Swellable Microneedles in Drug Delivery and Diagnostics
Abstract
1. Introduction
1.1. Overview of Polymers in Swellable Microneedles
1.2. Applications and Advancements of Swellable Microneedles
2. Drug Delivery Systems and Technologies
3. Potential Applications
3.1. Swellable Microneedles in Insulin Delivery
3.2. Swellable Microneedles in Psoriasis Management
3.3. Swellable Microneedles in Pain and Arthritis Management
3.4. Swellable Microneedles in Specific Therapeutic Areas
4. Biomedical Applications, Sensing, and Monitoring
4.1. Biomarker Sampling and Disease Diagnosis
4.2. Real-Time Monitoring and Sensor Integration
4.3. Minimally Invasive Sampling and Quantification
5. Miscellaneous Applications
5.1. Scar Management and Treatment
5.2. Wound Healing (Diabetic and Chronic Wounds)
5.3. Surgical Applications and Infection Control
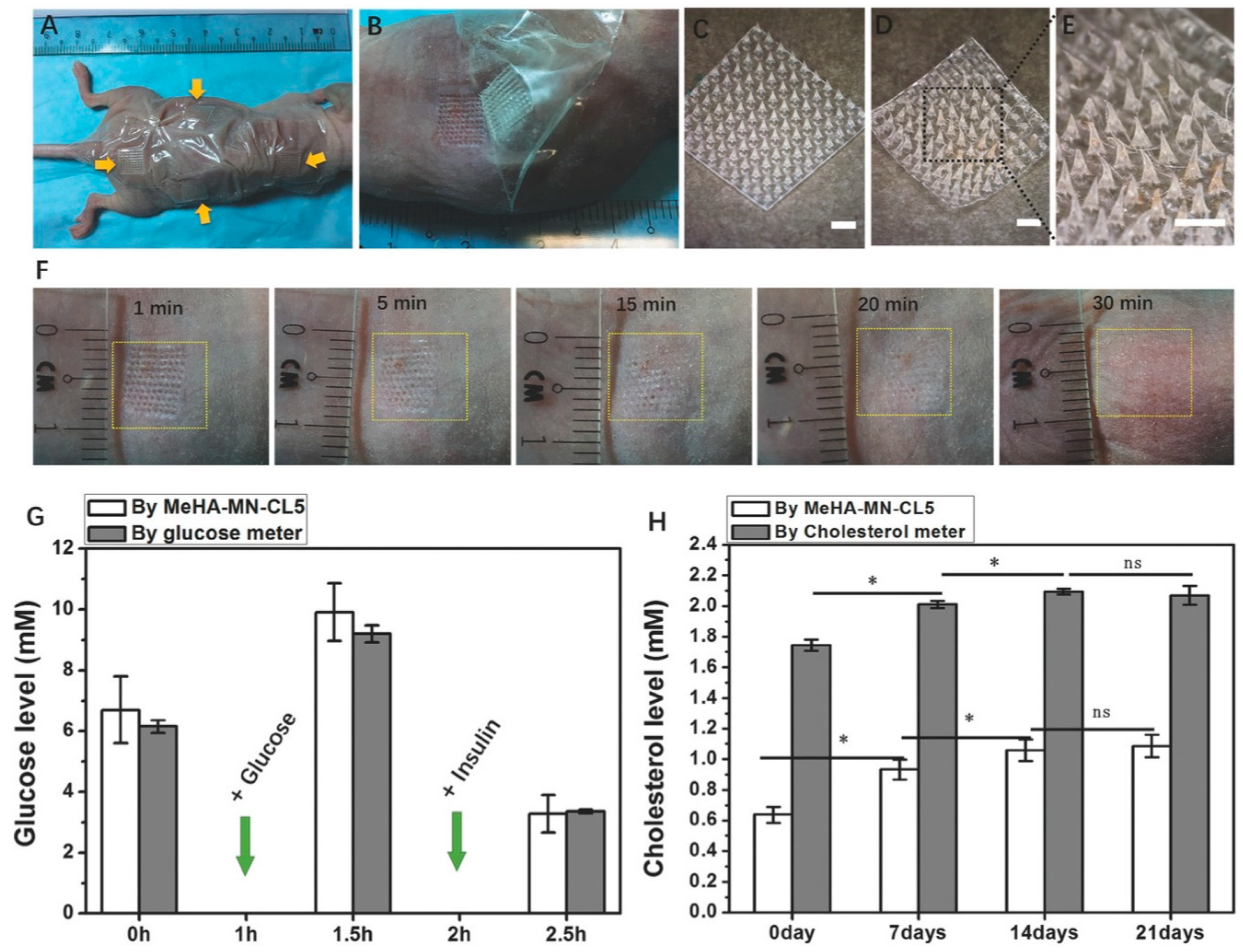
5.4. Immunotherapy and Other Innovations
5.5. Treating Alopecia
6. User Experience and Safety
7. Processes Involved in the Manufacturing of Swellable Microneedles
7.1. 3D Printing
7.2. Molding, Micromolding, and Injection Molding
7.3. Photothermal Microneedle Etching
7.4. Two-Layer Fabrication Strategy
7.5. Drop/Dry Procedure
7.6. Crosslinking Techniques
- Chemical Crosslinking: This prevalent method creates swellable microneedles, such as hydrogel MNs based on cerium–metal organic framework composite nanozymes, for biomarker detection [24]. Additionally, hydrogel MNs integrated with aptamer probes and fluorescence detection for reagentless biomarker quantification have been developed using chemical attachment during crosslinking [59].
- Microwave-Assisted Crosslinking: A novel technique for preparing hydrogel-forming MN arrays for transdermal drug delivery, offering rapid and efficient crosslinking [34].
- UV Irradiation: Utilized for the crosslinking of methacrylated hyaluronic acid to produce swellable MN patches designed for rapid extraction of skin interstitial fluid for metabolic analysis [23]. A glucose-responsive insulin-releasing hydrogel for MN dressing fabrication developed using biocompatible gelatin methacrylate (GelMa), glucose-responsive monomer AFPBA, and gluconic insulin demonstrated adequate mechanical properties and high biocompatibility. A specific method involved preparing a PDMS-negative mold by pouring PDMS over a stainless-steel MN master structure, followed by degassing and curing. The MeHA polymer and photo-initiator solution was cast into the PDMS mold and centrifuged to fill the needle cavities. After drying, the MeHA MN patches were exposed to UV light for crosslinking, resulting in variously crosslinked MeHA MN patches with different durations of UV exposure [66].
8. Tests and Assessments
9. Outcomes, Limitations, and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclosure
References
- Omidian, H.; Park, K. Engineered High Swelling Hydrogels. In Biomedical Applications of Hydrogels Handbook; Springer: Berlin/Heidelberg, Germany, 2010; pp. 351–374. [Google Scholar] [CrossRef]
- Omidian, H.; Park, K. Introduction to Hydrogels. In Biomedical Applications of Hydrogels Handbook; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–16. [Google Scholar] [CrossRef]
- Omidian, H.; Park, K. Hydrogels. In Fundamentals and Applications of Controlled Release Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2012; pp. 75–105. [Google Scholar] [CrossRef]
- Omidian, H.; Mastropietro, D.; Kandalam, U. Swelling, strength, and biocompatibility of acrylate-based superporous hydrogel hybrids. J. Bioact. Compat. Pol. 2014, 29, 66–80. [Google Scholar] [CrossRef]
- Liu, G.D.; Li, Z.; Hao, L.M.; Gong, J.X.; Zhang, J.F.; Zhang, Y.J. Research progress in hydrogel microneedle. Cailiao Gongcheng 2023, 51, 52–65. [Google Scholar] [CrossRef]
- Lutton, R.E.; Larraneta, E.; Kearney, M.C.; Boyd, P.; Woolfson, A.D.; Donnelly, R.F. A novel scalable manufacturing process for the production of hydrogel-forming microneedle arrays. Int. J. Pharm. 2015, 494, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Moffatt, K.; Alkilani, A.Z.; Vicente-Perez, E.M.; Barry, J.; McCrudden, M.T.; Woolfson, A.D. Hydrogel-forming microneedle arrays can be effectively inserted in skin by self-application: A pilot study centred on pharmacist intervention and a patient information leaflet. Pharm. Res. 2014, 31, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Perez, E.M.; Quinn, H.L.; McAlister, E.; O’Neill, S.; Hanna, L.A.; Barry, J.G.; Donnelly, R.F. The Use of a Pressure-Indicating Sensor Film to Provide Feedback upon Hydrogel-Forming Microneedle Array Self-Application In Vivo. Pharm. Res. 2016, 33, 3072–3080. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G.; Larraneta, E.; Donnelly, R.F.; McGoldrick, N.; Migalska, K.; McCrudden, M.T.; Irwin, N.J.; Donnelly, L.; McCoy, C.P. Hydrogel-Forming Microneedle Arrays Made from Light-Responsive Materials for On-Demand Transdermal Drug Delivery. Mol. Pharm. 2016, 13, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Bi, D.; Qu, F.; Xiao, W.; Wu, J.; Liu, P.; Du, H.; Xie, Y.; Liu, H.; Zhang, L.; Tao, J.; et al. Reactive Oxygen Species-Responsive Gel-Based Microneedle Patches for Prolonged and Intelligent Psoriasis Management. ACS Nano 2023, 17, 4346–4357. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Matsumoto, H.; Moro-Oka, Y.; Tanaka, M.; Miyahara, Y.; Suganami, T.; Matsumoto, A. Smart Microneedle Fabricated with Silk Fibroin Combined Semi-interpenetrating Network Hydrogel for Glucose-Responsive Insulin Delivery. ACS Biomater. Sci. Eng. 2019, 5, 5781–5789. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Miyazaki, T.; Itoh, M.; Matsumoto, H.; Moro-oka, Y.; Tanaka, M.; Miyahara, Y.; Suganami, T.; Matsumoto, A. Temperature-Stable Boronate Gel-Based Microneedle Technology for Self-Regulated Insulin Delivery. ACS Appl. Polym. Mater. 2020, 2, 2781–2790. [Google Scholar] [CrossRef]
- Khorshidian, A.; Sharifi, N.; Choupani Kheirabadi, F.; Rezaei, F.; Sheikholeslami, S.A.; Ariyannejad, A.; Esmaeili, J.; Basati, H.; Barati, A. In Vitro Release of Glycyrrhiza Glabra Extract by a Gel-Based Microneedle Patch for Psoriasis Treatment. Gels 2024, 10, 87. [Google Scholar] [CrossRef]
- Wang, J.; Ye, Y.; Yu, J.; Kahkoska, A.R.; Zhang, X.; Wang, C.; Sun, W.; Corder, R.D.; Chen, Z.; Khan, S.A.; et al. Core-Shell Microneedle Gel for Self-Regulated Insulin Delivery. ACS Nano 2018, 12, 2466–2473. [Google Scholar] [CrossRef] [PubMed]
- Nurul Fitri, A.M.; Elim, D.; Sya’ban Mahfud, M.A.; Fitri Sultan, N.A.; Saputra, M.D.; Afika, N.; Friandini, R.A.; Natsir Djide, N.J.; Permana, A.D. Polymeric hydrogel forming microneedle-mediated transdermal delivery of sildenafil citrate from direct-compressed tablet reservoir for potential improvement of pulmonary hypertension therapy. Int. J. Pharm. 2023, 631, 122549. [Google Scholar] [CrossRef] [PubMed]
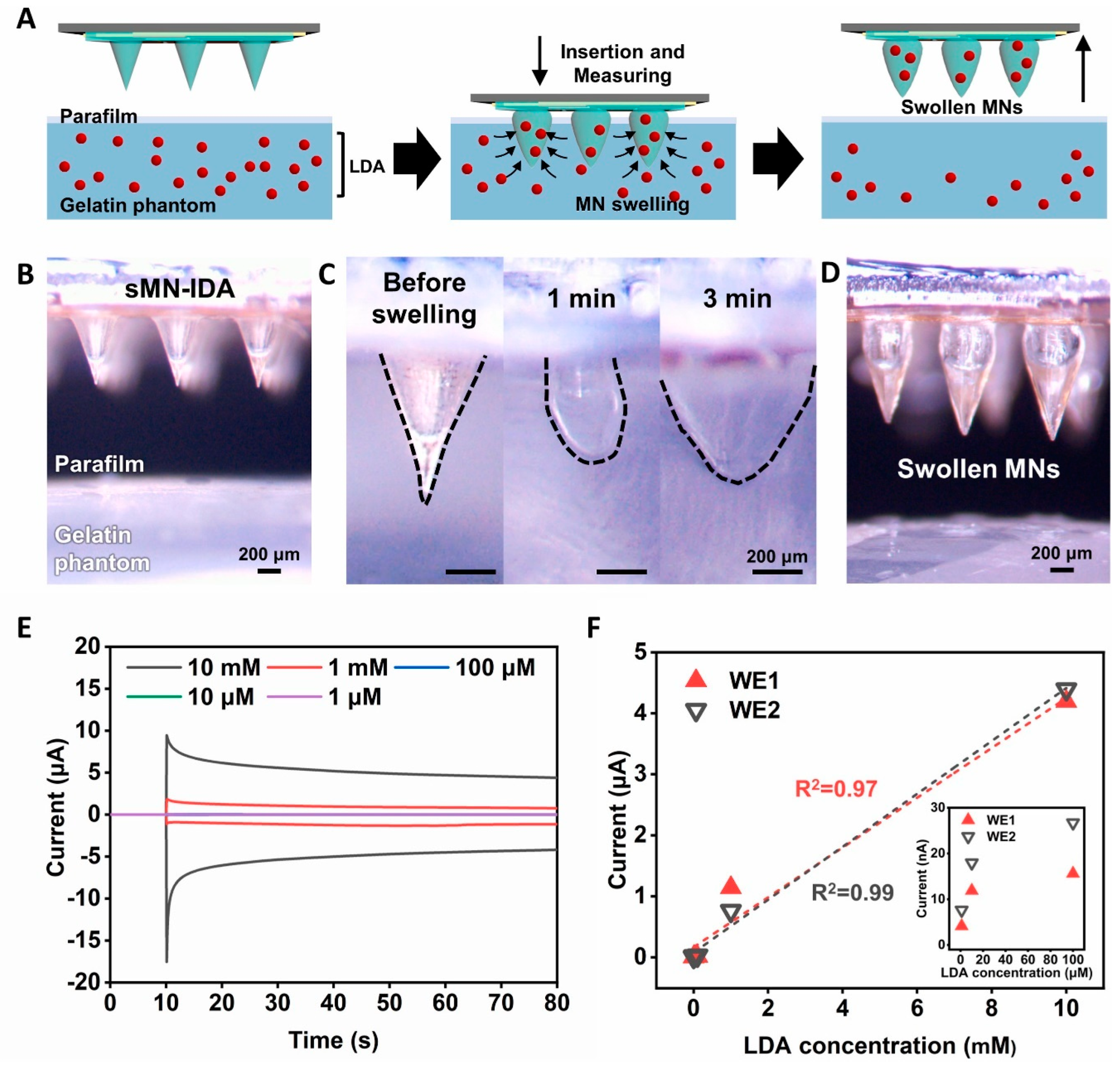
- Tekko, I.A.; Chen, G.; Dominguez-Robles, J.; Thakur, R.R.S.; Hamdan, I.M.N.; Vora, L.; Larraneta, E.; McElnay, J.C.; McCarthy, H.O.; Rooney, M.; et al. Development and characterisation of novel poly (vinyl alcohol)/poly (vinyl pyrrolidone)-based hydrogel-forming microneedle arrays for enhanced and sustained transdermal delivery of methotrexate. Int. J. Pharm. 2020, 586, 119580. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, H.; Yasuda, K. Photothermal microneedle etching: Improved three-dimensional microfabrication method for agarose gel for topographical control of cultured cell communities. Jpn. J. Appl. Phys. Part 2 Lett. Express Lett. 2006, 45, L796–L799. [Google Scholar] [CrossRef]
- Oh, N.G.; Hwang, S.Y.; Na, Y.H. Fabrication of a PVA-Based Hydrogel Microneedle Patch. ACS Omega 2022, 7, 25179–25185. [Google Scholar] [CrossRef] [PubMed]
- GhavamiNejad, P.; GhavamiNejad, A.; Zheng, H.; Dhingra, K.; Samarikhalaj, M.; Poudineh, M. A Conductive Hydrogel Microneedle-Based Assay Integrating PEDOT:PSS and Ag-Pt Nanoparticles for Real-Time, Enzyme-Less, and Electrochemical Sensing of Glucose. Adv. Healthc. Mater. 2023, 12, e2202362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.P.; Zhu, J.C.; Zhao, B.J.; Xu, S.H.; Wang, L.; Luo, X.L. Wearable transdermal microneedle patch based on photonic crystal hydrogel for glucose monitoring. Chin. J. Anal. Chem. 2022, 50, 5. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, G.; Zheng, M.; Hu, T.; Yang, C.; Xu, C. A nanometallic conductive composite-hydrogel core-shell microneedle skin patch for real-time monitoring of interstitial glucose levels. Nanoscale 2023, 15, 16493–16500. [Google Scholar] [CrossRef] [PubMed]
- Caffarel-Salvador, E.; Brady, A.J.; Eltayib, E.; Meng, T.; Alonso-Vicente, A.; Gonzalez-Vazquez, P.; Torrisi, B.M.; Vicente-Perez, E.M.; Mooney, K.; Jones, D.S.; et al. Hydrogel-Forming Microneedle Arrays Allow Detection of Drugs and Glucose In Vivo: Potential for Use in Diagnosis and Therapeutic Drug Monitoring. PLoS ONE 2015, 10, e0145644. [Google Scholar] [CrossRef]
- Chang, H.; Zheng, M.; Yu, X.; Than, A.; Seeni, R.Z.; Kang, R.; Tian, J.; Khanh, D.P.; Liu, L.; Chen, P.; et al. A Swellable Microneedle Patch to Rapidly Extract Skin Interstitial Fluid for Timely Metabolic Analysis. Adv. Mater. 2017, 29, 8. [Google Scholar] [CrossRef]
- Zhao, J.; Lv, J.; Ling, G.; Zhang, P. A swellable hydrogel microneedle based on cerium-metal organic frame composite nanozyme for detection of biomarkers. Int. J. Biol. Macromol. 2024, 254, 127745. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Jiang, X.; Zeng, Y.N.; Zhang, W.; Yuan, Q.Q.; Yin, M.R.; Wu, G.S.; Li, W. A swellable bilateral microneedle patch with core-shell structure for rapid lactate analysis and early melanoma diagnosis. Chem. Eng. J. 2023, 455, 8. [Google Scholar] [CrossRef]
- Odinotski, S.; Dhingra, K.; GhavamiNejad, A.; Zheng, H.; GhavamiNejad, P.; Gaouda, H.; Mohammadrezaei, D.; Poudineh, M. A Conductive Hydrogel-Based Microneedle Platform for Real-Time pH Measurement in Live Animals. Small 2022, 18, e2200201. [Google Scholar] [CrossRef] [PubMed]
- Al-Kasasbeh, R.; Brady, A.J.; Courtenay, A.J.; Larraneta, E.; McCrudden, M.T.C.; O’Kane, D.; Liggett, S.; Donnelly, R.F. Evaluation of the clinical impact of repeat application of hydrogel-forming microneedle array patches. Drug Deliv. Transl. Res. 2020, 10, 690–705. [Google Scholar] [CrossRef] [PubMed]
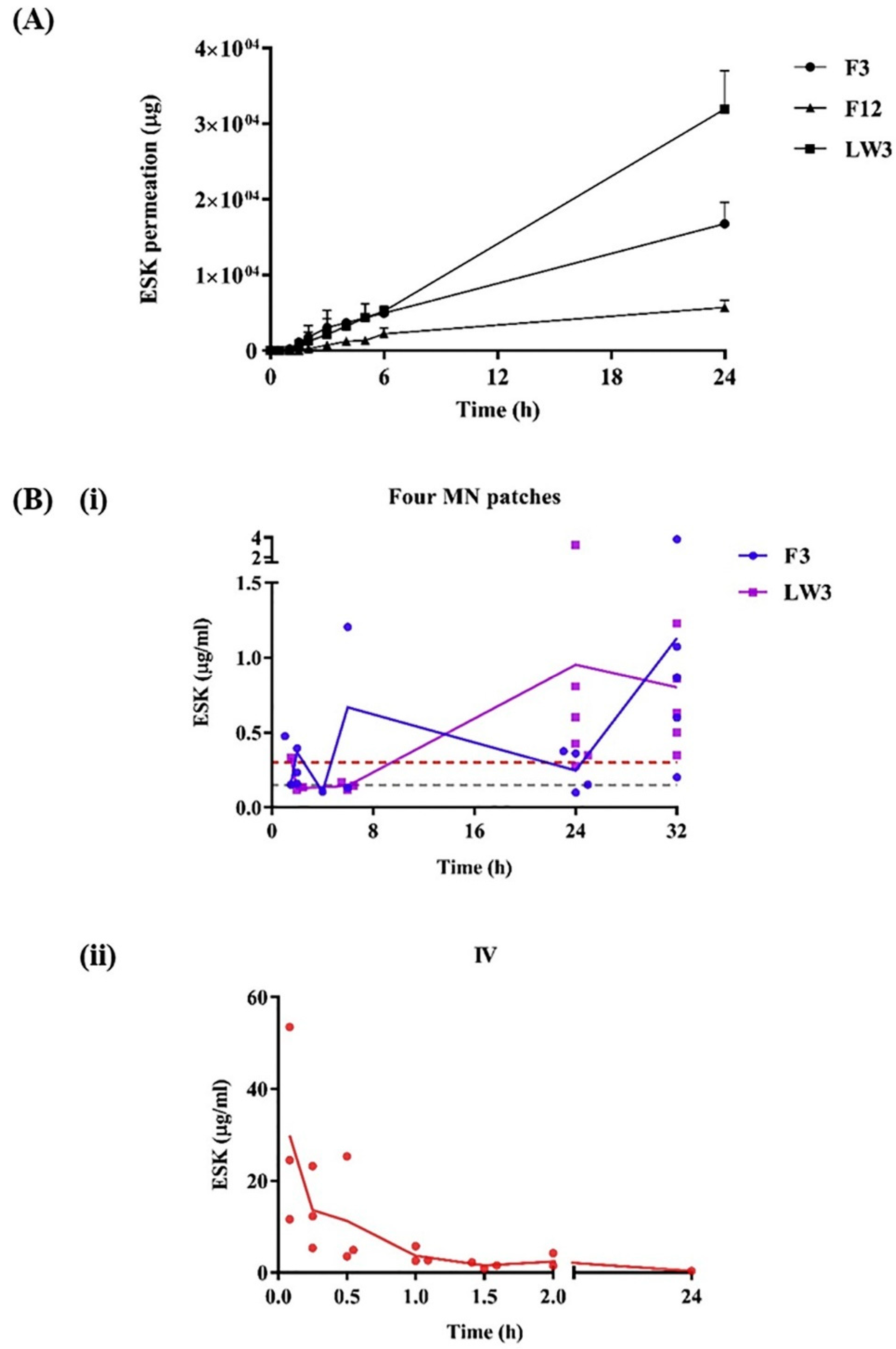
- Courtenay, A.J.; McAlister, E.; McCrudden, M.T.C.; Vora, L.; Steiner, L.; Levin, G.; Levy-Nissenbaum, E.; Shterman, N.; Kearney, M.C.; McCarthy, H.O.; et al. Hydrogel-forming microneedle arrays as a therapeutic option for transdermal esketamine delivery. J. Control. Release 2020, 322, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Al Sulaiman, D.; Chang, J.Y.H.; Bennett, N.R.; Topouzi, H.; Higgins, C.A.; Irvine, D.J.; Ladame, S. Hydrogel-Coated Microneedle Arrays for Minimally Invasive Sampling and Sensing of Specific Circulating Nucleic Acids from Skin Interstitial Fluid. ACS Nano 2019, 13, 9620–9628. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, Y.; Kwong, H.K.; Zheng, M.; Wu, J.; Cui, C.; Chan, K.W.Y.; Xu, C.; Chen, T.H. On-Site Melanoma Diagnosis Utilizing a Swellable Microneedle-Assisted Skin Interstitial Fluid Sampling and a Microfluidic Particle Dam for Visual Quantification of S100A1. Adv. Sci. 2024, 11, e2306188. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, C.; Ping, J.; Ying, Y. Recent advances in hydrogel microneedle-based biofluid extraction and detection in food and agriculture. Biosens. Bioelectron. 2024, 250, 116066. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; He, D.; Fan, L.; Ren, S.; Wang, L.; Sun, J. Application of hydrogel microneedles in the oral cavity. Biopolymers 2024, 115, e23573. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Singh, T.R.; Garland, M.J.; Migalska, K.; Majithiya, R.; McCrudden, C.M.; Kole, P.L.; Mahmood, T.M.; McCarthy, H.O.; Woolfson, A.D. Hydrogel-Forming Microneedle Arrays for Enhanced Transdermal Drug Delivery. Adv. Funct. Mater. 2012, 22, 4879–4890. [Google Scholar] [CrossRef]
- Larraneta, E.; Lutton, R.E.; Brady, A.J.; Vicente-Perez, E.M.; Woolfson, A.D.; Thakur, R.R.; Donnelly, R.F. Microwave-Assisted Preparation of Hydrogel-Forming Microneedle Arrays for Transdermal Drug Delivery Applications. Macromol. Mater. Eng. 2015, 300, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Elim, D.; Fitri, A.M.N.; Mahfud, M.A.S.; Afika, N.; Sultan, N.A.F.; Hijrah; Asri, R.M.; Permana, A.D. Hydrogel forming microneedle-mediated transdermal delivery of sildenafil citrate from polyethylene glycol reservoir: An ex vivo proof of concept study. Colloids Surf. B Biointerfaces 2023, 222, 113018. [Google Scholar] [CrossRef] [PubMed]
- Mahfufah, U.; Fitri Sultan, N.A.; Nurul Fitri, A.M.; Elim, D.; Sya’ban Mahfud, M.A.; Wafiah, N.; Ardita Friandini, R.; Chabib, L.; Aliyah; Permana, A.D. Application of multipolymers system in the development of hydrogel-forming microneedle integrated with polyethylene glycol reservoir for transdermal delivery of albendazole. Eur. Polym. J. 2023, 183, 111762. [Google Scholar] [CrossRef]
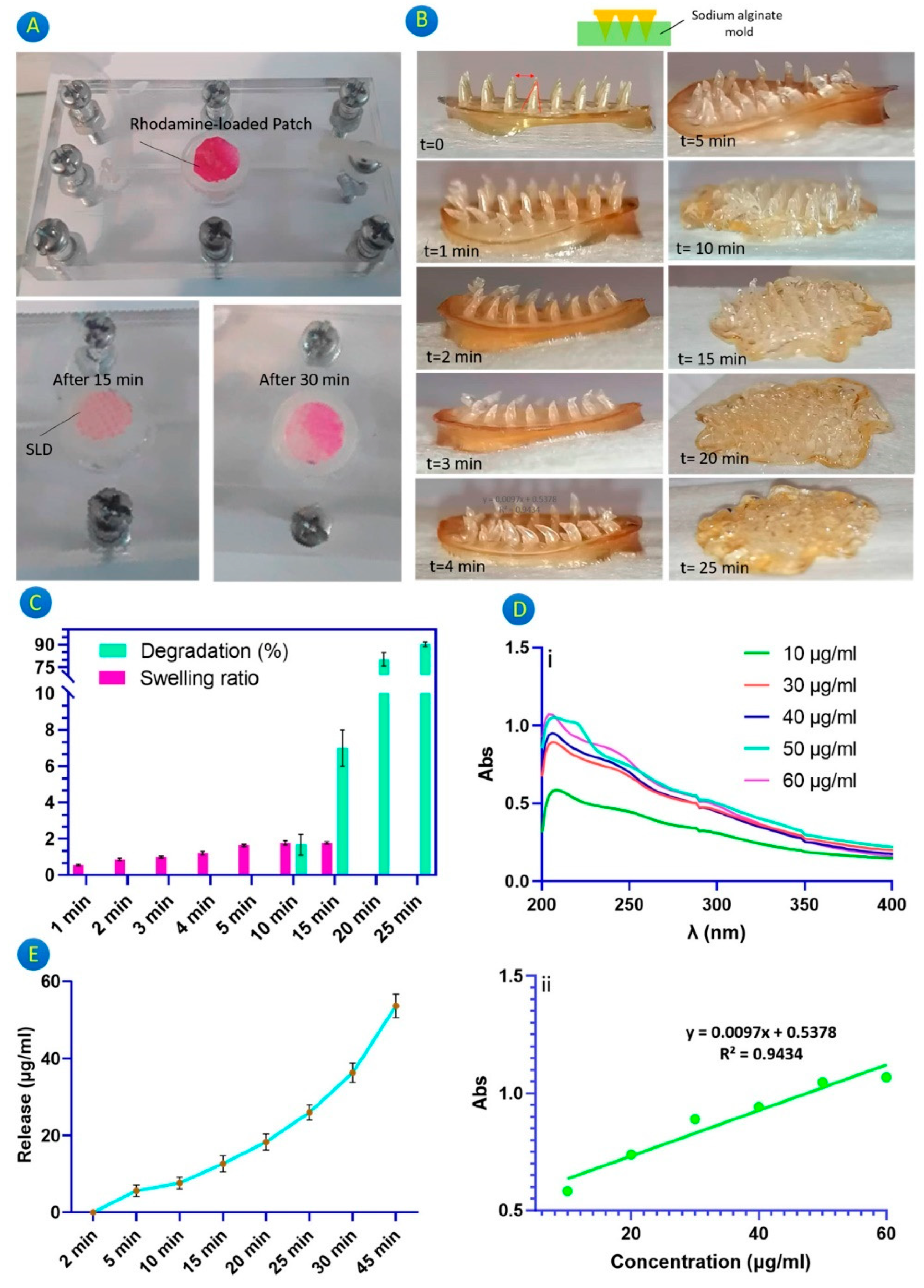
- Chew, S.W.T.; Shah, A.H.; Zheng, M.; Chang, H.; Wiraja, C.; Steele, T.W.J.; Xu, C. A self-adhesive microneedle patch with drug loading capability through swelling effect. Bioeng. Transl. Med. 2020, 5, e10157. [Google Scholar] [CrossRef] [PubMed]
- McAlister, E.; Dutton, B.; Vora, L.K.; Zhao, L.; Ripolin, A.; Zahari, D.; Quinn, H.L.; Tekko, I.A.; Courtenay, A.J.; Kelly, S.A.; et al. Directly Compressed Tablets: A Novel Drug-Containing Reservoir Combined with Hydrogel-Forming Microneedle Arrays for Transdermal Drug Delivery. Adv. Healthc. Mater. 2021, 10, e2001256. [Google Scholar] [CrossRef] [PubMed]
- Al-Badry, A.S.; Al-Mayahy, M.H.; Scurr, D.J. Enhanced Transdermal Delivery of Acyclovir via Hydrogel Microneedle Arrays. J. Pharm. Sci. 2023, 112, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Ranjan Yadav, P.; Iqbal Nasiri, M.; Vora, L.K.; Larraneta, E.; Donnelly, R.F.; Pattanayek, S.K.; Bhusan Das, D. Super-swelling hydrogel-forming microneedle based transdermal drug delivery: Mathematical modelling, simulation and experimental validation. Int. J. Pharm. 2022, 622, 121835. [Google Scholar] [CrossRef] [PubMed]
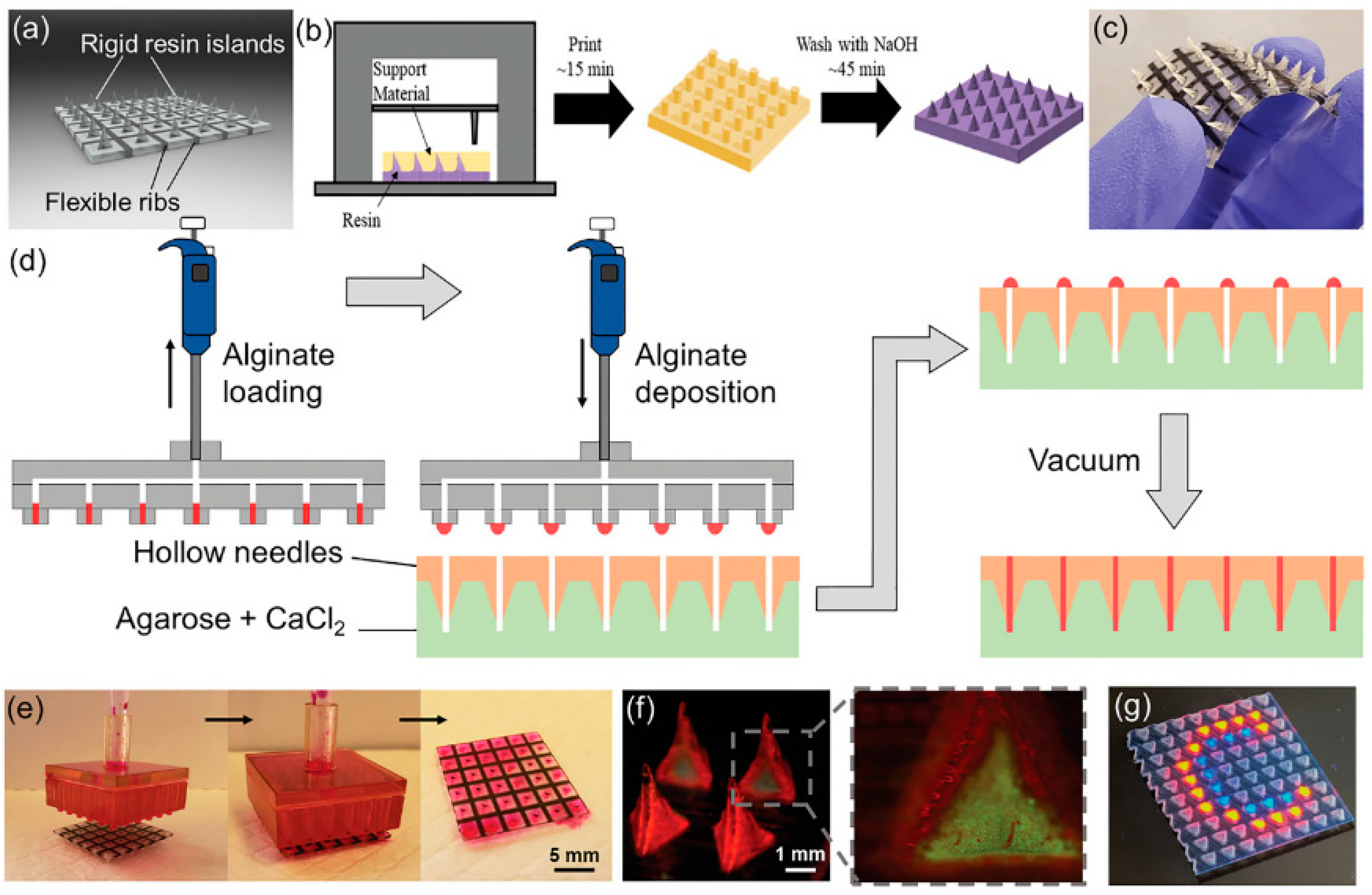
- Barnum, L.; Quint, J.; Derakhshandeh, H.; Samandari, M.; Aghabaglou, F.; Farzin, A.; Abbasi, L.; Bencherif, S.; Memic, A.; Mostafalu, P.; et al. 3D-Printed Hydrogel-Filled Microneedle Arrays. Adv. Healthc. Mater. 2021, 10, e2001922. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Miyazaki, T.; Itoh, M.; Matsumoto, H.; Moro-Oka, Y.; Tanaka, M.; Miyahara, Y.; Suganami, T.; Matsumoto, A. A Porous Reservoir-Backed Boronate Gel Microneedle for Efficient Skin Penetration and Sustained Glucose-Responsive Insulin Delivery. Gels 2022, 8, 74. [Google Scholar] [CrossRef]
- Chen, X.; Yu, H.; Wang, L.; Wang, N.; Zhang, Q.; Zhou, W.; Uddin, M.A. Preparation of phenylboronic acid-based hydrogel microneedle patches for glucose-dependent insulin delivery. J. Appl. Polym. Sci. 2020, 138, 11. [Google Scholar] [CrossRef]
- Chen, X.; Yu, H.; Wang, L.; Shen, D.; Li, C.; Zhou, W. Cross-Linking-Density-Changeable Microneedle Patch Prepared from a Glucose-Responsive Hydrogel for Insulin Delivery. ACS Biomater. Sci. Eng. 2021, 7, 4870–4882. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, H.J.; Wang, L.; Shen, D.; ul Amin, B.; Feng, J.Y.; Zhang, Q.; Xiong, W. Microneedle Patch Prepared from a Hydrogel by a Mild Method for Insulin Delivery. ChemNanoMat 2021, 7, 1230–1240. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, X.; Cai, L.; Gan, J.; Wang, J.; Wang, Y.; Zhao, Y. Black phosphorus hydrogel inverse opal microneedle patches for psoriasis treatment. Nano Today 2024, 54, 8. [Google Scholar] [CrossRef]
- Zhao, P.; Li, Z.; Ling, Z.; Zheng, Y.; Chang, H. Efficient Loading and Sustained Delivery of Methotrexate Using a Tip-Swellable Microneedle Array Patch for Psoriasis Treatment. ACS Biomater. Sci. Eng. 2024, 10, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Indermun, S.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Modi, G.; Luttge, R.; Govender, M.; Pillay, V. In Vitro and In Vivo Evaluation of a Hydrogel-Based Microneedle Device for Transdermal Electro-Modulated Analgesia. J. Pharm. Sci. 2017, 106, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Su, J.; An, M.; Yang, Y.; Zhang, Y.; Zuo, J.; Zhang, N.; Zhao, Y. Novel DEK-Targeting Aptamer Delivered by a Hydrogel Microneedle Attenuates Collagen-Induced Arthritis. Mol. Pharm. 2021, 18, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, W.; Ke, J.; Huang, S.; Wang, J.; Luo, C.; Li, X.; Zhang, K.; Liu, H.; Zheng, W.; et al. A mechanically tough and ultra-swellable microneedle for acute gout arthritis. Biomater. Sci. 2023, 11, 1714–1724. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fan, L.; Peng, N.; Yin, Y.; Mu, D.; Wang, J.; Meng, R.; Xie, J. Galunisertib-Loaded Gelatin Methacryloyl Hydrogel Microneedle Patch for Cardiac Repair after Myocardial Infarction. ACS Appl. Mater. Interfaces 2022, 14, 40491–40500. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.Q.; Wu, Z.R.; Wu, D.; Hou, B.; Bian, L.K.; Zhou, T.; Hou, Y.C.; Wang, H.Y.; Zheng, Z.G. Hydrogel microneedle patch for treatment of liver fibrosis. Mater. Today Adv. 2023, 20, 13. [Google Scholar] [CrossRef]
- Han, M.; Yang, H.; Lu, X.; Li, Y.; Liu, Z.; Li, F.; Shang, Z.; Wang, X.; Li, X.; Li, J.; et al. Three-Dimensional-Cultured MSC-Derived Exosome-Hydrogel Hybrid Microneedle Array Patch for Spinal Cord Repair. Nano Lett. 2022, 22, 6391–6401. [Google Scholar] [CrossRef]
- Zhu, W.B.; Liu, Q.; Zhang, Z.H.; Wang, Y.J.; Mei, J.W.; Xu, D.D.; Zhou, J.; Su, Z.; Zhang, X.Z.; Zhu, C.; et al. Photothermal Microneedle Hydrogel Patch for Refractory Soft Tissue Injuries through Thermosensitized Anti-Inflammaging Modulation. Small Struct. 2024, 5, 18. [Google Scholar] [CrossRef]
- Martins, C.F.; Garcia-Astrain, C.; Conde, J.; Liz-Marzan, L.M. Nanocomposite hydrogel microneedles: A theranostic toolbox for personalized medicine. Drug Deliv. Transl. Res. 2024, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi, M.; Seyfoori, A.; Pagan, E.; Askari, E.; Hassani Najafabadi, A.; Akbari, M. 3D Printed Hydrogel Microneedle Arrays for Interstitial Fluid Biomarker Extraction and Colorimetric Detection. Polymers 2023, 15, 1389. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, Y.J.; Kostal, E.; Matylitskaya, V.; Partel, S.; Ryu, W. Highly-sensitive single-step sensing of levodopa by swellable microneedle-mounted nanogap sensors. Biosens. Bioelectron. 2023, 220, 114912. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Zhang, M.; Xu, W.; Ling, G.; Yu, J.; Zhang, P. Swellable PVA/PVP hydrogel microneedle patches for the extraction of interstitial skin fluid toward minimally invasive monitoring of blood glucose level. Analyst 2022, 147, 1478–1491. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; GhavamiNejad, A.; GhavamiNejad, P.; Samarikhalaj, M.; Giacca, A.; Poudineh, M. Hydrogel Microneedle-Assisted Assay Integrating Aptamer Probes and Fluorescence Detection for Reagentless Biomarker Quantification. ACS Sens. 2022, 7, 2387–2399. [Google Scholar] [CrossRef] [PubMed]
- Eltayib, E.; Brady, A.J.; Caffarel-Salvador, E.; Gonzalez-Vazquez, P.; Zaid Alkilani, A.; McCarthy, H.O.; McElnay, J.C.; Donnelly, R.F. Hydrogel-forming microneedle arrays: Potential for use in minimally-invasive lithium monitoring. Eur. J. Pharm. Biopharm. 2016, 102, 123–131. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Niu, Y.; Li, Z.; Li, A.; Yang, H.; Xu, F.; Li, F. A Hydrogel Microneedle Patch for Point-of-Care Testing Based on Skin Interstitial Fluid. Adv. Healthc. Mater. 2020, 9, e1901201. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.C.; Lin, C.Y.; Lin, H.Y.; Kuo, C.T.; Yin, S.Y.; Hsu, Y.H.; Yeh, H.F.; Wang, J.; Wan, D. Controllable-Swelling Microneedle-Assisted Ultrasensitive Paper Sensing Platforms for Personal Health Monitoring. Adv. Healthc. Mater. 2023, 12, e2300321. [Google Scholar] [CrossRef]
- Keyvani, F.; Zheng, H.; Kaysir, M.R.; Mantaila, D.F.; Ghavami Nejad, P.; Rahman, F.A.; Quadrilatero, J.; Ban, D.; Poudineh, M. A Hydrogel Microneedle Assay Combined with Nucleic Acid Probes for On-Site Detection of Small Molecules and Proteins. Angew. Chem. Int. Ed. Engl. 2023, 62, e202301624. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, X.; Lin, Z.; Mao, H.; Qiu, Z.; Xiang, K.; Ke, T.; Li, L.; Lu, L.; Xiao, L. Layered GelMA/PEGDA Hydrogel Microneedle Patch as an Intradermal Delivery System for Hypertrophic Scar Treatment. ACS Appl. Mater. Interfaces 2023, 15, 43309–43320. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.X.; You, C.A.; Zhou, L.M.; Liu, H.L.; Zhou, S.H.; Wang, X.A.; Guo, R. Antimicrobial hydrogel microneedle loading verteporfin promotes skin regeneration by blocking mechanotransduction signaling. Chem. Eng. J. 2023, 472, 12. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, H.; Shi, Z.; Lin, L.; Li, Y.; Wang, M.; Pan, G.; Lei, Y.; Xue, L. Responsive hydrogel-based microneedle dressing for diabetic wound healing. J. Mater. Chem. B 2022, 10, 3501–3511. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gao, Y.; Liu, Q.; Li, W.; Li, Z.; Zhang, D.; Xie, R.; Zheng, Y.; Chen, H.; Zeng, X. A Bacterial Responsive Microneedle Dressing with Hydrogel Backing Layer for Chronic Wound Treatment. Small 2024, 20, e2307104. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhong, X.; Dai, M.; Feng, X.; Tang, C.; Cao, L.; Liu, L. Antibacterial microneedle patch releases oxygen to enhance diabetic wound healing. Mater. Today Bio 2024, 24, 100945. [Google Scholar] [CrossRef] [PubMed]
- Jeon, E.Y.; Lee, J.; Kim, B.J.; Joo, K.I.; Kim, K.H.; Lim, G.; Cha, H.J. Bio-inspired swellable hydrogel-forming double-layered adhesive microneedle protein patch for regenerative internal/external surgical closure. Biomaterials 2019, 222, 119439. [Google Scholar] [CrossRef]
- Zheng, G.; Xie, J.; Yao, Y.; Shen, S.; Weng, J.; Yang, Q.; Yan, Q. MgO@polydopamine Nanoparticle-Loaded Photothermal Microneedle Patches Combined with Chitosan Gel Dressings for the Treatment of Infectious Wounds. ACS Appl. Mater. Interfaces 2024, 16, 12202–12216. [Google Scholar] [CrossRef]
- Courtenay, A.J.; Rodgers, A.M.; McCrudden, M.T.C.; McCarthy, H.O.; Donnelly, R.F. Novel Hydrogel-Forming Microneedle Array for Intradermal Vaccination in Mice Using Ovalbumin as a Model Protein Antigen. Mol. Pharm. 2019, 16, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Qiu, Y.; Chen, J.; Zhang, S.; Xu, B.; Gao, Y. Effective transcutaneous immunization against hepatitis B virus by a combined approach of hydrogel patch formulation and microneedle arrays. Biomed. Microdevices 2013, 15, 1077–1085. [Google Scholar] [CrossRef]
- Younis, N.; Puigmal, N.; Kurdi, A.E.; Badaoui, A.; Zhang, D.; Morales, C.; Saad, A.; Cruz, D.; Rahy, N.A.; Daccache, A.; et al. Microneedle-mediated Delivery of Immunomodulators Restores Immune Privilege in Hair Follicles and Reverses Immune-Mediated Alopecia. Adv. Mater. 2024, e2312088. [Google Scholar] [CrossRef]
- Ding, Y.W.; Li, Y.; Zhang, Z.W.; Dao, J.W.; Wei, D.X. Hydrogel forming microneedles loaded with VEGF and Ritlecitinib/polyhydroxyalkanoates nanoparticles for mini-invasive androgenetic alopecia treatment. Bioact. Mater. 2024, 38, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Hutton, A.R.J.; Kirkby, M.; Larraneta, E.; Donnelly, R.F. Designing a unique feedback mechanism for hydrogel-forming microneedle array patches: A concept study. Drug Deliv. Transl. Res. 2022, 12, 838–850. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Singh, T.R.; Alkilani, A.Z.; McCrudden, M.T.; O’Neill, S.; O’Mahony, C.; Armstrong, K.; McLoone, N.; Kole, P.; Woolfson, A.D. Hydrogel-forming microneedle arrays exhibit antimicrobial properties: Potential for enhanced patient safety. Int. J. Pharm. 2013, 451, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; O’Cearbhaill, E.D.; Sisk, G.C.; Park, K.M.; Cho, W.K.; Villiger, M.; Bouma, B.E.; Pomahac, B.; Karp, J.M. A bio-inspired swellable microneedle adhesive for mechanical interlocking with tissue. Nat. Commun. 2013, 4, 1702. [Google Scholar] [CrossRef] [PubMed]
- Luzuriaga, M.A.; Berry, D.R.; Reagan, J.C.; Smaldone, R.A.; Gassensmith, J.J. Biodegradable 3D printed polymer microneedles for transdermal drug delivery. Lab Chip 2018, 18, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.G.; Laabei, M.; Li, S.; Estrela, P.; Leese, H.S. Antimicrobial releasing hydrogel forming microneedles. Biomater. Adv. 2023, 151, 213467. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, H.; Yu, Z.; Yu, H.; Meng, D.; Zhu, L.; Li, H. Direct 3D printing of triple-responsive nanocomposite hydrogel microneedles for controllable drug delivery. J. Colloid Interface Sci. 2024, 670, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Detamornrat, U.; McAlister, E.; Hutton, A.R.J.; Larraneta, E.; Donnelly, R.F. The Role of 3D Printing Technology in Microengineering of Microneedles. Small 2022, 18, e2106392. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jung, B.; Park, J.H. Hydrogel swelling as a trigger to release biodegradable polymer microneedles in skin. Biomaterials 2012, 33, 668–678. [Google Scholar] [CrossRef]
- Zeng, Z.; Jiang, G.; Liu, T.; Song, G.; Sun, Y.; Zhang, X. Fabrication of gelatin methacryloyl hydrogel microneedles for transdermal delivery of metformin in diabetic rats. Bio-Des. Manuf. 2021, 4, 902–911. [Google Scholar] [CrossRef]
- Sammoura, F.; Kang, J.J.; Heo, Y.M.; Jung, T.S.; Lin, L.W. Polymeric microneedle fabrication using a microinjection molding technique. In Microsystem Technologies—Micro-and Nanosystems-Information Storage and Processing Systems; Springer: Berlin/Heidelberg, Germany, 2007; Volume 13, pp. 517–522. [Google Scholar] [CrossRef]
- Trichur, R.; Kim, S.; Zhu, X.; Suk, J.; Hong, C.; Choi, J.-W.; Ahn, C. Development of Plastic Microneedles for Transdermal Interfacing Using Injection Molding Techniques; Springer: Dordrecht, The Netherlands, 2002; pp. 395–397. [Google Scholar] [CrossRef]
- Seong, K.Y.; Seo, M.S.; Hwang, D.Y.; O’Cearbhaill, E.D.; Sreenan, S.; Karp, J.M.; Yang, S.Y. A self-adherent, bullet-shaped microneedle patch for controlled transdermal delivery of insulin. J. Control. Release 2017, 265, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, Q.; Xiao, J.; Zhang, J.; Lin, J.; Wang, J. Microneedle-assisted transdermal delivery of perfluorotripropylamine-based oxygenated emulsion gel loaded with 5-aminolevulinic acid for enhanced photodynamic therapy of cutaneous squamous cell carcinoma. Eur. J. Pharm. Sci. 2023, 188, 106493. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Babla, H.; Han, T.; Das, D.B. Lidocaine carboxymethylcellulose with gelatine co-polymer hydrogel delivery by combined microneedle and ultrasound. Drug Deliv. 2016, 23, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, P.; Herzog, G.; O’Mahony, C.; O’Brien, J.; Scully, J.; Blake, A.; O’Mathuna, C.; Galvin, P. Microscopic gel-liquid interfaces supported by hollow microneedle array for voltammetric drug detection. Sens. Actuator B-Chem. 2014, 201, 572–578. [Google Scholar] [CrossRef]
- Zhang, D.; Das, D.B.; Rielly, C.D. Microneedle assisted micro-particle delivery from gene guns: Experiments using skin-mimicking agarose gel. J. Pharm. Sci. 2014, 103, 613–627. [Google Scholar] [CrossRef]

| Aim and Microneedle Composition | Potential Limitations and Challenges | Ref |
|---|---|---|
| Scalable production of hydrogel MN arrays. Hydrogel-forming MN arrays prepared with Gantrez® S-97 and PEG 10,000 using a novel manufacturing process with custom metal MN master templates and silicone MN molds via injection molding. | Lack of discussion of clinical application; unaddressed issues in real-world use, like patient compliance and microneedle stability. | [6] |
| On-demand transdermal drug delivery of ibuprofen. MN arrays prepared from 2-hydroxyethyl methacrylate (HEMA) and ethylene glycol dimethacrylate (EGDMA) through micromolding. System loaded with up to 5% (w/w) ibuprofen included in a light-responsive 3,5-dimethoxybenzoin conjugate. | Need for external light for drug release; limitations in practical application where light exposure is inconsistent; complexity may affect manufacturing and scalability. | [9] |
| Transdermal delivery of sildenafil citrate for pulmonary hypertension therapy. Hydrogel-forming MN arrays using polyvinyl alcohol (PVA) and polyvinyl pyrrolidone (PVP) with citric acid as the crosslinking agent, integrated with sildenafil citrate (SC) tablet reservoir into a silicon microneedle mold. | Challenges in ensuring consistent drug delivery with varying patient skin types. Improvement in therapeutic efficacy and patient compliance in clinical settings needs extensive validation. | [15] |
| Sustained transdermal delivery of methotrexate for rheumatoid arthritis and juvenile idiopathic arthritis. Hydrogel-forming MN arrays from PVA/PVP crosslinked with citric acid, combined with hydroxypropyl methylcellulose (HPMC) and glycerol reservoir for methotrexate (MTX) delivery using the micromolding technique. | Long-term skin irritation potential; need for comprehensive evaluation of systemic absorption consistency. | [16] |
| Transdermal drug delivery with high permeability and non-toxic properties. PVA-based hydrogel MN patch with adjustable disassembly time prepared using a molding process. Various ratios of lower saponification (PVA6 to PVA10) were tested. | Saponification variation affects drug release and degradation times. Need for more comprehensive in vivo studies to validate safety and efficacy; variability in mechanical properties depending on saponification ratio. | [18] |
| Transdermal delivery of esketamine for treatment-resistant depression. Hydrogel-forming MN arrays in conjunction with ESK-containing reservoir. The HFMNAs was prepared with Gantrez® S-97, PEG 10,000 and anhydrous sodium carbonate using laser-engineered silicone micro-molds. | Potential issues with the stability and consistency of the polymeric film and lyophilized reservoirs; uncertain long-term effects and patient acceptance; challenges in achieving consistent therapeutic plasma levels. | [28] |
| Efficient preparation of hydrogel MN arrays for transdermal drug delivery. Microwave-assisted crosslinking process for hydrogel-forming MN arrays, compared with conventional thermal crosslinking. The MN prepared with aqueous solution of Gantrez® S-97 and PEG 10,000. | Microwave process may have scalability limitations and could affect crosslink uniformity in larger batches; need for further in vivo validation; potential issues with patient compliance and comfort. | [34] |
| Transdermal delivery of sildenafil citrate for erectile dysfunction therapy. Hydrogel-forming microneedles (HFM) made using PVA and PVP with tartaric acid as the crosslinking agent, integrated with a polyethylene glycol (PEG) reservoir in a silicone mold and centrifugation. | Limited in vivo validation and long-term stability data for sildenafil citrate delivery challenges in ensuring consistent drug delivery and patient compliance; need for further optimization of PEG reservoir properties. | [35] |
| Transdermal delivery of albendazole for cystic echinococcosis therapy. HFM made from PVA and PVP crosslinked with citric acid, combined with a PEG reservoir for albendazole (ABZ) delivery. | Need for further optimization of PEG reservoir and crosslinking properties; variability in swelling properties affect drug delivery. | [36] |
| Broadly applicable platform for transdermal drug delivery. Methacrylated hyaluronic acid (MeHA) hydrogel MN patch with post-fabrication drug loading capability through swelling effect. | Burst release profile from the microneedles may not suit applications requiring controlled or prolonged drug release. | [37] |
| Transdermal delivery of various drugs (amoxicillin, levodopa/carbidopa, levofloxacin). Hydrogel-forming MN arrays prepared with Gantrez S-97, PEG10000, and Na2CO3, combined with directly compressed tablets (DCTs) for drug delivery in a silicone laser-engineered MN array mold. | Limited drug range due to non-aqueous reservoirs; complexity could challenge manufacturing and regulatory approval. | [38] |
| Enhanced transdermal delivery of acyclovir. Hydrogel MN arrays prepared through the micromolding method using PEG and PMVE/MA co-polymer with Na₂CO₃ for acyclovir delivery. | Potential for local skin reactions or sensitivities due to prolonged polymer exposure remains unaddressed. | [39] |
| Controlled transdermal drug delivery (TDD) of ibuprofen sodium. Super-swelling hydrogel-forming microneedles (HFMNs) made from a three-phase system (reservoir, microneedle, skin) with a mathematical model for drug transport. | Complex manufacturing may limit scalability and increase costs; accurate mathematical modeling required before production. | [40] |
| Controlled transdermal delivery of various drugs. 3D-printed microneedle arrays (MNAs) with a rigid outer layer filled with drug-eluting hydrogels (alginate, PEGDA), fabricated on a conformal backing. | Limited choice of hydrogel materials due to compatibility needs; challenges in ensuring consistent drug release profiles and mechanical strength; need for more comprehensive in vivo validation. | [41] |
| Aim and Microneedle Composition | Potential Limitations and Challenges | Ref |
|---|---|---|
| Insulin Delivery | ||
| Glucose-responsive insulin delivery for diabetes management. Smart MN composed of semi-interpenetrating network hydrogel with biocompatible silk fibroin (SF) and phenylboronic acid/acrylamide. Fabricated using a two-layer strategy. | Potential for delayed or inadequate insulin release in rapid-onset hyperglycemic episodes. | [11] |
| On-demand and convenient insulin delivery for diabetes. Glucose-responsive, temperature-stable, boronate-containing hydrogel MN patch. | Effectiveness and patient adherence to self-regulated system under diverse environmental conditions need thorough investigation. | [12] |
| Self-regulated insulin delivery. Core–shell microneedle array patch with PVA, TSPBA, and CAT-NG for insulin delivery, triggered by H2O2 generated during glucose oxidation. Coated with a thin layer embedding H2O2-scavenging enzyme. | Complexity impacts manufacturing scalability and cost-effectiveness; long-term stability of enzyme activity within the gel needs thorough investigation. | [14] |
| Sustained glucose-responsive transdermal insulin delivery. Polyvinyl alcohol (PVA)-coated microneedles with an interconnected porous gel drug reservoir containing boronate for glucose-responsive insulin delivery. | Issues with boronate gel’s responsiveness to glucose fluctuations may affect insulin dosing precision under varied conditions. | [42] |
| Glucose-dependent insulin delivery. Phenylboronic-acid-based hydrogel microneedle patch prepared through copolymerization, with insulin loaded using a mild drop/dry procedure. | Performance is pH-sensitive; requires improvement to function effectively in physiological environments; limits immediate clinical application. | [43] |
| Simplified preparation and glucose-responsive insulin delivery. Crosslinking density-changeable microneedle patch made from phenylboronic-acid-grafted polyallylamine and PVA, with insulin loading by mixing with gel. | Reliability of glucose-triggered changes in crosslinking density questioned; potential inconsistency in insulin release rates with rapid glucose changes; potential issues with mechanical strength and repeated freezing/thawing process | [44] |
| Insulin delivery with glucose-responsive behavior. Hydrogel microneedle patch made from phenylboronic-acid-grafted sodium hyaluronate and PVA, with cellulose nanofiber for mechanical strength. | Long-term stability of gel’s responsiveness and durability during extended wear could be challenging. | [45] |
| Psoriasis Management | ||
| Prolonged and intelligent psoriasis management. Detachable H(2)O(2)-responsive gel-based MN patches containing methotrexate (MTX) and epigallocatechin gallate (EGCG). EGCG used as both crosslinker and anti-inflammatory drug. | Complexity may challenge consistent manufacturing; long-term effects of continuous ROS exposure on skin health unexplored. | [10] |
| Psoriasis treatment by controlling cell proliferation. Chitosan-based MN patch fabricated using a CO2 laser cutter. Evaluated for delivery of Glycyrrhiza glabra extract (GgE). | Long-term effects and safety of Glycyrrhiza extract via microneedle need further investigation. | [13] |
| Psoriasis treatment with photothermal-responsive capacity and real-time monitoring. Black-phosphorus-loaded hydrogel inverse opal microneedles composed of N-isopropyl acrylamide (NIPAM)/PEGDA scaffold and gelatin/agarose filler. | Limited long-term stability and biocompatibility data for black phosphorus and photothermal materials; challenges in ensuring consistent photothermal drug release, need for more extensive clinical validation; potential issues with optical properties and real-time monitoring accuracy. | [46] |
| Sustained MTX delivery for psoriasis treatment. Tip-swellable microneedle array patch (TSMAP) made from photo-crosslinked methacrylated hyaluronic acid (MeHA) and biocompatible resin for methotrexate (MTX) delivery. | Challenges in controlling swelling and drug release rates; consistency in penetration depth across different skin types. | [47] |
| Pain Management | ||
| Treatment of chronic pain through electro-modulated analgesia. Hydrogel-based microneedle device (EMHM) evaluated for transdermal electro-modulated analgesia. | Complexity in integrating electrical components may challenge manufacturing, cost, and user handling; could limit widespread adoption. | [48] |
| Attenuation of collagen-induced arthritis. Hydrogel microneedle (hMN) for transdermal delivery of DEK-targeting aptamer DTA6 for rheumatoid arthritis treatment. | Need for more extensive in vivo validation; potential issues with aptamer stability and efficacy in diverse patient populations; limited data on long-term joint protection. | [49] |
| Management of acute gout arthritis. Mechanically tough and ultra-swellable hydrogel microneedles (HMNs) prepared with N,N′-Bis(acrylyl)cysteamine, N,N-Methylenebisacrylamide, and Irgacure 2959, a UV-responsive crosslinker for percutaneous delivery of colchicine (Col). | Swelling capacity may cause discomfort or pressure on the skin, potentially affecting patient compliance. | [50] |
| Specific Therapeutic Areas | ||
| Inhibit fibrosis and improve cardiac function post-MI. Gelatin methacryloyl hydrogel microneedle patch loaded with galunisertib for cardiac repair post-myocardial infarction. | Challenges in ensuring consistent drug release and cardiac repair effects; need for more comprehensive in vivo validation; potential issues with mechanical support for ventricular wall. | [51] |
| Treatment of chronic liver fibrosis. Hydrogel microneedle patch (MNP) based on GelMA for sustained release of pirfenidone (PFD) for liver fibrosis treatment. | Variability in degradation rates and drug release could affect treatment consistency; needs further validation in human studies. | [52] |
| Spinal cord injury repair. 3D-cultured MSC-derived exosome–hydrogel hybrid microneedle array patch for spinal cord repair. | Scale-up and clinical translation challenges; maintaining consistent exosome quality and performance. | [53] |
| Treatment of soft tissue injuries through anti-inflammaging modulation. Photothermal microneedle hydrogel patch with hyaluronic acid methacryloyl, LAP, and taurine-loaded Prussian blue nanoparticles for treating refractory soft tissue injuries. | Need for precise temperature control to avoid burns, or insufficient therapeutic effects could limit application outside of controlled settings. | [54] |
| Aim and Microneedle Composition | Potential Limitations and Challenges | Ref |
|---|---|---|
| 3D control of cell community topography in agarose gel. Three-dimensional (3D) microfabrication method for agarose gel, photothermal microneedle etching (PTMNE), using a 1064 nm laser beam and photoabsorbent chromium layer. | Limited to specific types of cells and agarose gel; potential challenges in scaling up the process for larger studies or clinical applications; potential issues with cell viability and growth in the microchambers over extended periods. | [17] |
| Real-time, enzyme-less electrochemical sensing of glucose. Hydrogel MN-CGM assay with swellable dopamine–hyaluronic acid hydrogel, incorporating platinum and silver nanoparticles, and PEDOT for electrochemical sensing of glucose. | Long-term reliability and sensor performance stability, including accuracy in varying conditions, need further validation. | [19] |
| Non-invasive glucose detection via color changes in the hydrogel film. Wearable transdermal MN patch with photonic crystal hydrogel functionalized with phenylboronic acid for glucose monitoring. | Reliance on colorimetric analysis may limit accuracy; influenced by ambient light and user interpretation. | [20] |
| Minimally invasive real-time monitoring of physiological signals. Nanometallic conductive composite–hydrogel core–shell MN skin patch for real-time monitoring of interstitial glucose levels. Inner core coated with biomarker-specific enzymes, outer hydrogel extract biomarkers. | Integration of multiple materials and technologies may complicate manufacturing and raise costs; challenges in ensuring consistent extraction and sensing of biomarkers; could limit widespread adoption. | [21] |
| Minimally invasive extraction and quantification of drugs and glucose. MN arrays prepared from poly(methyl-vinylether-co-maleic anhydride) and poly(ethyleneglycol), crosslinked through esterification. Used for extraction and quantification of drug substances and glucose. | Potential for skin irritation or allergic reactions; variability in microneedle insertion depth and fluid uptake; need for further validation in diverse patient populations; challenges in developing mathematical algorithms for accurate blood level determination. | [22] |
| Timely metabolic analysis via rapid ISF extraction. Swellable microneedle patch made of methacrylated hyaluronic acid (MeHA), crosslinked through UV irradiation, for rapid ISF extraction and metabolic analysis. | Promising for rapid sampling, but the consistency and efficiency of biomarker recovery and analysis may need further evaluation. | [23] |
| Rapid detection of biomarkers in ISF. Swellable hydrogel microneedles composed of PVA and sodium alginate, integrated with cerium–metal organic frame composite nanozyme for biomarker detection. | Ensuring stability and activity of nanozyme in various conditions is challenging for consistent performance. | [24] |
| Rapid lactate analysis and early melanoma diagnosis. Bilateral core–shell microneedle patch with a shell layer for rapid ISF absorption and a core layer for lactate reaction and color change. MN prepared with PVA, lactate oxidase, and horseradish peroxidase | Dependence on visible color changes may not be reliable in all clinical conditions; challenges in interpreting results without specialized training. | [25] |
| Real-time pH measurement in live animals. Conductive hydrogel microneedle platform made from dopamine-conjugated hyaluronic acid (HA) hydrogel with PEDOT for real-time pH measurement. | Long-term stability and repeatability of pH measurements, response time, and calibration need thorough investigation for clinical applicability. | [26] |
| Minimally invasive sampling and sensing of nucleic acid biomarkers. Hydrogel-coated MN arrays with alginate–peptide nucleic acid hybrid material for sequence-specific sampling and detection of nucleic acid biomarkers from skin interstitial fluid. | Sensitivity and specificity of detecting a broad range of nucleic acid biomarkers need full validation in clinical settings. | [29] |
| On-site melanoma diagnosis. Swellable MN for extracting S100A1 from skin ISF, followed by visual quantification using antibody-conjugated magnetic microparticles and polystyrene microparticles in a microfluidic particle dam. | Complexity could pose scalability issues; accuracy of visual quantification might vary with operator experience. | [30] |
| ISF biomarker extraction and colorimetric detection for chronic disease management. 3D-printed hydrogel microneedle arrays made of crosslinked PEGDA, integrated with a multiplexed sensor for colorimetric detection of pH and glucose biomarkers. | Challenges in ensuring consistent biomarker detection and colorimetric analysis; need for more extensive in vivo validation. | [56] |
| Accurate and timely sensing of levodopa for Parkinson’s disease management. Swellable MN-mounted nanogap sensor made from hydrogel made of MeHA for single-step levodopa (LDA) sensing with redox cycling in nanogap electrodes. | Challenges in integrating nanogap sensors with microneedles include fabrication consistency, sensor durability, and system reliability in various conditions. | [57] |
| Minimally invasive monitoring of blood glucose levels. PVA/PVP hydrogel microneedle patches for ISF extraction and glucose level monitoring. | Challenges in ensuring consistent and complete recovery of ISF; maintaining accuracy in variable clinical conditions. | [58] |
| On-needle measurement of biomarkers in ISF. Hydrogel microneedles with fluorescently tagged aptamer probes for reagentless biomarker quantification. | Specificity and durability of aptamer binding in diverse clinical scenarios may affect reliability; requires further optimization. | [59] |
| Minimally invasive lithium monitoring. Hydrogel-forming microneedle arrays made from hydrolyzed poly(methyl-vinylether-co-maleic anhydride) crosslinked with PEG for lithium monitoring. | Correlation between extracted interstitial fluid lithium levels and blood serum levels needs further investigation. | [60] |
| Effective ISF extraction and biomarker recovery for POCT. Hydrogel microneedle patch made of PVA and chitosan for point-of-care testing (POCT) based on ISF. | Recovery of biomarkers from hydrogel may be challenging; might require optimization for efficient and complete extraction and analysis. | [61] |
| Painless biofluid analysis and health monitoring. Controllable swelling microneedle patch made from PEGDA/methacrylated hyaluronic acid hydrogel, combined with a paper-based sensing platform for ultrasensitive molecular recognition. | Reliance on paper-based sensors may limit detectable biomarker range and quantitative accuracy compared to standard lab equipment. | [62] |
| Minimally invasive detection of clinical biomarkers. Hydrogel microneedles combined with graphene oxide–nucleic-acid-based fluorescence biosensor for on-site detection of small molecules and proteins. | Specificity and sensitivity of the biosensor need further validation to reliably detect a wide range of biomarkers in complex biological matrices. | [63] |
| Intradermal delivery for HS treatment. Layered GelMA/PEGDA hydrogel microneedle patch loaded with compound betamethasone (CB) for hypertrophic scar treatment. | Challenges in achieving consistent corticosteroid delivery and hypertrophic scar treatment; need for more extensive in vivo validation; potential variability in drug release and efficacy. | [64] |
| Promote scar-free wound healing by blocking YAP signaling. Detachable hydrogel microneedle system with bismuth nanosheets and verteporfin for scarless wound healing. | Challenges in ensuring consistent YAP signaling inhibition and scarless wound healing; need for more comprehensive in vivo validation. | [65] |
| Controlled insulin release and diabetic wound management. Glucose-responsive hydrogel microneedle dressing made from GelMa, AFPBA, and gluconic insulin for diabetic wound healing. | Challenges in achieving consistent insulin release and diabetic wound healing; need for more extensive in vivo validation; potential variability in glucose-responsive behavior and adhesion to skin. | [66] |
| Treatment of chronic wounds. Bacterial-responsive microneedle dressing with hydrogel backing layer, composed of polycaprolactone (PCL) microspheres loaded with doxycycline hydrochloride (Dox). | Integration of multiple functionalities may complicate manufacturing and increase costs; long-term stability of the dressing needs further evaluation. | [67] |
| Regenerative internal/external surgical closure. Bio-inspired swellable hydrogel-forming double-layered adhesive microneedle patch made from mussel adhesive protein (MAP)-based shell and silk fibroin (SF)-based core. | Potential for insufficient adhesion in highly dynamic or moist internal environments; requires further optimization. | [69] |
| Treatment of infectious wounds. MgO@polydopamine nanoparticle-loaded photothermal microneedle patches combined with chitosan gel for infectious wound treatment. | Dependence on external NIR irradiation for optimal effects; potential discomfort or thermal damage from photothermal activity. | [70] |
| Comparison of hydrogel-forming and dissolving microneedles for vaccine delivery. Hydrogel-forming microneedle array for intradermal vaccination using ovalbumin as a model protein antigen. | May not deliver antigens as effectively as dissolving microneedles, which showed higher IgG titers in studies. | [71] |
| Effective transcutaneous immunization. Hydrogel patch formulation combined with microneedle arrays for transcutaneous immunization (TCI) against hepatitis B virus. | Challenges in achieving consistent antigen delivery and immune response; potential issues with antigen stability and adjuvant effects. | [72] |
| Aim and Microneedle Composition | Potential Limitations and Challenges | Ref |
|---|---|---|
| Self-application of hydrogel MN arrays. Hydrogel-forming MN arrays tested for self-application by human volunteers with pharmacist intervention and information leaflet. Assessed skin barrier disruption and penetration depth. | Variability in self-application effectiveness across wider, less controlled populations; long-term effects of repeated self-application need thorough evaluation. | [7] |
| Provide feedback on MN insertion in vivo. Hydrogel-forming MN arrays combined with a pressure-indicating sensor film. Self-applied by human volunteers and assessed using optical coherence tomography and colorimetric analysis. | Reliance on visual color change could be subjective; may not guarantee optimal drug delivery if penetration depth is insufficient. | [8] |
| Safe and repeatable clinical monitoring and treatment. Hydrogel-forming MN array patches for clinical monitoring and skin cancer management. Evaluated repeat application in human volunteers, monitoring systemic biomarkers. | Continuous long-term studies needed to fully understand implications of chronic use, especially in varying environmental conditions. | [27] |
| Improve patient acceptance and ensure consistent skin insertion. Hydrogel-forming microneedle array patches with a water-filled reservoir for feedback mechanism. | Feedback mechanism may complicate manufacturing and increase costs; robustness of reservoir needs further validation. | [75] |
| Enhanced patient safety through antimicrobial properties. Hydrogel-forming microneedle arrays with antimicrobial properties made from hydrolyzed poly(methyl-vinylether-co-maleic anhydride) crosslinked with PEG. | Need for specific pharmacopeial standards for microneedle products; regulatory gaps can affect clinical adoption speed. | [76] |
| Achieve significant adhesion to soft tissues with minimal tissue damage. Bio-inspired swellable microneedle adhesive made from poly(styrene)-block-poly(acrylic acid) tip and polystyrene core. | Specific swelling behavior might vary under different environmental conditions; potential issues with universal applicability and bioactive therapeutic delivery | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omidian, H.; Dey Chowdhury, S. Swellable Microneedles in Drug Delivery and Diagnostics. Pharmaceuticals 2024, 17, 791. https://doi.org/10.3390/ph17060791
Omidian H, Dey Chowdhury S. Swellable Microneedles in Drug Delivery and Diagnostics. Pharmaceuticals. 2024; 17(6):791. https://doi.org/10.3390/ph17060791
Chicago/Turabian StyleOmidian, Hossein, and Sumana Dey Chowdhury. 2024. "Swellable Microneedles in Drug Delivery and Diagnostics" Pharmaceuticals 17, no. 6: 791. https://doi.org/10.3390/ph17060791
APA StyleOmidian, H., & Dey Chowdhury, S. (2024). Swellable Microneedles in Drug Delivery and Diagnostics. Pharmaceuticals, 17(6), 791. https://doi.org/10.3390/ph17060791







