Promising Strategies to Reduce the SARS-CoV-2 Amyloid Deposition in the Brain and Prevent COVID-19-Exacerbated Dementia and Alzheimer’s Disease
Abstract
1. Introduction
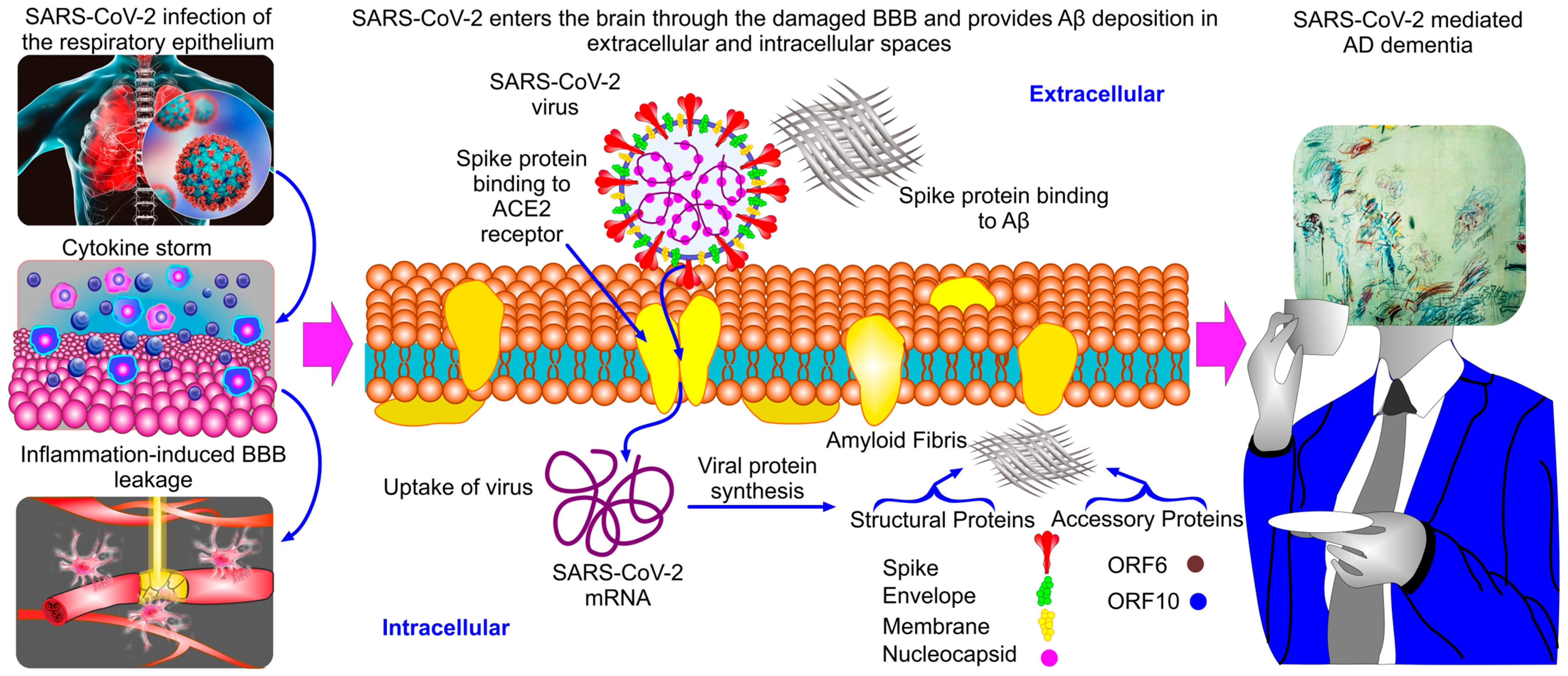
2. SARS-CoV-2 Amyloids and COVID-19-Mediated AD Dementia
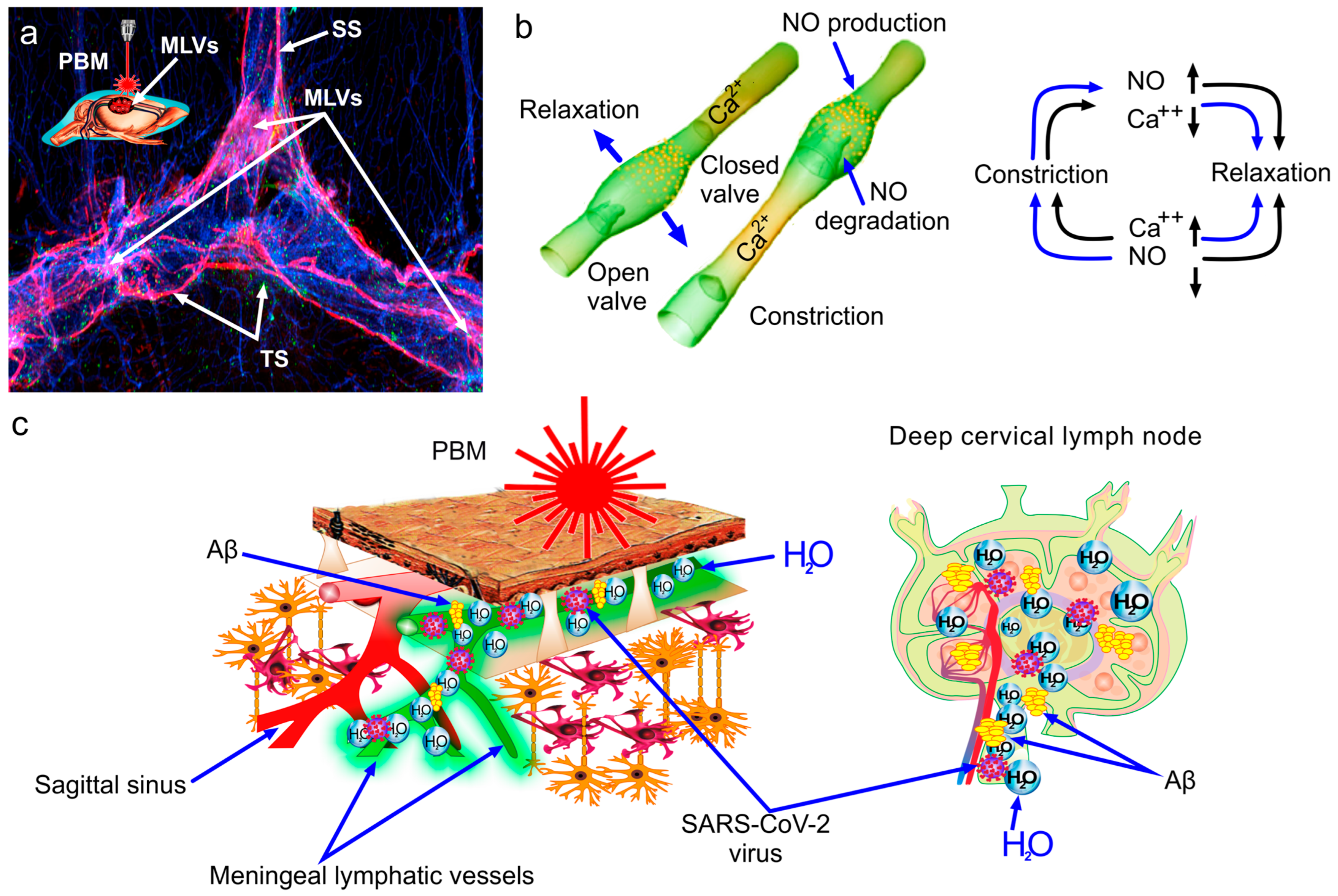
3. Meningeal Lymphatics as a Target for Anti-Amyloid Therapy
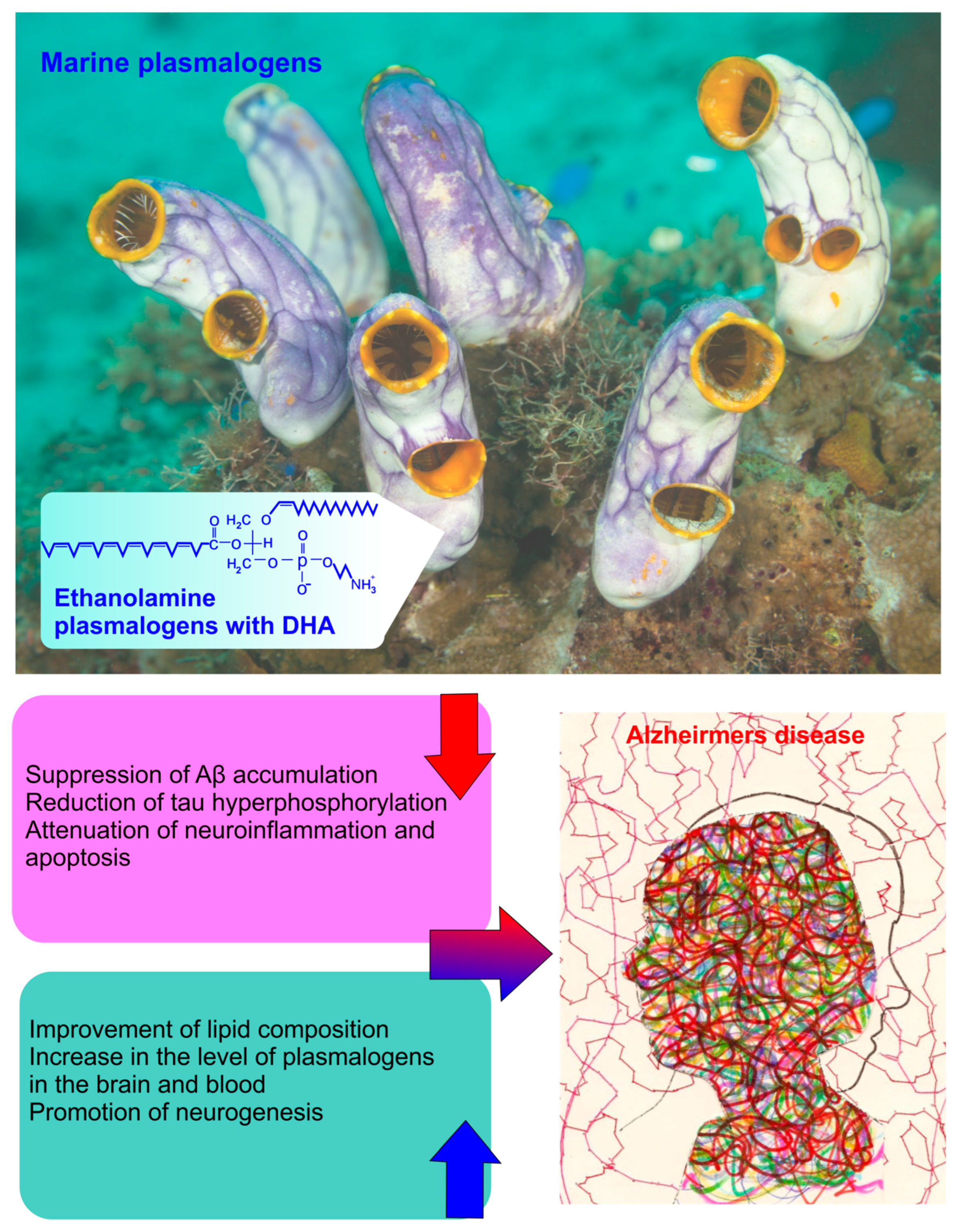
4. Plasmalogens as a Potential Therapy for AD
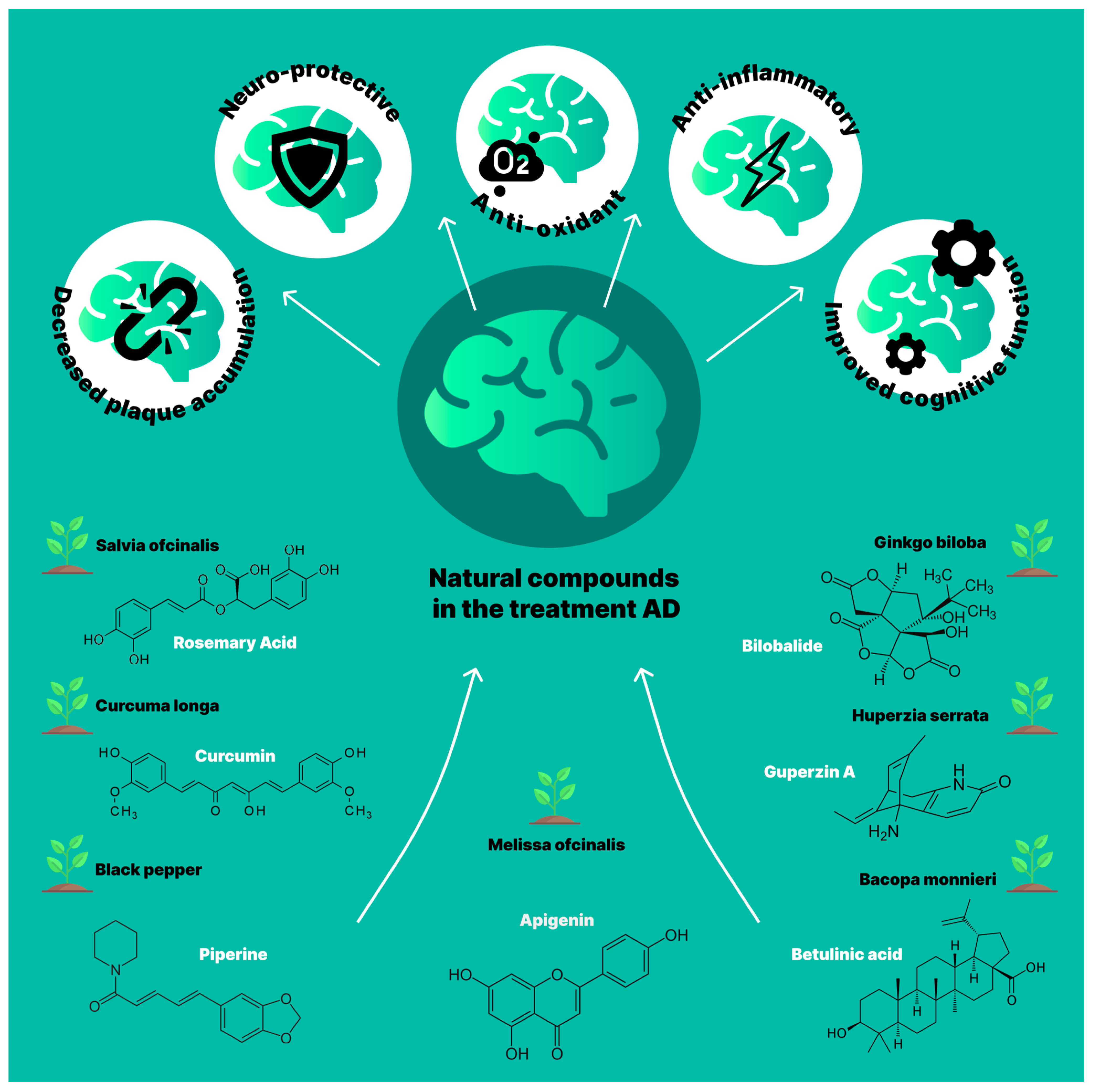
5. Natural Compounds as a Novel Treatment for AD
5.1. Curcuma longa and Piper nigrum
5.2. Ginkgo biloba
5.3. Bacopa monnieri
5.4. Salvia officinalis
5.5. Melissa officinalis
5.6. Huperzia serrata
5.7. Plants That Increase the Drainage Function of the Lymphatic System
6. Discussion
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Choi Matthews, K.A.; Xu, W.; Gaglioti, A.H.; Holt, J.B.; Croft, J.B.; Mack, D.; McGuire, L.C. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimer’s Dement. 2018, 15, 17–24. [Google Scholar] [CrossRef]
- 2023 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2023, 19, 1598–1695. [CrossRef]
- Milton, N. SARS-CoV-2 amyloid, is COVID-19-exacerbated dementia an amyloid disorder in the making? Front. Dement. 2023, 2, 1233340. [Google Scholar] [CrossRef]
- Charnley, M.; Islam, S.; Bindra, G.K.; Engwirda, J.; Ratcliffe, J.; Zhou, J.; Mezzenga, R.; Hulett, M.D.; Han, K.; Berryman, J.T.; et al. Neurotoxic amyloidogenic peptides in the proteome of SARS-Cov-2: Potential implications for neurological symptoms in COVID-19. Nat. Commun. 2022, 13, 3387. [Google Scholar] [CrossRef]
- Rudnicka-Drozak, E.; Drozak, P.; Mizerski, G.; Zaborowski, T.; S’lusarska, B.; Nowicki, G.; Drożak, M. Links between COVID-19 and Alzheimer’s Disease—What Do We Already Know? Int. J. Environ. Res. Public Health 2023, 20, 2146. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Davis, P.B.; Volkow, N.D.; Berger, N.A.; Kaelber, D.C.; Xu, R. Association of COVID-19 with New-Onset Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 89, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Jiang, X.; Li, H. The viral hypothesis in Alzheimer’s disease: SARS-CoV-2 on the cusp. Front. Aging Neurosci. 2023, 15, 1129640. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.R.; Akter, R.; Neelotpol, S.; Mayesha, I.I.; Afrose, A. The Neuropathological Impacts of COVID-19: Challenges and Alternative Treatment Options for Alzheimer’s Like Brain Changes on Severely SARS-CoV-2 Infected Patients. Am. J. Alzheimer’s Dis. Other Dement. 2023, 38, 15333175231214974. [Google Scholar] [CrossRef]
- Shahbaz, M.A.; Kuivanen, S.; Lampinen, R.; Mussalo, L.; Hron, T.; Závodná, T.; Ojha, R.; Krejčík, Z.; Saveleva, L.; Tahir, N.A.; et al. Human-derived air-liquid interface cultures decipher Alzheimer’s disease-SARS-CoV-2 crosstalk in the olfactory mucosa. J. Neuroinflamm. 2023, 20, 299. [Google Scholar] [CrossRef]
- Chen, F.; Chen, Y.; Wang, Y.; Ke, Q.; Cui, L. The COVID-19 pandemic and Alzheimer’s disease: Mutual risks and mechanisms. Transl. Neurodegener. 2022, 11, 40. [Google Scholar] [CrossRef]
- Amadoro, G.; Latina, V.; Stigliano, E.; Micera, A. COVID-19 and Alzheimer’s Disease Share Common Neurological and Ophthalmological Manifestations: A Bidirectional Risk in the Post-Pandemic Future. Cells 2023, 12, 2601. [Google Scholar] [CrossRef] [PubMed]
- Golzari-Sorkheh, M.; Weaver, D.F.; Reed, M.A. COVID-19 as a Risk Factor for Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 91, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, J.; Lei, Z. Amyloid precursor protein facilitates SARS-CoV-2 virus entry into cells and enhances amyloid-β-associated pathology in APP/PS1 mouse model of Alzheimer’s disease. Transl. Psychiatry 2023, 13, 396. [Google Scholar] [CrossRef] [PubMed]
- Baranova, A.; Cao, H.; Zhang, F. Causal effect of COVID-19 on Alzheimer’s disease: A Mendelian randomization study. J. Med. Virol. 2023, 95, e28107. [Google Scholar] [CrossRef] [PubMed]
- Monllor, P.; Kumar, P.; Lloret, M.Á.; Ftara, A.; Leon, J.L.; Lopez, B.; Cervera-Ferri, A.; Lloret, A. Multifactorial Causation of Alzheimer’s Disease Due to COVID-19. J. Alzheimer’s Dis. 2023, 96, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, L.; Yue, L.; Xiao, S. Alzheimer’s disease and COVID-19: Interactions, intrinsic linkages, and the role of immunoinflammatory responses in this process. Front. Immunol. 2023, 14, 1120495. [Google Scholar] [CrossRef] [PubMed]
- Itzhaki, R.F. COVID-19 and Alzheimer’s Disease: What Is the Connection? J. Alzheimer’s Dis. 2023, 91, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.A.; Yang, D.; Lewis, A.; Patel, P.; Medicherla, C. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J. Neurol. Sci. 2021, 426, 117486. [Google Scholar] [CrossRef]
- Ousseiran, Z.H.; Fares, Y.; Chamoun, W.T. Neurological manifestations of COVID-19: A systematic review and detailed comprehension. Int. J. Neurosci. 2023, 133, 754–769. [Google Scholar] [CrossRef]
- Hanganu, A.R.; Niculae, C.M.; Dulămea, A.O.; Moisă, E.; Constantin, R.; Neagu, G.; Hristea, A. The outcome and risk factors associated with central and peripheral nervous system involvement in hospitalized COVID-19 patients: A retrospective cohort study. Front. Neurol. 2024, 14, 1338593. [Google Scholar] [CrossRef]
- Priyal; Sehgal, V.; Kapila, S.; Taneja, R.; Mehmi, P.; Gulati, N. Review of Neurological Manifestations of SARS-CoV-2. Cureus 2023, 15, 38194. [Google Scholar]
- Jumagaliyeva, M.; Ayaganov, D.; Saparbayev, S.; Tuychibaeva, N.; Abdelazim, I.A.; Kurmambayev, Y.; Khamidullina, Z.; Yessenamanova, S. Possible mechanism of central nervous system targeting and neurological symptoms of the new-coronavirus (COVID-19): Literature review. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 9420–9428. [Google Scholar] [PubMed]
- Tyagi, K.; Rai, P.; Gautam, A.; Kaur, H.; Kapoor, S.; Suttee, A.; Jaiswal, P.K.; Sharma, A.; Singh, G.; Barnwal, R.P. Neurological manifestations of SARS-CoV-2: Complexity, mechanism and associated disorders. Eur. J. Med. Res. 2023, 28, 307. [Google Scholar] [CrossRef] [PubMed]
- Itzhaki, R.F.; Golde, T.E.; Heneka, M.T.; Readhead, B. Do infections have a role in the pathogenesis of Alzheimer disease? Nat. Rev. Neurol. 2020, 16, 193–197. [Google Scholar] [CrossRef]
- Wang, F.; Xu, J.; Xu, S.J.; Guo, J.J.; Wang, F.; Wang, Q.W. Analysis and identification genetic effect of SARS-CoV-2 infections to Alzheimer’s disease patients by integrated bioinformatics. J. Alzheimer’s Dis. 2022, 85, 729–744. [Google Scholar] [CrossRef]
- Tang, T.; Jia, J.; Garbarino, E.; Chen, L.; Ma, J.; Li, P. Human herpesvirus 6A U4 inhibits proteasomal degradation of the amyloid precursor protein. J. Virol. 2022, 96, 168821. [Google Scholar] [CrossRef]
- Linard, M.; Letenneur, L.; Garrigue, I.; Doize, A.; Dartigues, J.F.; Helmer, C. Interaction between ApoE4 and herpes simplex virus type 1 in Alzheimer’s disease. Alzheimer’s Dement. 2020, 16, 200–208. [Google Scholar] [CrossRef]
- Niklasson, B.; Lindquist, L.; Klitz, W.; Englund, E. Picornavirus identified in Alzheimer’s disease brains: A pathogenic path? J. Alzheimer’s Dis. Rep. 2020, 4, 141–146. [Google Scholar] [CrossRef]
- Nystrom, S.; Hammarstrom, P. Amyloidogenesis of SARS-CoV-2 spike protein. J. Am. Chem. Soc. 2022, 144, 8945–8950. [Google Scholar] [CrossRef]
- Tayeb-Fligelman, E.; Bowler, J.T.; Tai, C.E.; Sawaya, M.R.; Jiang, Y.X.; Garcia, G., Jr.; Griner, S.L.; Cheng, X.; Salwinski, L.; Lutter, L.; et al. Low complexity domains of the nucleocapsid protein of SARS-CoV-2 form amyloid fibrils. Nat. Commun. 2023, 14, 2379. [Google Scholar] [CrossRef]
- Galkin, A.P. Hypothesis: AA amyloidosis is a factor causing systemic complications after coronavirus disease. Prion 2021, 15, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z. A function of amyloid-beta in mediating activity-dependent Axon/Synapse competition may unify its roles in brain physiology and pathology. J. Alzheimer’s Dis. 2023, 92, 29–57. [Google Scholar] [CrossRef] [PubMed]
- Bourgade, K.; Dupuis, G.; Frost, E.H.; Fulop, T. Anti-viral properties of amyloid-beta peptides. J. Alzheimer’s Dis. 2016, 54, 859–878. [Google Scholar] [CrossRef] [PubMed]
- Powell-Doherty, R.D.; Abbott, A.R.N.; Nelson, L.A.; Bertke, A.S. Amyloid-β and p-Tau Anti-Threat Response to Herpes Simplex Virus 1 Infection in Primary Adult Murine Hippocampal Neurons. J. Virol. 2020, 94, 01874-19. [Google Scholar] [CrossRef] [PubMed]
- Pearson, H.A.; Peers, C. Physiological roles for amyloid beta peptides. J. Physiol. 2006, 575, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Passeri, E.; Elkhoury, K.; Morsink, M.; Broersen, K.; Linder, M.; Tamayol, A.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Alzheimer’s Disease: Treatment Strategies and Their Limitations. Int. J. Mol. Sci. 2022, 23, 13954. [Google Scholar] [CrossRef] [PubMed]
- Se Thoe, E.; Fauzi, A.; Tang, Y.Q.; Chamyuang, S.; Chia, A.Y.Y. A review on advances of treatment modalities for Alzheimer’s disease. Life Sci. 2021, 276, 119129. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C. Dementia Prevention, Intervention, and Care: 2020 Report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.; Liu, K.Y. Questions EMERGE as Biogen claims aducanumab turnaround. Nat. Rev. Neurol. 2020, 16, 63–64. [Google Scholar] [CrossRef]
- Panza, F.; Lozupone, M.; Seripa, D.; Imbimbo, B. Amyloid-β immunotherapy for Alzheimer disease: Is it now a long shot? Ann. Neurol. 2019, 85, 303–315. [Google Scholar] [CrossRef]
- Mullard, A. Landmark Alzheimer’s drug approval confounds research community. Nature 2021, 594, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Salloway, S.; Chalkias, S.; Barkhof, F.; Burkett, P.; Barakos, J.; Purcell, D.; Suhy, J.; Forrestal, F.; Tian, Y.; Umans, K. Amyloid-Related Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients with Early Alzheimer Disease. JAMA Neurol. 2022, 79, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Da Mesquita, S.; Papadopoulos, Z.; Dykstra, T.; Brase, L.; Farias, F.G.; Wall, M.; Jiang, H.; Kodira, C.D.; de Lima, K.A.; Herz, J.; et al. Meningeal lymphatics affect microglia responses and anti-Aβ immunotherapy. Nature 2021, 593, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Da Mesquita, S.; Louveau, A.; Vaccari, A.; Smirnov, I.; Cornelison, R.C.; Kingsmore, K.M.; Contarino, C.; Onengut-Gumuscu, S.; Farber, E.; Raper, D.; et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 2018, 560, 185–191. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Shirokov, A.; Blokhina, I.; Fedosov, I.; Terskov, A.; Dubrovsky, A.; Tsoy, M.; Zlatogosrskaya, D.; Adushkina, V.; Evsukova, A.; et al. Mechanisms of phototherapy of Alzheimer’s disease during sleep and wakefulness: The role of the meningeal lymphatics. Front. Optoelectron. 2023, 16, 22. [Google Scholar]
- Li, D.; Lin, H.; Sun, S.; Liu, S.; Liu, Z.; He, Y.; Zhu, J.; Xu, J.; Semyachkina-Glushkovskaya, O.; Yu, T.; et al. Photostimulation of lymphatic clearance of β- amyloid from mouse brain: New strategy for the therapy of Alzheimer’s disease. Front. Optoelectron. 2023, 16, 45. [Google Scholar] [CrossRef]
- Dupont, G.; Iwanaga, J.; Yilmaz, E.; Tubbs, R.S. Connections Between Amyloid Beta and the Meningeal Lymphatics As a Possible Route for Clearance and Therapeutics. Lymphat. Res. Biol. 2020, 18, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Rockson, S.G. Alzheimer’s Disease: Can the Meningeal Lymphatics Provide the Answer? Lymphat. Res. Biol. 2020, 18, 1. [Google Scholar] [CrossRef]
- Chen, J.; Pan, Y.; Liu, Q.; Li, G.; Chen, G.; Li, W.; Zhao, W.; Wang, Q. The Interplay between Meningeal Lymphatic Vessels and Neuroinflammation in Neurodegenerative Diseases. Curr. Neuropharmacol. 2024, 22, 1016–1032. [Google Scholar] [CrossRef]
- Lemprière, S. Meningeal lymphatics mediate drainage of viruses from the CNS. Nat. Rev. Neurol. 2022, 18, 382. [Google Scholar] [CrossRef]
- Goodman, J.R.; Adham, Z.O.; Woltjer, R.L.; Lund, A.W.; Iliff, J.J. Characterization of dural sinus-associated lymphatic vasculature in human Alzheimer’s dementia subjects. Brain Behav. Immun. 2018, 73, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Pagano, K.; Tomaselli, S.; Molinari, H.; Ragona, L. Natural Compounds as Inhibitors of Aβ Peptide Aggregation: Chemical Requirements and Molecular Mechanisms. Front. Neurosci. 2020, 14, 619667. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Chen, L.; Sun, R.; Wang, J.-L.; Wang, J.; Lin, Y.; Lei, S.; Zhang, Y.; Lv, D.; Jiang, F.; et al. Plasmalogens Eliminate Aging-Associated Synaptic Defects and Microglia-Mediated Neuroinflammation in Mice. Front. Mol. Biosci. 2022, 9, 815320. [Google Scholar] [CrossRef]
- Ezzat, K.; Pernemalm, M.; Palsson, S.; Roberts, T.C.; Jarver, P.; Dondalska, A.; Bestas, B.; Sobkowiak, M.J.; Levänen, B.; Sköld, M.; et al. The viral protein corona directs viral pathogenesis and amyloid aggregation. Nat. Commun. 2019, 10, 2331. [Google Scholar] [CrossRef] [PubMed]
- Leger, P.; Nachman, E.; Richter, K.; Tamietti, C.; Koch, J.; Burk, R.; Kummer, S.; Xin, Q.; Stanifer, M.; Bouloy, M.; et al. NSs amyloid formation is associated with the virulence of Rift Valley fever virus in mice. Nat. Commun. 2020, 11, 3281. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Challis, C.; Jain, N.; Moiseyenko, A.; Ladinsky, M.S.; Shastri, G.G.; Thron, T.; Needham, B.D.; Horvath, I.; Debelius, J.W.; et al. A gut bacterial amyloid promotes alpha-synuclein aggregation and motor impairment in mice. eLife 2020, 9, e53111. [Google Scholar] [CrossRef] [PubMed]
- Saumya, K.U.; Gadhave, K.; Kumar, A.; Giri, R. Zika virus capsid anchor forms cytotoxic amyloid-like fibrils. Virology 2021, 560, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Chaudhary, J.K.; Jain, N.; Chaudhary, P.K.; Khanra, S.; Dhamija, P.; Sharma, A.; Kumar, A.; Handu, S. Role of structural and non-structural proteins and therapeutic targets of SARS-CoV-2 for COVID-19. Cells 2021, 10, 821. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.N.; Ovsyannikova, I.G.; Kennedy, R.B.; Poland, G.A. Immunoinformatic identification of B cell and T cell epitopes in the SARS-CoV-2 proteome. Sci. Rep. 2020, 10, 14179. [Google Scholar] [CrossRef]
- Cao, S.; Song, Z.; Rong, J.; Andrikopoulos, N.; Liang, X.; Wang, Y.; Peng, G.; Ding, F.; Ke, P.C. Spike Protein Fragments Promote Alzheimer’s Amyloidogenesis. ACS Appl. Mater. Interfaces 2023, 15, 40317–40329. [Google Scholar] [CrossRef]
- Hassan, S.S.; Choudhury, P.P.; Dayhoff, G.W., 2nd; Aljabali, A.A.A.; Uhal, B.D.; Lundstrom, K.; Rezaei, N.; Pizzol, D.; Adadi, P.; Lal, A. The importance of accessory protein variants in the pathogenicity of SARS-CoV-2. Arch. Biochem. Biophys. 2022, 717, 109124. [Google Scholar] [CrossRef] [PubMed]
- Abavisani, M.; Rahimian, K.; Mahdavi, B.; Tokhanbigli, S.; Mollapour Siasakht, M.; Farhadi, A.; Kodori, M.; Mahmanzar, M.; Meshkat, Z. Mutations in SARS-CoV-2 structural proteins: A global analysis. Virol. J. 2022, 19, 220. [Google Scholar] [CrossRef] [PubMed]
- Idrees, D.; Kumar, V. SARS-CoV-2 spike protein interactions with amyloidogenic proteins: Potential clues to neurodegeneration. Biochem. Biophys. Res. Commun. 2021, 554, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulos, A.M.; Nigh, G.; Mccullough, P.A.; Seneff, S. Mitogen activated protein kinase (MAPK) activation, p53, and autophagy inhibition characterize the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein induced neurotoxicity. Cureus 2022, 14, e32361. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Huang, W.; Lee, H.; Van De Leemput, J.; Kane, M.A.; Han, Z. Characterization of SARS-CoV-2 proteins reveals Orf6 pathogenicity, subcellular localization, host interactions and attenuation by Selinexor. Cell. Biosci. 2021, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.; Lundstrom, K.; Serrano-Aroca, A.; Adadi, P.; Aljabali, A.A.A.; Redwan, E.M.; Lal, A.; Kandimalla, R.; Abd El-Aziz, T.M.; Choudhury, P.P.; et al. Emergence of unique SARS-CoV-2 ORF10 variants and their impact on protein structure and function. Int. J. Biol. Macromol. 2022, 194, 128–143. [Google Scholar] [CrossRef]
- Patel, N.S.; Paris, D.; Mathura, V.; Quadros, A.N.; Crawford, F.C.; Mullan, M.J. Inflammatory cytokine levels correlate with amyloid load in transgenic mouse models of Alzheimer’s disease. J. Neuroinflamm. 2005, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Tayeb-Fligelman, E.; Cheng, X.; Tai, C.; Bowler, J.T.; Griner, S.; Sawaya, M.R.; Seidler, P.M.; Jiang, Y.X.; Lu, J.; Rosenberg, G.M.; et al. Inhibition of amyloid formation of the Nucleoprotein of SARS-CoV-2. bioRxiv 2021, 5, 434000. [Google Scholar]
- Chen, H.; Cui, Y.; Han, X.; Hu, W.; Sun, M.; Zhang, Y.; Wang, P.H.; Song, G.; Chen, W.; Lou, J. Liquid–liquid phase separation by SARS-CoV-2 nucleocapsid protein and RNA. Cell Res. 2020, 30, 1143–1145. [Google Scholar] [CrossRef]
- Savastano, A.; Ibáñez de Opakua, A.; Rankovic, M.; Zweckstetter, M. Nucleocapsid protein of SARS-CoV-2 phase separates into RNA-rich polymerase-containing condensates. Nat. Commun. 2020, 11, 6041. [Google Scholar] [CrossRef]
- Pham, C.L.; Shanmugam, N.; Strange, M.; O’Carroll, A.; Brown, J.W.; Sierecki, E.; Gambin, Y.; Steain, M.; Sunde, M. Viral M45 and necroptosis-associated proteins form heteromeric amyloid assemblies. EMBO Rep. 2019, 20, e46518. [Google Scholar] [CrossRef] [PubMed]
- Koralnik, I.J.; Tyler, K.L. COVID-19: A global threat to the nervous system. Ann. Neurol. 2020, 88, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Divani, A.A.; Andalib, S.; Biller, J.; Di Napoli, M.; Moghimi, N.; Rubinos, C.A.; Nobleza, C.O.; Sylaja, P.N.; Toledano, M.; Lattanzi, S.; et al. Central nervous system manifestations associated with COVID-19. Curr. Neurol. Neurosci. Rep. 2020, 20, 60. [Google Scholar] [CrossRef]
- Iadecola, C.; Anrather, J.; Kamel, H. Effects of COVID-19 on the nervous system. Cell 2020, 183, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; Mccarthy, P.; Lange, F.; Andersson, J.L.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef]
- Serrano-Castro, P.J.; Garzon-Maldonado, F.J.; Casado-Naranjo, I.; Ollero-Ortiz, A.; Minguez-Castellanos, A.; Iglesias-Espinosa, M.; Baena-Palomino, P.; Sánchez-Sanchez, V.; Sánchez-Pérez, R.M.; Rubi-Callejon, J.; et al. The cognitive and psychiatric subacute impairment in severe Covid-19. Sci. Rep. 2022, 12, 3563. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Geng, D.; Mei, N.; Wu, P.Y.; Huang, C.C.; Jia, T.; Zhao, Y.; Wang, D.; Xiao, A.; et al. Cerebral micro-structural changes in COVID-19 patients—An MRI-based 3-month follow-up study: A brief title: Cerebral changes in COVID-19. eClinicalMedicine 2020, 25, 100484. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Roy, S.; Iktidar, M.A.; Chowdhury, S.; Akter, S.; Islam, A.; Hawlader, M. Post-COVID-19 memory complaints: Prevalence and associated factors. Neurología, 2022; Online ahead of print. [Google Scholar]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; Lange, F.; Andersson, J.L.; Griffanti, L.; Duff, E.; Jbabdi, S. Brain imaging before and after COVID-19 in UK Biobank. MedRxiv: Prepr. Serv. Health Sci. 2021. [Google Scholar] [CrossRef]
- Köhncke, Y.; Kühn, S.; Düzel, S.; Sander, M.C.; Brandmaier, A.M.; Lindenberger, U. Grey-matter structure in cortical and limbic regions correlates with general cognitive ability in old age. Aging Brain 2023, 5, 100103. [Google Scholar] [CrossRef]
- Poole, V.N.; Oveisgharan, S.; Yu, L.; Dawe, R.J.; Leurgans, S.E.; Zhang, S.; Arfanakis, K.; Buchman, A.S.; Bennett, D.A. Volumetric brain correlates of gait associated with cognitive decline in community-dwelling older adults. Front. Aging Neurosci. 2023, 15, 1194986. [Google Scholar] [CrossRef]
- Han, A.Y.; Mukdad, L.; Long, J.L.; Lopez, I.A. Anosmia in COVID-19: Mechanisms and significance. Chem. Sens. 2020, 45, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, K.; Chan, D.; Watermeyer, T. The cognitive consequences of the COVID-19 epidemic: Collateral damage? Brain Commun. 2020, 2, fcaa069. [Google Scholar] [CrossRef] [PubMed]
- Eichenbaum, H. The hippocampus and declarative memory: Cognitive mechanisms and neural codes. Behav. Brain Res. 2001, 127, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Fortin, N.J.; Agster, K.L.; Eichenbaum, H.B. Critical role of the hippocampus in memory for sequences of events. Nat. Neurosci. 2002, 5, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Furcila, D.; Defelipe, J.; Alonso-Nanclares, L. A study of amyloid-beta and phosphotau in plaques and neurons in the hippocampus of Alzheimer’s disease patients. J. Alzheimer’s Dis. 2018, 64, 417–435. [Google Scholar] [CrossRef] [PubMed]
- Lopez, O.L.; Becker, J.T.; Chang, Y.; Klunk, W.E.; Mathis, C.; Price, J.; Aizenstein, H.J.; Snitz, B.; Cohen, A.D.; DeKosky, S.T.; et al. Amyloid deposition and brain structure as long-term predictors of MCI, dementia, and mortality. Neurology 2018, 90, e1920–e1928. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Zhang, D.F.; Zou, Q.C.; Xie, X.; Xu, L.; Feng, X.L.; Li, X.; Han, J.B.; Yu, D.; Deng, Z.H.; et al. SARS-CoV-2 Spike protein S2 subunit modulates gamma-secretase and enhances amyloid-beta production in COVID-19 neuropathy. Cell Discov. 2022, 8, 99. [Google Scholar] [CrossRef]
- Priemer, D.S.; Rhodes, C.H.; Karlovich, E.; Perl, D.P.; Goldman, J.E. Abeta deposits in the neocortex of adult and infant hypoxic brains, including in cases of COVID-19. J. Neuropathol. Exp. Neurol. 2022, 81, 988–995. [Google Scholar] [CrossRef]
- Ziff, O.J.; Ashton, N.J.; Mehta, P.R.; Brown, R.; Athauda, D.; Heaney, J.; Heslegrave, A.J.; Benedet, A.L.; Blennow, K.; Checkley, A.M.; et al. Amyloid processing in COVID-19-associated neurological syndromes. J. Neurochem. 2022, 161, 146–157. [Google Scholar] [CrossRef]
- Chiricosta, L.; Gugliandolo, A.; Mazzon, E. SARS-CoV-2 exacerbates beta-amyloid neurotoxicity, inflammation and oxidative stress in Alzheimer’s disease patients. Int. J. Mol. Sci. 2021, 22, 13603. [Google Scholar] [CrossRef]
- Gordon, M.N.; Heneka, M.T.; Le Page, L.M.; Limberger, C.; Morgan, D.; Tenner, A.J.; Terrando, N.; Willette, A.A.; Willette, S.A. Impact of COVID-19 on the onset and progression of Alzheimer’s disease and related dementias: A roadmap for future research. Alzheimer’s Dement. 2022, 18, 1038–1046. [Google Scholar] [CrossRef]
- Solomon, I.H.; Normandin, E.; Bhattacharyya, S.; Mukerji, S.S.; Keller, K.; Ali, A.S.; Adams, G.; Hornick, J.L.; Padera, R.F., Jr.; Sabeti, P. Neuropathological Features of Covid-19. N. Engl. J. Med. 2020, 383, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Thakur, K.T.; Miller, E.H.; Glendinning, M.D.; Al-Dalahmah, O.; Banu, M.A.; Boehme, A.K.; Boubour, A.; Bruce, S.; Chong, A.M.; Claassen, J.; et al. COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain 2021, 144, 2696–2708. [Google Scholar] [CrossRef] [PubMed]
- Adingupu, D.D.; Soroush, A.; Hansen, A.; Twomey, R.; Dunn, J.F. Brain hypoxia, neurocognitive impairment, and quality of life in people post-COVID-19. J. Neurol. 2023, 270, 3303–3314. [Google Scholar] [CrossRef] [PubMed]
- Balsak, S.; Atasoy, B.; Donmez, Z.; Yabul, F.C.; Daşkaya, H.; Akkoyunlu, Y.; Yurtsever, İ.; Sarı, L.; Sijahovic, S.; Akcay, A.; et al. Microstructural alterations in hypoxia-related BRAIN centers after COVID-19 by using DTI: A preliminary study. J. Clin. Ultrasound 2023, 51, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Lall, R.; Mohammed, R.; Ojha, U. What are the links between hypoxia and Alzheimer’s disease? Neuropsychiatr. Dis. Treat. 2019, 15, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Raz, L.; Knoefel, J.; Bhaskar, K. The neuropathology and cerebrovascular mechanisms of dementia. J. Cereb. Blood Flow Metab. 2016, 36, 172–186. [Google Scholar] [CrossRef]
- Hassan, H.; Chen, R. Hypoxia in Alzheimer’s disease: Effects of hypoxia inducible factors. Neural Regen. Res. 2021, 16, 310–311. [Google Scholar]
- Shobatake, R.; Ota, H.; Takahashi, N.; Ueno, S.; Sugie, K.; Takasawa, S. The impact of intermittent hypoxia on metabolism and cognition. Int. J. Mol. Sci. 2022, 23, 12957. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, Y.; Chen, G.; Lu, Y.; Wang, D.; Zhu, L. Intermittent hypoxia therapy ameliorates beta-amyloid pathology via TFEB-mediated autophagy in murine Alzheimer’s disease. J. Neuroinflamm. 2023, 20, 240. [Google Scholar] [CrossRef]
- Xie, J.C.; Ma, X.Y.; Liu, X.H.; Yu, J.; Zhao, Y.C.; Tan, Y.; Liu, X.Y.; Zhao, Y.X. Hypoxia increases amyloid-β level in exosomes by enhancing the interaction between CD147 and Hook1. Am. J. Transl. Res. 2018, 10, 150–163. [Google Scholar] [PubMed]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. Hypoxia/ischemia activate processing of Amyloid Precursor Protein: Impact of vascular dysfunction in the pathogenesis of Alzheimer’s disease. J. Neurochem. 2017, 140, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Mamedova, A.; Vinnik, V.; Klimova, M.; Saranceva, E.; Ageev, V.; Yu, T.; Zhu, D.; Penzel, T.; Kurths, J. Brain Mechanisms of COVID-19-Sleep Disorders. Int. J. Mol. Sci. 2021, 22, 6917. [Google Scholar] [CrossRef] [PubMed]
- Montazersaheb, S.; Hosseiniyan Khatibi, S.M.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Sorbeni, F.G.; Farahzadi, R.; Ghasemnejad, T. COVID-19 infection: An overview on cytokine storm and related interventions. Virol. J. 2022, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Zanza, C.; Romenskaya, T.; Manetti, A.C.; Franceschi, F.; La Russa, R.; Bertozzi, G.; Maiese, A.; Savioli, G.; Volonnino, G.; Longhitano, Y. Cytokine Storm in COVID-19: Immunopathogenesis and Therapy. Medicina 2022, 58, 144. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Parra, H.; Reyes-Hernández, O.D.; Figueroa-González, G.; González-Del Carmen, M.; González-Torres, M.; Peña-Corona, S.I.; Florán, B.; Cortés, H.; Leyva-Gómez, G. Alteration of the blood-brain barrier by COVID-19 and its implication in the permeation of drugs into the brain. Front. Cell. Neurosci. 2023, 17, 1125109. [Google Scholar] [CrossRef] [PubMed]
- Suprewicz, Ł.; Fiedoruk, K.; Czarnowska, A.; Sadowski, M.; Strzelecka, A.; Galie, P.A.; Janmey, P.A.; Kułakowska, A.; Bucki, R. Blood-brain barrier function in response to SARS-CoV-2 and its spike protein. Neurol. Neurochir. Pol. 2023, 57, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.A.; Rhea, E.M.; Knopp, R.C.; Banks, W.A. Interactions of SARS-CoV-2 with the Blood–Brain Barrier. Int. J. Mol. Sci. 2021, 22, 2681. [Google Scholar] [CrossRef] [PubMed]
- Crunfli, F.; Carregari, V.C.; Veras, F.P.; Silva, L.S.; Nogueira, M.H.; Antunes, A.; Vendramini, P.H.; Valença, A.G.; Brandão-Teles, C.; Zuccoli, G.D.; et al. Morphological, cellular, and molecular basis of brain infection in COVID-19 patients. Proc. Natl. Acad. Sci. USA 2022, 119, e2200960119. [Google Scholar] [CrossRef]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef]
- Hosp, J.A.; Dressing, A.; Blazhenets, G.; Bormann, T.; Rau, A.; Schwabenland, M.; Thurow, J.; Wagner, D.; Waller, C.; Niesen, W.D.; et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain 2021, 144, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Sillett, R.; Zhu, L.; Mendel, J.; Camplisson, I.; Dercon, Q.; Harrison, P.J. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: An analysis of 2-year retrospective cohort studies including 1,284,437 patients. Lancet Psychiatry 2022, 9, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qi, L.; Yang, D.; Hao, S.; Zhang, F.; Zhu, X.; Sun, Y.; Chen, C.; Ye, J.; Yang, J.; et al. Meningeal lymphatic vessels mediate neurotropic viral drainage from the central nervous system. Nat. Neurosci. 2022, 25, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Rebejac, J.; Eme-Scolan, E.; Rua, R. Role of meningeal immunity in brain function and protection against pathogens. J. Inflamm. 2024, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, M.A.; Cowan, M.N.; Babcock, I.W.; Sibley, L.A.; Still, K.; Batista, S.J.; Labuzan, S.A.; Sethi, I.; Harris, T.H. Meningeal lymphatic drainage promotes T cell responses against Toxoplasma gondii but is dispensable for parasite control in the brain. eLife 2022, 11, e80775. [Google Scholar] [CrossRef] [PubMed]
- Abbaoui, A.; Fatoba, O.; Yamashita, T. Meningeal T cells function in the central nervous system homeostasis and neurodegenerative Diseases. Front. Cell. Neurosci. 2023, 17, 1181071. [Google Scholar] [CrossRef]
- Song, E.; Mao, T.; Dong, H.; Boisserand, L.S.B.; Antila, S.; Bosenberg, M.; Alitalo, K.; Thomas, J.L.; Iwasaki, A. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 2020, 577, 689–694. [Google Scholar] [CrossRef]
- Hu, X.T.; Deng, Q.; Ma, L.; Li, Q.; Chen, Y.; Liao, Y.; Zhou, F.; Zhang, C.; Shao, L.; Feng, J.; et al. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res. 2020, 30, 229–243. [Google Scholar] [CrossRef]
- Silva, M.T.T. Viral encephalitis. Arq. Neuropsiquiatr. 2013, 71, 703–709. [Google Scholar] [CrossRef]
- Solomon, T. Flavivirus encephalitis—Reply. N. Engl. J. Med. 2004, 351, 1804. [Google Scholar] [CrossRef]
- Tyler, K.L. Herpes simplex virus infections of the central nervous system: Encephalitis and meningitis, including Mollaret’s. Herpes 2004, 11, 57A–64A. [Google Scholar]
- Moseman, E.A.; Blanchard, A.C.; Nayak, D.; McGavern, D.B. T cell engagement of cross-presenting microglia protects the brain from a nasal virus infection. Sci. Immunol. 2020, 5, eabb1817. [Google Scholar] [CrossRef] [PubMed]
- Raval, U.; Trageser, K.J.; Naughton, S.X.; Griggs, E.; Iqbal, U.H.; Wu, H.; Rahim, M.A.; Harary, J.M.; Gursahai, S.; Pasinetti, G.M. COVID-19 and Alzheimer’s disease: Meninges-mediated neuropathology. Alzheimer’s Dement. 2021, 17, e056418. [Google Scholar]
- Wostyn, P. COVID-19 and chronic fatigue syndrome: Is the worst yet to come? Med. Hypotheses 2021, 146, 110469. [Google Scholar] [CrossRef]
- Patabendige, A.; Michael, B.D.; Craig, A.G.; Solomon, T. Brain microvascular endothelial–astrocyte cell responses following Japanese encephalitis virus infection in an in vitro human blood–brain barrier model. Mol. Cell Neurosci. 2018, 89, 60–70. [Google Scholar] [CrossRef]
- Mustafa, Y.M.; Meuren, L.M.; Coelho, S.V.A.; de Arruda, L.B. Pathways exploited by flaviviruses to counteract the blood–brain barrier and invade the central nervous system. Front. Microbiol. 2019, 10, 525. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Penzel, T.; Poluektov, M.; Fedosov, I.; Tzoy, M.; Terskov, A.; Blokhina, I.; Sidorov, V.; Kurths, J. Phototherapy of Alzheimer’s Disease: Photostimulation of Brain Lymphatics during Sleep: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 10946. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Fedosov, I.; Penzel, T.; Li, D.; Yu, T.; Telnova, V.; Kaybeleva, E.; Saranceva, E.; Terskov, A.; Khorovodov, A.; et al. Brain Waste Removal System and Sleep: Photobiomodulation as an Innovative Strategy for Night Therapy of Brain Diseases. Int. J. Mol. Sci. 2023, 24, 3221. [Google Scholar] [CrossRef]
- Lim, L. The Growing Evidence for Photobiomodulation as a Promising Treatment for Alzheimer’s Disease. J. Biosci. Med. 2018, 6, 100–110. [Google Scholar] [CrossRef]
- Berman, M.H.; Nichols, T.W. Treatment of Neurodegeneration: Integrating Photobiomodulation and Neurofeedback in Alzheimer’s Dementia and Parkinson’s: A Review. Photobiomodul. Photomed. Laser Surg. 2019, 37, 623–634. [Google Scholar] [CrossRef]
- Caldieraro, M.A.; Laufer-Silva, T.; Cassano, P. Dosimetry and Clinical Efficacy of Transcranial Photobiomodulation for Major Depression Disorder: Could they Guide Dosimetry for Alzheimer’s Disease? J. Alzheimer’s Dis. 2021, 83, 1453–1469. [Google Scholar] [CrossRef]
- Pan, W.T.; Liu, P.M.; Ma, D.; Yang, J.J. Advances in photobiomodulation for cognitive improvement by near-infrared derived multiple strategies. J. Transl. Med. 2023, 21, 135. [Google Scholar] [CrossRef] [PubMed]
- Saltmarche, A.E.; Naeser, M.A.; Ho, K.F.; Hamblin, M.R.; Lim, L. Significant Improvement in Cognition in Mild to Moderately Severe Dementia Cases Treated with Transcranial Plus Intranasal Photobiomodulation: Case Series Report. Photomed. Laser Surg. 2017, 35, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.L. Effects of Home Photobiomodulation Treatments on Cognitive and Behavioral Function, Cerebral Perfusion, and Resting-State Functional Connectivity in Patients with Dementia: A Pilot Trial. Photobiomodul. Photomed. Laser Surg. 2019, 37, 133–141. [Google Scholar] [CrossRef]
- Maksimovich, I.V. Dementia and cognitive impairment reduction after laser transcatheter treatment of Alzheimer’s disease. World J. Neurosci. 2015, 5, 189–203. [Google Scholar] [CrossRef]
- Nizamutdinov, D.; Qi, X.; Berman, M.; Dougal, G.; Dayawansa, S.; Wu, E.; Yi, S.; Stevens, A.; Huang, J. Transcranial near infrared light stimulations improve cognition in patients with dementia. Aging Dis. 2021, 12, 954–963. [Google Scholar] [CrossRef]
- Hamblin, M.R. Photobiomodulation for Alzheimer’s Disease: Has the Light Dawned? Photonics 2019, 6, 77. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Z.; Wang, P.; Sun, Z. Current application and future directions of photobiomodulation in central nervous diseases. Neural Regen. Res. 2021, 16, 1177–1185. [Google Scholar] [CrossRef]
- Stephan, W.; Banas, L.; Hamblin, M. Treatment Efficacy of Photobiomodulation for Moderate and Advanced Dementia or Alzheimer’s Disease: Case Studies. Adv. Alzheimer’s Dis. 2022, 11, 39–47. [Google Scholar] [CrossRef]
- Enengl, J.; Hamblin, M.; Dungel, P. Photobiomodulation for Alzheimer’s Disease: Translating Basic Research to Clinical Application. J. Alzheimer’s Dis. 2020, 75, 1073–1082. [Google Scholar] [CrossRef]
- Cho, G.; Lee, S.; Park, J.; Kim, M.; Park, M.; Choi, B.; Shin, Y.; Kim, N.; Shin, H. Photobiomodulation using a low-level light-emitting diode improves cognitive dysfunction in the 5XFAD mouse model of Alzheimer’s disease. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020, 75, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.S.; Lee, T.; Yeung, M.; Hamblin, M. Photobiomodulation improves the frontal cognitive function of older adults. Int. J. Geriatr. Psychiatry 2019, 2, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Jahan, A.; Nazari, M.; Mahmoudi, J. Transcranial near-infrared photobiomodulation could modulate brain electrophysiological features and attentional performance in healthy young adults. Lasers Med. Sci. 2019, 34, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Maksimovich, I.V. Laser Technologies as a New Direction in Transcatheter Interventions. Photobiomodul. Photomed. Laser Surg. 2019, 37, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Chao, L. Impact of Photobiomodulation (PBM) on Biomarkers of Alzheimer’s Disease (PBMbiomarker). 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03405662 (accessed on 28 April 2024).
- Lah, J. Stimulating Neural Activity to Improve Blood Flow and Reduce Amyloid: Path to Clinical Trials. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03543878 (accessed on 28 April 2024).
- Salehpour, F.; Hamblin, M.R.; Di Duro, J. Rapid Reversal of Cognitive Decline, Olfactory Dysfunction, and Quality of Life Using Multi-Modality Photobiomodulation Therapy: Case Report. Photobiomodul. Photomed. Laser Surg. 2019, 37, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Nizamutdinov, D.; Berman, M.; Dougal, G.; Chazot, P.; Wu, E.; Stevens, A.; Yi, S.; Huang, J. Gender Differences of Dementia in Response to Intensive Self-Administered Transcranial and Intraocular Near-Infrared Stimulation. Cureus 2021, 13, e16188. [Google Scholar] [CrossRef] [PubMed]
- Horner, S.; Berger, L.; Gibas, K. Nutritional Ketosis and photobiomodulation remediate mitochondria warding off Alzheimer’s disease in a diabetic, ApoE4+ patient with mild cognitive impairment: A case report. Photodiagn. Photodyn. Ther. 2020, 30, 101777. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Penzel, T.; Blokhina, I.; Khorovodov, A.; Fedosov, I.; Yu, T.; Karandin, G.; Evsukova, A.; Elovenko, D.; Adushkina, V.; et al. Night Photostimulation of Clearance of Beta-Amyloid from Mouse Brain: New Strategies in Preventing Alzheimer’s Disease. Cells 2021, 10, 3289. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Postnov, D.; Lavrova, A.; Fedosov, I.; Borisova, E.; Nikolenko, V.; Penzel, T.; Kurths, J.; Tu-chin, V. Biophotonic Strategies of Measurement and Stimulation of the Cranial and the Extracranial Lymphatic Drainage Function. IEEE J. Sel. Top. 2021, 27, 7400313. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Abdurashitov, A.; Dubrovsky, A.; Klimova, M.; Agranovich, I.; Terskov, A.; Shirokov, A.; Vinnik, V.; Kuznecova, A.; Lezhnev, N.; et al. Photobiomodulation of lymphatic drainage and clearance: Perspective strategy for augmentation of meningeal lymphatic functions. Biomed. Opt. Express 2020, 11, 725–734. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Fedosov, I.; Shirokov, A.; Vodovozov, E.; Alekseev, A.; Khorovodov, A.; Blokhina, I.; Ter-skov, A.; Mamedova, A.; Klimova, M.; et al. Photomodulation of lymphatic delivery of liposomes to the brain bypassing the blood-brain barrier: New perspectives for glioma therapy. Nanophotonics 2021, 12, 3215–3227. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Diduk, S.; Anna, E.; Elina, D.; Artem, K.; Khorovodov, A.; Shirokov, A.; Fedosov, I.; Du-brovsky, A.; Blokhina, I.; et al. Photomodulation of Lymphatic Delivery of Bevacizumab to the Brain: The Role of Singlet Oxygen. Adv. Exp. Med. Biol. 2022, 1395, 53–57. [Google Scholar] [PubMed]
- Semyachkina-Glushkovskaya, O.; Shirokov, A.; Blokhina, I.; Telnova, V.; Vodovozova, E.; Alekseeva, A.; Boldyrev, I.; Fedosov, I.; Dubrovsky, A.; Khorovodov, A.; et al. Intranasal delivery of liposomes to glioblastoma by photostimulation of the lymphatic system. Pharmaceutics 2023, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Fedosov, I.; Zaikin, A.; Ageev, V.; Ilyukov, E.; Myagkov, D.; Tuktarov, D.; Blokhina, I.; Shirokov, A.; Terskov, A.; et al. Technology of the photobiostimulation of the brain’s drainage system during sleep for improvement of learning and memory in male mice. Biomed. Opt. Express 2023, 15, 44–58. [Google Scholar] [CrossRef] [PubMed]
- LIA The Laser Institute. Available online: https://www.lia.org/resources/laser-safety-information/laser-safety-standards/ansi-z136-standards/z136-3 (accessed on 28 April 2024).
- Webstore International Electrotechnical Commission. IEC TR 60825. Available online: https://webstore.iec.ch/publication/63122 (accessed on 28 April 2024).
- Fehervari, Z. Brain lymphatic (dys)function. Nat. Immunol. 2018, 19, 901. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wei, H.; Zhou, Y. Overview of the meningeal lymphatic vessels in aging and central nervous system disorders. Cell Biosci. 2022, 12, 202. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, S.; Yu, T.-T.; Liu, Z.; Sub, S.; Bragin, D.; Shirokov, A.; Navolokin, N.; Bragina, O.; Zheng-Wu, H.; et al. Photostimulation of brain lymphatics in male newborn and adult rodents for therapy of intraventricular hemorrhage. Nat. Commun. 2023, 14, 6104. [Google Scholar] [CrossRef] [PubMed]
- Bohlen, H.; Gasheva, O.; Zawieja, D. Nitric oxide formation by lymphatic bulb and valves is a major regulatory component of lymphatic pumping. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1897–H1906. [Google Scholar] [CrossRef]
- Bohlen, H.; Wang, W.; Gashev, A.; Gasheva, O.; Zawieja, D. Phasic contractions of rat mesenteric lymphatics increase basal and phasic nitric oxide generation in vivo. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1319–H1328. [Google Scholar] [CrossRef]
- Kunert, C.; Baish, J.W.; Liao, S.; Padera, T.P.; Munn, L.L. Mechanobiological oscillators control lymph flow. Proc. Natl. Acad. Sci. USA 2015, 112, 10938–10943. [Google Scholar] [CrossRef]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of Animal and Marine Origin: Structure, Function, and Anti-Inflammatory Properties. Molecules 2017, 22, 1964. [Google Scholar] [CrossRef] [PubMed]
- Ahmmed, M.K.; Ahmmed, F.; Tian, H.S.; Carne, A.; Bekhit, A.E. Marine omega-3 (n-3) phospholipids: A comprehensive review of their properties, sources, bioavailability, and relation to brain health. Compr. Rev. Food Sci. Food Saf. 2020, 19, 64–123. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.; Mendes, I.; Duarte, M.P.; Bandarra, N.M.; Gomes-Bispo, A. New Forms of Neuroactive Phospholipids for DHA Enrichment in Brain. Mar. Drugs 2024, 22, 116. [Google Scholar] [CrossRef]
- Ahmmed, M.K.; Hachem, M.; Ahmmed, F.; Rashidinejad, A.; Oz, F.; Bekhit, A.A.; Carne, A.; Bekhit, A.E.A. Marine Fish-Derived Lysophosphatidylcholine: Properties, Extraction, Quantification, and Brain Health Application. Molecules 2023, 28, 3088. [Google Scholar] [CrossRef]
- Bennett, S.A.; Valenzuela, N.; Xu, H.; Franko, B.; Fai, S.; Figeys, D. Using neurolipidomics to identify phospholipid mediators of synaptic (dys)function in Alzheimer’s Disease. Front. Physiol. 2013, 4, 168. [Google Scholar] [CrossRef]
- Pelucchi, S.; Gardoni, F.; Di Luca, M.; Marcello, E. Synaptic dysfunction in early phases of Alzheimer’s Disease. Handb. Clin. Neurol. 2022, 184, 417–438. [Google Scholar] [PubMed]
- Meftah, S.; Gan, J. Alzheimer’s disease as a synaptopathy: Evidence for dysfunction of synapses during disease progression. Front. Synaptic Neurosci. 2023, 15, 1129036. [Google Scholar] [CrossRef]
- Li, J.; Huang, X.; An, Y.; Chen, X.; Chen, Y.; Xu, M.; Shan, H.; Zhang, M. The role of snapin in regulation of brain homeostasis. Neural Regen. Res. 2024, 19, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- Senanayake, V.; Goodenowe, D.B. Plasmalogen deficiency and neuropathology in Alzheimer’s disease: Causation or coincidence? Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 524–532. [Google Scholar] [CrossRef]
- Su, X.Q.; Wang, J.; Sinclair, A.J. Plasmalogens and Alzheimer’s disease: A review. Lipids Health Dis. 2019, 18, 100. [Google Scholar] [CrossRef]
- Yamashita, S.; Miyazawa, T.; Higuchi, O.; Kinoshita, M.; Miyazawa, T. Marine Plasmalogens: A Gift from the Sea with Benefits for Age-Associated Diseases. Molecules 2023, 28, 6328. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Sheikh, A.M.; Haque, M.A.; Osago, H.; Sakai, H.; Shibly, A.Z.; Yano, S.; Michikawa, M.; Hossain, S.; Tabassum, S.; et al. Time-Dependent Analysis of Plasmalogens in the Hippocampus of an Alzheimer’s Disease Mouse Model: A Role of Ethanolamine Plasmalogen. Brain Sci. 2021, 11, 1603. [Google Scholar] [CrossRef]
- Hossain, M.S.; Mawatari, S.; Fujino, T. Biological Functions of Plasmalogens. Adv. Exp. Med. Biol. 2020, 1299, 171–193. [Google Scholar] [PubMed]
- Honsho, M.; Fujiki, Y. Asymmetric Distribution of Plasmalogens and Their Roles-A Mini Review. Membranes 2023, 13, 764. [Google Scholar] [CrossRef] [PubMed]
- West, A.; Zoni, V.; Teague, W.E.; Leonard, A.N.; Vanni, S.; Gawrisch, K.; Tristram-Nagle, S.; Sachs, J.N.; Klauda, J.B. How do ethanolamine plasmalogens contribute to order and structure of neurological membranes? J. Phys. Chem. B 2020, 124, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Bozelli, J.C., Jr.; Azher, S.; Epand, R.M. Plasmalogens and Chronic Inflammatory Diseases. Front. Physiol. 2021, 12, 730829. [Google Scholar] [CrossRef] [PubMed]
- Kytikova, O.Y.; Novgorodtseva, T.P.; Antonyuk, M.V.; Gvozdenko, T.A. Plasmalogens in the Pathophysiology and Therapy of Age-Specific Diseases. Adv. Gerontol. 2020, 10, 272–281. [Google Scholar]
- Kling, M.A.; Goodenowe, D.B.; Senanayake, V.; Mahmoudian Dehkordi, S. Circulating ethanolamine plasmalogen indices in Alzheimer’s disease: Relation to diagnosis, cognition, and CSF tau. Alzheimer’s Dement. 2020, 16, 1234–1247. [Google Scholar] [CrossRef] [PubMed]
- Goodenowe, D. Breaking Alzheimer’s—A 15 Year Crusade to Expose the Cause and Deliver the Cure; Amazon Digital Services LLC—Kdp: Seattle, WA, USA, 2021; p. 244. [Google Scholar]
- Rouser, G.; Yamamoto, A. Curvilinear regression course of human brain lipid composition changes with age. Lipids 1968, 3, 284–287. [Google Scholar] [CrossRef]
- Goodenowe, D.B.; Haroon, J.; Kling, M.A.; Zielinski, M.; Mahdavi, K.; Habelhah, B.; Shtilkind, L.; Jordan, S. Targeted Plasmalogen Supplementation: Effects on Blood Plasmalogens, Oxidative Stress Biomarkers, Cognition, and Mobility in Cognitively Impaired Persons. Front. Cell. Dev. Biol. 2022, 10, 864842. [Google Scholar] [CrossRef]
- Hugo, J.; Ganguli, M. Dementia and cognitive impairment: Epidemiology, diagnosis, and treatment. Clin. Geriatr. Med. 2014, 30, 421–442. [Google Scholar] [CrossRef] [PubMed]
- Vermunt, L.; Sikkes, S.A.M.; van den Hout, A.; Handels, R.; Bos, I.; van der Flier, W.M.; Kern, S.; Ousset, P.J.; Maruff, P.; Skoog, I.; et al. Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex, and APOE genotype. Alzheimer’s Dement. 2019, 15, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.S.; Yu, L.; Schneider, J.A.; Bennett, D.A.; Boyle, P.A. Risk Aversion and Alzheimer Disease in Old Age. Am. J. Geriatr. Psychiatry 2019, 27, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Meletis, C.D. Alkyl-Acylglycerols and the Important Clinical Ramifications of Raising Plasmalogens in Dementia and Alzheimer’s Disease. Integr. Med. 2020, 19, 12e–16e. [Google Scholar]
- Jenkins, C.M.; Yang, K.; Liu, G.; Moon, S.H.; Dilthey, B.G.; Gross, R.W. Cytochrome c is an oxidative stress-activated plasmalogenase that cleaves plasmenylcholine and plasmenylethanolamine at the sn-1 vinyl ether linkage. J. Biol. Chem. 2018, 293, 8693–8709. [Google Scholar] [CrossRef] [PubMed]
- Dorninger, F.; Forss-Petter, S.; Wimmer, I.; Berger, J. Plasmalogens, platelet-activating factor and beyond—Ether lipids in signaling and neurodegeneration. Neurobiol. Dis. 2020, 145, 105061. [Google Scholar] [CrossRef] [PubMed]
- Braverman, N.E.; Moser, A.B. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta 2012, 1822, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Sindona, C.; Schepici, G.; Contestabile, V.; Bramanti, P.; Mazzon, E. NOX2 Activation in COVID-19: Possible Implications for Neurodegenerative Diseases. Medicina 2021, 57, 604. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.S.Y.; Loo, E.X.L.; Kang, A.Y.H.; Lau, H.X.; Tambyah, P.A.; Tham, E.H. Age-related differences in immunological responses to SARS-CoV-2. J. Allergy Clin. Immunol. Pract. 2020, 8, 3251–3258. [Google Scholar] [CrossRef]
- Feng, T.; Hu, X.; Fukui, Y.; Tadokoro, K.; Bian, Z.; Morihara, R.; Yamashita, T.; Abe, K. Neuroprotective effects of Scallop-derived plasmalogen in a mouse model of ischemic stroke. Brain Res. 2021, 1766, 147516. [Google Scholar] [CrossRef]
- Yamashita, S.; Hashimoto, M.; Haque, A.M.; Nakagawa, K.; Kinoshita, M.; Shido, O.; Miyazawa, T. Oral Administration of Ethanolamine Glycerophospholipid Containing a High Level of Plasmalogen Improves Memory Impairment in Amyloid beta-Infused Rats. Lipids 2017, 52, 575–585. [Google Scholar] [CrossRef]
- Hossain, M.S.; Mawatari, S.; Fujino, T. Plasmalogens inhibit neuroinflammation and promote cognitive function. Brain Res. Bull. 2023, 192, 56–61. [Google Scholar] [CrossRef]
- Hossain, M.S.; Tajima, A.; Kotoura, S.; Katafuchi, T. Oral ingestion of plasmalogens can attenuate the LPS-induced memory loss and microglial activation. Biochem. Biophys. Res. Commun. 2018, 496, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L.; Mankidy, R.; Ritchie, S.; Heath, D.; Wood, J.A.; Flax, J.; Goodenowe, D.B. Circulating plasmalogen levels and Alzheimer disease Assessment Scale-Cognitive scores in Alzheimer patients. J. Psychiatry Neurosci. 2010, 35, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Mawatari, S.; Ohara, S.; Taniwaki, Y.; Tsuboi, Y.; Maruyama, T.; Fujino, T. Improvement of Blood Plasmalogens and Clinical Symptoms in Parkinson’s Disease by Oral Administration of Ether Phospholipids: A Preliminary Report. Park. Dis. 2020, 2020, 2671070. [Google Scholar] [CrossRef]
- Fujino, T.; Yamada, T.; Mawatari, S.; Shinfuku, N.; Tsuboi, Y.; Wakana, C.; Kono, S. Effects of Plasmalogen on Patients with Moderate-to-Severe Alzheimer’s Disease and Blood Plasmalogen Changes: A Multi-Center, Open-Label Study. J. Alzheimer’s Dis. Park. 2019, 9, 474. [Google Scholar]
- Yamamoto, A.; Aizawa, T.; Kubomura, D.; Akahori, Y.; Yamashita, S.; Nakagawa, K.; Miyazawa, T. Effects of Ascidian-Derived Ethanolamine Plasmalogen on Cognitive Function and Its Safety—A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study. Pharmacometrics 2023, 104, 17–24. [Google Scholar]
- Watanabe, H.; Okawara, M.; Matahira, Y.; Mano, T.; Wada, T.; Suzuki, N.; Takara, T. The Impact of Ascidian (Halocynthia roretzi)-derived Plasmalogen on Cognitive Function in Healthy Humans: A Randomized, Double-blind, Placebo-controlled Trial. J. Oleo Sci. 2020, 69, 1597–1607. [Google Scholar] [CrossRef]
- Kawamura, J.; Kotoura, S.; Ando, T.; Kawasaki, Y.; Ebihara, S. The Evaluation Test of Brain Function by Oral Consumption of the Food Which Contain Plasmalogen—Randomized, Placebo-controlled, Double-blind Parallel-group Study. Jpn. Pharmacol. Ther. 2019, 47, 739–749. [Google Scholar]
- Olivera-Pueyo, J.; Pelegrin-Valero, C. Dietary supplements for cognitive impairment. Actas Esp. Psiquiatr. 2017, 45, 37–47. [Google Scholar]
- Liu, Y.; Cong, P.; Zhang, T.; Wang, R.; Wang, X.; Liu, J.; Wang, X.; Xu, J.; Wang, Y.; Wang, J.; et al. Plasmalogen attenuates the development of hepatic steatosis and cognitive deficit through mechanism involving p75NTR inhibition. Redox Biol. 2021, 43, 102002. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Mawatari, S.; Fujino, T. Plasmalogens, the Vinyl Ether-Linked Glycerophospholipids, Enhance Learning and Memory by Regulating Brain-Derived Neurotrophic Factor. Front. Cell Dev. Biol. 2022, 10, 828282. [Google Scholar] [CrossRef] [PubMed]
- Sejimo, S.; Hossain, M.S.; Akashi, K. Scallop-derived plasmalogens attenuate the activation of PKCδ associated with the brain inflammation. Biochem. Biophys. Res. Commun. 2018, 503, 837–842. [Google Scholar] [CrossRef]
- Che, H.; Li, Q.; Zhang, T.; Ding, L.; Zhang, L.; Shi, H.; Yanagita, T.; Xue, C.; Chang, Y.; Wang, Y. A comparative study of EPA-enriched ethanolamine plasmalogen and EPA-enriched phosphatidylethanolamine on Aβ42 induced cognitive deficiency in a rat model of Alzheimer’s disease. Food Funct. 2018, 9, 3008–3017. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, M.; Katafuchi, T.; Mawatari, S.; Noda, M.; Miake, K.; Sugiyama, M.; Fujino, T. Anti-inflammatory/anti-amyloidogenic effects of plasmalogens in lipopolysaccharide-induced neuroinflammation in adult mice. J. Neuroinflamm. 2012, 9, 197. [Google Scholar] [CrossRef]
- Yamashita, S.; Kiko, T.; Fujiwara, H.; Hashimoto, M.; Nakagawa, K.; Kinoshita, M.; Furukawa, K.; Arai, H.; Miyazawa, T. Alterations in the Levels of Amyloid-beta, Phospholipid Hydroperoxide, and Plasmalogen in the Blood of Patients with Alzheimer’s Disease: Possible Interactions between Amyloid-beta and These Lipids. J. Alzheimer’s Dis. 2016, 50, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.; Ibrahim, A.; Akashi, K.; Hossain, M.S. PUFA-Plasmalogens Attenuate the LPS-Induced Nitric Oxide Production by Inhibiting the NF-kB, p38 MAPK and JNK Pathways in Microglial Cells. Neuroscience 2019, 397, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Onodera, T.; Futai, E.; Kan, E.; Abe, N.; Uchida, T.; Kamio, Y.; Kaneko, J. Phosphatidylethanolamine plasmalogen enhances the inhibiting effect of phosphatidylethanolamine on γ-secretase activity. J. Biochem. 2014, 157, 301–309. [Google Scholar] [CrossRef]
- Miyazawa, T.; Itaya, M.; Burdeos, G.C.; Nakagawa, K.; Miyazawa, T. A Critical Review of the Use of Surfactant-Coated Nanoparticles in Nanomedicine and Food Nanotechnology. Int. J. Nanomed. 2021, 16, 3937–3999. [Google Scholar] [CrossRef]
- Hino, K.; Kaneko, S.; Harasawa, T.; Kimura, T.; Takei, S.; Shinohara, M.; Yamazaki, F.; Morita, S.-y.; Sato, S.; Kubo, Y.; et al. Change in Brain Plasmalogen Composition by Exposure to Prenatal Undernutrition Leads to Behavioral Impairment of Rats. J. Neurosci. 2019, 39, 7689–7702. [Google Scholar] [CrossRef]
- Katafuchi, T.; Ifuku, M.; Mawatari, S.; Noda, M.; Miake, K.; Sugiyama, M.; Fujino, T. Effects of plasmalogens on systemic lipopolysaccharide-induced glial activation and β-amyloid accumulation in adult mice. Ann. N. Y. Acad. Sci. 2012, 1262, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Rothhaar, T.L.; Grösgen, S.; Haupenthal, V.J.; Burg, V.K.; Hundsdörfer, B.; Mett, J.; Riemenschneider, M.; Grimm, H.S.; Hartmann, T.; Grimm, M.O. Plasmalogens inhibit APP processing by directly affecting γ-secretase activity in Alzheimer’s disease. Sci. World J. 2012, 2012, 141240. [Google Scholar] [CrossRef] [PubMed]
- Conti Filho, C.E.; Loss, L.B.; Marcolongo-Pereira, C.; Rossoni Junior, J.V.; Barcelos, R.M.; Chiarelli-Neto, O.; da Silva, B.S.; Passamani Ambrosio, R.; Castro, F.C.A.Q.; Teixeira, S.F.; et al. Advances in Alzheimer’s disease’s pharmacological treatment. Front. Pharmacol. 2023, 14, 1101452. [Google Scholar] [CrossRef] [PubMed]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Jin, H.; Xue, Y.; Chen, Q.; Yao, S.; Du, M.; Liu, S. Current and future therapeutic strategies for Alzheimer’s disease: An overview of drug development bottlenecks. Front. Aging Neurosci. 2023, 15, 1206572. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 10284, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Botchway, B.O.A.; Moore, M.K.; Akinleye, F.O.; Iyer, I.C.; Fang, M. Nutrition: Review on the possible treatment for Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 3, 867–883. [Google Scholar] [CrossRef] [PubMed]
- Mafi, J.N.; Leng, M.; Arbanas, J.C.; Tseng, C.H.; Damberg, C.L.; Sarkisian, C.; Landon, B.E. Estimated annual spending on aducanumab in the US medicare program. JAMA Health Forum 2022, 1, e214495. [Google Scholar] [CrossRef] [PubMed]
- Biogen. Biogen Plans Regulatory Filing for Aducanumab in Alzheimer’s Disease Based on New Analysis of Larger Dataset from Phase 3 Studies. Available online: http://investors.biogen.com/news-releases/news-release-details/biogen-plans-regulatory-filing-aducanumab-alzheimers-disease (accessed on 25 April 2024).
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H.; et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef]
- The Lancet. Lecanemab for Alzheimer’s disease: Tempering hype and hope. Lancet 2022, 400, 1899. [Google Scholar] [CrossRef]
- Silva, T.; Reis, J.; Teixeira, J.; Borges, F. Alzheimer’s disease, enzyme targets and drug discovery struggles: From natural products to drug prototypes. Ageing Res. Rev. 2014, 15, 116–145. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Huang, N.; Mao, Q.; Shi, J.; Qiu, Y. Natural bioactive compounds in Alzheimer’s disease: From the perspective of type 3 diabetes mellitus. Front. Aging Neurosci. 2023, 15, 1130253. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.d.C. Natural Compounds for Alzheimer’s Disease Therapy: A Systematic Review of Preclinical and Clinical Studies. Int. J. Mol. Sci. 2019, 20, 2313. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, Z.; Singh, T.D. An Insight in Pathophysiological Mechanism of Alzheimer’s Disease and its Management Using Plant Natural Products. Mini Rev. Med. Chem. 2021, 21, 35–57. [Google Scholar] [CrossRef]
- Nagori, K.; Nakhate, K.T.; Yadav, K.; Ajazuddin; Pradhan, M. Unlocking the Therapeutic Potential of Medicinal Plants for Alzheimer’s Disease: Preclinical to Clinical Trial Insights. Future Pharmacol. 2023, 3, 877–907. [Google Scholar] [CrossRef]
- Singh, A.K.; Gupta, A.; Mishra, A.K.; Gupta, V.; Bansal, P.; Kumar, S. Medicinal Plant for Curing Alzheimer’s Disease. Int. J. Pharm. Biol. Sci. Arch. 2010, 1, 108–114. [Google Scholar]
- Hatab, H.M.; Abdel Hamid, F.F.; Soliman, A.F.; Al-Shafie, T.A.; Ismail, Y.M.; El-Houseini, M.E. A combined treatment of curcumin, piperine, and taurine alters the circulating levels of IL-10 and miR-21 in hepatocellular carcinoma patients: A pilot study. J. Gastrointest. Oncol. 2019, 10, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, A.; Aggarwal, A. Therapeutic potentials of herbal drugs for Alzheimer’s disease—An overview. Indian J. Exp. Biol. 2017, 55, 63–73. [Google Scholar]
- Rane, J.S.; Bhaumik, P.; Panda, D. Curcumin inhibits tau aggregation and disintegrates preformed tau filaments in vitro. J. Alzheimer’s Dis. 2017, 60, 999–1014. [Google Scholar] [CrossRef]
- Voulgaropoulou, S.D.; van Amelsvoort, T.A.M.J.; Prickaerts, J.; Vingerhoets, C. The effect of curcumin on cognition in Alzheimer’s disease and healthy aging: A systematic review of pre-clinical and clinical studies. Brain Res. 2019, 1725, 146476. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Palanivelu, K. The effect of curcumin (turmeric) on Alzheimer’s disease: An overview. Ann. Indian Acad. Neurol. 2008, 11, 13–19. [Google Scholar] [CrossRef]
- Kesarwani, K.; Gupta, R. Bioavailability enhancers of herbal origin: An overview. Asian Pac. J. Trop. Biomed. 2013, 3, 253–266. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Ahmad, S.; Hafeez, A. Formulation and Development of Curcumin–Piperine-Loaded S-SNEDDS for the Treatment of Alzheimer’s Disease. Mol. Neurobiol. 2023, 60, 1067–1082. [Google Scholar] [CrossRef] [PubMed]
- Canevelli, M.; Adali, N.; Kelaiditi, E.; Cantet, C.; Ousset, P.J.; Cesari, M. Efects of Gingko biloba supplementation in Alzheimer disease patients receiving cholinesterase inhibitors: Data from the ICTUS study. Phytomedicine 2014, 21, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Kojder, K.; Zielonka-Brzezicka, J.; Wróbel, J.; Bosiacki, M.; Fabiańska, M.; Wróbel, M.; Sołek-Pastuszka, J.; Klimowicz, A. The use of Ginkgo biloba as a neuroprotective agent in the Alzheimer disease. Front. Pharmacol. 2021, 12, 775034. [Google Scholar] [CrossRef]
- Xie, L.; Zhu, Q.; Lu, J. Can we use Ginkgo biloba extract to treat Alzheimer disease? Lessons from preclinical and clinical studies. Cells 2022, 11, 479. [Google Scholar] [CrossRef]
- Oken, B.S.; Storzbach, D.M.; Kaye, J.A. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch. Neurol. 1998, 55, 1409–1415. [Google Scholar] [CrossRef]
- Dubey, T.; Chinnathambi, S. Brahmi (Bacopa monnieri): An ayurvedic herb against the Alzheimer disease. Arch. Biochem. Biophys. 2019, 676, 108153. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L. Salvia (Sage): A review of its potential cognitive-enhancing and protective effects. Drugs R&D 2017, 17, 53–64. [Google Scholar]
- Miraj, S.; Kiani, S. A review study of therapeutic effects of Salvia officanalis L. Pharm Lett. 2016, 8, 299–303. [Google Scholar]
- Datta, S.; Patil, S. Evaluation of traditional herb extract Salvia officanalis in treatment of Alzheimer disease. Pharmacogn. J. 2020, 12, 131–143. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Noroozian, M.; Mohammadi, M.; Ohadinia, S.; Jamshidi, A.H.; Khani, M. Melissa offcinalis extract in the treatment of patients with mild to moderate Alzheimer disease: A double blind, randomised, placebo controlled trial. J. Neurol. Neurosurg. Psychiatry 2003, 74, 863–866. [Google Scholar] [CrossRef] [PubMed]
- John, O.O.; Ihim, S.A.; Agbo, C.P.; Echezona, A. Phytotherapy: A promising approach for the treatment of Alzheimer disease. Pharmacol. Res. Mod. Chin. Med. 2022, 2, 100030. [Google Scholar] [CrossRef]
- López, V.; Martín, S.; Gómez-Serranillos, M.P.; Carretero, M.E.; Jäger, A.K.; Calvo, M.I. Neuroprotective and neurological properties of Melissa officinalis. Neurochem. Res. 2009, 34, 1955–1961. [Google Scholar] [CrossRef] [PubMed]
- Obulesu, M.; Rao, D.M. Effect of plant extracts on Alzheimer disease: An insight into therapeutic avenues. J. Neurosci. Rural Pract. 2011, 2, 56–61. [Google Scholar]
- Miraj, S.; Azizi, N.; Kiani, S. A review of chemical components and pharmacological effects of Melissa officinalis L. Pharm. Lett. 2016, 8, 229–237. [Google Scholar]
- Beheshti, S.; Shahmoradi, B. Therapeutic effect of Melissa offcinalis in an amyloid-β rat model of Alzheimer disease. J. Herbmed Pharmacol. 2018, 7, 193–199. [Google Scholar] [CrossRef]
- Mahboubi, M. Melissa offcinalis and rosmarinic acid in management of memory functions and Alzheimer disease. Asian Pac. J. Trop. Biomed. 2019, 9, 47–52. [Google Scholar] [CrossRef]
- Friedli, M.J.; Inestrosa, N.C. Huperzine A and Its Neuroprotective Molecular Signaling in Alzheimer’s Disease. Molecules 2021, 26, 6531. [Google Scholar] [CrossRef] [PubMed]
- Callizot, N.; Campanari, M.L.; Rouvière, L.; Jacquemot, G.; Henriques, A.; Garayev, E.; Poindron, P. Huperzia serrata Extract ‘NSP01’ with Neuroprotective Effects-Potential Synergies of Huperzine A and Polyphenols. Front. Pharmacol. 2021, 12, 681532. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.R.; Yang, J.H.; Wang, X.; Yao, Z.B. Induced dural lymphangiogenesis facilities soluble amyloid-beta clearance from brain in a transgenic mouse model of Alzheimer’s disease. Neural Regen. Res. 2018, 13, 709–716. [Google Scholar] [PubMed]
- Sheikhi-Mobarakeh, Z.; Yarmohammadi, H.; Mokhatri-Hesari, P.; Fahimi, S.; Montazeri, A.; Heydarirad, G. Herbs as old potential treatments for lymphedema management: A systematic review. Complement. Ther. Med. 2020, 55, 102615. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Dong, Y.; Fan, H.; Cao, M.; Wu, Q.; Wang, Y.; Zhou, C.; Li, S.; Zhao, C.; Wang, Y. Traditional Chinese Medicine Regulating Lymphangiogenesis: A Literature Review. Front. Pharmacol. 2020, 11, 1259. [Google Scholar] [CrossRef] [PubMed]
- Cacchio, A.; Prencipe, R.; Bertone, M.; De Benedictis, L.; Taglieri, L.; D’Elia, E.; Centoletti, C.; Di Carlo, G. Effectiveness and safety of a product containing diosmin, coumarin, and arbutin (Linfadren®) in addition to complex decongestive therapy on management of breast cancer-related lymphedema. Support. Care Cancer 2019, 27, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Mao, L.; Guan, L.; Zhang, Y.; Zhao, J. Ginsenoside Rg1 enhances lymphatic transport of intrapulmonary silica via VEGF-C/VEGFR-3 signaling in silicotic rats. Biochem. Biophys. Res. Commun. 2016, 472, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.G.; Shan, C.; Zhu, J.Z.; Bao, X.Y.; Tong, Q.; Wu, X.F.; Tang, X.C.; Xue, T.; Liu, J.; Zheng, G.Q.; et al. Additive Neuroprotective Effect of Borneol with Mesenchymal Stem Cells on Ischemic Stroke in Mice. Front. Physiol. 2018, 8, 1133. [Google Scholar] [CrossRef]
- Li, W.R.; Chen, R.Y.; Yang, L.; Huang, T.L.; Xu, Q.W.; Mi, S.Q.; Wang, N.S. Pharmacokinetics of natural borneol after oral administration in mice brain and its effect on excitation ratio. Eur. J. Drug Metab. Pharmacokinet. 2012, 37, 39–44. [Google Scholar] [CrossRef]
- Yu, B.; Ruan, M.; Cui, X.B.; Guo, J.M.; Xu, L.; Dong, X.P. Effects of borneol on the pharmacokinetics of geniposide in cortex, hippocampus, hypothalamus and striatum of conscious rat by simultaneous brain microdialysis coupled with UPLC-MS. J. Pharm. Biomed. Anal. 2013, 77, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.L.; Fu, B.M.; Zhang, Z.L. Borneol, a novel agent that improves central nervous system drug delivery by enhancing blood–brain barrier permeability. Drug Deliv. 2017, 24, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Tambe, R.F.; Jain, P.K.; Patil, S.; Ghumatkar, P.; Sathaye, S. Antiepileptogenic effects of borneol in pentylenetetrazole-induced kindling in mice. Naunyn. Schmiedebergs Arch. Pharmacol. 2016, 389, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.H.; Mak, H.; Zheng, Z.Y.; Xia, Y.J.; Xu, M.L.; Duan, R.; Dong, T.T.-X.; Li, S.-P.; Zhan, C.-S.; Shang, X.-H.; et al. Shexiang Baoxin Pill, a Traditional Chinese Herbal Formula, Rescues the Cognitive Impairments in APP/PS1 Transgenic Mice. Front. Pharmacol. 2020, 11, 1045. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.W.; Chen, N.; Ma, X.; Wang, J.; Wen, J.; Xie, Q.; Ma, R. The protective roles of L-borneolum, D-borneolum and synthetic borneol in cerebral ischaemia via modulation of the neurovascular unit. Biomed. Pharmacother. 2018, 102, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.T.; Wu, Y.; Shang, L.; Deng, X.Q.; Wang, S.J. Improved lymphatic targeting: Effect and mechanism of synthetic borneol on lymph node uptake of 7-ethyl-10-hydroxycamptothecin nanoliposomes following subcutaneous administration. Drug Deliv. 2018, 25, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, T.; Li, X.; Wei, Y.; Li, X.; Wang, S.; Liu, J.; Li, D.; Wang, S.; Ye, T. Borneol-driven meningeal lymphatic drainage clears amyloid-β peptide to attenuate Alzheimer-like phenotype in mice. Theranostics 2023, 13, 106–124. [Google Scholar] [CrossRef]
- Ding, X.; Wang, X.; Xia, D.; Liu, H.; Tian, H.; Fu, Y.; Chen, Y.; Qin, C.; Wang, J.; Xiang, Z.; et al. Impaired meningeal lymphatic drainage in patients with idiopathic Parkinson’s disease. Nat. Med. 2021, 27, 411–418. [Google Scholar] [CrossRef]
- Bolte, A.C.; Dutta, A.B.; Hurt, M.E. Meningeal lymphatic dysfunction exacerbates traumatic brain injury pathogenesis. Nat. Commun. 2020, 11, 4524. [Google Scholar] [CrossRef]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef]
- Fultz, N.E.; Bonmassar, G.; Setsompop, K.; Stickgold, R.; Rosen, B.; Polimeni, J.; Lewis, L. Coupled electrophysio-logical, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 2019, 366, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Shirokov, A.; Blokhina, I.; Fedosov, I.; Ilyukov, E.; Terskov, A.; Myagkov, D.; Tuktarov, D.; Tzoy, M.; Adushkina, V.; Zlatogosrkaya, D.; et al. Different Effects of Phototherapy for Rat Glioma during Sleep andWakefulness. Biomedicines 2024, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Sultanov, R.M.; Poleshchuk, T.S.; Ermolenko, E.V.; Kasyanov, S.P. Protective Properties of Marine Alkyl Glycerol Ethers in Chronic Stress. Mar. Drugs 2023, 21, 202. [Google Scholar] [CrossRef] [PubMed]
- Sultanov, R.; Ermolenko, E.; Poleshchuk, T.; Kasyanov, S. Alkyl Glycerol Ethers as Adaptogens. Mar. Drugs 2023, 21, 4. [Google Scholar] [CrossRef]
- Carvalho, D.; Knopman, D.; Boeve, D.; Lowe, V.; Roberts, R.; Mielke, M.; Przybelski, S.; Machulda, M.; Petersen, R.; Jack, C.; et al. association of excessive daytime sleepiness with longitudinal beta-amyloid accumulation in elderly persons without dementia. JAMA Neurol. 2018, 75, 672–680. [Google Scholar] [CrossRef]
- Sabia, S.; Fayosse, A.; Dumurgier, J.; Van Hees, V.; Paquet, C.; Sommerlad, A.; Kivimäki, M.; Dugravot, A.; Singh-Manoux, A. Association of sleep duration in middle and old age with incidence of dementia. Nat. Commun. 2021, 12, 2289. [Google Scholar] [CrossRef]
- Fessel, J. Analysis of Why Alzheimer’s Dementia Never Spontaneously Reverses, Suggests the Basis for Curative Treatment. J. Clin. Med. 2023, 12, 4873. [Google Scholar] [CrossRef]
- Li, Z.; Heckman, M.G.; Kanekiyo, T.; Martens, Y.A.; Day, G.S.; Vassilaki, M.; Liu, C.-C.; Bennett, D.A.; Petersen, R.C.; Zhao, N. Clinicopathologic Factors Associated with Reversion to Normal Cognition in Patients with Mild Cognitive Impairment. Neurology 2022, 98, e2036–e2045. [Google Scholar] [CrossRef] [PubMed]
- Pandya, S.Y.; Lacritz, L.H.; Weiner, M.F.; Deschner, M.; Woon, F.L. Predictors of reversion from mild cognitive impairment to normal cognition. Dement. Geriatr. Cogn. Disord. 2017, 43, 204–214. [Google Scholar] [CrossRef]
- Overton, M.; Pihlsgård, M.; Elmståhl, S. Diagnostic stability of mild cognitive impairment, and predictors of reversion to normal cognitive functioning. Dement. Geriatr. Cogn. Disord. 2020, 48, 317–329. [Google Scholar] [CrossRef]
- Malek-Ahmadi, M. Reversion from mild cognitive impairment to normal cognition. Alzheimer Dis. Assoc. Disord. 2016, 30, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Iraniparast, M.; Shi, Y.; Wu, Y.; Zeng, L.; Maxwell, C.J.; Kryscio, R.J.; St John, P.D.; SantaCruz, K.S.; Tyas, S.L. Cognitive Reserve and Mild Cognitive Impairment: Predictors and Rates of Reversion to Intact Cognition vs. Progression to Dementia. Neurology 2022, 98, e1114–e1123. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.; Brennan, S.; Keon, M.; Ooi, L. The role of amyloid oligomers in neurodegenerative pathologies. Int. J. Biol. Macromol. 2021, 181, 582–604. [Google Scholar] [CrossRef] [PubMed]
- Savage, M.; Trusko, S.; Howland, D.; Pinsker, L.; Mistretta, S.; Reaume, A.; Greenberg, B.; Siman, R.; Scott, R. Turnover of amyloid beta-protein in mouse brain and acute reduction of its level by phorbol ester. J. Neurosci. 1998, 18, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Mezey, E.; Szalayova, I.; Hogden, C.; Brady, A.; Dosa, A.; Sotonui, P.; Palkovits, M. An immunohistochemical study of lymphatic elements in the human brain. Proc. Natl. Acad. Sci. USA 2021, 118, e2002574118. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Guo, B.; Gao, Y.; Li, W.; Tong, X.; Feng, Y.; Abumaria, N. Characteristic Features of Deep Brain Lymphatic Vessels and Their Regulation by Chronic Stress. Research 2023, 6, 0120. [Google Scholar] [CrossRef] [PubMed]
- Prineas, L.W. Multiple sclerosis: Presence of lymphatic capillaries and lymphoid tissue in the brain and spinal cord. Science 1979, 203, 1123–1125. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Fedosov, I.; Navolokin, N.; Shirokov, A.; Maslyakova, G.; Bucharskaya, A.; Blokhina, I.; Terskov, A.; Khorovodov, A.; Postnov, D.; et al. Pilot identification of the Live-1/Prox-1 expressing lymphatic vessels and lymphatic elements in the unaffected and affected human brain. bioRxiv 2021. [Google Scholar] [CrossRef]
- Ahn, J.; Cho, H.; Kim, J.; Kim, S.; Ham, J.; Park, I.; Suh, S.; Hong, S.; Song, J.; Hong, Y.; et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 2019, 572, 62–66. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef]
- Zhinchenko, E.; Klimova, M.; Mamedova, A.; Agranovich, I.; Blokhina, I.; Antonova, T.; Terskov, A.; Shirokov, A.; Navolkin, N.; Morgun, A.; et al. Oxana Semyachkina-Glushkovskaya. Photostimulation of extravasation of beta-amyloid through the model of blood-brain barrier. Electronics 2020, 9, 1056. [Google Scholar] [CrossRef]
- Zhinchenko, E.; Navolokin, N.; Shirokov, A.; Khlebcov, B.; Dubrovsky, A.; Saranceva, E.; Abdurashitov, A.; Khorovodov, A.; Terskov, A.; Mamedova, A.; et al. Pilot study of transcranial photobiomodulation of lymphatic clearance of beta-amyloid from the mouse brain: Breakthrough strategies for nonpharmacologic therapy of Alzheimer’s disease. Biomed. Opt. Exp. 2019, 10, 4003–4017. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Terskov, A.; Khorovodov, A.; Telnova, V.; Blokhina, I.; Saranceva, E.; Kurths, J. Photodynamic Opening of the Blood–Brain Barrier and the Meningeal Lymphatic System: The New Niche in Immunotherapy for Brain Tumors. Pharmaceutics 2022, 14, 2612. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navolokin, N.; Adushkina, V.; Zlatogorskaya, D.; Telnova, V.; Evsiukova, A.; Vodovozova, E.; Eroshova, A.; Dosadina, E.; Diduk, S.; Semyachkina-Glushkovskaya, O. Promising Strategies to Reduce the SARS-CoV-2 Amyloid Deposition in the Brain and Prevent COVID-19-Exacerbated Dementia and Alzheimer’s Disease. Pharmaceuticals 2024, 17, 788. https://doi.org/10.3390/ph17060788
Navolokin N, Adushkina V, Zlatogorskaya D, Telnova V, Evsiukova A, Vodovozova E, Eroshova A, Dosadina E, Diduk S, Semyachkina-Glushkovskaya O. Promising Strategies to Reduce the SARS-CoV-2 Amyloid Deposition in the Brain and Prevent COVID-19-Exacerbated Dementia and Alzheimer’s Disease. Pharmaceuticals. 2024; 17(6):788. https://doi.org/10.3390/ph17060788
Chicago/Turabian StyleNavolokin, Nikita, Viktoria Adushkina, Daria Zlatogorskaya, Valeria Telnova, Arina Evsiukova, Elena Vodovozova, Anna Eroshova, Elina Dosadina, Sergey Diduk, and Oxana Semyachkina-Glushkovskaya. 2024. "Promising Strategies to Reduce the SARS-CoV-2 Amyloid Deposition in the Brain and Prevent COVID-19-Exacerbated Dementia and Alzheimer’s Disease" Pharmaceuticals 17, no. 6: 788. https://doi.org/10.3390/ph17060788
APA StyleNavolokin, N., Adushkina, V., Zlatogorskaya, D., Telnova, V., Evsiukova, A., Vodovozova, E., Eroshova, A., Dosadina, E., Diduk, S., & Semyachkina-Glushkovskaya, O. (2024). Promising Strategies to Reduce the SARS-CoV-2 Amyloid Deposition in the Brain and Prevent COVID-19-Exacerbated Dementia and Alzheimer’s Disease. Pharmaceuticals, 17(6), 788. https://doi.org/10.3390/ph17060788








