GRB7 Plays a Vital Role in Promoting the Progression and Mediating Immune Evasion of Ovarian Cancer
Abstract
1. Introduction
2. Results
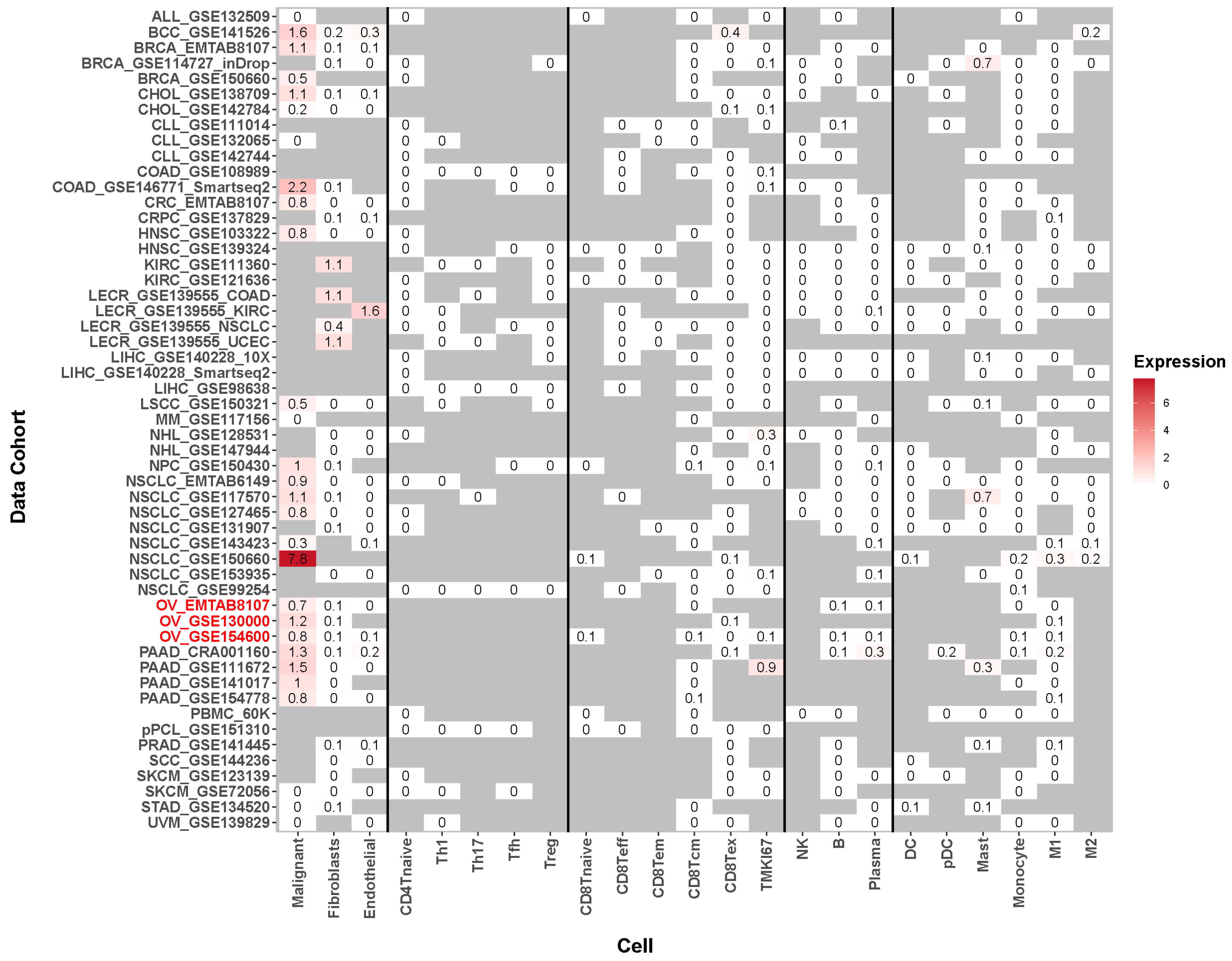
2.1. GRB7’s Expression Is Upregulated in Ovarian Cancer Tissue
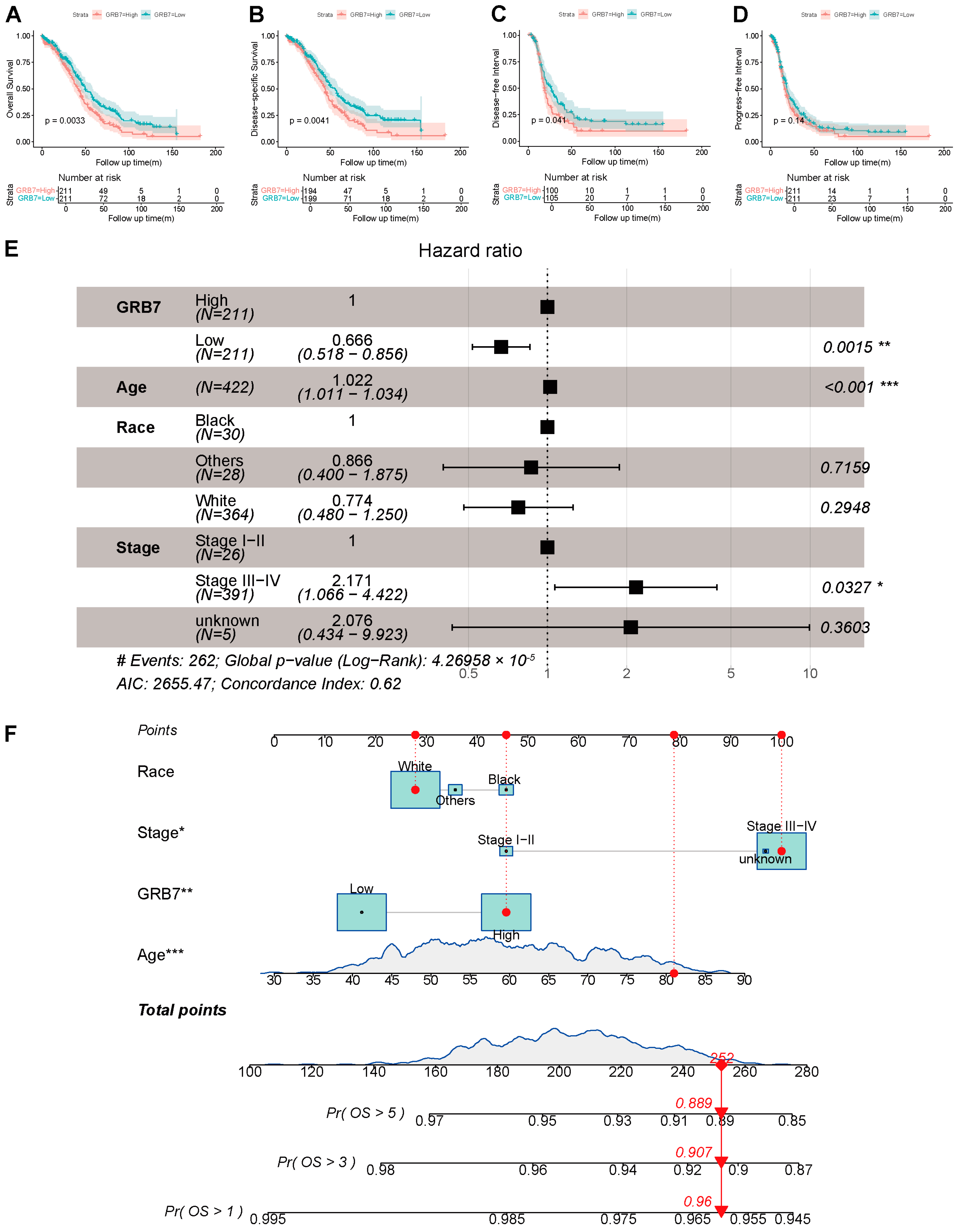
2.2. GRB7’s Expression Is Independently Associated with a Poorer Outcome and Is Valuable for Predicting OS in OC Patients
2.3. Network Establishment for GRB7-Correlated Genes in OC
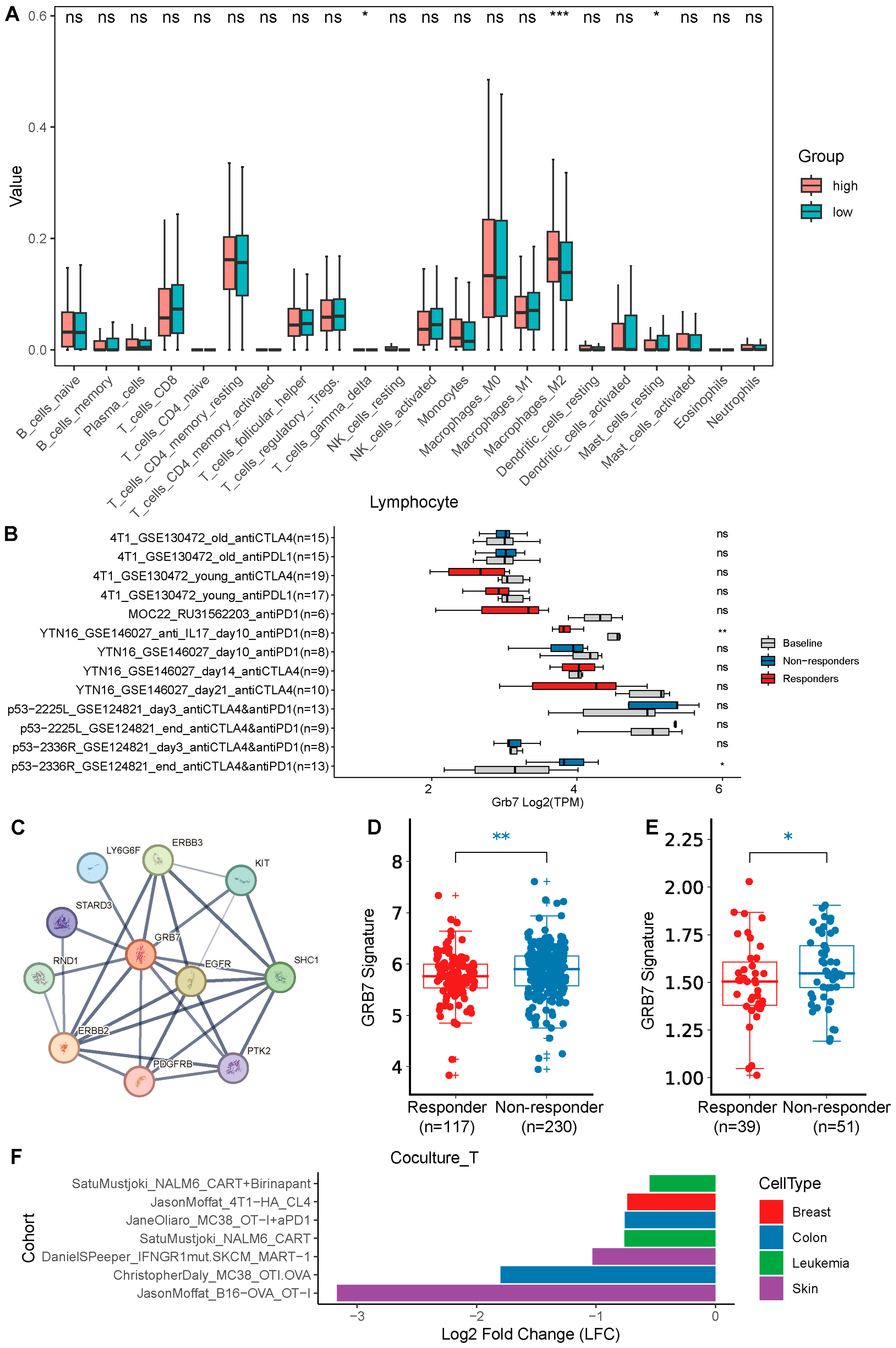
2.4. GRB7’s Expression Correlates with Immune Infiltration and Immunotherapy Response and Has Potential to Be a Therapeutic Target
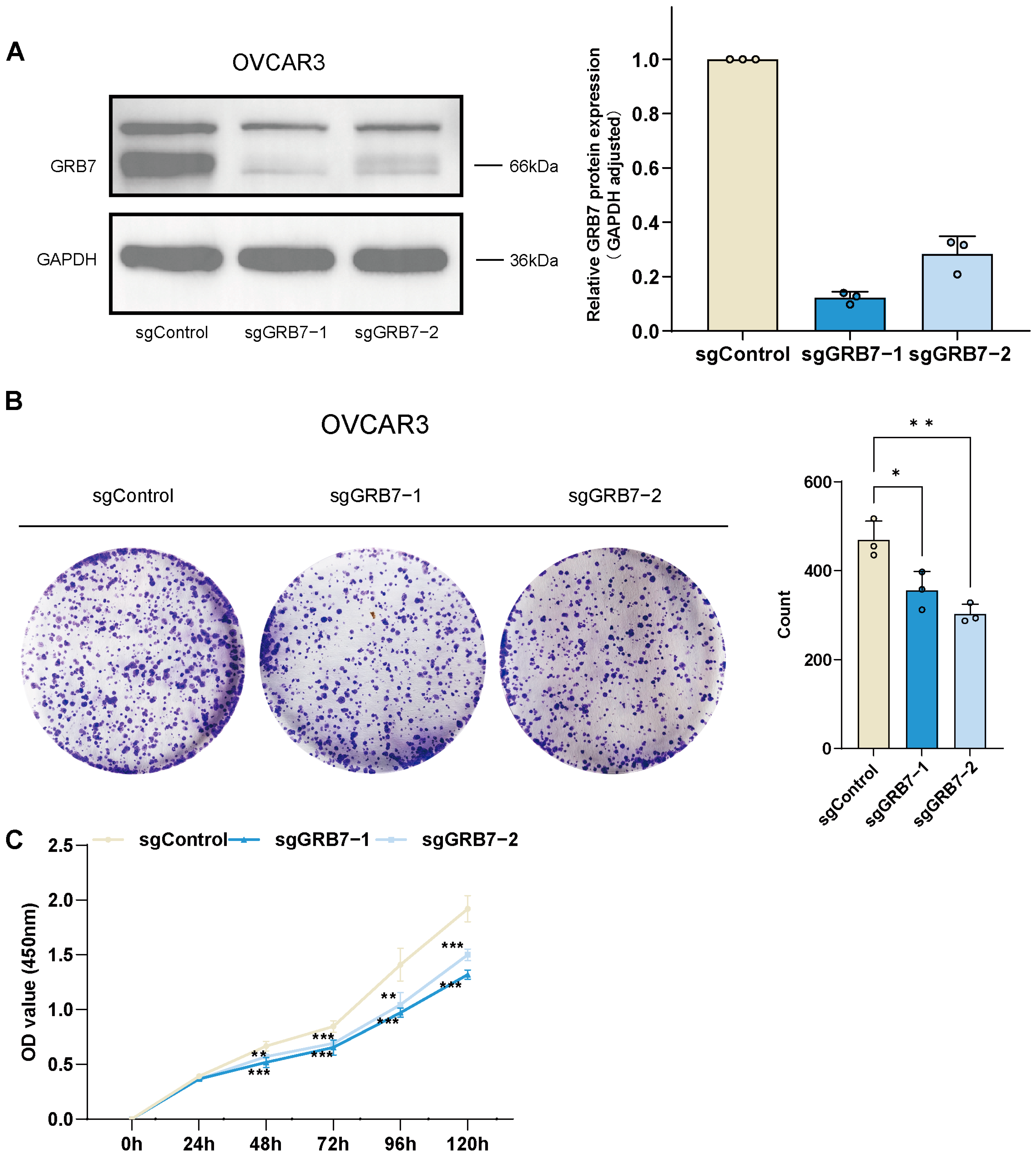
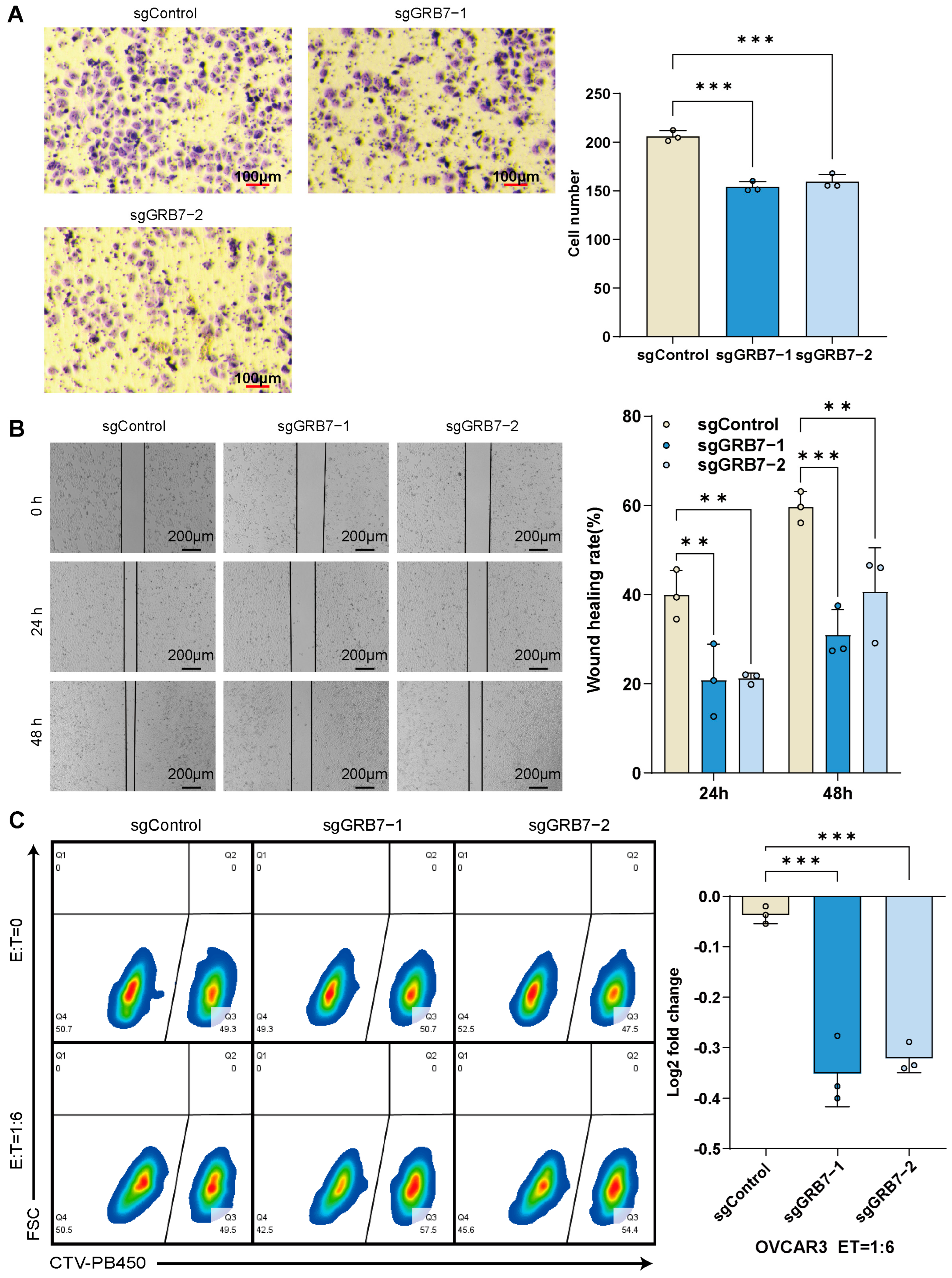
2.5. GRB7 Knockout Inhibits OC Cell Proliferation and Migration
2.6. Enhanced Susceptibility of OC Cells to T Cell-Mediated Cytotoxicity Post-GRB7 Knockout
3. Discussion
4. Materials and Methods
4.1. Expression of GRB7 and Clinicopathological Character Analysis
4.2. Correlation Analysis of GRB7 and Prognosis
4.3. Analyses of Univariate and Multivariate Cox Regression
4.4. Correlation of Related Genes and Gene Set Enrichment Analysis
4.5. Immune Cell Infiltration and Association with Immunotherapy
4.6. GRB7’s Expression Level in Single Cells of Tumor Tissue
4.7. Cell Culture
4.8. Cell Proliferation
4.9. Cell Migration
4.10. In Vitro Cancer-Killing Assay by Antigen-Specific T Cells
4.11. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Huang, H.; Ruan, T.; Yang, H.; Hu, J.; Xu, S.; Ling, S.; Yu, Y. Global, regional, and national burden of ovarian cancer and the attributable risk factors in all 194 countries and territories during 2007–2017: A systematic analysis of the Global Burden of Disease Study 2017. J. Obstet. Gynaecol. Res. 2021, 47, 4389–4402. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, E.; Yang, B.; Xiao, R.; Lu, F.; You, L.; Chen, G. Trends and age-period-cohort effects on mortality of the three major gynecologic cancers in China from 1990 to 2019: Cervical, ovarian and uterine cancer. Gynecol. Oncol. 2021, 163, 358–363. [Google Scholar] [CrossRef]
- Caetano Dos Santos, F.L.; Wojciechowska, U.; Michalek, I.M.; Didkowska, J. Progress in cancer survival across last two decades: A nationwide study of over 1.2 million Polish patients diagnosed with the most common cancers. Cancer Epidemiol. 2022, 78, 102147. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Shapira-Frommer, R.; Santin, A.D.; Lisyanskaya, A.S.; Pignata, S.; Vergote, I.; Raspagliesi, F.; Sonke, G.S.; Birrer, M.; Provencher, D.M.; et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: Results from the phase II KEYNOTE-100 study. Ann. Oncol. 2019, 30, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.Y.; Tai, Y.L.; Shen, T.L. Grb7, a Critical Mediator of EGFR/ErbB Signaling, in Cancer Development and as a Potential Therapeutic Target. Cells 2019, 8, 435. [Google Scholar] [CrossRef]
- Yu, C.; Luo, D.; Yu, J.; Zhang, M.; Zheng, X.; Xu, G.; Wang, J.; Wang, H.; Xu, Y.; Jiang, K.; et al. Genome-wide CRISPR-cas9 knockout screening identifies GRB7 as a driver for MEK inhibitor resistance in KRAS mutant colon cancer. Oncogene 2022, 41, 191–203. [Google Scholar] [CrossRef]
- Giricz, O.; Calvo, V.; Pero, S.C.; Krag, D.N.; Sparano, J.A.; Kenny, P.A. GRB7 is required for triple-negative breast cancer cell invasion and survival. Breast Cancer Res. Treat. 2012, 133, 607–615. [Google Scholar] [CrossRef]
- Dufva, O.; Koski, J.; Maliniemi, P.; Ianevski, A.; Klievink, J.; Leitner, J.; Pölönen, P.; Hohtari, H.; Saeed, K.; Hannunen, T.; et al. Integrated drug profiling and CRISPR screening identify essential pathways for CAR T-cell cytotoxicity. Blood 2020, 135, 597–609. [Google Scholar] [CrossRef]
- Lawson, K.A.; Sousa, C.M.; Zhang, X.; Kim, E.; Akthar, R.; Caumanns, J.J.; Yao, Y.; Mikolajewicz, N.; Ross, C.; Brown, K.R.; et al. Functional genomic landscape of cancer-intrinsic evasion of killing by T cells. Nature 2020, 586, 120–126. [Google Scholar] [CrossRef]
- Kearney, C.J.; Vervoort, S.J.; Hogg, S.J.; Ramsbottom, K.M.; Freeman, A.J.; Lalaoui, N.; Pijpers, L.; Michie, J.; Brown, K.K.; Knight, D.A.; et al. Tumor immune evasion arises through loss of TNF sensitivity. Sci. Immunol. 2018, 3, eaar3451. [Google Scholar] [CrossRef] [PubMed]
- Vredevoogd, D.W.; Kuilman, T.; Ligtenberg, M.A.; Boshuizen, J.; Stecker, K.E.; de Bruijn, B.; Krijgsman, O.; Huang, X.; Kenski, J.C.N.; Lacroix, R.; et al. Augmenting Immunotherapy Impact by Lowering Tumor TNF Cytotoxicity Threshold. Cell 2019, 178, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Young, T.M.; Reyes, C.; Pasnikowski, E.; Castanaro, C.; Wong, C.; Decker, C.E.; Chiu, J.; Song, H.; Wei, Y.; Bai, Y.; et al. Autophagy protects tumors from T cell-mediated cytotoxicity via inhibition of TNFα-induced apoptosis. Sci. Immunol. 2020, 5, eabb9561. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Liu, M.X.; Mak, C.S.; Yung, M.M.; Leung, T.H.; Xu, D.; Ngu, S.F.; Chan, K.K.; Yang, H.; Ngan, H.Y.; et al. Methylation-associated silencing of miR-193a-3p promotes ovarian cancer aggressiveness by targeting GRB7 and MAPK/ERK pathways. Theranostics 2018, 8, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Kasus-Jacobi, A.; Béréziat, V.; Perdereau, D.; Girard, J.; Burnol, A.F. Evidence for an interaction between the insulin receptor and Grb7. A role for two of its binding domains, PIR and SH2. Oncogene 2000, 19, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Han, D.C.; Shen, T.L.; Guan, J.L. The Grb7 family proteins: Structure, interactions with other signaling molecules and potential cellular functions. Oncogene 2001, 20, 6315–6321. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Pei, Y.; Yang, L.; Zeng, Z.; Wang, J.; Xie, G.; Wang, L.; Yuan, J. Upregulated GRB7 promotes proliferation and tumorigenesis of Bladder Cancer via Phospho-AKT Pathway. Int. J. Biol. Sci. 2020, 16, 3221–3230. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Yang, P.; Yang, X.; Peng, S.; Hu, X.; Bao, G. Growth factor receptor bound protein-7 regulates proliferation, cell cycle, and mitochondrial apoptosis of thyroid cancer cells via MAPK/ERK signaling. Mol. Cell Biochem. 2020, 472, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Pero, S.C.; Shukla, G.S.; Cookson, M.M.; Flemer, S., Jr.; Krag, D.N. Combination treatment with Grb7 peptide and Doxorubicin or Trastuzumab (Herceptin) results in cooperative cell growth inhibition in breast cancer cells. Br. J. Cancer 2007, 96, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Yang, Z.; Hu, X.; Liu, Y.; Yang, X.; Ran, H.; Li, Y.; Li, X.; Yu, Q. Grb7 gene amplification and protein expression by FISH and IHC in ovarian cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11296–11304. [Google Scholar]
- Xu, Q.; Liu, Z.; Zhu, Z.Q.; Fan, Y.; Chen, R.; Xie, X.H.; Cheng, M. Knockdown of growth factor receptor bound protein 7 suppresses angiogenesis by inhibiting the secretion of vascular endothelial growth factor A in ovarian cancer cells. Bioengineered 2021, 12, 12179–12190. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.; Wu, J.; Fuqua, S.A.; Roonprapunt, C.; Yajnik, V.; D’Eustachio, P.; Moskow, J.J.; Buchberg, A.M.; Osborne, C.K.; Margolis, B. The SH2 domain protein GRB-7 is co-amplified, overexpressed and in a tight complex with HER2 in breast cancer. Embo. J. 1994, 13, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, B.; Bai, T.; Hanlon Newell, A.; Troxell, M.; Park, B.; Olson, S.; Keenan, E.; Luoh, S.W. GRB7 protein over-expression and clinical outcome in breast cancer. Breast Cancer Res. Treat. 2011, 127, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Liang, G.; Lin, Q.; Fang, X.; Luo, Q.; Cen, Y.; Mehrpour, M.; Hamai, A.; Liu, Z.; Shi, Y.; et al. circCDYL2 promotes trastuzumab resistance via sustaining HER2 downstream signaling in breast cancer. Mol. Cancer 2022, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Schubert, L.; Elliott, A.; Le, A.T.; Estrada-Bernal, A.; Doebele, R.C.; Lou, E.; Borghaei, H.; Demeure, M.J.; Kurzrock, R.; Reuss, J.E.; et al. ERBB family fusions are recurrent and actionable oncogenic targets across cancer types. Front. Oncol. 2023, 13, 1115405. [Google Scholar] [CrossRef] [PubMed]
- Lesurf, R.; Griffith, O.L.; Griffith, M.; Hundal, J.; Trani, L.; Watson, M.A.; Aft, R.; Ellis, M.J.; Ota, D.; Suman, V.J.; et al. Genomic characterization of HER2-positive breast cancer and response to neoadjuvant trastuzumab and chemotherapy-results from the ACOSOG Z1041 (Alliance) trial. Ann. Oncol. 2017, 28, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.Y.; Li, T.K.; Ding, S.T.; Lai, I.R.; Shen, T.L. EGF-induced Grb7 recruits and promotes Ras activity essential for the tumorigenicity of Sk-Br3 breast cancer cells. J. Biol. Chem. 2010, 285, 29279–29285. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.B.; Zhang, X.F.; Jia, X.L.; Wang, H.B. Grb7 is over-expressed in cervical cancer and facilitate invasion and inhibit apoptosis in cervical cancer cells. Pathol. Res. Pract. 2017, 213, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Roberts-Thomson, S.J.; Chalmers, S.B.; Monteith, G.R. The Calcium-Signaling Toolkit in Cancer: Remodeling and Targeting. Cold Spring Harb. Perspect. Biol. 2019, 11, a035204. [Google Scholar] [CrossRef]
- Heldin, C.H.; Lu, B.; Evans, R.; Gutkind, J.S. Signals and Receptors. Cold Spring Harb. Perspect. Biol. 2016, 8, a005900. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell 2015, 161, 205–214. [Google Scholar] [CrossRef]
- Goode, E.L.; Block, M.S.; Kalli, K.R.; Vierkant, R.A.; Chen, W.; Fogarty, Z.C.; Gentry-Maharaj, A.; Tołoczko, A.; Hein, A.; Bouligny, A.L.; et al. Dose-Response Association of CD8+ Tumor-Infiltrating Lymphocytes and Survival Time in High-Grade Serous Ovarian Cancer. JAMA Oncol. 2017, 3, e173290. [Google Scholar] [CrossRef]
- Rajtak, A.; Ostrowska-Leśko, M.; Żak, K.; Tarkowski, R.; Kotarski, J.; Okła, K. Integration of local and systemic immunity in ovarian cancer: Implications for immunotherapy. Front. Immunol. 2022, 13, 1018256. [Google Scholar] [CrossRef]
- Kandalaft, L.E.; Dangaj Laniti, D.; Coukos, G. Immunobiology of high-grade serous ovarian cancer: Lessons for clinical translation. Nat. Rev. Cancer 2022, 22, 640–656. [Google Scholar] [CrossRef]
- López-Cade, I.; García-Barberán, V.; Cabañas Morafraile, E.; Díaz-Tejeiro, C.; Saiz-Ladera, C.; Sanvicente, A.; Pérez Segura, P.; Pandiella, A.; Győrffy, B.; Ocaña, A. Genomic mapping of copy number variations influencing immune response in breast cancer. Front. Oncol. 2022, 12, 975437. [Google Scholar] [CrossRef]
- Feng, Q.; Wei, H.; Morihara, J.; Stern, J.; Yu, M.; Kiviat, N.; Hellstrom, I.; Hellstrom, K.E. Th2 type inflammation promotes the gradual progression of HPV-infected cervical cells to cervical carcinoma. Gynecol. Oncol. 2012, 127, 412–419. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, J.; Luo, Y.; Liu, J.; Wang, X.; Feng, R.; Huang, J.; Du, H.; Li, Q.; Tan, J.; et al. Pharmaceutical targeting Th2-mediated immunity enhances immunotherapy response in breast cancer. J. Transl. Med. 2022, 20, 615. [Google Scholar] [CrossRef]
- Liu, J.; Geng, X.; Hou, J.; Wu, G. New insights into M1/M2 macrophages: Key modulators in cancer progression. Cancer Cell Int. 2021, 21, 389. [Google Scholar] [CrossRef]
- Hou, J.; He, Z.; Liu, T.; Chen, D.; Wang, B.; Wen, Q.; Zheng, X. Evolution of Molecular Targeted Cancer Therapy: Mechanisms of Drug Resistance and Novel Opportunities Identified by CRISPR-Cas9 Screening. Front. Oncol. 2022, 12, 755053. [Google Scholar] [CrossRef]
- Lei, Y.; Tang, R.; Xu, J.; Wang, W.; Zhang, B.; Liu, J.; Yu, X.; Shi, S. Applications of single-cell sequencing in cancer research: Progress and perspectives. J. Hematol. Oncol. 2021, 14, 91. [Google Scholar] [CrossRef]
- Mustachio, L.M.; Roszik, J. Single-Cell Sequencing: Current Applications in Precision Onco-Genomics and Cancer Therapeutics. Cancers 2022, 14, 657. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.; Wang, P.; Zenklusen, J.C. SnapShot: TCGA-Analyzed Tumors. Cell 2018, 173, 530. [Google Scholar] [CrossRef] [PubMed]
- Consortium, G. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef]
- Wang, D.; Qian, X.; Du, Y.-C.N.; Sanchez-Solana, B.; Chen, K.; Park, B.; Chen, B.; Jenkins, L.; Luo, J.; Tripathi, B.K.; et al. Abstract 3912: cProSite: A web based interactive platform for on-line proteomics and phosphoproteomics data analysis. Cancer Res. 2022, 82, 3912. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.M.; Steen, C.B.; Liu, C.L.; Gentles, A.J.; Chaudhuri, A.A.; Scherer, F.; Khodadoust, M.S.; Esfahani, M.S.; Luca, B.A.; Steiner, D.; et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat. Biotechnol. 2019, 37, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Wong, C.J.; Yang, L.; Ouardaoui, N.; Li, D.; Zhang, W.; Gu, S.; Zhang, Y.; Liu, Y.; Wang, X.; et al. TISMO: Syngeneic mouse tumor database to model tumor immunity and immunotherapy response. Nucleic Acids Res. 2022, 50, D1391–D1397. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E., III.; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [PubMed]
- Joung, J.; Kirchgatterer, P.C.; Singh, A.; Cho, J.H.; Nety, S.P.; Larson, R.C.; Macrae, R.K.; Deasy, R.; Tseng, Y.Y.; Maus, M.V.; et al. CRISPR activation screen identifies BCL-2 proteins and B3GNT2 as drivers of cancer resistance to T cell-mediated cytotoxicity. Nat. Commun. 2022, 13, 1606. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, L.; Hu, W.; Hou, S.; Luo, C.; Jin, Y.; Zeng, Z.; Zhang, Z.; Meng, Y. GRB7 Plays a Vital Role in Promoting the Progression and Mediating Immune Evasion of Ovarian Cancer. Pharmaceuticals 2024, 17, 1043. https://doi.org/10.3390/ph17081043
Wen L, Hu W, Hou S, Luo C, Jin Y, Zeng Z, Zhang Z, Meng Y. GRB7 Plays a Vital Role in Promoting the Progression and Mediating Immune Evasion of Ovarian Cancer. Pharmaceuticals. 2024; 17(8):1043. https://doi.org/10.3390/ph17081043
Chicago/Turabian StyleWen, Liang, Wei Hu, Sen Hou, Ce Luo, Yiteng Jin, Zexian Zeng, Zhe Zhang, and Yuanguang Meng. 2024. "GRB7 Plays a Vital Role in Promoting the Progression and Mediating Immune Evasion of Ovarian Cancer" Pharmaceuticals 17, no. 8: 1043. https://doi.org/10.3390/ph17081043
APA StyleWen, L., Hu, W., Hou, S., Luo, C., Jin, Y., Zeng, Z., Zhang, Z., & Meng, Y. (2024). GRB7 Plays a Vital Role in Promoting the Progression and Mediating Immune Evasion of Ovarian Cancer. Pharmaceuticals, 17(8), 1043. https://doi.org/10.3390/ph17081043






