Nuclear Receptors and Stress Response Pathways Associated with the Development of Oral Mucositis Induced by Antineoplastic Agents
Abstract
1. Introduction
2. Results
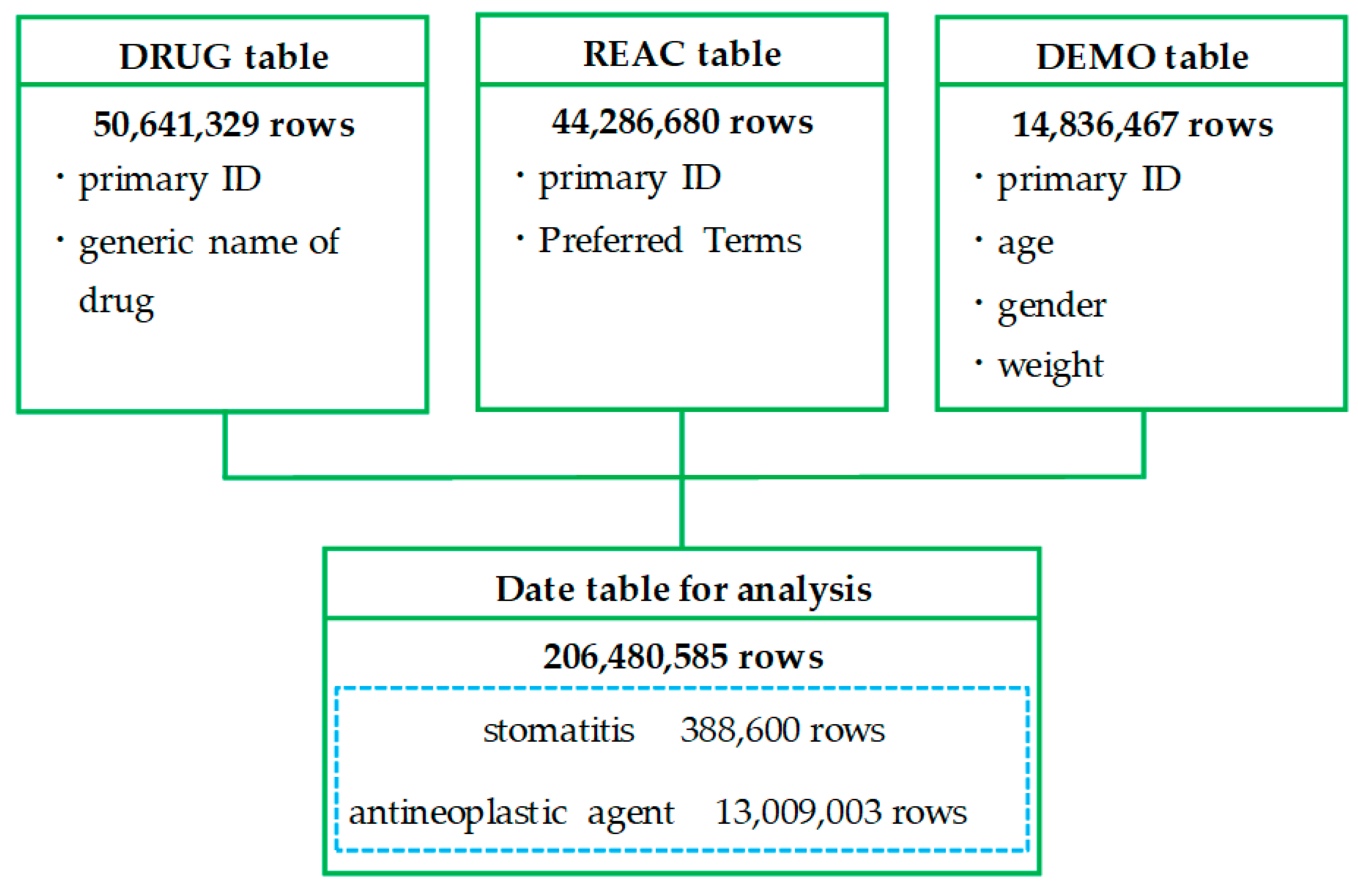
2.1. Creation of a Data Table
2.2. Association of OM with Patient Characteristics
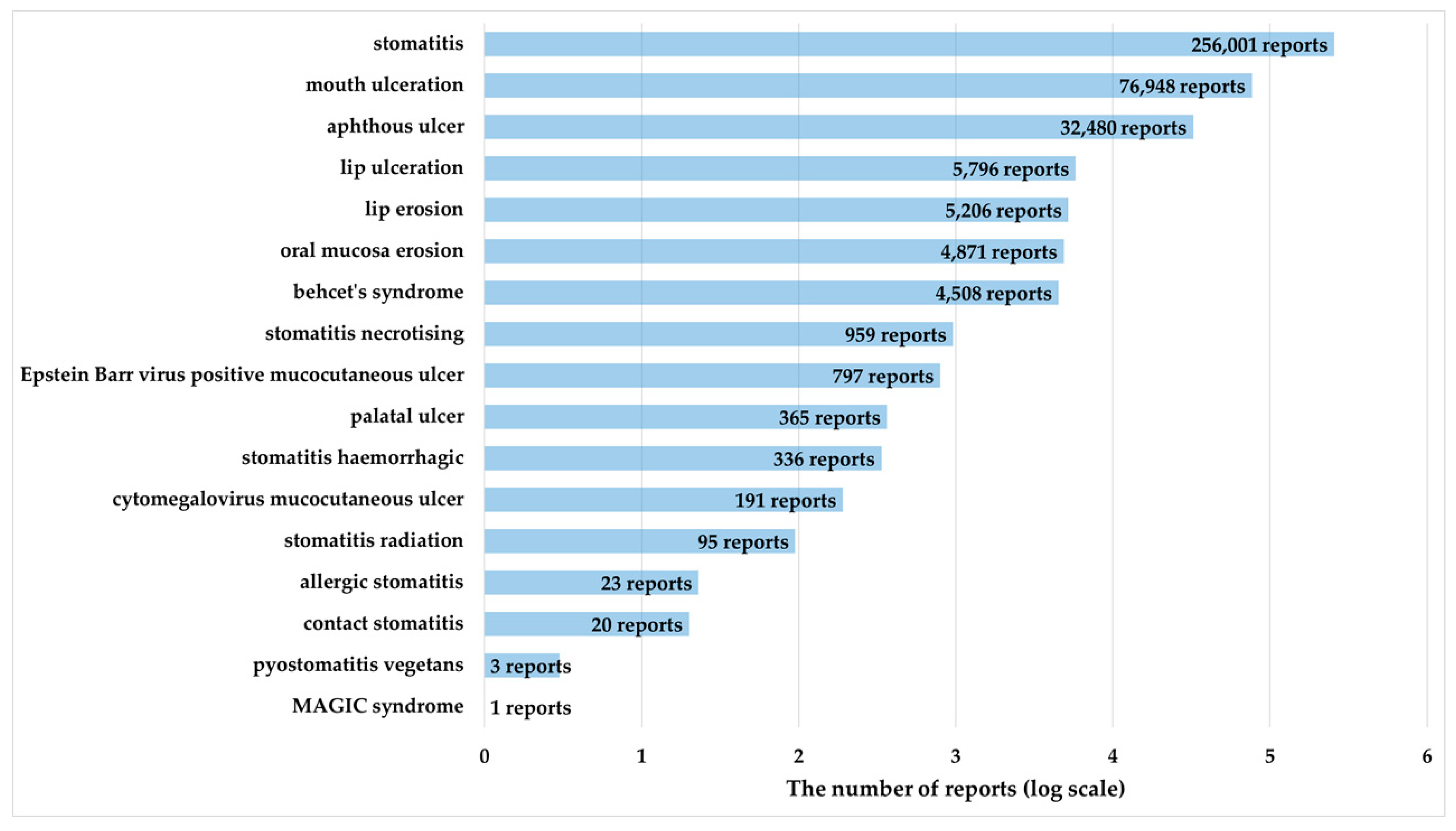
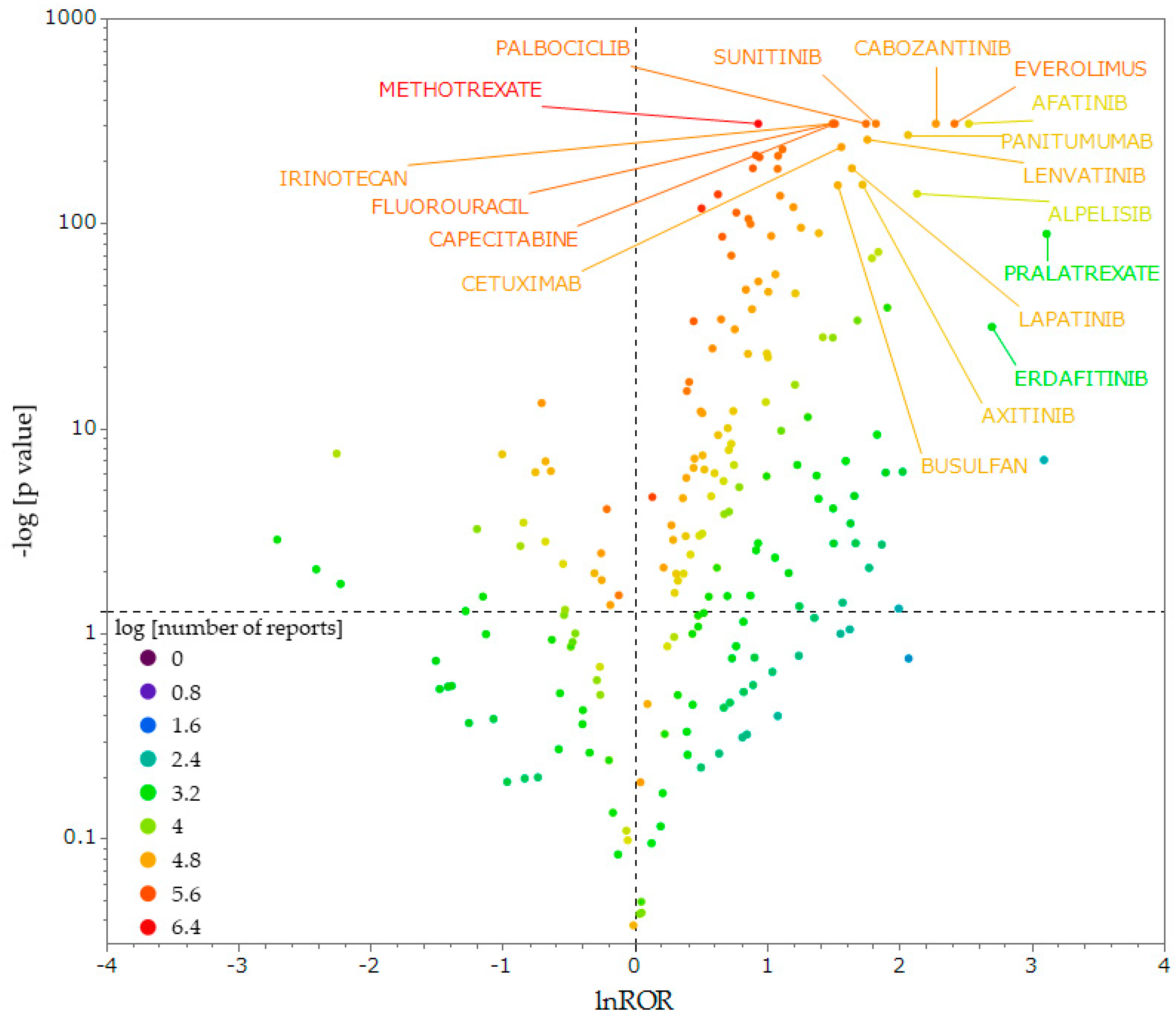
2.3. Antineoplastic Agents That Induce OM
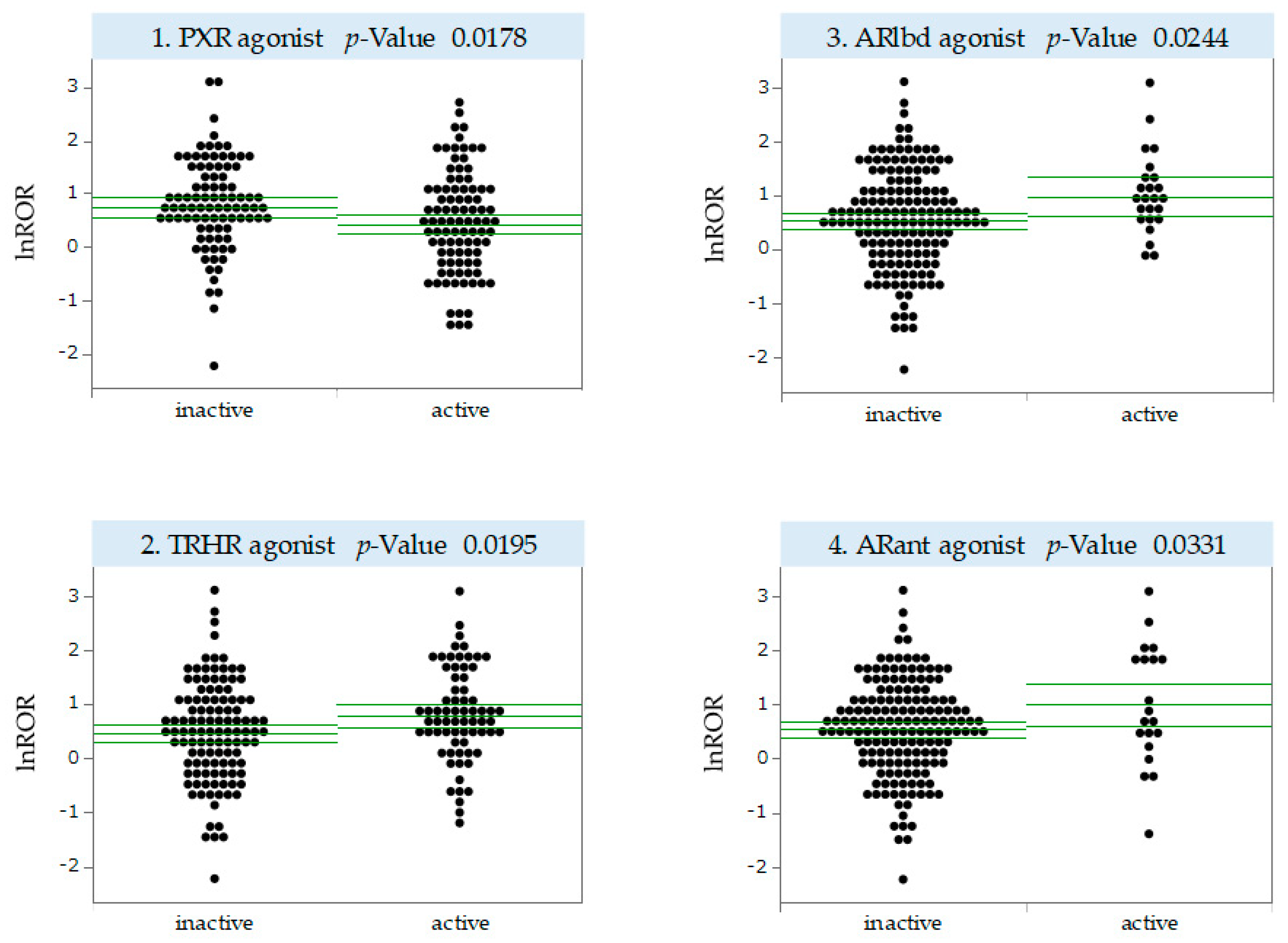
2.4. MIEs Associated with the Development of Antineoplastic Agent-Induced OM
3. Discussion
3.1. OM and Patient Characteristics
3.2. Antineoplastic Agents That Induce OM
3.3. MIEs Associated with Antineoplastic Agent-Induced OM Development
3.4. Limitations
4. Materials and Methods
4.1. FAERS Database
4.2. Drugs Analyzed and Definitions of ADEs
4.3. Creation of a Data Table for Analysis
4.4. Characteristics of Patients with Antineoplastic Agent-Induced OM
4.5. Calculation of RORs
4.6. Generation of a Scatter Plot
4.7. MIE Activity Prediction Using QSAR Toxicity Predictor
4.8. Univariate Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lalla, R.V.; Peterson, D.E. Oral mucositis. Dent. Clin. N. Am. 2005, 49, 167–184. [Google Scholar] [CrossRef]
- Lalla, R.V.; Brennan, M.T.; Gordon, S.M.; Sonis, S.T.; Rosenthal, D.I.; Keefe, D.M. Oral Mucositis Due to High-Dose Chemotherapy and/or Head and Neck Radiation Therapy. J. Natl. Cancer Inst. Monogr. 2019, 2019, lgz011. [Google Scholar] [CrossRef]
- Fidan, Ö.; Arslan, S. Development and Validation of the Oral Mucositis Risk Assessment Scale in Hematology Patients. Semin. Oncol. Nurs. 2021, 37, 151159. [Google Scholar] [CrossRef]
- Pulito, C.; Cristaudo, A.; Porta, C.; Zapperi, S.; Blandino, G.; Morrone, A.; Strano, S. Oral mucositis: The hidden side of cancer therapy. J. Exp. Clin. Cancer Res. 2020, 39, 210. [Google Scholar] [CrossRef]
- Villa, A.; Sonis, S.T. Pharmacotherapy for the management of cancer regimen-related oral mucositis. Expert Opin. Pharmacother. 2016, 17, 1801–1807. [Google Scholar] [CrossRef]
- Murphy, B.A. Clinical and economic consequences of mucositis induced by chemotherapy and/or radiation therapy. J. Support Oncol. 2007, 5 (Suppl. S4), 13–21. [Google Scholar] [PubMed]
- Kim, J.W.; Cha, Y.; Kim, S.J.; Han, S.W.; Oh, D.Y.; Lee, S.H.; Kim, D.W.; Im, S.A.; Kim, T.Y.; Heo, D.S.; et al. Association of oral mucositis with quality of life and symptom clusters in patients with solid tumors receiving chemotherapy. Support. Care Cancer 2012, 20, 395–403. [Google Scholar] [CrossRef]
- Elting, L.S.; Cooksley, C.; Chambers, M.; Cantor, S.B.; Manzullo, E.; Rubenstein, E.B. The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer 2003, 98, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Colella, G.; Boschetti, C.E.; Vitagliano, R.; Colella, C.; Jiao, L.; King-Smith, N.; Li, C.; Nuoh Lau, Y.; Lai, Z.; Mohammed, A.I.; et al. Interventions for the Prevention of Oral Mucositis in Patients Receiving Cancer Treatment: Evidence from Randomised Controlled Trials. Curr. Oncol. 2023, 30, 967–980. [Google Scholar] [CrossRef]
- Hao, S.; Jin, Y.; Yu, Y.; Wang, J.; Zou, J.; Wang, Y. Identification of potential molecular mechanisms and candidate drugs for radiotherapy- and chemotherapy-induced mucositis. Support. Care Cancer 2023, 31, 223. [Google Scholar] [CrossRef]
- Cinausero, M.; Aprile, G.; Ermacora, P.; Basile, D.; Vitale, M.G.; Fanotto, V.; Parisi, G.; Calvetti, L.; Sonis, S.T. New Frontiers in the Pathobiology and Treatment of Cancer Regimen-Related Mucosal Injury. Front. Pharmacol. 2017, 8, 354. [Google Scholar] [CrossRef]
- Sonis, S.T.; Elting, L.S.; Keefe, D.; Peterson, D.E.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.N.; Raber-Durlacher, J.; Donnelly, J.P.; Rubenstein, E.B.; et al. Perspectives on cancer therapy-induced mucosal injury: Pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004, 100 (Suppl. S9), 1995–2025. [Google Scholar] [CrossRef]
- Sonis, S.T. New thoughts on the initiation of mucositis. Oral Dis. 2010, 16, 597–600. [Google Scholar] [CrossRef]
- Bian, L.; Han, G.; Zhao, C.W.; Garl, P.J.; Wang, X.J. The role of Smad7 in oral mucositis. Protein Cell 2015, 6, 160–169. [Google Scholar] [CrossRef]
- Hosoya, R.; Ishii-Nozawa, R.; Terajima, T.; Kagaya, H.; Uesawa, Y. The Association between Molecular Initiating Events and Drug-Induced Hiccups. Pharmaceuticals 2024, 17, 379. [Google Scholar] [CrossRef] [PubMed]
- Leist, M.; Ghallab, A.; Graepel, R.; Marchan, R.; Hassan, R.; Bennekou, S.H.; Limonciel, A.; Vinken, M.; Schildknecht, S.; Waldmann, T.; et al. Adverse outcome pathways: Opportunities, limitations and open questions. Arch. Toxicol. 2017, 91, 3477–3505. [Google Scholar] [CrossRef]
- Villeneuve, D.L.; Crump, D.; Garcia-Reyero, N.; Hecker, M.; Hutchinson, T.H.; LaLone, C.A.; Landesmann, B.; Lettieri, T.; Munn, S.; Nepelska, M.; et al. Adverse outcome pathway (AOP) development I: Strategies and principles. Toxicol. Sci. 2014, 142, 312–320. [Google Scholar] [CrossRef]
- Wedlake, A.J.; Folia, M.; Piechota, S.; Allen, T.E.H.; Goodman, J.M.; Gutsell, S.; Russell, P.J. Structural Alerts and Random Forest Models in a Consensus Approach for Receptor Binding Molecular Initiating Events. Chem. Res. Toxicol. 2020, 33, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Attene-Ramos, M.S.; Miller, N.; Huang, R.; Michael, S.; Itkin, M.; Kavlock, R.J.; Austin, C.P.; Shinn, P.; Simeonov, A.; Tice, R.R.; et al. The Tox21 robotic platform for the assessment of environmental chemicals—From vision to reality. Drug Discov. Today 2013, 18, 716–723. [Google Scholar] [CrossRef]
- Kurosaki, K.; Wu, R.; Uesawa, Y. A Toxicity Prediction Tool for Potential Agonist/Antagonist Activities in Molecular Initiating Events Based on Chemical Structures. Int. J. Mol. Sci. 2020, 21, 7853. [Google Scholar] [CrossRef]
- Cheng, K.K.; Lee, V.; Li, C.H.; Goggins, W.; Thompson, D.R.; Yuen, H.L.; Epstein, J.B. Incidence and risk factors of oral mucositis in paediatric and adolescent patients undergoing chemotherapy. Oral Oncol. 2011, 47, 153–162. [Google Scholar] [CrossRef]
- Elad, S.; Yarom, N.; Zadik, Y.; Kuten-Shorrer, M.; Sonis, S.T. The broadening scope of oral mucositis and oral ulcerative mucosal toxicities of anticancer therapies. CA Cancer J. Clin. 2022, 72, 57–77. [Google Scholar] [CrossRef]
- Goldberg, S.L.; Chiang, L.; Selina, N.; Hamarman, S. Patient perceptions about chemotherapy-induced oral mucositis: Implications for primary/secondary prophylaxis strategies. Support. Care Cancer 2004, 12, 526–530. [Google Scholar] [CrossRef]
- Chansky, K.; Benedetti, J.; Macdonald, J.S. Differences in toxicity between men and women treated with 5-fluorouracil therapy for colorectal carcinoma. Cancer 2005, 103, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Vokurka, S.; Bystrická, E.; Koza, V.; Scudlová, J.; Pavlicová, V.; Valentová, D.; Visokaiová, M.; Misaniová, L. Higher incidence of chemotherapy induced oral mucositis in females: A supplement of multivariate analysis to a randomized multicentre study. Support. Care Cancer 2006, 14, 974–976. [Google Scholar] [CrossRef] [PubMed]
- Sloan, J.A.; Loprinzi, C.L.; Novotny, P.J.; Okuno, S.; Nair, S.; Barton, D.L. Sex differences in fluorouracil-induced stomatitis. J. Clin. Oncol. 2000, 18, 412–420. [Google Scholar] [CrossRef]
- Sloan, J.A.; Goldberg, R.M.; Sargent, D.J.; Vargas-Chanes, D.; Nair, S.; Cha, S.S.; Novotny, P.J.; Poon, M.A.; O’Connell, M.J.; Loprinzi, C.L. Women experience greater toxicity with fluorouracil-based chemotherapy for colorectal cancer. J. Clin. Oncol. 2002, 20, 1491–1498. [Google Scholar] [CrossRef]
- Wuketich, S.; Hienz, S.A.; Marosi, C. Prevalence of clinically relevant oral mucositis in outpatients receiving myelosuppressive chemotherapy for solid tumors. Support. Care Cancer 2012, 20, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Grazziutti, M.L.; Dong, L.; Miceli, M.H.; Krishna, S.G.; Kiwan, E.; Syed, N.; Fassas, A.; van Rhee, F.; Klaus, H.; Barlogie, B.; et al. Oral mucositis in myeloma patients undergoing melphalan-based autologous stem cell transplantation: Incidence, risk factors and a severity predictive model. Bone Marrow Transplant. 2006, 38, 501–506. [Google Scholar] [CrossRef]
- Nishii, M.; Soutome, S.; Kawakita, A.; Yutori, H.; Iwata, E.; Akashi, M.; Hasegawa, T.; Kojima, Y.; Funahara, M.; Umeda, M.; et al. Factors associated with severe oral mucositis and candidiasis in patients undergoing radiotherapy for oral and oropharyngeal carcinomas: A retrospective multicenter study of 326 patients. Support. Care Cancer 2020, 28, 1069–1075. [Google Scholar] [CrossRef]
- Stein, B.N.; Petrelli, N.J.; Douglass, H.O.; Driscoll, D.L.; Arcangeli, G.; Meropol, N.J. Age and sex are independent predictors of 5-fluorouracil toxicity. Analysis of a large scale phase III trial. Cancer 1995, 75, 11–17. [Google Scholar] [CrossRef]
- Gebri, E.; Kiss, A.; Tóth, F.; Hortobágyi, T. Female sex as an independent prognostic factor in the development of oral mucositis during autologous peripheral stem cell transplantation. Sci. Rep. 2020, 10, 15898. [Google Scholar] [CrossRef]
- Lee, C.; Kasa-Vubu, J.; Supiano, M. Androgenicity and obesity are independently associated with insulin sensitivity in postmenopausal women. Metab. Clin. Exp. 2004, 53, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Kashiwazaki, H.; Matsushita, T.; Sugita, J.; Shigematsu, A.; Kasashi, K.; Yamazaki, Y.; Kanehira, T.; Kondo, T.; Endo, T.; Tanaka, J.; et al. A comparison of oral mucositis in allogeneic hematopoietic stem cell transplantation between conventional and reduced-intensity regimens. Support. Care Cancer 2012, 20, 933–939. [Google Scholar] [CrossRef]
- Vagliano, L.; Feraut, C.; Gobetto, G.; Trunfio, A.; Errico, A.; Campani, V.; Costazza, G.; Mega, A.; Matozzo, V.; Berni, M.; et al. Incidence and severity of oral mucositis in patients undergoing haematopoietic SCT—Results of a multicentre study. Bone Marrow Transplant. 2011, 46, 727–732. [Google Scholar] [CrossRef]
- Sonis, S.T. Mucositis as a biological process: A new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol. 1998, 34, 39–43. [Google Scholar] [CrossRef]
- Çakmak, S.; Nural, N. Incidence of and risk factors for development of oral mucositis in outpatients undergoing cancer chemotherapy. Int. J. Nurs. Pract. 2019, 25, e12710. [Google Scholar] [CrossRef] [PubMed]
- Zalcberg, J.; Kerr, D.; Seymour, L.; Palmer, M. Haematological and non-haematological toxicity after 5-fluorouracil and leucovorin in patients with advanced colorectal cancer is significantly associated with gender, increasing age and cycle number. Tomudex International Study Group. Eur. J. Cancer 1998, 34, 1871–1875. [Google Scholar] [CrossRef]
- Wardill, H.R.; Sonis, S.T.; Blijlevens, N.M.A.; Van Sebille, Y.Z.A.; Ciorba, M.A.; Loeffen, E.A.H.; Cheng, K.K.F.; Bossi, P.; Porcello, L.; Castillo, D.A.; et al. Prediction of mucositis risk secondary to cancer therapy: A systematic review of current evidence and call to action. Support. Care Cancer 2020, 28, 5059–5073. [Google Scholar] [CrossRef]
- Naidu, M.U.; Ramana, G.V.; Rani, P.U.; Mohan, I.K.; Suman, A.; Roy, P. Chemotherapy-induced and/or radiation therapy-induced oral mucositis--complicating the treatment of cancer. Neoplasia 2004, 6, 423–431. [Google Scholar] [CrossRef]
- Hosonaka, K.; Yamaoka, K.; Ikeda, N.; Uchida, M.; Uesawa, Y.; Takahashi, K.; Shimizu, T. Disproportionality analysis of stomatitis associated with anticancer drugs using Japanese Adverse Drug Event Reporting Database. Oncology 2024, 102, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Vigarios, E.; Epstein, J.B.; Sibaud, V. Oral mucosal changes induced by anticancer targeted therapies and immune checkpoint inhibitors. Support. Care Cancer 2017, 25, 1713–1739. [Google Scholar] [CrossRef]
- Abdel-Rahman, O.; Fouad, M. Risk of mucocutaneous toxicities in patients with solid tumors treated with lapatinib: A systematic review and meta-analysis. Curr. Med. Res. Opin. 2015, 31, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Califano, R.; Tariq, N.; Compton, S.; Fitzgerald, D.A.; Harwood, C.A.; Lal, R.; Lester, J.; McPhelim, J.; Mulatero, C.; Subramanian, S.; et al. Expert Consensus on the Management of Adverse Events from EGFR Tyrosine Kinase Inhibitors in the UK. Drugs 2015, 75, 1335–1348. [Google Scholar] [CrossRef]
- Carrozzo, M.; Eriksen, J.G.; Bensadoun, R.J.; Boers-Doets, C.B.; Lalla, R.V.; Peterson, D.E. Oral Mucosal Injury Caused by Targeted Cancer Therapies. J. Natl. Cancer Inst. Monogr. 2019, 2019, lgz012. [Google Scholar] [CrossRef]
- Dietrich, E.; Antoniades, K. Molecularly targeted drugs for the treatment of cancer: Oral complications and pathophysiology. Hippokratia 2012, 16, 196–199. [Google Scholar] [PubMed]
- Kliewer, S.A.; Goodwin, B.; Willson, T.M. The nuclear pregnane X receptor: A key regulator of xenobiotic metabolism. Endocr. Rev. 2002, 23, 687–702. [Google Scholar] [CrossRef]
- Oladimeji, P.O.; Chen, T. PXR: More Than Just a Master Xenobiotic Receptor. Mol. Pharmacol. 2018, 93, 119–127. [Google Scholar] [CrossRef]
- Lv, Y.; Luo, Y.Y.; Ren, H.W.; Li, C.J.; Xiang, Z.X.; Luan, Z.L. The role of pregnane X receptor (PXR) in substance metabolism. Front. Endocrinol. 2022, 13, 959902. [Google Scholar] [CrossRef]
- Zhou, C.; Tabb, M.M.; Nelson, E.L.; Grün, F.; Verma, S.; Sadatrafiei, A.; Lin, M.; Mallick, S.; Forman, B.M.; Thummel, K.E.; et al. Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. J. Clin. Investig. 2006, 116, 2280–2289. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Ma, X.; Gonzalez, F.J. Pregnane X receptor- and CYP3A4-humanized mouse models and their applications. Br. J. Pharmacol. 2011, 163, 461–468. [Google Scholar] [CrossRef]
- Deuring, J.J.; Li, M.; Cao, W.; Chen, S.; Wang, W.; de Haar, C.; van der Woude, C.J.; Peppelenbosch, M. Pregnane X receptor activation constrains mucosal NF-κB activity in active inflammatory bowel disease. PLoS ONE 2019, 14, e0221924. [Google Scholar] [CrossRef]
- Shah, Y.M.; Ma, X.; Morimura, K.; Kim, I.; Gonzalez, F.J. Pregnane X receptor activation ameliorates DSS-induced inflammatory bowel disease via inhibition of NF-kappaB target gene expression. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1114–G1122. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Shah, Y.M.; Gonzalez, F.J. Pregnane X receptor as a target for treatment of inflammatory bowel disorders. Trends Pharmacol. Sci. 2012, 33, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, M.; Song, M.; Wang, J.; Cai, J.; Lin, C.; Li, Y.; Jin, X.; Shen, C.; Chen, Z.; et al. Patchouli alcohol activates PXR and suppresses the NF-κB-mediated intestinal inflammatory. J. Ethnopharmacol. 2020, 248, 112302. [Google Scholar] [CrossRef]
- Garg, A.; Zhao, A.; Erickson, S.L.; Mukherjee, S.; Lau, A.J.; Alston, L.; Chang, T.K.; Mani, S.; Hirota, S.A. Pregnane X Receptor Activation Attenuates Inflammation-Associated Intestinal Epithelial Barrier Dysfunction by Inhibiting Cytokine-Induced Myosin Light-Chain Kinase Expression and c-Jun N-Terminal Kinase 1/2 Activation. J. Pharmacol. Exp. Ther. 2016, 359, 91–101. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Bioassay Record for AID 1347033, Human Pregnane X receptor (PXR) Small Molecule Agonists: Summary, Source: Tox21. PubChem. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/bioassay/1347033 (accessed on 27 May 2024).
- National Center for Biotechnology Information. PubChem Bioassay Record for AID 1347030, Thyrotropin-Releasing Hormone Receptor (TRHR) Small Molecule Agonists: Summary, Source: Tox21. PubChem. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/bioassay/1347030 (accessed on 17 May 2024).
- Gary, K.A.; Sevarino, K.A.; Yarbrough, G.G.; Prange, A.J., Jr.; Winokur, A. The thyrotropin-releasing hormone (TRH) hypothesis of homeostatic regulation: Implications for TRH-based therapeutics. J. Pharmacol. Exp. Ther. 2003, 305, 410–416. [Google Scholar] [CrossRef]
- Kamath, J.; Yarbrough, G.G.; Prange, A.J., Jr.; Winokur, A. The thyrotropin-releasing hormone (TRH)-immune system homeostatic hypothesis. Pharmacol. Ther. 2009, 121, 20–28. [Google Scholar] [CrossRef]
- Brod, S.A.; Bauer, V. Ingested (oral) thyrotropin releasing factor (TRH) inhibits EAE. Cytokine 2013, 61, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Thiefin, G.; Taché, Y.; Leung, F.W.; Guth, P.H. Central nervous system action of thyrotropin-releasing hormone to increase gastric mucosal blood flow in the rat. Gastroenterology 1989, 97, 405–411. [Google Scholar] [CrossRef]
- Lai, J.J.; Lai, K.P.; Zeng, W.; Chuang, K.H.; Altuwaijri, S.; Chang, C. Androgen receptor influences on body defense system via modulation of innate and adaptive immune systems: Lessons from conditional AR knockout mice. Am. J. Pathol. 2012, 181, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.J.; Lai, K.P.; Chuang, K.H.; Chang, P.; Yu, I.C.; Lin, W.J.; Chang, C. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-alpha expression. J. Clin. Investig. 2009, 119, 3739–3751. [Google Scholar] [CrossRef] [PubMed]
- Ojanotko-Harri, A.; Forssell, H.; Laine, M.; Hurttia, H.; Bläuer, M.; Tuohimaa, P. Immunohistochemical detection of androgen receptors in human oral mucosa. Arch. Oral Biol. 1992, 37, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T. Role and Applicability of Spontaneous Reporting Databases in Medical Big Data. Yakugaku Zasshi 2021, 141, 165–168. [Google Scholar] [CrossRef]
- Alemayehu, D. Evaluation of Reporting Bias in Postmarketing Risk Assessment Based on Spontaneous Reporting Systems. Pharm. Med. 2009, 23, 195–200. [Google Scholar] [CrossRef]
- Pariente, A.; Avillach, P.; Salvo, F.; Thiessard, F.; Miremont-Salamé, G.; Fourrier-Reglat, A.; Haramburu, F.; Bégaud, B.; Moore, N.; Association Française des Centres Régionaux de Pharmacovigilance (CRPV). Effect of competition bias in safety signal generation: Analysis of a research database of spontaneous reports in France. Drug Saf. 2012, 35, 855–864. [Google Scholar] [CrossRef]
- Okunaka, M.; Kano, D.; Uesawa, Y. Nuclear Receptor and Stress Response Pathways Associated with Antineoplastic Agent-Induced Diarrhea. Int. J. Mol. Sci. 2022, 23, 12407. [Google Scholar] [CrossRef]
- FDA Adverse Event Reporting System (FAERS). FDA. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-adverse-event-reporting-system-faers (accessed on 8 July 2024).
- Chen, L.; Liu, T.; Zhao, X. Inferring anatomical therapeutic chemical (ATC) class of drugs using shortest path and random walk with restart algorithms. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2228–2240. [Google Scholar] [CrossRef]
- MedDRA Japanese Maintenance Organization. JMO. Available online: https://www.jmo.pmrj.jp/ (accessed on 8 July 2024).
- Weber, F.; Knapp, G.; Ickstadt, K.; Kundt, G.; Glass, Ä. Zero-cell corrections in random-effects meta-analyses. Res. Synth. Methods 2020, 11, 913–919. [Google Scholar] [CrossRef]
- Chen, J.J.; Wang, S.J.; Tsai, C.A.; Lin, C.J. Selection of differentially expressed genes in microarray data analysis. Pharmacogenom. J. 2007, 7, 212–220. [Google Scholar] [CrossRef]
- Li, C.; Chen, J.; Qin, G. Partial Youden index and its inferences. J. Biopharm. Stat. 2019, 29, 385–399. [Google Scholar] [CrossRef] [PubMed]
| Patient Background | Stomatitis (25,115) | Without Stomatitis (1,297,554) | Odds Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|---|---|
| Sex | Female | 16,078 | 735,315 | 0.735 | 0.716−0.754 | <0.0001 |
| Male | 9037 | 562,239 | ||||
| Age | <70 years old | 18,300 | 919,739 | 0.907 | 0.881−0.932 | <0.0001 |
| ≥70 years old | 6815 | 377,815 |
| Antineoplastic Agents | ROR | 95% Confidence Interval | p-Value | Number of Reports | ATC Code | ATC Name |
|---|---|---|---|---|---|---|
| Afatinib | 12.48 | 11.44–13.6 | <0.0001 | 23,023 | L01EB | EGFR tyrosine kinase inhibitors |
| Everolimus | 11.20 | 10.85–11.56 | <0.0001 | 191,356 | L01EG | mTOR kinase inhibitors |
| Cabozantinib | 9.72 | 9.26–10.22 | <0.0001 | 89,600 | L01EX | Other protein kinase inhibitors |
| Alpelisib | 8.43 | 7.45–9.54 | <0.0001 | 16,464 | L01EM | Pi3K inhibitors |
| Panitumumab | 7.88 | 7.23–8.59 | <0.0001 | 35,861 | L01FE | EGFR inhibitors |
| Sunitinib | 6.18 | 5.9–6.47 | <0.0001 | 163,120 | L01EX | Other protein kinase inhibitors |
| Lenvatinib | 5.79 | 5.36–6.26 | <0.0001 | 59,076 | L01EX | Other protein kinase inhibitors |
| Palbociclib | 5.73 | 5.5–5.98 | <0.0001 | 211,026 | L01EF | CDK inhibitors |
| Axitinib | 5.58 | 5.06–6.16 | <0.0001 | 38,095 | L01EK | VEGFR tyrosine kinase inhibitors |
| Lapatinib | 5.15 | 4.72–5.62 | <0.0001 | 53,535 | L01EH | HER2 tyrosine kinase inhibitors |
| Stomatitis | Without Stomatitis | |
|---|---|---|
| Reports with the suspected medicine | a | b |
| All other reports | c | d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kagaya, M.; Uesawa, Y. Nuclear Receptors and Stress Response Pathways Associated with the Development of Oral Mucositis Induced by Antineoplastic Agents. Pharmaceuticals 2024, 17, 1086. https://doi.org/10.3390/ph17081086
Kagaya M, Uesawa Y. Nuclear Receptors and Stress Response Pathways Associated with the Development of Oral Mucositis Induced by Antineoplastic Agents. Pharmaceuticals. 2024; 17(8):1086. https://doi.org/10.3390/ph17081086
Chicago/Turabian StyleKagaya, Moena, and Yoshihiro Uesawa. 2024. "Nuclear Receptors and Stress Response Pathways Associated with the Development of Oral Mucositis Induced by Antineoplastic Agents" Pharmaceuticals 17, no. 8: 1086. https://doi.org/10.3390/ph17081086
APA StyleKagaya, M., & Uesawa, Y. (2024). Nuclear Receptors and Stress Response Pathways Associated with the Development of Oral Mucositis Induced by Antineoplastic Agents. Pharmaceuticals, 17(8), 1086. https://doi.org/10.3390/ph17081086









