Superhydrophobicity Effects on Spheroid Formation and Polarization of Macrophages
Abstract
1. Introduction
2. Results
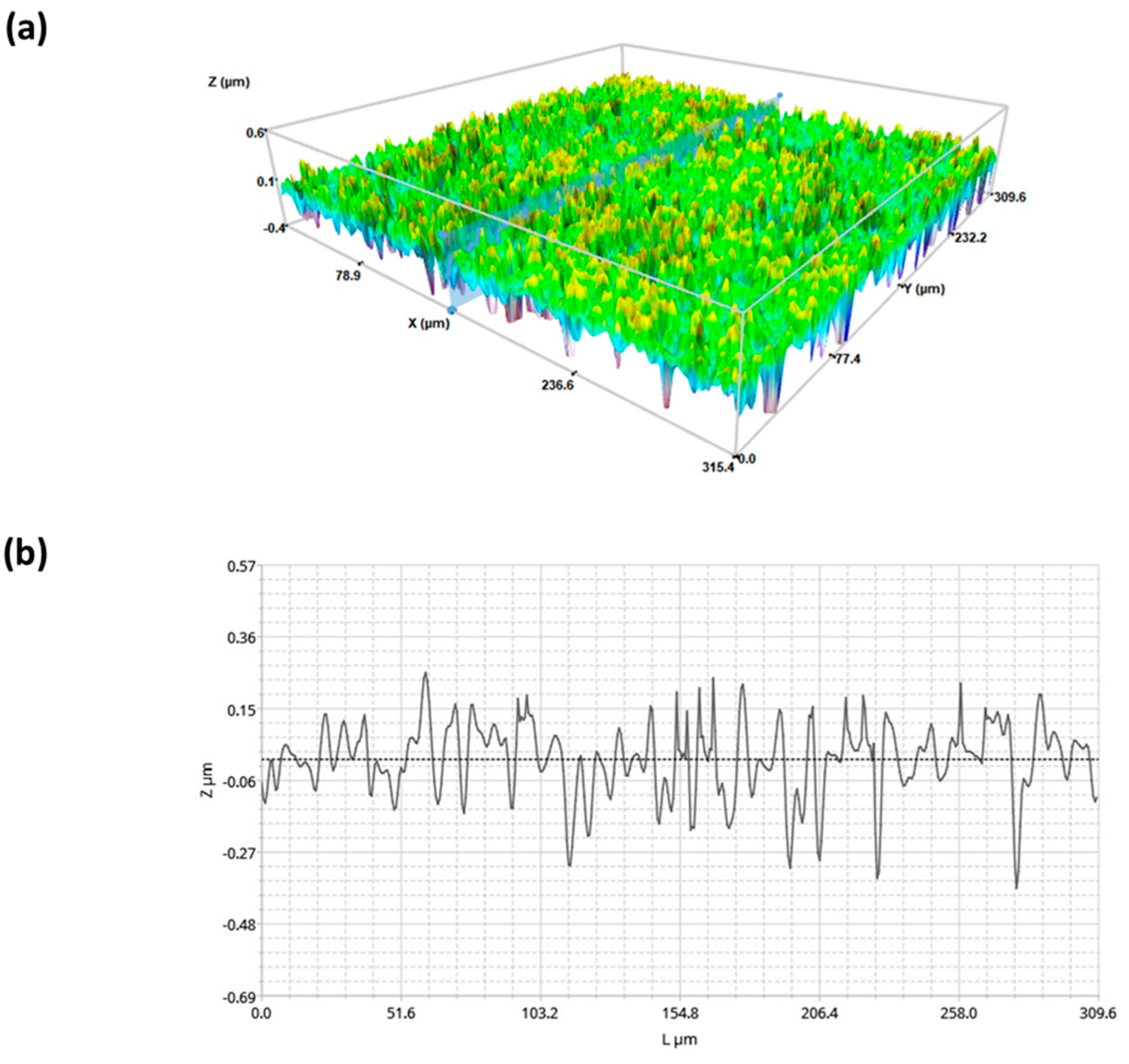
2.1. Surface Characterization of Superhydophobic Coating
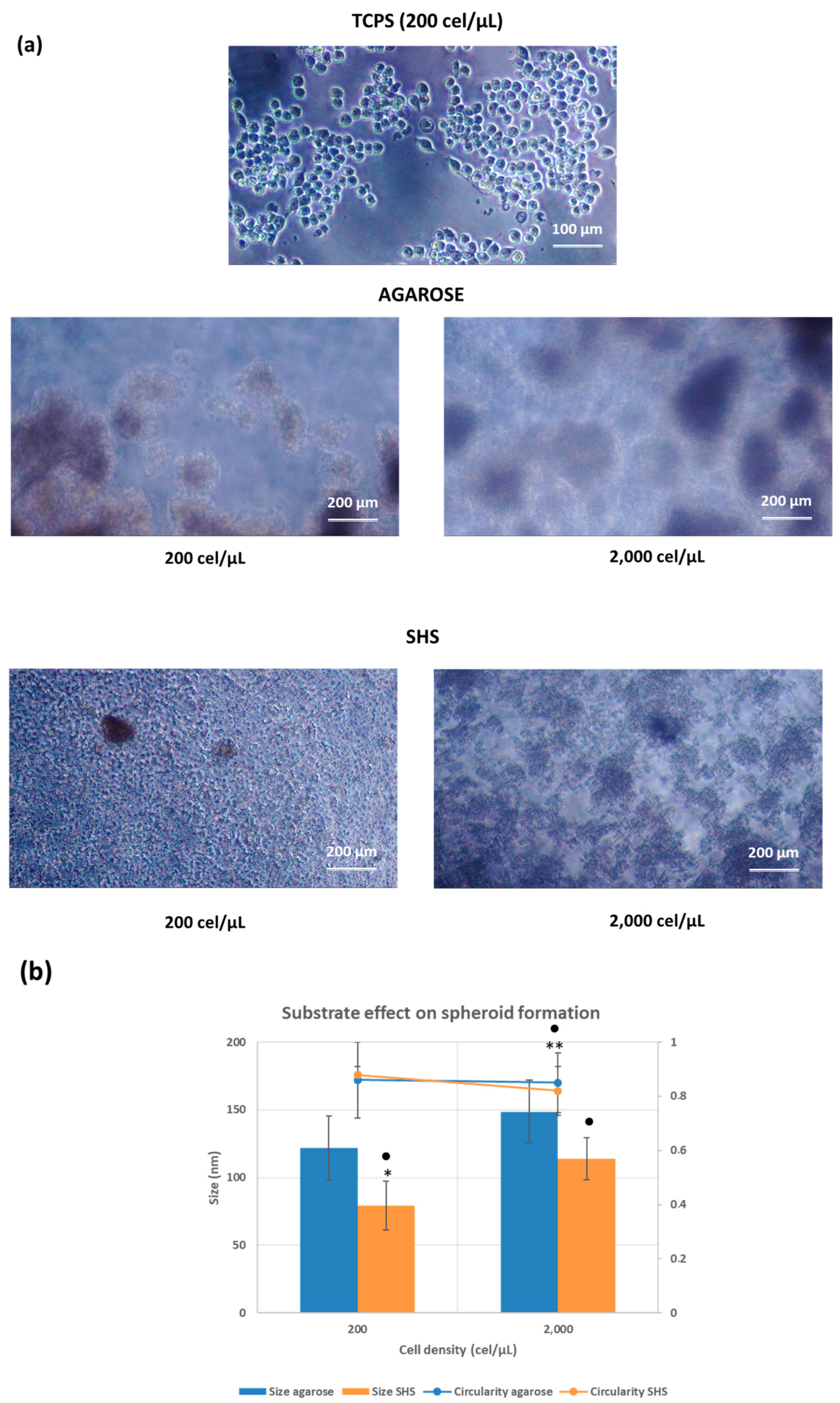
2.2. Cell Behavior in Different Substrates
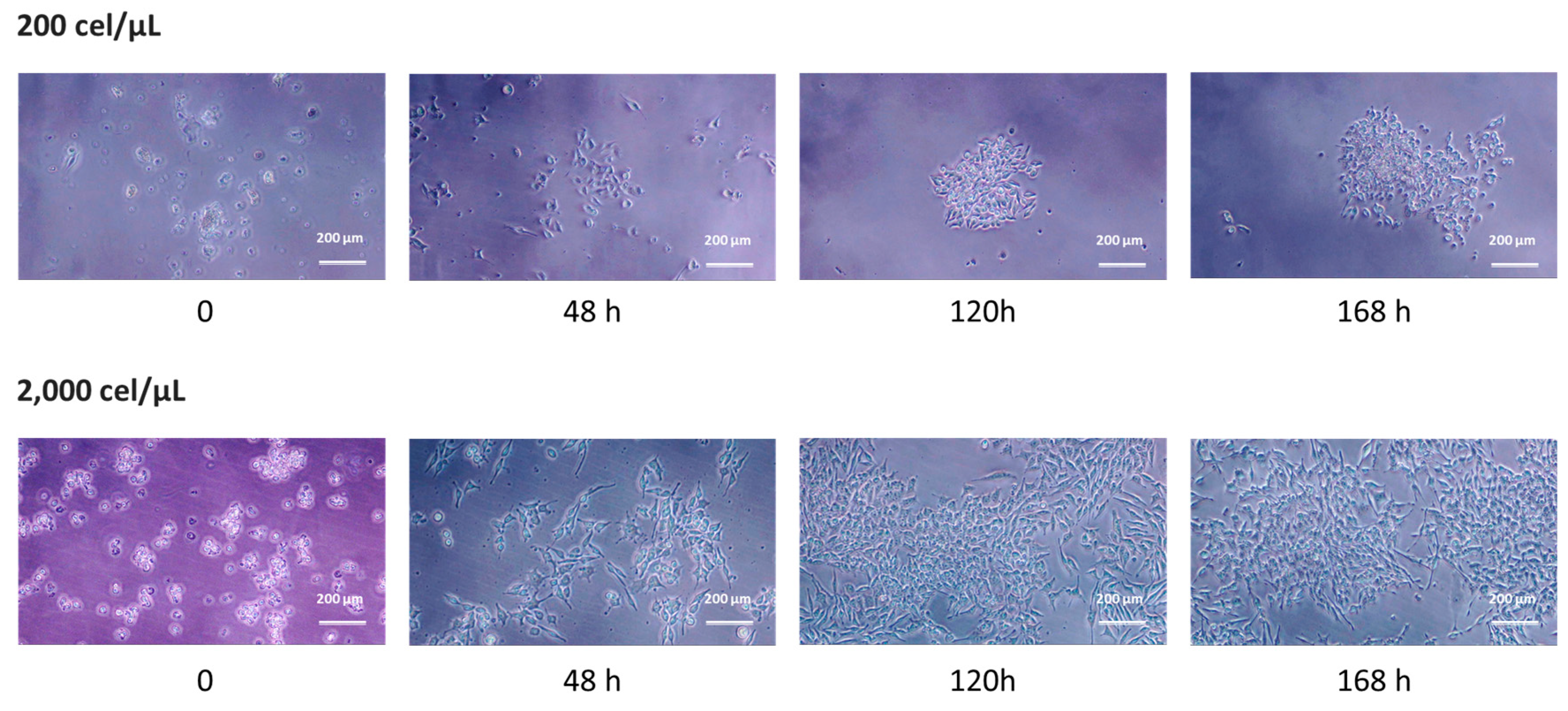
2.3. 2D Growth as a Measure of Cell Viability
2.4. Morphological Characterization of Migrated Cells from SHS
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Surface Preparation and Characterization
4.2.2. Cell Cultures
4.2.3. Conventional 2D Culture
4.2.4. Cell Culture in Agarose-Induced 3D Culture
4.2.5. Cell Culture in Superhydrophobic Substrates
4.2.6. Spheroid Recovery and Growth under 2D Conditions
4.2.7. Profilometry Studies
4.2.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pierres, A.; Benoliel, A.M.; Bongrand, P. Cell-cell interaction. In Physical Chemistry of Biological Interfaces; Baszkin, A., Norde, W., Eds.; Marcel Dekker: New York, NY, USA, 1999; pp. 459–522. [Google Scholar]
- Brochard-Wyart, F.; de Gennes, P.G. Adhesion induced by mobile binders: Dynamics. Proc. Natl. Acad. Sci. USA 2002, 99, 7854–7859. [Google Scholar] [CrossRef] [PubMed]
- Schakenraad, J.M.; Busscher, H.J. Cell polymer interactions—The influence of protein adsorption. Colloids Surf. 1989, 42, 331–343. [Google Scholar] [CrossRef]
- Harnett, E.M.; Alderman, J.; Wood, T. The surface energy of various biomaterials coated with adhesion molecules used in cell culture. Colloids Surf. B 2007, 55, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Nagase, K.; Kobayashi, J.; Okano, T. Temperature-responsive intelligent interfaces for biomolecular separation and cell sheet engineering. J. R. Soc. Interface 2009, 6, S293–S309. [Google Scholar] [CrossRef] [PubMed]
- Vagaska, B.; Bacakova, L.; Filova, E.; Balik, K. Osteogenic cells on bio-inspired materials for bone tissue engineering. Physiol. Res. 2010, 59, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M. Biocompatibility of materials. In Biomaterials and Tissue Engineering. Biological and Medical Physics, Biomedical Engineering; Shi, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Ratner, B.D. The biocompatibility of implant materials. In Host Response to Biomaterials. The Impact of Host Response on Biomaterial Selection; BadylaK, S.F., Ed.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Kulinets, I. Biomaterials and their applications in medicine. In Regulatory Affairs for Biomaterials and Medical Devices. A Volume in Woodhead Publishing Series in Biomaterials; Amato, S.F., Ezzell, R.M., Eds.; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- Oliveira, S.M.; Alves, N.A.; Mano, J.F. Cell interactions with superhydrophilic and superhydrophobic surfaces. J. Adhes. Sci. Technol. 2012, 28, 843–863. [Google Scholar] [CrossRef]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants—A review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef]
- Li, B.; Cao, H.; Zhao, Y.; Cheng, M.; Qin, H.; Cheng, T.; Hu, Y.; Zhang, X.; Liu, X. In vitro and in vivo responses of macrophages to magnesium-doped titanium. Sci. Rep. 2017, 7, 42707. [Google Scholar]
- Brown, B.N.; Ratner, B.D.; Goodman, S.B.; Amar, S.; Badylak, S.F. Macrophage polarization: An opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 2012, 33, 3792–3802. [Google Scholar] [CrossRef]
- Liu, Y.; Minze, L.J.; Mumma, L.; Li, X.C.; Ghobrial, R.M.; Kloc, M. Mouse macrophage polarity and ROCK1 activity depend on RhoA and non-apoptotic caspase 3. Exp. Cell Res. 2016, 341, 225–236. [Google Scholar] [CrossRef]
- Liu, Y.; Tejpal, N.; You, J.; Li, X.C.; Ghobrial, R.M.; Kloc, M. ROCK inhibition impedes macrophage polarity and functions. Cell Immunol. 2016, 300, 54–62. [Google Scholar] [CrossRef]
- Boersema, G.S.; Grotenhuis, N.; Bayon, Y.; Lange, J.F.; Bastiaansen-Jenniskens, Y.M. The Effect of Biomaterials Used for Tissue Regeneration Purposes on Polarization of Macrophages. BioRes. Open Access 2016, 5, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.U.; Gott, S.C.; Woo, B.W.; Rao, M.P.; Liu, W.F. Micro- and Nanopatterned Topographical Cues for Regulating Macrophage Cell Shape and Phenotype. ACS Appl. Mater. Interfaces 2015, 7, 28665–28672. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, R.; Cameron, A.R.; Kelly, D.J.; Kearney, C.J.; O’Brien, F.J. Biomaterial based modulation of macrophage polarization: A review and suggested design principles. Mater. Today 2015, 18, 313–325. [Google Scholar] [CrossRef]
- Hsieh, J.Y.; Keating, M.T.; Smith, T.D.; Meli, V.S.; Botvinick, E.L.; Liu, W.F. Matrix crosslinking enhances macrophage adhesion, migration, and inflammatory activation. APL Bioeng. 2019, 3, 016103. [Google Scholar] [CrossRef] [PubMed]
- Adlerz, K.M.; Aranda-Espinoza, H.; Hayenga, H.N. Substrate elasticity regulates the behavior of human monocyte-derived macrophages. Eur. Biophys. J. 2016, 45, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Meli, V.S.; Atcha, H.; Veerasubramanian, P.K.; Nagalla, R.R.; Luu, T.U.; Chen, E.Y.; Guerrero-Juarez, C.F.; Yamaga, K.; Pandori, W.; Hsieh, J.Y.; et al. YAP453 mediated mechanotransduction tunes the macrophage inflammatory response. Sci. Adv. 2020, 6, eabb8471. [Google Scholar] [CrossRef] [PubMed]
- Blakney, A.K.; Swartzlander, M.D.; Bryant, S.J. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels. J. Biomed. Mater. Res. A 2012, 100, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Dutta, B.; Goswami, R.; Rahaman, S.O. TRPV4 Plays a Role in Matrix Stiffness-Induced Macrophage Polarization. Front. Immunol. 2020, 11, 570195. [Google Scholar] [CrossRef]
- Friedemann, M.; Kalbitzer, L.; Franz, S.; Moeller, S.; Schnabelrauch, M.; Simon, J.C.; Pompe, T.; Franke, K. Instructing Human Macrophage Polarization by Stiffness and Glycosaminoglycan Functionalization in 3D Collagen Networks. Adv. Healthc. Mater. 2017, 6, 1600967. [Google Scholar] [CrossRef]
- Taufalele, P.V.; Wang, W.; Simmons, A.J.; Southard-Smith, A.N.; Chen, B.; Greenlee, J.D.; King, M.R.; Lau, K.J.S.; Hassane, D.C.; Bordeleau, F.; et al. Matrix stiffness enhances cancer-macrophage interactions and M2-like macrophage accumulation in the breast tumor microenvironment. Acta Biomater. 2023, 163, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Larsen, A.M.H.; Kuczek, D.E.; Kalvisa, A.; Siersbæk, M.S.; Thorseth, M.L.; Johansen, A.Z.; Carretta, M.; Grøntved, L.; Vang, O.; Madsen, D.H. Collagen Density Modulates the Immunosuppressive Functions of Macrophages. J. Immunol. 2020, 205, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.Z.; Chang, H.Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008, 3, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D tumor spheroids: An overview on the tools and techniques used for their analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Cirisano, F.; Morán, M.C. Super Liquid-repellent Surfaces and 3D Spheroids Growth. Front. Biosci. 2022, 27, 144. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Cirisano, F.; Morán, M.C. Mammalian Cell Spheroids on Mixed Organic–Inorganic Superhydrophobic Coating. Molecules 2022, 27, 1247. [Google Scholar] [CrossRef] [PubMed]
- Morán, M.C.; Cirisano, F.; Ferrari, M. Spheroid Formation and Recovery Using Superhydrophobic Coating for Regenerative Purposes. Pharmaceutics 2023, 15, 2226. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Kumacheva, E. Hydrogel microenvironments for cancer spheroid growth and drug screening. Sci. Adv. 2018, 4, eaas8998. [Google Scholar] [CrossRef] [PubMed]
- Karp, J.M.; Yeh, J.; Eng, G.; Fukuda, J.; Blumling, J.; Suh, K.Y.; Cheng, J.; Mahdavi, A.; Borenstein, J.; Langer, R.; et al. Controlling size, shape and homogeneity of embryoid bodies using poly(ethylene glycol) microwells. Lab Chip 2007, 7, 786–794. [Google Scholar] [CrossRef]
- Napolitano, A.P.; Dean, D.M.; Man, A.J.; Youssef, J.; Ho, D.N.; Rago, A.P.; Lech, M.P.; Morgan, J.R. Scaffold-free three-dimensional cell culture utilizing micromolded nonadhesive hydrogels. Biotechniques 2007, 43, 494–500. [Google Scholar] [CrossRef]
- Fukuda, J.; Khademhosseini, A.; Yeo, Y.; Yang, X.Y.; Yeh, J.; Eng, G.; Blumling, J.; Wang, C.F.; Kohane, D.S.; Langer, R. Micromolding of photocrosslinkable chitosan hydrogel for spheroid microarray and co-cultures. Biomaterials 2006, 27, 5259–5267. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.D.; Liu, J.M.; Chen, Y. Agarose multi-wells for tumour spheroid formation and anti-cancer drug test. Microelectron. Eng. 2016, 158, 41–45. [Google Scholar] [CrossRef]
- Siva Sankar, P.; Che Mat, M.F.; Muniandy, K.; Xiang, B.L.S.; Ling, P.S.; Hoe, S.L.L.; Khoo, A.S.; Mohana-Kumaran, N. Modeling nasopharyngeal carcinoma in three dimensions. Oncol. Lett. 2017, 13, 2034–2044. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Kwon, S.; Kim, K.S. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int. 2021, 21, 152. [Google Scholar] [CrossRef] [PubMed]
- Caiado Decarli, M.; Amaral, R.; Peres dos Santos, D.; Bueno Tofani, L.; Katayama, E.; Alvarenga Rezende, R.; Lopes da Silva, J.V.; Swiech, K.; Torres Suazo, C.A.; Mota, C.; et al. Cell spheroids as a versatile research platform: Formation mechanisms, high throughput production, characterization and applications. Biofabrication 2021, 13, 032002. [Google Scholar] [CrossRef] [PubMed]
- Morán, M.C.; Cirisano, F.; Ferrari, M. 3D profilometry and cell viability studies for drug response screening. Mater. Sci. Eng. C 2020, 115, 111142. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.; Chun, J.; Zangle, T.A.; Kalim, S.; Hong, J.S.; Pefley, S.E.; Zheng, X.; Gimzewski, J.K.; Teitell, M.A. Rapid, massively parallel single-cell drug response measurements via live cell interferometry. Biophys. J. 2011, 101, 1025. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, S.; Honig, F.; Vasilevich, A.; Roumans, N.; Romero, M.; Eren, A.D. Expanding Biomaterial Surface Topographical Design Space through Natural Surface Reproduction. Adv. Mater. 2021, 33, 2102084. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.K.; Lambert, I.H.; Pedersen, S.F. Physiology of Cell Volume Regulation in Vertebrates. Physiol. Rev. 2009, 89, 193–277. [Google Scholar] [CrossRef]
- McWhorter, F.Y.; Wang, T.; Nguyen, P.; Chung, T.; Liu, W.F. Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. USA 2013, 110, 17253–17258. [Google Scholar] [CrossRef]
- Menzyanova, N.G.; Pyatina, S.A.; Shabanov, A.V.; Nemtsev, I.V.; Stolyarov, D.P.; Dryganov, D.B.; Sakhnov, E.V.; Shishatskaya, E.I. The Morphology and Phenotype of Monocyte-Macrophages When Cultured on Bionanofilms Substrates with Different Surface Relief Profiles. Biomolecules 2019, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Wosik, J.; Suarez-Villagran, M.; Miller, J.H., Jr.; Ghobrial, R.M.; Kloc, M. Macrophage phenotype bioengineered by magnetic, genetic, or pharmacologic interference. Immunol. Res. 2019, 67, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Park, M.D.; Silvin, A.; Ginhoux, F.; Merad, M. Macrophages in health and disease. Cell 2022, 185, 4259–4279. [Google Scholar] [CrossRef] [PubMed]
- Benien, P.; Swami, A. 3D tumor models: History, advances and future perspectives. Future Oncol. 2014, 10, 1311–1327. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, S.P.; Pinto, C.; Martins, T.R.; Harrer, N.; Estrada, M.F.; Loza-Alvarez, P.; Cabecadas, J.; Alves, P.M.; Gualda, E.J.; Sommergruber, W.; et al. 3D-3-culture: A tool to unveil macrophage plasticity in the tumour microenvironment. Biomaterials 2018, 163, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Burchett, A.; Siri, S.; Li, J.; Lu, X.; Datta, M. Novel 3-D macrophage spheroid model reveals reciprocal regulation of immunomechanical stress and mechano-immunological response. bioRxiv 2024. [Google Scholar] [CrossRef]
- Ferrari, M.; Benedetti, A.; Santini, E.; Ravera, F.; Liggieri, L.; Guzman, E.; Cirisano, F. Biofouling control by superhydrophobic surfaces in shallow euphotic seawater. Colloids Surf. A-Physicochem. Eng. Asp. 2015, 480, 369–375. [Google Scholar] [CrossRef]
- Schmitz, T.; Jannasch, M.; Weigel, T.; Moseke, C.; Gbureck, U.; Groll, J.; Walles, H.; Hansmann, J. Nanotopographical Coatings Induce an Early Phenotype-Specific Response of Primary Material-Resident M1 and M2 Macrophages. Materials 2020, 13, 1142. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, T.; Saito, N.; Takai, O. Correlation of Cell Adhesive Behaviors on Superhydrophobic, Superhydrophilic, and Micropatterned Superhydrophobic/Superhydrophilic Surfaces to Their Surface Chemistry. Langmuir 2010, 26, 8147–8154. [Google Scholar] [CrossRef]
- Ribeiro, A.R.B.; Silva, E.C.O.; Araújo, P.M.C.; Souza, S.T.; Fonseca, E.J.D.S.; Barreto, E. Application of Raman spectroscopy for characterization of the functional polarization of macrophages into M1 and M2 cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 265, 20328. [Google Scholar] [CrossRef]
- Case Study. Morphological Surface Analysis to Study Cell Viability Induced by Proliferative and Toxic Treatments. Available online: https://www.sensofar.com/cs28-morphological-analysis-cell-viability/ (accessed on 6 March 2023).
- Liggieri, L.; Passerone, A. An automatic technique for measuring the surface tension of liquid metals. High Temp. Technol. 1989, 7, 82–86. [Google Scholar] [CrossRef]
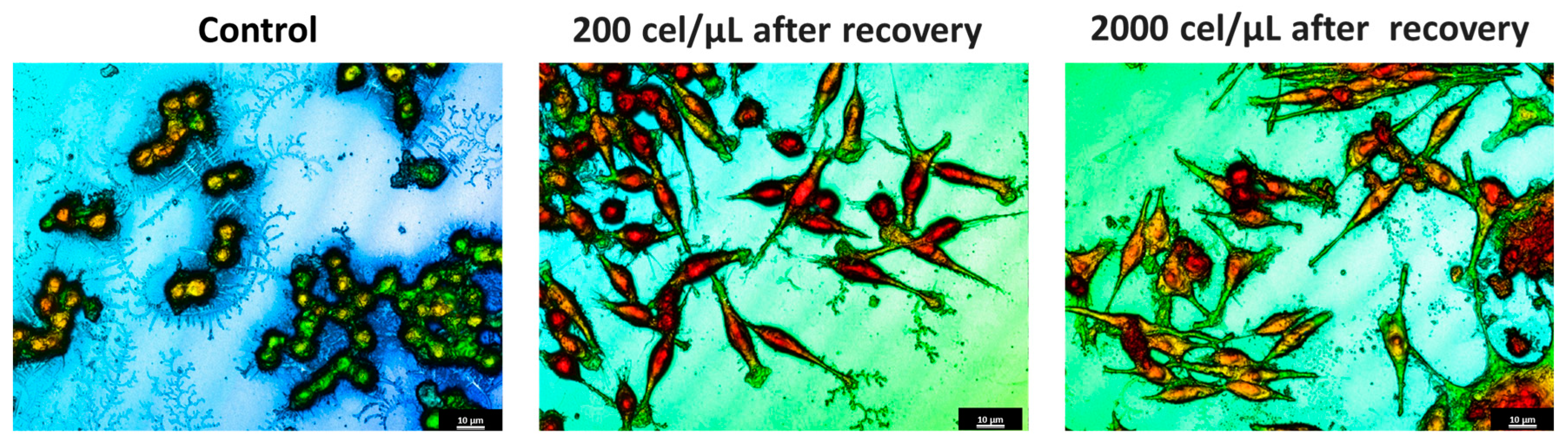
| Profile | Surface Factor | Height (μm) | Volume (μm3) |
|---|---|---|---|
Control cells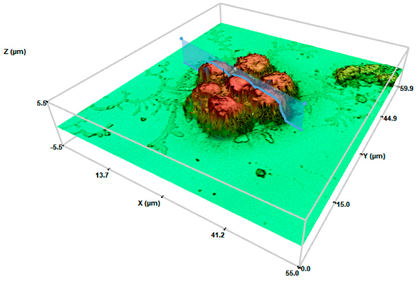 | 1.063 ± 0.031 | 4.50 ± 0.21 | 184 ± 0.201 |
SHS 200 cel/μL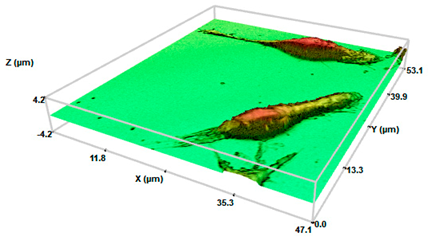 | 5.007 ± 0.193 * | 3.2 ± 0.30 * | 205 ± 0.471 |
SHS 2000 cel/μL | 10.02 ± 2.21 **● | 2.15 ± 0.65 ** | 223 ± 0.163 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Carmen Morán, M.; Cirisano, F.; Ferrari, M. Superhydrophobicity Effects on Spheroid Formation and Polarization of Macrophages. Pharmaceuticals 2024, 17, 1042. https://doi.org/10.3390/ph17081042
del Carmen Morán M, Cirisano F, Ferrari M. Superhydrophobicity Effects on Spheroid Formation and Polarization of Macrophages. Pharmaceuticals. 2024; 17(8):1042. https://doi.org/10.3390/ph17081042
Chicago/Turabian Styledel Carmen Morán, María, Francesca Cirisano, and Michele Ferrari. 2024. "Superhydrophobicity Effects on Spheroid Formation and Polarization of Macrophages" Pharmaceuticals 17, no. 8: 1042. https://doi.org/10.3390/ph17081042
APA Styledel Carmen Morán, M., Cirisano, F., & Ferrari, M. (2024). Superhydrophobicity Effects on Spheroid Formation and Polarization of Macrophages. Pharmaceuticals, 17(8), 1042. https://doi.org/10.3390/ph17081042








