The Infusion of Piperacillin/Tazobactam with an Elastomeric Device: A Combined 24-H Stability Study and Drug Solution Flow Rate Analysis
Abstract
1. Introduction
2. Results
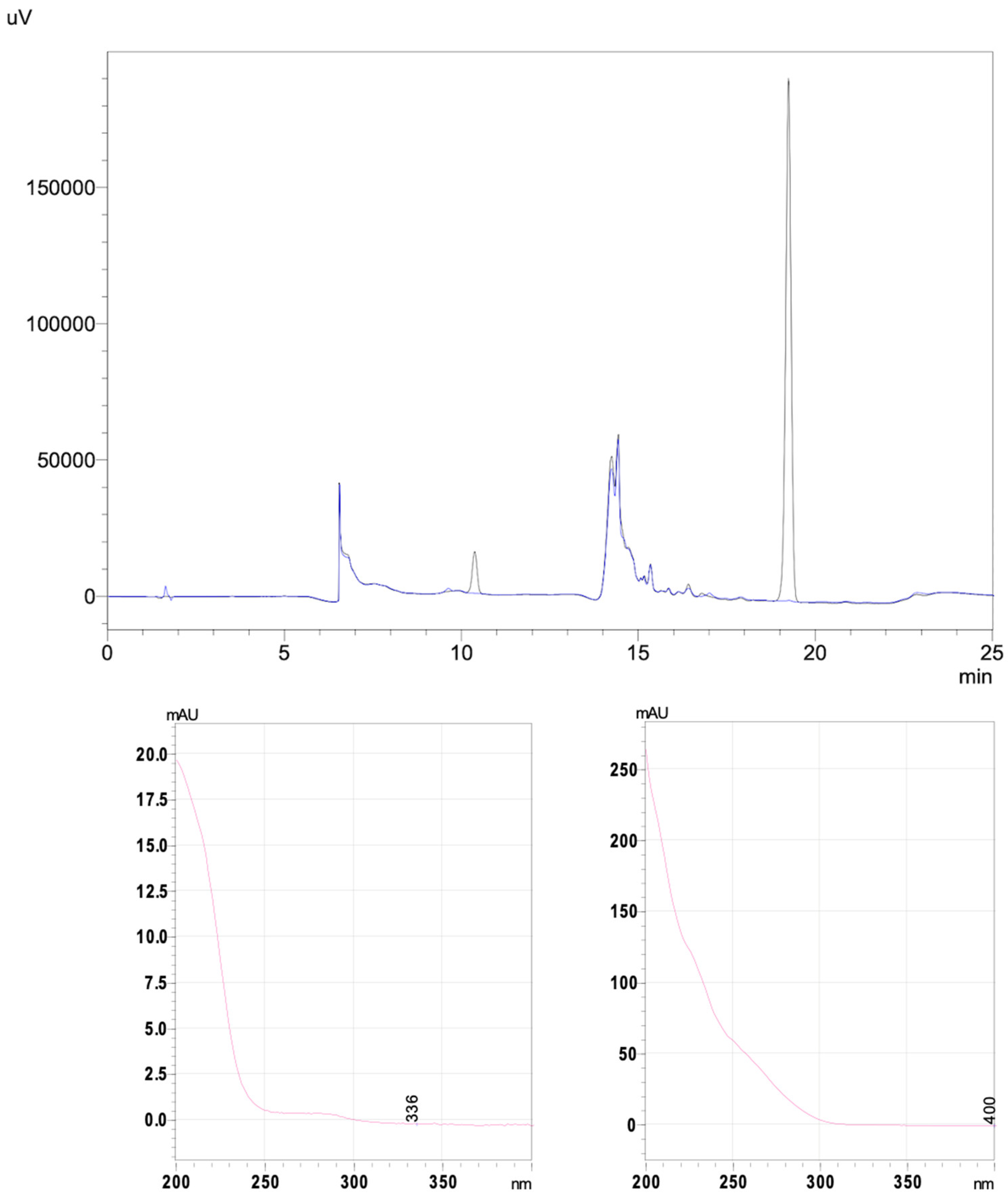
2.1. Validation Variables for the HPLC-UV Method
2.2. The Forced Degradation Study
2.3. The Stability Study
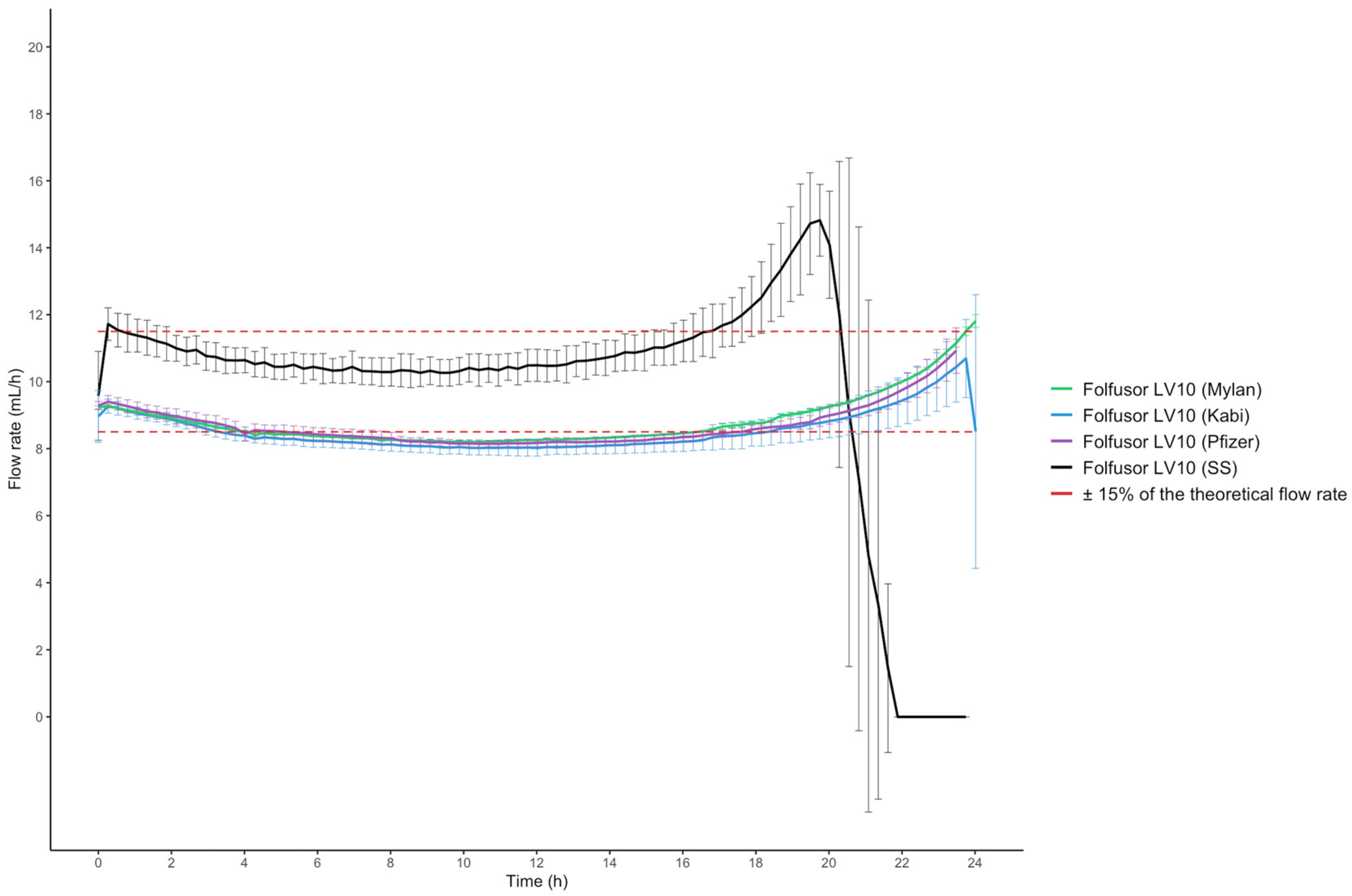
2.4. Drug Solution Flow Rates and Viscosity
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents and Diluents
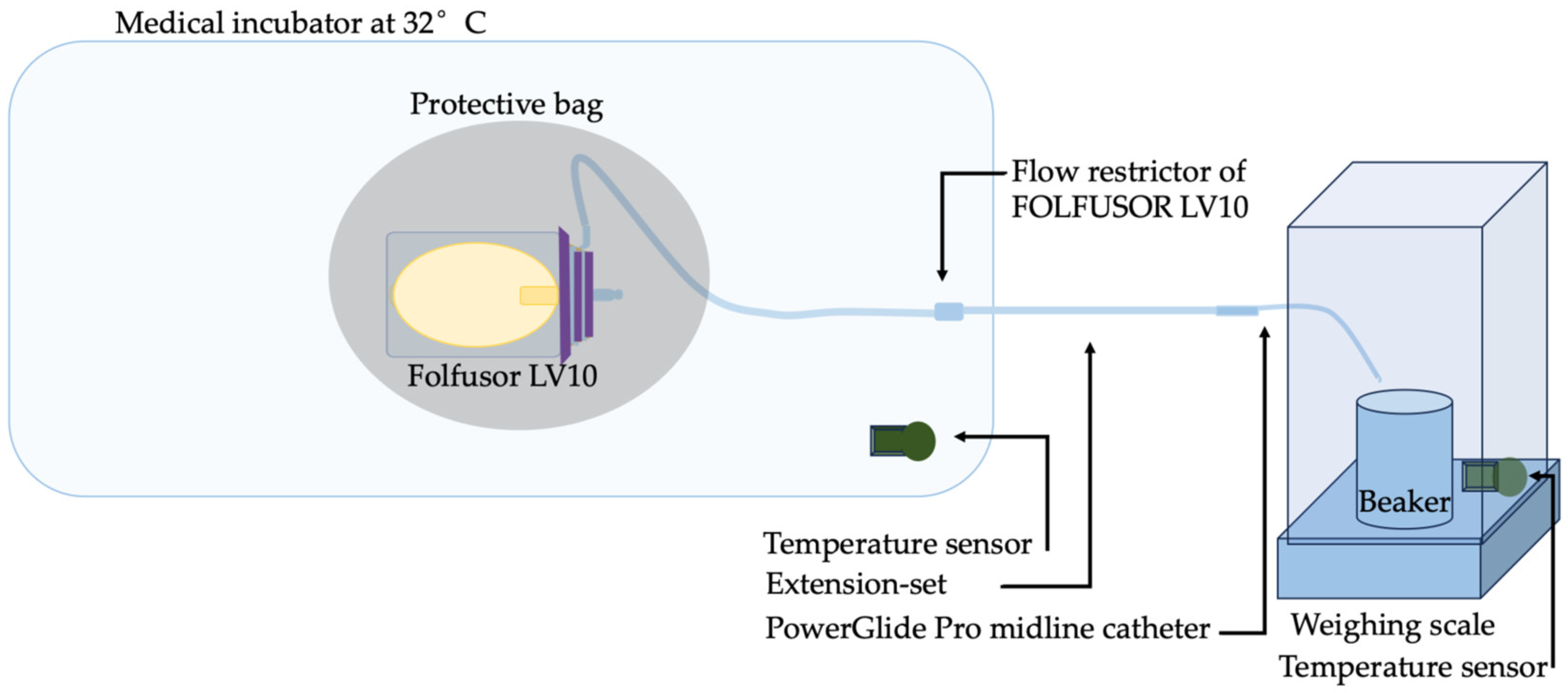
4.2. Preparation of Solutions and the In Vitro Infusion Set-Up
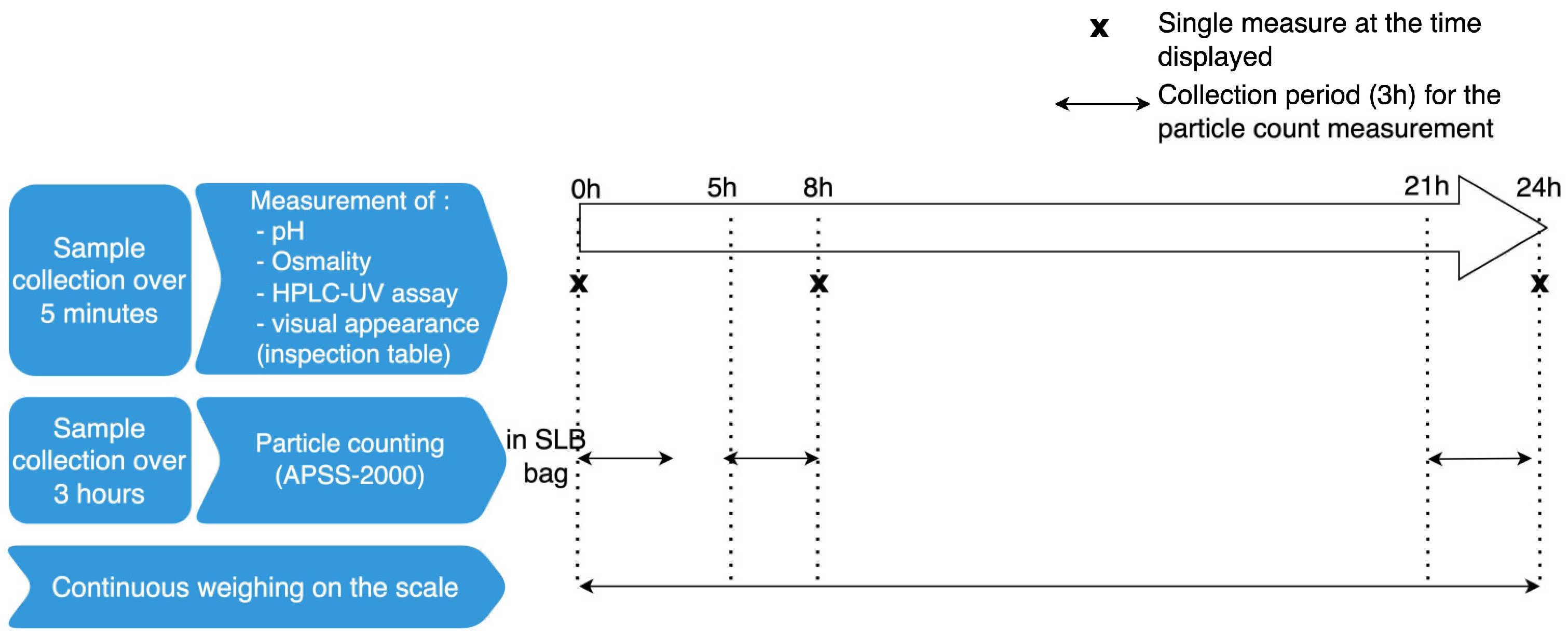
4.3. The 24-H Stability Study
4.3.1. Chromatographic Apparatus and Conditions
4.3.2. The HPLC-UV Forced Degradation Study
4.3.3. Validation of the HPLC-UV Stability-Indicating Method
4.3.4. Measurements of pH and Osmolality
4.3.5. Visual Assessment
4.3.6. Particle Counts
4.4. Viscosity Measurements
4.5. Expression of the Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamieson, C.; Ozolina, L.; Seaton, R.A.; Gilchrist, M.; Hills, T.; Drummond, F.; Wilkinson, A.S. Assessment of the Stability of Citrate-Buffered Piperacillin/Tazobactam for Continuous Infusion When Stored in Two Commercially Available Elastomeric Devices for Outpatient Parenteral Antimicrobial Chemotherapy: A Study Compliant with the NHS Yellow Cover Document Requirements. Eur. J. Hosp. Pharm. 2022, 29, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Loeuille, G.; D’Huart, E.; Vigneron, J.; Nisse, Y.-E.; Beiler, B.; Polo, C.; Ayari, G.; Sacrez, M.; Demoré, B.; Charmillon, A. Stability Studies of 16 Antibiotics for Continuous Infusion in Intensive Care Units and for Performing Outpatient Parenteral Antimicrobial Therapy. Antibiotics 2022, 11, 458. [Google Scholar] [CrossRef]
- Base de données publique des médicaments Summary of Product Characteristics- Pipéracilline/Tazobactam VIATRIS 4 g/0.5 g, Powder for Solution for Infusion. Available online: https://base-donnees-publique.medicaments.gouv.fr/affichageDoc.php?specid=61008378&typedoc=R (accessed on 26 July 2022).
- Monographie-Piperacillin Sodium/Tazobactam-Stabilis 4.0. Available online: https://www.stabilis.org/Monographie.php?IdMolecule=215 (accessed on 1 August 2024).
- Calbiac, P.D.; Lamoureux, F.; Pourrat, X.; Bretault, L.; Marchand, S.; Grassin, J.; Antier, D. Traitement Des Surinfections Bronchiques: Stabilité Des Antibiotiques Dans Les Diffuseurs Portatifs. Therapies 2006, 61, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Commission d’évaluation des Produits et Prestations, Haute Autorité de Santé Avis de la Commission du 21 mai 2003: Diffuseurs Portables. Available online: https://www.has-sante.fr/jcms/c_398549/fr/diffuseurs-portables (accessed on 22 May 2024).
- Guiffant, G.; Durussel, J.-J.; Flaud, P.; Vigier, J.-P.; Dupont, C.; Bourget, P.; Merckx, J. Mechanical Performances of Elastomers Used in Diffusers. Med. Devices 2011, 4, 71–76. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fernández-Rubio, B.; Del Valle-Moreno, P.; Herrera-Hidalgo, L.; Gutiérrez-Valencia, A.; Luque-Márquez, R.; López-Cortés, L.E.; Gutiérrez-Urbón, J.M.; Luque-Pardos, S.; Fernández-Polo, A.; Gil-Navarro, M.V. Stability of Antimicrobials in Elastomeric Pumps: A Systematic Review. Antibiotics 2021, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Baxter. Baxter Elastomeric Pumps: Technical Guide 2019; Baxter: Guyancourt, France, 2024. [Google Scholar]
- Vygon. Elastomeric Brochure of Accufuser; Vygon: Swindon, UK, 2018. [Google Scholar]
- Song, W.; Zhong, F.; Calautit, J.K.; Li, J. Exploring the Role of Skin Temperature in Thermal Sensation and Thermal Comfort: A Comprehensive Review. Energy Built Environ. 2024. [Google Scholar] [CrossRef]
- Abe, T.; Matsuzaka, K.; Nakayama, T.; Otsuka, M.; Sagara, A.; Sato, F.; Yumoto, T. Impact of Air Temperature and Drug Concentration on Liquid Emission from Elastomeric Pumps. J. Pharm. Health Care Sci. 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Quintens, C.; Spriet, I. 4CPS-049 Underdosing with High Dose Piperacillin/Tazobactam Administered via Continuous Infusion in Outpatient Parenteral Antimicrobial Therapy: A Stability or Viscosity Problem? Eur. J. Hosp. Pharm. 2020, 27, A70. [Google Scholar] [CrossRef]
- Akahane, M.; Enoki, Y.; Saiki, R.; Hayashi, Y.; Hiraoka, K.; Honma, K.; Itagaki, M.; Gotoda, M.; Shinoda, K.; Hanyu, S.; et al. Stability of Antimicrobial Agents in an Elastomeric Infusion Pump Used for Outpatient Parenteral Antimicrobial Therapy. Int. J. Infect. Dis. 2021, 103, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Bourget, P.; Amin, A.; Dupont, C.; Abely, M.; Desmazes-Dufeu, N.; Dubus, J.C.; Jouani, B.-L.; Merlette, C.; Nové-Josserand, R.; Pages, J.; et al. How To Minimize Toxic Exposure to Pyridine during Continuous Infusion of Ceftazidime in Patients with Cystic Fibrosis? Antimicrob. Agents Chemother. 2014, 58, 2849–2855. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.; Shanu, S.; Jamieson, C.; Santillo, M. Systematic Review of the Stability of Antimicrobial Agents in Elastomeric Devices for Outpatient Parenteral Antimicrobial Therapy Services Based on NHS Yellow Cover Document Standards. Eur. J. Hosp. Pharm. 2022, 29, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Diamantis, S.; Dawudi, Y.; Cassard, B.; Longuet, P.; Lesprit, P.; Gauzit, R. Home Intravenous Antibiotherapy and the Proper Use of Elastomeric Pumps: Systematic Review of the Literature and Proposals for Improved Use. Infect. Dis. Now 2021, 51, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Viaene, E.; Chanteux, H.; Servais, H.; Mingeot-Leclercq, M.-P.; Tulkens, P.M. Comparative Stability Studies of Antipseudomonal Beta-Lactams for Potential Administration through Portable Elastomeric Pumps (Home Therapy for Cystic Fibrosis Patients) and Motor-Operated Syringes (Intensive Care Units). Antimicrob. Agents Chemother. 2002, 46, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Voumard, R.; Van Neyghem, N.; Cochet, C.; Gardiol, C.; Decosterd, L.; Buclin, T.; de Valliere, S. Antibiotic Stability Related to Temperature Variations in Elastomeric Pumps Used for Outpatient Parenteral Antimicrobial Therapy (OPAT). J. Antimicrob. Chemother. 2017, 72, 1462–1465. [Google Scholar] [CrossRef] [PubMed]
- Perks, S.J.; Lanskey, C.; Robinson, N.; Pain, T.; Franklin, R. Systematic Review of Stability Data Pertaining to Selected Antibiotics Used for Extended Infusions in Outpatient Parenteral Antimicrobial Therapy (OPAT) at Standard Room Temperature and in Warmer Climates. Eur. J. Hosp. Pharm. 2020, 27, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Loeuille, G. Réalisation d’études de Stabilité d’antibiotiques, Ceftolozane/Tazobactam, Méropénem, Témocilline, Céfidérocol, Ceftazidime, Ceftazidime/Avibactam et Pipéracilline/Tazobactam En Seringue et En Diffuseur Portable Dans Le Cadre de l’optimisation de Ces Molécules En Administration Continue En Réanimation et En Ambulatoire. PhD Thesis, Université de Lorraine, Nancy, France, 2021. [Google Scholar]
- El Saghir, F.; Meier, J.; Bornand, D.; Senn, M.; Deuster, S. Stability Testing of Piperacilline/Tazobactam in Elastomeric Infusion Pumps. Pharm. Hosp. Clin. 2017, 52, e32. [Google Scholar] [CrossRef]
- Fernández-Rubio, B.; Herrera-Hidalgo, L.; de Alarcón, A.; Luque-Márquez, R.; López-Cortés, L.E.; Luque, S.; Gutiérrez-Urbón, J.M.; Fernández-Polo, A.; Gutiérrez-Valencia, A.; Gil-Navarro, M.V. Stability Studies of Antipseudomonal Beta Lactam Agents for Outpatient Therapy. Pharmaceutics 2023, 15, 2705. [Google Scholar] [CrossRef] [PubMed]
- NHS Pharmaceutical Quality Assurance Committee (PQAC) A Standard Protocol for Deriving and Assessment of Stability. Part 1-Aseptic Preparations (Small Molecules). 2015. Available online: https://pasg.nhs.uk/downloads.php?did=288 (accessed on 1 August 2024).
- NHS Pharmaceutical Quality Assurance Committee (PQAC) A Standard Protocol for Deriving and Assessment of Stability. Part 1-Aseptic Preparations (Small Molecules). 2019. Available online: https://www.sps.nhs.uk/articles/a-standard-protocol-for-deriving-and-assessment-of-stability-part-1-aseptic-preparation/ (accessed on 1 August 2024).
- Salman, D.; Biliune, J.; Kayyali, R.; Ashton, J.; Brown, P.; McCarthy, T.; Vikman, E.; Barton, S.; Swinden, J.; Nabhani-Gebara, S. Evaluation of the Performance of Elastomeric Pumps in Practice: Are We under-Delivering on Chemotherapy Treatments? Curr. Med. Res. Opin. 2017, 33, 2153–2159. [Google Scholar] [CrossRef]
- Perks, S.J.; Pain, T.; Franklin, R. Total Intended Antibiotic Delivery Related to Drug Concentration Affecting the Flow Rate of Elastomeric Devices Used in Outpatient Parenteral Antimicrobial Therapy (OPAT). J. Pharm. Pract. Res. 2019, 49, 349–355. [Google Scholar] [CrossRef]
- Pandya, K.H.; Eaton, V.; Kowalski, S.; Sluggett, J.K. Safety of Continuous Antibiotic Infusions Administered through an Australian Hospital in the Home Service: A Pilot Study. J. Pharm. Pract. Res. 2017, 47, 333–339. [Google Scholar] [CrossRef]
- Kawabata, Y. Effect of Coefficient of Viscosity and Ambient Temperature on the Flow Rate of Drug Solutions in Infusion Pumps. Pharm. Dev. Technol. 2012, 17, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, S.D.; Reed, L.D.; Nolan, R.W.; Cattroll, S.W. Measurement of Torso Skin Temperature under Clothing. Europ. J. Appl. Physiol. 1988, 57, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Pinewood Laboratories, Ireland Patient Information Leaflet for Piperacillin/Tazocillin Powder for Solution for Infusion 2022.
- Cassettari, V.; Novato, N.; Onuchic, M.H.F. Antimicrobial Stewardship in the Outpatient Parenteral Antimicrobial Therapy (OPAT) Setting: The Impact of Prescription Assessment by an Infectious Diseases Specialist. Braz. J. Infect. Dis. 2021, 25, 101560. [Google Scholar] [CrossRef] [PubMed]
- Base de données publique des médicaments Summary of Product Characteristics-Tazocilline 4 g/0.5 g, Powder for Solution for Infusion-Base de Données Publique Des Médicaments. Available online: https://base-donnees-publique.medicaments.gouv.fr/affichageDoc.php?specid=60445784&typedoc=R (accessed on 3 June 2024).
- European Medicines Agency. Summary of Product Characteristics of Tazocin, Powder for Solution for Infusion, Labelling and Package Leaflet, Annex III. Available online: https://www.ema.europa.eu/en/documents/referral/tazocin-article-30-referral-annex-iii_en.pdf (accessed on 1 August 2024).
- SFPC (French Society of Clinical Pharmacy); GERPAC (Evaluation and Research Group on Protection in Controlled Atmosphere) Methodological Guidelines for Stability Studies of Hospital Pharmaceuticals Preparations, Part 1: Liquid Preparations 2013. Available online: https://www.gerpac.eu/IMG/pdf/guide_stabilite_anglais.pdf (accessed on 1 August 2024).
- Chapuzet, E.; Mercier, N.; Bervoas-Martin, S.; Boulanger, B.; Chevalier, P.; Chiap, P.; Grandjean, D.; Hubert, P.; Lagorge, P.; Lallier, M.; et al. Méthodes Chromatographiques de Dosage Dans Les Milieux Biologiques: Stratégie de Validation-Rapport d’une Commission SFSTP. S.T.P. Pharma Prat. 1997, 7, 169–194. [Google Scholar]
- European Pharmacopoeia 11.5 2.2.3. Potentiometric Determination of pH. Available online: https://pheur-edqm-eu.ressources-electroniques.univ-lille.fr/app/11-5/content/default/20203E.htm (accessed on 24 May 2024).
- European Pharmacopoeia 11.5 2.2.35. Osmolality. Available online: https://pheur-edqm-eu.ressources-electroniques.univ-lille.fr/app/11-5/content/11-5/20235E.htm?highlight=on&terms=2.2.35 (accessed on 24 May 2024).
- Gorski, L.A. The 2016 Infusion Therapy Standards of Practice. Home Healthc Now 2017, 35, 10–18. [Google Scholar] [CrossRef]
- European Pharmacopoeia 11.5 2.9.20. Particulate Contamination: Visible Particles. Available online: https://pheur-edqm-eu.ressources-electroniques.univ-lille.fr/app/11-5/content/11-5/20920E.htm?highlight=on&terms=visual&terms=visual (accessed on 24 May 2024).
- European Pharmacopoeia Commission 2.9.19. Particulate Contamination: Sub-Visible Particles. In European Pharmacopoeia 10.8. 2020, pp. 5079–5081. Available online: https://pheur.edqm.eu (accessed on 1 August 2024).

| Validation Variables | Piperacillin (Mylan, Fresenius Kabi) | Tazobactam (Mylan, Fresenius Kabi) | Piperacillin (Pfizer) | Tazobactam (Pfizer) | |
|---|---|---|---|---|---|
| Calibration range | 150–350 µg/mL | 18.75–43.75 µg/mL | 150–350 µg/mL | 18.75–43.75 µg/mL | |
| Determination coefficient | 0.997 | 0.999 | 0.999 | 0.996 | |
| Regression coefficients | Slope | 867.85 | 4897.28 | 842.07 | 4763.45 |
| Intercept | 7535.48 | 1247.50 | 3264.58 | 3560.96 | |
| Limit of detection (µg/mL) | 8.227 | 0.576 | 4.488 | 1.279 | |
| Limit of quantification (µg/mL) | 16.454 | 1.152 | 8.976 | 2.558 | |
| Cochran’s test | Experimental value | 0.3966 | 0.334 | 0.4067 | 0.3155 |
| Theoretical value (α = 5%; 5; 8) | 0.4564 | 0.4564 | 0.4564 | 0.4564 | |
| ANOVA (non-linearity) | Experimental value (α = 5%; 1; 3) | 13,310.91 | 43,118.28 | 43,334.05 | 8284.46 |
| Theoretical value | 4.11 | 4.11 | 4.11 | 4.11 | |
| Generic P/T (Fresenius Kabi) | T0 h (Bag Preparation) | T0 h | T8 h | T24 h |
|---|---|---|---|---|
| Appearance of the solution | Clear and colorless | Clear and colorless | Clear and colorless | Clear and colorless |
| P concentration% | / | 100.0 ± 2.27 | 99.08 ± 0.64 | 97.91 ± 1.44 |
| T concentration% | / | 100.0 ± 9.62 | 98.64 ± 7.79 | 97.39 ± 6.13 |
| pH | 5.4 ± 0.0 | 5.3 ± 0.1 | 5.1 ± 0.1 | 5.0 ± 0.0 |
| Osmolality (mOsm/kg) | 417 ± 4 | 417 ± 4 | 415 ± 3 | 418 ± 2 |
| Particles ≥ 10 µm/mL | 396 ± 103 | 5 ± 4 | 2 ± 1 | 5 ± 4 |
| Particles ≥ 25 µm/mL | 3 ± 2 | 0 ± 0 | 0 ± 1 | 0 ± 0 |
| Generic P/T (Mylan) | T0 h (Bag Preparation) | T0 h | T8 h | T24 h |
|---|---|---|---|---|
| Appearance of the solution | Clear and colorless | Clear and colorless | Clear and colorless | Clear and colorless |
| P concentration% | / | 100.0 ± 1.17 | 98.36 ± 0.70 | 95.40 ± 1.16 |
| T concentration% | / | 100.0 ± 1.47 | 98.76 ± 0.80 | 99.88 ± 1.94 |
| pH | 5.4 ± 0.0 | 5.4 ± 0.0 | 5.2 ± 0.1 | 4.9 ± 0.0 |
| Osmolality (mOsm/kg) | 414 ± 3 | 415 ± 2 | 416 ± 3 | 415 ± 1 |
| Particles ≥ 10 (µm/mL) | 31 ± 12 | 3 ± 3 | 2 ± 2 | 6 ± 3 |
| Particles ≥ 25 (µm/mL) | 1 ± 1 | 0 ± 1 | 0 ± 0 | 1 ± 1 |
| Proprietary Drug (Pfizer) | T0 h (Bag Preparation) | T0 h | T8 h | T24 h |
|---|---|---|---|---|
| Appearance of the solution | Clear and colorless | Clear and colorless | Clear and colorless | Clear and colorless |
| P concentration% | / | 100.0 ± 1.04 | 97.06 ± 1.18 | 96.04 ± 0.87 |
| T concentration% | / | 100.0 ± 3.45 | 103.01 ± 0.68 | 99.38 ± 0.16 |
| pH | 6.1 ± 0.0 | 6.1 ± 0.0 | 6.0 ± 0.0 | 5.8 ± 0.0 |
| Osmolality (mOsm/kg) | 439 ± 3 | 442 ± 1 | 442 ± 4 | 441 ± 4 |
| Particles ≥ 10 (µm/mL) | 90 ± 8 | 7 ± 5 | 3 ± 1 | 5 ± 1 |
| Particles ≥ 25 (µm/mL) | 1 ± 1 | 1 ± 1 | 0 ± 0 | 0 ± 1 |
| Generic P/T (Mylan) in the Elastomeric Device | Generic P/T (Fresenius Kabi) in the Elastomeric Device | Proprietary Drug (Pfizer) in the Elastomeric Device | |
|---|---|---|---|
| Weight of the empty Folfusor® LV10 (g) | 62.04 ± 0.35 | 61.94 ± 0.30 | 61.76 ± 0.73 |
| Weight of the filled Folfusor® LV10, before infusion (g) | 307.58 ± 0.61 | 305.30 ± 0.78 | 308.27 ± 1.28 |
| Calculated weight of solution before infusion (g) | 245.55 ± 0.95 | 243.36 ± 0.48 | 246.52 ± 1.72 |
| Calculated volume of solution before infusion (mL) | 239.09 ± 0.92 | 236.96 ± 0.46 | 239.34 ± 1.67 |
| Calculated weight of solution after the 24-h infusion (g) | 20.02 ± 3.60 | 20.29 ± 4.59 | 24.84 ± 5.01 |
| Calculated volume of solution after the 24-h infusion (mL) | 19.50 ± 3.50 | 19.76 ± 5.47 | 24.12 ± 4.86 |
| Proportion of infusion solution not infused (volume, %) | 8.12 ± 1.46 | 8.23 ± 1.86 | 10.04 ± 2.03 |
| Analysis Time (min) | Organic Phase/Aqueous Phase % |
|---|---|
| 0–3 | 0/100 |
| 3.5–11 | 8/92 |
| 11.5–20 | 30/70 |
| 20.5–25 | 0/100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Négrier, L.; Mena, A.M.; Dupont, C.; Gamache, P.; Zimbril, J.-O.; Abdoune, Y.; Karrout, Y.; Odou, P.; Genay, S.; Décaudin, B. The Infusion of Piperacillin/Tazobactam with an Elastomeric Device: A Combined 24-H Stability Study and Drug Solution Flow Rate Analysis. Pharmaceuticals 2024, 17, 1085. https://doi.org/10.3390/ph17081085
Négrier L, Mena AM, Dupont C, Gamache P, Zimbril J-O, Abdoune Y, Karrout Y, Odou P, Genay S, Décaudin B. The Infusion of Piperacillin/Tazobactam with an Elastomeric Device: A Combined 24-H Stability Study and Drug Solution Flow Rate Analysis. Pharmaceuticals. 2024; 17(8):1085. https://doi.org/10.3390/ph17081085
Chicago/Turabian StyleNégrier, Laura, Anthony Martin Mena, Christian Dupont, Philémon Gamache, Jeanne-Olive Zimbril, Yasmine Abdoune, Youness Karrout, Pascal Odou, Stéphanie Genay, and Bertrand Décaudin. 2024. "The Infusion of Piperacillin/Tazobactam with an Elastomeric Device: A Combined 24-H Stability Study and Drug Solution Flow Rate Analysis" Pharmaceuticals 17, no. 8: 1085. https://doi.org/10.3390/ph17081085
APA StyleNégrier, L., Mena, A. M., Dupont, C., Gamache, P., Zimbril, J.-O., Abdoune, Y., Karrout, Y., Odou, P., Genay, S., & Décaudin, B. (2024). The Infusion of Piperacillin/Tazobactam with an Elastomeric Device: A Combined 24-H Stability Study and Drug Solution Flow Rate Analysis. Pharmaceuticals, 17(8), 1085. https://doi.org/10.3390/ph17081085






