Silkworm Cocoon: Dual Functions as a Traditional Chinese Medicine and the Raw Material of Promising Biocompatible Carriers
Abstract
1. Introduction
2. Chemical Composition
2.1. Silk Fibroin
2.2. Silk Sericin
2.3. Flavonoids
2.4. Other Components
3. Biological Activities
3.1. Hypoglycemic Action
3.2. Cardioprotective Effect
3.3. Hypolipidemic Activity
3.4. Anti-Inflammatory Effect
3.5. Antioxidant Effect
3.6. Antiviral and Antimicrobial Effects
| Biological Activities | Sample | Animal | Model | Dose | Results | Mechanisms | Refs. |
|---|---|---|---|---|---|---|---|
| hypoglycemic effect | fibroin, sericin | silkworm /in vivo | diets containing glucose or sucrose | 5% added to the diets | exhibited postprandial antihyperglycemic activity | inhibition of the expression of intestinal glucose transporters, promotion of the regeneration of pancreatic β cells, or activation of the insulin-like signaling pathway | [42] |
| ethanolic extract from the green cocoon sericin layer of silkworm | ICR mice /in vivo | fed with a high-fat diet and injected with streptozotocin | 150, 250, and 350 mg/kg | ameliorated glucose metabolism and regulated the balance between glycolysis and gluconeogenesis | reduction of the levels of NF-κB, IL-6, and TNF-α; enhancement of the expression levels of IR, IRS, PI3K, p-Akt, and p-GSK3β involved in insulin signalling; activation of AMPK and GLUT4; reduction of the levels of G6pase and PEPCK; improvement of the GK level | [43] | |
| flavonoid-rich ethanolic extract from silkworm green cocoon | ICR mice /in vivo | induced by high-fat and streptozotocin | 150, 250, and 350 mg/kg | regulated the glucose level and body weight and improved renal dysfunction | inhibition of the TNF-α-p38 MAP kinase signaling pathway | [39] | |
| hydroalcoholic extract of silk cocoon | Wistar rats /in vivo | induced by streptozotocin | 200, 400, and 800 mg/kg | decreased prolactin and inhibin; increased leptin, IGF-2, activin A, insulin, LH, testosterone, FSH, and GnRH levels; improved gonadal weight, the diameter of tunica albuginea, and seminiferous tubules as well as increased the numbers of spermatocytes and Sertoli–Leydig cells | NA | [44] | |
| cardioprotective effect | sericin | Wistar rats /in vivo | isoproterenol induced cardiac toxicity and hypertrophy | 500 and 1000 mg/kg | significantly increased the non-enzymatic antioxidant markers in serum and heart tissue; significantly decreased the myocyte size | prevented the myocardial tissue from enzymatic leakage from the cell sites; reduced the synthesis of collagen in myocardiocytes, thus reducing the incidence of fibrosis; reduction of fibrosis and synthesis of collagens contributed to the protective effect against hypertrophy; decreased inflammatory reactions and oxidative stress, which led to improved myocardial activity, and reduced cardiac damage after myocardial ischemia | [9] |
| sericin | Wistar rats /in vivo | cholesterol diet-induced hypercholesterolaemia model | 1000 mg/kg | improved cardiac muscle contraction under hypercholesterolaemia, restored the cardiac mitochondrial structure, increased mitochondrial fusion in the heart, and inhibited the progression of apoptosis at the last stage of dysmorphic mitochondria | upregulation of OPA1 and reduction of NADH-ubiquin-one oxidoreductase 75 kDa subunit expression; improvement of mitochondrial energy production by upregulating acetyl-CoA acetyltransferase and NADH dehydrogenase 1a subcomplex subunit 10 expression | [48] | |
| ethanolic extract of silk cocoons | Albino Wistar rats /in vivo | isoprenaline-induced myocardial infarction | 250 and 500 mg/kg | significantly prevented myocardial damage and hypertrophy, and decreased the levels of various cardiac enzymes | NA | [8] | |
| an emulsion formulation composed of methanol extract of silk cocoons, flaxseed oil, and coenzyme Q10 | Sprague Dawley rats /in vivo | doxorubicin induced myocardial toxicity | 500 mg/kg methanol extract of silk cocoons, 1.8 mL/kg flaxseed oil, and 5 mg/kg coenzyme Q10 | significantly prevented the increase in serum levels of AST, ALT, LDH, and creatinine and the lipid profile, increased the levels of HDL, SOD, GSH, and CAT in heart tissue, and lowered the increase in heart weight due to hypertrophy | may be mainly due to the high protein content of sericin, flavonoids, and n-3 fatty acids that have potential free radical scavenging and antioxidant activities (the author speculated; needs to be experimentally confirmed) | [49] | |
| hypolipidemic effect | 1% NaCl solution extract of silk cocoons | New Zealand white rabbits /in vivo | cholesterol powder mixed with coconut oil | 50 mg/ 100 g | reduced the levels of total cholesterol, triglycerides, and low-density lipoprotein, as well as the size of atherosclerotic plaque in the aorta; increased the high-density lipoprotein level and body weight | probably inhibited the second step of lipid implantation in the injured arterial wall by its lipid-lowering and antioxidant properties (the author speculated; needs to be experimentally confirmed) | [51] |
| sericin | C57BL/6 mice /in vivo | fed with fat-rich diets | 1000 mg/kg | increased lipid excretion in feces and restored intestinal wall morphometry in obese mice | NA | [11] | |
| anti-inflammatory effect | silkworm cocoon-derived carbon dots | C57 black mice and Kunming mice /in vivo | (1) dimethylbenzene-induced ear oedema; (2) vascular permeability induced by acetic acid; (3) lipopolysaccharide- induced sepsis model | 0.35, 0.7, and 1.4 mg/kg | significantly lowered the percentage inflammation at the doses of 0.7 and 1.4 mg/kg, and the plasma extravasation of the test groups was similar to that of the dexamethasone group | inhibition of the expressions of IL-6 and TNF-α | [3] |
| sericin | Sprague–Dawley rats /in vivo | imiquimod-induced skin psoriasis | 2.5, 5, and 10% sericin cream applied topically | 10% sericin had the desired effect of improving skin psoriasis, similar to that of betamethasone and calcitriol treatments | reduction in cytokine production of Th17 cells by interfering with the JAK-STAT signaling pathway; modulation of immune response via upregulation of galectin-3 and downregulation of sphingosine-1-phosphate lyase1 | [12] | |
| antioxidant effect | ethanolic extract of the green cocoons | in vitro | DPPH and ABTS assay | in DPPH test: IC50 = 296.95 ± 13.24 μg/mL; in ABTS test: IC50 = 94.31 ± 9.13 μg/mL | showed excellent antioxidation | NA | [57] |
| diazo cocoon extracts | in vitro | DPPH and ABTS assay | NA | exhibited high antioxidant activities | NA | [58] | |
| silk sericin and associated secondary metabolites (polyphenols and flavonoids) | in vitro | human dermal fibroblast cells | NA | the human dermal fibroblast cells treated with silk sericin exposed to UVA1 showed a significant increase in total collagen content | upregulates the expression of MMP-1 in human dermal fibroblast cells along with MMP-3, resulting in the degradation of collagen, and leads to the loss of the structural integrity of the skin | [59] | |
| antiviral and antimicrobial effect | 95% ethanol extract of silk cocoon | in vitro | HSV-1 and HSV-2 | NA | the inactivation of HSV-1 and HSV-2 | drastically reduced HSV-induced cell death and prevented inflammation by reducing the production of inflammatory cytokine genes | [21] |
| silkworm cocoon | in vitro | three different species of fungi: Candida albicans, Beauveria bassiana, and Saccharomyces cerevisiae | NA | strongly suppressed the sporular growth of the three fungal species | BmSPI51 attaches to mannan and β-D-glucan on the surface of fungal cells, thus inhibiting fungal growth | [61] |
3.7. Other Effects
4. Practical Applications from Laboratories to Clinics and Markets
4.1. Laboratory Investigations
4.1.1. Application in Drug Delivery Systems
4.1.2. Application in Tissue Engineering
4.1.3. Application in Regenerative Medicine
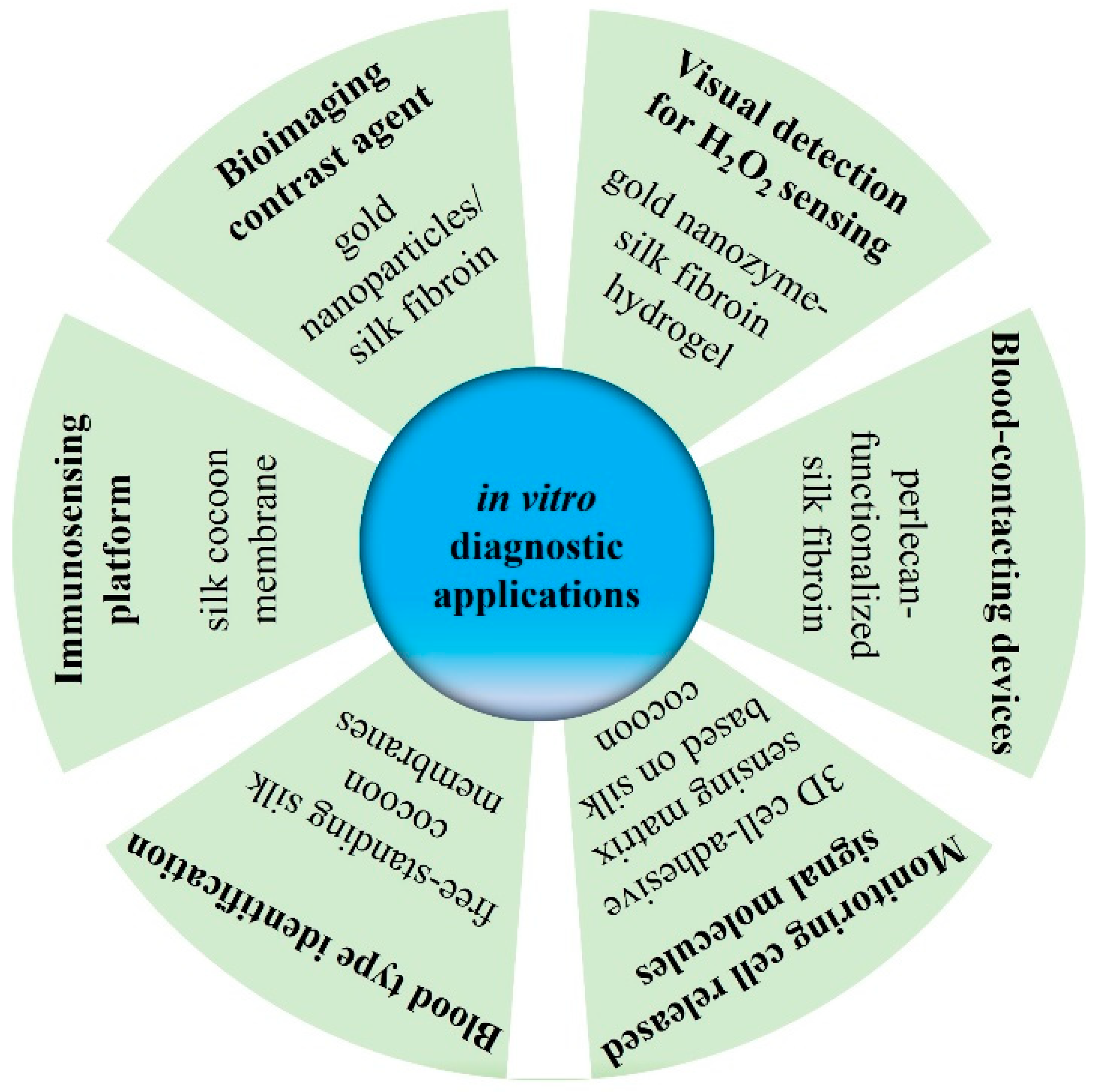
4.1.4. Application in In Vitro Diagnosis
4.2. Clinical Studies
4.3. Products on the Market
5. Safety
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zhang, L.; Zhang, L.; Li, Y.; Guo, X.-F.; Liu, X.-S. Biotransformation effect of Bombyx mori L. may play an important role in treating diabetic nephropathy. Chin. J. Integr. Med. 2016, 22, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.; Zhang, J.; Zhou, L.; Zhang, Y.; Gu, X. Clinical observation of silkworm cocoon shell decoction combined with salmeterol in the treatment of bronchial asthma. J. Clin. Pulm. Med. 2018, 23, 404–408. [Google Scholar]
- Wang, X.; Zhang, Y.; Kong, H.; Cheng, J.; Zhang, M.; Sun, Z.; Wang, S.; Liu, J.; Qu, H.; Zhao, Y. Novel mulberry silkworm cocoon-derived carbon dots and their anti-inflammatory properties. Artif. Cells Nanomed. Biotechnol. 2020, 48, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Xia, Q.; Zhao, P. Antimicrobial components in the cocoon silk of silkworm, Bombyx mori. Int. J. Biol. Macromol. 2023, 224, 68–78. [Google Scholar] [CrossRef] [PubMed]
- DeBari, M.K.; King, C.I., III; Altgold, T.A.; Abbott, R.D. Silk fibroin as a green material. ACS Biomater. Sci. Eng. 2021, 7, 3530–3544. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Cao, G.; Renyu, X.; Zhonghua, P.; Xiaojian, Z.; Zhou, W.; Gong, C. Reducing blood glucose level in TIDM mice by orally administering the silk glands of transgenic hIGF-I silkworms. Biochem. Biophys. Res. Commun. 2011, 410, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Choi, J.W.; Ra, K.S.; Kim, J.-M.; Suh, H.J. Novel tripeptides with α-glucosidase inhibitory activity isolated from silk cocoon hydrolysate. J. Agric. Food Chem. 2011, 59, 11522–11525. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, R.K.; Siddiqui, H.H.; Mahmood, T.; Ahsan, F. Evaluation of cardioprotective effect of silk cocoon (Abresham) on isoprenaline-induced myocardial infarction in rats. Avicenna J. Phytomed. 2013, 3, 216–223. [Google Scholar] [PubMed]
- Ahsan, F.; Mahmood, T.; Wani, T.A.; Zargar, S.; Siddiqui, M.H.; Usmani, S.; Shamim, A.; Wahajuddin, M. Effectual Endeavors of silk protein sericin against isoproterenol induced cardiac toxicity and hypertrophy in wistar rats. Life 2022, 12, 1063. [Google Scholar] [CrossRef]
- Shariq, M.; Mahmood, T.; Kushwaha, P.; Parveen, S.; Shamim, A.; Ahsan, F.; Wani, T.A.; Zargar, S.; Wasim, R.; Wahajuddin, M. Fabrication of nanoformulation containing carvedilol and silk protein sericin against doxorubicin induced cardiac damage in rats. Pharmaceuticals 2023, 16, 561. [Google Scholar] [CrossRef]
- Kunz, R.I.; Capelassi, A.N.; Alegre-Maller, A.C.P.; Bonfleur, M.L.; Ribeiro, L.d.F.C.; Costa, R.M.; Natali, M.R.M. Sericin as treatment of obesity: Morphophysiological effects in obese mice fed with high-fat diet. Einstein (Sao Paulo) 2020, 18, eAO4876. [Google Scholar] [CrossRef] [PubMed]
- Rujimongkon, K.; Ampawong, S.; Reamtong, O.; Buaban, T.; Aramwit, P. The therapeutic effects of Bombyx mori sericin on rat skin psoriasis through modulated epidermal immunity and attenuated cell proliferation. J. Tradit. Complement. Med. 2021, 11, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Ritprajak, P.; Sirithanakorn, C.; NY Nguyen, T.N.; Sereemaspun, A.; Aramwit, P. Biosynthetic sericin 1-like protein skews dendritic cells to tolerogenic-like phenotype. Biotechnol. Appl. Biochem. 2021, 68, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Tuentam, K.; Aramwit, P.; Reamtong, O.; Supasai, S.; Chaisri, U.; Fongsodsri, K.; Yamdech, R.; Tirawanchai, N.; Sukphopetch, P.; Ampawong, S. Sericin-based poly(Vinyl) alcohol relieves plaque and epidermal pesions in Psoriasis; a chance for dressing development in a specific area. Int. J. Mol. Sci. 2022, 24, 145. [Google Scholar] [CrossRef]
- Seyedaghamiri, F.; Farajdokht, F.; Vatandoust, S.M.; Mahmoudi, J.; Khabbaz, A.; Sadigh-Eteghad, S. Sericin modulates learning and memory behaviors by tuning of antioxidant, inflammatory, and apoptotic markers in the hippocampus of aged mice. Mol. Biol. Rep. 2021, 48, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.; Acharya, C.; Bindu, P.C.; Kundu, S.C. Antioxidant potential of silk protein sericin against hydrogen peroxide-induced oxidative stress in skin fibroblasts. BMB Rep. 2008, 41, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, R.Y.; Zhong, D.B.; Zhao, P.; Xia, Q.Y. Highly efficient expression of human extracellular superoxide dismutase (rhEcSOD) with ultraviolet-B-induced damage-resistance activity in transgenic silkworm cocoons. Insect Sci. 2023, 30, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, S.; Ali, S.; Pervaiz, A.; Awan, U.A.; Nauroze, T.; Kanwal, L.; Summer, M.; Mumtaz, S.; Mughal, T.A.; Tahir, H.M. Toxicological effects of dimethlybenzeneanthracene in Balb C mice and pharmacological intervention by silk sericin–conjugated silver nanoparticles. Sci. Prog. 2024, 107, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, M.; Dong, Z.; Zhao, D.; An, L.; Zhu, H.; Xia, Q.; Zhao, P. Synthesis, secretion, and antifungal mechanism of a phosphatidylethanolamine-binding protein from the silk gland of the silkworm Bombyx mori. Int. J. Biol. Macromol. 2020, 149, 1000–1007. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, C.; Zhu, Y.; Liu, W.; Li, H.; Wang, L.; Chen, W.; Wang, Z.; Wang, L. Lamprey-teeth-inspired oriented antibacterial sericin microneedles for infected wound healing improvement. Nano Lett. 2022, 22, 2702–2711. [Google Scholar] [CrossRef]
- Jantakee, K.; Prangkio, P.; Panya, A.; Tragoolpua, Y. Anti-herpes simplex virus efficacy of silk cocoon, silkworm pupa and non-sericin extracts. Antibiotics 2021, 10, 1553. [Google Scholar] [CrossRef]
- Tan, H.; Ji, Y.; Lei, H.; Wang, F.; Dong, H.; Yang, S.; Zhou, H.; Deng, H.; Chen, S.; Kaplan, D.L.; et al. Large-scale and cost-effective production of recombinant human serum albumin (rHSA) in transgenic Bombyx mori cocoons. Int. J. Biol. Macromol. 2023, 245, 125527. [Google Scholar] [CrossRef] [PubMed]
- Lian, A.A.; Yamaji, Y.; Kajiwara, K.; Takaki, K.; Mori, H.; Liew, M.W.O.; Kotani, E.; Maruta, R. A bioengineering approach for the development of fibroblast growth factor-7-functionalized sericin biomaterial applicable for the cultivation of keratinocytes. Int. J. Mol. Sci. 2022, 23, 9953. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xiang, P.; Yu, B.; Sun, Z.; Li, X.; Qv, A.; Sohail, M.; Li, Y. Research progress of novel drug delivery systems of Chinese medicine monomers based on natural silk fibroin: A mini-review. Curr. Drug Deliv. 2023, 20, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yang, Q.; Wang, R.; Tian, C.; Ji, Y.; Tan, H.; Zhao, P.; Kaplan, D.L.; Wang, F.; Xia, Q. Genetically engineered pH-responsive silk sericin nanospheres with efficient therapeutic effect on ulcerative colitis. Acta Biomater. 2022, 144, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Tan, H.; Yang, Q.; Wang, R.; Tian, C.; Ji, Y.; Zhao, P.; Xia, Q.; Wang, F. Fabrication of a silk sericin hydrogel system delivering human lactoferrin using genetically engineered silk with improved bioavailability to alleviate chemotherapy-induced immunosuppression. ACS Appl. Mater. Interfaces 2021, 13, 45175–45190. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, L.; Deng, Y.; Zou, M.; Cai, B.; Song, Y.; Wang, Z.; Wang, L. Silk sericin-based materials for biomedical applications. Biomaterials 2022, 287, 121638. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.S.; Costa, E.C.; Reis, S.; Spencer, C.; Calhelha, R.C.; Miguel, S.P.; Ribeiro, M.P.; Barros, L.; Vaz, J.A.; Coutinho, P. Silk sericin: A promising sustainable biomaterial for biomedical and pharmaceutical applications. Polymers 2022, 14, 4931. [Google Scholar] [CrossRef] [PubMed]
- Luetchford, K.A.; Chaudhuri, J.B.; De Bank, P.A. Silk fibroin/gelatin microcarriers as scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater Biol Appl 2020, 106, 110116. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Shi, X.; Qin, S.; Liu, J.; Lv, Q.; Liu, J.; Li, Q.; Wang, Z.; Wang, L. Development and application of an advanced biomedical material-silk sericin. Adv. Mater. 2024, 36, e2311593. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Nooeaid, P.; Thanyacharoen, T.; Techasakul, S.; Pavasant, P.; Kanjanamekanant, K. Injectable eggshell-derived hydroxyapatite-incorporated fibroin-alginate composite hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2021, 193, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Santi, S.; Mancini, I.; Dirè, S.; Callone, E.; Speranza, G.; Pugno, N.; Migliaresi, C.; Motta, A. A bio-inspired multifunctionalized silk fibroin. ACS Biomater. Sci. Eng. 2021, 7, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Yazawa, K.; Tsuchiya, K.; Numata, K.; Guan, J. Molecular Interactions and toughening mechanisms in silk fibroin-epoxy resin blend films. Biomacromolecules 2019, 20, 2295–2304. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Li, M.; Wang, T.; Sun, F.; Su, C.; Shi, T. Viscoelastic silk fibroin hydrogels with tunable strength. ACS Biomater. Sci. Eng. 2021, 7, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Baptista-Silva, S.; Borges, S.; Costa-Pinto, A.R.; Costa, R.; Amorim, M.; Dias, J.R.; Ramos, Ó.; Alves, P.; Granja, P.L.; Soares, R.; et al. In situ forming silk sericin-based hydrogel: A novel wound healing biomaterial. ACS Biomater. Sci. Eng. 2021, 7, 1573–1586. [Google Scholar] [CrossRef] [PubMed]
- Manesa, K.C.; Kebede, T.G.; Dube, S.; Nindi, M.M. Fabrication and characterization of sericin-PVA composite films from Gonometa postica, Gonometa rufobrunnea, and Argema mimosae: Potentially applicable in biomaterials. ACS Omega 2022, 7, 19328–19336. [Google Scholar] [CrossRef] [PubMed]
- Kurioka, A.; Yamazaki, M. Purification and identification of flavonoids from the yellow green cocoon shell (Sasamayu) of the silkworm, Bombyx mori. Biosci. Biotechnol. Biochem. 2002, 66, 1396–1399. [Google Scholar] [CrossRef] [PubMed]
- Bungthong, C.; Siriamornpun, S. Changes in amino acid profiles and bioactive compounds of Thai silk cocoons as affected by water extraction. Molecules 2021, 26, 2033. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Zhao, J.-G.; Wei, Z.-G.; Zhang, Y.-Q. The renal protection of flavonoid-rich ethanolic extract from silkworm green cocoon involves in inhibiting TNF-α-p38 MAP kinase signalling pathway in type 2 diabetic mice. Biomed. Pharmacother. 2019, 118, 109379. [Google Scholar] [CrossRef]
- Kaur, J.; Rajkhowa, R.; Tsuzuki, T.; Wang, X. Crystals in Antheraea assamensis silkworm cocoon: Their removal, recovery and roles. Mater. Des. 2015, 88, 236–244. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, P.; Wang, Q.; Zhang, Y.; Xu, H.; Zhao, P. Overexpression of Gloverin2 in the Bombyx mori silk gland enhances cocoon/silk antimicrobial activity. Dev. Comp. Immunol. 2019, 98, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Aznar-Cervantes, S.D.; Monteagudo Santesteban, B.; Cenis, J.L. Products of sericulture and their hypoglycemic action evaluated by using the silkworm, Bombyx mori (Lepidoptera: Bombycidae), as a model. Insects 2021, 12, 1059. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-G.; Wang, H.-Y.; Wei, Z.-G.; Zhang, Y.-Q. Therapeutic effects of ethanolic extract from the green cocoon shell of silkworm Bombyx mori on type 2 diabetic mice and its hypoglycaemic mechanism. Toxicol. Res. 2019, 8, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Jangir, R.N.; Jain, G.C. Diabetes mellitus induced impairment of male reproductive functions: A review. Curr. Diabetes Rev. 2014, 10, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Ahi, S.; Ebrahimi, F.; Abedi, H.A.; Kargar Jahromi, H.; Zarei, S. The effects of hydroalcoholic extract of silk cocoon on hypothalamic-pituitary–gonadal axis in streptozotocin-induced diabetic male rats. Autoimmune Dis. 2022, 2022, 7916159. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, F.; Ansari, T.M.; Usmani, S.; Bagga, P. An insight on silk protein sericin: From processing to biomedical application. Drug Res. 2018, 68, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Investig. 2018, 128, 3716–3726. [Google Scholar] [CrossRef] [PubMed]
- Rujimongkon, K.; Ampawong, S.; Isarangkul, D.; Reamtong, O.; Aramwit, P. Sericin-mediated improvement of dysmorphic cardiac mitochondria from hypercholesterolaemia is associated with maintaining mitochondrial dynamics, energy production, and mitochondrial structure. Pharm. Biol. 2022, 60, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Tarique, M.; Badruddeen; Ahsan, F.; Akhtar, J.; Khan, M.I.; Khalid, M. Formulation development and pharmacological evaluation of fixed dose combination of Bombyx mori coccon shell extract, Flaxseed oil and coenzyme Q10 against doxorubicin induced cardiomyopathy in rats. Orient. Pharm. Exp. Med. 2019, 19, 469–483. [Google Scholar] [CrossRef]
- Nasiruddin, M.; Ahmad, N.; Tajuddin. Effect of an unani formulation on lipid profile in rat. Indian J. Pharmacol. 2006, 38, 56–57. [Google Scholar] [CrossRef]
- Ali, M.M.; Arumugam, S.B. Effect of crude extract of Bombyx mori coccoons in hyperlipidemia and atherosclerosis. J. Ayurveda Integr. Med. 2011, 2, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Kakehi, S.; Xu, Y.; Tsujimoto, K.; Sasaki, M.; Ogawa, H.; Kato, N. Consumption of sericin reduces serum lipids, ameliorates glucose tolerance and elevates serum adiponectin in rats fed a high-fat diet. Biosci. Biotechnol. Biochem. 2010, 74, 1534–1538. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.; Beraldi, E.J.; Ferreira, P.E.B.; Bazotte, R.B.; Buttow, N.C. Intestinal and neuronal myenteric adaptations in the small intestine induced by a high-fat diet in mice. BMC Gastroenterol. 2015, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Pei, M.; Zhao, X.; Tian, K.; Zhou, T.; Liu, P. PEGylated oxidized alginate-DOX prodrug conjugate nanoparticles cross-linked with fluorescent carbon dots for tumor theranostics. ACS Biomater. Sci. Eng. 2016, 2, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Ogura, H.; Shimizu, K.; Ikeda, M.; Hirose, T.; Matsuura, H.; Kang, S.; Takahashi, K.; Tanaka, T.; Shimazu, T. The clinical importance of a cytokine network in the acute phase of sepsis. Sci. Rep. 2018, 8, 13995. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Towiwat, P.; Srichana, T. Anti-inflammatory potential of silk sericin. Nat. Prod. Commun. 2013, 8, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Zhao, J.-G.; Zhang, Y.-Q. The flavonoid-rich ethanolic extract from the green cocoon shell of silkworm has excellent antioxidation, glucosidase inhibition, and cell protective effects in vitro. Food Nutr. Res. 2020, 64, 1637. [Google Scholar] [CrossRef]
- Tsvetkova, M.; Hristova-Avakumova, N.; Atanasova, L.; Panayotov, M.; Hadjimitova, V. Effect of extraction conditions on the antioxidant activity of diazo cocoon extracts effect of extraction conditions on the antioxidant activity of diazo cocoon extracts. In Proceedings of the 10th Jubilee International Conference of the Balkan Physical Union, Sofia, Bulgaria, 26–30 August 2018. [Google Scholar]
- Kumar, J.P.; Mandal, B.B. Inhibitory role of silk cocoon extract against elastase, hyaluronidase and UV radiation-induced matrix metalloproteinase expression in human dermal fibroblasts and keratinocytes. Photochem. Photobiol. Sci. 2019, 18, 1259–1274. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, P.; Liu, H.; Guo, X.; He, H.; Zhu, R.; Xiang, Z.; Xia, Q. TIL-type protease inhibitors may be used as targeted resistance factors to enhance silkworm defenses against invasive fungi. Insect Biochem. Mol. Biol. 2015, 57, 11–19. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, K.; Dong, Z.; Chen, Z.; Zhu, H.; Zhang, Y.; Xia, Q.; Zhao, P. Kunitz-type protease inhibitor BmSPI51 plays an antifungal role in the silkworm cocoon. Insect Biochem. Mol. Biol. 2020, 116, 103258. [Google Scholar] [CrossRef]
- Kaewkorn, W.; Limpeanchob, N.; Tiyaboonchai, W.; Pongcharoen, S.; Sutheerawattananonda, M. Effects of silk sericin on the proliferation and apoptosis of colon cancer cells. Biol. Res. 2012, 45, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Micheli, L.; Parisio, C.; Lucarini, E.; Carrino, D.; Ciampi, C.; Toti, A.; Ferrara, V.; Pacini, A.; Ghelardini, C.; Di Cesare Mannelli, L. Restorative and pain-relieving effects of fibroin in preclinical models of tendinopathy. Biomed. Pharmacother. 2022, 148, 112693. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, M.; Zhu, L.; Zhu, Y. Honeysuckle flowers extract loaded Bombyx mori silk fibroin films for inducing apoptosis of HeLa cells. Microsc. Res. Tech. 2017, 80, 1297–1303. [Google Scholar] [CrossRef]
- Lin, M.-J.; Lu, M.-C.; Chang, H.-Y. Sustained Release of Insulin-Like Growth Factor-1 from Bombyx mori L. Silk fibroin delivery for diabetic wound therapy. Int. J. Mol. Sci. 2021, 22, 6267. [Google Scholar] [CrossRef]
- Montalbán, M.G.; Coburn, J.M.; Lozano-Pérez, A.A.; Cenis, J.L.; Víllora, G.; Kaplan, D.L. Production of curcumin-loaded silk fibroin nanoparticles for cancer therapy. Nanomaterials 2018, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Holanda, F.H.; Ribeiro, A.N.; Sánchez-Ortiz, B.L.; de Souza, G.C.; Borges, S.F.; Ferreira, A.M.; Florentino, A.C.; Yoshioka, S.A.; Moraes, L.S.; Carvalho, J.C.T.; et al. Anti-inflammatory potential of baicalein combined with silk fibroin protein in a zebrafish model (Danio rerio). Biotechnol. Lett. 2023, 45, 235–253. [Google Scholar] [CrossRef]
- Liu, J.; Sun, H.; Peng, Y.; Chen, L.; Xu, W.; Shao, R. Preparation and Characterization of natural silk fibroin hydrogel for protein drug delivery. Molecules 2022, 27, 3418. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, M.; Zhao, L.; Kuang, D.; Kundu, S.C.; Lu, S. Insulin-loaded silk fibroin microneedles as sustained release system. ACS Biomater. Sci. Eng. 2019, 5, 1887–1894. [Google Scholar] [CrossRef]
- Stinson, J.A.; Raja, W.K.; Lee, S.; Kim, H.B.; Diwan, I.; Tutunjian, S.; Panilaitis, B.; Omenetto, F.G.; Tzipori, S.; Kaplan, D.L. Silk fibroin microneedles for transdermal vaccine delivery. ACS Biomater. Sci. Eng. 2017, 3, 360–369. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Z.; Jiang, J.; Shi, Z.; Mao, Y.; Qin, N.; Tao, T.H. Silk microneedle patch capable of on-demand multidrug delivery to the brain for glioblastoma treatment. Adv. Mater. 2022, 34, 2106606. [Google Scholar] [CrossRef]
- Yin, Z.; Kuang, D.; Wang, S.; Zheng, Z.; Yadavalli, V.K.; Lu, S. Swellable silk fibroin microneedles for transdermal drug delivery. Int. J. Biol. Macromol. 2018, 106, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Lujerdean, C.; Baci, G.-M.; Cucu, A.-A.; Dezmirean, D.S. The contribution of silk fibroin in biomedical engineering. Insects 2022, 13, 286. [Google Scholar] [CrossRef] [PubMed]
- Bucciarelli, A.; Motta, A. Use of Bombyx mori silk fibroin in tissue engineering: From cocoons to medical devices, challenges, and future perspectives. Mater. Sci. Eng. C 2022, 139, 212982. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Bi, X.; Ye, F.; Shu, X.; Sun, L.; Guan, J.; Ritchie, R.O.; Wu, S. Controlled Cryogelation and catalytic cross-linking yields highly elastic and robust silk fibroin scaffolds. ACS Biomater. Sci. Eng. 2020, 6, 4512–4522. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Rasheed, S.; Yougen, C. Silk fibroin/hydroxyapatite scaffold: A highly compatible material for bone regeneration. Sci. Technol. Adv. Mater. 2020, 21, 242–266. [Google Scholar] [CrossRef] [PubMed]
- Mejia, M.L.; Moncada, M.E.; Ossa-Orozco, C.P. Poly (vinyl alcohol)/Silk Fibroin/Ag NPs composite nanofibers for bone tissue engineering. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Guadalajara, Mexico, 1–5 November 2021; pp. 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Ran, J.; Yan, P.; Zheng, L.; Shen, X.; Tong, H. Rational design of a high-strength bone scaffold platform based on in situ hybridization of bacterial cellulose/nano-hydroxyapatite framework and silk fibroin reinforcing phase. J. Biomater. Sci. Polym. Ed. 2018, 29, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Asadpour, S.; Kargozar, S.; Moradi, L.; Ai, A.; Nosrati, H.; Ai, J. Natural biomacromolecule based composite scaffolds from silk fibroin, gelatin and chitosan toward tissue engineering applications. Int. J. Biol. Macromol. 2020, 154, 1285–1294. [Google Scholar] [CrossRef]
- Bon, S.B.; Chiesa, I.; Degli Esposti, M.; Morselli, D.; Fabbri, P.; De Maria, C.; Morabito, A.; Coletta, R.; Calamai, M.; Pavone, F.S.; et al. Carbon nanotubes/regenerated silk composite as a three-dimensional printable bio-adhesive ink with self-powering properties. ACS Appl. Mater. Interfaces 2021, 13, 21007–21017. [Google Scholar] [CrossRef]
- Chakraborty, J.; Mu, X.; Pramanick, A.; Kaplan, D.L.; Ghosh, S. Recent advances in bioprinting using silk protein-based bioinks. Biomaterials 2022, 287, 121672. [Google Scholar] [CrossRef]
- Mu, X.; Gonzalez-Obeso, C.; Xia, Z.; Sahoo, J.K.; Li, G.; Cebe, P.; Zhang, Y.S.; Kaplan, D.L. 3D printing of monolithic proteinaceous cantilevers using regenerated silk fibroin. Molecules 2022, 27, 2148. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Fraile, H.; Amoros, S.; Mendizabal, I.; Galvez-Monton, C.; Prat-Vidal, C.; Bayes-Genis, A.; Navajas, D.; Farre, R.; Otero, J. Silk-reinforced collagen hydrogels with raised multiscale stiffness for mesenchymal cells 3D culture. Tissue Eng. Part A 2020, 26, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Yan, L.; Liu, S.; Tan, Y.; Xiao, J.; Cao, Y.; Chen, K.; Xiao, W.; Li, B.; Liao, X. Preparation of silk fibroin/hyaluronic acid hydrogels with enhanced mechanical performance by a combination of physical and enzymatic crosslinking. J. Biomater. Sci. Polym. Ed. 2021, 32, 1635–1653. [Google Scholar] [CrossRef] [PubMed]
- Khosropanah, M.H.; Vaghasloo, M.A.; Shakibaei, M.; Mueller, A.L.; Kajbafzadeh, A.M.; Amani, L.; Haririan, I.; Azimzadeh, A.; Hassannejad, Z.; Zolbin, M.M. Biomedical applications of silkworm (Bombyx mori) proteins in regenerative medicine (a narrative review). J. Tissue Eng. Regen. Med. 2022, 16, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Manjubala, I.; Akila, R.; Priya, G.; Anitha, R.; Madhan, B.; Narendrakumar, U. Fabrication of biocomposite sheets from silk cocoons for tissue engineering applications. Int. J. Nanotechnol. 2021, 18, 561–571. [Google Scholar] [CrossRef]
- Lam, Y.T.; Tan, R.P.; Michael, P.L.; Lau, K.; Yang, N.; Rnjak-Kovacina, J.; Wise, S.G. Bioengineering silk into blood vessels. Biochem. Soc. Trans. 2021, 49, 2271–2286. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Lorentz, K.L.; Haskett, D.G.; Cunnane, E.M.; Ramaswamy, A.K.; Weinbaum, J.S.; Vorp, D.A.; Mandal, B.B. Bioresorbable silk grafts for small diameter vascular tissue engineering applications: In vitro and in vivo functional analysis. Acta Biomater. 2020, 105, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Balcão, V.M.; Harada, L.K.; Jorge, L.R., Jr.; Oliveira, J.M.; Tubino, M.; Vila, M.M.D.C. Structural and functional stabilization of sericin from Bombyx mori cocoons in a biopolysaccharide film: Bioorigami for skin regeneration. J. Braz. Chem. Soc. 2020, 31, 833–848. [Google Scholar] [CrossRef]
- Yang, C.; Chen, S.; Su, H.; Zhang, H.; Tang, J.; Guo, C.; Song, F.; Zhang, W.; Gu, J.; Liu, Q. Biocompatible, small-sized and well-dispersed gold nanoparticles regulated by silk fibroin fiber from Bombyx mori cocoons. Front. Mater. Sci. 2019, 13, 126–132. [Google Scholar] [CrossRef]
- Hu, F.X.; Xie, X.; Wang, D.; Bin Yang, H.; Gu, Y.; Chen, B.; Zhang, C.; Rao, Q.; Li, Q.; Guo, C. Three-dimensional cell-adhesive matrix of silk cocoon derived carbon fiber assembled with iron-porphyrin for monitoring cell released signal molecules. Sens. Actuators B Chem. 2021, 334, 129594. [Google Scholar] [CrossRef]
- Wang, H.; Duan, S.; Chen, Y.; Liu, H.; Tian, J.; Wu, F.; Du, Z.; Tang, L.; Li, Y.; Ding, S. Study on a natural silk cocoon membrane-based versatile and stable immunosensing platform via directional immunoaffinity recognition. ACS Omega 2022, 7, 35297–35304. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Duan, S.; Wang, M.; Wei, S.; Chen, Y.; Chen, W.; Li, Y.; Ding, S. Silk cocoon membrane-based immunosensing assay for red blood cell antigen typing. Sens. Actuators B Chem. 2020, 320, 128376. [Google Scholar] [CrossRef]
- Lau, K.; Waterhouse, A.; Akhavan, B.; Gao, L.; Na Kim, H.; Tang, F.; Whitelock, J.M.; Bilek, M.M.; Lord, M.S.; Rnjak-Kovacina, J. Biomimetic silk biomaterials: Perlecan-functionalized silk fibroin for use in blood-contacting devices. Acta Biomater. 2021, 132, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-L.; Shen, Y.-H.; Sun, X.; Li, Z.-H.; Wang, X.-Y.; Zhao, Z. High biocompatible AuNCs-silk fibroin hydrogel system for visual detection of H2O2. Microchem. J. 2020, 157, 105036. [Google Scholar] [CrossRef]
- Available online: www.clinicaltrials.gov (accessed on 13 November 2023).
- Louiselle, A.E.; Niemiec, S.; Azeltine, M.; Mundra, L.; French, B.; Zgheib, C.; Liechty, K.W. Evaluation of skin care concerns and patient’s perception of the effect of NanoSilk Cream on facial skin. J. Cosmet. Dermatol. 2022, 21, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Egan, G.; Phuagkhaopong, S.; Matthew, S.A.L.; Connolly, P.; Seib, F.P. Impact of silk hydrogel secondary structure on hydrogel formation, silk leaching and in vitro response. Sci. Rep. 2022, 12, 3729. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.E.; Gulka, C.P.; Giordano, J.E.M.; Montero, M.P.; Hoang, A.; Carroll, T.L. Injectable silk protein microparticle-based fillers: A novel material for potential use in glottic insufficiency. J. Voice 2019, 33, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Kijanska, M.; Marmaras, A.; Hegglin, A.; Kurtcuoglu, V.; Giovanoli, P.; Lindenblatt, N. In vivo characterization of the integration and vascularization of a silk-derived surgical scaffold. JPRAS J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, D.-K.; Park, H.S.; Jeong, J.Y.; Yeon, Y.K.; Kumar, V.; Bae, S.H.; Lee, J.M.; Moon, B.M.; Park, C.H. A prospective cohort study of the silk fibroin patch in chronic tympanic membrane perforation. Laryngoscope 2016, 126, 2798–2803. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.S.; Kim, D.-K.; Park, C.H.; Lee, H.R. Clinical outcomes of silk patch in acute tympanic membrane perforation. Clin. Exp. Otorhinolaryngol. 2015, 8, 117–122. [Google Scholar] [CrossRef]
- Mazurek, Ł.; Szudzik, M.; Rybka, M.; Konop, M. Silk fibroin biomaterials and their beneficial role in skin wound healing. Biomolecules 2022, 12, 1852. [Google Scholar] [CrossRef] [PubMed]
- Yigit, S.; Hallaj, N.S.; Sugarman, J.L.; Chong, L.C.; Roman, S.E.; Abu-Taleb, L.M.; Goodman, R.E.; Johnson, P.E.; Behrens, A.M. Toxicological assessment and food allergy of silk fibroin derived from Bombyx mori cocoons. Food Chem. Toxicol. 2021, 151, 112117. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhang, J.; Yang, H.; Yao, S.; He, L.; Liang, H.; Wang, Y.; Chen, H.; Zhao, P.; Qin, G. Safety assessment of water-extract sericin from silkworm (Bombyx mori) cocoons using different model approaches. BioMed Res. Int. 2020, 2020, 9689386. [Google Scholar] [CrossRef] [PubMed]
- Acharya, C.; Ghosh, S.K.; Kundu, S.C. Silk fibroin protein from mulberry and non-mulberry silkworms: Cytotoxicity, biocompatibility and kinetics of L929 murine fibroblast adhesion. J. Mater. Sci. Mater. Med. 2008, 19, 2827–2836. [Google Scholar] [CrossRef] [PubMed]

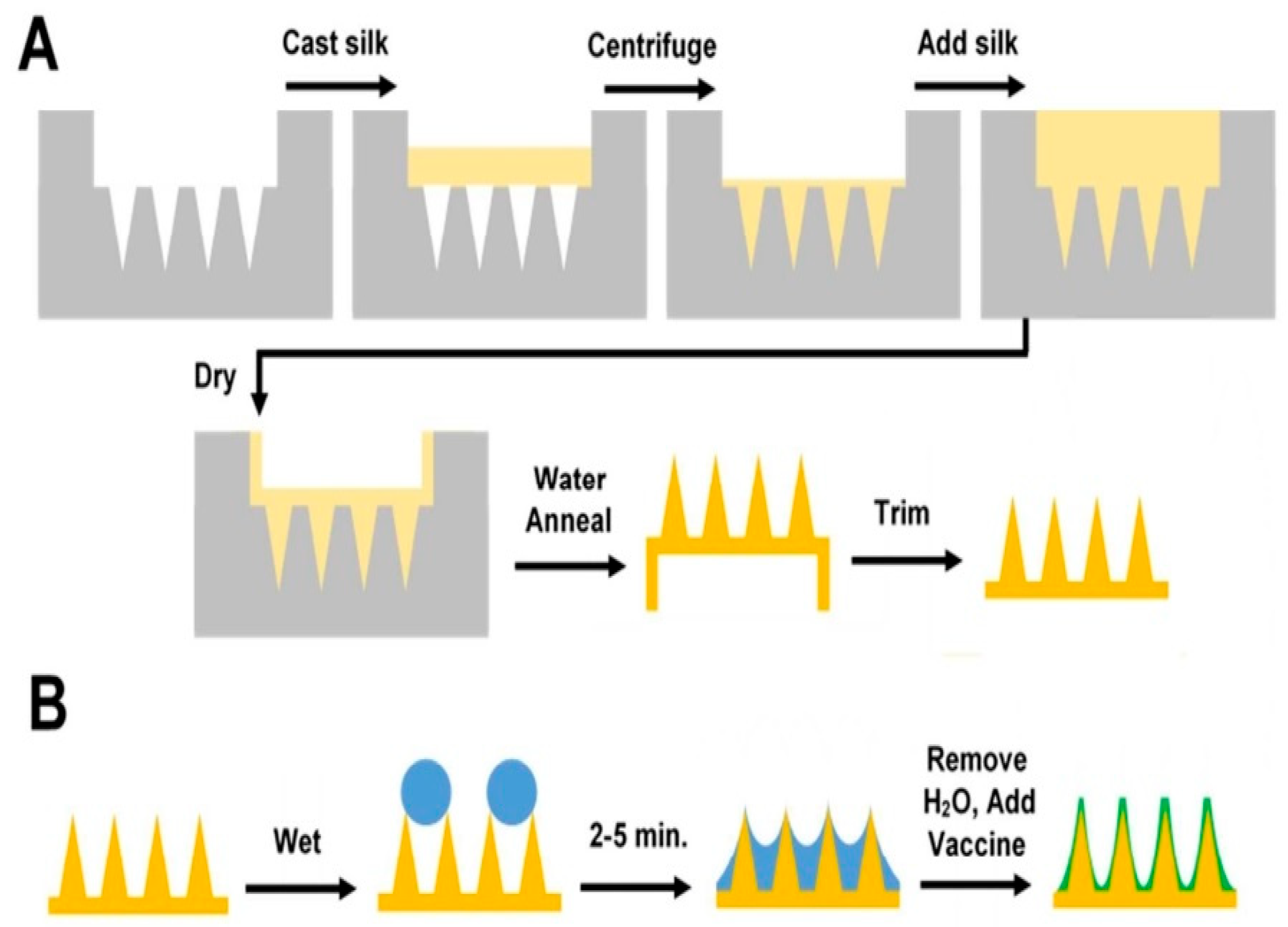
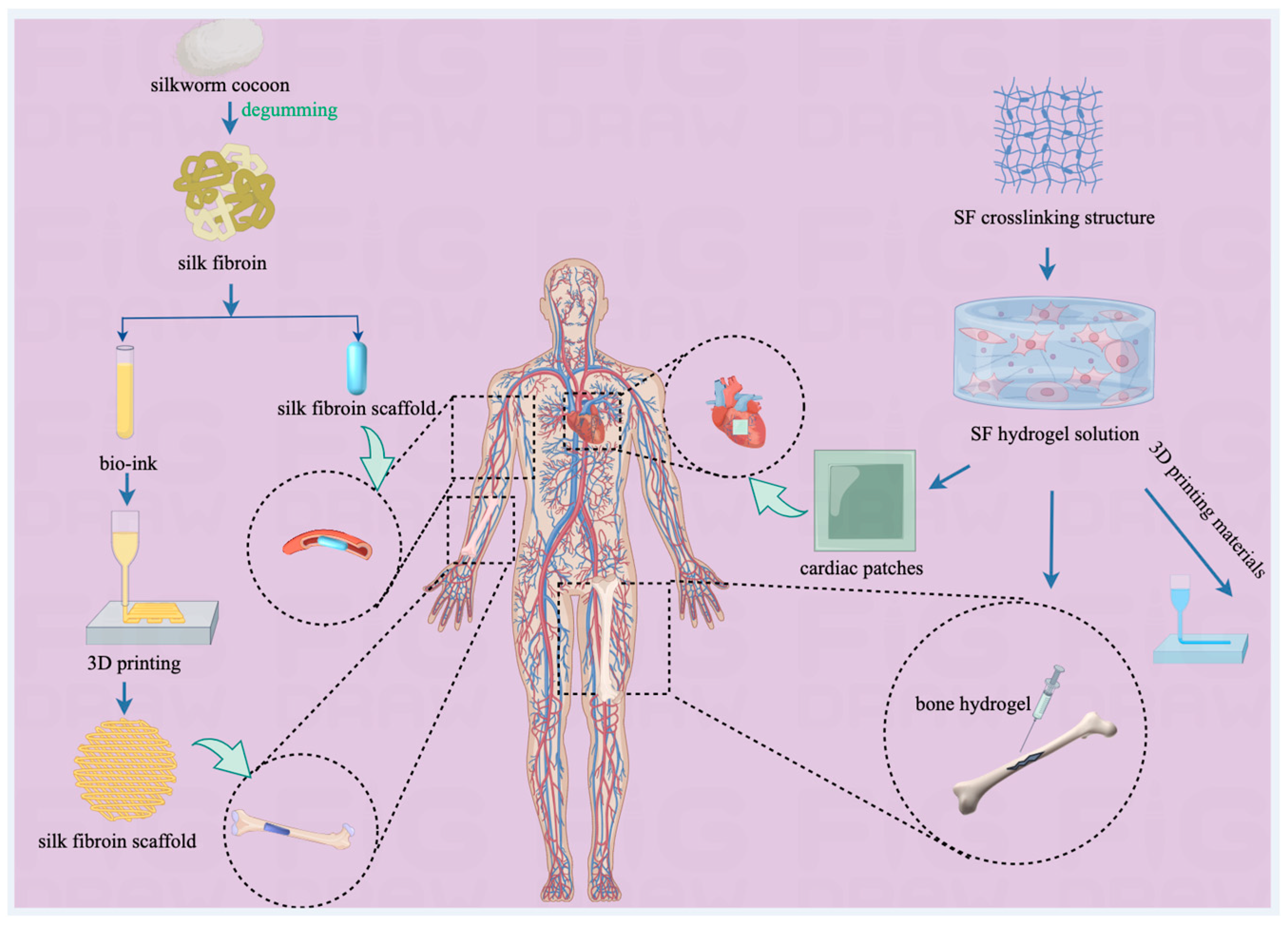
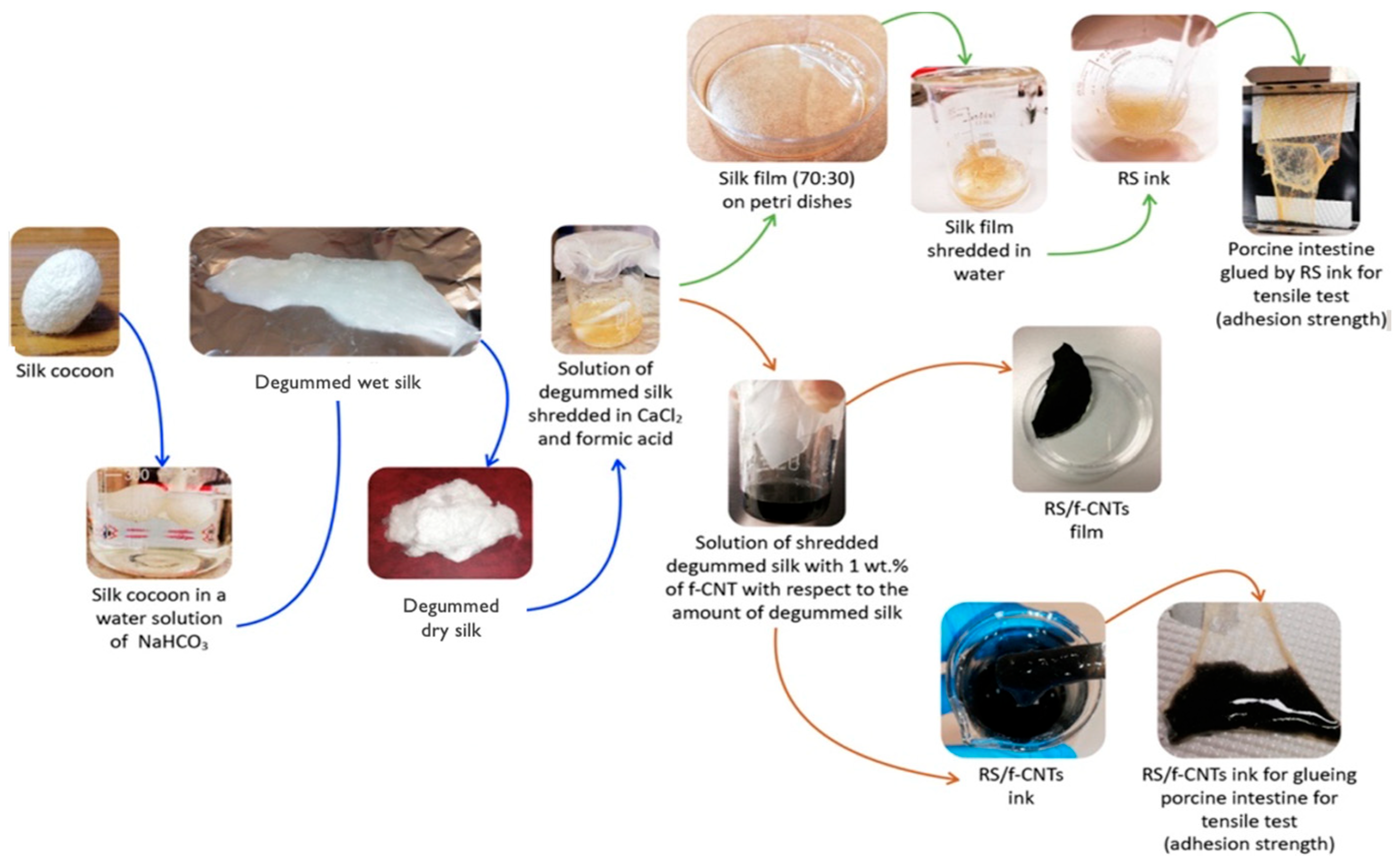
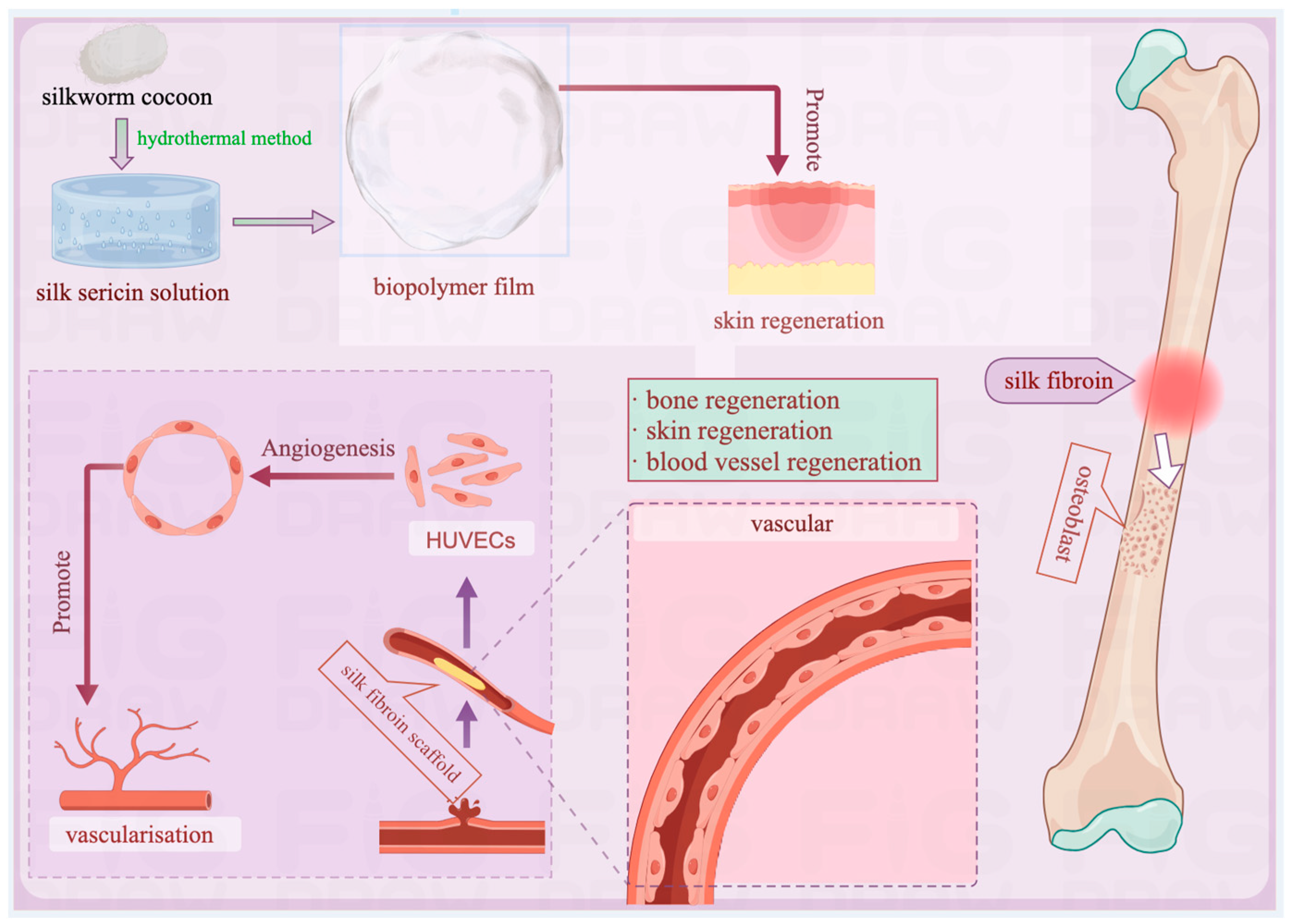
| Main Compound | Extraction Method | Content |
|---|---|---|
| silk fibroin | soda boiling process (degumming) | 70–80% |
| silk sericin | hydrothermal method; chemical method; enzymatic method | 20–30% |
| flavonoids | solvent extraction process | <1% |
| calcium oxalate | acid dissolution method | |
| other peptides | enzymolysis method |
| Official Title | Sponsor | Enrollment | Objective | Dosage | Study Phase | Condition | Location | Current Status |
|---|---|---|---|---|---|---|---|---|
| A Comparative Evaluation of Subgingivally Delivered Chlorhexidine, Silk Fibroin and Combination of Fibroin and Chlorhexidine as Local Drug Delivery in Periodontitis—A Randomized Control Trial | Krishnadevaraya College of Dental Sciences & Hospital | 15 (Estimated) | To evaluate the effect of silk fibroin as a drug delivery system while simultaneously assessing the efficacy of silk fibroin in comparison to chlorhexidine | Films | Phase 1 | Periodontal Pocket | Not provided | Not Yet Recruiting |
| Efficacy and Safety of Wound Dressing Containing Silk Fibroin With Bioactive Coating Layer Versus Medicated Paraffin Gauze Dressing in the Treatment of Split-thickness Skin Graft Donor Sites | Chulalongkorn University | 29 (Actual) | To compare wound dressing containing silk fibroin with bioactive coating layer with Bactigras®, with regard to healing time, patients’ pain intensity, skin’s transepidermal water loss after healing and evidence of infection in the treatment of split-thickness skin graft donor sites | Films | Phase 1 Phase 2 | Impaired Wound Healing; Infection of Skin Donor Site; Late Complication From Skin Graft; Intractable Pain | Thailand | Completed |
| Manufacturing, Characterization and Evaluation of the Effect of Silk Fibroin Membranes, Loaded or Not With Neurotensins on Open Wounds in the Palate: Randomized Clinical Study | Universidade Estadual Paulista Júlio de Mesquita Filho | 66 (Estimated) | To manufacture and characterize silk fibroin membranes loaded or not with neurotensin and to evaluate clinical, patient-centered, and immunological parameters to determine the effect of using these membranes on open wounds on the human palate | Films | Not Applicable | Wound Healing; Palate Wound | Brazil | Recruiting |
| A New Drug Delivery System—Silk Fibroin Film Loaded or Not With Insulin on Palatal Mucosa Wound Healing: in Vitro Study and a Randomized Clinical Trial | Universidade Estadual Paulista Júlio de Mesquita Filho | 75 (Estimated) | To evaluate the effect of silk fibroin films loaded or not with insulin in the repair of palatal mucosa open wounds | Films | Not Applicable | Wound Healing; Palate Wound | Brazil | Unknown |
| A Pilot Study to Evaluate the Reconstruction of Digital Nerve Defects in Humans Using an Implanted Silk Nerve Guide | Silk Biomaterials srl | 4 (Actual) | To ascertain the feasibility and safety of the procedure using SilkBridge—a biocompatible silk fibroin-based scaffold—for the regeneration of sensory nerve fibers | Scaffold | Not Applicable | Peripheral Nerve Injury Digital Nerve Hand | Switzerland | Unknown |
| NanoSilk Cosmo: Evaluation of a Novel Silk Complex on Biophysical Parameters Related to Skin Aging | University of Colorado, Denver | 46 (Actual) | To evaluate a novel silk complex on biophysical parameters related to skin aging including skin resilience, elasticity, and hydration | Nanosolution | Not Applicable | Aging | United States | Completed |
| Multi-center, Randomized, Active-controlled, Single-blind, Parallel Two-group Trial of HQ® Matrix Soft Tissue Mesh and ULTRAPRO® Partially Absorbable Lightweight Mesh for the Treatment of Inguinal Hernia | Zhejiang Xingyue Biotechnology Co., Ltd. | 144 (Estimated) | To evaluate the safety and effectiveness of HQ® Matrix Soft Tissue Mesh for the Treatment of Inguinal Hernia | Scaffold | Not Applicable | Inguinal Hernia | China | Unknown |
| Pilot Evaluation of Cosmetic Outcome and Surgical Site Infection Rates of Coated VICRYL* Plus Antibacterial (Polyglactin 910) Suture Compared to Chinese Silk in Scheduled Breast Cancer Surgery | Ethicon, Inc. | 101 (Actual) | To evaluate the cosmetic outcome and surgical site infection in approximately 100 patients from 6 centers in China undergoing scheduled modified radical mastectomy for breast cancer | Silk Suture | Phase 4 | Breast Cancer | China | Completed |
| Product Name | Main Compositions | Indications | Approval Year | Nation |
|---|---|---|---|---|
| Silk Voice® | A silk fibroin injection | To treat vocal cord-mediated and vocal cord dysfunction. | 2018 | USA |
| SERI® | Surgical stent device based on silk fibroin | Abdominal wall reconstruction and plastic surgery. | Unknown | USA |
| Tympasil® | A silk fibroin patch | To treat ear drum perforations. | Unknown | Korea |
| Antibacterial wound dressing patch | A silk fibroin patch | Postoperative incisions, skin surface abrasions, and ulcer coverage. | 2020 | China |
| Functional healing wound dressing patch | Silk fibroin, surface coated with a composite silicon-based powder | Promoting healing, repair, and coverage of postoperative wounds, abrasions, and non-healing wounds. | 2021 | China |
| Absorbable silk fibroin repair film | Composed of silk fibroin, glycerol, and water | Used in conjunction with bone meal as a physical barrier for preserving the extraction site of adult patients after tooth extraction. | 2022 | China |
| Silk fibroin film dressing | Silk fibroin with an amino acid content of ≥90% | For skin area coverage. | 2020 | China |
| Silk fibroin vaginal packing gel | Gel consists of silk fibroin, carbomer, triethanolamine, sodium ethyl paraben, glycerin, and purified water | To block HPV infection in the reproductive tract and prevent cervical lesions caused by HPV infection. To improve the vaginal microenvironment, alleviate itching, pain, congestion, edema, increased secretion, purulent discharge symptoms caused by chronic cervicitis, and reduce the surface of cervical erosion. | 2023 | China |
| Silk fibroin hydrogel dressing | Gel composed of silk fibroin and purified water | Coverage and care of non-chronic wounds after laser surgery. | 2022 | China |
| Light guide gel | Composed of carbomer 940, glycerol, silk fibroin, pentanediol, sodium hydroxide, and purified water | For thermal insulation and light guidance during photon therapy, in conjunction with photon therapy equipment. | 2022 | China |
| Liquid dressing | A solution composed of silk fibroin, sodium alginate, sodium carboxymethyl cellulose, glycerol, and purified water | For the care of superficial wounds and surrounding skin such as small wounds, abrasions, and cuts. | 2021 | China |
| Liquid wound dressing | A solution composed of silk fibroin, sodium chloride, glycerol, and carbomer | For the care of superficial wounds and surrounding skin such as small wounds, abrasions, and cuts. | 2020 | China |
| Liquid dressing | A solution composed of silk fibroin, glycerol, sodium benzoate, potassium sorbate, and carboxymethyl cellulose | For the care of superficial wounds and surrounding skin such as small wounds, abrasions, and cuts. | 2020 | China |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Z.; Zhao, C.; Huang, T.; Yu, L.; Sun, Y.; Tao, Y.; Cao, Y.; Du, R.; Lin, W.; Zeng, J. Silkworm Cocoon: Dual Functions as a Traditional Chinese Medicine and the Raw Material of Promising Biocompatible Carriers. Pharmaceuticals 2024, 17, 817. https://doi.org/10.3390/ph17070817
Tian Z, Zhao C, Huang T, Yu L, Sun Y, Tao Y, Cao Y, Du R, Lin W, Zeng J. Silkworm Cocoon: Dual Functions as a Traditional Chinese Medicine and the Raw Material of Promising Biocompatible Carriers. Pharmaceuticals. 2024; 17(7):817. https://doi.org/10.3390/ph17070817
Chicago/Turabian StyleTian, Zhijie, Chuncao Zhao, Ting Huang, Lining Yu, Yijie Sun, Yian Tao, Yunfeng Cao, Ruofei Du, Wenhui Lin, and Jia Zeng. 2024. "Silkworm Cocoon: Dual Functions as a Traditional Chinese Medicine and the Raw Material of Promising Biocompatible Carriers" Pharmaceuticals 17, no. 7: 817. https://doi.org/10.3390/ph17070817
APA StyleTian, Z., Zhao, C., Huang, T., Yu, L., Sun, Y., Tao, Y., Cao, Y., Du, R., Lin, W., & Zeng, J. (2024). Silkworm Cocoon: Dual Functions as a Traditional Chinese Medicine and the Raw Material of Promising Biocompatible Carriers. Pharmaceuticals, 17(7), 817. https://doi.org/10.3390/ph17070817






