Ceftazidime and Usnic Acid Encapsulated in Chitosan-Coated Liposomes for Oral Administration against Colorectal Cancer-Inducing Escherichia coli
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Liposomes
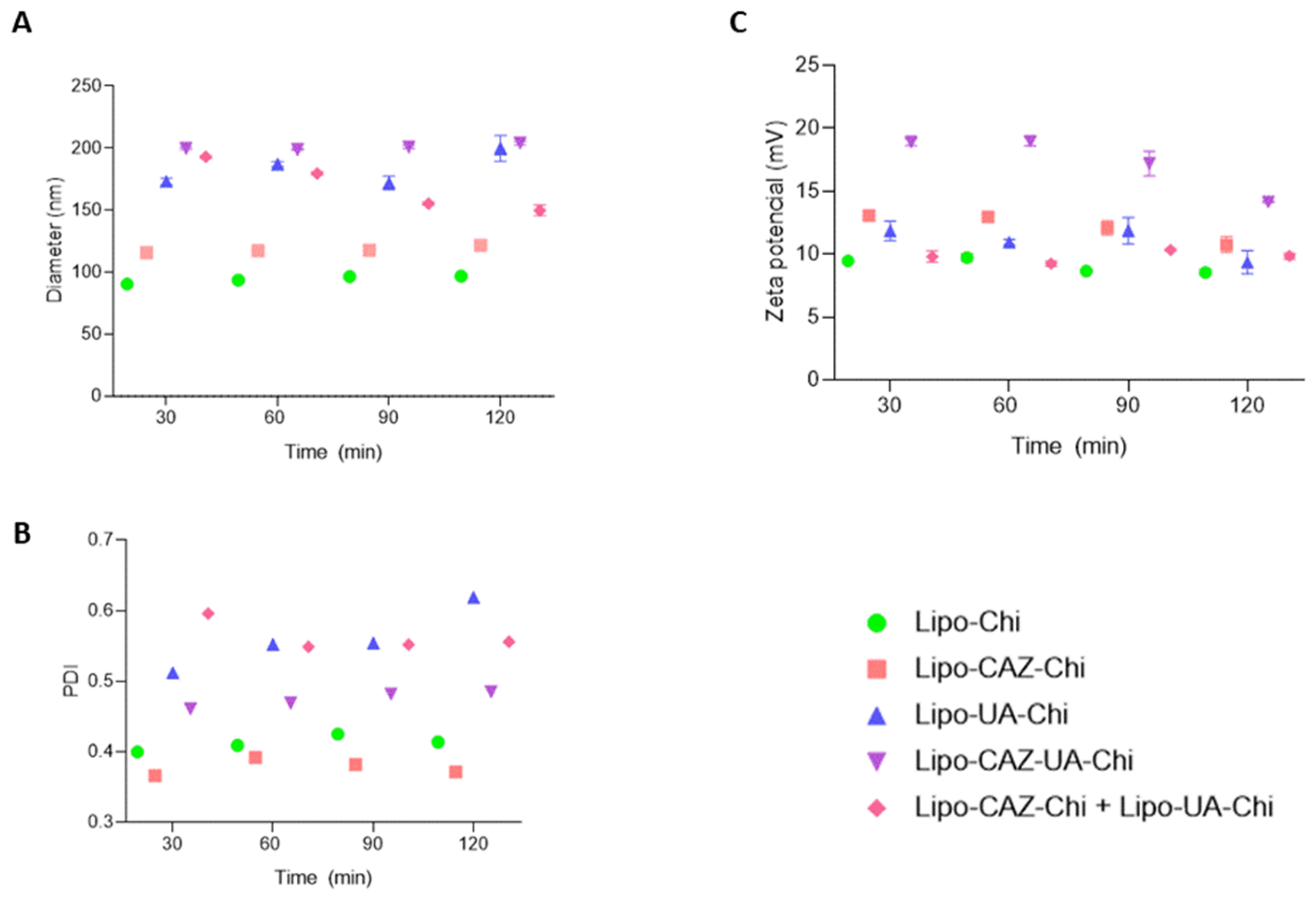
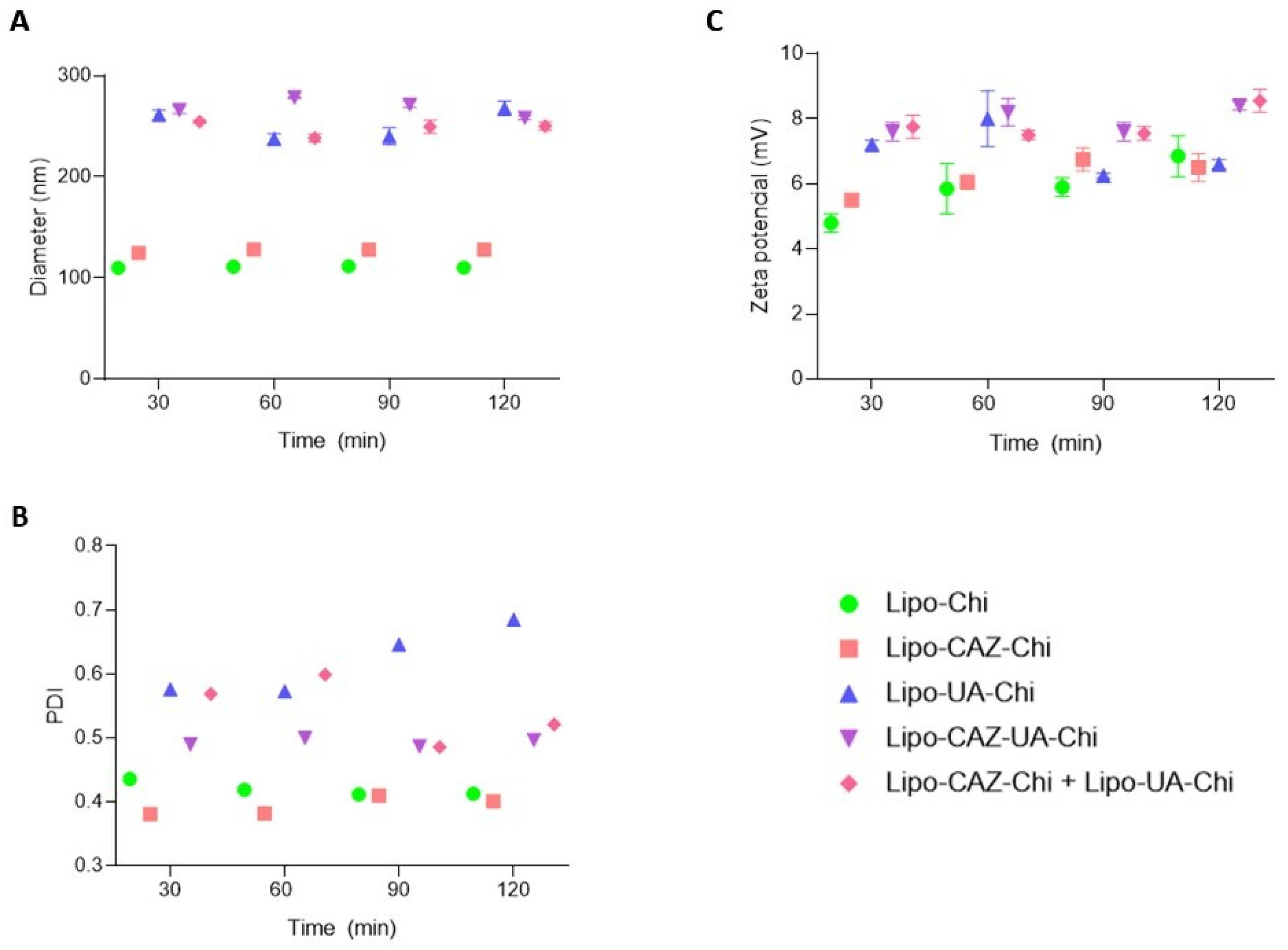
2.1.1. Particle Size, Polydispersity Index, Zeta Potential, pH, and Encapsulation Efficiency of the Liposomes
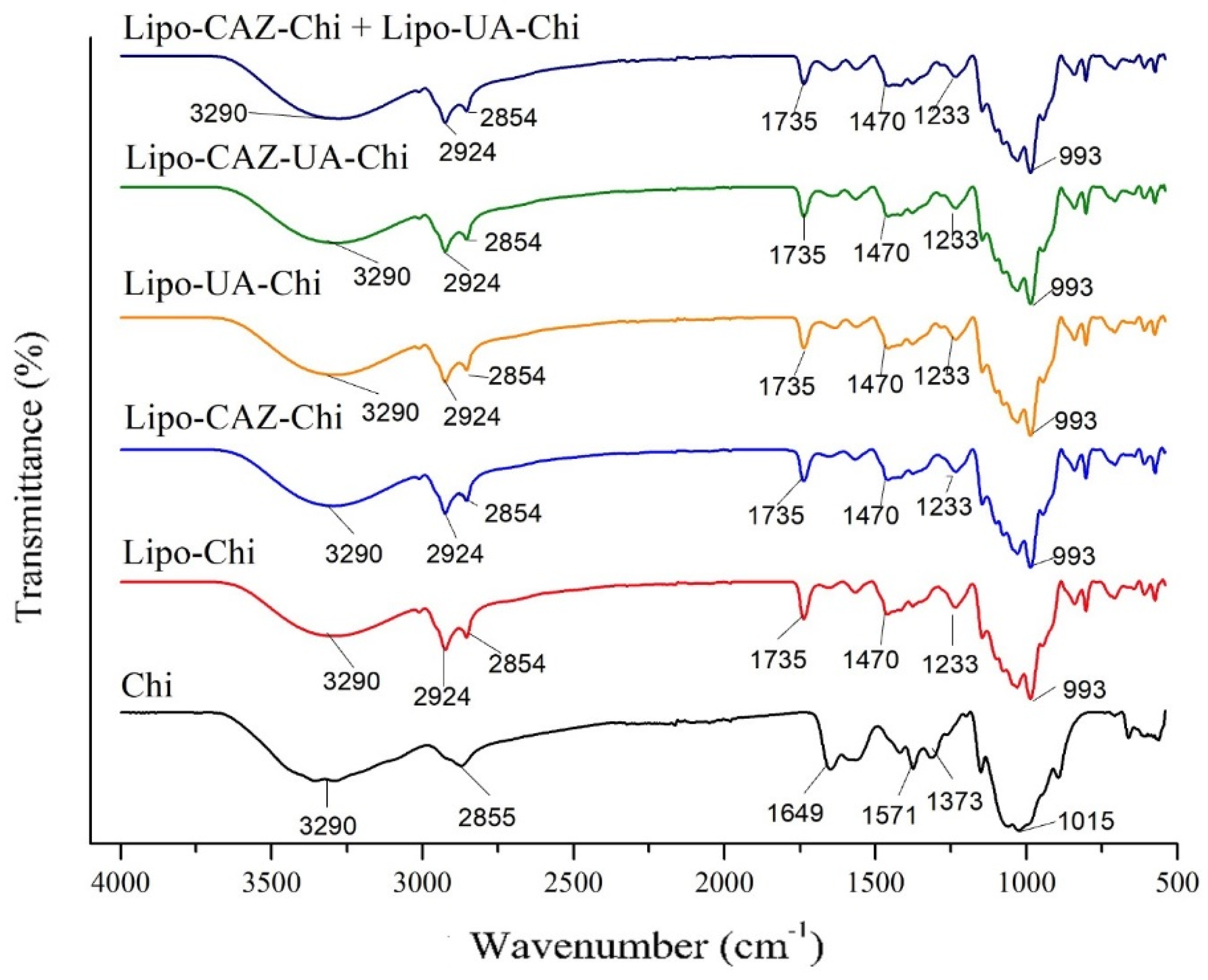
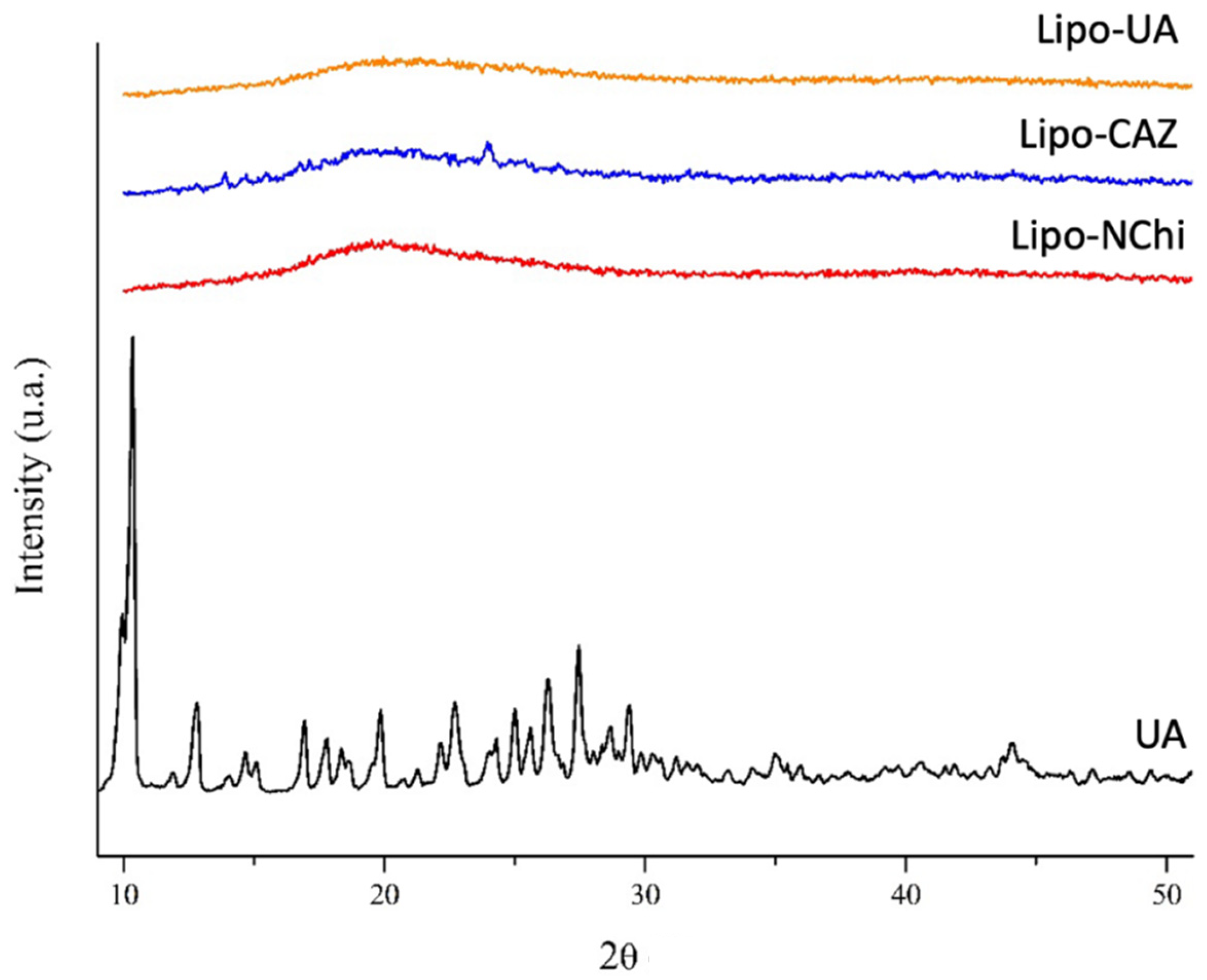
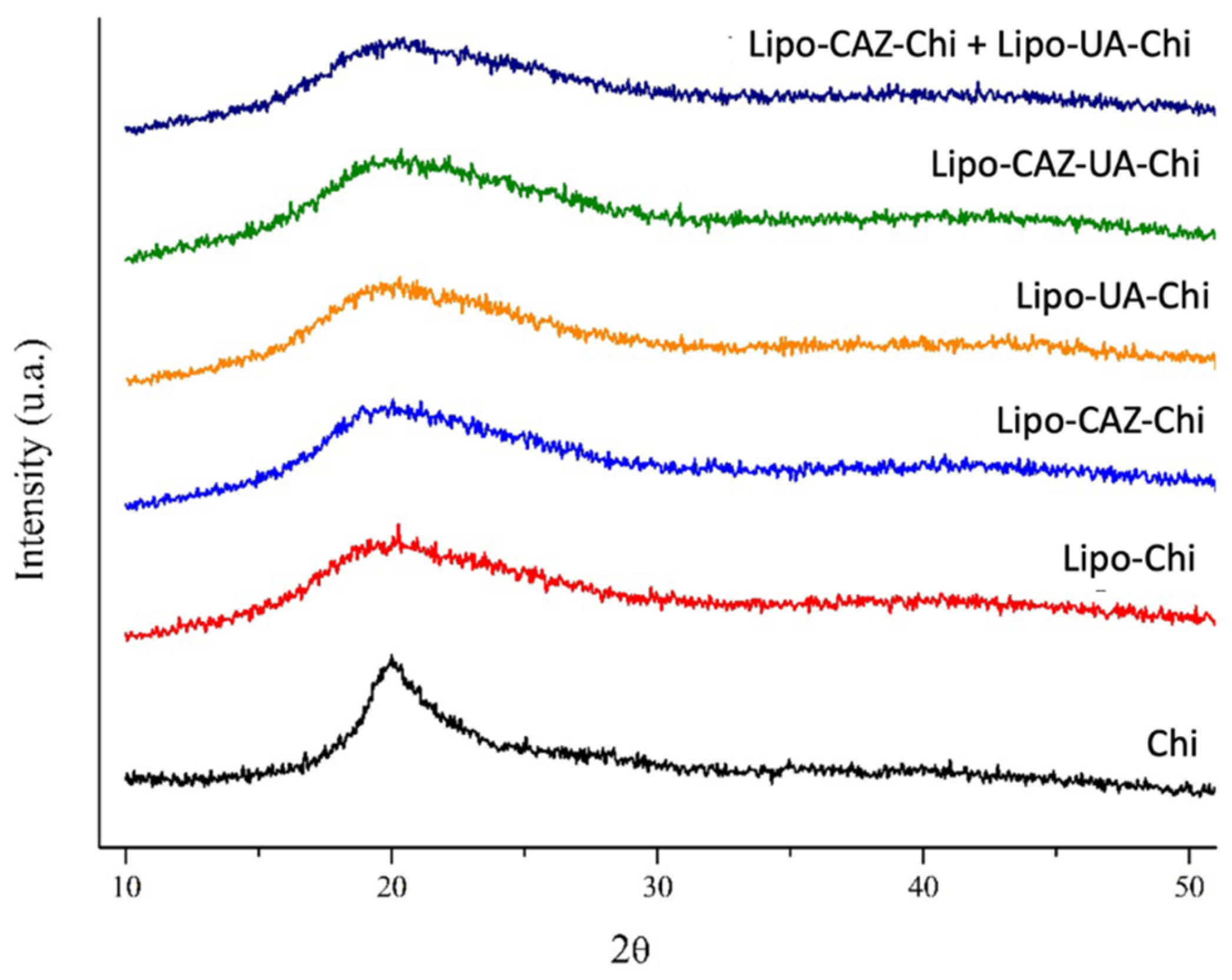
2.1.2. FTIR and XRD of the Liposomes
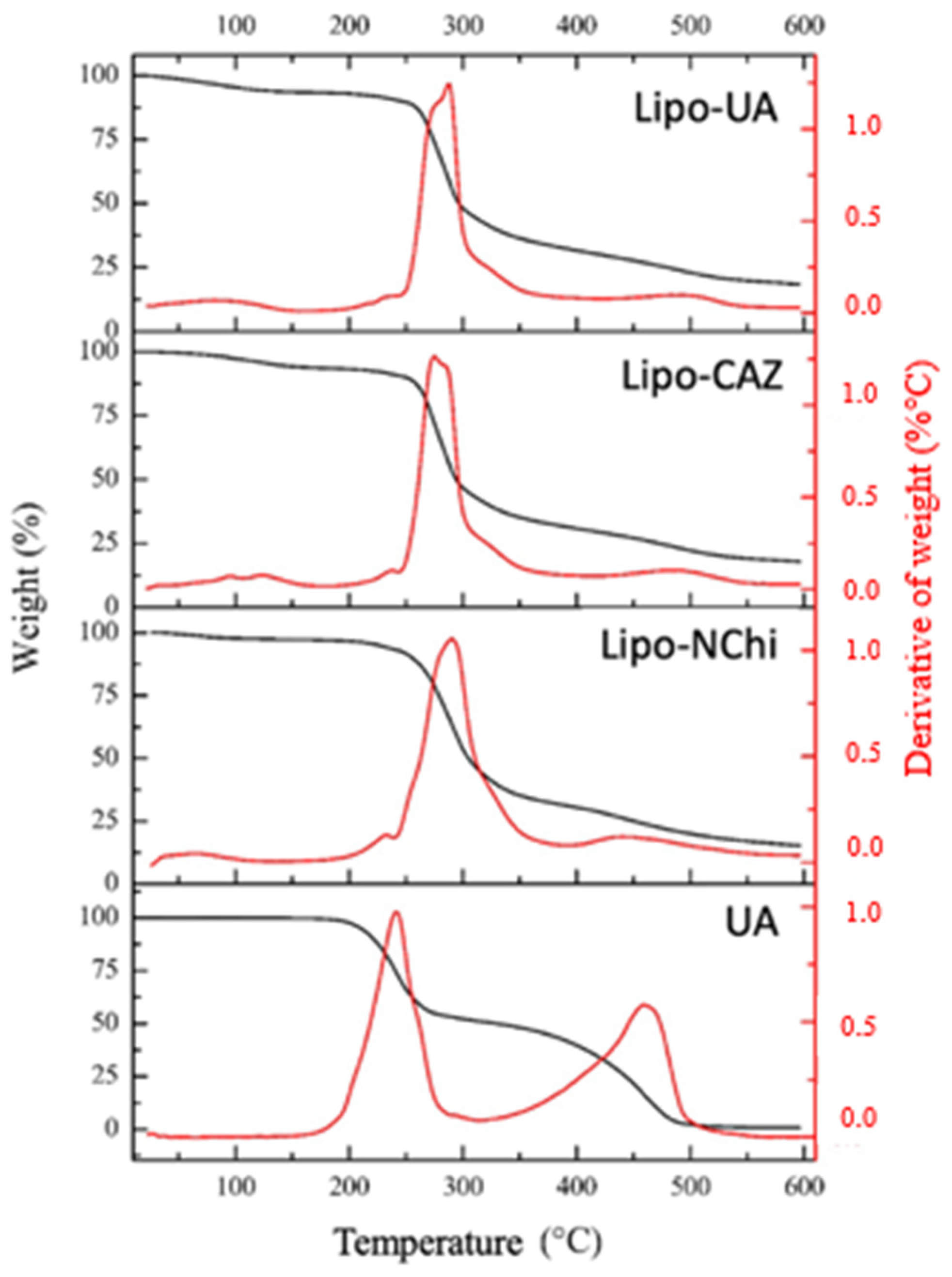
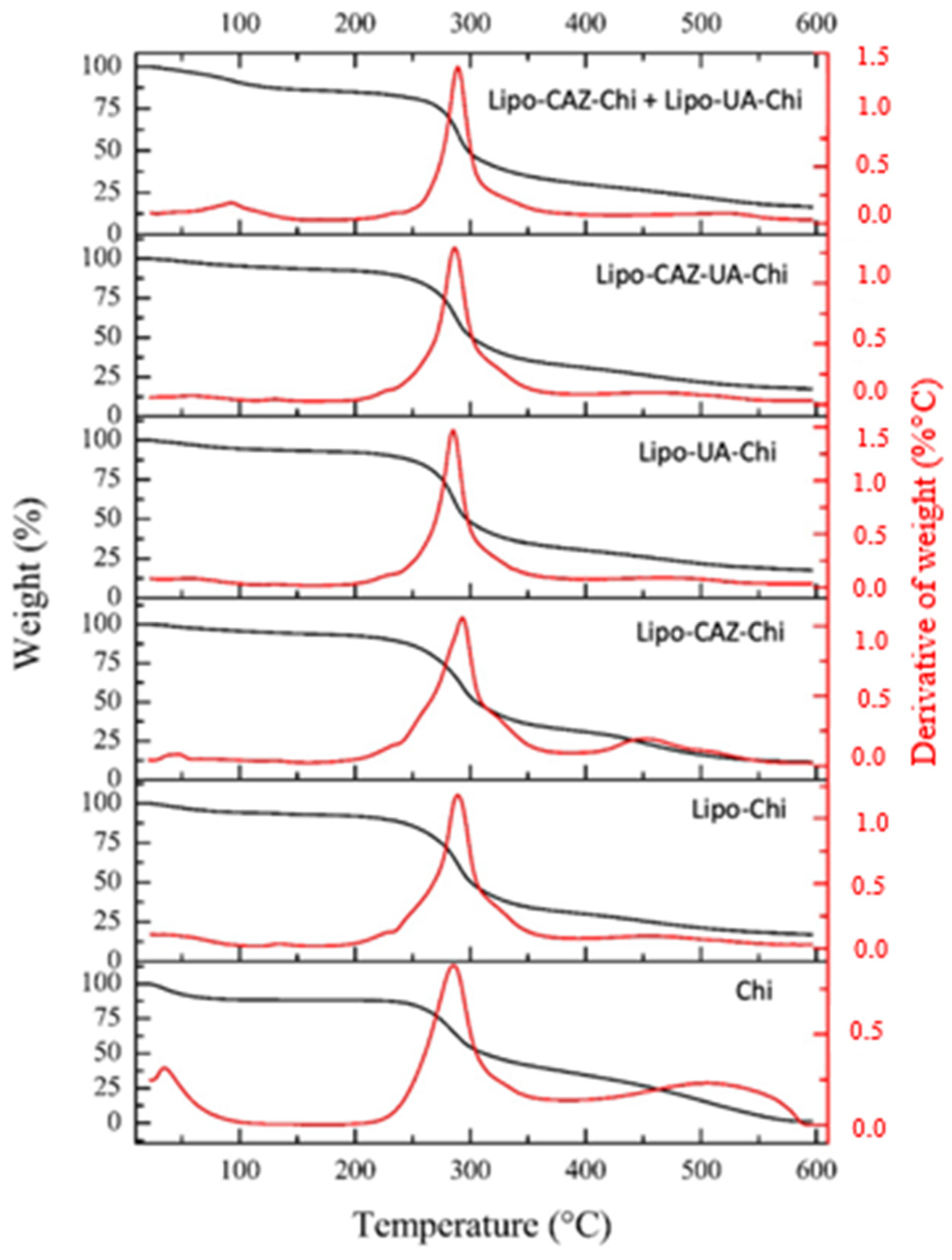
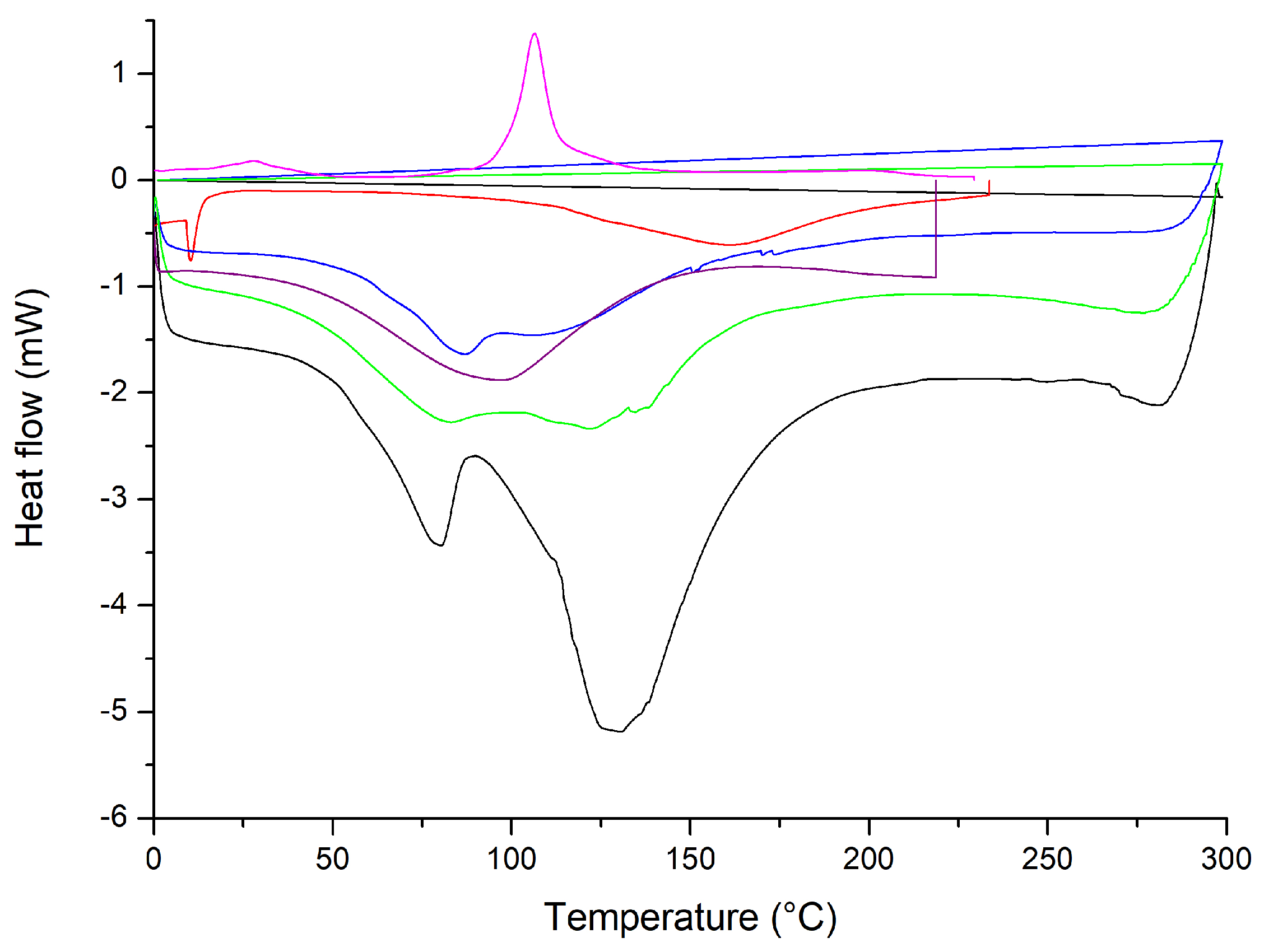
2.1.3. Thermal Analysis through Thermogravimetry Analysis and Differential Scanning Calorimetry of the Liposomes
2.1.4. Stability of the Liposomes under Simulated Gastrointestinal pH Conditions
2.1.5. Stability of the Liposomal Dispersions
2.2. Microbiological Analyses
2.2.1. Evaluation of Antibacterial Activity
2.2.2. Evaluation of Antibiofilm Activity
Assessment of Biofilm Inhibition
Evaluation of Preformed Biofilm Inhibition
3. Material and Methods
3.1. Material
3.1.1. Drugs and Reagents
3.1.2. Bacteria
3.2. Methods
3.2.1. Preparation of Chitosan-Coated Liposomes Containing CAZ, UA, and CAZ+UA
3.2.2. Characterization of the Liposomes
Particle Size, Polydispersity Index, Zeta Potential, and pH of the Liposomes
Determination of the Content and Encapsulation Efficiency of CAZ in Liposomes
Determination of the Content and Efficiency of the Encapsulation of UA in Liposomes
Fourier-Transform Infrared Spectroscopy and X-ray Diffraction of Liposomes
Thermal Analysis by Thermogravimetry and Differential Scanning Calorimetry of the Liposomes
3.2.3. Stability of the Liposomes
Stability of Liposomes under Simulated Gastrointestinal pH Conditions
Stability of the Liposomal Dispersions
3.3. Evaluation of Antibacterial Activity
3.4. Evaluation of Antibiofilm Activity
3.4.1. Determination of Biofilm Formation Inhibition Concentration
3.4.2. Determination of Preformed Biofilm Inhibition Concentration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durães, R.O.; Berardinelli, G.N.; da Costa, A.M.; Scapulatempo-Neto, C.; Pereira, R.; Oliveira, M.A.; Guimarães, D.P.; Reis, R.M. Role of Genetic Ancestry in 1,002 Brazilian Colorectal Cancer Patients from Barretos Cancer Hospital. Front. Oncol. 2020, 10, 145. [Google Scholar] [CrossRef]
- Wild, C.P.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020; pp. 23–33. [Google Scholar]
- Butt, J.; Jenab, M.; Werner, J.; Fedirko, V.; Weiderpass, E.; Dahm, C.C.; Tjonneland, A.; Olsen, A.; Boutron-Ruault, M.-C.; Rothwell, J.A.; et al. Association of pre-diagnostic antibody responses to Escherichia coli and Bacteroides fragilis toxin proteins with colorectal cancer in a European cohort. Gut Microbes 2021, 13, 1–14. [Google Scholar] [CrossRef]
- Veziant, J.; Villéger, R.; Barnich, N.; Bonnet, M. Gut Microbiota as Potential Biomarker and/or Therapeutic Target to Improve the Management of Cancer: Focus on Colibactin-Producing Escherichia coli in Colorectal Cancer. Cancers 2021, 13, 2215–2225. [Google Scholar] [CrossRef]
- Lucas, C.; Salesse, L.; Hoang, M.H.T.; Bonnet, M.; Sauvanet, P.; Larabi, A.; Godfraind, C.; Gagnière, J.; Pezet, D.; Rosenstiel, P.; et al. Autophagy of Intestinal Epithelial Cells Inhibits Colorectal Carcinogenesis Induced by Colibactin-producing Escherichia coli in ApcMin/+ mice. Gastroenterology 2020, 158, 1373–1388. [Google Scholar] [CrossRef]
- Mohamed, A.; Menon, H.; Chulkina, M.; Yee, N.S.; Pinchuk, I.V. Drug–Microbiota Interaction in Colon Cancer Therapy: Impact of Antibiotics. Biomedicines 2021, 9, 259. [Google Scholar] [CrossRef]
- Sadecki, P.W.; Balboa, S.J.; Lopez, L.R.; Kedziora, K.M.; Arthur, J.C.; Hicks, L.M. Evolution of polymyxin resistance regulates colibactin production in Escherichia coli. ACS Chem. Biol. 2021, 16, 1243–1254. [Google Scholar] [CrossRef]
- Hattori, N.; Niwa, T.; Ishida, T.; Kobayashi, K.; Imai, T.; Mori, A.; Kimura, K.; Mori, T.; Asami, Y.; Ushijima, T. Antibiotics suppress colon tumorigenesis through inhibition of aberrant DNA methylation in an azoxymethane and dextran sulfate sodium colitis model. Cancer Sci. 2018, 110, 147–156. [Google Scholar] [CrossRef]
- Pogue, J.M.; Bonomo, R.A.; Kaye, K.S. Ceftazidime/avibactam, meropenem/vaborbactam, or both? Clinical and formulary considerations. Clin. Infect. Dis. 2018, 68, 519–524. [Google Scholar] [CrossRef]
- Nguyen, T.; Menten, L.; Spriet, I.; Quintens, C.; Van Schepdael, A.; Adams, E. Liquid chromatographic method to follow-up ceftazidime and pyridine in portable elastomeric infusion pumps over 24 h. Electrophoresis 2021, 43, 970–977. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Mallmann, V.; Costa, É.R.; Simionatto, E.; Coutinho, E.J.; De Lara Da Silva, R.C.; Ribeiro, S.M.; Franco, O.L.; Migliolo, L.; Croda, J.; et al. Antibacterial activity and synergism of the essential oil of Nectandra megapotamica (L.) flowers against OXA-23-producing Acinetobacter baumannii. J. Essent. Oil Res. 2020, 32, 260–268. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Bronstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Ferraz-Carvalho, R.S.; Pereira, M.A.; Linhares, L.A.; Lira-Nogueira, M.C.; Cavalcanti, I.M.; Santos-Magalhães, N.S.; Montenegro, L.M. Effects of the encapsulation of usnic acid into liposomes and interactions with antituberculous agents against multidrug-resistant tuberculosis clinical isolates. Mem. Inst. Oswaldo Cruz 2016, 111, 330–334. [Google Scholar] [CrossRef]
- Júnior, S.D.d.C.; da Silva, W.R.C.; da Silva, A.M.C.M.; Maciel, M.A.V.; Cavalcanti, I.M.F. Synergistic effect between usnic acid and polymyxin B against resistant clinical isolates of Pseudomonas aeruginosa. Evid. Based Complement Altern. Med. 2020, 2020, 9852145. [Google Scholar]
- Francolini, I.; Giansanti, L.; Piozzi, A.; Altieri, B.; Mauceri, A.; Mancini, G. Glucosylated liposomes as drug delivery systems of usnic acid to address bacterial infections. Colloids Surf. B Biointerfaces 2019, 181, 632–638. [Google Scholar] [CrossRef]
- Battista, S.; Bellio, P.; Celenza, G.; Galantini, L.; Franceschini, I.; Mancini, G.; Giansanti, L. Correlation of Physicochemical and Antimicrobial Properties of Liposomes Loaded with (+)-Usnic Acid. Chempluschem 2020, 85, 1014–1021. [Google Scholar] [CrossRef]
- Aghdam, M.A.; Bagheri, R.; Mosafer, J.; Baradaran, B.; Hashemzaei, M.; Baghbanzadeh, A.; de la Guardia, M.; Mokhtarzadeh, A. Recent advances on thermosensitive and pH-sensitive liposomes employed in controlled release. J. Control. Release 2019, 315, 1–22. [Google Scholar] [CrossRef]
- Shukla, S.; Hernandez, C. Liposome based drug delivery as a potential treatment option for Alzheimer’s disease. Neural Regen. Res. 2022, 17, 1190–1198. [Google Scholar] [CrossRef]
- William, B.; Noémie, P.; Brigitte, E.; Géraldine, P. Supercritical fluid methods: An alternative to conventional methods to prepare liposomes. Chem. Eng. J. 2020, 383, 123106. [Google Scholar] [CrossRef]
- Mokdad, R.; Seguin, C.; Fournel, S.; Frisch, B.; Heurtault, B.; Hadjsadok, A. Anti-inflammatory effects of free and liposome-encapsulated natural Algerian thermal water in RAW 264.7 macrophages. Int. J. Pharm. 2022, 614, 121452. [Google Scholar] [CrossRef]
- Ajeeshkumar, K.K.; Aneesh, P.A.; Raju, N.; Suseela, M.; Ravishankar, C.N.; Benjakul, S. Advancements in liposome technology: Preparation techniques and applications in food, functional foods, and bioactive delivery: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1280–1306. [Google Scholar] [CrossRef]
- Zhou, F.; Xu, T.; Zhao, Y.; Song, H.; Zhang, L.; Wu, X.; Lu, B. Chitosan-coated liposomes as delivery systems for improving the stability and oral bioavailability of acteoside. Food Hydrocoll. 2018, 83, 17–24. [Google Scholar] [CrossRef]
- Dong, W.; Tang, C.; Xia, M.; Sheng, L.; Cai, Z. Preparation and characterization of egg yolk immunoglobulin loaded chitosan-liposome assisted by supercritical carbon dioxide. Food Chem. 2021, 369, 130934. [Google Scholar] [CrossRef]
- Alavi, S.; Haeri, A.; Dadashzadeh, S. Utilization of chitosan-caged liposomes to push the boundaries of therapeutic delivery. Carbohydr. Polym. 2017, 157, 991–1012. [Google Scholar] [CrossRef]
- Colino, C.I.; Gomez, D.V.; Horcajo, E.A.; Gutierrez-Millan, C. A comparative study of liposomes and chitosomes for topical quercetin antioxidant therapy. J. Drug Deliv. Sci. Technol. 2022, 68, 103094. [Google Scholar] [CrossRef]
- Tang, L.; Zhou, Y.J.; Zhu, S.; Liang, G.D.; Zhuang, H.; Zhao, M.F.; Chang, X.Y.; Li, H.N.; Liu, Z.; Guo, Z.R.; et al. E. coli diversity: Low in colorectal cancer. BMC Med. Genom. 2020, 13, 1–17. [Google Scholar] [CrossRef]
- Crossman, L.C.; Chaudhuri, R.R.; Beatson, S.A.; Wells, T.J.; Desvaux, M.; Cunningham, A.F.; Petty, N.K.; Mahon, V.; Brinkley, C.; Hobman, J.L.; et al. A commensal gone bad: Complete genome sequence of the prototypical enterotoxigenic Escherichia coli strain H10407. J. Bacteriol. 2010, 192, 5822–5831. [Google Scholar] [CrossRef]
- Zhao, M.; Lee, S.H.; Song, J.G.; Kim, H.Y.; Han, H.K. Enhanced oral absorption of sorafenib via the layer-by-layer deposition of a pH-sensitive polymer and glycol chitosan on the liposome. Int. J. Pharm. 2018, 544, 14–20. [Google Scholar] [CrossRef]
- Yu, T.; Wu, C.; Zhu, C.; He, Y.; Yang, D.; Cheng, Y.; Gao, X. Oral administration of liposome-apatinib and locally delivery of docetaxel/MPEG-PCL by fibrin glue synergistically improve therapeutic effect in colorectal cancer. J. Biomed. Nanotechnol. 2018, 14, 2077–2091. [Google Scholar] [CrossRef]
- Khurana, R.K.; Mahajan, M.; Teenu; Kapoor, S.; Jain, S.; Singh, B. The sojourn from parenteral to oral taxanes using nanocarrier systems: A patent review. Recent Patents Drug Deliv. Formul. 2016, 10, 44–58. [Google Scholar] [CrossRef]
- Qu, C.; Zhang, L.; Du, X.; Zhang, X.; Zheng, J.; Zhao, Y.; Tu, P. Preparation and evaluation of wet-milled usnic acid nanocrystal suspension for better bioaffinity. Drug Dev. Ind. Pharm. 2017, 44, 707–712. [Google Scholar] [CrossRef]
- Soni, K.; Rizwanullah, M.; Kohli, K. Development and optimization of sulforaphane-loaded nanostructured lipid carriers by the Box-Behnken design for improved oral efficacy against cancer: In vitro, ex vivo and in vivo assessments. Artif. Cells Nanomed. Biotechnol. 2018, 46, 15–31. [Google Scholar] [CrossRef]
- Kim, J.H.; Shin, D.H.; Kim, J.S. Preparation, characterization, and pharmacokinetics of liposomal docetaxel for oral administration. Arch. Pharm. Res. 2018, 41, 765–775. [Google Scholar] [CrossRef]
- Vahedikia, N.; Garavand, F.; Tajeddin, B.; Cacciotti, I.; Jafari, S.M.; Omidi, T.; Zahedi, Z. Biodegradable zein film composites reinforced with chitosan nanoparticles and cinnamon essential oil: Physical, mechanical, structural and antimicrobial attributes. Colloids Surf. B Biointerfaces 2019, 177, 25–32. [Google Scholar] [CrossRef]
- Bashiri, S.; Ghanbarzadeh, B.; Ayaseh, A.; Dehghannya, J.; Ehsani, A. Preparation and characterization of chitosan-coated nanostructured lipid carriers (CH-NLC) containing cinnamon essential oil for enriching milk and anti-oxidant activity. LWT 2020, 119, 108836. [Google Scholar] [CrossRef]
- Ramezanzade, L.; Hosseini, S.F.; Akbari-Adergani, B.; Yaghmur, A. Cross-linked chitosan-coated liposomes for encapsulation of fish-derived peptide. LWT 2021, 150, 112057. [Google Scholar] [CrossRef]
- Nahr, F.K.; Ghanbarzadeh, B.; Hamishehkar, H.; Kafil, H.S. Food grade nanostructured lipid carrier for cardamom essential oil: Preparation, characterization and antimicrobial activity. J. Funct. Foods 2018, 40, 1–8. [Google Scholar] [CrossRef]
- Ezzat, H.M.; Elnaggar, Y.S.R.; Abdallah, O.Y. Improved oral bioavailability of the anticancer drug catechin using chitosomes: Design, in-vitro appraisal and in-vivo studies. Int. J. Pharm. 2019, 565, 488–498. [Google Scholar] [CrossRef]
- Seyedabadi, M.M.; Rostami, H.; Jafari, S.M.; Fathi, M. Development and characterization of chitosan-coated nanoliposomes for encapsulation of caffeine. Food Biosci. 2020, 40, 100857. [Google Scholar] [CrossRef]
- Sharilatinia, Z.; Jalali, A.M. Chitosan-based hydrogels: Preparation, properties and applications. Int. J. Biol. Macromol. 2018, 115, 194–2020. [Google Scholar] [CrossRef]
- Thanou, M.; Verhoef, J.; Junginger, H. Oral drug absorption enhancement by chitosan and its derivatives. Adv. Drug Deliv. Rev. 2001, 52, 117–126. [Google Scholar] [CrossRef]
- Martinez, M.N.; Amidon, G.L. A mechanistic approach to understanding the factors affecting drug absorption: A review of Fundamentals. J. Clin. Pharmacol. 2002, 42, 620–643. [Google Scholar] [CrossRef]
- Abuhelwa, A.Y.; Foster, D.J.R.; Upton, R.N. A quantitative review and metamodels of the variability and factors affecting oral drug absorption-part I: Gastrointestinal pH. AAPS J. 2016, 18, 1309–1321. [Google Scholar] [CrossRef]
- Abuhelwa, A.Y.; Williams, D.B.; Upton, R.N.; Foster, D.J. Food, gastrointestinal pH, and models of oral drug absorption. Eur. J. Pharm. Biopharm. 2017, 112, 234–248. [Google Scholar] [CrossRef]
- Niu, M.; Lu, Y.; Hovgaard, L.; Guan, P.; Tan, Y.; Lian, R.; Qi, J.; Wu, W. Hypoglycemic activity and oral bioavailability of insulin-loaded liposomes containing bile salts in rats: The effect of cholate type, particle size and administered dose. Eur. J. Pharm. Biopharm. 2012, 81, 265–272. [Google Scholar] [CrossRef]
- He, H.; Lu, Y.; Qi, J.; Zhu, Q.; Chen, Z.; Wu, W. Adapting liposomes for oral drug delivery. Acta Pharm. Sin. B 2018, 9, 36–48. [Google Scholar] [CrossRef]
- Torres, I.M.S.; Bento, E.B.; Almeida, L.D.C.; Sá, L.Z.C.M.D.; Lima, E.M. Preparation, characterization and in vitro antimicrobial activity of liposomal ceftazidime and cefepime against Pseudomonas aeruginosa strains. Braz. J. Microbiol. 2012, 43, 984–992. [Google Scholar] [CrossRef]
- Budai, M.; Budai, L.; Szilasi, M.; Petrikovics, I.; Wijesooriya, C. Optimization of liposomal encapsulation for ceftazidime for developing a potential eye drop formulation. J. Basic Clin. Pharm. 2013, 4, 73–85. [Google Scholar] [CrossRef]
- Farzampanah, L.; Chitsaz, M.; Tabandeh, H.; Hajihosseini, R.; Mansouri, S. Preparation and Investigation of In vitro Effect of Liposomal Ceftazidime on the Resistant Pseudomonas Aeruginosa. Adv. Stud. Biol. 2017, 9, 105–111. [Google Scholar] [CrossRef]
- Zhou, L.; Tong, X.; Sharma, P.; Xu, H.; Al-Huniti, N.; Zhou, D. Physiologically based pharmacokinetic modelling to predict exposure differences in healthy volunteers and subjects with renal impairment: Ceftazidime case study. Basic Clin. Pharmacol. Toxicol. 2019, 125, 100–107. [Google Scholar] [CrossRef]
- Alshamsan, A.; Aleanizy, F.S.; Badran, M.; Alqahtani, F.Y.; Alfassam, H.; Almalik, A.; Alosaimy, S. Exploring anti-MRSA activity of chitosan-coated liposomal dicloxacillin. J. Microbiol. Methods 2018, 156, 23–28. [Google Scholar] [CrossRef]
- Chen, H.; Pan, H.; Li, P.; Wang, H.; Wang, X.; Pan, W.; Yuan, Y. The potential use of novel chitosan-coated deformable liposomes in an ocular drug delivery system. Colloids Surf. B Biointerfaces 2016, 143, 455–462. [Google Scholar] [CrossRef]
- Alomrani, A.; Badran, M.; Harisa, G.I.; Alshehry, M.; Alhariri, M.; Alshamsan, A.; Alkholief, M. The use of chitosan-coated flexible liposomes as a remarkable carrier to enhance the antitumor efficacy of 5-fluorouracil against colorectal cancer. Saudi Pharm. J. 2019, 27, 603–611. [Google Scholar] [CrossRef]
- Lira, M.C.; Siqueira-Moura, M.P.; Rolim-Santos, H.M.; Galetti, F.C.; Simioni, A.R.; Santos, N.P.; Tabosa Do Egito, E.S.; Silva, C.L.; Tedesco, A.C.; Santos-Magalhães, N.S. In vitro uptake and antimycobacterial activity of liposomal usnic acid formulation. J. Liposome Res. 2009, 19, 49–58. [Google Scholar] [CrossRef]
- Cavalcanti, I.M.F.; Menezes, T.G.C.; Campos, L.A.d.A.; Ferraz, M.S.; Maciel, M.A.V.; Caetano, M.N.P.; Santos-Magalhães, N.S. Interaction study between vancomycin and liposomes containing natural compounds against methicillin-resistant Staphylococcus aureus clinical isolates. Braz. J. Pharm. Sci. 2018, 54, e00203. [Google Scholar] [CrossRef]
- Nunes, P.S.; Rabelo, A.S.; de Souza, J.C.C.; Santana, B.V.; da Silva, T.M.M.; Serafini, M.R.; Menezes, P.d.P.; Lima, B.d.S.; Cardoso, J.C.; Alves, J.C.S.; et al. Gelatin-based membrane containing usnic acid-loaded liposome improves dermal burn healing in a porcine model. Int. J. Pharm. 2016, 513, 473–482. [Google Scholar] [CrossRef]
- Sağıroğlu, A.A. Chitosan-coated liposome-containing carbamazepine and coenzyme Q10: Design, optimization and evaluation. J. Liposome Res. 2020, 31, 389–398. [Google Scholar] [CrossRef]
- Ran, L.; Chi, Y.; Huang, Y.; He, Q.; Ren, Y. Synergistic antioxidant effect of glutathione and edible phenolic acids and improvement of the activity protection by coencapsulation into chitosan-coated liposomes. LWT 2020, 127, 109409. [Google Scholar] [CrossRef]
- Eftekhari, R.B.; Maghsoudnia, N.; Samimi, S.; Zamzami, A.; Dorkoosh, F.A. Co-delivery nanosystems for cancer treatment: A review. Pharm. Nanotechnol. 2019, 7, 90–112. [Google Scholar] [CrossRef]
- Meng, R.; Wu, Z.; Xie, Q.-T.; Cheng, J.-S.; Zhang, B. Preparation and characterization of zein/carboxymethyl dextrin nanoparticles to encapsulate curcumin: Physicochemical stability, antioxidant activity and controlled release properties. Food Chem. 2020, 340, 127893. [Google Scholar] [CrossRef]
- Yokota, D.; Moraes, M.; Pinho, S.C. Characterization of lyophilized liposomes produced with non-purified soy lecithin: A case study of casein hydrolysate microencapsulation. Braz. J. Chem. Eng. 2012, 29, 325–335. [Google Scholar] [CrossRef]
- Umar, A.K.; Sriwidodo, S.; Maksum, I.P.; Wathoni, N. Film-Forming spray of water-soluble chitosan containing liposome-coated human epidermal growth factor for wound healing. Molecules 2021, 26, 5326. [Google Scholar] [CrossRef]
- Dafale, N.A.; Semwal, U.P.; Agarwal, P.K.; Sharma, P.; Singh, G.N. Quantification of ceftriaxone sodium in pharmaceutical preparations by a new validated microbiological bioassay. Anal. Methods 2012, 4, 2490–2498. [Google Scholar] [CrossRef]
- Osório, L.R.; Meneguin, A.B.; da Silva, H.B.; Barreto, H.M.; Osajima, J.A.; Filho, E.C.D.S. Evaluation of physico-chemical properties and antimicrobial synergic effect of ceftazidime-modified chitosan. J. Therm. Anal. Calorim. 2018, 134, 1629–1636. [Google Scholar] [CrossRef]
- Nunes, P.S.; Bezerra, M.S.; Costa, L.P.; Cardoso, J.C.; Albuquerque, R.L.C.; Rodrigues, M.O.; Barin, G.B.; da Silva, F.A.; Araújo, A.A.S. Thermal characterization of usnic acid/collagen-based films. J. Therm. Anal. Calorim. 2010, 99, 1011–1014. [Google Scholar] [CrossRef]
- Maulidiyah, M.; Darmawan, A.; Wahyu, W.; Musdalifah, A.; Salim, L.O.A.; Nurdin, M. Potential of Usnic Acid Compound from Lichen Genus Usnea sp. as Antidiabetic Agents. J. Oleo Sci. 2022, 71, 127–134. [Google Scholar] [CrossRef]
- Hasan, M.; Messaoud, G.B.; Michaux, F.; Tamayol, A.; Kahn, C.J.F.; Belhaj, N.; Linder, M.; Arab-Tehrany, E. Chitosan-coated liposomes encapsulating curcumin: Study of lipid–polysaccharide interactions and nanovesicle behavior. RSC Adv. 2016, 6, 45290–45304. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, T.; Liu, Y.; Wang, Q.; Xing, S.; Li, L.; Wang, L.; Liu, L.; Gao, D. Ursolic acid liposomes with chitosan modification: Promising antitumor drug delivery and efficacy. Mater. Sci. Eng. C 2017, 71, 1231–1240. [Google Scholar] [CrossRef]
- Hamedinasab, H.; Rezayan, A.H.; Mellat, M.; Mashreghi, M.; Jaafari, M.R. Development of chitosan-coated liposome for pulmonary delivery of N-acetylcysteine. Int. J. Biol. Macromol. 2019, 156, 1455–1463. [Google Scholar] [CrossRef]
- Huang, M.; Liang, C.; Tan, C.; Huang, S.; Ying, R.; Wang, Y.; Wang, Z.; Zhang, Y. Liposome co-encapsulation as a strategy for the delivery of curcumin and resveratrol. Food Funct. 2019, 10, 6447–6458. [Google Scholar] [CrossRef]
- Feuser, P.E.; Cordeiro, A.P.; Silveira, G.d.B.; Corrêa, M.E.A.B.; Silveira, P.C.L.; Sayer, C.; de Araújo, P.H.H.; Machado-De-Ávila, R.A.; Bó, A.G.D. Co-encapsulation of sodium diethyldithiocarbamate (DETC) and zinc phthalocyanine (ZnPc) in liposomes promotes increases phototoxic activity against (MDA-MB 231) human breast cancer cells. Colloids Surf. B Biointerfaces 2020, 197, 111434. [Google Scholar] [CrossRef]
- Somasekhar, T.; Javadi, M.; Sistla, R.; Mallavadhani, U.V. Synthesis of novel anti-inflammatory usnic acid-based imidazolium salts. Eur. Chem. Bull. 2021, 10, 67–72. [Google Scholar] [CrossRef]
- Khan, F.; Yu, H.; Kim, Y.-M. Bactericidal activity of usnic acid-chitosan nanoparticles against persister cells of biofilm-forming pathogenic bacteria. Mar. Drugs 2020, 18, 270. [Google Scholar] [CrossRef]
- Pereira, L.A.; da Silva Reis, L.; Batista, F.A.; Mendes, A.N.; Osajima, J.A.; Silva-Filho, E.C. Biological properties of chitosan derivatives associated with the ceftazidime drug. Carbohydr. Polym. 2019, 222, 115002. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, T.; Wei, S.; Zhou, C.; Lan, Y.; Cao, A.; Yang, J.; Wang, W. Mucus adhesion-and penetration-enhanced liposomes for paclitaxel oral delivery. Int. J. Pharm. 2018, 537, 245–256. [Google Scholar] [CrossRef]
- Caddeo, C.; Díez-Sales, O.; Pons, R.; Carbone, C.; Ennas, G.; Puglisi, G.; Fadda, A.M.; Manconi, M. Cross-linked chitosan/liposome hybrid system for the intestinal delivery of quercetin. J. Colloid Interface Sci. 2016, 461, 69–78. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Q.; Chu, L.; Xia, Q. Liposome-chitosan hydrogel bead delivery system for the encapsulation of linseed oil and quercetin: Preparation and in vitro characterization studies. LWT 2019, 117, 108615. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, X.; Fan, X.; Li, H.; Xu, B.; Li, X. Whey protein isolate coated liposomes as novel carrier systems for astaxanthin. Eur. J. Lipid Sci. Technol. 2020, 122, 1900325. [Google Scholar] [CrossRef]
- de Santana, M.S.A.; de Oliveira, Y.S.; de Castro Fonseca, J.; Ferreira, W.C.; Neto, V.S.; Ayala, A.P. Stability of ceftazidime pentahydrate investigated by thermal analysis techniques. J. Pharm. Sci. 2020, 109, 1324–1329. [Google Scholar] [CrossRef]
- Nkanga, C.I.; Krause, R.W.; Noundou, X.S.; Walker, R.B. Preparation and characterization of isoniazid-loaded crude soybean lecithin liposomes. Int. J. Pharm. 2017, 526, 466–473. [Google Scholar] [CrossRef]
- Moussout, H.; Ahlafi, H.; Aazza, M.; Bourakhouadar, M. Kinetics and mechanism of the thermal degradation of biopolymers chitin and chitosan using thermogravimetric analysis. Polym. Degrad. Stab. 2016, 130, 1–9. [Google Scholar] [CrossRef]
- Niaz, T.; Shabbir, S.; Noor, T.; Rahman, A.; Bokhari, H.; Imran, M. Potential of polymer stabilized nano-liposomes to enhance antimicrobial activity of nisin Z against foodborne pathogens. LWT 2018, 96, 98–110. [Google Scholar] [CrossRef]
- Liu, W.; Liu, W.; Ye, A.; Peng, S.; Wei, F.; Liu, C.; Han, J. Environmental stress stability of microencapsules based on liposomes decorated with chitosan and sodium alginate. Food Chem. 2016, 196, 396–404. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, C.; Ma, L.; Zou, L.; Liu, W.; Li, R.; Cao, Y.; Liu, Y.; Ruan, R.; Li, J. The formation of chitosan-coated rhamnolipid liposomes containing curcumin: Stability and in vitro digestion. Molecules 2021, 26, 560. [Google Scholar] [CrossRef]
- Chen, D.; Xia, D.; Li, X.; Zhu, Q.; Yu, H.; Zhu, C.; Gan, Y. Comparative study of Pluronic® F127-modified liposomes and chitosan-modified liposomes for mucus penetration and oral absorption of cyclosporine A in rats. Int. J. Pharm. 2013, 449, 1–9. [Google Scholar] [CrossRef]
- Hui, C.; Huang, H. A study on chitosan-coated liposomes as a carrier of bovine serum albumin as oral protein drug. J. Dispers. Sci. Technol. 2021, 42, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, G.M.; Ali, Y.; Boehm, B.O.; Huang, Y.Y.; Venkatraman, S. Layer-by-layer coated nanoliposomes for oral delivery of insulin. Nanoscale 2020, 13, 776–789. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, J.; Jiang, S.; Zeng, M. Effect of chitosan coating on the properties of nanoliposomes loaded with oyster protein hydrolysates: Stability during spray-drying and freeze-drying. Food Chem. 2022, 385, 132603. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, H.; Qin, Y.; Li, D.; Liu, Y.; Wang, H.; Gan, L. Overcoming drug-resistant lung cancer by paclitaxel-loaded hyaluronic acid-coated liposomes targeted to mitochondria. Drug Dev. Ind. Pharm. 2018, 44, 2071–2082. [Google Scholar] [CrossRef]
- Maciąg-Dorszyńska, M.; Węgrzyn, G.; Guzow-Krzemińska, B. Antibacterial activity of lichen secondary metabolite usnic acid is primarily caused by inhibition of RNA and DNA synthesis. FEMS Microbiol. Lett. 2014, 353, 57–62. [Google Scholar] [CrossRef]
- Luzina, O.A.; Salakhutdinov, N.F. Biological activity of usnic acid and its derivatives: Part 1. Activity against unicellular organisms. Russ. J. Bioorg. Chem. 2016, 42, 115–132. [Google Scholar] [CrossRef]
- Acosta-Gutiérrez, S.; Bodrenko, I.V.; Ceccarelli, M. The Influence of Permeability through Bacterial Porins in Whole-Cell Compound Accumulation. Antibiotics 2021, 10, 635. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Cotar, A.I.; Andronescu, E.; Ficai, A.; Ghitulica, C.D.; Grumezescu, V.; Vasile, B.S.; Chifiriuc, M.C. In vitro activity of the new water-dispersible Fe3O4@ usnic acid nanostructure against planktonic and sessile bacterial cells. J. Nanoparticle Res. 2013, 15, 1–10. [Google Scholar] [CrossRef]
- Pu, C.; Tang, W. A chitosan-coated liposome encapsulating antibacterial peptide, Apep10: Characterisation, triggered-release effects and antilisterial activity in thaw water of frozen chicken. Food Funct. 2016, 7, 4310–4322. [Google Scholar] [CrossRef]
- Jamil, K. Health effects of pharmaceuticals and personal care products. Pharm. Pers. Care Prod. Waste Manag. Treat. Technol. 2019, 6, 115–128. [Google Scholar]
- Sun, Q.; Zhou, Z.; Qiu, N.; Shen, Y. Rational design of cancer nanomedicine: Nanoproperty integration and synchronization. Adv. Mater. 2017, 29, 1606628. [Google Scholar] [CrossRef]
- Low, W.L.; Martin, C.; Hill, D.J.; Kenward, M.A. Antimicrobial efficacy of liposome-encapsulated silver ions and tea tree oil against Pseudomonas aeruginosa, Staphylococcus aureus and Candida albicans. Lett. Appl. Microbiol. 2013, 57, 33–39. [Google Scholar] [CrossRef]
- Aljihani, S.A.; Alehaideb, Z.; Alarfaj, R.E.; Alghoribi, M.F.; Akiel, M.A.; Alenazi, T.H.; Al-Fahad, A.J.; Al Tamimi, S.M.; Albakr, T.M.; Alshehri, A.; et al. Enhancing azithromycin antibacterial activity by encapsulation in liposomes/liposomal-N-acetylcysteine formulations against resistant clinical strains of Escherichia coli. Saudi J. Biol. Sci. 2020, 27, 3065–3071. [Google Scholar] [CrossRef]
- Alarfaj, R.E.; Alkhulaifi, M.M.; Al-Fahad, A.J.; Aljihani, S.; Yassin, A.E.B.; Alghoribi, M.F.; Halwani, M.A. Antibacterial Efficacy of Liposomal Formulations Containing Tobramycin and N-Acetylcysteine against Tobramycin-Resistant Escherichia coli, Klebsiella pneumoniae, and Acinetobacter baumannii. Pharmaceutics 2022, 14, 130. [Google Scholar] [CrossRef]
- Liou, J.M.; Lee, Y.C.; El-Omar, E.M.; Wu, M.S. Efficacy and long-term safety of H. pylori eradication for gastric cancer prevention. Cancers 2019, 11, 593. [Google Scholar] [CrossRef]
- Nouri, H.R.; Karkhah, A.; Varasteh, A.; Sankian, M. Expression of a chimeric allergen with high rare codons content in codon bias-adjusted Escherichia coli: Escherichia coli BL21 (DE3)-codon plus RIL as an efficient host. Curr. Microbiol. 2016, 73, 91–98. [Google Scholar] [CrossRef]
- Buc, E.; Dubois, D.; Sauvanet, P.; Raisch, J.; Delmas, J.; Darfeuille-Michaud, A.; Pezet, D.; Bonnet, R. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS ONE 2013, 8, e56964. [Google Scholar]
- Suzuki, H.; Mori, H. World trends for H. pylori eradication therapy and gastric cancer prevention strategy by H. pylori test-and-treat. J. Gastroenterol. 2017, 53, 354–361. [Google Scholar] [CrossRef]
- Costello, S.P.; Hughes, P.A.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Andrews, J.M. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: A randomized clinical trial. JAMA 2019, 321, 156–164. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; Xue, Z.; Zhai, Q.; et al. Lactobacillus plantarum CCFM8610 alleviates irritable bowel syndrome and prevents gut microbiota dysbiosis: A randomized, double-blind, placebo-controlled, pilot clinical trial. Engineering 2020, 7, 376–385. [Google Scholar] [CrossRef]
- Strati, F.; Pujolassos, M.; Burrello, C.; Giuffrè, M.R.; Lattanzi, G.; Caprioli, F.; Troisi, J.; Facciotti, F. Antibiotic-associated dysbiosis affects the ability of the gut microbiota to control intestinal inflammation upon fecal microbiota transplantation in experimental colitis models. Microbiome 2021, 9, 1–15. [Google Scholar] [CrossRef]
- Huang, F.; Li, S.; Chen, W.; Han, Y.; Yao, Y.; Yang, L.; Li, Q.; Xiao, Q.; Wei, J.; Liu, Z.; et al. Postoperative probiotics administration attenuates gastrointestinal complications and gut microbiota dysbiosis caused by chemotherapy in colorectal cancer patients. Nutrients 2023, 15, 356. [Google Scholar] [CrossRef]
- Heidari, F.; Akbarzadeh, I.; Nourouzian, D.; Mirzaie, A.; Bakhshandeh, H. Optimization and characterization of tannic acid loaded niosomes for enhanced antibacterial and anti-biofilm activities. Adv. Powder Technol. 2020, 31, 4768–4781. [Google Scholar] [CrossRef]
- Lu, C.; Liu, H.; Shangguan, W.; Chen, S.; Zhong, Q. Antibiofilm activities of the cinnamon extract against Vibrio parahaemolyticus and Escherichia coli. Arch. Microbiol. 2020, 203, 125–135. [Google Scholar] [CrossRef]
- Malaekeh-Nikouei, B.; Bazzaz, B.S.F.; Mirhadi, E.; Tajani, A.S.; Khameneh, B. The role of nanotechnology in combating biofilm-based antibiotic resistance. J. Drug Deliv. Sci. Technol. 2020, 60, 101880. [Google Scholar] [CrossRef]
- Alam, K.; Al Farraj, D.A.; Mah-E-Fatima, S.; Yameen, M.A.; Elshikh, M.S.; Alkufeidy, R.M.; Mustafa, A.E.-Z.M.; Bhasme, P.; Alshammari, M.K.; Alkubaisi, N.A.; et al. Anti-biofilm activity of plant derived extracts against infectious pathogen-Pseudomonas aeruginosa PAO1. J. Infect. Public Health 2020, 13, 1734–1741. [Google Scholar] [CrossRef]
- Thyagarajan, R.; Namasivayam, S.K.R.; Narendrakumar, G.; Singh, V.; Samydurai, S. Evaluation of in vitro drug controlled release of biocompatible metallic and non metallic nanoparticles incorporated anti bacterial antibiotics and their anti biofilm activity against E. coli. Res. J. Pharm. Technol. 2015, 8, 316–319. [Google Scholar] [CrossRef]
- Díez-Aguilar, M.; Ekkelenkamp, M.; Morosini, M.-I.; Huertas, N.; del Campo, R.; Zamora, J.; Fluit, A.C.; Tunney, M.M.; Obrecht, D.; Bernardini, F.; et al. Anti-biofilm activity of murepavadin against cystic fibrosis Pseudomonas aeruginosa isolates. J. Antimicrob. Chemother. 2021, 76, 2578–2585. [Google Scholar] [CrossRef]
- Wan, B.; Zhu, Y.; Tao, J.; Zhu, F.; Chen, J.; Li, L.; Zhao, J.; Wang, L.; Sun, S.; Yang, Y.; et al. Alginate lyase guided silver nanocomposites for eradicating Pseudomonas aeruginosa from lungs. ACS Appl. Mater. Interfaces 2020, 12, 9050–9061. [Google Scholar] [CrossRef]
- Cavalcanti, I.M.; Mendonça, E.A.; Lira, M.C.; Honrato, S.B.; Camara, C.A.; Amorim, R.V.; Filho, J.M.; Rabello, M.M.; Hernandes, M.Z.; Ayala, A.P.; et al. The encapsulation of β-lapachone in 2-hydroxypropyl-β-cyclodextrin inclusion complex into liposomes: A physicochemical evaluation and molecular modeling approach. Eur. J. Pharm. Sci. 2011, 44, 332–340. [Google Scholar] [CrossRef]
- Chen, S.; Han, Y.; Jian, L.; Liao, W.; Zhang, Y.; Gao, Y. Fabrication, characterization, physicochemical stability of zein-chitosan nanocomplex for co-encapsulating curcumin and resveratrol. Carbohydr. Polym. 2020, 236, 116090. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Wang, X.; Zhou, Y.; Gao, X.; Puligundla, P.; Wan, X. Encapsulation of epigallocatechin gallate in zein/chitosan nanoparticles for controlled applications in food systems. Food Chem. 2017, 231, 19–24. [Google Scholar] [CrossRef]
- Rosasco, M.A.; Bonafede, S.L.; Faudone, S.N.; Segall, A.I. Compatibility study of tobramycin and pharmaceutical excipients using differential scanning calorimetry, FTIR, DRX, and HPLC. J. Therm. Anal. Calorim. 2018, 134, 1929–1941. [Google Scholar] [CrossRef]
- Park, C.-E.; Park, D.-J.; Kim, B.-K. Effects of a chitosan coating on properties of retinol-encapsulated zein nanoparticles. Food Sci. Biotechnol. 2015, 24, 1725–1733. [Google Scholar] [CrossRef]
- Cavalcanti, I.D.L.; Ximenes, R.M.; Pessoa, O.D.L.; Magalhães, N.S.S.; Lira-Nogueira, M.C.D.B. Fucoidan-coated PIBCA nanoparticles containing oncocalyxone A: Activity against metastatic breast cancer cells. J. Drug Deliv. Sci. Technol. 2021, 65, 102698. [Google Scholar] [CrossRef]
- Campos, L.A.d.A.; Neto, A.F.S.; Scavuzzi, A.M.L.; Lopes, A.C.D.S.; Santos-Magalhães, N.S.; Cavalcanti, I.M.F. Ceftazidime/Tobramycin Co-Loaded Chitosan-Coated Zein Nanoparticles against Antibiotic-Resistant and Biofilm-Producing Pseudomonas aeruginosa and Klebsiella pneumoniae. Pharmaceuticals 2024, 17, 320. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; (M100-S31); Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- Peeters, E.; Hooyberghs, G.; Robijns, S.; Waldrant, K.; De Weerdt, A.; Delattin, N.; Liebens, V.; Kucharíková, S.; Tournu, H.; Verstraeten, N.; et al. Modulation of the Substitution Pattern of 5-Aryl-2-Aminoimidazoles Allows Fine-Tuning of Their Antibiofilm Activity Spectrum and Toxicity. Antimicrob. Agents Chemother. 2016, 60, 6483–6497. [Google Scholar] [CrossRef]





| Formulation | Ø (nm) | PDI | ζ (mV) | pH | Content (%) | %EE |
|---|---|---|---|---|---|---|
| Lipo-NChi | 106.1 ± 12.5 | 0.244 | −2.2 ± 1.5 | 7.7 | - | - |
| Lipo-CAZ-Chi | 116.5 ± 5.3 | 0.401 | +16.4 ± 0.6 | 5.2 | 98.5 ± 0.4 | 51.5 ± 0.2 |
| Lipo-UA-Chi | 136.6 ± 5.1 | 0.465 | +28 ± 0.8 | 5.0 | 98.9 ± 0.3 | 99.9 ± 0.1 |
| Lipo-CAZ-UA-Chi | 240.3 ± 3.5 | 0.411 | +24.9 ± 0.1 | 5.0 | 98.3 ± 0.1 (CAZ) | 45.2 ± 0.5 (CAZ) |
| 98.7 ± 0.6 (UA) | 95.6 ± 0.3 (UA) | |||||
| Lipo-CAZ-Chi + Lipo-UA-Chi | 212.8 ± 0.7 | 0.563 | +22.9 ± 1.2 | 5.2 | 98.2 ± 0.2 (CAZ) | 50.7 ± 3 (CAZ) |
| 98.5 ± 0.4% (UA) | 98.9 ± 0.5 (UA) |
| Months | |||
|---|---|---|---|
| Parameters | 1 | 2 | 3 |
| Ø (nm) | 118.4 ± 4.3 | 159.9 ± 7.1 | 201.8 ± 2.0 |
| PDI | 0.412 | 0.612 | 0.612 |
| ζ (mV) | +17.6 ± 1.0 | +12.5 ± 3.5 | +9.1 ± 2.3 |
| pH | 5.1 | 4.5 | 4.5 |
| CAZ content (%) | 98.1 ± 0.2 | 95.1 ± 0.9 | 95.4 ± 1.0 |
| CAZ %EE | 50.9 ± 0.5 | 47.4 ± 0.2 | 40.2 ± 0.7 |
| Months | |||
|---|---|---|---|
| Parameters | 1 | 2 | 3 |
| Ø (nm) | 141.3 ± 4.3 | 289.9 ± 7.1 | 303.5 ± 6.4 |
| PDI | 0.456 | 0.636 | 0.726 |
| ζ (mV) | +24.6 ± 2.2 | +15.8 ± 4.5 | +7.8 ± 3.1 |
| AU content (%) | 95.2 ± 1.2 | 94.2 ± 1.5 | 92.7 ± 0.2 |
| AU %EE | 99.9 ± 0.3 | 90.2 ± 1.0 | 80.1 ± 0.6 |
| Months | |||
|---|---|---|---|
| Lipo-CAZ-AU-Chi | |||
| Parameters | 1 | 2 | 3 |
| Ø (nm) | 235.2 ± 7.4 | 256.4 ± 2.4 | 345.1 ± 2.0 |
| PDI | 0.419 | 0.471 | 0.526 |
| ζ (mV) | +19.1 ± 4.3 | +17.1 ± 2.2 | +9.1 ± 2.0 |
| pH | 5.0 | 4.9 | 4.7 |
| CAZ content (%) | 98.1 ± 0.5 | 95.8 ± 0.1 | 92.6 ± 0.3 |
| CAZ %EE | 45.1 ± 0.2 | 42.4 ± 1.0 | 38.4 ± 0.6 |
| AU content (%) | 96.9 ± 0.5 | 91.2 ± 0.4 | 91.6 ± 0.3 |
| AU %EE | 94.9 ± 0.4 | 79.2 ± 0.1 | 48.4 ± 0.6 |
| Lipo-CAZ-Chi + Lipo-AU-Chi | |||
| Parameters | 1 | 2 | 3 |
| Ø (nm) | 232.4 ± 2.5 | 91.5 ± 0.1 | 326.2 ± 2.7 |
| PDI | 0.632 | 0.701 | 0.821 |
| ζ (mV) | +19.3 ± 1.6 | +7.1 ± 0.6 | +4.1 ± 1.0 |
| pH | 5.0 | 4.8 | 3.6 |
| CAZ content (%) | 91.2 ± 0.6 | 90.6 ± 1.0 | 92.4 ± 2.6 |
| CAZ %EE | 45.6 ± 2.1 | 30.1 ± 2.0 | 25.1 ± 0.2 |
| AU content (%) | 97.5 ± 1.0 | 96.4 ± 0.2 | 94.9 ± 0.9 |
| AU %EE | 91.5 ± 0.1 | 68.1 ± 3.0 | 54.6 ± 1.2 |
| Bacteria | CAZ | UA | Lipo-CAZ-Chi | Lipo-UA-Chi | Lipo-CAZ-UA-Chi | Lipo-CAZ-Chi + Lipo-UA-Chi | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| µg/mL | ||||||||||||
| Escherichia coli ATCC 25922 | 1.95 | 1.95 | 500 | 1000 | 0.062 | 0.062 | 62.5 | 62.5 | 0.975/0.975 | 0.975/0.975 | 0.031/0.488 | 0.031/0.488 |
| E. coli NCTC 13846 | 1.95 | 1.95 | 1000 | 1000 | 0.125 | 0.250 | 62.5 | 62.5 | 1.95/1.95 | 1.95/1.95 | 0.062/0.976 | 0.062/0.976 |
| E. coli H10407 | 1.95 | 1.95 | 250 | 1000 | 0.125 | 0.125 | 62.5 | 62.5 | 0.975/0.975 | 0.975/0.975 | 0.062/0.975 | 0.062/0.975 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, J.B.; de Lacerda Coriolano, D.; dos Santos Silva, R.C.; da Costa Júnior, S.D.; de Almeida Campos, L.A.; Cavalcanti, I.D.L.; Lira Nogueira, M.C.d.B.; Pereira, V.R.A.; Brelaz-de-Castro, M.C.A.; Cavalcanti, I.M.F. Ceftazidime and Usnic Acid Encapsulated in Chitosan-Coated Liposomes for Oral Administration against Colorectal Cancer-Inducing Escherichia coli. Pharmaceuticals 2024, 17, 802. https://doi.org/10.3390/ph17060802
de Souza JB, de Lacerda Coriolano D, dos Santos Silva RC, da Costa Júnior SD, de Almeida Campos LA, Cavalcanti IDL, Lira Nogueira MCdB, Pereira VRA, Brelaz-de-Castro MCA, Cavalcanti IMF. Ceftazidime and Usnic Acid Encapsulated in Chitosan-Coated Liposomes for Oral Administration against Colorectal Cancer-Inducing Escherichia coli. Pharmaceuticals. 2024; 17(6):802. https://doi.org/10.3390/ph17060802
Chicago/Turabian Stylede Souza, Jaqueline Barbosa, Davi de Lacerda Coriolano, Rayza Camila dos Santos Silva, Sérgio Dias da Costa Júnior, Luís André de Almeida Campos, Iago Dillion Lima Cavalcanti, Mariane Cajubá de Britto Lira Nogueira, Valéria Rêgo Alves Pereira, Maria Carolina Accioly Brelaz-de-Castro, and Isabella Macário Ferro Cavalcanti. 2024. "Ceftazidime and Usnic Acid Encapsulated in Chitosan-Coated Liposomes for Oral Administration against Colorectal Cancer-Inducing Escherichia coli" Pharmaceuticals 17, no. 6: 802. https://doi.org/10.3390/ph17060802
APA Stylede Souza, J. B., de Lacerda Coriolano, D., dos Santos Silva, R. C., da Costa Júnior, S. D., de Almeida Campos, L. A., Cavalcanti, I. D. L., Lira Nogueira, M. C. d. B., Pereira, V. R. A., Brelaz-de-Castro, M. C. A., & Cavalcanti, I. M. F. (2024). Ceftazidime and Usnic Acid Encapsulated in Chitosan-Coated Liposomes for Oral Administration against Colorectal Cancer-Inducing Escherichia coli. Pharmaceuticals, 17(6), 802. https://doi.org/10.3390/ph17060802








