Comprehensive Review of Cyclamen: Development, Bioactive Properties, and Therapeutic Applications
Abstract
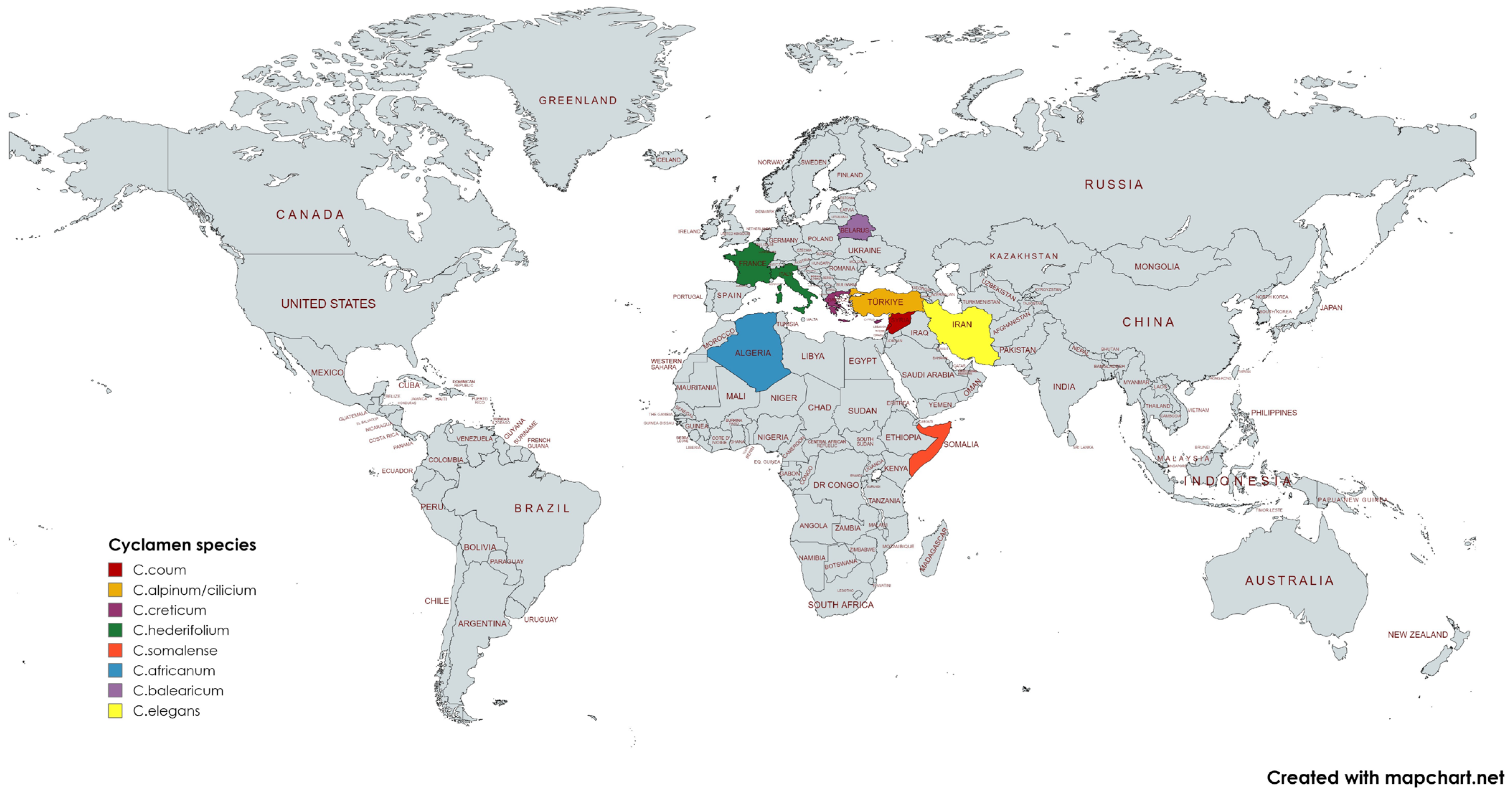
1. Introduction
2. Major Properties of Cyclamen
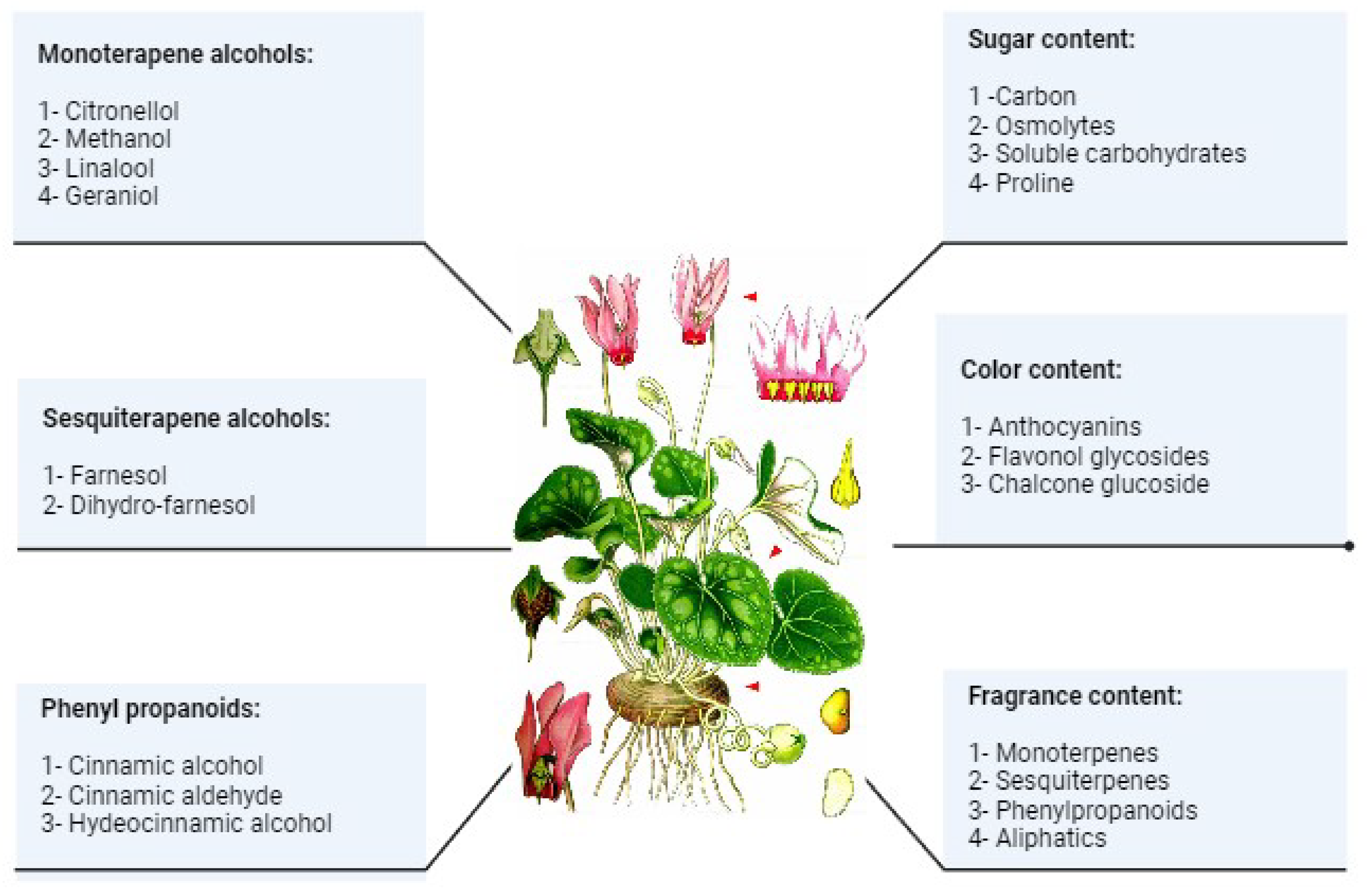
3. Cyclamen Composition
3.1. Cyclamen Somatic Embryogenesis
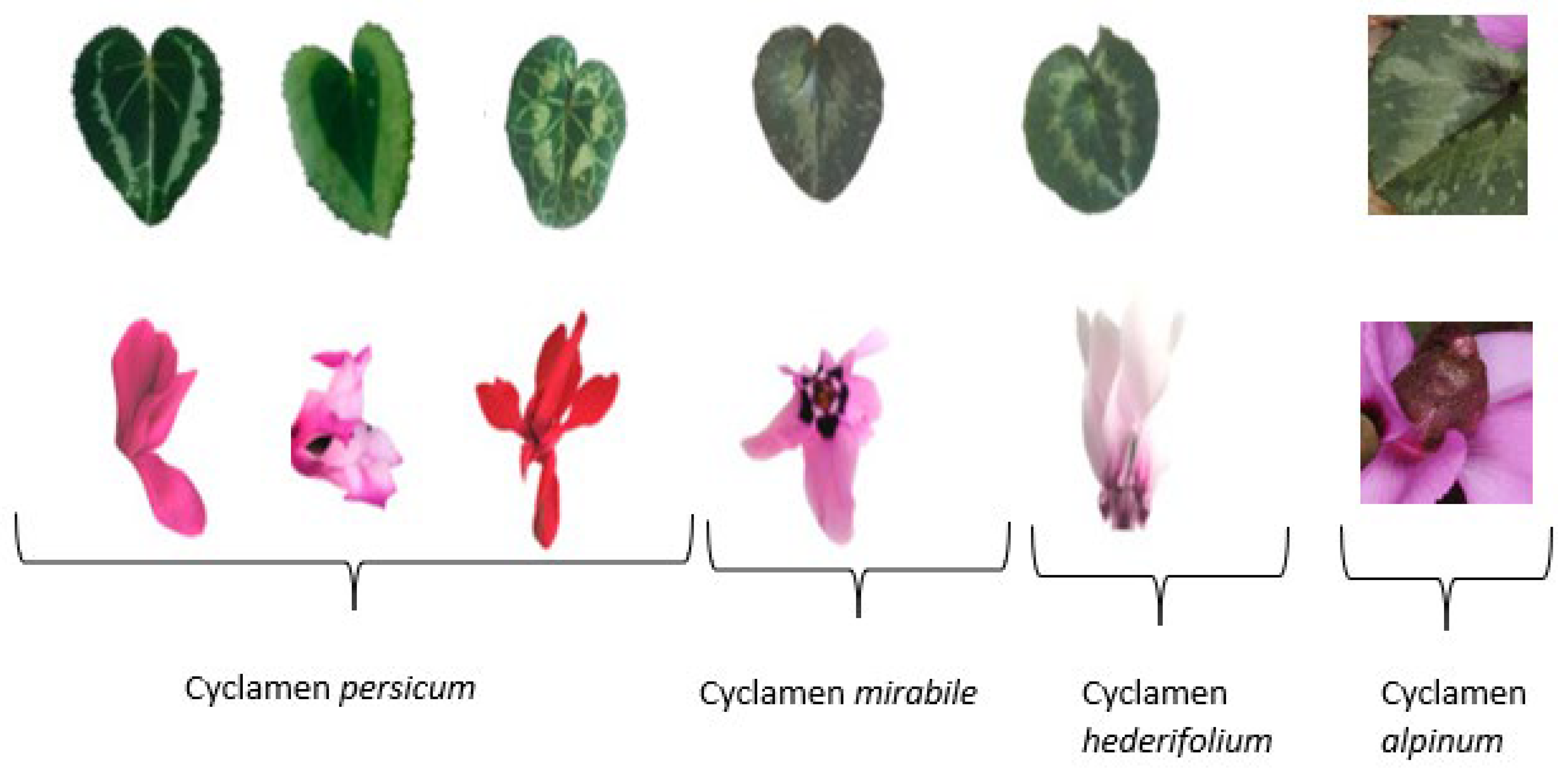
3.2. Color Distribution among Different Parts of Cyclamen Leaves and Flowers
3.3. Sugar Content
3.4. Flower Fragrance and Volatile Compounds
4. Bioactive Potential Efficiency of Cyclamen Plant
4.1. Antioxidant Activity
4.2. Anti-Inflammatory Effect
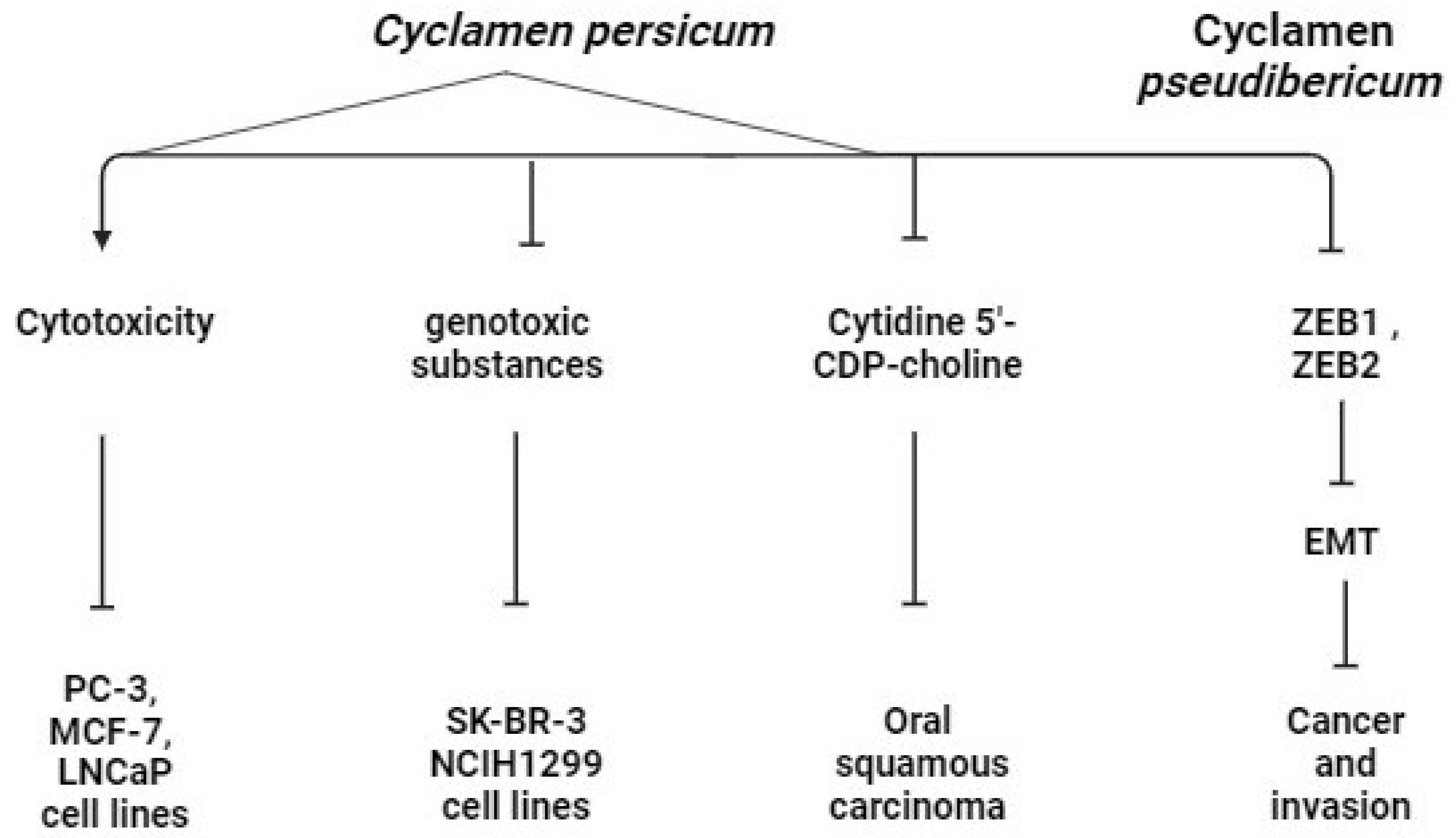
4.3. Anti-Cancerous Effect
5. Therapeutic Applications and Clinical Trials of Cyclamen
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pant, B. Application of Plant Cell and Tissue Culture for the Production of Phytochemicals in Medicinal Plants. In Infectious Diseases and Nanomedicine II; Adhikari, R., Thapa, S., Eds.; Advances in Experimental Medicine and Biology; Springer: New Delhi, India, 2014; Volume 808, pp. 25–39. ISBN 978-81-322-1773-2. [Google Scholar]
- Welz, A.N.; Emberger-Klein, A.; Menrad, K. The Importance of Herbal Medicine Use in the German Health-Care System: Prevalence, Usage Pattern, and Influencing Factors. BMC Health Serv. Res. 2019, 19, 952. [Google Scholar] [CrossRef] [PubMed]
- Illamola, S.M.; Amaeze, O.U.; Krepkova, L.V.; Birnbaum, A.K.; Karanam, A.; Job, K.M.; Bortnikova, V.V.; Sherwin, C.M.T.; Enioutina, E.Y. Use of Herbal Medicine by Pregnant Women: What Physicians Need to Know. Front. Pharmacol. 2020, 10, 1483. [Google Scholar] [CrossRef]
- Martin, K.W.; Ernst, E. Herbal Medicines for Treatment of Bacterial Infections: A Review of Controlled Clinical Trials. J. Antimicrob. Chemother. 2003, 51, 241–246. [Google Scholar] [CrossRef]
- Zengin, G.; Mahomoodally, M.F.; Sinan, K.I.; Picot-Allain, M.C.N.; Yildiztugay, E.; Cziáky, Z.; Jekő, J.; Saleem, H.; Ahemad, N. Chemical Characterization, Antioxidant, Enzyme Inhibitory and Cytotoxic Properties of Two Geophytes: Crocus pallasii and Cyclamen cilicium. Food Res. Int. 2020, 133, 109129. [Google Scholar] [CrossRef] [PubMed]
- Saboora, A.; Sajjadi, S.-T.; Mohammadi, P. Comparison of Aglycon and Glycosidic Saponin Extracts of Cyclamen coum Tuber against Candida spp. Curr. Med. Mycol. 2016, 2, 40–44. [Google Scholar] [CrossRef][Green Version]
- Damke, E.; Tsuzuki, J.K.; Cortez, D.A.; Ferreira, I.C.; Bertoni, T.A.; Batista, M.R.; Donati, L.; Svidzinski, T.I.; Consolaro, M.E. In Vivo Activity of Sapindus saponaria against Azole-Susceptible and -Resistant Human Vaginal Candida Species. BMC Complement. Altern. Med. 2011, 11, 35. [Google Scholar] [CrossRef]
- Sparg, S.G.; Light, M.E.; Van Staden, J. Biological Activities and Distribution of Plant Saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Barrett, B.; Kiefer, D.; Rabago, D. Assessing the Risks and Benefits of Herbal Medicine: An Overview of Scientific Evidence. Altern. Ther. Health Med. 1999, 5, 40–49. [Google Scholar]
- Nabi, F.; Shi, D.; Wu, Q.; Baloch, D.M. Editorial: Treatment of Animal Diseases with Veterinary Phytotherapy. Front. Vet. Sci. 2023, 10, 1171987. [Google Scholar] [CrossRef]
- Bent, S. Herbal Medicine in the United States: Review of Efficacy, Safety, and Regulation: Grand Rounds at University of California, San Francisco Medical Center. J. Gen. Intern. Med. 2008, 23, 854–859. [Google Scholar] [CrossRef]
- Fitzgerald, M.; Heinrich, M.; Booker, A. Medicinal Plant Analysis: A Historical and Regional Discussion of Emergent Complex Techniques. Front. Pharmacol. 2020, 10, 1480. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Li, S.; Tan, H.; Wang, N.; Tsao, S.-W.; Feng, Y. Current Status of Herbal Medicines in Chronic Liver Disease Therapy: The Biological Effects, Molecular Targets and Future Prospects. Int. J. Mol. Sci. 2015, 16, 28705–28745. [Google Scholar] [CrossRef] [PubMed]
- Silva Sofrás, F.M.; Alonso, R.; Retta, D.S.; Di Leo Lira, P.; Desimone, M.F.; Van Baren, C.M. Development and Validation of a Simple, Fast, and Accessible HPLC-UVMethod for Cannabinoids Determination in Cannabis sativa L. Extracts andMedicinal Oils. Curr. Pharm. Des. 2023, 29, 1918–1928. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Pei, G.; Wang, Z.; Chen, N. Neurotrophic Basis to the Pathogenesis of Depression and Phytotherapy. Front. Pharmacol. 2023, 14, 1182666. [Google Scholar] [CrossRef] [PubMed]
- Boase, M.R.; Lewis, D.H.; Davies, K.M.; Marshall, G.B.; Patel, D.; Schwinn, K.E.; Deroles, S.C. Isolation and Antisense Suppression of Flavonoid 3′, 5′-Hydroxylase Modifies Flower Pigments and Colour in Cyclamen. BMC Plant Biol. 2010, 10, 107. [Google Scholar] [CrossRef]
- Webby, R.F.; Boase, M.R. Peonidin 3-O-Neohesperidoside and Other Flavonoids from Cyclamen Persicum Petals. Phytochemistry 1999, 52, 939–941. [Google Scholar] [CrossRef]
- Ishizaka, H. Breeding of Fragrant Cyclamen by Interspecific Hybridization and Ion-Beam Irradiation. Breed. Sci. 2018, 68, 25–34. [Google Scholar] [CrossRef]
- Reinhardt, S.; Ewalda, A.; Hellwig, F. The Anatomy of the Stigma and Style from Cyclamen persicum (Mill.) Cv. “Pure White” and Its Relation to Pollination Success. Plant Biol. 2007, 9, 158–162. [Google Scholar] [CrossRef]
- Bolhaar, S.T.H.P.; Ginkel, C.J.W.V.A.N. Occupational Allergy to Cyclamen. Allergy 2000, 55, 411–412. [Google Scholar] [CrossRef]
- Yesson, C.; Culham, A. A Phyloclimatic Study of Cyclamen. BMC Evol. Biol. 2006, 6, 72. [Google Scholar] [CrossRef][Green Version]
- Yan, X.; Liu, T.; Yuan, X.; Xu, Y.; Yan, H.; Hao, G. Chloroplast Genomes and Comparative Analyses among Thirteen Taxa within Myrsinaceae s.Str. Clade (Myrsinoideae, Primulaceae). Int. J. Mol. Sci. 2019, 20, 4534. [Google Scholar] [CrossRef] [PubMed]
- Thulin, M.; Warfa, A.M. Cyclamen (Primulaceae) in Tropical Africa. Plant Syst. Evol. 1989, 166, 249–252. [Google Scholar] [CrossRef]
- Mao, L.; Zou, Q.; Sun, Z.; Dong, Q.; Cao, X. Insights into Chloroplast Genome Structure, Intraspecific Variation, and Phylogeny of Cyclamen Species (Myrsinoideae). Sci. Rep. 2023, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Cornea-Cipcigan, M.; Bunea, A.; Bouari, C.M.; Pamfil, D.; Páll, E.; Urcan, A.C.; Mărgăoan, R. Anthocyanins and Carotenoids Characterization in Flowers and Leaves of Cyclamen Genotypes Linked with Bioactivities Using Multivariate Analysis Techniques. Antioxidants 2022, 11, 1126. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Fu, A.; Aluru, M.; Park, S.; Xu, Y.; Liu, H.; Liu, X.; Foudree, A.; Nambogga, M.; Rodermel, S. Variegation Mutants and Mechanisms of Chloroplast Biogenesis: Variegation Mutants. Plant Cell Environ. 2007, 30, 350–365. [Google Scholar] [CrossRef] [PubMed]
- El Hosry, L.; Di Giorgio, C.; Birer, C.; Habib, J.; Tueni, M.; Bun, S.-S.; Herbette, G.; De Meo, M.; Ollivier, E.; Elias, R. In Vitro Cytotoxic and Anticlastogenic Activities of Saxifragifolin B and Cyclamin Isolated from Cyclamen persicum and Cyclamen libanoticum. Pharm. Biol. 2014, 52, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Heringer, A.S.; Santa-Catarina, C.; Silveira, V. Insights from Proteomic Studies into Plant Somatic Embryogenesis. Proteomics 2018, 18, 1700265. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.G. Diversity and Classification of Flowering Plants: Eudicots. In Plant Systematics; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Goldberg, R.B.; De Paiva, G.; Yadegari, R. Plant Embryogenesis: Zygote to Seed. Science 1994, 266, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, T. Clonal Propagation of Cyclamen persicum Via Somatic Embryogenesis. In Protocols for In Vitro Propagation of Ornamental Plants; Jain, S.M., Ochatt, S.J., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 589, pp. 281–290. ISBN 978-1-60327-390-9. [Google Scholar]
- Xia, D.; He, G.; Wang, K.; Wang, T.; Zhu, Z.; Niu, Z.; Shi, G.; Liu, G. Anthocyanins Profiling Analysis and RNA-Seq Revealed the Dominating Pigments and Coloring Mechanism in Cyclamen Flowers. Biology 2022, 11, 1721. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of Plant Pigments: Anthocyanins, Betalains and Carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Wong, D.C.J.; Perkins, J.; Peakall, R. Anthocyanin and Flavonol Glycoside Metabolic Pathways Underpin Floral Color Mimicry and Contrast in a Sexually Deceptive Orchid. Front. Plant Sci. 2022, 13, 860997. [Google Scholar] [CrossRef]
- Tarone, A.G.; Cazarin, C.B.B.; Marostica Junior, M.R. Anthocyanins: New Techniques and Challenges in Microencapsulation. Food Res. Int. 2020, 133, 109092. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, Pharmacology and Health Benefits of Anthocyanins: Anthocyanins and Human Health. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Q.; Cheung, L.S.; Feng, L.; Tanner, W.; Frommer, W.B. Transport of Sugars. Annu. Rev. Biochem. 2015, 84, 865–894. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.-L. Sucrose Metabolism: Gateway to Diverse Carbon Use and Sugar Signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef] [PubMed]
- Pouris, J.; Levizou, E.; Karatassiou, M.; Meletiou-Christou, M.-S.; Rhizopoulou, S. The Influence of the Partitioning of Sugars, Starch, and Free Proline in Various Organs of Cyclamen graecum on the Biology of the Species and Its Resistance to Abiotic Stressors. Plants 2022, 11, 1254. [Google Scholar] [CrossRef] [PubMed]
- Khodorova, N.; Boitel-Conti, M. The Role of Temperature in the Growth and Flowering of Geophytes. Plants 2013, 2, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Ishizaka, H. Interspecific Hybrids of Cyclamen persicum and C. Graecum. Euphytica 1996, 91, 109–117. [Google Scholar] [CrossRef]
- Funck, D.; Winter, G.; Baumgarten, L.; Forlani, G. Requirement of Proline Synthesis during Arabidopsis Reproductive Development. BMC Plant Biol. 2012, 12, 191. [Google Scholar] [CrossRef]
- Carfagna, S.; Salbitani, G.; Innangi, M.; Menale, B.; De Castro, O.; Di Martino, C.; Crawford, T.W. Simultaneous Biochemical and Physiological Responses of the Roots and Leaves of Pancratium maritimum (Amaryllidaceae) to Mild Salt Stress. Plants 2021, 10, 345. [Google Scholar] [CrossRef]
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Floral Scents and Fruit Aromas: Functions, Compositions, Biosynthesis, and Regulation. Front. Plant Sci. 2022, 13, 860157. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Pichersky, E. Biochemical and Molecular Genetic Aspects of Floral Scents. Plant Physiol. 2000, 122, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Sezgin-Bayindir, Z.; Paiva-Martins, F.; Bravo-Díaz, C. Biochemistry of Antioxidants: Mechanisms and Pharmaceutical Applications. Biomedicines 2022, 10, 3051. [Google Scholar] [CrossRef]
- Zoral, F.B.; Turgay, Ö. Çeşitli Gıda Atıklarının Toplam Fenolik Madde İçeriğinin, Antioksidan ve Antimikrobiyal Etkilerinin Araştırılması. KSÜ Doğ. Bil. Derg 2014, 17, 24. Available online: https://dergipark.org.tr/tr/pub/ksudobil/issue/22841/243836 (accessed on 21 June 2024). [CrossRef]
- Kehrer, J.P.; DiGiovanni, J. Comparison of Lung Injury Induced in 4 Strains of Mice by Butylated Hydroxytoluene. Toxicol. Lett. 1990, 52, 55–61. [Google Scholar] [CrossRef]
- Pasqualone, A.; Bianco, A.M.; Paradiso, V.M.; Summo, C.; Gambacorta, G.; Caponio, F.; Blanco, A. Production and Characterization of Functional Biscuits Obtained from Purple Wheat. Food Chem. 2015, 180, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Freitas, K.H.G.; Fatibello-Filho, O. Simultaneous Determination of Butylated Hydroxyanisole (BHA) and Butylated Hydroxytoluene (BHT) in Food Samples Using a Carbon Composite Electrode Modified with Cu3(PO4)2 Immobilized in Polyester Resin. Talanta 2010, 81, 1102–1108. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Borland, A.; Elliott, S.; Patterson, S.; Taybi, T.; Cushman, J.; Pater, B.; Barnes, J. Are the Metabolic Components of Crassulacean Acid Metabolism Up-Regulated in Response to an Increase in Oxidative Burden? J. Exp. Bot. 2006, 57, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Tabata, K.; Ôba, K.; Suzuki, K.; Esaka, M. Generation and Properties of Ascorbic Acid-Deficient Transgenic Tobacco Cells Expressing Antisense RNA for L-Galactono-1,4-Lactone Dehydrogenase: GalLDH in Ascorbic Acid-Deficient Transgentic Tobacco. Plant J. 2001, 27, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Horemans, N.; Foyer, C.H.; Asard, H. Transport and Action of Ascorbate at the Plant Plasma Membrane. Trends Plant Sci. 2000, 5, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G. Metabolic Signalling in Defence and Stress: The Central Roles of Soluble Redox Couples. Plant Cell Environ. 2006, 29, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Gang, W.; Zhen-Kuan, W.; Yong-Xiang, W.; Li-Ye, C.; Hong-Bo, S. The Mutual Responses of Higher Plants to Environment: Physiological and Microbiological Aspects. Colloids Surf. B Biointerfaces 2007, 59, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, F.; Wang, X.; Kim, H.-J.; He, G.; Haley-Zitlin, V.; Huang, G. Antioxidant Constituents in Feverfew (Tanacetum parthenium) Extract and Their Chromatographic Quantification. Food Chem. 2006, 96, 220–227. [Google Scholar] [CrossRef]
- Turan, M.; Mammadov, R. Antioxidant, Antimicrobial, Cytotoxic, Larvicidal and Anthelmintic Activities and Phenolic Contents of Cyclamen alpinum. Pharmacol. Pharm. 2018, 9, 100–116. [Google Scholar] [CrossRef]
- Weih, M.; Karlsson, P.S. Growth Response of Altitudinal Ecotypes of Mountain Birch to Temperature and Fertilisation. Oecologia 1999, 119, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Marxen, K.; Vanselow, K.; Lippemeier, S.; Hintze, R.; Ruser, A.; Hansen, U.-P. Determination of DPPH Radical Oxidation Caused by Methanolic Extracts of Some Microalgal Species by Linear Regression Analysis of Spectrophotometric Measurements. Sensors 2007, 7, 2080–2095. [Google Scholar] [CrossRef]
- Maya, M.A.; Matsubara, Y. Influence of Arbuscular Mycorrhiza on the Growth and Antioxidative Activity in Cyclamen under Heat Stress. Mycorrhiza 2013, 23, 381–390. [Google Scholar] [CrossRef]
- Signore, A.; Glaudemans, A.W.J.M. The Molecular Imaging Approach to Image Infections and Inflammation by Nuclear Medicine Techniques. Ann. Nucl. Med. 2011, 25, 681–700. [Google Scholar] [CrossRef] [PubMed]
- Speroni, E.; Schwaiger, S.; Egger, P.; Berger, A.-T.; Cervellati, R.; Govoni, P.; Guerra, M.C.; Stuppner, H. In Vivo Efficacy of Different Extracts of Edelweiss (Leontopodium alpinum Cass.) in Animal Models. J. Ethnopharmacol. 2006, 105, 421–426. [Google Scholar] [CrossRef]
- Pagnacco, M.C.; Maksimović, J.P.; Nikolić, N.T.; Bajuk Bogdanović, D.V.; Kragović, M.M.; Stojmenović, M.D.; Blagojević, S.N.; Senćanski, J.V. Indigo Carmine in a Food Dye: Spectroscopic Characterization and Determining Its Micro-Concentration through the Clock Reaction. Molecules 2022, 27, 4853. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Hasmeda, M.; Kweifio-Okai, G.; Macrides, T.; Polya, G.M. Selective Inhibition of Eukaryote Protein Kinases by Anti-Inflammatory Triterpenoids. Planta Med. 1999, 65, 14–18. [Google Scholar] [CrossRef]
- Speroni, E.; Cervellati, R.; Costa, S.; Dall’Acqua, S.; Guerra, M.C.; Panizzolo, C.; Utan, A.; Innocenti, G. Analgesic and Antiinflammatory Activity of Cyclamen repandum S. et S. Phytother. Res. 2007, 21, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Mashkova, T.A.; Matveeva, V.N.; Panchenko, I.G. Comparative characteristic of different methods of conservative treatment of exudative rhinosinusitis. Vestn. Otorinolaringol. 2010, 5, 13–14. [Google Scholar]
- Zalmanovici Trestioreanu, A.; Barua, A.; Pertzov, B. Cyclamen europaeum Extract for Acute Sinusitis. Cochrane Database Syst. Rev. 2018, 5, CD011341. [Google Scholar] [CrossRef] [PubMed]
- Mullol, J. Trends on Rhinosinusitis Diagnosis and Treatment. Otolaryngol. Pol. 2009, 63, 3–4. [Google Scholar] [CrossRef][Green Version]
- Momenimovahed, Z.; Salehiniya, H. Epidemiological Characteristics of and Risk Factors for Breast Cancer in the World. Breast Cancer 2019, 11, 151–164. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Shelton, L.M. Cancer as a Metabolic Disease. Nutr. Metab. 2010, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J.; Kalluri, R. Cancer without Disease. Nature 2004, 427, 787. [Google Scholar] [CrossRef] [PubMed]
- Blackadar, C.B. Historical Review of the Causes of Cancer. World J. Clin. Oncol. 2016, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Khalilia, W.M. Cytotoxic Activity of Cyclamen persicum Ethanolic Extract on MCF-7, PC-3 and LNCaP Cancer Cell Lines. J. Nat. Sci. 2020, 10, 42–46. [Google Scholar] [CrossRef]
- Karagur, E.R.; Ozay, C.; Mammadov, R.; Akca, H. Anti-Invasive Effect of Cyclamen Pseudibericum Extract on A549 Non-Small Cell Lung Carcinoma Cells via Inhibition of ZEB1 Mediated by miR-200c. J. Nat. Med. 2018, 72, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-β-Induced Epithelial to Mesenchymal Transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-H.; Chen, C.-L.; Chau, G.-Y.; Chiou, S.-H.; Su, C.-W.; Chou, T.-Y.; Peng, W.-L.; Wu, J.-C. Comprehensive Analysis of the Independent Effect of Twist and Snail in Promoting Metastasis of Hepatocellular Carcinoma. Hepatology 2009, 50, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, M.; Bozcu, H.; Tokgun, O.; Karagur, E.R.; Akyurt, O.; Akca, H. Cyclamen Exerts Cytotoxicity in Solid Tumor Cell Lines: A Step Toward New Anticancer Agents? Asian Pac. J. Cancer Prev. 2013, 14, 5911–5913. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Ma, L. MicroRNA Control of Epithelial–Mesenchymal Transition and Metastasis. Cancer Metastasis Rev. 2012, 31, 653–662. [Google Scholar] [CrossRef]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The miR-200 Family and miR-205 Regulate Epithelial to Mesenchymal Transition by Targeting ZEB1 and SIP1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Aigner, K.; Dampier, B.; Descovich, L.; Mikula, M.; Sultan, A.; Schreiber, M.; Mikulits, W.; Brabletz, T.; Strand, D.; Obrist, P.; et al. The Transcription Factor ZEB1 (δEF1) Promotes Tumour Cell Dedifferentiation by Repressing Master Regulators of Epithelial Polarity. Oncogene 2007, 26, 6979–6988. [Google Scholar] [CrossRef] [PubMed]
- Çaliş, İ.; Şatana, M.E.; Yürüker, A.; Kelican, P.; Demirdamar, R.; Alaçam, R.; Tanker, N.; Rüegger, H.; Sticher, O. Triterpene Saponins from Cyclamen mirabile and Their Biological Activities. J. Nat. Prod. 1997, 60, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.E.; Marnett, L.J.; Ames, B.N. Spontaneous and Mutagen-Induced Deletions: Mechanistic Studies in Salmonella Tester Strain TA102. Proc. Natl. Acad. Sci. USA 1984, 81, 4457–4461. [Google Scholar] [CrossRef] [PubMed]
- Krishna, G.; Kropko, M.L.; Theiss, J.C. Use of the Cytokinesis-Block Method for the Analysis of Munuclei in V79 Chinese Hamster Lung Cells: Results with Mitomycin C and Cyclophosphamide. Mutat. Res. Genet. Toxicol. 1989, 222, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Murugappan, M.N.; King-Kallimanis, B.L.; Bhatnagar, V.; Kanapuru, B.; Farley, J.F.; Seifert, R.D.; Stenehjem, D.D.; Chen, T.-Y.; Horodniceanu, E.G.; Kluetz, P.G. Measuring Frailty Using Patient-Reported Outcomes (PRO) Data: A Feasibility Study in Patients with Multiple Myeloma. Qual. Life Res. 2023, 32, 2281–2292. [Google Scholar] [CrossRef] [PubMed]
- Elson, C.E.; Peffley, D.M.; Hentosh, P.; Mo, H. Isoprenoid-Mediated Inhibition of Mevalonate Synthesis: Potential Application to Cancer. Proc. Soc. Exp. Biol. Med. 1999, 221, 294–311. [Google Scholar] [CrossRef] [PubMed]
- Burke, Y.D.; Stark, M.J.; Roach, S.L.; Sen, S.E.; Crowell, P.L. Inhibition of Pancreatic Cancer Growth by the Dietary Isoprenoids Farnesol and Geraniol. Lipids 1997, 32, 151. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Elson, C.E. Studies of the Isoprenoid-Mediated Inhibition of Mevalonate Synthesis Applied to Cancer Chemotherapy and Chemoprevention. Exp. Biol. Med. 2004, 229, 567–585. [Google Scholar] [CrossRef]
- Duncan, R.E.; Archer, M.C. Farnesol Decreases Serum Triglycerides in Rats: Identification of Mechanisms Including Up-Regulation of PPARα and Down-Regulation of Fatty Acid Synthase in Hepatocytes. Lipids 2008, 43, 619–627. [Google Scholar] [CrossRef]
- Goto, T.; Kim, Y.-I.; Funakoshi, K.; Teraminami, A.; Uemura, T.; Hirai, S.; Lee, J.-Y.; Makishima, M.; Nakata, R.; Inoue, H.; et al. Farnesol, an Isoprenoid, Improves Metabolic Abnormalities in Mice via Both PPARα-Dependent and -Independent Pathways. Am. J. Physiol.-Endocrinol. Metab. 2011, 301, E1022–E1032. [Google Scholar] [CrossRef] [PubMed]
- Scheper, M.A.; Shirtliff, M.E.; Meiller, T.F.; Peters, B.M.; Jabra-Rizk, M.A. Farnesol, a Fungal Quorum-Sensing Molecule Triggers Apoptosis in Human Oral Squamous Carcinoma Cells. Neoplasia 2008, 10, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Mihci-Gaidi, G.; Ozbey, S.; Orhan, I.; Sener, B.; Miyamoto, T.; Mirjolet, J.-F.; Duchamp, O.; Mitaine-Offer, A.-C.; Lacaille-Dubois, M.-A. Triterpene Saponins from Cyclamen trocopteranthum. Planta Med. 2010, 76, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Amin Jaradat, N.; Al-Masri, M.; Hussen, F.; Zaid, A.N.; Ali, I.; Tammam, A.; Mostafa Odeh, D.; Hussein Shakarneh, O.; Rajabi, A. Preliminary Phytochemical and Biological Screening of Cyclamen coum a Member of Palestinian Flora. Pharm. Sci. 2017, 23, 231–237. [Google Scholar] [CrossRef]
- Altunkeyik, H.; Gülcemal, D.; Masullo, M.; Alankus-Caliskan, O.; Piacente, S.; Karayildirim, T. Triterpene Saponins from Cyclamen hederifolium. Phytochemistry 2012, 73, 127–133. [Google Scholar] [CrossRef]
- Kojicic, K.; Arsenijevic, A.; Markovic, M.; Stankov-Jovanovic, V.; Simic, Z.; Tadic, V.; Cupara, S. Chemical and Pharmacological Characterization of Aqueous and Ethanolic Extracts of Cyclamen hederifolium Ait. (Primulaceae) Tuber. Vojnosanit. Pregl. 2021, 78, 532–541. [Google Scholar] [CrossRef]
- Yang, X.; Chung, K.F.; Huang, K. Worldwide Prevalence, Risk Factors and Burden of Chronic Cough in the General Population: A Narrative Review. J. Thorac Dis. 2023, 15, 2300–2313. [Google Scholar] [CrossRef] [PubMed]
- Steć, Z.; Burska, Z.; Brożek-Mądry, E.; Straburzyński, M.; Waliszewska-Prosół, M.; Krzeski, A. Clinical Characteristics of Acute Rhinosinusitisin COVID-19 a Post-Hoc Analysis of a Longitudinalstudy. Otolaryngol. Pol. 2022, 77, 12–18. [Google Scholar] [CrossRef]
- Ponikau, J.U.; Hamilos, D.L.; Barreto, A.; Cecil, J.; Jones, S.W.; Manthei, S.E.; Collins, J. An Exploratory Trial of Cyclamen europaeum Extract for Acute Rhinosinusitis. The Laryngoscope 2012, 122, 1887–1892. [Google Scholar] [CrossRef]
- Welge-Lussen, A.; Hilgenfeld, A.; Meusel, T.; Hummel, T. Long-Term Follow-up of Posttraumatic Olfactory Disorders. Rhin 2012, 50, 67–72. [Google Scholar] [CrossRef]
- Wasserfallen, J.-B.; Livio, F.; Zanetti, G. Acute Rhinosinusitis: A Pharmacoeconomic Review of Antibacterial Use. PharmacoEconomics 2004, 22, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Mullol, J.; Crespo, C.; Carré, C.; Brosa, M. Pharmacoeconomics of Cyclamen europaeum in the Management of Acute Rhinosinusitis. Laryngoscope 2013, 123, 2620–2625. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Campos; Clares; Rodríguez-Lagunas; Jauregui; Casals; Calpena Ex-Vivo and In-Vivo Assessment of Cyclamen europaeum Extract After Nasal Administration. Pharmaceutics 2019, 11, 426. [CrossRef] [PubMed]
- Lopatin, A.S.; Ivanchenko, O.A.; Soshnikov, S.S.; Mullol, J. Cyclamen europaeum Improves the Effect of Oral Antibiotics on Exacerbations and Recurrences of Chronic Rhinosinusitis: A Real-Life Observational Study (CHRONOS). Acta Otorhinolaryngol. Ital. 2018, 38, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Wine, J.J. Parasympathetic Control of Airway Submucosal Glands: Central Reflexes and the Airway Intrinsic Nervous System. Auton. Neurosci. 2007, 133, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Böttger, S.; Hofmann, K.; Melzig, M.F. Saponins Can Perturb Biologic Membranes and Reduce the Surface Tension of Aqueous Solutions: A Correlation? Bioorganic Med. Chem. 2012, 20, 2822–2828. [Google Scholar] [CrossRef] [PubMed]
- Jurkiewicz, D.; Hassmann-Poznańska, E.; Kaźmierczak, H.; Składzień, J.; Pietruszewska, W.; Burduk, P.; Rapiejko, P. Lyophilized Cyclamen europaeum Tuber Extract in the Treatment of Rhinosinusitis. Otolaryngol. Pol. 2016, 70, 1–9. [Google Scholar] [CrossRef]
- Al-Rimawi, F.; Khalid, M.; Salah, Z.; Zawahreh, M.A.A.; Alnasser, S.M.; Alshammari, S.O.; Wedian, F.; Karimulla, S.; Almutairi, A.; Alanazi, F.I.B.; et al. Anticancer, Antioxidant, and Antibacterial Activity of Chemically Fingerprinted Extract from Cyclamen persicum Mill. Sci. Rep. 2024, 14, 8488. [Google Scholar] [CrossRef] [PubMed]
- Bokov Do, B.; Krasikova Mk, K.; Sergunova Ev, S.; Bobkova Nv, B.; Kovaleva TYu, K.; Bondar Aa, B.; Marakhova Ai, M.; Morokhina Sl, M.; Krasnyuk Ii, K.; Moiseev Dv, M. Pharmacognostic, Phytochemical and Ethnopharmacological Potential of Cyclamen coum Mill. Pharmacogn. J. 2020, 12, 204–212. [Google Scholar] [CrossRef]
- Arslan, S.; Ozgun, O. Cyclamen trochopteranthum: Cytotoxic Activity and Possible Adverse Interactions Including Drugs and Carcinogens. Chin. J. Integr. Med. 2012. [Google Scholar] [CrossRef]

| C. alpinum | C. cilicium | C. coum | C. criticum | C. hederif-olium | C. mirable | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| December | |||||||||||||
| November | |||||||||||||
| October | |||||||||||||
| September | |||||||||||||
| August | |||||||||||||
| July | |||||||||||||
| June | |||||||||||||
| May | |||||||||||||
| April | Summer | ||||||||||||
| March | Spring | ||||||||||||
| February | Winter | ||||||||||||
| January | Autumn | ||||||||||||
| Cyclamen Species | Plant Part | Biological Pathway Effect | Cancer Cell Line Effect | Reference |
|---|---|---|---|---|
| C. alpinum Dammann ex. spreng | Tubers | ↑ toxic LC50 in T. tubifex | ↓ cytotoxicity in HCT 116 and HT29 | [60,96] |
| C. coum Mill | Multiple parts | ↓ antibacterial activity | ↑ cytotoxicity HeLa and NSCLC H1299 | [82,97] |
| C. hederifolium Aiton | Tubers | ↓ cytotoxic activity | ↓ apoptosis in Hela, H-446, HT-29, U937 | [98] |
| C. persicum Mill | Tubers | ↑ cytotoxic activity | ↑ apoptosis in human carcinoma nasopharynx | [99] |
| C. pseudibericum Hildebr. | Tubers | ↓ cytotoxic activity | ↑ cytotoxicity on A549 cells ↓ number of A549 cells | [79] |
| Species | Active Component | Therapeutic Effect | Mechanism of Action | Reference |
|---|---|---|---|---|
| Cyclamen persicum | Saponins (repanoside) | Anti-inflammatory | Stimulate nasal receptors Release inflammatory sinus exudates Relieves congestion | [111] |
| Cyclamen persicum | Flavonoids polyphenols | Antioxidant | Free radical scavengers hydrogen donors, reducing agents, and singlet oxygen quenchers | [111] |
| Cyclamen europaeum | Tannins Carotenoids (lutein and β-carotene) | Anti-cancerous | Anticancer effect on MCF7 breast cancer and colorectal adenocarcinoma (HT29) colon cancer | [111] |
| Cyclamen europaeum | Anthocyanins (Malvidin 3-O-glucoside cyanidin 3,5-di-O-glucoside) with values ranging from 40.5% to 75.7% | Anti-cancerous | Inhibitory activities against the cell proliferation of stomach, colon, lung, breast, and CNS cancer cells | [25] |
| Cyclamen repandum | Triterpenoids Polyphenols Flavonoids Saponins | Antioxidant Anti-inflammatory | Inhibit eicosanoid and cytokine production, hydrolytic enzyme activity, lipid peroxidation, and interaction with some serine/threonine kinases | [69] |
| C. europaeum | Triterpene saponins | Anti-inflammatory | Secretory Drainage stimulation Mucosa secretion Sufficient safety profile | [106] |
| C. coum | Saponins Phenolic compounds | Antifungal | The interaction of aglycone fragments of saponins and fungal membrane sterols is the main mechanism that causes the formation of transmembrane pores, it destroys the integrity and leads to membrane lysis. | [112] |
| C. coum | n-butanolic extract |
Antibacterial Antibiofilm | Prevents the Pseudomonas aeruginosa biofilm from growing, which is a significant factor for individuals with cystic fibrosis. | [6] |
| C. trochopteranthum | Water extract | Anti-cancerous | Increases CYP1A1/2 mRNA level Decreases CYP2B6 mRNA level Human hepatocellular liver carcinoma cell line (HepG2) and human epithelial colorectal adenocarcinoma (Caco2). | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharara, A.; Badran, A.; Hijazi, A.; Albahri, G.; Bechelany, M.; Mesmar, J.E.; Baydoun, E. Comprehensive Review of Cyclamen: Development, Bioactive Properties, and Therapeutic Applications. Pharmaceuticals 2024, 17, 848. https://doi.org/10.3390/ph17070848
Sharara A, Badran A, Hijazi A, Albahri G, Bechelany M, Mesmar JE, Baydoun E. Comprehensive Review of Cyclamen: Development, Bioactive Properties, and Therapeutic Applications. Pharmaceuticals. 2024; 17(7):848. https://doi.org/10.3390/ph17070848
Chicago/Turabian StyleSharara, Aya, Adnan Badran, Akram Hijazi, Ghosoon Albahri, Mikhael Bechelany, Joelle Edward Mesmar, and Elias Baydoun. 2024. "Comprehensive Review of Cyclamen: Development, Bioactive Properties, and Therapeutic Applications" Pharmaceuticals 17, no. 7: 848. https://doi.org/10.3390/ph17070848
APA StyleSharara, A., Badran, A., Hijazi, A., Albahri, G., Bechelany, M., Mesmar, J. E., & Baydoun, E. (2024). Comprehensive Review of Cyclamen: Development, Bioactive Properties, and Therapeutic Applications. Pharmaceuticals, 17(7), 848. https://doi.org/10.3390/ph17070848







