Pharmaceutical Potential of Remedial Plants and Helminths for Treating Inflammatory Bowel Disease
Abstract
1. Introduction
2. Plant-Derived Anti-Inflammatory Compounds
| Botanical Name and Family | Plant Parts and Traditional Uses | Anti-Inflammatory Compounds/Extracts. | Model/Cell Used for Testing | Main Effect on Inflammation | Ref |
|---|---|---|---|---|---|
| Abrus precatorius L. (Fabaceae) | Seeds; abortion | Olean-12-ene-3β, 16β,23,28-tetrahydroxy-3-O-{[β-D-glucopyranosyl-(→ 4)-β-D-glucopyranosyl-( 1→ 3)]-[β-D-glucopyranosyl-(→ 2)]-β-D-fucopyranose} | Croton oil ear model | ↓ Inflammation on the ear tissue of rats | [4] |
| (S)-8-Hydroxy 6,7, 5′-trimethoxyisoflavan-1′,4′-quino Abruquinone A and B | Polymorphonuclear cells (PMNs) | ↓ TNF and ROS | [84] | ||
| Acacia melanoxylon R.Br. (Fabaceae) | Bark: headache, cold and fever | Kolavic acid 15-methyl ester | LPS- LPS-stimulated J774 cell | ↓IL-6 | [85] |
| Acalypha wilkesiana Müll.Arg. (Euphorbiaceae) | Shoot: sores/skin lesions/wounds/cuts | Polyphenol-enriched fraction | LPS-stimulated RAW264.7 macrophage | ↓ TNF, IL- 1β, and IL-6 | [78,86] |
| Ageratum conyzoides L. (Asteraceae) | Whole plant: sores/skin lesions/wounds/cuts | 5′-Methoxy nobiletin 1, 2-benzopyrone | Carrageenan-induced pleurisy | ↓ p-p65 NF-κB and p-p38 MAPK ↑ IL-17A, IL-6, TNF and IFN-γ levels | [87,88] |
| Leaves extract and aerial extract | Cotton pellet-induced granuloma and formaldehyde-induced arthritis models | ↓ Paw edema | |||
| Alphitonia petriei Braid and C.T. White (Rhamnaceae) | Bark, leaves, stem; Body pain | Embolic acid | LPS + IFN-γ activated RAW 264.7 cells | ↓ NO and TNF production | [89] |
| Alphitonia excelsa Reissek ex Endl (Rhamnaceae). | Whole plants: headache, colds, fever, stomach upset, skin lesions, wounds, cuts | Betulinic acid | λ-carrageenan-induced paw edema mice | ↓ COX-2, NO, TNF, and IL1-β | [90] |
| Angophora costata Britten (Myrtaceae) | Costatamins A-C | LPS-stimulated RAW264.7 cell | ↓ NO production and TNF | [91] | |
| Alstonia scholaris (L.) R.Br. (Apocynaceae) | Juice, sap, bark; toothache, fever, sores, skin lesions, wounds, and cuts | 12-ursene-2,3,18,19-tetrol-28 Acetate | Carrageenan-induced. paw edema (Wistar rats) | ↓ Paw edema | [92] |
| Picrinine, vallesamine, and scholaricine | Mice air pouch model (In vivo) | ↑ SOD activity ↓ NO production, PGE2, and MD | [93] | ||
| Antidesma bunius Wall. (Phyllanthaceae) | Fruit: colds, fever, and headache | Antidesoside, podocarpusflavone A, byzantionoside B, (6S,9R)-roseoside | LPS-stimulated BV2 cells and RAW264.7 macrophages | ↓ NO production | [94] |
| Arivela viscosa (L.) Raf. (Cleomaceae) | Whole plants; Colds and fever | Quercetin 3-O-(2″-acetyl)-glucoside | Carrageenan-induced rat paw edema | ↓ Carrageenan-induced rat paw edema | [80] |
| Coumarinolignoid cleomiscosins A, B and C | Female Swiss albino mice | ↓ IL-6, TNF NO production ↑ IL-4 in a dose-dependent manner | [81] | ||
| ↓ Carrageenan-induced rat paw edema ↓ IL-4, TNF, and NO production ↓ COX-1 | |||||
| Cembrenoid diterpene Malabaric acid | Cyclooxygenase enzyme (COX-1 and -2) inhibitory assays | ↓ COX-1 and COX-2 enzyme | [95] | ||
| Stigmast-4-ene-3,6-dione, cleomaldeic acid, stigmast-4-en-3-one, and lupeol | ↓ COX-1 and COX-2 enzyme | ||||
| Barringtonia racemose (L.) Spreng. (Lecythidaceae) | Bark, fruits; tonic, pain and inflammatory | Ethyl acetate fraction | Carrageenan-induced rat paw edema model | Demonstrated dose-dependent anti-inflammatory activity | [96] |
| Barringosides I | LPS-stimulated RAW264.7 cell | ↓ Production of NO | [97] | ||
| Blainvillea acmella (L.) Philipson (Asteraceae) | Bark, fruits, and roots; muscle sprain, bone aches, dislocation, broken bones | Spilanthol | LPS and IFNγ induced RAW264.7 cells | ↓ iNOS expression, NO production, and TFN | [48] |
| Methanol extracts | LPS-stimulated RAW264.7 macrophages | ↓ IL-1β and IL-6 | [47] | ||
| Boerhavia diffusa L. (Nyctaginaceae) | Whole plants; asthma | Boeravinone B and N | Carrageenan-induced paw edema | ↓ COX-1 and COX-2 Exhibited anti-inflammatory activity | [98] |
| Rotenoid-rich fraction | Sprague–Dawley rats | Exhibited ↑ anti-inflammatory potential and ↑ plasma level | [99] | ||
| Brasenia schreberi J.F.Gmel. (Cabombaceae) | Leaves: stomach cancer, boil, dysentery, and tuberculosis | Quercetin 7-0-β-d-glucopyranoside | LPS-stimulated RAW 264.7 cells | ↓ Expression of iNOS and NO Overexpression of COX-2 ↓ Granulocyte macrophage-colony-stimulating factor | [100] |
| Brucea javanica (L.) Merr. (Simaroubaceae) | Leaves and roots; pain | Ethyl acetate fraction of seeds | LPS-stimulated RAW264.7 macrophages | ↓ NO, PGE2, TNF, IL-1β, and IL-6 ↑ IL-10 | [101] |
| Carrageenan-induced paw edema | Inhibited carrageenan-induced paw edema | ||||
| Oil emulsion | DSS-induced colitis mice | ↓ TNF, IL-1β, IL-6, IL-8, IL-17, and IFN-γ ↓ mRNA expression of MPO, iNOS, and COX-2 | [102] | ||
| Cleomiscosin A and E | LPS-stimulated RAW264.7 macrophages | ↓ NO production | [103] | ||
| Brujavanoid E | LPS-induced RAW264.7 cells | ↓ Caspase1, CD206, IL-1β, IL-18, MCP-1, TNF, NIK, NLRP3, and p65 in a dose-dependent manner | [104] | ||
| Brusatol | LPS-induced RAW 264.7 cells TNBS-induced colitis mice | ↓ TNF, IL-1β, PGE2, and NO levels ↓ NF-κB signaling pathway. ↓ IL-1β and IL-18 levels ↓ Catalase, glutathione, and superoxide dismutase enzymes in the colon tissue | [105,106,107] | ||
| Calophyllum inophyllum L. (Clusiaceae) | Fruits; body pain and purgative | Acetone extract | LPS-stimulated RAW 264.7 cells | ↓ NO production, iNOS, COX-2, and NF-κB | [108] |
| Calophyllolide | Albino male mice | ↓ IL-1β, IL-6, TNF ↑IL-10 | [109] | ||
| E-coumaroyl triterpenoid (27-[(E)-p-coumaroyloxy] canophyllic acid) | LPS-induced RAW 264.7 cells | ↓ NO production, IL-1β, and TNF, iNOS ↓ NF-κB signaling pathway | [110] | ||
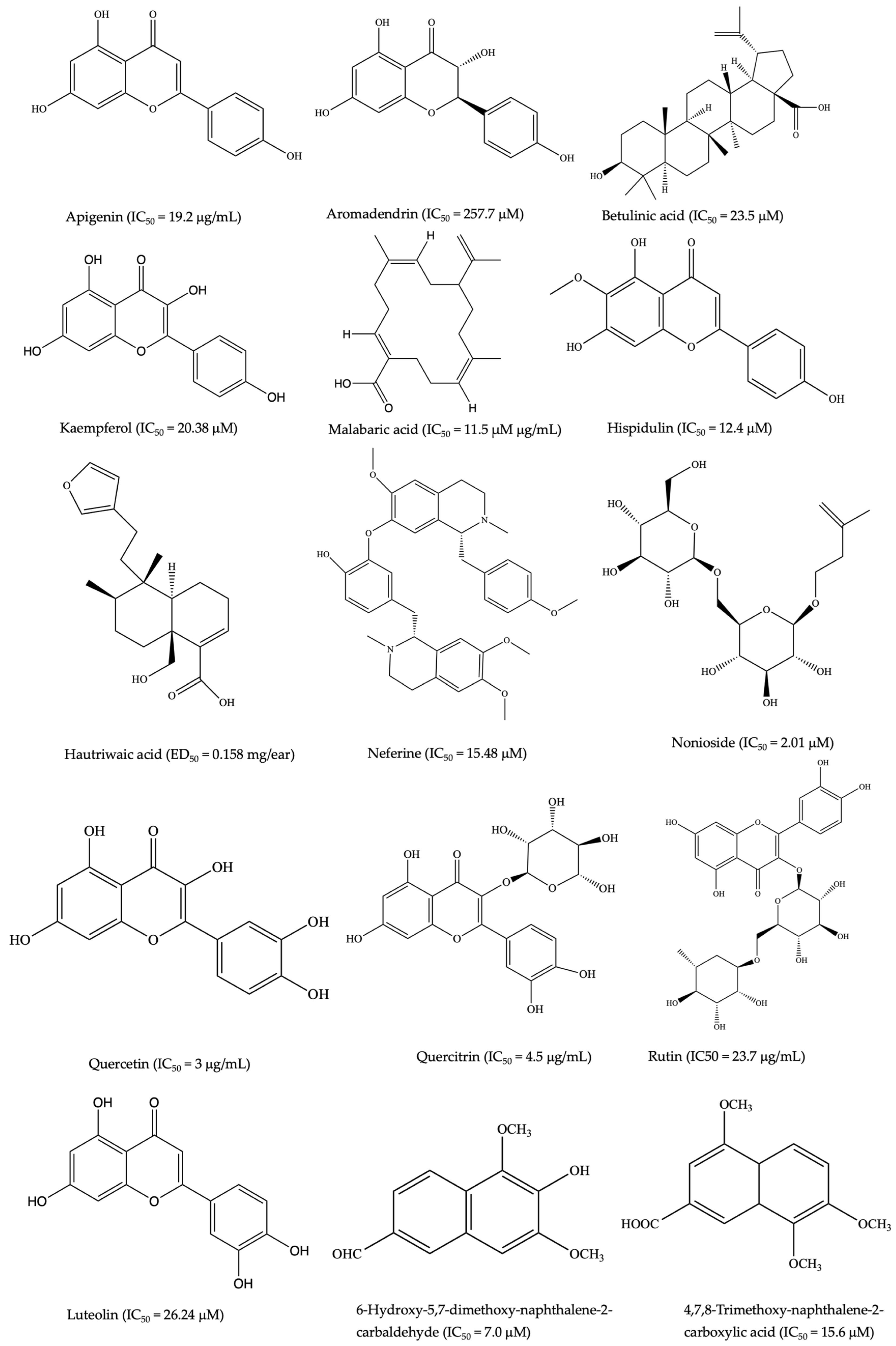
| Capparis mitchellii Lindl. (Capparaceae) | Bark: cuts, wounds, and skin lesions | Luteolin, kaempferol, apigenin and catechin | LPS-induced RAW 264.7 cells | Inhibition with IC50 values of 26.24 ± 1.78, 20.38 ± 1.36, 22.8 ± 1.57, and 42.5 ± 2.24 μM, respectively ↓ NO production | [111] |
| Salvia plebeian R.Br. (Labiatae) | Colds and tumors | Ethanol extract | BALB/c mice | ↓ IL-4, IL-17, MMP-1, and MMP-3 ↓ Akt and MAPKs pathways, ↓ Akt and ERK p38 expression | [112] |
| Carpobrotus rossii Schwantes (Aizoaceae) | Leaves: (extract juice) respiratory tract and throat infections, gastrointestinal discomfort, insect bites, wounds burns, eczema, bluebottle, and jellyfish stings | Aqueous extract | PBMC | ↓ IL-10, TNF, and MCP-1 | [113] |
| Clematis pickeringii A. Gray (Ranunculaceae) | Headache, pain, rheumatism, infections, common colds. | Ethanolic extract | HepG2 cells | ↑ PPARa and PPARy at the dose of 60 µg/mL | [114] |
| Centipeda minima (L.) A.Br. (Apiaceae) | Nasal allergy, diarrhea, asthma | Whole-plant extract | LPS-induced RAW 264.7 cells and λ-carrageenan-induced paw edema | ↓ NO production | [115] |
| DSS-induced colitis mouse model | ↓ TNF-α and IL-1β | [116,117] | |||
| Brevilin A, centiplide A, centiplide H, and Helenalin isova lerate | LPS-induced RAW 264.7 cells | Attenuated NF-κB pathway activation and oxidative stress ↓ NO production | [76,77] | ||
| 6-O-angeloylplenolin | LPS-induced macrophage cells (BV2 cells) | ↓ TNF and IL-1β ↓ Phosphorylation of NF-κB ↓ IκB-α, NO, and PGE2 | [76,118] | ||
| Centella asiatica (L.) Urb. (Apiaceae) | Whole plants; lupus, varicose ulcers, eczema, and psoriasis | Triterpenoid saponin-rich fraction | LPS-stimulated RAW 264.7 cells | ↓ IL-13, NF-κB pathway | [119] |
| Asiatic acid, isomadecassoside, asiaticoside B and G, rosmarinic acid | LPS-stimulated RAW 264.7 cells | ↓ NO production | [120,121] | ||
| Clematis microphylla DC. (Ranunculaceae) | Whole plants; headache, colds, sores, gastric disorder and fever | Ethanolic plant extracts | HepG2 cells | ↓ COX-1, 5-LOX | [114] |
| Clerodendrum inerme R.Br. (Lamiaceae) | Leaves and roots; sores, skin lesions, wounds, cuts, and sprains | Ethanol leaf extract | LPS-induced RAW 264.7 cells | ↓ NO production ↓ mRNA and protein expressions of iNOS | [122] |
| Hispidulin | LPS-stimulated RAW 264.7 cells | ↓ NO production, NF-κB DNA-binding activity, and JNK signaling pathway ↓ iNOS and COX-2 expression | |||
| Methanolic extract | Formalin-induced hind-paw edema | ↑ Anti-inflammatory activity at dose 200 mg/kg | [123] | ||
| Ethyl acetate fraction | LPS-stimulated RAW264.7 macrophage cells | ↓ NO production ↓ iNOS | [122,124] | ||
| Cleome viscosa L. (Capparidaceae) | Whole plants; diarrhea, fever, cuts, and ulcers | Quercetin 3-O-(2″-acetyl)-glucoside | Carrageenan-induced paw edema | ↓ Carrageenan-induced rat paw edema | [80,124] |
| Cleomiscosins A-C | LPS-stimulated Peritoneal Macrophages (Swiss albino mice) | ↓ IL-4, TNF, and NO production | [81] | ||
| Malabaric acid | ↓ COX-1 and two activities | [95] | |||
| Hispidulin | ↓ PGE2 production, and iNOS and COX-2 expressions ↓ NF-κB DNA-binding activity and JNK pathway | [122] | |||
| Crinum pedunculatum R.Br. (Amaryllidoideae) | Whole plants; stings from marine life | Methanol, ethanol, and ethyl acetate extracts | Carrageenin-induced Wistar albino paw edema | ↓ Carrageenin-induced rat paw edema | [124,125] |
| Morinda citrifolia (Rubiaceae) | Fruits: cough and cold, sore throat | LPS-stimulated human monocyte | ↓ Matrix metalloproteinase-9 (MMP-9) production | [126] | |
| Fruit juice | DSS-induced colitis model | ↓ TNF and IFN-γ, NO and IL-17 | [127] | ||
| Ethyl acetate extract | Caco-2 cells | ↓ COX-2, IL-8, and prostaglandin E2 production and neutrophil chemotaxis | [128] | ||
| Fruit extract | LPS-stimulated RAW 264.7 cells | ↓ NO synthase and TNF | [129] | ||
| Rutin, nonioside A, (2E,4E,7Z)-deca-2,4,7-trienoate-2-O-β-d-glucopyranosyl-β-d-glucopyranoside, and tricetin | LPS-stimulated RAW 264.7 cells | ↓ NO production, expression of IKKα/β, I-κBα, and NF-κB p65 | [126,130] | ||
| Eucalyptus camaldulensis Dehnh. (Myrtaceae) | Leaves and gum from barks; diarrhea | Ethanol extract | Carrageenan-induced paw edema | ↓ Carrageenan-induced paw edema | [124,131] |
| 12-O -tetradecanoyl-phorbol-13-acetate | ↓ Edema and leukocyte infiltration | ||||
| Acalypha wilkesiana Mull.Arg. (Euphorbiaceae) | Shoots: skin lesions, sores, cuts, wounds | Leaf extract | LPS-stimulated RAW 264.7 cells | ↓ NO and PGE2 ↓ iNOS productions and COX-2 expression ↓ TNF, IL-1β) and IL-6 | [2] |
| Corymbia terminalis (F.Muell.) K.D.Hill and L.A.S.Johnson (Myrtaceae) | Bark; dysentery | Methanol leaves extracts | LPS- stimulated mammalian keratinocyte (HaCaT) cell | ↓ IL-6, IL-8, and COX-1 and 2 enzyme activities | [49,50] |
| Axifolin, aromadendrin, cianidanol, and farrerol | ↓ IL-6, IL-8, and COX-1 and 2 enzyme activities | [49] | |||
| Dodonaea polyandra Merr. and L.M.Perry (Sapindaceae) | Roots: sores, skin lesions, wounds, cuts, and toothache | Nonpolar hexane and methylene chloride/methanol | TPA-induced mouse ear edema | ↓ Inflammation in TPA-induced mouse ear edema by 72.12 and 79.81%, respectively, at 0.4 mg/ear; 12 and 79.81%, respectively, at 0.4 mg/ear | [132] |
| Polyandric acid A | Mouse Ear Tissue (male BALB/c mice) | ↓ IL-1β | [133] | ||
| 5,16-Epoxy-8α-(benzyloxy) methyl-2α-hydroxycleroda-3,13(16),14-tried-18-oic acid and 15,16-Epoxy-2α-benzoyloxycleroda-3,13(16),14-tried-18-oic acid | TPA-induced mouse ear edema | Exhibited optimum inhibition of inflammation (70–76%) at doses of 0.22 and 0.9 μmol/ear, respectively | [134] | ||
| Dodonaea viscosa Jacq. (Sapindaceae) | Leaves, rheumatism, skin infections, and diarrhea | Hydroalcoholic extract | Carrageenin-induced paw edema | ↓ Edema induced in rats by carrageenin | [135] |
| Dichloromethane extract | TPA-induced edema model in CD-1 mice | ↓ 97.8% of the edema | [136] | ||
| Hautriwaic acid | 12-O-tetradecanoylphorbol 13-acetate (TPA) mice ear edema models | 97% of edema inhibition with an ED50 = 0.158 mg/ear | |||
| Excoecaria agallocha L. (Euphorbiaceae) | Latex; stings (marine) | Agallochanin K | RAW264.7 cells | ↓ NF-κB | [137] |
| Agallolides I and J | NF-κB (p65) RAW264.7 cells | ↓ NF-κB with inhibition rates of 23.4% and 19.4%, respectively, at the concentration of 100.0 µM | [138] | ||
| Eleocharis dulcis Hensch. (Cyperaceae) | Whole plants; antiseptic, wounds | Ethyl acetate extract | LPS-induced RAW 264.7 macrophages | ↓ TNF, iNOS, and COX-2 | [124,139] |
| Ipomoea pes-caprae (L.) R.Br. (Convolvulaceae) | Whole plants; diseases, boils, and swelling | Ethanol extract from leaves and stems | Trypsin-, histamine-, and bradykinin-induced paw edema in mice | ↓ Trypsin-, histamine-, and bradykinin-induced paw edema in mice | [140] |
| Heliotropium ovalifolium Forssk. (Boraginaceae) | Body wash and fever | 4,7,8-Trimethoxy-naphthalene-2-carboxylic acid and 6-hydroxy-5,7-dimethoxy-naphthalene-2-carbaldehyde | LPS-stimulated THP-1 cell | ↓ IL-6 and TNF | [141] |
| Euphorbia hirta L. (Euphorbiaceae) | Leaves; hypertension, respiratory ailments, tumors, and wounds | Ethanol extract | LPS-induced inflamed rat knees | ↓ TNF and NO production | [142] |
| Butanol extract | LPS-stimulated RAW 264.7 cells | ↓ NO production and iNO protein expressions | [143] | ||
| Melaleuca leucadendra L. (Myrtaceae) | Leaves and bark; cough and cold; wounds, cuts, and sores | Butanol extract | LPS-stimulated RAW 264.7 cells | ↓ NO and PGE2 production ↓ Expression of COX-2 and iNOS protein ↓ NF-κB transcriptional activity in a dose-dependent manner | [144] |
| Casuarinin | Ethanol-induced gastric ulceration in rats | ↓ NF-κB, COX-2, iNOS | [145] | ||
| Euphorbia tirucalli L. (Euphorbiaceae) | Latex; skin cancer | Ethanol extract from roots | Carrageenan-induced acute-inflammation in albino rat model | ↓ TNF and IFN-γ production | [51] |
| Manihot esculenta Crantz (Euphorbiaceae) | Roots: diarrhea and stomachache | Carrageenan-induced paw edema in rats and xylene-induced ear edema in mice | ↓ Carrageenan-induced rat paw edema and xylene-induced ear swelling in mice | [146] | |
| Flueggea virosa (Roxb. Ex willd.) Royle (Phyllanthaceae) | Root; toothache | Flueggrenes A | N-formylmethionyl-leucyl-phenylalanine (FMLP)/cytochalasin B (CB) activated-human neutrophils | ↓ Superoxide anion generation and elastase release | [147] |
| Litsea glutinosa (Lour.) C.B.Rob. (Auraceae) | Leaves and bark; fever, scabies, gastritis pain, cuts, and wounds | Hydroalcoholic extracts of leaves | Carrageenan-induced edema | Carrageenan-induced rat paw edema | [148] |
| Merremia tridentata (L.) Hallier f. (Convolaceae) | Whole plants; sores, antiseptic | Apigenin | LPS-stimulated BV2 microglia | ↓ TNF, IL-1β, and IL-6 production ↓ NF-κB activation | [83,124] |
| Quercetin, quercitrin | LPS-induced RAW264.7 Cells | ↓ NO production, TNF, IL-1β, and IL-6 | [82] | ||
| Ocimum tenuiflorum L. (Lamiaceae) | Leaves and stems; pain and stomachache | Leaf extract | LPS-induced RAW264.7 Cells | ↓ Lipopolysaccharide-induced inflammation in RAW 264.7 cells | [149] |
| Nauclea orientalis (L.) L. (Rubiaceae) | Bark; colds, stomachache, and snake bite | Ethyl acetate (EA) and ethyl alcohol (ET) lotus petal extracts | LPS-stimulated human monocyte-derived macrophages | ↓ TNF, NF-κB | [150] |
| Nelumbo nucifera Gaertn. (Nelumbonaceae) | Ethanol extract from fruits | Carrageenan-induced paw edema (Wistar male rats) | ↓ Carrageenan-induced rat paw edema | [151] | |
| Leaf extract | 293T cells | ↑ IL-10 and IL-12 ↓ IL-6, IL-1β, TNF-α, and IFN-γ | [152] | ||
| Neferine | DSS-induce colitis mice model (C57BL/6J male mice) | TNF, IL-1β, and IL-6 ↑ IL-10 | [153,154] | ||
| Murine macrophage RAW264.7 cells | IL-6 and TNF production ↑ Peroxisome proliferator-activated receptor (PPARα and PPARγ) | [155] | |||
| LPS-stimulated RAW 264.7 cells | ↓ NO release | [156] | |||
| Ochrosia elliptica Labill. (Apocynaceae) | Bark; malaria | Quercetin-3-O-β-d-glucuronide | LPS-stimulated RAW 264.7 cells and human peripheral blood monocytes | ↓ NO production and TNF | [157,158] |
| Phyllanthus urinaria L. (Phyllanthaceae) | Leaves; colds | Corilagin | IB3-1 cells | ↓ IL-8 gene expression in TNF-treated IB3-1 cells TNF-alpha-induced secretion of MCP-1 | [159] |
| Scoparia dulcis L. (Plantaginaceae) | Whole plants; sores, stomachache, skin lesions, wounds, and cuts | Ethanol extract | Carrageenan-induced paw edema | ↓ COX-2, NO, TNF and IL1-β | [160] |
| Betulinic acid | ↓ TNF, IL-1β, COX-2, and NO production | ||||
| Terminalia catappa L. (Combretaceae) | Bark, young green fruit; sore throat and thrush | Ethanol extract of leaves | 12-O-TPA-induced edema (Male ICR mice) | ↓ Myeloperoxidase (MPO) activity | [161] |
| Ursolic acid and 2α,3β,23-trihydroxyurs-12-en-28-oic acid | ↓ TPA-induced ear edema and inhibited MPO activity | ||||
| Terminalia muelleri Benth. (Combretaceae) | Leaves: Scabies, sores, skin lesions, wounds, and cuts | Polyphenol-rich fraction | Carrageenan-induced paw edema model | ↓ PGE2 and IL-6, IL-1β and TNF, IL-1β | [162] |
| Uromyrtus metrosideros (F.M.Bailey) A.J.Scott (Myrtaceae) | Leaves and branches | (4S)-α-terpineol 8-O-β-D-(6-O-galloyl) glucopyranoside | Human PBMCs stimulated by P/I or anti-CD3/anti-CD28 | ↓ IFN-γ, IL-17A and IL-8 | [64] |
| Vitex trifolia L. (Labiatae) | Leaves; inflammatory ailments | Aqueous extract | LPS-induced RAW 264.7 cells | ↓ COX-2, NF-κB, L-1 β and caspase-3 | [163] |
| Galloyl-lawsoniaside | ↓IFN-γ and IL-17A | ||||
| Lophostemon suaveolens (Soland. ex Gaertn.) Peter G.Wilson and J.T.Waterh. (Myrtaceae) | N-hexane and dichloromethane extracts from leaves | LPS-stimulated RAW264.7 cells | ↓ NO production ↓ PGE2 in 3T3 murine fibroblasts | [164] | |
| Betulinic acid | LPS-induced lung inflammation in Sprague-Dawley rats | Inhibit cell recruitment, ↓ TNF, NO, and TGF-β1 expression ↓ MDA production | [165] | ||
| Tasmannia lanceolate (Poir.) A.C.Sm. (Winteraceae) | Berries, bark, and leaves | Polyphenolic-rich extracts (gallic acid, 2-hydroxybenzoic acid and chlorogenic acid were identified through their MS/MS spectra) | LPS-stimulated RAW264.7 cells | ↓ iNOS and COX-2 ↓ NO and prostaglandin E2 (PGE2) levels | [166] |
3. Helminth-Derived Anti-Inflammatory Compounds
| Helminth Species | SMs/ESP/Somatic Tissue/Larva/Egg/EVs/Protein | Animal Model or Cell Line | Mechanism of Action | Reference |
|---|---|---|---|---|
| Ancylostoma caninum | Low-molecular-weight somatic tissue extract metabolites | TNBS-induced colitis model | ↓ TNF, IL-1β, and IL-13 | [188] |
| Low-molecular-weight excretory-secretory product metabolites | LPS-stimulated PBMC | ↓ TNF, IL-1β, and IL-13 | ||
| Dichloromethane-acetonitrile somatic extract | ↓ TNF, IL-1β, IL-6 and the chemokine MCP-1 | |||
| DSS-induced colitis model mice | ↓ Proinflammatory mediators iNOS, IL-6, and IL-17A ↑ IL-4 and IL-10 levels | [200] | ||
| Ancylostoma ceylanicum | Crude extracts and excretory-secretory products | DSS-induced colitis model | ↓ Th1 and Th17 cytokines ↓ Colonic microscopic ↓ EPO and MPO activity | [201] |
| Trichinella spiralis | 53 kDa ES protein | TNBS-induced colitis model | ↑ IL-4, IL-13, IL-10, TGF-β, AAM | [202] |
| ↓ IFN-γ, TNF, IL-6 ↓ inflammatory score | ||||
| Extracellular vesicles | TNBS-induced colitis model | ↓ IL 1β, TNF IFN-γ, and IL-17A ↑ IL-10 and TGF-β, IL-4, and IL-13 | [203] | |
| Nippostrongylus brasiliensis | Extracellular vesicles | Murine small intestinal organoids | ↓ IL-6, IL-1β, IFN γ, and IL-17a ↑ IL-10 | [204] |
| Spirometra erinaceieuropaei | Extracellular vesicles | LPS-stimulated RAW 264.7 cell | ↓ NO production ↓ TNF, IL-1β, and IL-6) | [205] |
| Schistosoma mansoni | Egg | TNBS-induced colitis model | ↓ IFN-γ ↑ IL-10 mRNA expression | [172] |
| Egg | DSS-induced colitis model. | ↑ FoxP3+ T regulatory cells and Th2 cytokines. | [206] | |
| Trichinella spiralis | Infective larvae | TNBS-induced colitis model | ↓ DAI score and weight | [207] |
| ↓ IFN-γ ↑ IL-4 | ||||
| T. spiralis | Infective larvae | TNBS-induced colitis model | ↑ IL-4, IL-13- induction of a Th2 response. | [182] |
| ↓ IL-12, IFN-γ, MPO activity | ||||
| Heligmosomoides polygyrus | Infective larvae | Piroxicam- induced IL-10–/– mice | ↓ IL-12 and IFN-γ production ↑ IL-13, | [170] |
| Infective larvae | TNBS-induced colitis model | ↑ IL-4, IL-5, IL-10, IL-13 | [208] | |
| ↓ IL-12p40, IFN-γ ↑ IL-4, IL-5, IL-13, and IL-10 secretion | ||||
| Infective larvae | IL-10−/− mice | ↓ IL-17 ↓ IL-4 and IL-10 | [209] | |
| Infective third-stage larvae | TNBS-induced colitis model | ↑ IL-4, IL-13, mucosal mast cells, and resistance | [210] | |
| ↓ IFN-γ, TNF | ||||
| Trichuris muris | Embryonated eggs | Mdr1a−/− mice | ↑ IFNγ, TNF ↑ IL-13 and IL-5 | [211] |
| Infective larvae | IL-10−/− mice | ↑ IFN-γ, IL-17 | [212] | |
| ↓ IL-13 ↑ IL-13Rα2 | ||||
| Necator americanus | Netrin-domain-containing proteins (prophylactic Na-AIP-1) | TNBS-induced colitis model | ↓ TNF | [213] |
4. Anti-Inflammatory Agents in Clinical Trials
5. Advances and Recent Approaches in SM Drug Lead Discovery
6. Challenges and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tung, B.T.; Linh, T.V.; Thao, T.P.; Thuan, N.D. Anti-inflammatory Agents from Medicinal Plants. In Phytochemical Drug Discovery for Central Nervous System Disorders: Biochemistry and Therapeutic Effects, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2023; pp. 219–250. [Google Scholar]
- Morgan, R.E.; Ahn, S.; Nzimiro, S.; Fotie, J.; Phelps, M.A.; Cotrill, J.; Yakovich, A.J.; Sackett, D.L.; Dalton, J.T.; Werbovetz, K.A. Inhibitors of tubulin assembly identified through screening a compound library. Chem. Biol. Drug. 2008, 72, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Vishal, V.; Ganesh, S.; Mukesh, G.; Ranjan, B. A review on some plants having anti-inflammatory activity. J. Phytopharmacol. 2014, 3, 214–221. [Google Scholar]
- Yeshi, K.; Turpin, G.; Jamtsho, T.; Wangchuk, P. Indigenous uses, phytochemical analysis, and anti-inflammatory properties of Australian tropical medicinal plants. Molecules 2022, 27, 3849. [Google Scholar] [CrossRef] [PubMed]
- Hijos-Mallada, G.; Sostres, C.; Gomollón, F. NSAIDs, gastrointestinal toxicity and inflammatory bowel disease. Gastroenterol. Hepatol. 2022, 45, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Lin, Y.; Farag, M.A.; Li, Z.; Shao, P. Dendrobium as a new natural source of bioactive for the prevention and treatment of digestive tract diseases: A comprehensive review with future perspectives. Phytomedicine 2023, 114, 154784. [Google Scholar] [CrossRef] [PubMed]
- Zurba, Y.; Gros, B.; Shehab, M. Exploring the pipeline of novel therapies for inflammatory bowel disease; state of the art review. Biomedicines 2023, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Yeshi, K.; Ruscher, R.; Hunter, L.; Daly, N.L.; Loukas, A.; Wangchuk, P. Revisiting inflammatory bowel Ddsease: Pathology, treatments, challenges and emerging therapeutics including drug leads from natural products. J. Clin. Med. 2020, 9, 1273. [Google Scholar] [CrossRef] [PubMed]
- Braus, N.A.; Elliott, D.E. Advances in the pathogenesis and treatment of IBD. Clin. Immunol. 2009, 132, 1–9. [Google Scholar] [CrossRef]
- de Souza, H.S.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef]
- Talley, N.J.; Abreu, M.T.; Achkar, J.P.; Bernstein, C.N.; Dubinsky, M.C.; Hanauer, S.B.; Kane, S.V.; Sandborn, W.J.; Ullman, T.A.; Moayyedi, P. An evidence-based systematic review on medical therapies for inflammatory bowel disease. Am. J. Gastroenterol. 2011, 106 (Suppl. S1), S2–S25; quiz S26. [Google Scholar] [CrossRef]
- Coskun, M.; Steenholdt, C.; de Boer, N.K.; Nielsen, O.H. Pharmacology and optimization of thiopurines and methotrexate in inflammatory bowel disease. Clin. Pharmacokinet. 2016, 55, 257–274. [Google Scholar] [CrossRef]
- Zhao, L.; Xiao, H.T.; Mu, H.X.; Huang, T.; Lin, Z.S.; Zhong, L.L.D.; Zeng, G.Z.; Fan, B.M.; Lin, C.Y.; Bian, Z.X. Magnolol, a natural polyphenol, attenuates dextran sulfate sodium-induced colitis in mice. Molecules 2017, 22, 1218. [Google Scholar] [CrossRef] [PubMed]
- Madasu, C.; Xu, Y.-M.; Wijeratne, E.K.; Liu, M.X.; Molnár, I.; Gunatilaka, A.L. Semi-synthesis and cytotoxicity evaluation of pyrimidine, thiazole, and indole analogues of argentatins A–C from guayule (Parthenium argentatum) resin. Med. Chem. Res. 2022, 31, 1088–1098. [Google Scholar] [CrossRef]
- Shyni, G.; Renjitha, J.; B Somappa, S.; Raghu, K. Zerumin A attenuates the inflammatory responses in LPS-stimulated H9c2 cardiomyoblasts. J. Biochem. Mol. Toxicol. 2021, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jalaja, R.; Leela, S.G.; Mohan, S.; Nair, M.S.; Gopalan, R.K.; Somappa, S.B. Anti-hyperlipidemic potential of natural product based labdane-pyrroles via inhibition of cholesterol and triglycerides synthesis. Bioorg. Chem. 2021, 108, 104664. [Google Scholar] [CrossRef] [PubMed]
- El-Senduny, F.F.; Altouhamy, M.; Zayed, G.; Harsha, C.; Jalaja, R.; Somappa, S.B.; Nair, M.S.; Kunnumakkara, A.B.; Alsharif, F.M.; Badria, F.A. Azadiradione-loaded liposomes with improved bioavailability and anticancer efficacy against triple negative breast cancer. J. Drug Deliv. Sci. Technol. 2021, 65, 102665. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Olivera, P.; Danese, S.; Peyrin-Biroulet, L. Next generation of small molecules in inflammatory bowel disease. Gut 2017, 66, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tabakman, S.; Welsher, K.; Dai, H. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery. Nano Res. 2009, 2, 85–120. [Google Scholar] [CrossRef]
- Razzacki, S.Z.; Thwar, P.K.; Yang, M.; Ugaz, V.M.; Burns, M.A. Integrated microsystems for controlled drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 185–198. [Google Scholar] [CrossRef]
- Vasir, J.K.; Tambwekar, K.; Garg, S. Bioadhesive microspheres as a controlled drug delivery system. Int. J. Pharm. 2003, 255, 13–32. [Google Scholar] [CrossRef]
- Pellá, M.C.; Lima-Tenório, M.K.; Tenório-Neto, E.T.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Anabousi, S.; Ehrhardt, C.; Ravi Kumar, M. Liposomes as targeted drug delivery systems in the treatment of breast cancer. J. Drug Target. 2006, 14, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, W.S.; Almughem, F.A.; Almehmady, A.M.; Jarallah, S.J.; Alsharif, W.K.; Alzahrani, N.M.; Alshehri, A.A. Phytosomes as an emerging nanotechnology platform for the topical delivery of bioactive phytochemicals. Pharmaceutics 2021, 13, 1475. [Google Scholar] [CrossRef] [PubMed]
- Haba, Y.; Kojima, C.; Harada, A.; Ura, T.; Horinaka, H.; Kono, K. Preparation of poly (ethylene glycol)-modified poly (amido amine) dendrimers encapsulating gold nanoparticles and their heat-generating ability. Langmuir 2007, 23, 5243–5246. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Sun, K.; Baker, J.R., Jr. Spontaneous formation of functionalized dendrimer-stabilized gold nanoparticles. J. Phys. Chem. C 2008, 112, 8251–8258. [Google Scholar] [CrossRef] [PubMed]
- De Villiers, M.M.; Aramwit, P.; Kwon, G.S. Nanotechnology in Drug Delivery; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Dent, M.; Matoba, N. Cancer biologics made in plants. Curr. Opin. Biotechnol. 2020, 61, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-derived natural products for drug discovery: Current approaches and prospects. Nucleus 2022, 65, 399–411. [Google Scholar] [CrossRef]
- Muema, F.W.; Nanjala, C.; Oulo, M.A.; Wangchuk, P. Phytochemical content and antidiabetic properties of most commonly used antidiabetic medicinal plants of Kenya. Molecules 2023, 28, 7202. [Google Scholar] [CrossRef]
- Cláudia da Costa Rocha, A.; José de Andrade, C.; de Oliveira, D. Perspective on integrated biorefinery for valorization of biomass from the edible insect Tenebrio molitor. Trends Food Sci. Technol. 2021, 116, 480–491. [Google Scholar] [CrossRef]
- Inanoglu, S.; Goksen, G.; Nayik, G.A.; Mozaffari Nejad, A.S. Chapter 11—Essential oils from Lamiaceae family (rosemary, thyme, mint, basil). In Essential Oils; Nayik, G.A., Ansari, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 309–324. [Google Scholar]
- Fürst, R.; Zündorf, I. Plant-derived anti-inflammatory compounds: Hopes and disappointments regarding the translation of preclinical knowledge into clinical progress. Mediat. Inflamm. 2014, 2014, 146832. [Google Scholar] [CrossRef]
- Cao, Z.; Deng, Z. De novo assembly, annotation, and characterization of root transcriptomes of three caladium cultivars with a focus on necrotrophic pathogen resistance/defense-related genes. Int. J. Mol. Sci. 2017, 18, 712. [Google Scholar] [CrossRef]
- Shazhni, J.A.; Renu, A.; Vijayaraghavan, P. Insights of antidiabetic, anti-inflammatory and hepatoprotective properties of antimicrobial secondary metabolites of corm extract from Caladium x hortulanum. Saudi J. Biol. Sci. 2018, 25, 1755–1761. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Chalmers, A.C.; Jyoti Bhuyan, D.; Bowyer, M.C.; Scarlett, C.J. Botanical, phytochemical, and anticancer properties of the Eucalyptus Species. Chem. Biodivers. 2015, 12, 907–924. [Google Scholar] [CrossRef]
- Jamtsho, T.; Yeshi, K.; Samten; Wangchuk, P. Comparative analysis of two Himalayan Aconitum species for their phytopharmaceutical properties. J. Herb. Med. 2022, 32, 100497. [Google Scholar] [CrossRef]
- Wangchuk, P. Phytochemical Analysis, Bioassays and the Identification of Drug Lead Compounds from Seven Bhutanese Medicinal Plants. Ph.D. Thesis, University of Wollongong, Wollongong, Australia, 2014. [Google Scholar]
- Wangchuk, P.; Navarro, S.; Shepherd, C.; Keller, P.A.; Pyne, S.G.; Loukas, A. Diterpenoid alkaloids of Aconitum laciniatum and mitigation of inflammation by 14-O-acetylneoline in a murine model of ulcerative colitis. Sci. Rep. 2015, 5, 12845. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllidi, A.; Xanthos, T.; Papalois, A.; Triantafillidis, J.K. Herbal and plant therapy in patients with inflammatory bowel disease. Ann. Gastroenterol. 2015, 28, 210. [Google Scholar]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837, 837a–837d. [Google Scholar] [CrossRef]
- Maroyi, A. Albizia Adianthifolia: Botany, Medicinal Uses, Phytochemistry, and Pharmacological Properties. Sci. World J. 2018, 2018, 7463584. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.C.; Kim, B.R.; Vuong, H.L.; Cho, S. Spilanthes acmella inhibits inflammatory responses via inhibition of NF-κB and MAPK signaling pathways in RAW 264.7 macrophages. Mol. Med. Rep. 2017, 16, 339–346. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bakondi, E.; Singh, S.B.; Hajnády, Z.; Nagy-Pénzes, M.; Regdon, Z.; Kovács, K.; Hegedűs, C.; Madácsy, T.; Maléth, J.; Hegyi, P.; et al. Spilanthol Inhibits Inflammatory Transcription Factors and iNOS Expression in Macrophages and Exerts Anti-inflammatory Effects in Dermatitis and Pancreatitis. Int. J. Mol. Sci. 2019, 20, 4308. [Google Scholar] [CrossRef] [PubMed]
- Negahban, M. The Medicinal Effects of Two Australian Native Plants. Ph.D. Thesis, Queensland University of Technology, Queensland, Australia, 2020. [Google Scholar]
- Turpin, G.; Ritmejerytė, E.; Jamie, J.; Crayn, D.; Wangchuk, P. Aboriginal medicinal plants of Queensland: Ethnopharmacological uses, species diversity, and biodiscovery pathways. J. Ethnobiol. Ethnomed. 2022, 18, 54. [Google Scholar] [CrossRef] [PubMed]
- Palit, P.; Mukherjee, D.; Mahanta, P.; Shadab, M.; Ali, N.; Roychoudhury, S.; Asad, M.; Mandal, S.C. Attenuation of nociceptive pain and inflammatory disorders by total steroid and terpenoid fraction of Euphorbia tirucalli Linn root in experimental in vitro and in vivo model. Inflammopharmacology 2018, 26, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Nikbakht, M.; Rahimi-Nasrabadi, M.; Ahmadi, F.; Gandomi, H.; Abbaszadeh, S.; Batooli, H. The chemical composition and in vitro antifungal activities of essential oils of five Eucalyptus species. J. Essent. Oil-Bear. Plants 2015, 18, 666–677. [Google Scholar] [CrossRef]
- Venkataraman, B.; Almarzooqi, S.; Raj, V.; Bhongade, B.A.; Patil, R.B.; Subramanian, V.S.; Attoub, S.; Rizvi, T.A.; Adrian, T.E.; Subramanya, S.B. Molecular docking Identifies 1,8-Cineole (Eucalyptol) as A novel PPARγ agonist that alleviates colon inflammation. Int. J. Mol. Sci. 2023, 24, 6160. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, M.; Wang, G.; Ma, H.; Mu, Y.; Zheng, D.; Huang, X.; Li, L. New monoterpene acid and gallic acid glucose esters with anti-Inflammatory activity from Blue gum (Eucalyptus globulus) Leaves. J. Agric. Food Chem. 2022, 70, 4981–4994. [Google Scholar] [CrossRef]
- Qabaha, K.; Ras, S.A.; Abbadi, J.; Al-Rimawi, F. Anti-inflammatory activity of Eucalyptus spp. and Pistascia lentiscus leaf extracts. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 1–6. [Google Scholar] [CrossRef]
- Yoshimura, M.; Ito, H.; Miyashita, K.; Hatano, T.; Taniguchi, S.; Amakura, Y.; Yoshida, T. Flavonol glucuronides and C-glucosidic ellagitannins from Melaleuca squarrosa. Phytochemistry 2008, 69, 3062–3069. [Google Scholar] [CrossRef]
- Hussein, S.A.; Hashim, A.N.; El-Sharawy, R.T.; Seliem, M.A.; Linscheid, M.; Lindequist, U.; Nawwar, M.A. Ericifolin: An eugenol 5-O-galloylglucoside and other phenolics from Melaleuca ericifolia. Phytochemistry 2007, 68, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Wollenweber, E.; Wehde, R.; Dörr, M.; Lang, G.; Stevens, J.F. C-methyl-flavonoids from the leaf waxes of some Myrtaceae. Phytochemistry 2000, 55, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Abdel Bar, F.M.; Zaghloul, A.M.; Bachawal, S.V.; Sylvester, P.W.; Ahmad, K.F.; El Sayed, K.A. Antiproliferative triterpenes from Melaleuca ericifolia. J. Nat. Prod. 2008, 71, 1787–1790. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Chang, M.H. Four new triterpenes from the heartwood of Melaleuca leucadendron. J. Nat. Prod. 1999, 62, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K. A New Norlupene from the Leaves of Melaleuca leucadendron. J. Nat. Prod. 1998, 61, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Tsuruga, T.; Chun, Y.T.; Ebizuka, Y.; Sankawa, U. Biologically active constituents of Melaleuca leucadendron: Inhibitors of induced histamine release from rat mast cells. Chem. Pharm. Bull. 1991, 39, 3276–3278. [Google Scholar] [CrossRef]
- Bar, F.M.A. Genus Melaleuca—A review on the phytochemistry and pharmacological activities of the non-volatile components. Rec. Nat. Prod. 2021, 15, 219–242. [Google Scholar] [CrossRef]
- Ritmejerytė, E.; Ryan, R.Y.; Byatt, B.J.; Peck, Y.; Yeshi, K.; Daly, N.L.; Zhao, G.; Crayn, D.; Loukas, A.; Pyne, S.G. Anti-inflammatory properties of novel galloyl glucosides isolated from the Australian tropical plant Uromyrtus metrosideros. Chem. Biol. Interact. 2022, 368, 110124. [Google Scholar] [CrossRef]
- Vogel, A.; Pelletier, J. Examen chimique de la racine de Curcuma. J. Pharm. 1815, 1, 289–300. [Google Scholar]
- Karamanou, M.; Tsoucalas, G.; Pantos, K.; Androutsos, G. Isolating colchicine in 19th century: An old drug revisited. Curr. Pharm. Des. 2018, 24, 654–658. [Google Scholar] [CrossRef]
- Pezzuto, J.M. Resveratrol: Twenty years of growth, development and controversy. Biomol. Ther. 2019, 27, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Escogido Mde, L.; Gonzalez-Mondragon, E.G.; Vazquez-Tzompantzi, E. Chemical and pharmacological aspects of capsaicin. Molecules 2011, 16, 1253–1270. [Google Scholar] [CrossRef] [PubMed]
- Basiricò, L.; Morera, P.; Dipasquale, D.; Bernini, R.; Santi, L.; Romani, A.; Lacetera, N.; Bernabucci, U. (-)-Epigallocatechin-3-gallate and hydroxytyrosol improved antioxidative and anti-inflammatory responses in bovine mammary epithelial cells. Animal 2019, 13, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Takahara, M.; Takaki, A.; Hiraoka, S.; Adachi, T.; Shimomura, Y.; Matsushita, H.; Nguyen, T.T.T.; Koike, K.; Ikeda, A.; Takashima, S. Berberine improved experimental chronic colitis by regulating interferon-γ-and IL-17A-producing lamina propria CD4+ T cells through AMPK activation. Sci. Rep. 2019, 9, 11934. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Hu, Z.; Song, X.; Cui, Q.; Fu, Q.; Jia, R.; Zou, Y.; Li, L.; Yin, Z. Analgesic and anti-inflammatory activities of resveratrol through classic models in mice and rats. Evid. Based Complement. Altern. Med. 2017, 2017, 5197567. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-T.; Xu, Y.-F.; Huang, Y.-F.; Qu, C.; Xu, L.-Q.; Su, Z.-R.; Zeng, H.-F.; Zheng, L.; Yi, T.-G.; Li, H.-L. Berberrubine attenuates mucosal lesions and inflammation in dextran sodium sulfate-induced colitis in mice. PLoS ONE 2018, 13, e0194069. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Bose, M.; Ju, J.; Ryu, J.-H.; Chen, X.; Sang, S.; Lee, M.-J.; Yang, C.S. Modulation of arachidonic acid metabolism by curcumin and related β-diketone derivatives: Effects on cytosolic phospholipase A2, cyclooxygenases and 5-lipoxygenase. Carcinogenesis 2004, 25, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-Y.; Kim, K.-H.; Lee, S.-H.; Yoon, M.-S.; Lee, H.-J.; Moon, D.-O.; Lee, C.-M.; Ahn, S.-C.; Park, Y.C.; Park, Y.-M. Curcumin Inhibits Immunostimulatory Function of Dendritic Cells: MAPKs and Translocation of NF-κB as Potential Targets1. J. Immunol. 2005, 174, 8116–8124. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.O.; Ferguson, J.E.; Hunsaker, L.A.; Deck, L.M.; Vander Jagt, D.L. Natural products inhibit LPS-induced activation of pro-inflammatory cytokines in peripheral blood mononuclear cells. Nat. Prod. Res. 2010, 24, 1177–1188. [Google Scholar] [CrossRef]
- Zhou, Y.-L.; Yan, Y.-M.; Li, S.-Y.; He, D.-H.; Xiong, S.; Wei, S.-F.; Liu, W.; Hu, L.; Wang, Q.; Pan, H.-F. 6-O-angeloylplenolin exerts neuroprotection against lipopolysaccharide-induced neuroinflammation in vitro and in vivo. Acta Pharmacol. Sin. 2020, 41, 10–21. [Google Scholar] [CrossRef]
- Xue, P.-H.; Zhang, N.; Liu, D.; Zhang, Q.-R.; Duan, J.-S.; Yu, Y.-Q.; Li, J.-Y.; Cao, S.-J.; Zhao, F.; Kang, N. Cytotoxic and anti-inflammatory sesquiterpenes from the whole plants of Centipeda minima. J. Nat. Prod. 2021, 84, 247–258. [Google Scholar] [CrossRef]
- Wu, H.; Pang, H.; Chen, Y.; Huang, L.; Liu, H.; Zheng, Y.; Sun, C.; Zhang, G.; Wang, G. Anti-inflammatory effect of a polyphenol-enriched fraction from Acalypha wilkesiana on lipopolysaccharide-stimulated RAW 264.7 macrophages and acetaminophen-induced liver injury in mice. Oxid. Med. Cell. Longev. 2018, 2018, 7858094. [Google Scholar] [CrossRef]
- Vigil de Mello, S.V.G.; da Rosa, J.S.; Facchin, B.M.; Luz, A.B.G.; Vicente, G.; Faqueti, L.G.; Rosa, D.W.; Biavatti, M.W.; Fröde, T.S. Beneficial effect of Ageratum conyzoides Linn (Asteraceae) upon inflammatory response induced by carrageenan into the mice pleural cavity. J. Ethnopharmacol. 2016, 194, 337–347. [Google Scholar] [CrossRef]
- Senthamilselvi, M.M.; Kesavan, D.; Sulochana, N. An anti-inflammatory and anti-microbial flavone glycoside from flowers of Cleome viscosa. Org. Med. Chem. Lett. 2012, 2, 19. [Google Scholar] [CrossRef]
- Bawankule, D.; Chattopadhyay, S.; Pal, A.; Saxena, K.; Yadav, S.; Faridi, U.; Darokar, M.; Gupta, A.; Khanuja, S.P.S. Modulation of inflammatory mediators by coumarinolignoids from Cleome viscosa in female swiss albino mice. Inflammopharmacology 2008, 16, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Diao, P.; Shu, X.; Li, L.; Xiong, L. Quercetin and quercitrin attenuates the inflammatory response and oxidative stress in LPS-induced RAW264. 7 cells: In vitro assessment and a theoretical model. BioMed Res. Int. 2019, 2019, 7039802. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Huo, X.; Liu, W.; Li, K.; Sun, Z.; Tian, J. Apigenin exhibits anti-inflammatory effects in LPS-stimulated BV2 microglia through activating GSK3β/Nrf2 signaling pathway. Immunopharmacol. Immunotoxicol. 2020, 42, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Okoro, E.E.; Maharjan, R.; Jabeen, A.; Ahmad, M.S.; Azhar, M.; Shehla, N.; Zaman, W.; Shams, S.; Osoniyi, O.R.; Onajobi, F.D.; et al. Isoflavanquinones from Abrus precatorius roots with their antiproliferative and anti-inflammatory effects. Phytochemistry 2021, 187, 112743. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.; Duarte, S.; Arsénio, P.; Gonçalves, J.; Rodrigues, C.; Lourenço, A.; Máximo, P. Exploring the phytochemicals of Acacia melanoxylon R. Br. Plants. 2021, 10, 2698. [Google Scholar] [CrossRef]
- Kim, J.E.; Min, S.K.; Hong, J.-M.; Kim, K.H.; Han, S.J.; Yim, J.H.; Park, H.; Kim, I.-C. Anti-inflammatory effects of methanol extracts from the Antarctic lichen, Amandinea sp. in LPS-stimulated raw 264.7 macrophages and zebrafish. Fish Shellfish. Immunol. 2020, 107, 301–308. [Google Scholar] [CrossRef]
- Vyas, A.V.; Mulchandani, N.B. Polyoxygenated flavones from Ageratum conyzoides. Phytochemistry 1986, 25, 2625–2627. [Google Scholar] [CrossRef]
- Moreira, M.D.; Picanço, M.C.; Barbosa, L.C.; Guedes, R.N.; Barros, E.C.; Campos, M.R. Compounds from Ageratum conyzoides: Isolation, structural elucidation and insecticidal activity. Pest Manag. Sci. 2007, 63, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Gunawardena, D.; Ahktar, M.A.; Low, M.; Reddell, P.; Münch, G. Anti-Inflammatory Chemical Profiling of the Australian Rainforest Tree Alphitonia petriei (Rhamnaceae). Molecules 2016, 21, 1521. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, A.Y.; Kiendrebeogo, M.; Kehoe, P.G.; Sombie, P.A.; Lamien, C.E.; Millogo, J.F.; Nacoulma, O.G. Antioxidant and anti-inflammatory effects of Scoparia dulcis L. J. Med. Food 2011, 14, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Singh, A.; Bodkin, F.; Münch, G. Costatamins A–C, new 4-phenylcoumarins with anti-inflammatory activity from the Australian woodland tree Angophora costata (Myrtaceae). Fitoterapia 2019, 133, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Qazi, M.S.; Kamal, M. New anti-inflammatory triterpene esters and glycosides from Alstonia scholaris. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2020, 19, 370–386. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.-H.; Cai, X.-H.; Feng, T.; Zhao, Y.-L.; Wang, J.-K.; Zhang, L.-Y.; Yan, M.; Luo, X.-D. Pharmacological evaluation of Alstonia scholaris: Anti-inflammatory and analgesic effects. J. Ethnopharmacol. 2010, 129, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Trang, D.T.; Huyen, L.T.; Nhiem, N.X.; Quang, T.H.; Hang, D.T.T.; Yen, P.H.; Tai, B.H.; Anh, H.L.T.; Binh, N.Q.; Van Minh, C. Tirucallane glycoside from the leaves of Antidesma bunius and inhibitory NO production in BV2 cells and RAW264.7 macrophages. Nat. Prod. Commun. 2016, 11, 1934578X1601100717. [Google Scholar] [CrossRef]
- Dissanayake, A.A.; Georges, K.; Nair, M.G. Cyclooxygenase enzyme and lipid peroxidation inhibitory terpenoids and steroidal compounds as major constituents in Cleome viscosa leaves. Planta Med. 2022, 88, 1287–1292. [Google Scholar] [CrossRef]
- Patil, K.R.; Patil, C.R. Anti-inflammatory activity of bartogenic acid containing fraction of fruits of Barringtonia racemosa Roxb. in acute and chronic animal models of inflammation. J. Tradit. Complement. Med. 2017, 7, 86–93. [Google Scholar] [CrossRef]
- Van, Q.T.T.; Vien, L.T.; Hanh, T.T.H.; Huong, P.T.T.; Cuong, N.T.; Thao, N.P.; Thuan, N.H.; Dang, N.H.; Thanh, N.V.; Cuong, N.X. Acylated flavonoid glycosides from Barringtonia racemosa. Nat. Prod. Res. 2020, 34, 1276–1281. [Google Scholar] [CrossRef]
- Bairwa, K.; Singh, I.N.; Roy, S.K.; Grover, J.; Srivastava, A.; Jachak, S.M. Rotenoids from Boerhaavia diffusa as potential anti-inflammatory agents. J. Nat. Prod. 2013, 76, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Bairwa, K.; Jachak, S.M. Anti-inflammatory potential of a lipid-based formulation of a rotenoid-rich fraction prepared from Boerhavia diffusa. Pharm. Biol. 2015, 53, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Legault, J.; Perron, T.; Mshvildadze, V.; Girard-Lalancette, K.; Perron, S.; Laprise, C.; Sirois, P.; Pichette, A. Antioxidant and anti-inflammatory activities of quercetin 7-O-β-D-glucopyranoside from the leaves of Brasenia schreberi. J. Med. Food 2011, 14, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, S.; Xie, C.; Ye, H.; Tang, H.; Chen, L.; Peng, A. Anti-inflammatory activity of ethyl acetate fraction of the seeds of Brucea javanica. J. Ethnopharmacol. 2013, 147, 442–446. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Zhou, J.-T.; Qu, C.; Dou, Y.-X.; Huang, Q.-H.; Lin, Z.-X.; Xian, Y.-F.; Xie, J.-H.; Xie, Y.-L.; Lai, X.-P.; et al. Anti-inflammatory effects of Brucea javanica oil emulsion by suppressing NF-κB activation on dextran sulfate sodium-induced ulcerative colitis in mice. J. Ethnopharmacol. 2017, 198, 389–398. [Google Scholar] [CrossRef]
- Yang, J.; Liu, W.; Li, S.; Ye, H.; Tang, H.; Chen, L.; Peng, A. Coumarinolignans isolated from the seeds of Brucea javanica. Helv. Chim. Acta 2014, 97, 278–282. [Google Scholar] [CrossRef]
- Hu, Z.-F.; Su, J.-C.; Sun, X.; Xia, R.-F.; Wu, J.-L.; Fu, X.-N.; Zhang, B.-Z.; Chen, J.-C.; Wan, L.-S. Brujavanoids A–U, structurally diverse apotirucallane-type triterpenoids from Brucea javanica and their anti-inflammatory effects. Bioorg. Chem. 2022, 127, 106012. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, T.; Dou, Y.; Huang, Y.; Qu, C.; Gao, J.; Huang, Z.; Xie, Y.; Huang, P.; Lin, Z.; et al. Brusatol ameliorates 2, 4, 6-trinitrobenzenesulfonic acid-induced experimental colitis in rats: Involvement of NF-κB pathway and NLRP3 inflammasome. Int. Immunopharmacol. 2018, 64, 264–274. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, G.; Yang, Y.; Zhang, S.; Jiang, H.; Tian, K.; Arenbaoligao; Chen, D. The treatment of inflammatory bowel disease with monoclonal antibodies in Asia. Biomed. Pharmacother. 2023, 157, 114081. [Google Scholar] [CrossRef]
- Dalavaye, N.; Erridge, S.; Nicholas, M.; Pillai, M.; Bapir, L.; Holvey, C.; Coomber, R.; Rucker, J.J.; Hoare, J.; Sodergren, M.H. The effect of medical cannabis in inflammatory bowel disease: Analysis from the UK Medical Cannabis Registry. Expert Rev. Gastroenterol. Hepatol. 2023, 17, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-C.; Liang, Y.-H.; Chiang, J.-H.; Liu, F.-C.; Lin, W.-H.; Chang, S.-J.; Lin, W.-Y.; Wu, C.-H.; Weng, J.-R. Anti-inflammatory effects of Calophyllum inophyllum L. in RAW264.7 cells. Oncol. Rep. 2012, 28, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-L.; Truong, C.-T.; Nguyen, B.C.Q.; Vo, T.-N.V.; Dao, T.-T.; Nguyen, V.-D.; Trinh, D.-T.T.; Huynh, H.K.; Bui, C.-B. Anti-inflammatory and wound healing activities of calophyllolide isolated from Calophyllum inophyllum Linn. PLoS ONE 2017, 12, e0185674. [Google Scholar] [CrossRef]
- Van Thanh, N.; Jang, H.-J.; Vinh, L.B.; Linh, K.T.P.; Huong, P.T.T.; Cuong, N.X.; Nam, N.H.; Van Minh, C.; Kim, Y.H.; Yang, S.Y. Chemical constituents from Vietnamese mangrove Calophyllum inophyllum and their anti-inflammatory effects. Bioorg. Chem. 2019, 88, 102921. [Google Scholar] [CrossRef]
- Wu, F.-S.; Huang, C.-M.; Chen, L.-C.; Chang, T.-H.; Shu, C.-W.; Sung, P.-J.; Chu, Y.-C.; Cheng, M.-J.; Hsiao, J.-W.; Chen, J.-J. A new cinnamic acid derivative and anti-inflammatory constituents from Capparis lanceolaris. Chem. Nat. Compd. 2023, 59, 493–496. [Google Scholar] [CrossRef]
- Choi, J.K.; Oh, H.-M.; Park, J.H.; Choi, J.H.; Sa, K.H.; Kang, Y.M.; Park, P.-H.; Shin, T.-Y.; Rho, M.-C.; Kim, S.-H. Salvia plebeia extract inhibits the inflammatory response in human rheumatoid synovial fibroblasts and a murine model of arthritis. Phytomedicine 2015, 22, 415–422. [Google Scholar] [CrossRef]
- Geraghty, D.P.; Ahuja, K.D.; Pittaway, J.; Shing, C.; Jacobson, G.A.; Jager, N.; Jurković, S.; Narkowicz, C.; Saunders, C.I.; Ball, M. In vitro antioxidant, antiplatelet and anti-inflammatory activity of Carpobrotus rossii (pigface) extract. J. Ethnopharmacol. 2011, 134, 97–103. [Google Scholar] [CrossRef]
- Li, R.W.; Lin, G.D.; Leach, D.N.; Waterman, P.G.; Myers, S.P. Inhibition of COXs and 5-LOX and activation of PPARs by Australian Clematis species (Ranunculaceae). J. Ethnopharmacol. 2006, 104, 138–143. [Google Scholar] [CrossRef]
- Huang, S.-S.; Chiu, C.-S.; Lin, T.-H.; Lee, M.-M.; Lee, C.-Y.; Chang, S.-J.; Hou, W.-C.; Huang, G.-J.; Deng, J.-S. Antioxidant and anti-inflammatory activities of aqueous extract of Centipeda minima. J. Ethnopharmacol. 2013, 147, 395–405. [Google Scholar] [CrossRef]
- Li, S.-Y.; Zhou, Y.-L.; He, D.-H.; Liu, W.; Fan, X.-Z.; Wang, Q.; Pan, H.-F.; Cheng, Y.-X.; Liu, Y.-Q. Centipeda minima extract exerts antineuroinflammatory effects via the inhibition of NF-κB signaling pathway. Phytomedicine 2020, 67, 153164. [Google Scholar] [CrossRef]
- Chan, B.D.; Wong, W.-Y.; Lee, M.M.-L.; Leung, T.-W.; Shum, T.-Y.; Cho, W.C.-S.; Chen, S.; Tai, W.C.-S. Centipeda minima extract attenuates dextran sodium sulfate-induced acute colitis in mice by inhibiting macrophage activation and monocyte chemotaxis. Front. Pharmacol. 2021, 12, 738139. [Google Scholar] [CrossRef] [PubMed]
- Chittasupho, C.; Chaobankrang, K.; Sarawungkad, A.; Samee, W.; Singh, S.; Hemsuwimon, K.; Okonogi, S.; Kheawfu, K.; Kiattisin, K.; Chaiyana, W. Antioxidant, Anti-Inflammatory and Attenuating Intracellular Reactive Oxygen Species Activities of Nicotiana tabacum var. Virginia Leaf Extract Phytosomes and Shape Memory Gel Formulation. Gels 2023, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Tiwari, A.; Şahin, K.; Küçük, Ö.; Ali, S. Triterpenoid saponin-rich fraction of Centella asiatica decreases IL-1ß and NF-κB, and augments tissue regeneration and excision wound repair. Turk. J. Biol. 2016, 40, 399–409. [Google Scholar] [CrossRef]
- Nhiem, N.X.; Tai, B.H.; Quang, T.H.; Van Kiem, P.; Van Minh, C.; Nam, N.H.; Kim, J.-H.; Im, L.-R.; Lee, Y.-M.; Kim, Y.H. A new ursane-type triterpenoid glycoside from Centella asiatica leaves modulates the production of nitric oxide and secretion of TNF-α in activated RAW 264.7 cells. Bioorg. Med. Chem. Lett. 2011, 21, 1777–1781. [Google Scholar] [CrossRef] [PubMed]
- Chianese, G.; Masi, F.; Cicia, D.; Ciceri, D.; Arpini, S.; Falzoni, M.; Pagano, E.; Taglialatela-Scafati, O. Isomadecassoside, a new ursane-type triterpene glycoside from Centella asiatica leaves, reduces nitrite levels in LPS-stimulated macrophages. Biomolecules 2021, 11, 494. [Google Scholar] [CrossRef] [PubMed]
- Srisook, K.; Srisook, E.; Nachaiyo, W.; Chan-In, M.; Thongbai, J.; Wongyoo, K.; Chawsuanthong, S.; Wannasri, K.; Intasuwan, S.; Watcharanawee, K. Bioassay-guided isolation and mechanistic action of anti-inflammatory agents from Clerodendrum inerme leaves. J. Ethnopharmacol. 2015, 165, 94–102. [Google Scholar] [CrossRef]
- Al Haidari, R.; Alshali, K.; Elkhayat, E.; Fouad, M.; Ibrahim, S.; Mohamed, G. Chemical constituents and biological investigations of the aerial parts of Egyptian Clerodendrum inerme. Bull. Fac. Pharm. Cairo Univ. 2014, 52, 165–170. [Google Scholar] [CrossRef]
- Cock, I.E. Medicinal and aromatic plants–Australia. In Ethnopharmacology, Encyclopedia of Life Support Systems (EOLSS); Developed under the Auspices of the UNESCO; EOLSS Publishers: Oxford, UK, 2011. [Google Scholar]
- Doe, P.; Danquah, C.A.; Ohemeng, K.A.; Opare, A.E.; Sharif, A.; Akua-Abora, D.; Akuoko, A.K.; Kpabitey, A.; Quarshie, E.; Asante, O.O. Analgesic, anti-inflammatory, and anti-pyretic activities of Crinum pedunculatum R. Br. Bulb extracts. Pharmacogn. Res. 2022, 14, 24–29. [Google Scholar] [CrossRef]
- Deng, S.; Palu, A.K.; West, B.J.; Su, C.X.; Zhou, B.-N.; Jensen, J.C. Lipoxygenase inhibitory constituents of the fruits of noni (Morinda citrifolia) collected in Tahiti. J. Nat. Prod. 2007, 70, 859–862. [Google Scholar] [CrossRef]
- Coutinho de Sousa, B.; Reis Machado, J.; da Silva, M.V.; da Costa, T.A.; Lazo-Chica, J.E.; Degasperi, T.d.P.; Rodrigues Junior, V.; Sales-Campos, H.; Uber Bucek, E.; Freire Oliveira, C.J. Morinda citrifolia (noni) fruit juice reduces inflammatory cytokines expression and contributes to the maintenance of intestinal mucosal integrity in DSS experimental colitis. Mediat. Inflamm. 2017, 2017, 6567432. [Google Scholar] [CrossRef]
- Huang, H.-L.; Liu, C.-T.; Chou, M.-C.; Ko, C.-H.; Wang, C.-K. Noni (Morinda citrifolia L.) fruit extracts improve colon microflora and exert anti-inflammatory activities in Caco-2 cells. J. Med. Food 2015, 18, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, T.; Kitamura, M.; Hayashi, Y.; Tomida, N.; Uwaya, A.; Isami, F.; Inoue, Y. Anti-inflammatory effects of Morinda citrifolia extract against lipopolysaccharide-induced inflammation in RAW264 cells. Medicines 2021, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Yu, J.S.; Huang, P.; Qader, M.; Manavalan, A.; Wu, X.; Kim, J.-C.; Pang, C.; Cao, S.; Kang, K.S. Identification of anti-inflammatory compounds from Hawaiian noni (Morinda citrifolia L.) fruit juice. Molecules 2020, 25, 4968. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.; Quispe, C.; Sarkar, C.; Bepari, T.C.; Alam, M.J.; Saha, S.; Ray, P.; Rahim, M.A.; Islam, M.T.; Setzer, W.N. Analgesic and anti-inflammatory potential of essential oil of Eucalyptus camaldulensis leaf: In vivo and in silico studies. Nat. Prod. Commun. 2021, 16, 1934578X211007634. [Google Scholar] [CrossRef]
- Simpson, B.; Claudie, D.; Smith, N.; Wang, J.; McKinnon, R.; Semple, S. Evaluation of the anti-inflammatory properties of Dodonaea polyandra, a Kaanju traditional medicine. J. Ethnopharmacol. 2010, 132, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Simpson, B.S.; Luo, X.; Costabile, M.; Caughey, G.E.; Wang, J.; Claudie, D.J.; McKinnon, R.A.; Semple, S.J. Polyandric acid A, a clerodane diterpenoid from the Australian medicinal plant Dodonaea polyandra, attenuates pro-inflammatory cytokine secretion in vitro and in vivo. J. Nat. Prod. 2014, 77, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Simpson, B.S.; Claudie, D.J.; Gerber, J.P.; Pyke, S.M.; Wang, J.; McKinnon, R.A.; Semple, S.J. In vivo activity of benzoyl ester clerodane diterpenoid derivatives from Dodonaea polyandra. J. Nat. Prod. 2011, 74, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.; Sperotto, J.; Manfron, M. Antiinflammatory activity and acute toxicity of Dodonaea viscosa. Fitoterapia 2006, 77, 478–480. [Google Scholar] [CrossRef]
- Salinas-Sánchez, D.O.; Herrera-Ruiz, M.; Pérez, S.; Jiménez-Ferrer, E.; Zamilpa, A. Anti-inflammatory activity of hautriwaic acid isolated from Dodonaea viscosa leaves. Molecules 2012, 17, 4292–4299. [Google Scholar] [CrossRef]
- Jiang, Z.-P.; Zou, B.-H.; Li, X.-J.; Liu, J.-J.; Shen, L.; Wu, J. Ent-kauranes from the Chinese Excoecaria agallocha L. and NF-κB inhibitory activity. Fitoterapia 2019, 133, 159–170. [Google Scholar] [CrossRef]
- Jiang, Z.-P.; Yu, Y.; Shen, L. Agallolides AM, including two rearranged ent-atisanes featuring a bicyclo [3.2. 1] octane motif, from the Chinese Excoecaria agallocha. Bioorg. Chem. 2020, 104, 104206. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tang, X.; Shuai, L.; Kwon, Y.S.; Kim, M.J. Chemical characterization, antioxidant properties and anti-inflammatory activity of Chinese water chestnut extracts. Sci. Asia 2020, 46, 151–156. [Google Scholar] [CrossRef]
- da Silva Barth, C.; de Souza, H.G.T.; Rocha, L.W.; da Silva, G.F.; Dos Anjos, M.F.; Pastor, V.D.A.; Bresolin, T.M.B.; Couto, A.G.; Santin, J.R.; Quintão, N.L.M. Ipomoea pes-caprae (L.) R. Br (Convolvulaceae) relieved nociception and inflammation in mice—A topical herbal medicine against effects due to cnidarian venom-skin contact. J. Ethnopharmacol. 2017, 200, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni-Almeida, A.; Suthar, A.; Goswami, H.; Vishwakarma, R.; Chauhan, V.S.; Balakrishnan, A.; Sharma, S. Novel leads from Heliotropium ovalifolium, 4, 7, 8-trimethoxy-naphthalene-2-carboxylic acid and 6-hydroxy-5, 7-dimethoxy-naphthalene-2-carbaldehyde show specific IL-6 inhibitory activity in THP-1 cells and primary human monocytes. Phytomedicine 2008, 15, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.F.; Bani, S.; Sultan, P.; Ali, S.A.; Bakheet, S.A.; Attia, S.M.; Abd-Allah, A.R. TNF-α inhibitory effect of Euphorbia hirta in rats. Pharm. Biol. 2013, 51, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.-F.; Cheng, Y.-D.; Shen, C.-R.; Cherng, J.-Y. A molecular pharmacology study into the anti-inflammatory actions of Euphorbia hirta L. on the LPS-induced RAW 264.7 cells through selective iNOS protein inhibition. J. Nat. Med. 2010, 64, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Surh, J.; Yun, J.-M. Antioxidant and anti-inflammatory activities of butanol extract of Melaleuca leucadendron L. Prev. Nutr. Food Sci. 2012, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Al-Sayed, E.; Michel, H.E.; Khattab, M.A.; El-Shazly, M.; Singab, A.N. Protective role of casuarinin from Melaleuca leucadendra against ethanol-induced gastric ulcer in rats. Planta Med. 2020, 86, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, O.O.; Yemitan, O.K.; Afolabi, L. Inhibition of chemically induced inflammation and pain by orally and topically administered leaf extract of Manihot esculenta Crantz in rodents. J. Ethnopharmacol. 2008, 119, 6–11. [Google Scholar] [CrossRef]
- Chao, C.-H.; Cheng, J.-C.; Hwang, T.-L.; Shen, D.-Y.; Wu, T.-S. Trinorditerpenes from the roots of Flueggea virosa. Bioorg. Med. Chem. Lett. 2014, 24, 447–449. [Google Scholar] [CrossRef]
- Bhowmick, R.; Sarwar, M.S.; RahmanDewan, S.M.; Das, A.; Das, B.; NasirUddin, M.M.; Islam, M.S.; Islam, M.S. In vivo analgesic, antipyretic, and anti-inflammatory potential in Swiss albino mice and in vitro thrombolytic activity of hydroalcoholic extract from Litsea glutinosa leaves. Biol. Res. 2014, 47, 56. [Google Scholar] [CrossRef] [PubMed]
- Narasimhulu, C.A.; Vardhan, S. Therapeutic potential of Ocimum tenuiflorum as MPO inhibitor with implications for atherosclerosis prevention. J. Med. Food 2015, 18, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Sranujit, R.P.; Noysang, C.; Tippayawat, P.; Kooltheat, N.; Luetragoon, T.; Usuwanthim, K. Phytochemical’s and immunomodulatory effect of Nelumbo nucifera flower extracts on human macrophages. Plants 2021, 10, 2007. [Google Scholar] [CrossRef] [PubMed]
- Rajput, M.A.; Zehra, T.; Fizzah, A.; Kumar, G. Evaluation of antiinflammatory activity of ethanol extract of Nelumbo nucifera fruit. Turk. J. Pharm. Sci. 2021, 18, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, Y.; Yang, Y.; Gou, Y.; Li, S.; Wang, R.; Zeng, S.; Zhao, X. Antioxidant and inflammatory effects of Nelumbo nucifera Gaertn. leaves. Oxid. Med. Cell. Longev. 2021, 2021, 8375961. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Guo, Y.; Min, X.; Pei, L.; Chen, X. Neferine, a bisbenzylisoquinoline alkaloid, ameliorates dextran sulfate sodium-induced ulcerative colitis. Am. J. Chin. Med. 2018, 46, 1263–1279. [Google Scholar] [CrossRef] [PubMed]
- Min, X.; Guo, Y.; Zhou, Y.; Chen, X. Protection against dextran sulfate sodium-induced ulcerative colitis in mice by neferine, a natural product from Nelumbo nucifera Gaertn. Cell J. 2021, 22, 523. [Google Scholar] [CrossRef]
- Zhang, C.; Deng, J.; Liu, D.; Tuo, X.; Yu, Y.; Yang, H.; Wang, N. Nuciferine inhibits proinflammatory cytokines via the PPARs in LPS-induced RAW264.7 cells. Molecules 2018, 23, 2723. [Google Scholar] [CrossRef]
- Li, F.; Sun, X.-Y.; Li, X.-W.; Yang, T.; Qi, L.-W. Enrichment and separation of quercetin-3-O-β-d-glucuronide from lotus leaves (Nelumbo nucifera gaertn.) and evaluation of its anti-inflammatory effect. J. Chromatogr. B Biomed. Appl. 2017, 1040, 186–191. [Google Scholar] [CrossRef]
- Fua, Y.-H. Structurally diverse indole alkaloids from Ochrosia elliptica. Heterocycles 2017, 94, 743–749. [Google Scholar] [CrossRef]
- Tian, L.-X.; Li, X.-Y.; Tang, X.; Zhou, X.-Y.; Luo, L.; Ma, X.-Y.; Tang, W.-Q.; Yu, J.; Ma, W.; Yang, X. Ellipticine conveys protective effects to lipopolysaccharide-activated macrophages by targeting the JNK/AP-1 signaling pathway. Inflammation 2020, 43, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Gambari, R.; Borgatti, M.; Lampronti, I.; Fabbri, E.; Brognara, E.; Bianchi, N.; Piccagli, L.; Yuen, M.C.-W.; Kan, C.-W.; Hau, D.K.-P. Corilagin is a potent inhibitor of NF-kappaB activity and downregulates TNF-alpha induced expression of IL-8 gene in cystic fibrosis IB3-1 cells. Int. Immunopharmacol. 2012, 13, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.-C.; Peng, W.-H.; Chiu, T.-H.; Lai, S.-C.; Lee, C.-Y. Anti-inflammatory effects of Scoparia dulcis L. and betulinic acid. Am. J. Chin. Med. 2011, 39, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xu, L.; Gao, J.; Wang, Y.; Tang, X.; Zhao, X.; Zhang, Z. Phytochemical and antiinflammatory studies on Terminalia catappa. Fitoterapia 2004, 75, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, N.M.; Al-Sayed, E.; Abdel-Daim, M.M.; Singab, A.N. Anti-inflammatory and analgesic activities of Terminalia muelleri benth.(combretaceae). Drug Dev. Res. 2017, 78, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Adib-Conquy, M.; Coste, A.; Kumar-Roiné, S.; Pipy, B.; Laurent, D.; Pauillac, S. Aqueous extract of Vitex trifolia L. (Labiatae) inhibits LPS-dependent regulation of inflammatory mediators in RAW 264.7 macrophages through inhibition of Nuclear Factor kappa B translocation and expression. J. Ethnopharmacol. 2012, 143, 24–32. [Google Scholar] [CrossRef]
- Naz, T.; Packer, J.; Yin, P.; Brophy, J.J.; Wohlmuth, H.; Renshaw, D.E.; Smith, J.; Elders, Y.C.; Vemulpad, S.R.; Jamie, J.F. Bioactivity and chemical characterisation of Lophostemon suaveolens—An endemic Australian Aboriginal traditional medicinal plant. Nat. Prod. Res. 2016, 30, 693–696. [Google Scholar] [CrossRef]
- Oliveira-Costa, J.F.; Meira, C.S.; Neves, M.; Dos Reis, B.; Soares, M.B.P. Anti-Inflammatory Activities of Betulinic Acid: A Review. Front. Pharmacol. 2022, 13, 883857. [Google Scholar] [CrossRef]
- Guo, Y.; Sakulnarmrat, K.; Konczak, I. Anti-inflammatory potential of native Australian herbs polyphenols. Toxicol. Rep. 2014, 1, 385–390. [Google Scholar] [CrossRef]
- Aykul, S.; Martinez-Hackert, E. Determination of half-maximal inhibitory concentration using biosensor-based protein interaction analysis. Anal. Biochem. 2016, 508, 97–103. [Google Scholar] [CrossRef]
- Graziani, V.; Scognamiglio, M.; Belli, V.; Esposito, A.; D’Abrosca, B.; Chambery, A.; Russo, R.; Panella, M.; Russo, A.; Ciardiello, F. Metabolomic approach for a rapid identification of natural products with cytotoxic activity against human colorectal cancer cells. Sci. Rep. 2018, 8, 5309. [Google Scholar] [CrossRef] [PubMed]
- Atagozli, T.; Elliott, D.E.; Ince, M.N. Helminth lessons in inflammatory bowel diseases (IBD). Biomedicines 2023, 11, 1200. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.E.; Setiawan, T.; Metwali, A.; Blum, A.; Urban, J.F., Jr.; Weinstock, J.V. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur. J. Immunol. 2004, 34, 2690–2698. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, H.-L.; Bannick, N.; Henry, M.; Holm, A.N.; Metwali, A.; Urban, J.F.; Rothman, P.B.; Weiner, G.J.; Blazar, B.R. Intestinal helminths regulate lethal acute graft-versus-host disease and preserve the graft-versus-tumor effect in mice. J. Immunol. 2015, 194, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.E.; Li, J.; Blum, A.; Metwali, A.; Qadir, K.; Urban, J.F., Jr.; Weinstock, J.V. Exposure to Schistosome eggs protects mice from TNBS-induced colitis. Am. J. Physiol. Gastrointest. Liver. Physiol. 2003, 284, G385–G391. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, W.; Guan, X.; Truscott, J.; Creemers, J.W.; Chen, H.-L.; Pesu, M.; El Abiad, R.G.; Karacay, B.; Urban, J.F. STAT6 and Furin Are Successive Triggers for the Production of TGF-β by T Cells. J. Immunol. 2018, 201, 2612–2623. [Google Scholar] [CrossRef] [PubMed]
- Metwali, A.; Winckler, S.; Urban, J.F., Jr.; Kaplan, M.H.; Ince, M.N.; Elliott, D.E. Helminth-induced regulation of T-cell transfer colitis requires intact and regulated T cell Stat6 signaling in mice. Eur. J. Immunol. 2021, 51, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhu, F.; Zheng, W.; Jacques, M.L.; Huang, J.; Guan, F.; Lei, J. Protective effect of Schistosoma japonicum eggs on TNBS-induced colitis is associated with regulating Treg/Th17 balance and reprogramming glycolipid metabolism in mice. Front. Cell. Infect. Microbiol. 2022, 12, 1028899. [Google Scholar] [CrossRef]
- Kitagaki, K.; Businga, T.R.; Racila, D.; Elliott, D.E.; Weinstock, J.V.; Kline, J.N. Intestinal helminths protect in a murine model of asthma. J. Immunol. 2006, 177, 1628–1635. [Google Scholar] [CrossRef]
- Metenou, S.; Dembele, B.; Konate, S.; Dolo, H.; Coulibaly, S.Y.; Coulibaly, Y.I.; Diallo, A.A.; Soumaoro, L.; Coulibaly, M.E.; Sanogo, D. At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. J. Immunol. 2010, 184, 5375–5382. [Google Scholar] [CrossRef]
- Smith, K.A.; Löser, S.; Varyani, F.; Harcus, Y.; McSorley, H.J.; McKenzie, A.N.; Maizels, R.M. Concerted IL-25R and IL-4Rα signaling drive innate type 2 effector immunity for optimal helminth expulsion. eLife 2018, 7, e38269. [Google Scholar] [CrossRef] [PubMed]
- Lechner, A.; Bohnacker, S.; Esser-von Bieren, J. Macrophage regulation & function in helminth infection. Semin. Immunol. 2021, 53, 101526. [Google Scholar] [CrossRef] [PubMed]
- Hang, L.; Setiawan, T.; Blum, A.M.; Urban, J.; Stoyanoff, K.; Arihiro, S.; Reinecker, H.-C.; Weinstock, J.V. Heligmosomoides polygyrus infection can inhibit colitis through direct interaction with innate immunity. J. Immunol. 2010, 185, 3184–3189. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.M.; Hang, L.; Setiawan, T.; Urban, J.P.; Stoyanoff, K.M.; Leung, J.; Weinstock, J.V. Heligmosomoides polygyrus bakeri induces tolerogenic dendritic cells that block colitis and prevent antigen-specific gut T cell responses. J. Immunol. 2012, 189, 2512–2520. [Google Scholar] [CrossRef]
- Khan, W.; Blennerhasset, P.; Varghese, A.; Chowdhury, S.; Omsted, P.; Deng, Y.; Collins, S. Intestinal nematode infection ameliorates experimental colitis in mice. Infect. Immun. 2002, 70, 5931–5937. [Google Scholar] [CrossRef] [PubMed]
- Melon, A.; Wang, A.; Phan, V.; McKay, D.M. Infection with Hymenolepis diminuta is more effective than daily corticosteroids in blocking chemically induced colitis in mice. J. Biomed. Biotechnol. 2009, 2010, 384523. [Google Scholar] [CrossRef]
- Summers, R.W.; Elliott, D.; Urban, J.; Thompson, R.; Weinstock, J. Trichuris suis therapy in Crohn’s disease. Gut 2005, 54, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Xu, N.; Wang, X.; Vallée, I.; Liu, M.; Liu, X. Helminth therapy for immune-mediated inflammatory diseases: Current and future perspectives. J. Inflamm. Res. 2022, 15, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, A.J.; Levy, C.W.; Jowitt, T.A.; Hayes, K.S.; Thompson, S.; McKenzie, E.A.; Ball, M.D.; Dubaissi, E.; France, A.P.; Bellina, B.; et al. The major secreted protein of the whipworm parasite tethers to matrix and inhibits interleukin-13 function. Nat. Commun. 2019, 10, 2344. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Wangchuk, P.; Shepherd, C.; Constantinoiu, C.; Ryan, R.Y.M.; Kouremenos, K.A.; Becker, L.; Jones, L.; Buitrago, G.; Giacomin, P.; Wilson, D.; et al. Hookworm-derived metabolites suppress pathology in a mouse model of colitis and inhibit secretion of key inflammatory cytokines in primary human leukocytes. Infect. Immun. 2019, 87. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.E.; Urban, J.F., Jr.; Argo, C.K.; Weinstock, J.V. Does the failure to acquire helminthic parasites predispose to Crohn’s disease? FASEB J. 2000, 14, 1848–1855. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, P.; Kouremenos, K.; Eichenberger, R.M.; Pearson, M.; Susianto, A.; Wishart, D.S.; McConville, M.J.; Loukas, A. Metabolomic profiling of the excretory–secretory products of hookworm and whipworm. Metabolomics 2019, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, P.; Constantinoiu, C.; Eichenberger, R.M.; Field, M.; Loukas, A. Characterization of tapeworm metabolites and their reported biological activities. Molecules 2019, 24, 1480. [Google Scholar] [CrossRef]
- Wangchuk, P.; Anderson, D.; Yeshi, K.; Loukas, A. Identification of small molecules of the infective stage of human hookworm using LCMS-based metabolomics and lipidomics protocols. ACS Infect. Dis. 2021, 7, 3264–3276. [Google Scholar] [CrossRef]
- Wu, T.; Wang, C.; Ding, L.; Shen, Y.; Cui, H.; Wang, M.; Wang, H. Arginine relieves the inflammatory response and enhances the casein Expression in bovine mammary epithelial cells induced by lipopolysaccharide. Mediat. Inflamm. 2016, 2016, 9618795. [Google Scholar] [CrossRef]
- Unnikrishnan, M.; Rao, M. Antiinflammatory activity of methionine, methionine sulfoxide and methionine sulfone. Agents Actions 1990, 31, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Glushakova, O.; Kosugi, T.; Roncal, C.; Mu, W.; Heinig, M.; Cirillo, P.; Sanchez-Lozada, L.G.; Johnson, R.J.; Nakagawa, T. Fructose induces the inflammatory molecule ICAM-1 in endothelial cells. J. Am. Soc. Nephrol. 2008, 19, 1712. [Google Scholar] [CrossRef]
- Tang, X.; Liu, J.; Dong, W.; Li, P.; Li, L.; Lin, C.; Zheng, Y.; Hou, J.; Li, D. The cardioprotective effects of citric acid and L-malic acid on myocardial ischemia/reperfusion injury. Evid. Based Complement. Altern. Med. 2013, 2013, 820695. [Google Scholar] [CrossRef]
- Ahmad, M.; Baba, W.N.; Gani, A.; Wani, T.A.; Gani, A.; Masoodi, F. Effect of extraction time on antioxidants and bioactive volatile components of green tea (Camellia sinensis), using GC/MS. Cogent Food Agric. 2015, 1, 1106387. [Google Scholar] [CrossRef]
- Hearps, A.; Tyssen, D.; Srbinovski, D.; Bayigga, L.; Diaz, D.; Aldunate, M.; Cone, R.; Gugasyan, R.; Anderson, D.; Tachedjian, G. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol. 2017, 10, 1480–1490. [Google Scholar] [CrossRef]
- Shepherd, C.; Giacomin, P.; Navarro, S.; Miller, C.; Loukas, A.; Wangchuk, P. A medicinal plant compound, capnoidine, prevents the onset of inflammation in a mouse model of colitis. J. Ethnopharmacol. 2018, 211, 17–28. [Google Scholar] [CrossRef]
- Ferreira, I.; Smyth, D.; Gaze, S.; Aziz, A.; Giacomin, P.; Ruyssers, N.; Artis, D.; Laha, T.; Navarro, S.; Loukas, A.; et al. Hookworm Excretory/Secretory Products Induce Interleukin-4 (IL-4)+ IL-10+ CD4+ T Cell Responses and Suppress Pathology in a Mouse Model of Colitis. Infect. Immun. 2013, 81, 2104–2111. [Google Scholar] [CrossRef]
- Cançado, G.G.L.; Fiuza, J.A.; de Paiva, N.C.N.; de Carvalho Dhom Lemos, L.; Ricci, N.D.; Gazzinelli-Guimarães, P.H.; Martins, V.G.; Bartholomeu, D.C.; Negrão-Corrêa, D.A.; Carneiro, C.M.; et al. Hookworm products ameliorate dextran sodium sulfate-induced colitis in BALB/c mice. J. Leukoc. Biol. 2011, 17, 2275–2286. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Tang, H.; Ma, Z.; Xu, J.; Gao, W.; Chen, J.; Gan, W.; Zhang, Z.; Yu, X.; Zhou, X. The protective effect of the recombinant 53-kDa protein of Trichinella spiralis on experimental colitis in mice. Dig. Dis. Sci. 2011, 56, 2810–2817. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, L.; Liu, X.; Zhang, Y.; Shi, H.; Jia, W.; Zhu, H.; Jia, H.; Liu, M.; Bai, X. Extracellular vesicles derived from Trichinella spiralis muscle larvae ameliorate TNBS-induced colitis in mice. Front. pharmacol. 2020, 11, 1174. [Google Scholar] [CrossRef]
- Eichenberger, R.M.; Ryan, S.; Jones, L.; Buitrago, G.; Polster, R.; Montes de Oca, M.; Zuvelek, J.; Giacomin, P.R.; Dent, L.A.; Engwerda, C.R. Hookworm secreted extracellular vesicles interact with host cells and prevent inducible colitis in mice. Front. pharmacol. 2018, 9, 850. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Ito, D.; Taniguchi, R.; Tademoto, S.; Horie, T.; Otsuki, H. Extracellular vesicles derived from Spirometra erinaceieuropaei plerocercoids inhibit activation of murine macrophage RAW264.7 cells. Parasitol. Int. 2023, 95, 102742. [Google Scholar] [CrossRef]
- Hasby, E.A.; Hasby Saad, M.A.; Shohieb, Z.; El Noby, K. FoxP3+ T regulatory cells and immunomodulation after Schistosoma mansoni egg antigen immunization in experimental model of inflammatory bowel disease. Cell. Immunol. 2015, 295, 67–76. [Google Scholar] [CrossRef]
- Xu, J.; Yu, P.; Wu, L.; Liu, M.; Lu, Y. Effect of Trichinella spiralis intervention on TNBS-induced experimental colitis in mice. Immunobiol. 2019, 224, 147–153. [Google Scholar] [CrossRef]
- Setiawan, T.; Metwali, A.; Blum, A.M.; Ince, M.N.; Urban, J.F., Jr.; Elliott, D.E.; Weinstock, J.V. Heligmosomoides polygyrus promotes regulatory T-cell cytokine production in the murine normal distal intestine. Infect. Immun. 2007, 75, 4655–4663. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.E.; Metwali, A.; Leung, J.; Setiawan, T.; Blum, A.M.; Ince, M.N.; Bazzone, L.E.; Stadecker, M.J.; Urban, J.F.; Weinstock, J.V. Colonization with Heligmosomoides polygyrus suppresses mucosal IL-17 production. J. Immunol. 2008, 181, 2414–2419. [Google Scholar] [CrossRef]
- Sutton, T.L.; Zhao, A.; Madden, K.B.; Elfrey, J.E.; Tuft, B.A.; Sullivan, C.A.; Urban, J.F., Jr.; Shea-Donohue, T. Anti-Inflammatory mechanisms of enteric Heligmosomoides polygyrus infection against trinitrobenzene sulfonic acid-induced colitis in a murine model. Infect. Immun. 2008, 76, 4772–4782. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, E.K.; Else, K.J.; Rogan, M.T.; Warhurst, G. Increased susceptibility to Trichuris muris infection and exacerbation of colitis in Mdr1a-/- mice. World J. Gastroenterol. 2014, 20, 1797–1806. [Google Scholar] [CrossRef]
- Wilson, M.S.; Ramalingam, T.R.; Rivollier, A.; Shenderov, K.; Mentink–Kane, M.M.; Madala, S.K.; Cheever, A.W.; Artis, D.; Kelsall, B.L.; Wynn, T.A. Colitis and intestinal inflammation in IL10−/− mice results from IL-13Rα2–mediated attenuation of IL-13 activity. Gastroenterology 2011, 140, 254–264. [Google Scholar] [CrossRef]
- Buitrago, G.; Pickering, D.; Ruscher, R.; Cobos Caceres, C.; Jones, L.; Cooper, M.; Van Waardenberg, A.; Ryan, S.; Miles, K.; Field, M.; et al. A netrin domain-containing protein secreted by the human hookworm Necator americanus protects against CD4 T cell transfer colitis. Transl. Res. 2021, 232, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.M.; Eichenberger, R.M.; Ruscher, R.; Giacomin, P.R.; Loukas, A. Harnessing helminth-driven immunoregulation in the search for novel therapeutic modalities. PLoS Pathog. 2020, 16, e1008508. [Google Scholar] [CrossRef] [PubMed]
- Jamtsho, T.; Yeshi, K.; Perry, M.J.; Loukas, A.; Wangchuk, P. Approaches, Strategies and Procedures for Identifying Anti-Inflammatory Drug Lead Molecules from Natural Products. Pharmaceuticals 2024, 17, 283. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, A.; Ojeda-Montes, M.J.; Tomás-Hernández, S.; Cereto-Massagué, A.; Beltrán-Debón, R.; Mulero, M.; Pujadas, G.; Garcia-Vallvé, S. The light and dark sides of virtual screening: What is there to know? Int. J. Mol. Sci. 2019, 20, 1375. [Google Scholar] [CrossRef]
- Lin, X.; Li, X.; Lin, X. A review on applications of computational methods in drug screening and design. Molecules 2020, 25, 1375. [Google Scholar] [CrossRef]
- Johnson, T.O.; Odoh, K.D.; Nwonuma, C.O.; Akinsanmi, A.O.; Adegboyega, A.E. Biochemical evaluation and molecular docking assessment of the anti-inflammatory potential of Phyllanthus nivosus leaf against ulcerative colitis. Heliyon 2020, 6, e03893. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, T.O.; Akinsanmi, A.O.; Ejembi, S.A.; Adeyemi, O.E.; Oche, J.-R.; Johnson, G.I.; Adegboyega, A.E. Modern drug discovery for inflammatory bowel disease: The role of computational methods. World J. Gastroenterol. 2023, 29, 310–331. [Google Scholar] [CrossRef] [PubMed]
- Grienke, U.; Foster, P.A.; Zwirchmayr, J.; Tahir, A.; Rollinger, J.M.; Mikros, E. 1H NMR-MS-based heterocovariance as a drug discovery tool for fishing bioactive compounds out of a complex mixture of structural analogues. Sci. Rep. 2019, 9, 11113. [Google Scholar] [CrossRef] [PubMed]
- Berlinck, R.G.; Monteiro, A.F.; Bertonha, A.F.; Bernardi, D.I.; Gubiani, J.R.; Slivinski, J.; Michaliski, L.F.; Tonon, L.A.; Venancio, V.A.; Freire, V.F. Approaches for the isolation and identification of hydrophilic, light-sensitive, volatile and minor natural products. Nat. Prod. Rep. 2019, 36, 981–1004. [Google Scholar] [CrossRef]
- Jones, C.G.; Martynowycz, M.W.; Hattne, J.; Fulton, T.J.; Stoltz, B.M.; Rodriguez, J.A.; Nelson, H.M.; Gonen, T. The CryoEM method MicroED as a powerful tool for small molecule structure determination. ACS Cent. Sci. 2018, 4, 1587–1592. [Google Scholar] [CrossRef]
- Tu, P.-C.; Chan, C.-J.; Liu, Y.-C.; Kuo, Y.-H.; Lin, M.-K.; Lee, M.-S. Bioactivity-guided fractionation and NMR-based identification of the immunomodulatory isoflavone from the roots of Uraria crinita (L.) Desv. ex DC. Foods 2019, 8, 543. [Google Scholar] [CrossRef]
| Plant Species | Natural Therapeutics | Diseases | Objective | Clinical Phase | Clinical Trial Number |
|---|---|---|---|---|---|
| Plant-derived compounds/drugs | |||||
| C. sinensis L. | Epigallocatechin3-gallate (Polyphenon E®) | Mild to moderately active UC | Assess safety of an oral dose of green tea extract as initial evidence to substantiate its effectiveness in UC (oral administration of placebo tablet) | Phase II | NCTT00718094 |
| C. longa L. | Curcumin | Both UC and CD | Assess the tolerability of curcumin in pediatric patients with IBD (double-blinded placebo-controlled study) | Phase I | NCT00889161 |
| C. longa L. | Curcumin | UC | Assess effectiveness of a combined therapy involving curcumin and 5ASA compared to that of 5ASA alone in patients with mild to moderate UC (randomized, double-blind, placebo-controlled study) | Phase III | NCT01320436 |
| Coptis chinensis Franch | Berberine (berberine chloride) | UC | Examine the safety profile of berberine in individuals with UC who are in clinical remission and are simultaneously undergoing maintenance therapy with mesalamine (oral administration of placebo) | Phase I | NCT02365480 |
| Tripterygium wilfordii Hook. F. | Triptolide (Tripterygium glycosides) | CD | Evaluate the efficacy of combining Tripterygium glycosides with enteral nutrition in inducing remission in patients with Crohn’s disease, comparing outcomes with those receiving either Tripterygium glycosides alone or enteral nutrition alone | Not applicable | NCT01820247 |
| Helminths products/drugs | |||||
| Trichuris suis | Trichuris suis ova (CNDO 201) | CD | Evaluate the safety and tolerability of single oral doses of CNDO 201 (double-blind, placebo-controlled) | Phase I | NCT01434693 |
| Trichuris suis | Trichuris suis ova (CNDO 201) | UC | Investigate the immune response activated in the human gastrointestinal tract (double-blind, placebo-controlled oral inoculation) | Phase I and II | NCT01433471 |
| Trichuris suis | Trichuris suis ova (TSO) | CD | Compare the efficacy of three doses of oral TSO suspension versus a placebo in inducing remission | Phase II | NCT01279577 |
| Schistosoma mansoni | P28GST (protein 28 Kd glutathion S Transferase) | CD | Assess the effectiveness in regulating immunologic and inflammatory markers in blood and tissue, as well as determining the occurrence of clinical recurrence using the disease activity index | Phase II | NCT02281916 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamtsho, T.; Loukas, A.; Wangchuk, P. Pharmaceutical Potential of Remedial Plants and Helminths for Treating Inflammatory Bowel Disease. Pharmaceuticals 2024, 17, 819. https://doi.org/10.3390/ph17070819
Jamtsho T, Loukas A, Wangchuk P. Pharmaceutical Potential of Remedial Plants and Helminths for Treating Inflammatory Bowel Disease. Pharmaceuticals. 2024; 17(7):819. https://doi.org/10.3390/ph17070819
Chicago/Turabian StyleJamtsho, Tenzin, Alex Loukas, and Phurpa Wangchuk. 2024. "Pharmaceutical Potential of Remedial Plants and Helminths for Treating Inflammatory Bowel Disease" Pharmaceuticals 17, no. 7: 819. https://doi.org/10.3390/ph17070819
APA StyleJamtsho, T., Loukas, A., & Wangchuk, P. (2024). Pharmaceutical Potential of Remedial Plants and Helminths for Treating Inflammatory Bowel Disease. Pharmaceuticals, 17(7), 819. https://doi.org/10.3390/ph17070819








