Critical Appraisal and Future Challenges of Artificial Intelligence and Anticancer Drug Development
Abstract
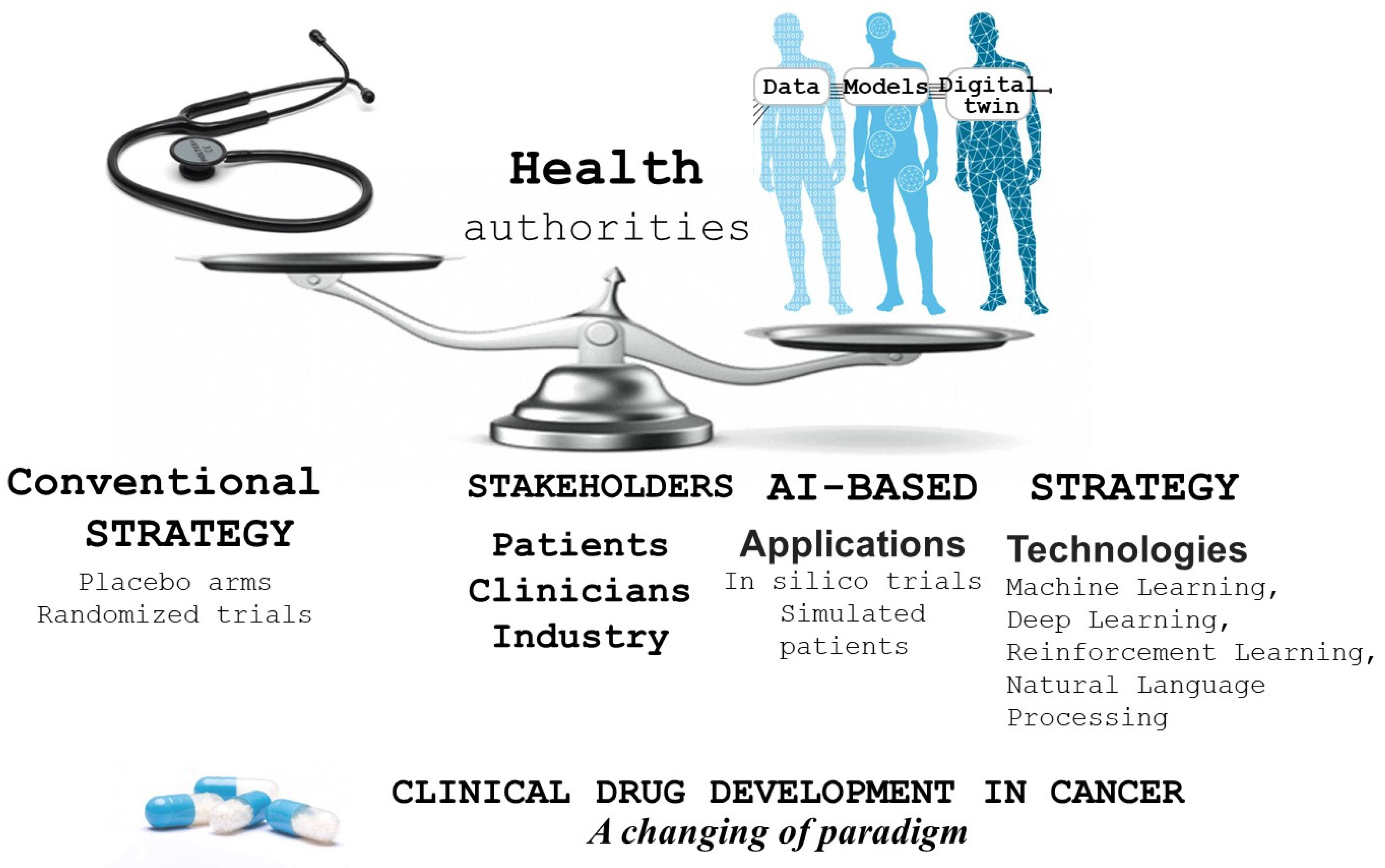
1. Introduction
2. Breaking the Dogma of Traditional Clinical Trial Designs
3. In Silico Clinical Trials
4. Critical Appraisal (Table 1)
| Strengths [14] |
|
| Limits and potential deficiencies [27,28] |
|
5. Future Challenges
Author Contributions
Funding
Conflicts of Interest
References
- Gupta, R.; Srivastava, D.; Sahu, M.; Tiwari, S.; Ambasta, R.K.; Kumar, P. Artificial intelligence to deep learning: Machine intelligence approach for drug discovery. Mol. Divers. 2021, 25, 1315–1360. [Google Scholar] [CrossRef]
- Gerstberger, S.; Jiang, Q.; Ganesh, K. Metastasis. Cell 2023, 186, 1564–1579. [Google Scholar] [CrossRef]
- Kortemme, T. De novo protein design-from new structures to programmable functions. Cell 2024, 187, 526–544. [Google Scholar] [CrossRef]
- Gal, J.; Milano, G.; Ferrero, J.M.; Saada-Bouzid, E.; Viotti, J.; Chabaud, S.; Gougis, P.; Le Tourneau, C.; Schiappa, R.; Paquet, A.; et al. Optimizing drug development in oncology by clinical trial simulation: Why and how? Brief. Bioinform. 2018, 19, 1203–1217. [Google Scholar] [CrossRef]
- Paule, I.; Tod, M.; Henin, E.; You, B.; Freyer, G.; Girard, P. Dose adaptation of capecitabine based on individual prediction of limiting toxicity grade: Evaluation by clinical trial simulation. Cancer Chemother. Pharmacol. 2012, 69, 447–455. [Google Scholar] [CrossRef]
- Claret, L.; Lu, J.F.; Bruno, R.; Hsu, C.P.; Hei, Y.J.; Sun, Y.N. Simulations using a drug-disease modeling framework and phase II data predict phase III survival outcome in first-line non-small-cell lung cancer. Clin. Pharmacol. Ther. 2012, 92, 631–634. [Google Scholar] [CrossRef]
- Mazzocco, P.; Honnorat, J.; Ducray, F.; Ribba, B. Increasing the Time Interval between PCV Chemotherapy Cycles as a Strategy to Improve Duration of Response in Low-Grade Gliomas: Results from a Model-Based Clinical Trial Simulation. Comput. Math. Methods Med. 2015, 2015, 297903. [Google Scholar] [CrossRef][Green Version]
- Zhang, Z.; Wei, X. Artificial intelligence-assisted selection and efficacy prediction of antineoplastic strategies for precision cancer therapy. Semin. Cancer Biol. 2023, 90, 57–72. [Google Scholar] [CrossRef]
- Perurena, N.; Situ, L.; Cichowski, K. Combinatorial strategies to target RAS-driven cancers. Nat. Rev. Cancer 2024, 24, 316–337. [Google Scholar] [CrossRef]
- Diaz, L.A., Jr.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): Final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef]
- Cohen, R.; Raeisi, M.; Chibaudel, B.; Yoshino, T.; Shi, Q.; Zalcberg, J.R.; Adams, R.; Cremolini, C.; Grothey, A.; Mayer, R.J.; et al. Efficacy of immune checkpoint inhibitors for metastatic colorectal cancer with microsatellite instability in second or latter line using synthetic control arms: A non-randomised evaluation. Eur. J. Cancer 2024, 199, 113537. [Google Scholar] [CrossRef]
- Ferri-García, R.; Rueda, M.D.M. Propensity score adjustment using machine learning classification algorithms to control selection bias in online surveys. PLoS ONE 2020, 15, e0231500. [Google Scholar] [CrossRef]
- Beaulieu-Jones, B.K.; Finlayson, S.G.; Yuan, W.; Altman, R.B.; Kohane, I.S.; Prasad, V.; Yu, K.H. Examining the Use of Real-World Evidence in the Regulatory Process. Clin. Pharmacol. Ther. 2020, 107, 843–852. [Google Scholar] [CrossRef]
- Creemers, J.H.A.; Ankan, A.; Roes, K.C.B.; Schröder, G.; Mehra, N.; Figdor, C.G.; de Vries, I.J.M.; Textor, J. In silico cancer immunotherapy trials uncover the consequences of therapy-specific response patterns for clinical trial design and outcome. Nat. Commun. 2023, 14, 2348. [Google Scholar] [CrossRef]
- Chopra, H.; Annu; Shin, D.K.; Munjal, K.; Priyanka; Dhama, K.; Emran, T.B. Revolutionizing clinical trials: The role of AI in accelerating medical breakthroughs. Int. J. Surg. 2023, 109, 4211–4220. [Google Scholar] [CrossRef]
- Swanson, K.; Wu, E.; Zhang, A.; Alizadeh, A.A.; Zou, J. From patterns to patients: Advances in clinical machine learning for cancer diagnosis, prognosis, and treatment. Cell 2023, 186, 1772–1791. [Google Scholar] [CrossRef]
- jinkō: A Complete Solution for Trial Simulation & Design Optimization. Available online: https://www.novadiscovery.com/jinko/ (accessed on 15 April 2024).
- EGFR-Mutant NSCLC: Chemo-TKI Bests TKI Alone. Cancer Discov. 2023, 13, 2298. [CrossRef]
- L’Hostis, A.; Palgen, J.L.; Perrillat-Mercerot, A.; Peyronnet, E.; Jacob, E.; Bosley, J.; Duruisseaux, M.; Toueg, R.; Lefevre, L.; Kahoul, R.; et al. Knowledge-based mechanistic modeling accurately predicts disease progression with gefitinib in EGFR-mutant lung adenocarcinoma. NPJ Syst. Biol. Appl. 2023, 9, 37. [Google Scholar] [CrossRef]
- Schiappa, R.; Contu, S.; Culie, D.; Chateau, Y.; Gal, J.; Pace-Loscos, T.; Bailleux, C.; Haudebourg, J.; Ferrero, J.M.; Barranger, E.; et al. Validation of RUBY for Breast Cancer Knowledge Extraction From a Large French Electronic Medical Record System. JCO Clin. Cancer Inf. 2023, 7, e2200130. [Google Scholar] [CrossRef] [PubMed]
- Schiappa, R.; Contu, S.; Culie, D.; Thamphya, B.; Chateau, Y.; Gal, J.; Bailleux, C.; Haudebourg, J.; Ferrero, J.M.; Barranger, E.; et al. RUBY: Natural Language Processing of French Electronic Medical Records for Breast Cancer Research. JCO Clin. Cancer Inform. 2022, 6, e2100199. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, J.N.; Hahn, W.; Rollig, C.; Stasik, S.; Platzbecker, U.; Muller-Tidow, C.; Serve, H.; Baldus, C.D.; Schliemann, C.; Schafer-Eckart, K.; et al. Mimicking clinical trials with synthetic acute myeloid leukemia patients using generative artificial intelligence. NPJ Digit. Med. 2024, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Sanford, N.N.; Hong, T.S.; Hall, W.A. Elucidating the Benefit of Radiation Therapy in GI Cancers: Rethinking Trial End Points and Patient Selection. J. Clin. Oncol. 2024, 42, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Musuamba, F.T.; Skottheim Rusten, I.; Lesage, R.; Russo, G.; Bursi, R.; Emili, L.; Wangorsch, G.; Manolis, E.; Karlsson, K.E.; Kulesza, A.; et al. Scientific and regulatory evaluation of mechanistic in silico drug and disease models in drug development: Building model credibility. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 804–825. [Google Scholar] [CrossRef] [PubMed]
- Karr, J.; Malik-Sheriff, R.S.; Osborne, J.; Gonzalez-Parra, G.; Forgoston, E.; Bowness, R.; Liu, Y.; Thompson, R.; Garira, W.; Barhak, J.; et al. Model Integration in Computational Biology: The Role of Reproducibility, Credibility and Utility. Front. Syst. Biol. 2022, 2. [Google Scholar] [CrossRef] [PubMed]
- Cobanaj, M.; Corti, C.; Dee, E.C.; McCullum, L.; Boldrini, L.; Schlam, I.; Tolaney, S.M.; Celi, L.A.; Curigliano, G.; Criscitiello, C. Advancing equitable and personalized cancer care: Novel applications and priorities of artificial intelligence for fairness and inclusivity in the patient care workflow. Eur. J. Cancer 2024, 198, 113504. [Google Scholar] [CrossRef] [PubMed]
- Poweleit, E.A.; Vinks, A.A.; Mizuno, T. Artificial Intelligence and Machine Learning Approaches to Facilitate Therapeutic Drug Management and Model-Informed Precision Dosing. Ther. Drug Monit. 2023, 45, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Kolanska, K.; Chabbert-Buffet, N.; Darai, E.; Antoine, J.M. Artificial intelligence in medicine: A matter of joy or concern? J. Gynecol. Obs. Hum. Reprod. 2021, 50, 101962. [Google Scholar] [CrossRef] [PubMed]
- Beaver, J.A.; Pazdur, R. The Wild West of Checkpoint Inhibitor Development. N. Engl. J. Med. 2022, 386, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- Leary, A.; Besse, B.; André, F. The need for pragmatic, affordable, and practice-changing real-life clinical trials in oncology. Lancet 2024, 403, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Moutinho, S. Dozens of precision cancer drugs tested at lower doses to reduce side effects and cut costs. Nat. Med. 2024, 30, 611–614. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamorey, E.; Gal, J.; Mograbi, B.; Milano, G. Critical Appraisal and Future Challenges of Artificial Intelligence and Anticancer Drug Development. Pharmaceuticals 2024, 17, 816. https://doi.org/10.3390/ph17070816
Chamorey E, Gal J, Mograbi B, Milano G. Critical Appraisal and Future Challenges of Artificial Intelligence and Anticancer Drug Development. Pharmaceuticals. 2024; 17(7):816. https://doi.org/10.3390/ph17070816
Chicago/Turabian StyleChamorey, Emmanuel, Jocelyn Gal, Baharia Mograbi, and Gérard Milano. 2024. "Critical Appraisal and Future Challenges of Artificial Intelligence and Anticancer Drug Development" Pharmaceuticals 17, no. 7: 816. https://doi.org/10.3390/ph17070816
APA StyleChamorey, E., Gal, J., Mograbi, B., & Milano, G. (2024). Critical Appraisal and Future Challenges of Artificial Intelligence and Anticancer Drug Development. Pharmaceuticals, 17(7), 816. https://doi.org/10.3390/ph17070816






