological modeling: A review of principles and methods. Bone Rep. 2017, 6, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Bastian, O.; Pillay, J.; Alblas, J.; Leenen, L.; Koenderman, L.; Blokhuis, T. Systemic inflammation and fracture healing. J. Leukoc. Biol. 2011, 89, 669–673. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Hak, D.; Sanders, D.; Donohoe, E.; Tosounidis, T.; Bahney, C. Inflammation, bone healing, and anti-inflammatory drugs: An update. J. Orthop. Trauma 2015, 29, S6–S9. [Google Scholar] [CrossRef]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.H.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef]
- Cheung, W.H.; Miclau, T.; Chow, S.K.H.; Yang, F.F.; Alt, V. Fracture healing in osteoporotic bone. Injury 2016, 47, S21–S26. [Google Scholar] [CrossRef] [PubMed]
- Arazi, M.; Canbora, M.K. Fracture healing. In Musculoskeletal Research and Basic Science; Elsevier Inc.: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Kostenuik, P.; Mirza, F.M. Fracture healing physiology and the quest for therapies for delayed healing and nonunion. J. Orthop. Res. 2017, 35, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Bahney, C.S.; Zondervan, R.L.; Allison, P.; Theologis, A.; Ashley, J.W.; Ahn, J.; Miclau, T.; Marcucio, R.S.; Hankenson, K.D. Cellular biology of fracture healing. J. Orthop. Res. 2019, 37, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Lipphaus, A.; Witzel, U. Finite-Element Syntheses of Callus and Bone Remodeling: Biomechanical Study of Fracture Healing in Long Bones. Anat. Rec. 2018, 301, 2112–2121. [Google Scholar] [CrossRef]
- Ramirez-GarciaLuna, J.L.; Rangel-Berridi, K.; Olasubulumi, O.O.; Rosenzweig, D.H.; Henderson, J.E.; Gawri, R.; Martineau, P.A. Enhanced Bone Remodeling After Fracture Priming. Calcif. Tissue Int. 2022, 110, 349–366. [Google Scholar] [CrossRef]
- Li, H.; Xiao, Z.; Quarles, L.D.; Li, W. Osteoporosis: Mechanism, Molecular Target and Current Status on Drug Development. Curr. Med. Chem. 2022, 28, 1489–1507. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Pepe, J.; Napoli, N.; Palermo, A.; Magopoulos, C.; Khan, A.A.; Zillikens, M.C.; Body, J.J. Response to Letter to the Editor From Taguchi: “Osteonecrosis of the Jaw and Antiresorptive Agents in Benign and Malignant Diseases: A Critical Review Organized by the ECTS”. J. Clin. Endocrinol. Metab. 2022, 107, E2651–E2652. [Google Scholar] [CrossRef]
- Rogers, M.J.; Mönkkönen, J.; Munoz, M.A. Molecular mechanisms of action of bisphosphonates and new insights into their effects outside the skeleton. Bone 2020, 139, 115493. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Q.; Yan, G.; Jin, X. Denosumab compared to bisphosphonates to treat postmenopausal osteoporosis: A meta-analysis. J. Orthop. Surg. Res. 2018, 13, 194. [Google Scholar] [CrossRef]
- De Mattia, G.; Maffi, M.; Mazzantini, M. Anabolic treatment for osteoporosis and fragility fracture risk: One year in review 2024. Clin. Exp. Rheumatol. 2024, 42, 1311–1316. [Google Scholar] [CrossRef]
- Haas, A.V.; LeBoff, M.S. Osteoanabolic agents for osteoporosis. J. Endocr. Soc. 2018, 2, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.; Gregson, C.L.; Tobias, J.H. Anabolic treatments for osteoporosis in postmenopausal women. Fac. Rev. 2021, 10, 44. [Google Scholar] [CrossRef]
- Skjødt, M.K.; Frost, M.; Abrahamsen, B. Side effects of drugs for osteoporosis and metastatic bone disease. Br. J. Clin. Pharmacol. 2019, 85, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Boy, H.I.A.; Rutilla, A.J.H.; Santos, K.A.; Ty, A.M.T.; Yu, A.I.; Mahboob, T.; Tangpoong, J.; Nissapatorn, V. Recommended Medicinal Plants as Source of Natural Products: A Review. Digit. Chin. Med. 2018, 1, 131–142. [Google Scholar] [CrossRef]
- Buza, J.A.; Einhorn, T. Bone healing in 2016. Clin. Cases Miner. Bone Metab. 2016, 13, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Yong, E.L.; Logan, S. Logan Susan Menopausal osteoporosis: Screening, prevention and treatment. Singap. Med. J. 2021, 62, 156–166. [Google Scholar] [CrossRef]
- Burr, D.B.; Phipps, R. Selective Estrogen Receptor Modulators (SERMs). In Osteoporotic Fracture and Systemic Skeletal Disorders: Mechanism, Assessment, and Treatment; Springer: Singapore, 2021; pp. 399–411. [Google Scholar] [CrossRef]
- Larsson, S.; Fazzalari, N.L. Anti-osteoporosis therapy and fracture healing. Arch. Orthop. Trauma Surg. 2014, 134, 291–297. [Google Scholar] [CrossRef]
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-Khoei, H. Medicinal plants: Past history and future perspective. J. Herbmed Pharmacol. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Fitzgerald, M.; Heinrich, M.; Booker, A. Medicinal plant analysis: A historical and regional discussion of emergent complex techniques. Front. Pharmacol. 2019, 10, 1480. [Google Scholar] [CrossRef]
- Lowe, H.; Steele, B.; Bryant, J.; Fouad, E.; Toyang, N.; Ngwa, W. Antiviral activity of jamaican medicinal plants and isolated bioactiv compounds. Molecules 2021, 26, 607. [Google Scholar] [CrossRef]
- Noor, F.; Tahir ul Qamar, M.; Ashfaq, U.A.; Albutti, A.; Alwashmi, A.S.; Aljasir, M.A. Network Pharmacology Approach for Medicinal Plants: Review and Assessment. Pharmaceuticals 2022, 15, 572. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Mah, J.; Amirkia, V. Alkaloids used as medicines: Structural phytochemistry meets biodiversity—An update and forward look. Molecules 2021, 26, 1836. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A Comprehensive Review on the Biological, Agricultural and Pharmaceutical Properties of Secondary Metabolites Based-Plant Origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef]
- Sytar, O.; Smetanska, I. Special Issue “Bioactive Compounds from Natural Sources (2020, 2021)”. Molecules 2022, 27, 1929. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Azwanida, N.N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 3–8. [Google Scholar] [CrossRef]
- Al-Ajalein, A.H.A.S.; Shafie, M.H.; Yap, P.G.; Kassim, M.A.; Naharudin, I.; Wong, T.W.; Gan, C.Y. Microwave-assisted extraction of polysaccharide from Cinnamomum cassia with anti-hyperpigmentation properties: Optimization and characterization studies. Int. J. Biol. Macromol. 2023, 226, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Eddouks, M.; Chattopadhyay, D.; De Feo, V.; Cho, W.C.S. Medicinal plants in the prevention and treatment of chronic diseases 2013. Evid.-Based Complement. Altern. Med. 2014, 2014, 180981. [Google Scholar] [CrossRef]
- Curate, F. Osteoporosis and paleopathology: A review. J. Anthropol. Sci. 2014, 92, 119–146. [Google Scholar] [CrossRef]
- Liu, M.J.; Li, Y.; Pan, J.H.; Liu, H.; Wang, S.J.; Teng, J.R.; Zhao, H.Y.; Ju, D.H. Effects of zuogui pill on Wnt singal transduction in rats with glucocorticoid-induced osteoporosis. J. Tradit. Chin. Med. 2011, 31, 98–102. [Google Scholar] [CrossRef][Green Version]
- Yin, H.; Wang, S.; Zhang, Y.; Wu, M.; Wang, J.W.; Ma, Y. Zuogui Pill improves the dexamethasone-induced osteoporosis progression in zebrafish larvae. Biomed. Pharmacother. 2018, 97, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, K.; Qi, B.; Feng, G.; Wang, W.; Sun, Q.; Zheng, C.; Wei, X.; Jia, Y. An evaluation of the effects and safety of Zuogui pill for treating osteoporosis: Current evidence for an ancient Chinese herbal formula. Phyther. Res. 2021, 35, 1754–1767. [Google Scholar] [CrossRef]
- Eun, S.Y.; Cheon, Y.H.; Do Park, G.; Chung, C.H.; Lee, C.H.; Kim, J.Y.; Lee, M.S. Anti-osteoporosis effects of the eleutherococcus senticosus, achyranthes japonica, and atractylodes japonica mixed extract fermented with nuruk. Nutrients 2021, 13, 3904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Li, X.; Wan, H.Y.; Wong, M.S. Erythrina variegata extract exerts osteoprotective effects by suppression of the process of bone resorption. Br. J. Nutr. 2010, 104, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.M.; Bayat, M.; Rahimi, R. Plant-derived natural medicines for the management of osteoporosis: A comprehensive review of clinical trials. J. Tradit. Complement. Med. 2024, 14, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chitme, H.R.; Muchandi, I.S.; Burli, S.C. Effect of Asparagus Racemosus Willd Root Extract on Ovariectomized Rats. Open Nat. Prod. J. 2009, 2, 16–23. [Google Scholar] [CrossRef]
- Shi, S.; Wang, F.; Huang, Y.; Chen, B.; Pei, C.; Huang, D.; Wang, X.; Wang, Y.; Kou, S.; Li, W.; et al. Epimedium for Osteoporosis Based on Western and Eastern Medicine: An Updated Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 782096. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, X.; Sun, K.; Guo, J.; Zhao, J.; Dong, Y.; Bao, Y. Onion (Allium cepa L.) Flavonoid Extract Ameliorates Osteoporosis in Rats Facilitating Osteoblast Proliferation and Differentiation in MG-63 Cells and Inhibiting RANKL-Induced Osteoclastogenesis in RAW 264.7 Cells. Int. J. Mol. Sci. 2024, 25, 6754. [Google Scholar] [CrossRef]
- Park, E.; Sozański, T.; Lee, C.G.; Kucharska, A.Z.; Przybylska, D.; Piórecki, N.; Jeong, S.Y. A Comparison of the Antiosteoporotic Effects of Cornelian Cherry (Cornus mas L.) Extracts from Red and Yellow Fruits Containing Different Constituents of Polyphenols and Iridoids in Osteoblasts and Osteoclasts. Oxid. Med. Cell. Longev. 2022, 2022, 4122253. [Google Scholar] [CrossRef]
- Xue, N.; Jia, L.; Li, Q. Potential of natural medicines for treatment of osteoporosis: A narrative review. J. Tradit. Chin. Med. 2023, 43, 198–204. [Google Scholar] [CrossRef]
- Kaur, J.; Sharma, G.; Mahajan, A.; Katare, O.P.; Bhadada, S.K.; Ghoshal, G. Role of Cissus quadrangularis in the Management of Osteoporosis: An Overview. Crit. Rev. Ther. Drug Carrier Syst. 2021, 38, 27–51. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, C.; Shah, O.P.; Chigurupati, S.; Ashokan, B.; Meerasa, S.S.; Rashid, S.; Behl, T.; Bungau, S.G. Understanding the mechanistic potential of plant based phytochemicals in management of postmenopausal osteoporosis. Biomed. Pharmacother. 2023, 163, 114850. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, J.; Chen, F.; Liu, T.; Li, J.; Jiang, L.; Jia, Y.; Hu, C.; Gao, Z.; Gan, C.; et al. Acanthopanax senticosus aqueous extract ameliorates ovariectomy-induced bone loss in middle-aged mice by inhibiting the receptor activator of nuclear factor-κB ligand-induced osteoclastogenesis. Food Funct. 2020, 11, 9696–9709. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Hu, X.; Li, T.; Wu, G.; Dou, P.; Ouyang, Z. Cimifugin Suppresses NF-κB Signaling to Prevent Osteoclastogenesis and Periprosthetic Osteolysis. Front. Pharmacol. 2021, 12, 724256. [Google Scholar] [CrossRef]
- Azam, Z.; Pandey, V.; Gupta, N.; Sapra, L.; Dar, H.Y.; Shokeen, N.; Bhardwaj, A.; Ahmad, A.; Soni, V.; Srivastava, R.K. Phytoconstituents as novel osteo-protective agents: Implications in bone health. Front. Biosci.—Landmark 2020, 25, 1259–1296. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, S.; Singh, S.; Mishra, C.; Tripathi, D.; SK Verma, V. Estrogenic Effect of Asparagus racemosus, Cissus quadrangularis, Punica granatum and Pueraria tuberosa in Post-menopausal Syndrome. Pharmacogn. Res. 2021, 13, 238–245. [Google Scholar] [CrossRef]
- Dou, C.; Chen, Y.; Ding, N.; Li, N.; Jiang, H.; Zhao, C.; Kang, F.; Cao, Z.; Quan, H.; Luo, F.; et al. Xanthotoxin prevents bone loss in ovariectomized mice through the inhibition of RANKL-induced osteoclastogenesis. Osteoporos. Int. 2016, 27, 2335–2344. [Google Scholar] [CrossRef]
- Ham, J.R.; Choi, R.Y.; Yee, S.T.; Hwang, Y.H.; Kim, M.J.; Lee, M.K. Methoxsalen supplementation attenuates bone loss and inflammatory response in ovariectomized mice. Chem. Biol. Interact. 2017, 278, 135–140. [Google Scholar] [CrossRef]
- Kang, I.S.; Agidigbi, T.S.; Kwon, Y.M.; Kim, D.G.; Kim, R.I.; In, G.; Lee, M.H.; Kim, C. Effect of co-administration of panax ginseng and brassica oleracea on postmenopausal osteoporosis in ovariectomized mice. Nutrients 2020, 12, 2415. [Google Scholar] [CrossRef]
- Laaraj, S.; Salmaoui, S.; Addi, M.; El-Rhouttais, C.; Tikent, A.; Elbouzidi, A.; Taibi, M.; Hano, C.; Noutfia, Y.; Elfazazi, K. Carob (Ceratonia siliqua L.) Seed Constituents: A Comprehensive Review of Composition, Chemical Profile, and Diverse Applications. J. Food Qual. 2023, 2023, 3438179. [Google Scholar] [CrossRef]
- Sharaf, H.; Shaffie, N.; Morsy, F.; Badawi, M.; Abbas, N. Role of some phytoestrogens in recovering bone loss: Histological results from experimental ovariectomized rat models. J. Arab. Soc. Med. Res. 2015, 10, 65. [Google Scholar] [CrossRef]
- Neri, A.A.; Galanis, D.; Galanos, A.; Pepe, A.E.; Soultanis, K.; Zervas, A.; Zoitsis, S.; Kourkoulis, S.K.; Pasiou, E.D.; Vontzalidou, A.; et al. The Effect of Ceratonia siliqua Supplement on Bone Mineral Density in Ovariectomy-induced Osteoporosis in Rats. In Vivo 2023, 37, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, H.J.; Lee, S.H.; Kim, S.Y. Potent inhibitory effect of Foeniculum vulgare Miller extract on osteoclast differentiation and ovariectomy-induced bone loss. Int. J. Mol. Med. 2012, 29, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Zhou, L.; Zheng, G.; Zheng, X.; Chen, Z.; He, W.; Wei, Q. A novel Glycyrrhiza glabra extract liquiritin targeting NFATc1 activity and ROS levels to counteract ovariectomy-induced osteoporosis and bone loss in murine model. Front. Pharmacol. 2023, 14, 1287827. [Google Scholar] [CrossRef]
- Galanis, D.; Soultanis, K.; Lelovas, P.; Zervas, A.; Papadopoulos, P.; Galanos, A.; Argyropoulou, K.; Makropoulou, M.; Patsaki, A.; Passali, C.; et al. Protective effect of Glycyrrhiza glabra roots extract on bone mineral density of ovariectomized rats. Biomed. 2019, 9, 2–12. [Google Scholar] [CrossRef]
- Kaczmarczyk-Sedlak, I.; Wojnar, W.; Zych, M.; Ozimina-Kamińska, E.; Taranowicz, J.; Siwek, A. Effect of formononetin on mechanical properties and chemical composition of bones in rats with ovariectomy-induced osteoporosis. Evid.-Based Complement. Altern. Med. 2013, 2013, 457052. [Google Scholar] [CrossRef]
- Abd El-Motelp, B.A.; Ebrahim, M.T.; Mohamed, H.K. Salvia officinalis Extract and 17 β -Estradiol Suppresses Ovariectomy Induced Osteoporosis in Female Rats. Pak. J. Biol. Sci. 2021, 24, 434–444. [Google Scholar] [CrossRef]
- Tao, Z.S.; Wu, X.J.; Yang, M.; Xu, H.G. Local administration with silymarin could increase osseointegration of hydroxyapatite-coated titanium implants in ovariectomized rats. J. Biomater. Appl. 2019, 34, 664–672. [Google Scholar] [CrossRef]
- Tao, Z.; Li, T.; Yang, M.; Xu, H. Silibinin Promotes Bone Regeneration Through HIF- 1 α / VEGF and Notch Signaling Pathway in Ovariectomized Rats. Res. Sq. 2021, 1–17. [Google Scholar] [CrossRef]
- Wang, T.; Cai, L.; Wang, Y.; Wang, Q.; Lu, D.; Chen, H.; Ying, X. The protective effects of silibinin in the treatment of streptozotocin-induced diabetic osteoporosis in rats. Biomed. Pharmacother. 2017, 89, 681–688. [Google Scholar] [CrossRef]
- Usmani, Q.I.; Jahan, N.; Aleem, M.; Hasan, S.A. Aatrilal (Ammi majus L.), an important drug of Unani system of medicine: A review. J. Ethnopharmacol. 2021, 276, 114144. [Google Scholar] [CrossRef]
- Treccarichi, S.; Ben Ammar, H.; Amari, M.; Cali, R.; Tribulato, A.; Branca, F. Molecular Markers for Detecting Inflorescence Size of Brassica oleracea L. Crops and B. oleracea Complex Species (n = 9) Useful for Breeding of Broccoli (B. oleracea var. italica) and Cauliflower (B. oleracea var. botrytis). Plants 2023, 12, 407. [Google Scholar] [PubMed]
- Moreb, N.; Murphy, A.; Jaiswal, S.; Jaiswal, A.K. Cabbage. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Elsevier Inc.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Lučić, D.; Pavlović, I.; Brkljačić, L.; Bogdanović, S.; Farkaš, V.; Cedilak, A.; Nanić, L.; Rubelj, I.; Salopek-Sondi, B. Antioxidant and Antiproliferative Activities of Kale (Brassica oleracea L. var. acephala DC.) and Wild Cabbage (Brassica incana Ten.) Polyphenolic Extracts. Molecules 2023, 28, 1840. [Google Scholar] [CrossRef]
- Kodama, J.; Kaito, T. Osteoclast multinucleation: Review of current literature. Int. J. Mol. Sci. 2020, 21, 5685. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jung, S.; You, H.; Lee, Y.; Park, Y.; Lee, H.; Hyun, S. Effect of Fermented Red Ginseng Concentrate Intake on Stool Characteristic, Biochemical Parameters, and Gut Microbiota in Elderly Korean Women. Nutrients 2022, 14, 1693. [Google Scholar] [CrossRef] [PubMed]
- Vedi, S.; Kaptoge, S.; Compston, J.E. Age-related changes in iliac crest cortical width and porosity: A histomorphometric study. J. Anat. 2011, 218, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Lerner, U.H. Osteoblasts, Osteoclasts, and Osteocytes: Unveiling Their Intimate-Associated Responses to Applied Orthodontic Forces. Semin. Orthod. 2012, 18, 237–248. [Google Scholar] [CrossRef]
- Nakayama, H.; Takakuda, K.; Matsumoto, H.N.; Miyata, A.; Baba, O.; Tabata, M.J.; Ushiki, T.; Oda, T.; McKee, M.D.; Takano, Y. Effects of altered bone remodeling and retention of cement lines on bone quality in osteopetrotic aged c-Src-deficient mice. Calcif. Tissue Int. 2010, 86, 172–183. [Google Scholar] [CrossRef]
- Manuha, M.I. Potent effects of some herbal supplements in the management of osteoporosis: A systematic review. ARCTIC Journal. 2022, 73, 117–134. [Google Scholar]
- Ghazanfarpour, M.; Amini, E.; Khadivzadeh, T.; Babakhanian, M.; Nouri, B.; Rakhshandeh, H.; Afiat, M. The effect of short-term treatment with fennel on bone density in postmenopausal women: A randomized controlled trial. J. Menopausal Med. 2017, 23, 124–130. [Google Scholar] [CrossRef]
- Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, M.F.; Kandasamy, G.; Vasudevan, R.; Ali, M.S.; Amir, M. Glycyrrhiza glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology. Plants 2021, 10, 2751. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarczyk-Sedlak, I.; Klasik-Ciszewska, S.; Wojnar, W. Glabridin and glycyrrhizic acid show no beneficial effect on the chemical composition and mechanical properties of bones in ovariectomized rats, when administered in moderate dose. Pharmacol. Rep. 2016, 68, 1036–1041. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Ghavami, V.; Niazi, A.; Shirvan, F.S.; Rasa, S. The effect of salvia officinalis on hot flashes in postmenopausal women: A systematic review and meta-analysis. Int. J. Community Based Nurs. Midwifery 2023, 11, 169–178. [Google Scholar] [CrossRef]
- Kayalar, E.; Goger, F.; Tas Deynek, G.; Tok, O.E.; Kucuk, S. New bone-generative effect of Salvia officinalis L. in the expanded midpalatal suture: An in vivo and in vitro study. J. Orofac. Orthop. 2022, 83, 85–95. [Google Scholar] [CrossRef]
- Zeidabadi, A.; Emamghoreishi, M.; Hosein Dabbaghmanesh, M.; Dejbakhat, M.; Reza Sasani, M.; Akbarzadeh, M. Comparison of the effect of vitex agnus and salvia officinalis extract at calcium, phosphorus and vitamin d levels in postmenopausal women referring. Bull. Pharm. Sci. 2021, 44, 367–376. [Google Scholar]
- Wang, X.; Zhang, Z.; Wu, S.C. Health Benefits of Silybum marianum: Phytochemistry, Pharmacology, and Applications. J. Agric. Food Chem. 2020, 68, 11644–11664. [Google Scholar] [CrossRef]
- Ballhause, T.M.; Jiang, S.; Baranowsky, A.; Brandt, S.; Mertens, P.R.; Frosch, K.H.; Yorgan, T.; Keller, J. Relevance of notch signaling for bone metabolism and regeneration. Int. J. Mol. Sci. 2021, 22, 1325. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Anand, V.; Roy, S. Vascular endothelial growth factor signaling in hypoxia and inflammation. J. Neuroimmune Pharmacol. 2014, 9, 142–160. [Google Scholar] [CrossRef]
- Trindade, A.; Djokovic, D.; Gigante, J.; Badenes, M.; Pedrosa, A.R.; Fernandes, A.C.; Lopes-da-Costa, L.; Krasnoperov, V.; Liu, R.; Gill, P.S.; et al. Low-Dosage inhibition of DLL4 signaling promotes wound healing by inducing functional Neo-Angiogenesis. PLoS ONE 2012, 7, e29863. [Google Scholar] [CrossRef]
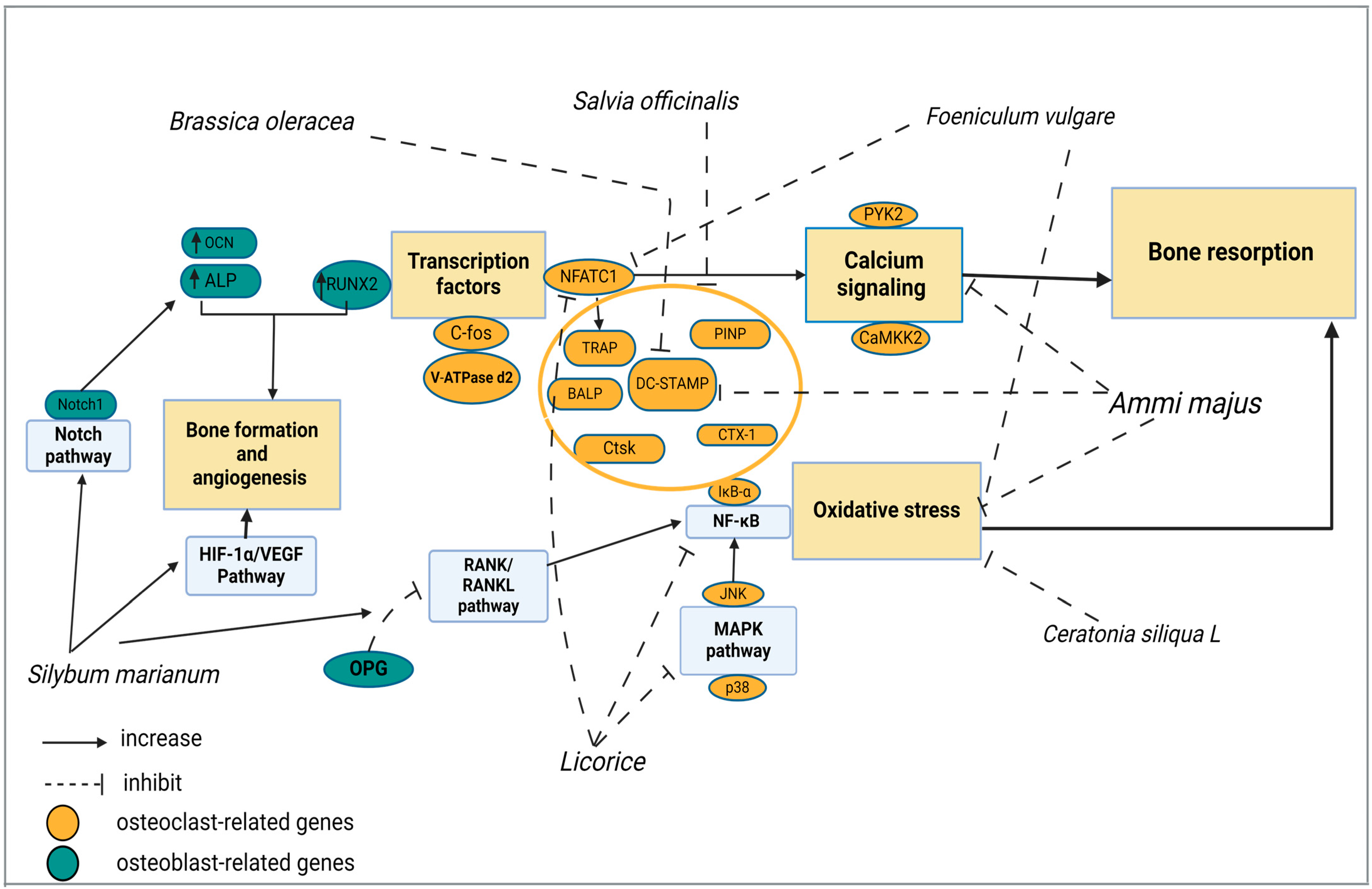
| Osteoclast-Regulating Factors | Functions | References |
|---|---|---|
| Dendritic-cell-specific transmembrane protein (DC-STAMP)/differentiation | Key regulator of the process of osteoclast formation. | [35] |
| Receptor activator of nuclear kappa-B (RANK) | The receptor for RANKL is responsible for regulating and activating osteoclasts. | [36] |
| Receptor activator of nuclear kappa-B ligand (RANKL) | Binds with the RANK receptor and plays a key part in the regulation and activation of osteoclasts. | [36] |
| c-fos transcriptional factor | Binds to the NFATc1 activator, leading to the activation of NFATc1, which, in response, starts the osteoclast differentiation. | [37] |
| Nuclear factor of activated T cells 1 (NFATc1) | Enhances osteoclast differentiation. | [37] |
| Tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6) | Acts as a mediator in the RANK/RANKL signaling pathway. | [38] |
| Macrophage colony-stimulating factor (M-CSF) | Important for differentiating osteoclasts from precursor cells to the mature form. | [39] |
| Osteoprotegerin (OPG) | Suppresses osteoclast formation by blocking the RANK/RANKL signaling pathway. | [36] |
| T cell immune regulator 1 (TCIRG1) gene | Mutations in this gene lead to the form of the a3 subunit in V-ATPase. | [40] |
| Vacuolar H+-ATPase (V-ATPase) | Is essential in enhancing the acidity of osteoclasts and promoting bone resorption. | [40] |
| Chloride channel 7 (CIC-7) | Increases osteoclast activity by promoting acidification. | [41] |
| Cathepsin K | Promotes the activity of osteoclasts, which stimulates organic-stage breakdown during resorption activity. | [42] |
| Osteoblast-Regulating Factors | Functions | References |
|---|---|---|
| Runt-related transcription factor 2 (RUNX2) | Synthesizes the proteins that play a role in the differentiation of osteoblasts. | [43] |
| Osterix (OSX/Sp7) | Responsible for the differentiation of osteoblasts during the embryonic stage. | [44] |
| Activating transcription factor 4 (ATF4) | Required for preserving bone mass by the accumulation of osteoblasts. | [45] |
| Collagen1-α1 (Col1a1) | Required to synthesize type 1 collagen, where type 1 collagen is secreted by osteoblasts to generate the bone matrix. | [46] |
| Bone γ-carboxyglutamate protein (BGLAP) | Is crucial in encoding osteocalcin. | [47] |
| LDL (low-density lipoprotein) receptor-related protein 5 (LRP5) | Found on the surface of osteoblasts and binds with the Wnt ligand to form a bond with osteoblasts. | [48] |
| SOST sclerostin (SOST) | Regulates osteoblasts by inhibiting canonical Wnt/β-catenin signaling. | [49] |
| β-Catenin | Controls osteoblasts by enhancing the expression of specific genes within cells (intracellular) after being activated by Wnt ligand. | [49] |
| Wingless-related integration site (Wnt) | The ligand binds to the surface of osteoblasts and activates β-Catenin, which regulates osteoblasts. | [49] |
| Core binding factor-α1 (Cbfa1) | Osteoblast differentiation. | [50] |
| Scientific Name | Type of Extract/Bioactive Compound | Administration (In Vivo and In Vitro) | Concentrations/Doses | Effects on Bone | References |
|---|---|---|---|---|---|
| Ammi majus | Xanthotoxin extract. | In vitro: Bone-marrow-derived macrophages (BMM). | 0.01 and 1 μM. |
| [112] |
| In vivo: Ovariectomized mice model. | 0.5 and 5 mg/kg (intraperitoneal injection). |
| |||
| Ammi majus | Methoxsalen extract | In vivo: Ovariectomized mice model. | 0.02% in diet. |
| [113] |
| Brassica oleracea (co-administration with Panax ginseng) | Hot water extract. | In vitro: RAW 264.7 cells. | 50, 100, and 200 μg/mL. |
| [114] |
| In vivo: Ovariectomized mice model. | 500 mg/kg (oral administration) |
| |||
| Ceratonia siliqua L. | Polyphenols | In vivo: Ovariectomized rat model. | The food was supplemented with 0.46% of the treatment. |
| [115,116] |
| Ceratonia siliqua L. | Flavonoids | In vivo: Ovariectomized rat model. | Ceratonia siliqua mixed with food at 3 g/kg/day/rat. |
| [117] |
| Foeniculum vulgare | Aqueous extract of fennel | In vitro: Bone marrow-derived macrophages (BMMs). | 0.5–10 μg/mL. (0.5, 1, and 2 μg/mL for the osteoclast formation test and 2 and 10 μg/mL for osteoclast activity test) |
| [118] |
| In vivo: Ovariectomized C57BL/6 mice model. | 30 and 100 mg/kg. (oral administration). |
| |||
| licorice | Liquiritin extract. | In vitro: Bone-marrow-derived macrophages (BMMs). | 0.01, 0.05, and 0.1 mM. |
| [119] |
| In vivo: Ovariectomized C57BL/6J mice models. | 20 mg/kg (intraperitoneal injection). |
| |||
| licorice | Glycyrrhiza extract (methanolic extract). | In vivo: Ovariectomized rat model. | 12.4 mg/kg (oral administration). |
| [120] |
| licorice | Formononetin extract. | In vivo: Ovariectomized rat model. | 10 mg/kg |
| [121] |
| Salvia officinalis | N. D | In vivo: Ovariectomized albino rat model. | 10 mg/kg (oral gavage administration). |
| [122] |
| Silybum marianum | silymarin | In vivo: Ovariectomized Sprague–Dawley rat model. | 100 mg/mL. (local administration). |
| [123] |
| Silybum marianum | Silibinin or silybin | In vitro: Preosteoblastic cell line (MC3TE-E1). | 20 and 50 μmol/L. |
| [124] |
| In vivo: Ovariectomized rat model. | 50 mg/kg (intraperitoneal injection). | ||||
| Silybum marianum | Silibinin or silybin | In vivo: Diabetic rat model. | 50 and 100 mg/kg/day (oral administration). |
| [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ajalein, A.A.; Ibrahim, N.‘I.; Fauzi, M.B.; Mokhtar, S.A.; Naina Mohamed, I.; Shuid, A.N.; Mohamed, N. Evaluating the Anti-Osteoporotic Potential of Mediterranean Medicinal Plants: A Review of Current Evidence. Pharmaceuticals 2024, 17, 1341. https://doi.org/10.3390/ph17101341
Al-Ajalein AA, Ibrahim N‘I, Fauzi MB, Mokhtar SA, Naina Mohamed I, Shuid AN, Mohamed N. Evaluating the Anti-Osteoporotic Potential of Mediterranean Medicinal Plants: A Review of Current Evidence. Pharmaceuticals. 2024; 17(10):1341. https://doi.org/10.3390/ph17101341
Chicago/Turabian StyleAl-Ajalein, Alhareth Abdulraheem, Nurul ‘Izzah Ibrahim, Mh Busra Fauzi, Sabarul Afian Mokhtar, Isa Naina Mohamed, Ahmad Nazrun Shuid, and Norazlina Mohamed. 2024. "Evaluating the Anti-Osteoporotic Potential of Mediterranean Medicinal Plants: A Review of Current Evidence" Pharmaceuticals 17, no. 10: 1341. https://doi.org/10.3390/ph17101341
APA StyleAl-Ajalein, A. A., Ibrahim, N. ‘I., Fauzi, M. B., Mokhtar, S. A., Naina Mohamed, I., Shuid, A. N., & Mohamed, N. (2024). Evaluating the Anti-Osteoporotic Potential of Mediterranean Medicinal Plants: A Review of Current Evidence. Pharmaceuticals, 17(10), 1341. https://doi.org/10.3390/ph17101341









