Anti-Inflammatory Activities of Yataprasen Thai Traditional Formulary and Its Active Compounds, Beta-Amyrin and Stigmasterol, in RAW264.7 and THP-1 Cells
Abstract
1. Introduction
2. Results
2.1. Total Phenolic and Flavonoid Content in the YTPS Formulary Extract
2.2. Identification and Quantification of Bioactive Compounds in the YTPS Formulary Extract Using HPLC Analysis
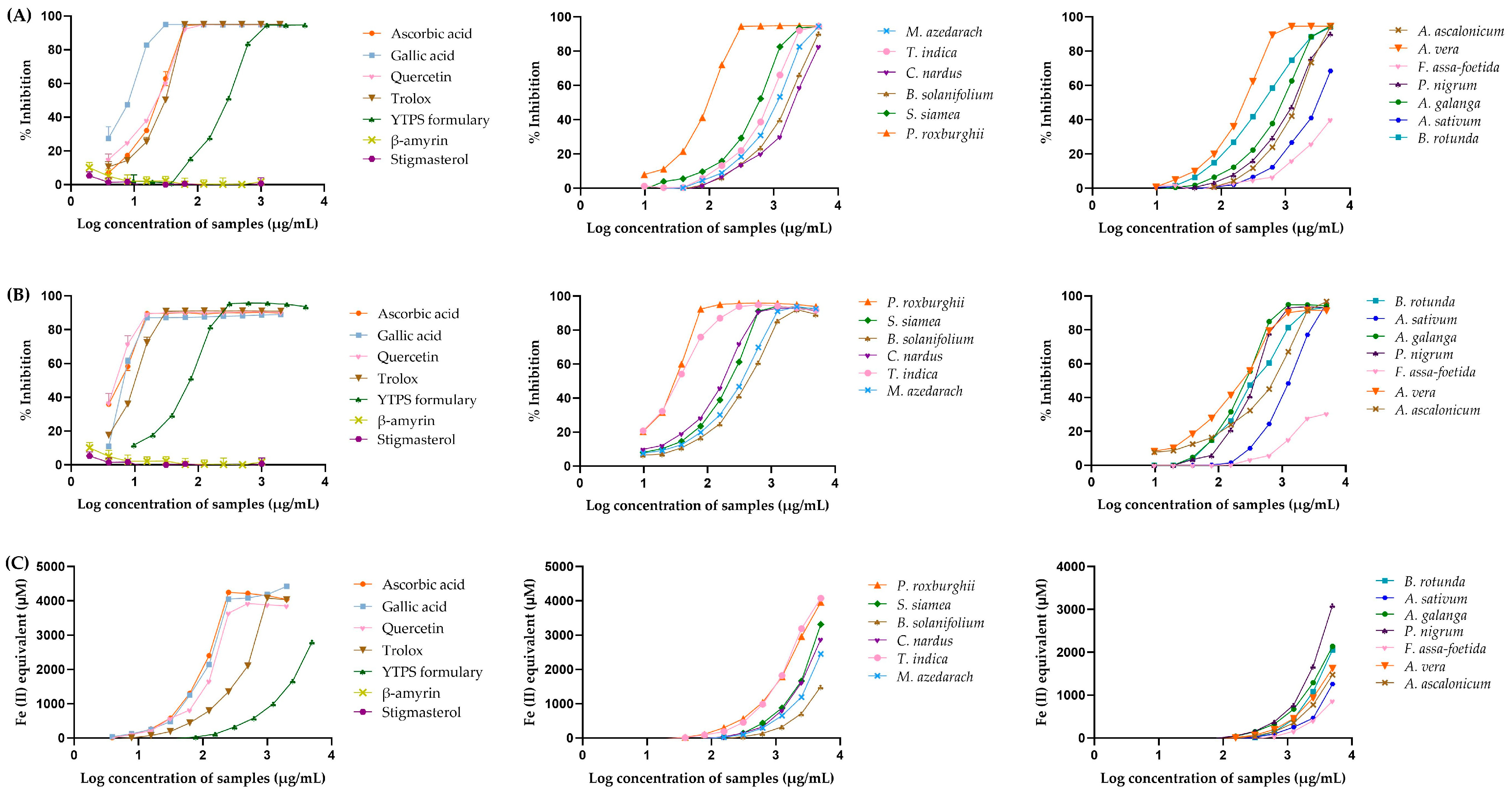
2.3. Anti-Oxidant Activities of the YTPS Formulary Extract
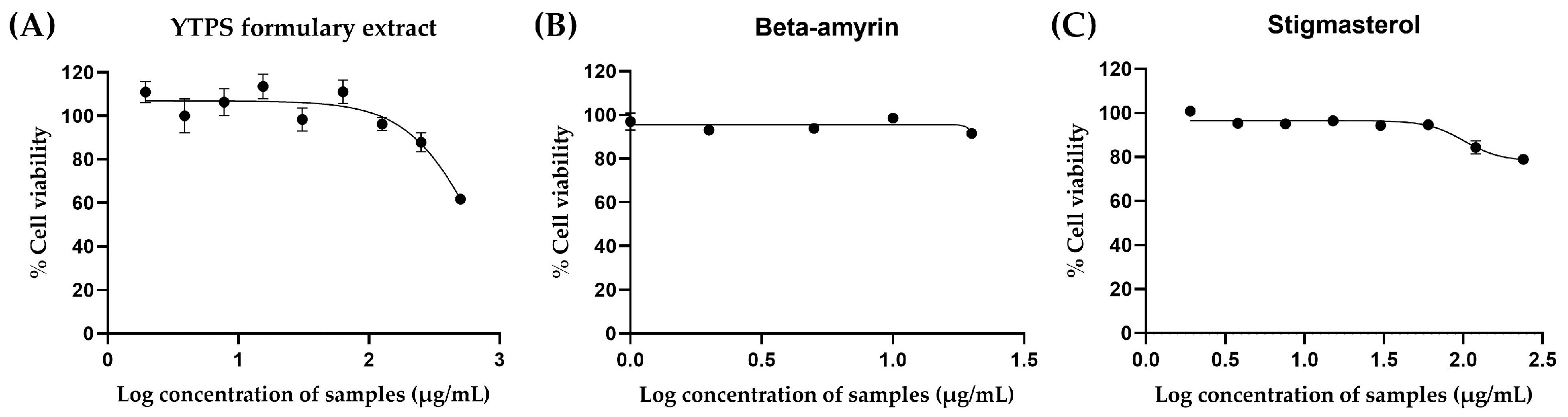
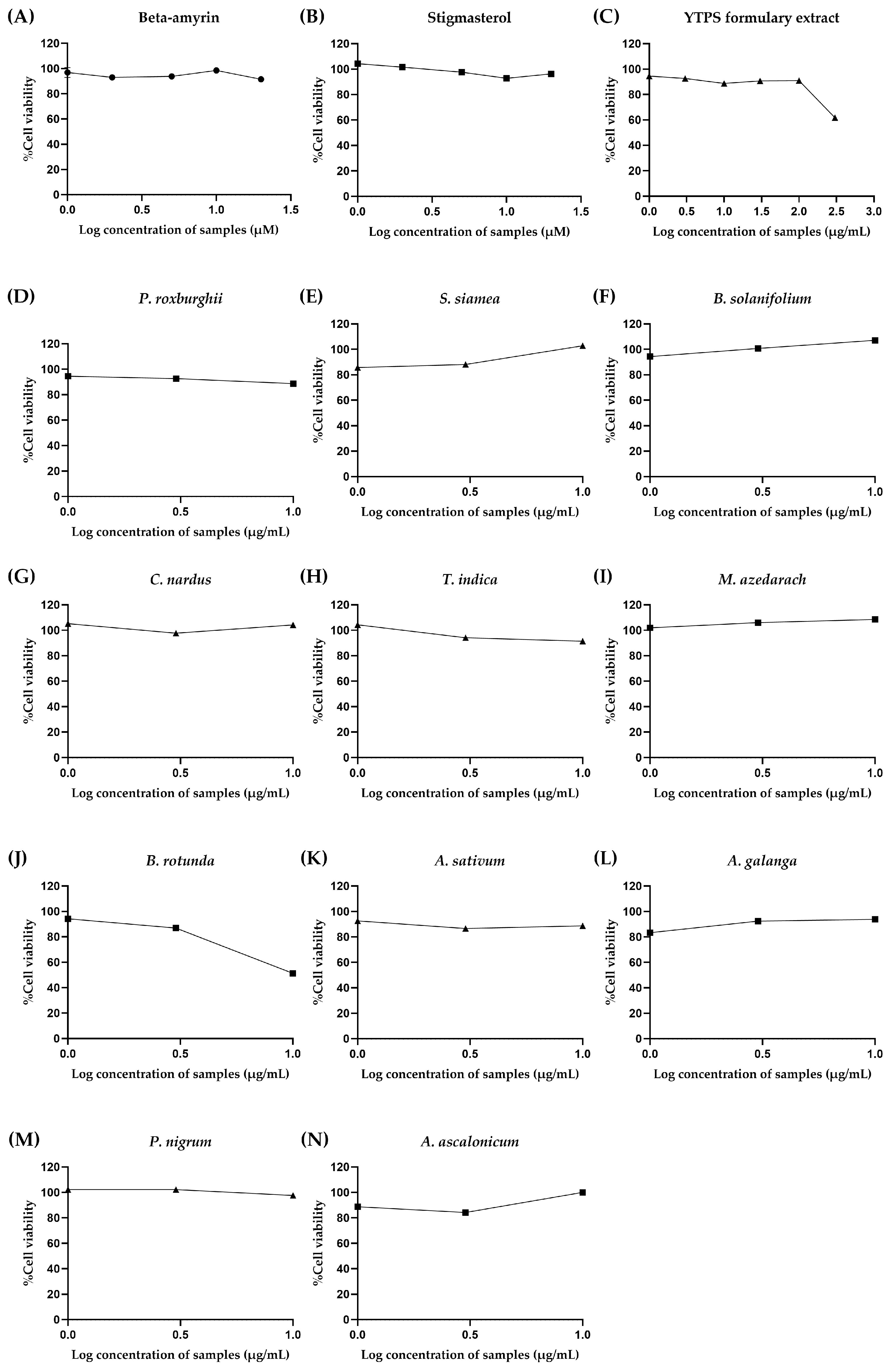
2.4. In Vitro Cytotoxicity of the YTPS Formulary Extract, β-Amyrin, Stigmasterol, and 11 Individual Extracts
2.4.1. Cytotoxicity of β-Amyrin, Stigmasterol, and the YTPS Formulary Extract on RAW 264.7 Cells
2.4.2. Cytotoxicity of β-Amyrin, Stigmasterol, the YTPS Formulary Extract, and 11 Distinct Extracts on THP-1 Cells
2.5. Anti-Inflammatory Effects of the YTPS Formulary Extract, β-Amyrin, Stigmasterol, and 13 Individual Extracts
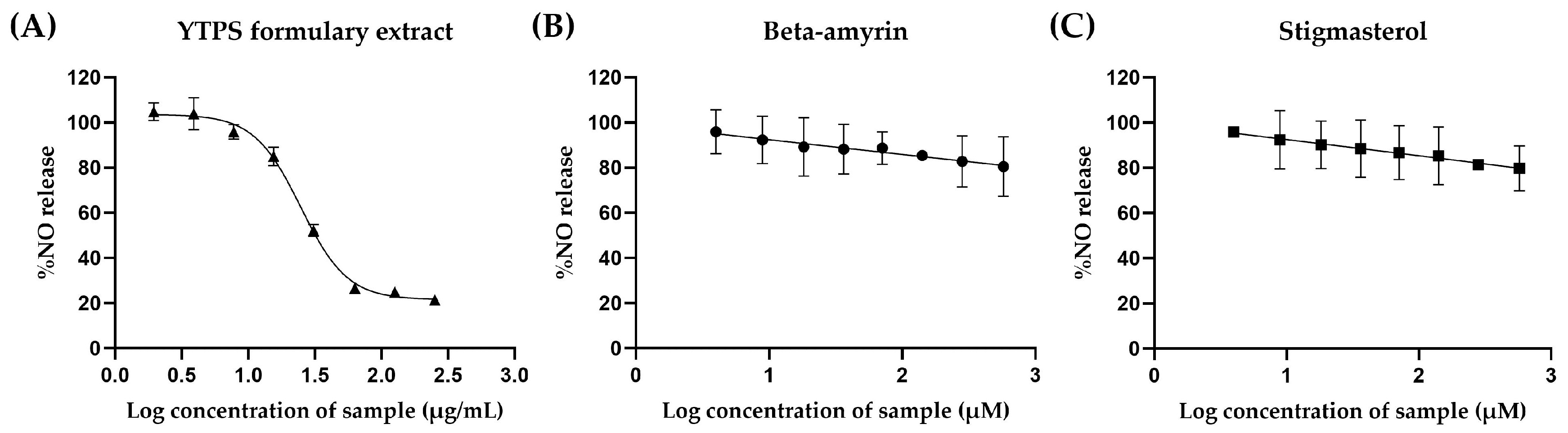
2.5.1. Effects of the YTPS Formulary Extract on Nitric Oxide Production
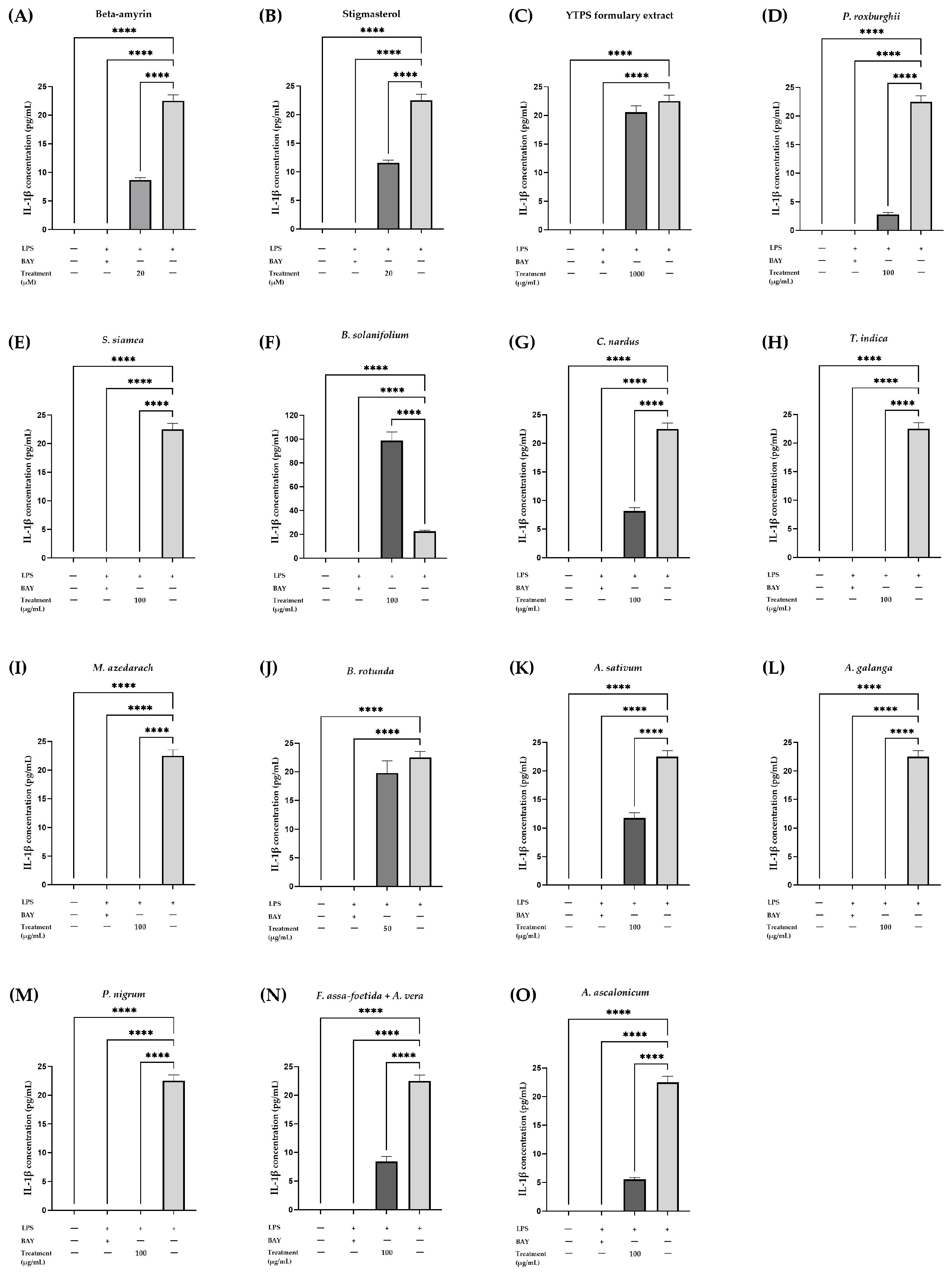
2.5.2. Effects of YTPS Formulary Extract, β-AMYRIN, Stigmasterol, and 13 Individual Extracts on IL-1β Secretion
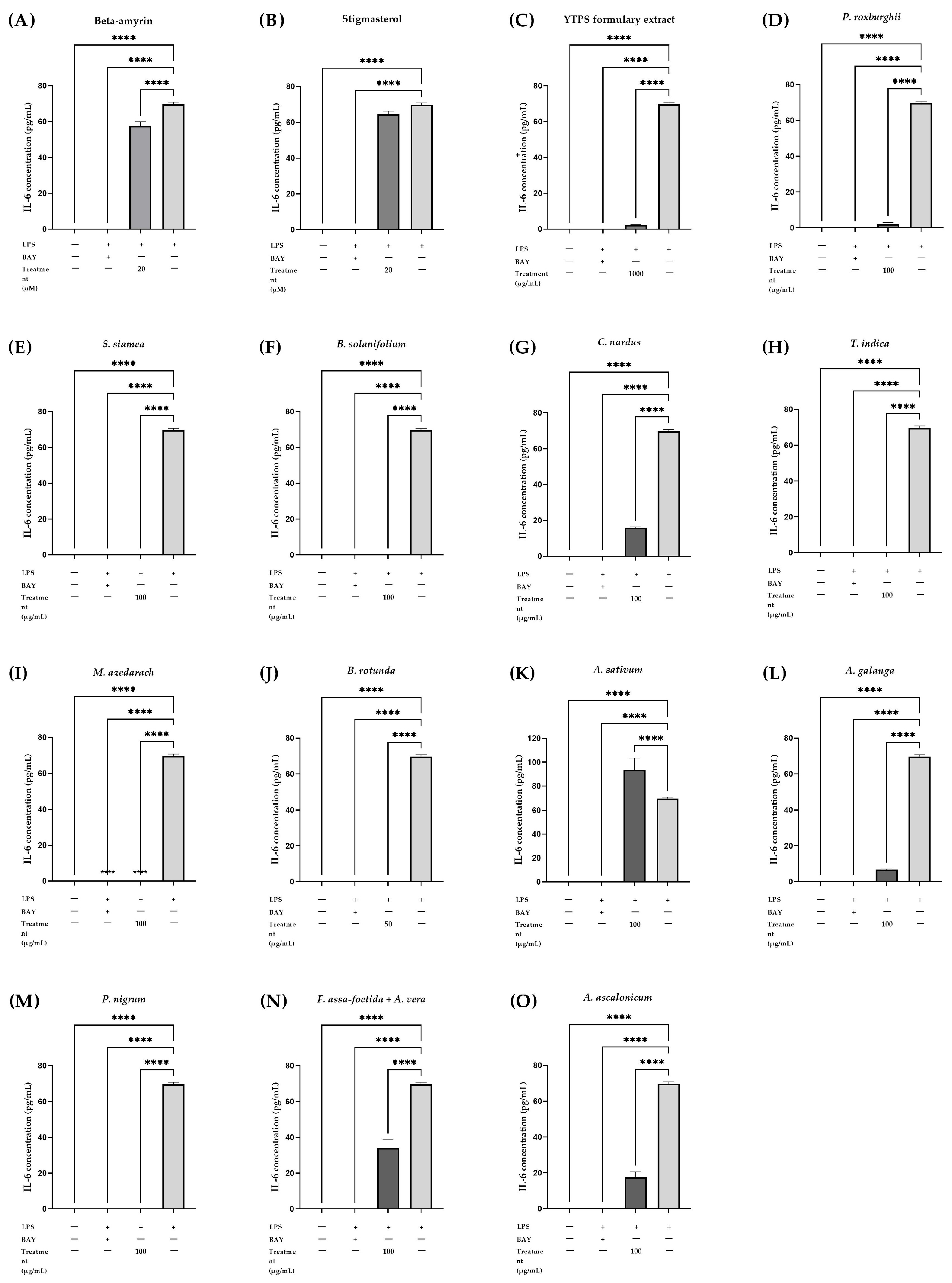
2.5.3. Effects of the YTPS Formulary Extract, β-Amyrin, Stigmasterol, and 13 Individual Extracts on IL-6 Secretion
2.5.4. Effects of YTPS Formulary Extract on TNF-α Secretion
2.6. Safety Evaluation of the YTPS Formulary Extract
2.6.1. Safety Evaluation on the Skin Using HaCaT Cells
2.6.2. Safety Evaluation on the Kidney Using HEK293 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
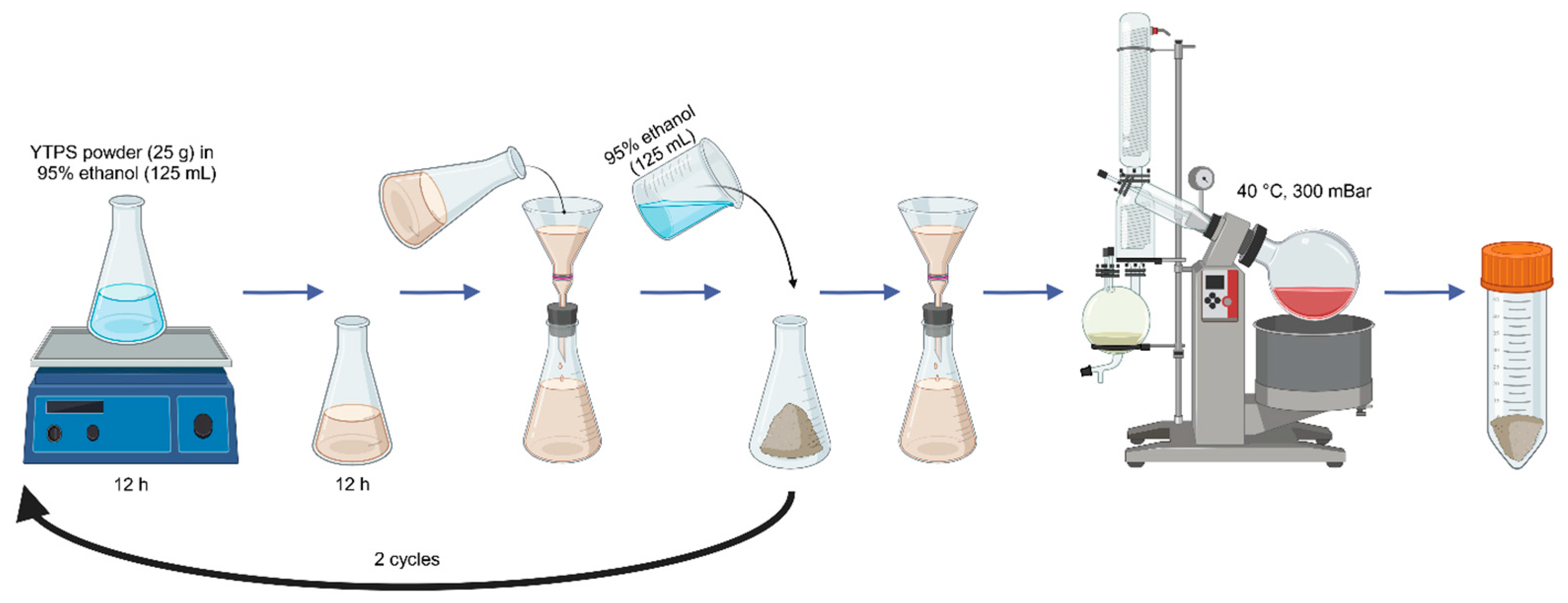
4.2.1. Plant Material and Extraction Procedure
4.2.2. Preparation of Plant Extracts
4.2.3. Determination of Total Phenolic Content of YTPS Formulary Extract
4.2.4. Determination of Total Flavonoid Content of YTPS Formulary Extract
4.2.5. HPLC Analysis for the Identification and Quantification of Bioactive Compounds in YTPS Formulary Extract
4.2.6. Antioxidant Assay
2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity Assay
2,2′-Azino-bis (3-Ethylbenzthiazoline-6-sulphonic Acid (ABTS) Radical Scavenging Activity Assay
Ferric Reducing Antioxidant Power (FRAP) Assay
4.2.7. In Vitro Cytotoxicity
Cell Culture
Measurement of Cell Viability by MTT Assay
Measurement of Cell Viability by AlamarBlue Assay
4.2.8. Anti-Inflammation Assay
Nitric Oxide Assay
Measurement of Cytokine Secretion by ELISA Assay
4.2.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamood, R.; Tirosh, M.; Fallach, N.; Chodick, G.; Eisenberg, E.; Lubovsky, O. Prevalence and Incidence of Osteoarthritis: A Population-Based Retrospective Cohort Study. J. Clin. Med. 2021, 10, 4282. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S.S.; Klotz, U.; Alscher, D.M.; Mais, A.; Lauer, G.; Schweer, H.; Seyberth, H.W.; Fritz, P.; Bierbach, U. Osteoarthritis of the knee—Clinical assessments and inflammatory. Osteoarthr. Cartil. 2004, 12, 469–475. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Derry, S.; Moore, R.A.; Gaskell, H.; McIntyre, M.; Wiffen, P.J. Topical NSAIDs for acute musculoskeletal pain in adults. Cochrane Database Syst. Rev. 2015, 2015, CD007402. [Google Scholar] [CrossRef] [PubMed]
- Barkin, R.L. The Pharmacology of Topical Analgesics. Postgrad. Med. 2013, 125, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Domper Arnal, M.-J.; Hijos-Mallada, G.; Lanas, A. Gastrointestinal and cardiovascular adverse events associated with NSAIDs. Expert. Opin. Drug Saf. 2022, 21, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Ansaripour, S.; Dehghan, M. Efficacy of some Herbal Medicines in Osteoarthritis with a Focus on Topical Agents: A Systematic Review. Curr. Pharm. Des. 2020, 26, 2676–2681. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.; Rodrigues, I.; Napoleão, L.; Lira, L.; Marques, D.; Veríssimo, M.; Andrade, J.P.; Dourado, M. Non-steroidal anti-inflammatory drugs (NSAIDs), pain and aging: Adjusting prescription to patient features. Biomed. Pharmacother. 2022, 150, 112958. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Public Health. National Thai Traditional Medicine Formulary 2021 Edition; Division of Protection and Promotion of Thai Traditional and Indigenous Medicine Knowledge, Department of Thai Traditional and Alternative Medicine: Chang Wat Nonthaburi, Thailand, 2021. [Google Scholar]
- Okoye, N.N.; Ajaghaku, D.L.; Okeke, H.N.; Ilodigwe, E.E.; Nworu, C.S.; Okoye, F.B.C. beta-Amyrin and alpha-amyrin acetate isolated from the stem bark of Alstonia boonei display profound anti-inflammatory activity. Pharm. Biol. 2014, 52, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.-H.; El Omari, N.; Sheikh, R.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Health Benefits and Pharmacological Properties of Stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef]
- Badole, M.; Dighe, V.; Charegaonkar, G. Simultaneous quantification of B-amyrin and stigmasterol in Putranjiva roxburghii wall. by high-performance thin-layer chromatography. Int. J. Pharma Bio Sci. 2011, 2, 346–352. [Google Scholar]
- Krishnan, K.; Mathew, L.E.; Vijayalakshmi, N.R.; Helen, A. Anti-inflammatory potential of β-amyrin, a triterpenoid isolated from Costus igneus. Inflammopharmacology 2014, 22, 373–385. [Google Scholar] [CrossRef]
- Ashraf, R.; Bhatti, H.N. Chapter 10—Stigmasterol. In A Centum of Valuable Plant Bioactives; Mushtaq, M., Anwar, F., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 213–232. [Google Scholar]
- Ahmad Khan, M.; Sarwar, A.H.M.G.; Rahat, R.; Ahmed, R.S.; Umar, S. Stigmasterol protects rats from collagen induced arthritis by inhibiting proinflammatory cytokines. Int. Immunopharmacol. 2020, 85, 106642. [Google Scholar] [CrossRef] [PubMed]
- Park, K.I.; Kang, S.R.; Park, H.S.; Lee, D.H.; Nagappan, A.; Kim, J.A.; Shin, S.C.; Kim, E.H.; Lee, W.S.; Chung, H.J.; et al. Regulation of Proinflammatory Mediators via NF-κB and p38 MAPK-Dependent Mechanisms in RAW 264.7 Macrophages by Polyphenol Components Isolated from Korea Lonicera japonica THUNB. Evid. Based Complement. Altern. Med. 2012, 2012, 828521. [Google Scholar] [CrossRef]
- Panighel, G.; Ferrarese, I.; Lupo, M.G.; Sut, S.; Dall’Acqua, S.; Ferri, N. Investigating the in vitro mode of action of okra (Abelmoschus esculentus) as hypocholesterolemic, anti-inflammatory, and antioxidant food. Food Chem. Mol. Sci. 2022, 5, 100126. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-K.; Park, S.H.; Kim, H.J.; Jang, S.; Choo, B.-K.; Kim, H.K.; Kim, T. Inhibitory effect of Sanguisorba hakusanensis Makino ethanol extract on atopic dermatitis-like responses in NC/Nga mice and human keratinocytes. Sci. Rep. 2023, 13, 14594. [Google Scholar] [CrossRef] [PubMed]
- Grauzdytė, D.; Pukalskas, A.; Viranaicken, W.; El Kalamouni, C.; Venskutonis, P.R. Protective effects of Phyllanthus phillyreifolius extracts against hydrogen peroxide induced oxidative stress in HEK293 cells. PLoS ONE 2018, 13, e0207672. [Google Scholar] [CrossRef] [PubMed]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ji, P.; Shang, X.; Zhou, Y. Connection between Osteoarthritis and Nitric Oxide: From Pathophysiology to Therapeutic Target. Molecules 2023, 28, 1683. [Google Scholar] [CrossRef] [PubMed]
- Schildberger, A.; Rossmanith, E.; Eichhorn, T.; Strassl, K.; Weber, V. Monocytes, Peripheral Blood Mononuclear Cells, and THP-1 Cells Exhibit Different Cytokine Expression Patterns following Stimulation with Lipopolysaccharide. Mediat. Inflamm. 2013, 2013, 697972. [Google Scholar] [CrossRef] [PubMed]
- Angsusing, J.; Samee, W.; Tadtong, S.; Chittasupho, C. Development and Validation of HPTLC Method for Determination of Beta-Amyrin and Stigmasterol in Ya-Ta-Pra-Sen Polyherbal Extract. In Proceedings of the 11th International Conference on Nutrition and Physical Activity in Ageing, Obesity and Cancer (NAPA 2022), Chiang Mai, Thailand, 15–17 December 2022; pp. 52–56. [Google Scholar]
- National Center for Biotechnology Information. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Beta-Amyrin (accessed on 26 March 2024).
- Li, Y.; Wang, J.; Li, L.; Song, W.; Li, M.; Hua, X.; Wang, Y.; Yuan, J.; Xue, Z. Natural products of pentacyclic triterpenoids: From discovery to heterologous biosynthesis. Nat. Prod. Rep. 2023, 40, 1303–1353. [Google Scholar] [CrossRef] [PubMed]
- Aragao, G.F.; Pinheiro, M.C.C.; Bandeira, P.N.; Lemos, T.L.G.; Viana, G.S.d.B. Analgesic and Anti-Inflammatory Activities of the Isomeric Mixture of Alpha- and Beta-Amyrin from Protium heptaphyllum (Aubl.) March. J. Herb. Pharmacother. 2008, 7, 31–47. [Google Scholar] [CrossRef]
- Karen Cardoso, B.; Line Marko de Oliveira, H.; Zonta Melo, U.; Mariano Fernandez, C.M.; Franco de Araújo Almeida Campo, C.; Gonçalves, J.E.; Laverde, A., Jr.; Barion Romagnolo, M.; Andrea Linde, G.; Cristiani Gazim, Z. Antioxidant activity of α and β-amyrin isolated from Myrcianthes pungens leaves. Nat. Prod. Res. 2020, 34, 1777–1781. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.A.; Al-Yousef, H.M.; Alhowiriny, T.A.; Alam, P.; Hassan, W.H.B.; Amina, M.; Hussain, A.; Abdelaziz, S.; Abdallah, R.H. Concurrent analysis of bioactive triterpenes oleanolic acid and β-amyrin in antioxidant active fractions of Hibiscus calyphyllus, Hibiscus deflersii and Hibiscus micranthus grown in Saudi Arabia by applying validated HPTLC method. Saudi Pharm. J. 2018, 26, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Vázquez, L.; Palazón Barandela, J.; Navarro-Ocaña, A. The pentacyclic triterpenes α, β-amyrins: A review of sources and biological activities. In Phytochemicals: A Global Perspective of Their Role in Nutrition and Health; Venketeshwer, R., Ed.; IntechOpen: London, UK, 2012; Chapter 23; pp. 487–502. ISBN 978-953-51-4317-8. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Stigmasterol (accessed on 26 March 2024).
- La Torre Fabiola, V.-D.; Ralf, K.; Gabriel, B.; Victor Ermilo, A.-A.; Martha, M.-G.; Mirbella, C.-F.; Rocio, B.-A. Anti-inflammatory and immunomodulatory effects of Critonia aromatisans leaves: Downregulation of pro-inflammatory cytokines. J. Ethnopharmacol. 2016, 190, 174–182. [Google Scholar] [CrossRef]
- Takale, N.; Kothawale, T.; Ghule, B.; Kotagale, N. Isolation, identification, and quantification of stigmasterol in Hygrophila schulli plant by a validated high-performance thin-layer chromatography–densitometric method. JPC J. Planar Chromatogr. Mod. TLC 2023, 36, 223–235. [Google Scholar] [CrossRef]
- Jain, P.; Bari, S. Isolation of lupeol, stigmasterol and campesterol from petroleum ether extract of woody stem of Wrightia tinctoria. Asian J. Plant Sci. 2010, 9, 163. [Google Scholar] [CrossRef]
- Mailafiya, M.; Pateh, U.U.; Hassan, H.; Sule, M.; Yusuf, A.; Bila, A. Isolation and Characterization of Stigmasterol glycoside from the root bark of Leptadenia hastata. FUW Trends Sci. Technol. J. 2020, 5, 394–398. [Google Scholar]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef] [PubMed]
- Chelombitko, M.A. Role of Reactive Oxygen Species in Inflammation: A Minireview. Mosc. Univ. Biol. Sci. Bull. 2018, 73, 199–202. [Google Scholar] [CrossRef]
- Elmarakby, A.A.; Sullivan, J.C. Relationship between Oxidative Stress and Inflammatory Cytokines in Diabetic Nephropathy. Cardiovasc. Ther. 2012, 30, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Nikoomanesh, F.; Khorasanchi, Z.; Mohamadian, M.; Ferns, G.A. The relationship between food quality score with inflammatory biomarkers, and antioxidant capacity in young women. Physiol. Rep. 2023, 11, e15590. [Google Scholar] [CrossRef] [PubMed]
- Sunil, C.; Irudayaraj, S.S.; Duraipandiyan, V.; Al-Dhabi, N.A.; Agastian, P.; Ignacimuthu, S. Antioxidant and free radical scavenging effects of β-amyrin isolated from S. cochinchinensis Moore. Leaves. Ind. Crops Prod. 2014, 61, 510–516. [Google Scholar] [CrossRef]
- Osuntokun, D.O.T.; Oluduro, A.O.; Idowu, T.O.; Omotuyi, A. Assessment of Nephrotoxicity, Anti-Inflammatory and Antioxidant Properties of Epigallocatechin Epicatechin and Stigmasterol Phytosterol (Synergy) Derived from Ethyl Acetate Stem Bark Extract of Spondias mombin on Wistar Rats Using Molecular Method of Analysis. J. Mol. Microbiol. 2017, 1, 103. [Google Scholar]
- Zahan, O.M.; Serban, O.; Gherman, C.; Fodor, D. The evaluation of oxidative stress in osteoarthritis. Med. Pharm. Rep. 2020, 93, 12–22. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodríguez, P.; Baquero, L.P.; Larrota, H.R. Chapter 14—Flavonoids: Potential Therapeutic Agents by Their Antioxidant Capacity. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 265–288. [Google Scholar]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- Riemann, A.; Wußling, H.; Loppnow, H.; Fu, H.; Reime, S.; Thews, O. Acidosis differently modulates the inflammatory program in monocytes and macrophages. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1862, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Sharif, O.; Bolshakov, V.N.; Raines, S.; Newham, P.; Perkins, N.D. Transcriptional profiling of the LPS induced NF-κB response in macrophages. BMC Immunol. 2007, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Gao, H.; Chen, S.; Wang, Q.; Li, X.; Du, L.-J.; Li, J.; Luo, Y.-Y.; Li, J.-X.; Zhao, L.-C.; et al. Procyanidin A1 Alleviates Inflammatory Response induced by LPS through NF-κB, MAPK, and Nrf2/HO-1 Pathways in RAW264.7 cells. Sci. Rep. 2019, 9, 15087. [Google Scholar] [CrossRef]
- Li, P.; Hao, Z.; Wu, J.; Ma, C.; Xu, Y.; Li, J.; Lan, R.; Zhu, B.; Ren, P.; Fan, D.; et al. Comparative Proteomic Analysis of Polarized Human THP-1 and Mouse RAW264. 7 Macrophages. Front. Immunol. 2021, 12, 700009. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, D.; Bai, X. Macrophages regulate the progression of osteoarthritis. Osteoarthr. Cartil. 2020, 28, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.-P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-B.; Hong, S.-C.; Jeong, H.-J.; Koo, J.-S. Anti-inflammatory effects of ethyl acetate fraction from Cnidium officinale Makino on LPS-stimulated RAW 264.7 and THP-1 cells. Korean J. Plant Resour. 2012, 25, 299–307. [Google Scholar] [CrossRef]
- Ahmad, N.; Ansari, M.Y.; Haqqi, T.M. Role of iNOS in osteoarthritis: Pathological and therapeutic aspects. J. Cell. Physiol. 2020, 235, 6366–6376. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric Oxide Production and Signaling in Inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- MacMacking, J.; Xie, Q.; Nathan, C. Nitric oxide and macrophages function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yuan, C.; Zhou, X.; Han, Y.; He, Y.; Ouyang, J.; Zhou, W.; Wang, Z.; Wang, H.; Li, G. Anti-Inflammatory Activity of Three Triterpene from Hippophae rhamnoides L. in Lipopolysaccharide-Stimulated RAW264.7 Cells. Int. J. Mol. Sci. 2021, 22, 12009. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.; Lee, S.; Hong, J.; Park, B.; Kim, D.; Kim, C. Anti-Inflammatory Effect of 4,5-Dicaffeoylquinic Acid on RAW264.7 Cells and a Rat Model of Inflammation. Nutrients 2021, 13, 3537. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kim, M.; Yuk, H.J.; Kim, S.-H.; Kim, D.-S. Siraitia grosvenorii Residual Extract Inhibits Inflammation in RAW264.7 Macrophages and Attenuates Osteoarthritis Progression in a Rat Model. Nutrients 2023, 15, 1417. [Google Scholar] [CrossRef] [PubMed]
- Molnar, V.; Matisic, V.; Kodvanj, I.; Bjelica, R.; Jelec, Z.; Hudetz, D.; Rod, E.; Cukelj, F.; Vrdoljak, T.; Vidovic, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Derler, M.; Basílio, J.; Schmid, J.A. NF-κB in monocytes and macrophages—An inflammatory master regulator in multitalented immune cells. Front. Immunol. 2023, 14, 1134661. [Google Scholar] [CrossRef] [PubMed]
- Nasu, K.; Nishida, M.; Ueda, T.; Yuge, A.; Takai, N.; Narahara, H. Application of the nuclear factor-κB inhibitor BAY 11-7085 for the treatment of endometriosis: An in vitro study. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E16–E23. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.R.; Jothi, G.; Mohana, T.; Vasconcelos, A.B.S.; Montalvão, M.M.; Hariharan, G.; Sridharan, G.; Kumar, P.M.; Gurgel, R.Q.; Li, H.-B.; et al. Anti-inflammatory natural products as potential therapeutic agents of rheumatoid arthritis: A systematic review. Phytomedicine 2021, 93, 153766. [Google Scholar] [CrossRef] [PubMed]
- Melo, C.M.; Carvalho, K.M.; Neves, J.C.; Morais, T.C.; Rao, V.S.; Santos, F.A.; Brito, G.A.; Chaves, M.H. alpha, beta-amyrin, a natural triterpenoid ameliorates L-arginine-induced acute pancreatitis in rats. World J. Gastroenterol. 2010, 16, 4272–4280. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Xu, P.; Li, H. Stigmasterol alleviates interleukin-1beta-induced chondrocyte injury by down-regulatingsterol regulatory element binding transcription factor 2 to regulateferroptosis. Bioengineered 2021, 12, 9332–9340. [Google Scholar] [CrossRef]
- Kangsamaksin, T.; Chaithongyot, S.; Wootthichairangsan, C.; Hanchaina, R.; Tangshewinsirikul, C.; Svasti, J. Lupeol and stigmasterol suppress tumor angiogenesis and inhibit cholangiocarcinoma growth in mice via downregulation of tumor necrosis factor-α. PLoS ONE 2017, 12, e0189628. [Google Scholar] [CrossRef] [PubMed]
- Gabay, O.; Sanchez, C.; Salvat, C.; Chevy, F.; Breton, M.; Nourissat, G.; Wolf, C.; Jacques, C.; Berenbaum, F. Stigmasterol: A phytosterol with potential anti-osteoarthritic properties. Osteoarthr. Cartil. 2010, 18, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Grazul, M.; Kwiatkowski, P.; Hartman, K.; Kilanowicz, A.; Sienkiewicz, M. How to naturally support the immune system in inflammation—Essential oils as immune boosters. Biomedicines 2023, 11, 2381. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Li, J.; Li, X. Luteolin regulates CLP-induced sepsis mice by inhibiting PPAR-gamma/STAT/MyD88 pathway. Int. J. Clin. Exp. Med. 2018, 11, 6941–6948. [Google Scholar]
- Patil, P.; Nene, S.; Shah, S.; Singh, S.B.; Srivastava, S. Exploration of novel drug delivery systems in topical management of osteoarthritis. Drug Deliv. Transl. Res. 2023, 13, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Van Loo, G.; Bertrand, M.J.M. Death by TNF: A road to inflammation. Nat. Rev. Immunol. 2023, 23, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Voon, F.L.; Sulaiman, M.R.; Akhtar, M.N.; Idris, M.F.; Akira, A.; Perimal, E.K.; Israf, D.A.; Ming-Tatt, L. Cardamonin (2’,4’-dihydroxy-6’-methoxychalcone) isolated from Boesenbergia rotunda (L.) Mansf. inhibits CFA-induced rheumatoid arthritis in rats. Eur. J. Pharmacol. 2017, 794, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Werawattanachai, N.; Kaewamatawong, R.; Junlatat, J.; Sripanidkulchai, B.-O. Anti-inflammatory potential of ethanolic bulb extract of Allium ascalonicum. J. Sci. Technol. 2015, 17, 63–68. [Google Scholar]
- George, G.; Shyni, G.L.; Abraham, B.; Nisha, P.; Raghu, K.G. Downregulation of TLR4/MyD88/p38MAPK and JAK/STAT pathway in RAW 264.7 cells by Alpinia galanga reveals its beneficial effects in inflammation. J. Ethnopharmacol. 2021, 275, 114132. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Xie, H.; Yu, S.; Wang, S.; Yang, H. Piperine Derived from Piper nigrum L. Inhibits LPS-Induced Inflammatory through the MAPK and NF-kappaB Signalling Pathways in RAW264.7 Cells. Foods 2022, 11, 2990. [Google Scholar] [CrossRef]
- Liu, S.; He, Y.; Shi, J.; Liu, L.; Ma, H.; He, L.; Guo, Y. Allicin Attenuates Myocardial Ischemia Reperfusion Injury in Rats by Inhibition of Inflammation and Oxidative Stress. Transplant. Proc. 2019, 51, 2060–2065. [Google Scholar] [CrossRef] [PubMed]
- Samra, Y.A.; Hamed, M.F.; El-Sheakh, A.R. Hepatoprotective effect of allicin against acetaminophen-induced liver injury: Role of inflammasome pathway, apoptosis, and liver regeneration. J. Biochem. Mol. Toxicol. 2020, 34, e22470. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Li, N.; Zhou, D.; Chen, G.; Jiao, K.; Wang, W.; Si, Y.; Hou, Y. Sesquiterpene Coumarins from Ferula sinkiangensis Act as Neuroinflammation Inhibitors. Planta Med. 2017, 83, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Yun, N.; Lee, C.-H.; Lee, S.-M. Protective effect of Aloe vera on polymicrobial sepsis in mice. Food Chem. Toxicol. 2009, 47, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Mangmool, S.; Duangrat, R.; Rujirayunyong, T.; Anantachoke, N. Anti-inflammatory effects of the Thai herbal remedy Yataprasen and biflavonoids isolated from Putranjiva roxburghii in RAW264.7 macrophages. J. Ethnopharmacol. 2024, 327, 117997. [Google Scholar] [CrossRef] [PubMed]
- Chittasupho, C.; Chaobankrang, K.; Sarawungkad, A.; Samee, W.; Singh, S.; Hemsuwimon, K.; Okonogi, S.; Kheawfu, K.; Kiattisin, K.; Chaiyana, W. Antioxidant, Anti-Inflammatory and Attenuating Intracellular Reactive Oxygen Species Activities of Nicotiana tabacum var. virginia Leaf Extract Phytosomes and Shape Memory Gel Formulation. Gels 2023, 9, 78. [Google Scholar] [CrossRef]
- Chittasupho, C.; Samee, W.; Tadtong, S.; Jittachai, W.; Managit, C.; Athikomkulchai, S. Cytotoxicity, Apoptosis Induction, Oxidative Stress, and Cell Cycle Arrest of Clerodendrum chinense Flower Extract Nanoparticles in HeLa Cells. Nat. Life Sci. Commun. 2023, 22, 978. [Google Scholar] [CrossRef]
- Athikomkulchai, S.; Tunit, P.; Tadtong, S.; Jantrawut, P.; Sommano, S.R.; Chittasupho, C. Moringa oleifera Seed Oil Formulation Physical Stability and Chemical Constituents for Enhancing Skin Hydration and Antioxidant Activity. Cosmetics 2021, 8, 2. [Google Scholar] [CrossRef]
- Chiangnoon, R.; Samee, W.; Uttayarat, P.; Jittachai, W.; Ruksiriwanich, W.; Sommano, S.R.; Athikomkulchai, S.; Chittasupho, C. Phytochemical Analysis, Antioxidant, and Wound Healing Activity of Pluchea indica L. (Less) Branch Extract Nanoparticles. Molecules 2022, 27, 635. [Google Scholar] [CrossRef] [PubMed]
- Chittasupho, C.; Manthaisong, A.; Okonogi, S.; Tadtong, S.; Samee, W. Effects of Quercetin and Curcumin Combination on Antibacterial, Antioxidant, In Vitro Wound Healing and Migration of Human Dermal Fibroblast Cells. Int. J. Mol. Sci. 2022, 23, 142. [Google Scholar] [CrossRef] [PubMed]
- Chittasupho, C.; Ditsri, S.; Singh, S.; Kanlayavattanakul, M.; Duangnin, N.; Ruksiriwanich, W.; Athikomkulchai, S. Ultraviolet Radiation Protective and Anti-Inflammatory Effects of Kaempferia galanga L. Rhizome Oil and Microemulsion: Formulation, Characterization, and Hydrogel Preparation. Gels 2022, 8, 639. [Google Scholar] [CrossRef] [PubMed]
- Bibič, L.; Stokes, L. Revisiting the Idea That Amyloid-β Peptide Acts as an Agonist for P2X7. Front. Mol. Neurosci. 2020, 13, 166. [Google Scholar] [CrossRef] [PubMed]




| Samples | Total Phenolic Contents (mg GAE/g) | Total Flavonoid Contents (mg QAE/g) |
|---|---|---|
| YTPS formulary extract | 117.73 ± 8.55 | 3148.06 ± 2.05 |
| Putranjiva roxburghii Wall. extract | 123.64 ± 5.17 | 2172.84 ± 6.74 |
| Senna siamea (Lam.) H.S. Irwin & Barneby extract | 114.29 ± 3.91 | 2546.67 ± 2.05 |
| Baliospermum solanifolium (Burm.) Suresh extract | 120.06 ± 2.45 | 2667.56 ± 14.37 |
| Cymbopogon nardus (L.) Rendle extract | 122.31 ± 5.71 | 1615.13 ± 11.73 |
| Tamarindus indica L. extract | 127.28 ± 1.47 | 2496.22 ± 2.05 |
| Melia azedarach L. extract | 109.94 ± 7.96 | 1659.49 ± 10.56 |
| Boesenbergia rotunda (L.) Mansf. extract | 142.04 ± 7.84 | 1340.00 ± 1.76 |
| Allium sativum L. extract | 100.65 ± 5.23 | 855.61 ± 2.35 |
| Alpinia galanga (L.) Willd. extract | 126.01 ± 0.42 | 636.86 ± 5.57 |
| Piper nigrum L. extract | 115.44 ± 1.34 | 1391.31 ± 0.88 |
| Ferula assa-foetida L. extract | 87.15 ± 0.45 | 1904.31 ± 4.40 |
| Aloe vera (L.) Burm.f. extract | 134.94 ± 4.56 | 2083.10 ± 7.04 |
| Allium ascalonicum L. extract | 115.89 ± 2.11 | 171.43 ± 11.73 |
| Treatment | IC50 Values of DPPH Assay (µg/mL) | IC50 Values of ABTS Assay (µg/mL) | FRAP Values (µM) |
|---|---|---|---|
| Ascorbic acid | 8.16 ± 0.30 | 24.23 ± 2.02 | 4150.33 ± 6.84 |
| Gallic acid | 6.84 ± 0.18 | 9.77 ± 0.77 | 4185.50 ± 2.77 |
| Quercetin | 6.71 ± 0.35 | 26.01 ± 1.31 | 3883.53 ± 13.58 |
| Trolox® | 10.72 ± 0.21 | 32.22 ± 0.32 | 4081.54 ± 10.56 |
| YTPS formulary extract | 79.61 ± 0.99 | 271.40 ± 10.02 | 100.99 ± 8.11 |
| P. roxburghii | 36.99 ± 1.53 | 96.98 ± 3.38 | 1788.21 ± 2.63 |
| S. siamea | 222.90 ± 1.60 | 564.50 ± 8.60 | 878.97 ± 18.66 |
| B. solanifolium | 417.80 ± 7.77 | 3903.00 ± 128.00 | 328.97 ± 8.47 |
| C. nardus | 176.80 ± 3.99 | 6419.50 ± 645.59 | 809.25 ± 7.12 |
| T. indica | 39.64 ± 1.01 | 858.0 ± 13.00 | 1821.25 ± 2.26 |
| M. azedarach | 311.80 ± 10.48 | 1334 ± 15.01 | 652.19 ± 4.40 |
| B. rotunda | 353.0 ± 10.05 | 446.30 ± 8.76 | 444.87 ± 16.35 |
| A. sativum | 1438.0 ± 8.49 | 8667 ± 95.46 | 253.35 ± 14.73 |
| A. galanga | 232.30 ± 24.59 | 962.90 ± 14.98 | 647.28 ± 8.20 |
| P. nigrum | 324.10 ± 28.85 | 1611 ± 15.28 | 774.16 ± 9.03 |
| A. vera | 226.90 ± 16.74 | 214.70 ± 6.01 | 457.65 ± 7.74 |
| A. ascalonicum | 1024.0 ± 8.72 | 2225 ± 77.93 | 353.86 ± 10.24 |
| Treatment | Concentration | IL-1β (pg/mL) | IL-6 (pg/mL) | TNF-α (pg/mL) |
|---|---|---|---|---|
| Control | - | 0.00 | 0.00 | 0.00 |
| LPS | 22.51 ± 1.03 | 69.71 ± 1.06 | 64.96 ± 0.51 | |
| LPS + BAY 11-7085 | 0.00 | 0.00 | 0.00 | |
| LPS + β-amyrin | 20 µM | 8.65 ± 0.42 **** | 57.57 ± 2.29 **** | 11.69 ± 1.64 **** |
| LPS + stigmasterol | 20 µM | 11.56 ± 0.47 **** | 64.54 ± 1.75 | 15.39 ± 0.72 **** |
| LPS + YTPS formulary | 1000 µg/mL | 20.54 ± 1.12 | 2.31 ± 0.14 **** | 57.32 ± 3.78 **** |
| LPS + P. roxburghii | 100 µg/mL | 2.77 ± 0.36 **** | 2.17±0.86 **** | 16.29 ± 0.74 **** |
| LPS + S. siamea | 100 µg/mL | 0.00 **** | 0.00 **** | 0.00 **** |
| LPS + B. solanifolium | 100 µg/mL | 98.73 ± 7.22 **** | 0.00 **** | 36.62 ± 1.36 **** |
| LPS + C. nardus | 100 µg/mL | 8.16 ± 0.57 **** | 16.02 ± 0.37 **** | 14.94 ± 1.80 **** |
| LPS + T. indica | 50 µg/mL | 0.00 **** | 0.00 **** | 0.00 **** |
| LPS + M. azedarach | 100 µg/mL | 0.00 **** | 0.00 **** | 0.00 **** |
| LPS + B. rotunda | 100 µg/mL | 19.77 ± 2.14 | 0.00 **** | 0.00 **** |
| LPS + A. sativum | 100 µg/mL | 11.80 ± 0.92 **** | 93.67 ± 9.70 **** | 24.42 ± 1.42 **** |
| LPS + A. galanga | 100 µg/mL | 0.00 **** | 6.79 ± 0.35 **** | 2.29 ± 0.62 **** |
| LPS + P. nigrum | 100 µg/mL | 0.00 **** | 0.00 **** | 0.00 **** |
| LPS + F. assa-foetida + A. vera | 100 µg/mL | 8.40 ± 0.89 **** | 34.21 ± 4.45 **** | 15.23 ± 1.86 **** |
| LPS + A. ascalonicum | 100 µg/mL | 5.51 ± 0.33 **** | 17.50 ± 3.14 **** | 6.15 ± 1.05 **** |
| No. | Plant Scientific Name | Parts Used | %w/w | Voucher Specimen |
|---|---|---|---|---|
| 1 | P. roxburghii | Leaves | 37 | TTM0005479 |
| 2 | S. siamea | Leaves | 9 | TTM0005489 |
| 3 | B. solanifolium | Leaves | 9 | TTM0005473 |
| 4 | C. nardus | Rhizomes and leaves | 9 | TTM0005477 |
| 5 | T. indica | Leaves | 9 | TTM0005469 |
| 6 | M. azedarach | Leaves | 9 | TTM0005488 |
| 7 | B. rotunda | Rhizomes and roots | 2 | TTM0005476 |
| 8 | A. sativum | Bulbs | 2 | TTM0005474 |
| 9 | A. galanga | Rhizomes | 2 | TTM0005475 |
| 10 | P. nigrum | Fruits | 2 | TTM0005490 |
| 11 | F. assa-foetida | Resin from roots | 2 | TTM1000743 |
| 12 | A. vera | Resin | 2 | TTM1000744 |
| 13 | A. ascalonicum | Bulbs | 2 | TTM0005478 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angsusing, J.; Singh, S.; Samee, W.; Tadtong, S.; Stokes, L.; O’Connell, M.; Bielecka, H.; Toolmal, N.; Mangmool, S.; Chittasupho, C. Anti-Inflammatory Activities of Yataprasen Thai Traditional Formulary and Its Active Compounds, Beta-Amyrin and Stigmasterol, in RAW264.7 and THP-1 Cells. Pharmaceuticals 2024, 17, 1018. https://doi.org/10.3390/ph17081018
Angsusing J, Singh S, Samee W, Tadtong S, Stokes L, O’Connell M, Bielecka H, Toolmal N, Mangmool S, Chittasupho C. Anti-Inflammatory Activities of Yataprasen Thai Traditional Formulary and Its Active Compounds, Beta-Amyrin and Stigmasterol, in RAW264.7 and THP-1 Cells. Pharmaceuticals. 2024; 17(8):1018. https://doi.org/10.3390/ph17081018
Chicago/Turabian StyleAngsusing, Jaenjira, Sudarshan Singh, Weerasak Samee, Sarin Tadtong, Leanne Stokes, Maria O’Connell, Hanna Bielecka, Nopparut Toolmal, Supachoke Mangmool, and Chuda Chittasupho. 2024. "Anti-Inflammatory Activities of Yataprasen Thai Traditional Formulary and Its Active Compounds, Beta-Amyrin and Stigmasterol, in RAW264.7 and THP-1 Cells" Pharmaceuticals 17, no. 8: 1018. https://doi.org/10.3390/ph17081018
APA StyleAngsusing, J., Singh, S., Samee, W., Tadtong, S., Stokes, L., O’Connell, M., Bielecka, H., Toolmal, N., Mangmool, S., & Chittasupho, C. (2024). Anti-Inflammatory Activities of Yataprasen Thai Traditional Formulary and Its Active Compounds, Beta-Amyrin and Stigmasterol, in RAW264.7 and THP-1 Cells. Pharmaceuticals, 17(8), 1018. https://doi.org/10.3390/ph17081018







