Oral Administration of Efavirenz Dysregulates the Tph2 Gene in Brain Serotonergic Areas and Alters Weight and Mood in Mice
Abstract
1. Introduction
2. Results
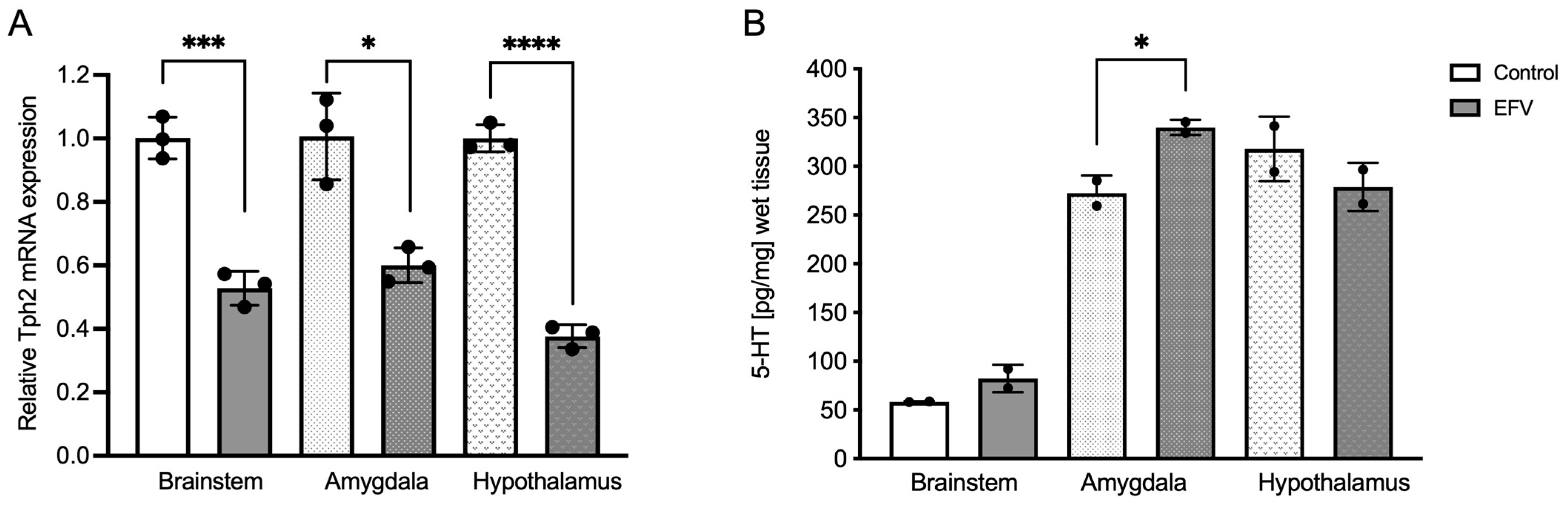
2.1. Effects of EFV on Tph2 Expression in the Brainstem, Hypothalamus, and Amygdala in Mice
2.2. Effects of EFV on 5-HT Levels in the Brainstem, Hypothalamus, and Amygdala in Mice
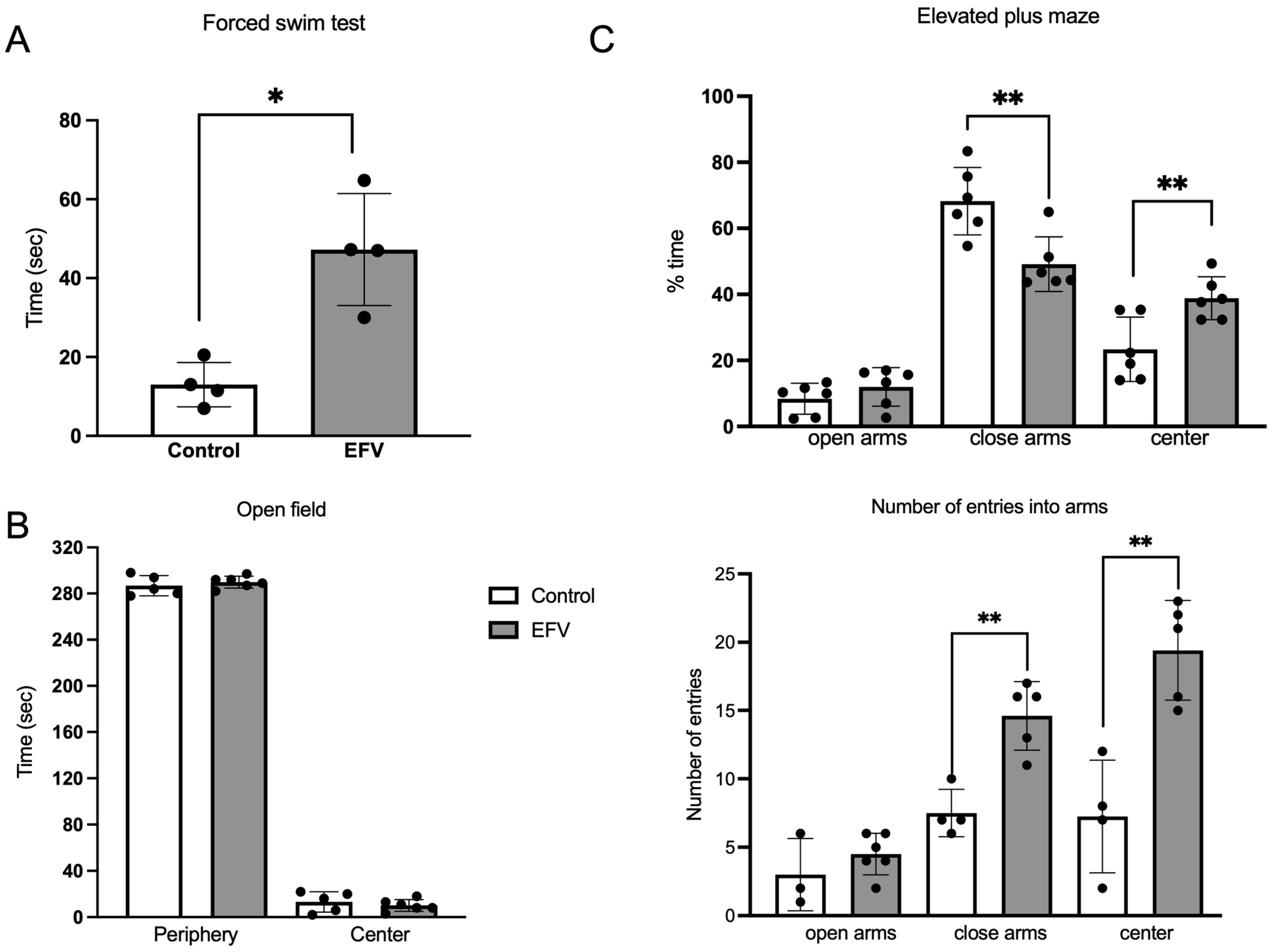
2.3. Effects of EFV on Behavior in Mice
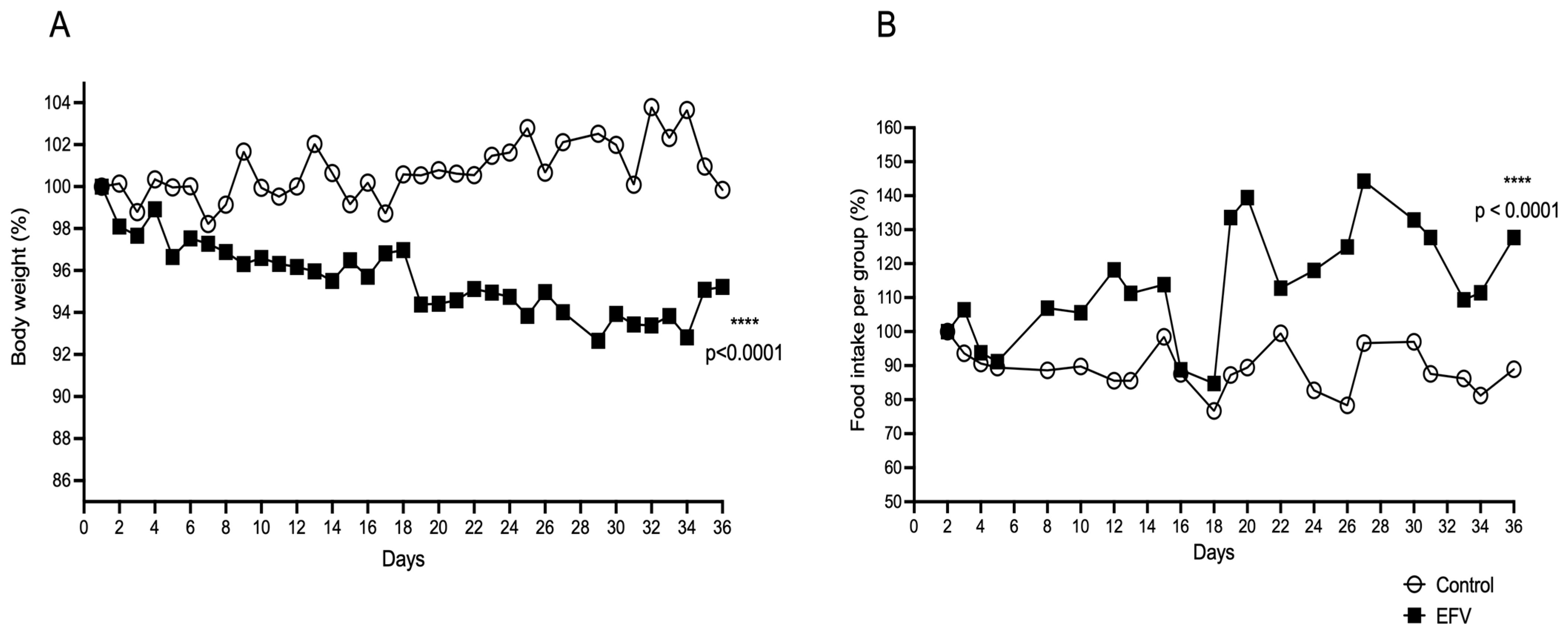
2.4. Effects of EFV on Body Weight and Food Intake in Mice
3. Discussion
4. Materials and Methods
4.1. Mice and Ethical Aspects
4.2. Pharmacological Treatment
4.3. RNA Preparation and Tph2 Expression Analysis by Quantitative Real-Time PCR
4.4. Brain 5-HT Measurements
4.5. Behavioral Assays
4.5.1. Forced Swim Test
4.5.2. Elevated Plus Maze
4.5.3. Open-Field Test
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutiérrez, F.; García, L.; Padilla, S.; Alvarez, D.; Moreno, S.; Navarro, G.; Gómez-Sirvent, J.; Vidal, F.; Asensi, V.; Masiá, M. Risk of clinically significant depression in HIV infected patients: Effect of antiretroviral drugs. HIV Med. 2014, 15, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Grimmig, B.; Izzo, J.; Brown, L.A.M.; Hudson, C.; Smith, A.J.; Tan, J.; Bickford, P.C.; Giunta, B. HIV Non-Nucleoside Reverse Transcriptase Inhibitor Efavirenz Reduces Neural Stem Cell Proliferation in Vitro and in Vivo. Cell Transplant. 2016, 25, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Sumari-de Boer, M.; Schellekens, A.; Duinmaijer, A.; Lalashowi, J.M.; Swai, H.J.; de Mast, Q.; van der Ven, A.; Kinabo, G. Efavirenz is related to neuropsychiatric symptoms among adults, but not among adolescents living with human immunodeficiency virus in Kilimanjaro, Tanzania. Trop. Med. Int. Health 2018, 23, 164–172. [Google Scholar] [CrossRef]
- Clifford, D.B.; Evans, S.; Yang, Y.; Acosta, E.P.; Ribaudo, H.; Gulick, R.M. A5097s Study Team. Long-term impact of efavirenz on neuropsychological performance and symptoms in HIV-infected individuals (ACTG 5097s). HIV Clin. Trials 2009, 10, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Vaida, F.; Wong, J.; Sanders, C.A.; Kao, Y.T.; Croteau, D.; Clifford, D.B.; Collier, A.C.; Gelman, B.B.; Marra, C.M.; et al. Long-term efavirenz use is associated with worse neurocognitive functioning in HIV-infected patients. J. Neurovirol. 2016, 22, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Dalwadi, D.A.; Kim, S.; Amdani, S.M.; Chen, Z.; Huang, R.Q.; Schetz, J.A. Molecular mechanisms of serotonergic action of the HIV-1 antiretroviral efavirenz. Pharmacol. Res. 2016, 110, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.W.; Ribaudo, H.J.; Kim, R.B.; Tierney, C.; Wilkinson, G.R.; Gulick, R.M.; Clifford, D.B.; Hulgan, T.; Marzolini, C.; Acosta, E.P. Pharmacogenetics of efavirenz and central nervous system side effects: An Adult AIDS Clinical Trials Group study. AIDS 2004, 18, 2391–2400. [Google Scholar] [PubMed]
- Rotger, M.; Colombo, S.; Furrer, H.; Bleiber, G.; Buclin, T.; Lee, B.L.; Keiser, O.; Biollaz, J.; Décosterd, L.; Telenti, A. Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenet. Genom. 2005, 15, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, S.; Stenz Justesen, U.; Von Lüttichau, H.R.; Eg Hansen, A.B. Genotyping of CYP2B6 and therapeutic drug monitoring in an HIV-infected patient with high efavirenz plasma concentrations and severe CNS side effects. Scand. J. Infect. Dis. 2006, 38, 733–735. [Google Scholar] [CrossRef]
- Desta, Z.; Saussele, T.; Ward, B.; Blievernicht, J.; Li, L.; Klein, K.; Flockhart, D.A.; Zanger, U.M. Impact of CYP2B6 polymorphism on hepatic efavirenz metabolism in vitro. Pharmacogenomics 2007, 8, 547–558. [Google Scholar] [CrossRef]
- Gounden, V.; Van Niekerk, C.; Snyman, T.; George, J.A. Presence of the CYP2B6 516G.T polymorphism, increased plasma efavirenz concentrations and early neuropsychiatric side effects in South African HIV-infected patients. AIDS Res. Ther. 2010, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.F.; Neiner, A.; Kharasch, E.D. Efavirenz Metabolism: Influence of Polymorphic CYP2B6 Variants and Stereochemistry. Drug Metab. Dispos. Biol. Fate Chem. 2019, 47, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Kwara, A.; Lartey, M.; Sagoe, K.W.; Rzek, N.L.; Court, M.H. CYP2B6 (c.516G-T) and CYP2A6 (*9B and/or *17) polymorphisms are independent predictors of efavirenz plasma concentrations in HIV-infected patients. Br. J. Clin. Pharmacol. 2009, 67, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Lowenhaupt, E.A.; Matson, K.; Qureishi, B.; Saitoh, A.; Pugatch, D. Psychosis in a 12-year-old HIV-positive girl with an increased serum concentration of efavirenz. Clin. Infect. Dis. 2007, 45, e128–e130. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, G.; Ramesh, K.; Hemanth Kumar, A.K.; Jagan, I.; Vasantha, M.; Padmapriyadarsini, C.; Narendran, G.; Rajasekaran, S.; Swaminathan, S. Association of high T allele frequency of CYP2B6 G516T polymorphism among ethnic south Indian HIV-infected patients with elevated plasma efavirenz and nevirapine. J. Antimicrob. Chemother. 2009, 63, 841–843. [Google Scholar] [CrossRef][Green Version]
- Saitoh, A.; Fletcher, C.V.; Brundage, R.; Alvero, C.; Fenton, T.; Hsia, K.; Spector, S.A. Efavirenz pharmacokinetics in HIV-1-infected children are associated with CYP2B6-G516T polymorphism. J. Acquir. Immune Defic. Syndr. 2007, 45, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Ribaudo, H.J.; Haas, D.W.; Tierney, C.; Kim, R.B.; Wilkinson, G.R.; Gulick, R.M.; Clifford, D.B.; Marzolini, C.; Fletcher, C.V.; Tashima, K.T.; et al. Pharmacogenetics of plasma efavirenz exposure after treatment discontinuation: An Adult AIDS Clinical Trials Group study. Clin. Infect. Dis. 2006, 42, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Swart, M.; Skelton, M.; Ren, Y.; Smith, P.; Takuva, S.; Dandara, C. High predictive value of CYP2B6 SNPs for steady-state plasma efavirenz levels in South African HIV/AIDS patients. Pharmacogenet. Genom. 2013, 23, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Streck, E.L.; Ferreira, G.K.; Scaini, G.; Rezin, G.T.; Gonçalves, C.L.; Jeremias, I.C.; Zugno, A.I.; Ferreira, G.C.; Moreira, J.; Fochesato, C.M.; et al. Non-nucleoside reverse transcriptase inhibitors efavirenz and nevirapine inhibit cytochrome C oxidase in mouse brain regions. Neurochem. Res. 2011, 36, 962–966. [Google Scholar] [CrossRef]
- Montenegro-Burke, J.R.; Woldstad, C.J.; Fang, M.; Bade, A.N.; McMillan, J.; Edagwa, B.; Boska, M.D.; Gendelman, H.E.; Siuzdak, G. Nanoformulated Antiretroviral Therapy Attenuates Brain Metabolic Oxidative Stress. Mol. Neurobiol. 2019, 56, 2896–2907. [Google Scholar] [CrossRef]
- Dalwadi, D.A.; Ozuna, L.; Harvey, B.H.; Viljoen, M.; Schetz, J.A. Adverse Neuropsychiatric Events and Recreational Use of Efavirenz and Other HIV-1 Antiretroviral Drugs. Pharmacol. Rev. 2018, 70, 684–711. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, G.I.; Chaves Filho, A.J.; Linhares, M.I.; de Carvalho Lima, C.N.; Venâncio, E.T.; Rios, E.R.; de Souza, F.C.; Vasconcelos, S.M.; Macêdo, D.; de França Fonteles, M.M. HIV antiretroviral drug Efavirenz induces anxiety-like and depression-like behavior in rats: Evaluation of neurotransmitter alterations in the striatum. Eur. J. Pharmacol. 2017, 799, 7–15. [Google Scholar] [CrossRef]
- Streck, E.L.; Scaini, G.; Rezin, G.T.; Moreira, J.; Fochesato, C.M.; Romão, P.R.T. Effects of the HIV treatment drugs nevirapine and efavirenz on brain creatine kinase activity. Metab. Brain Dis. 2008, 23, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Edagha, I.A.; Ekanem, A.U.; Usoh, I.F.; Umoh, V.A.; Ataben, A.M.; Akpan, A.A. Brain antioxidants and hippocampal microanatomical alterations following the administration of Efavirenz/Lamivudine/Tenofovir disoproxil fumarate and Lamivudine/Nevirapine/Zidovudine in adult male Wistar rats. IBRO Neurosci. Rep. 2022, 12, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Romão, P.R.; Lemos, J.C.; Moreira, J.; de Chaves, G.; Moretti, M.; Castro, A.A.; Andrade, V.M.; Boeck, C.R.; Quevedo, J.; Gavioli, E.C. Anti-HIV drugs nevirapine and efavirenz affect anxiety-related behavior and cognitive performance in mice. Neurotox. Res. 2011, 19, 73–80. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Myint, A.M.; Steinbusch, H.; Leonard, B.E. Efavirenz induces depressive-like behaviour, increased stress response and changes in the immune response in rats. Neuroimmunomodulation 2005, 12, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Ntshangase, S.; Mdanda, S.; Singh, S.D.; Naicker, T.; Kruger, H.G.; Baijnath, S.; Govender, T. Mass Spectrometry Imaging Demonstrates the Regional Brain Distribution Patterns of Three First-Line Antiretroviral Drugs. ACS Omega 2019, 4, 21169–21177. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, B.; Pratelli, M.; Migliarini, S.; Pacini, G.; Pasqualetti, M. Generation of a Tph2 Conditional Knockout Mouse Line for Time- and Tissue-Specific Depletion of Brain Serotonin. PLoS ONE 2015, 10, e0136422. [Google Scholar] [CrossRef] [PubMed]
- Tesoro-Cruz, E.; Oviedo, N.; Manuel-Apolinar, L.; Orozco-Suárez, S.; Pérez de la Mora, M.; Martínez-Pérez, G.; Guerra-Castillo, F.X.; Aguirre-Alvarado, C.; Bekker-Méndez, V.C. Ophthalmic Administration of a DNA Plasmid Harboring the Murine Tph2 Gene: Evidence of Recombinant Tph2-FLAG in Brain Structures. Mol. Biotechnol. 2020, 62, 200–209. [Google Scholar] [CrossRef]
- Tesoro-Cruz, E.; Manuel-Apolinar, L.; Oviedo, N.; Orozco-Suárez, S.; Crespo Ramírez, M.; Bekker-Méndez, V.C.; Aguirre-García, M.M.; Rojas-Osornio, S.A.; Paredes-Cervantes, V.; Pérez de la Mora, M. Increase of 5-HT levels is induced both in mouse brain and HEK-293 cells following their exposure to a non-viral tryptophan hydroxylase construct. Transl. Psychiatry 2021, 11, 515. [Google Scholar] [CrossRef]
- Gaida, R.; Truter, I.; Grobler, C.; Kotze, T.; Godman, B. A review of trials investigating efavirenz-induced neuropsychiatric side effects and the implications. Expert Rev. Anti-Infect. Ther. 2016, 14, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Mave, V.; Erlandson, K.M.; Gupte, N.; Balagopal, A.; Asmuth, D.M.; Campbell, T.B.; Smeaton, L.; Kumarasamy, N.; Hakim, J.; Santos, B.; et al. Inflammation and Change in Body Weight with Antiretroviral Therapy Initiation in a Multinational Cohort of HIV-Infected Adults. J. Infect. Dis. 2016, 214, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Erlandson, K.M.; Kitch, D.; Tierney, C.; Sax, P.E.; Daar, E.S.; Tebas, P.; Melbourne, K.; Ha, B.; Jahed, N.C.; McComsey, G.A. Weight and lean body mass change with antiretroviral initiation and impact on bone mineral density. AIDS 2013, 27, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.J.; Ho, M.W.; Lai, C.H.; Chou, C.H.; Li, J.P.; Cheng, C.F.; Wu, Y.C.; Liu, X.; Tsang, H.; Lin, T.H.; et al. Evaluation of Oral Antiretroviral Drugs in Mice with Metabolic and Neurologic Complications. Front. Pharmacol. 2018, 9, 1004. [Google Scholar] [CrossRef] [PubMed]
- Zareifopoulos, N.; Lagadinou, M.; Karela, A.; Pouliasi, F.; Economou, I.; Tsigkou, A.; Velissaris, D. Efavirenz as a psychotropic drug. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10729–10735. [Google Scholar] [PubMed]
- Frick, A.; Åhs, F.; Engman, J.; Jonasson, M.; Alaie, I.; Björkstrand, J.; Frans, Ö.; Faria, V.; Linnman, C.; Appel, L.; et al. Serotonin Synthesis and Reuptake in Social Anxiety Disorder: A Positron Emission Tomography Study. JAMA Psychiatry 2015, 72, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Hanley, N.R.; Hensler, J.G. Mechanisms of ligand-induced desensitization of the 5-hydroxytryptamine(2A) receptor. J. Pharmacol. Exp. Ther. 2002, 300, 468–477. [Google Scholar] [CrossRef]
- Waider, J.; Proft, F.; Langlhofer, G.; Asan, E.; Lesch, K.P.; Gutknecht, L. GABA concentration and GABAergic neuron populations in limbic areas are differentially altered by brain serotonin deficiency in Tph2 knockout mice. Histochem. Cell Biol. 2013, 139, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.D.; Zhu, Y.; Sun, Q.X.; Deng, F.; Wan, J.; Zheng, D.; Gong, W.; Xie, S.Z.; Shen, C.J.; Fu, J.Y.; et al. Distinct serotonergic pathways to the amygdala underlie separate behavioral features of anxiety. Nat. Neurosci. 2022, 25, 1651–1663. [Google Scholar] [CrossRef]
- de Oliveira, H.M.; Damiani, A.P.; Dias Rde, O.; Romão, P.R.; Andrade, V.M. Effect of antiretroviral drugs on the DNA damage in mice. Environ. Toxicol. Pharmacol. 2014, 37, 390–395. [Google Scholar] [CrossRef]
- Albert, P.R.; Vahid-Ansari, F. The 5HT1A receptor: Signaling to behaviour. Biochimie 2019, 161, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Gatch, M.B.; Kozlenkov, A.; Huang, R.Q.; Yang, W.; Nguyen, J.D.; González-Maeso, J.; Rice, K.C.; France, C.P.; Dillon, G.H.; Forster, M.J.; et al. The HIV antiretroviral drug efavirenz has LSD-like properties. Neuropsychopharmacology 2013, 38, 2373–2384. [Google Scholar] [CrossRef] [PubMed]
- Pérez de la Mora, M.; Borroto-Escuela, D.O.; Crespo-Ramírez, M.; Rejón-Orantes, J.D.C.; Palacios-Lagunas, D.A.; Martínez-Mata, M.K.; Sánchez-Luna, D.; Tesoro-Cruz, E.; Fuxe, K. Dysfunctional Heteroreceptor Complexes as Novel Targets for the Treatment of Major Depressive and Anxiety Disorders. Cells 2022, 11, 1826. [Google Scholar] [CrossRef] [PubMed]
- Borroto-Escuela, D.O.; Ambrogini, P.; Chruścicka, B.; Lindskog, M.; Crespo-Ramirez, M.; Hernández-Mondragón, J.C.; Perez de la Mora, M.; Schellekens, H.; Fuxe, K. The Role of Central Serotonin Neurons and 5-HT Heteroreceptor Complexes in the Pathophysiology of Depression: A Historical Perspective and Future Prospects. Int. J. Mol. Sci. 2021, 22, 1927. [Google Scholar] [CrossRef] [PubMed]
- Gutknecht, L.; Jacob, C.; Strobel, A.; Kriegebaum, C.; Müller, J.; Zeng, Y.; Markert, C.; Escher, A.; Wendland, J.; Reif, A.; et al. Tryptophan hydroxylase-2 gene variation influences personality traits and disorders related to emotional dysregulation. Int. J. Neuropsychopharmacol. 2007, 10, 309–320. [Google Scholar]
- Stamm, S.; Gruber, S.B.; Rabchevsky, A.G.; Emeson, R.B. The activity of the serotonin receptor 2C is regulated by alternative splicing. Hum. Genet. 2017, 136, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.W.; Woods, S.C.; Porte DJr Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef] [PubMed]
- van Galen, K.A.; Ter Horst, K.W.; Serlie, M.J. Serotonin, food intake, and obesity. Obes. Rev. 2021, 22, e13210. [Google Scholar] [CrossRef]
- Nogueiras, R.; Sabio, G. Brain JNK and metabolic disease. Diabetologia 2021, 64, 265–274. [Google Scholar] [CrossRef]
- Collazos, J.; Ibarra, S.; Loureiro, M. Cortisol serum levels and their relationship to certain antiretroviral drugs. Scand. J. Infect. Dis. 2004, 36, 480–482. [Google Scholar] [CrossRef]
- Sension, M.; Deckx, H. Lipid metabolism and lipodystrophy in HIV-1-infected patients: The role played by nonnucleoside reverse transcriptase inhibitors. AIDS Rev. 2015, 17, 21–36. [Google Scholar]
- Sinxadi, P.Z.; McIlleron, H.M.; Dave, J.A.; Smith, P.J.; Levitt, N.S.; Haas, D.W.; Maartens, G. Plasma Efavirenz Concentrations Are Associated With Lipid and Glucose Concentrations. Medicine 2016, 95, e2385. [Google Scholar] [CrossRef] [PubMed]
- Lagathu, C.; Béréziat, V.; Gorwood, J.; Fellahi, S.; Bastard, J.P.; Vigouroux, C.; Boccara, F.; Capeau, J. Metabolic complications affecting adipose tissue, lipid and glucose metabolism associated with HIV antiretroviral treatment. Expert Opin. Drug Saf. 2019, 18, 829–840. [Google Scholar] [CrossRef]
- Khemla, S.; Meesing, A.; Sribenjalux, W.; Chetchotisakd, P. Lipid profiles of people with human immunodeficiency virus with dyslipidemia after switching from efavirenz to dolutegravir. Drug Target Insights 2023, 17, 45–53. [Google Scholar] [CrossRef]
- Baza Caraciolo, B.; Pérez de Oteyza, C.; Carrió Montiel, D.; Carrió Montiel, J.C.; Salguero Aparicio, M.; del Romero Guerrero, J. Perfil lipídico en pacientes VIH (+) no tratados: Infección VIH: ¿factor de riesgo cardiovascular? An. De Med. Interna 2007, 24, 160–167. [Google Scholar]
- NOM-062-Z00-1999; Norma Oficial Mexicana Para la Producción, Cuidado y Uso de los Animales de Laboratorio. NOM: Mexico, Chapter 9.
- Apostolova, N.; Funes, H.A.; Blas-Garcia, A.; Galindo, M.J.; Alvarez, A.; Esplugues, J.V. Efavirenz and the CNS: What we already know and questions that need to be answered. J. Antimicrob. Chemother. 2015, 70, 2693–2708. [Google Scholar] [CrossRef]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The forced swim test as a model of depressive-like behavior. J. Vis. Exp. 2015, 97, 52587. [Google Scholar]
- Porsolt, R.D.; Le Pichon, M.; Jalfre, M. Depression: A new animal model sensitive to antidepressant treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open: Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Pérez de la Mora, M.; Gallegos-Cari, A.; Crespo-Ramírez, M.; Marcellino, D.; Chansson, A.C.; Fuxe, K. Distribution of dopamine D2-like receptors in the rat amygdale and their role in the modulation of unconditioned fear and anxiety. Neuroscience 2012, 201, 252–266. [Google Scholar] [CrossRef]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V. Measuring grooming in stress and comfort. Proced. Measur. Behav. 2000, 3, 148–149. [Google Scholar]
- Dunn, A.J.; Berridge, C.W.; Lai, Y.I.; Yachabach, T.L. CRF-induced excessive grooming behavior in rats and mice. Peptides 1987, 8, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.N. Exploratory behavior models of anxiety in mice. Neurosci. Biobehav. 1985, 9, 37–44. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Osornio, S.A.; Crespo-Ramírez, M.; Paredes-Cervantes, V.; Mata-Marín, A.; Martínez-Lara, R.; Pérez de la Mora, M.; Tesoro-Cruz, E. Oral Administration of Efavirenz Dysregulates the Tph2 Gene in Brain Serotonergic Areas and Alters Weight and Mood in Mice. Pharmaceuticals 2024, 17, 801. https://doi.org/10.3390/ph17060801
Rojas-Osornio SA, Crespo-Ramírez M, Paredes-Cervantes V, Mata-Marín A, Martínez-Lara R, Pérez de la Mora M, Tesoro-Cruz E. Oral Administration of Efavirenz Dysregulates the Tph2 Gene in Brain Serotonergic Areas and Alters Weight and Mood in Mice. Pharmaceuticals. 2024; 17(6):801. https://doi.org/10.3390/ph17060801
Chicago/Turabian StyleRojas-Osornio, Sandra Angélica, Minerva Crespo-Ramírez, Vladimir Paredes-Cervantes, Antonio Mata-Marín, Ricardo Martínez-Lara, Miguel Pérez de la Mora, and Emiliano Tesoro-Cruz. 2024. "Oral Administration of Efavirenz Dysregulates the Tph2 Gene in Brain Serotonergic Areas and Alters Weight and Mood in Mice" Pharmaceuticals 17, no. 6: 801. https://doi.org/10.3390/ph17060801
APA StyleRojas-Osornio, S. A., Crespo-Ramírez, M., Paredes-Cervantes, V., Mata-Marín, A., Martínez-Lara, R., Pérez de la Mora, M., & Tesoro-Cruz, E. (2024). Oral Administration of Efavirenz Dysregulates the Tph2 Gene in Brain Serotonergic Areas and Alters Weight and Mood in Mice. Pharmaceuticals, 17(6), 801. https://doi.org/10.3390/ph17060801





