Effect of Curcumin Plus Piperine on Redox Imbalance, Fecal Calprotectin and Cytokine Levels in Inflammatory Bowel Disease Patients: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
2.1.1. Effects of Supplementation on Gastrointestinal Symptoms
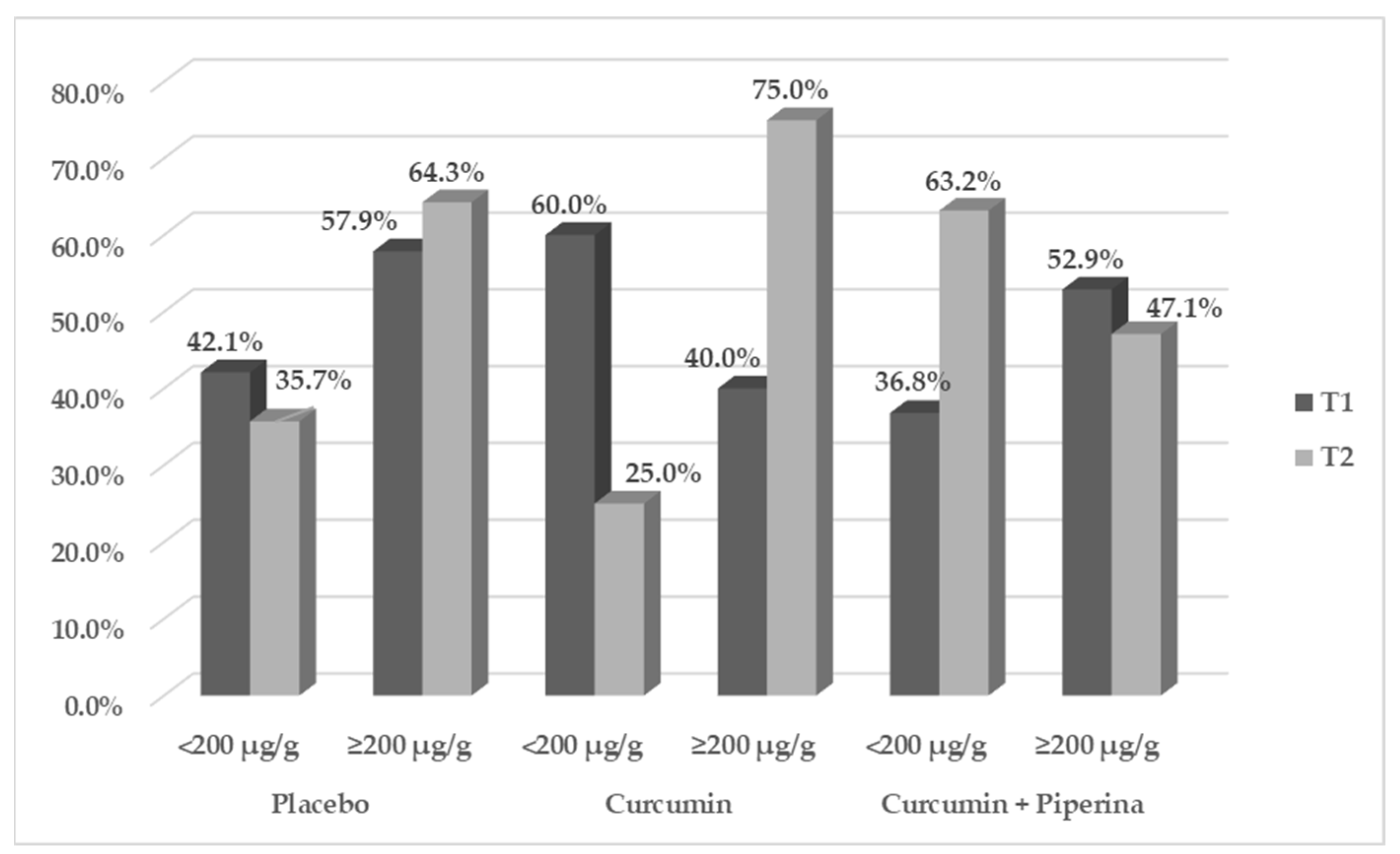
2.1.2. Effects of Supplementation on Redox Imbalance and Inflammation
3. Materials and Methods
3.1. Study Type and Design
3.2. Intervention
3.3. Ingredient Characterization
3.4. Blood Collection and Sample Preparation
3.5. General Data and Anthropometric Measurements
- (a)
- Socioeconomic information:
- education: less than four years (corresponding to an incomplete primary education) or more than four years;
- self-declared racial categories: white, black, brown, indigenous, and yellow;
- marital status: either in a stable relationship or not (divorced, widowed, or single).
- (b)
- Clinical background:
- nature of IBD and the date of diagnosis;
- existence of extraintestinal manifestations; and usage of certain medications for IBD treatment or not;
- historical information regarding COVID-19 infections was gathered.
- (c)
- Lifestyle:
- alcoholism and smoking;
- consistent exercise: physical activity was deemed consistent when it was done to preserve or enhance physical capacity.
- (d)
- Symptoms related to the stomach (secondary outcome)
- Anthropometric assessment: Performed by a qualified expert prior to and following the intervention period, it involved weight (kg) and height (m) measurements to determine the body mass index (BMI), which was expressed in kg/m2. The appropriate cutoffs were used according to age: adults [21] and elderly people [22].
3.6. Fecal Calprotectin Analysis (CalF)
3.7. Inflammation and Oxidative Stress Analyses
3.8. Sample Size
3.9. Randomization, Blinding and Allocation
3.10. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, W.; Chen, A.; Tiao, D.; Selinger, C.; Leong, R. Medication adherence in inflammatory bowel disease. Intest. Res. 2017, 15, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Na Young, K.; Moniruzzaman, M.; Beyene, A.M.; Do, K.; Kalaiselvi, S.; Min, T. Curcumin and Its Modified Formulations on Inflammatory Bowel Disease (IBD): The Story So Far and Future Outlook. Pharmaceutics 2021, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Flynn, S.; Eisenstein, S. Inflammatory Bowel Disease Presentation and Diagnosis. Surg. Clin. North Am. 2019, 99, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Ahmed, S.; Dryden, G. Immunopathophysiology of inflammatory bowel disease: How genetics link barrier dysfunction and innate immunity to inflammation. J. Endotoxin Res. 2017, 23, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Ciobica, A.; Balmus, I.M.; Trifan, A.; Stanciu, C. The implications of oxidative stress and antioxidant therapies in Inflammatory Bowel Disease: Clinical aspects and animal models. Saudi J. Gastroenterol. 2016, 22, 3–17. [Google Scholar] [CrossRef]
- Caër, C.; Wick, M.J. Human Intestinal Mononuclear Phagocytes in Health and Inflammatory Bowel Disease. Front. Immunol. 2020, 11, 410. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Ahuja, V.; Limdi, J.K. Optimal management of acute severe ulcerative colitis. Postgrad. Med. J. 2019, 95, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.S.P.; Campos, S.B.G.; Goulart, M.O.F.; Moura, F.A. Extraintestinal manifestations of inflammatory bowel disease, nitroxi-dative stress and dysbiosis: What is the link between them? Biocell 2021, 45, 461–481. [Google Scholar] [CrossRef]
- Sabir, S.; Zeb, A.; Mahmood, M.; Abbas, S.; Ahmad, Z.; Iqbal, N. Phytochemical analysis and biological activities of ethanolic extract of Curcuma longa rhizome. Braz. J. Biol. 2021, 81, 737–740. [Google Scholar] [CrossRef]
- de Cerqueira Alves, M.; Santos, M.O.; Bueno, N.B.; de Araújo, O.R.P.; Goulart, M.O.F.; Moura, F.A. Efficacy of oral consumption of curcumin/ for symptom improvement in inflammatory bowel disease: A systematic review of animal models and a meta-analysis of ran-domized clinical trials. Biocell 2022, 46, 2015–2047. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Reiner, Ž.; Majeed, M.; Sahebkar, A. Curcuminoids modify lipid profile in type 2 diabetes mellitus: A randomized controlled trial. Complement. Ther. Med. 2017, 33, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Quispe, C.; Javed, Z.; Iqbal, M.J.; Sadia, H.; Raza, S.; Irshad, A.; Salehi, B.; Reiner, Ž.; Sharifi-Rad, J. Resveratrol, curcumin, paclitaxel and miRNAs mediated regulation of PI3K/Akt/mTOR pathway: Go four better to treat bladder cancer. Cancer Cell Int. 2020, 20, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.S.D.P.; Alves, M.D.C.; DE Araújo, O.R.P.; Camatari, F.O.D.S.; Goulart, M.O.F.; Moura, F.A. Curcumin in inflammatory bowel diseases: Cellular targets and molecular mechanisms. Biocell 2023, 47, 2547–2566. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Heidari, H.; Bagherniya, M.; Majeed, M.; Sathyapalan, T.; Jamialahmadi, T.; Sahebkar, A. Curcumin-piperine co-supplementation and human health: A comprehensive review of preclinical and clinical studies. Phytotherapy Res. 2023, 37, 1462–1487. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.; Ghavidel, F.; Panahi, G.; Majeed, M.; Sahebkar, A. A systematic review and meta-analysis of randomized controlled trials investigating the effect of the curcumin and piperine combination on lipid profile in patients with metabolic syndrome and related disorders. Phytotherapy Res. 2023, 37, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Dziąbowska-Grabias, K.; Sztanke, M.; Zając, P.; Celejewski, M.; Kurek, K.; Szkutnicki, S.; Korga, P.; Bulikowski, W.; Sztanke, K. Antioxidant Therapy in Inflam-matory Bowel Disease: A Systematic Review and a Meta-Analysis of Randomized Clinical Trials. Pharmaceuticals 2023, 10, 412. [Google Scholar]
- Peterson, C.T.; Vaughn, A.R.; Sharma, V.; Chopra, D.; Mills, P.J.; Peterson, S.N.; Sivamani, R.K. Effects of Turmeric and Curcumin Dietary Supplementation on Human Gut Microbiota: A Double-Blind, Randomized, Placebo-Controlled Pilot Study. J. Evidence-Based Integr. Med. 2018, 23, 1–8. [Google Scholar] [CrossRef]
- Khismatrao, A.; Bhairy, S.; Hirlekar, R. Development and Validation of Rp-Hplc Method for Simultaneous Estimation of Cur-cumin and Piperine. Int. J. Appl. Pharm. 2018, 10, 43. [Google Scholar] [CrossRef]
- World Health Organization. Physical Status: The Use and Interpretation of Anthropometry, Report of a WHO Expert Committee; World Health Organization technical report series; WHO: Geneva, Switzerland, 1995; pp. 1–452. [Google Scholar]
- Lipschitz, D.A. Screening for nutritional status in the elderly. Prim. Care Clin. Off. Pract. 1994, 21, 55–67. [Google Scholar] [CrossRef]
- Karatas, F.; Karatepe, M.; Baysar, A. Determination of free malondialdehyde in human serum by high-performance liquid chromatography. Anal. Biochem. 2002, 311, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Pick, E.; Keisari, Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J. Immunol. Methods 1980, 38, 161–170. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Bradley, P.P.; Christensen, R.D.; Rothstein, G. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood 1982, 60, 618–622. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M.; Edeas, M.A. SOD, oxidative stress and human pathologies: A brief history and a future vision. Biomed. Pharmacother. 2005, 59, 139–142. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Liochev, S.I.; Fridovich, I. Superoxide and iron: Partners in crime. IUBMB Life 1999, 48, 157–161. [Google Scholar] [CrossRef]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Majeed, M.; Sahebkar, A. Antioxidant and anti-inflammatory effects of curcumi-noid-piperine combination in subjects with metabolic syndrome: A randomized controlled trial and an updated meta-analysis. Clin. Nutr. 2015, 34, 1101–1108. [Google Scholar] [CrossRef]
- Panahi, Y.; Ghanei, M.; Hajhashemi, A.; Sahebkar, A. Effects of Curcuminoids-Piperine Combination on Systemic Oxidative Stress, Clinical Symptoms and Quality of Life in Subjects with Chronic Pulmonary Complications Due to Sulfur Mustard: A Ran-domized Controlled Trial. J. Diet. Suppl. 2016, 13, 93–105. [Google Scholar] [CrossRef]
- Panahi, Y.; Rahimnia, A.R.; Sharafi, M.; Alishiri, G.; Saburi, A.; Sahebkar, A. Curcuminoid treatment for knee osteoarthritis: A ran-domized double-blind placebo-controlled trial. Phytother. Res. 2014, 28, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.K.; Sałaga, M.; Siwiński, P.; Włodarczyk, M.; Dziki, A.; Fichna, J. Oxidative Stress Does Not Influence Subjective Pain Sensation in Inflammatory Bowel Disease Patients. Antioxidants 2021, 10, 1237. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, E.; Qujeq, D.; Taheri, H.; Hajian-Tilaki, K. Evaluation of Serum Trace Element Levels and Superoxide Dismutase Activity in Patients with Inflammatory Bowel Disease: Translating Basic Research into Clinical Application. Biol. Trace Element Res. 2017, 177, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Kruidenier, L.; Kuiper, I.; van Duijn, W.; Marklund, S.L.; A van Hogezand, R.; Lamers, C.B.; Verspaget, H.W. Differential mucosal expression of three superoxide dismutase isoforms in inflammatory bowel disease. J. Pathol. 2003, 201, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]
- Seguí, J.; Gironella, M.; Sans, M.; Granell, S.; Gil, F.; Gimeno, M.; Coronel, P.; Piqué, J.M.; Panés, J. Superoxide dismutase ameliorates TNBS-induced colitis by reducing oxidative stress, adhesion molecule expression, and leukocyte recruitment into the inflamed intestine. J. Leukoc. Biol. 2004, 76, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.L.; Zhang, J.; Liu, X.T.; Liu, H.; Zeng, X.F.; Qiao, S.Y. Superoxide dismutase recombinant Lactobacillus fermentum ameliorates intestinal oxidative stress through inhibiting NF-kappaB activation in a trinitrobenzene sulphonic acid-induced colitis mouse model. J. Appl. Microbiol. 2014, 116, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, N.; Mansoori, A.; Shayesteh, A.; Hashemi, S.J. The effect of curcumin supplementation on clinical outcomes and in-flammatory markers in patients with ulcerative colitis. Phytother. Res. 2020, 34, 1123–1133. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef]
- Faki, Y.; Er, A. Different Chemical Structures and Physiological/Pathological Roles of Cyclooxygenases. Rambam Maimonides Med. J. 2021, 12, e0003. [Google Scholar] [CrossRef]

| Total | Groups | p-Value | ||||
|---|---|---|---|---|---|---|
| Placebo n = 19 | Curcumin n = 20 | Curcumin + Piperine n = 19 | ||||
| Age | Mean (SD) | 47.5 ± 15.5 | 50.9 ± 14.4 | 46.9 ± 18.9 | 44.7 ± 12.4 | 0.460 1 |
| Sex | Female | 38 (65.5) | 13 (68.4) | 11 (55.0) | 14 (73.7) | 0.447 2 |
| Male | 20 (34.5) | 6 (31.6) | 9 (45.0) | 5 (26.3) | ||
| Schooling | <4 years | 38 (65.5) | 8 (42.1) | 15 (75.0) | 15 (78.9) | 0.031 2 |
| ≥4 years | 20 (34.5) | 11 (57.9) a | 5 (25.0) b | 4 (21.1) b | ||
| Self-declared race | White | 14 (24.1) | 2 (10.5) | 7 (35.0) | 5 (26.3) | 0.196 2 |
| Black/Brown | 44 (75.9) | 17 (89.5) | 13 (65.0) | 14 (73.7) | ||
| Marital status | Single/Divorced | 19 (32.8) | 6 (31.6) | 8 (40.0) | 5 (26.3) | 0.668 2 |
| Stable union | 33 (67.2) | 10 (52.6) | 10 (50.0) | 13 (68.4) | ||
| Inflammatory Bowel Disease | Crohn’s disease | 19 (32.8) | 9 (47.4) b | 2 (10.0) a | 8 (42.1) ab | 0.026 2 |
| Ulcerative colitis | 39 (67.2) | 10 (52.6) | 18 (90.0) | 11 (57.9) | ||
| Diagnosis time | <10 years | 22 (37.9) | 11 (57.9) | 5 (25.0) | 6 (31.6) | 0.084 2 |
| ≥10 years | 36 (62.1) | 8 (42.1) | 15 (75.0) | 13 (68.4) | ||
| Chronic non-communicable diseases | No | 41 (70.7) | 14 (73.7) | 14 (70.0) | 13 (68.4) | 0.935 2 |
| Yes | 17 (29.3) | 5 (26.3) | 6 (30.0) | 6 (31.6) | ||
| History of COVID-19 | No | 47 (81.0) | 17 (89.5) | 15 (75.0) | 15 (78.9) | 0.495 2 |
| Yes | 11 (19.0) | 2 (10.5) | 5 (25.0) | 4 (21.1) | ||
| Extraintestinal manifestation | No | 53 (91.4) | 17 (89.5) | 19 (95.0) | 17 (89.5) | 0.776 2 |
| Yes | 5 (8.6) | 2 (10.5) | 1 (5.0) | 2 (10.5) | ||
| Pharmacologic treatment | Aminosalicylates | 24 (41.4) | 6 (31.6) | 8 (40.0) | 10 (52.6) | 0.520 2 |
| Immunosuppressant alone/combined with aminosalicylates | 3 (5.2) | 1 (5.3) | 2 (10.0) | 0 (0.0) | ||
| Biological therapy | 30 (51.7) | 11 (57.9) | 10 (50.0) | 9 (47.4) | ||
| No drug therapy | 1 (1.7) | 1 (10.5) | 0 (0.0) | 0 (0.0) | ||
| Smoking | No | 43 (74.1) | 13 (68.4) | 15 (75.0) | 15 (78.9) | 0.755 2 |
| Yes | 15 (25.9) | 6 (31.6) | 5 (25.0) | 4 (21.1) | ||
| Physical exercise | No | 42 (72.4) | 13 (68.4) | 15 (75.0) | 14 (73.7) | 0.890 2 |
| Yes | 16 (27.6) | 6 (31.6) | 5 (25.0) | 5 (26.3) | ||
| Body mass index | Low weight | 9 (15.5) | 1 (5.3) | 4 (20.0) | 4 (21.1) | 0.588 2 |
| Suitable weight | 24 (41.4) | 10 (52.6) | 7 (35.0) | 7 (36.8) | ||
| Overweight | 25 (43.1) | 8 (42.1) | 9 (45.0) | 8 (42.1) | ||
| Group | p-Value | ||||
|---|---|---|---|---|---|
| Placebo | Curcumin | Curcumin + Piperine | ANCOVA/Bonferroni * | ||
| MPO | T1 | 11.5 ± 2.5 | 10.8 ± 2.6 | 10.7 ± 2.4 | |
| T2 | 11.8 ± 2.6 | 11.8 ± 1.6 | 12.9 ± 1.5 | 0.162 | |
| Δ | −0.0 ± 3.7 | 1.0 ± 2.2 | 1.8 ± 1.9 | 0.199 | |
| TNF-α (pg/µL) Φ | T1 | 0.0 ± 0.6 | 0.1 ± 0.6 | 0.2 ± 0.5 | |
| T2 | 0.0 ± 0.6 | −0.4 ± 0.5 | −0.2 ± 0.5 | 0.126 | |
| IL-17A (pg/µL) Φ | T1 | 0.3 ± 0.2 | 0.1 ± 0.3 | 0.2 ± 0.4 | |
| T2 | 0.3 (0.2) | 0.1 (0.5) | 0.3 (0.2) | 0.334 K | |
| IL 22 (pg/µL) | T1 | 3.4 ± 1.8 | 3.7 ± 2.0 | 3.9 ± 2.8 | |
| T2 | 4.2 ± 1.9 | 4.7 ± 1.7 | 5.1 ± 2.9 | 0.498 | |
| Δ | 1.3 ± 1.8 | 1.0 ± 2.1 | 1.0 ± 3.0 | 0.853 | |
| IL-10 (pg/µL) | T1 | 0.9 ± 0.7 | 1.0 ± 0.8 | 1.1 ± 0.8 | |
| T2 | 1.5 ± 0.7 | 1.9 ± 1.0 | 1.9 ± 1.2 | 0.511 | |
| Δ | 0.6 ± 0.7 | 0.8 ± 1.3 | 0.7 ± 1.3 | 0.940 | |
| SOD (U/µL) | T1 | 3599.8 ± 684.4 | 3728.1 ± 714.5 | 3761.3 ± 638.8 | |
| T2 | 3614.5 ± 731.5 | 3910.2 ± 800.4 | 4346.9 ± 879.0 | 0.020 ## | |
| Δ | −126.8 ± 762.7 | 142.1 ± 906.9 | 538.8 ± 1040.1 | 0.027 ## | |
| Catalase (U/min) | T1 | 7.6 ± 2.9 | 8.5 ± 2.8 | 9.0 ± 3.2 | |
| T2 | 9.6 ± 4.2 | 9.2 ± 3.4 | 9.2 ± 3.4 | 0.781 | |
| Δ | 1.4 ± 6.1 | 0.6 ± 3.6 | 0.3 ± 2.6 | 0.576 | |
| Hydrogen Peroxide (nmol/mL) | T1 | 218.3 ± 155.7 | 224.7 ± 154.9 | 259.5 ± 149.7 | |
| T2 | 186.9 ± 118.7 | 215.5 ± 94.9 | 183.7 ± 98.1 | 0.307 | |
| Δ | −45.7 ± 220.0 | −9.1 ± 165.9 | −78.3 ± 168.3 | 0.476 | |
| MDA (ng/µL) Φ | T1 | 1.0 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.2 | |
| T2 | 0.8 ± 0.4 | 0.7 ± 0.4 | 0.7 ± 0.3 | 0.602 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, A.S.d.P.; Araújo, O.R.P.d.; Gomes, A.d.S.; Araujo, F.L.C.; Oliveira Junior, J.; Vasconcelos, J.K.G.d.; Rodrigues Junior, J.I.; Cerqueira, I.T.; Lins Neto, M.Á.d.F.; Bueno, N.B.; et al. Effect of Curcumin Plus Piperine on Redox Imbalance, Fecal Calprotectin and Cytokine Levels in Inflammatory Bowel Disease Patients: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Pharmaceuticals 2024, 17, 849. https://doi.org/10.3390/ph17070849
Martins ASdP, Araújo ORPd, Gomes AdS, Araujo FLC, Oliveira Junior J, Vasconcelos JKGd, Rodrigues Junior JI, Cerqueira IT, Lins Neto MÁdF, Bueno NB, et al. Effect of Curcumin Plus Piperine on Redox Imbalance, Fecal Calprotectin and Cytokine Levels in Inflammatory Bowel Disease Patients: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Pharmaceuticals. 2024; 17(7):849. https://doi.org/10.3390/ph17070849
Chicago/Turabian StyleMartins, Amylly Sanuelly da Paz, Orlando Roberto Pimentel de Araújo, Amanda da Silva Gomes, Fernanda Lívia Cavalcante Araujo, José Oliveira Junior, Joice Kelly Gomes de Vasconcelos, José Israel Rodrigues Junior, Islany Thaissa Cerqueira, Manoel Álvaro de Freitas Lins Neto, Nassib Bezerra Bueno, and et al. 2024. "Effect of Curcumin Plus Piperine on Redox Imbalance, Fecal Calprotectin and Cytokine Levels in Inflammatory Bowel Disease Patients: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial" Pharmaceuticals 17, no. 7: 849. https://doi.org/10.3390/ph17070849
APA StyleMartins, A. S. d. P., Araújo, O. R. P. d., Gomes, A. d. S., Araujo, F. L. C., Oliveira Junior, J., Vasconcelos, J. K. G. d., Rodrigues Junior, J. I., Cerqueira, I. T., Lins Neto, M. Á. d. F., Bueno, N. B., Goulart, M. O. F., & Moura, F. A. (2024). Effect of Curcumin Plus Piperine on Redox Imbalance, Fecal Calprotectin and Cytokine Levels in Inflammatory Bowel Disease Patients: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Pharmaceuticals, 17(7), 849. https://doi.org/10.3390/ph17070849








