In Vitro/In Vivo Correlation of Two Extended-Release Cilostazol Formulations
Abstract
1. Introduction
2. Results
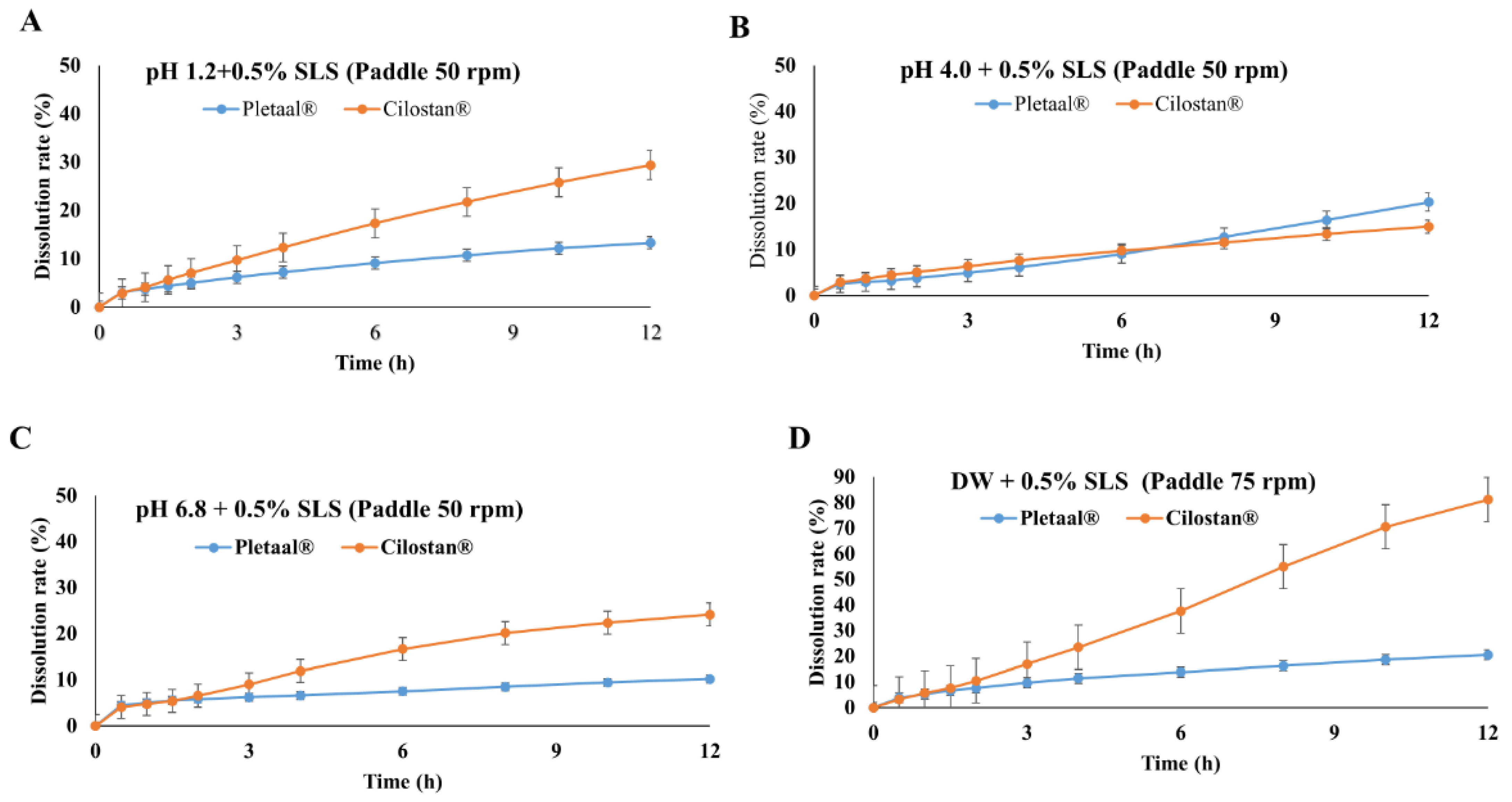
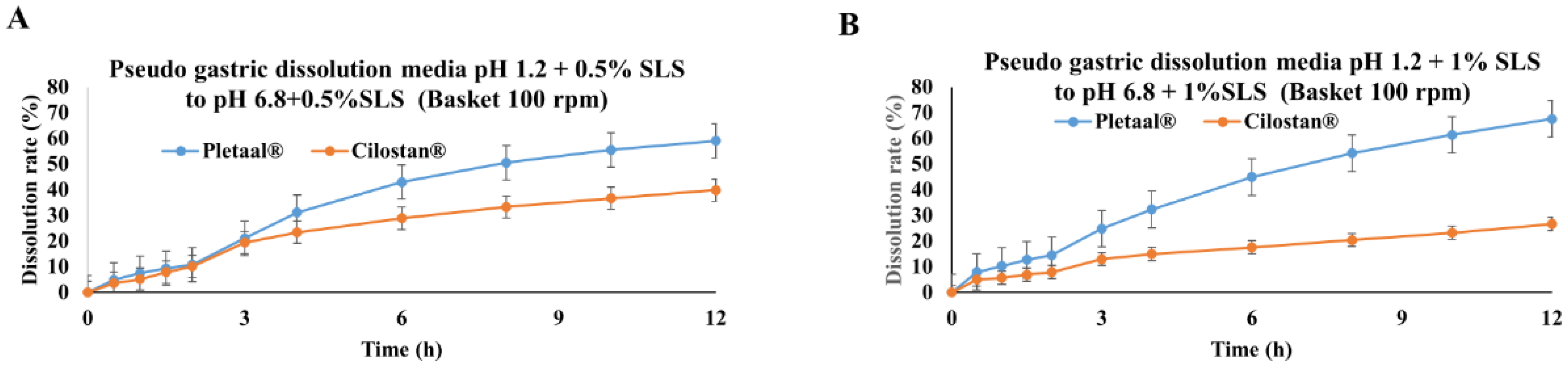
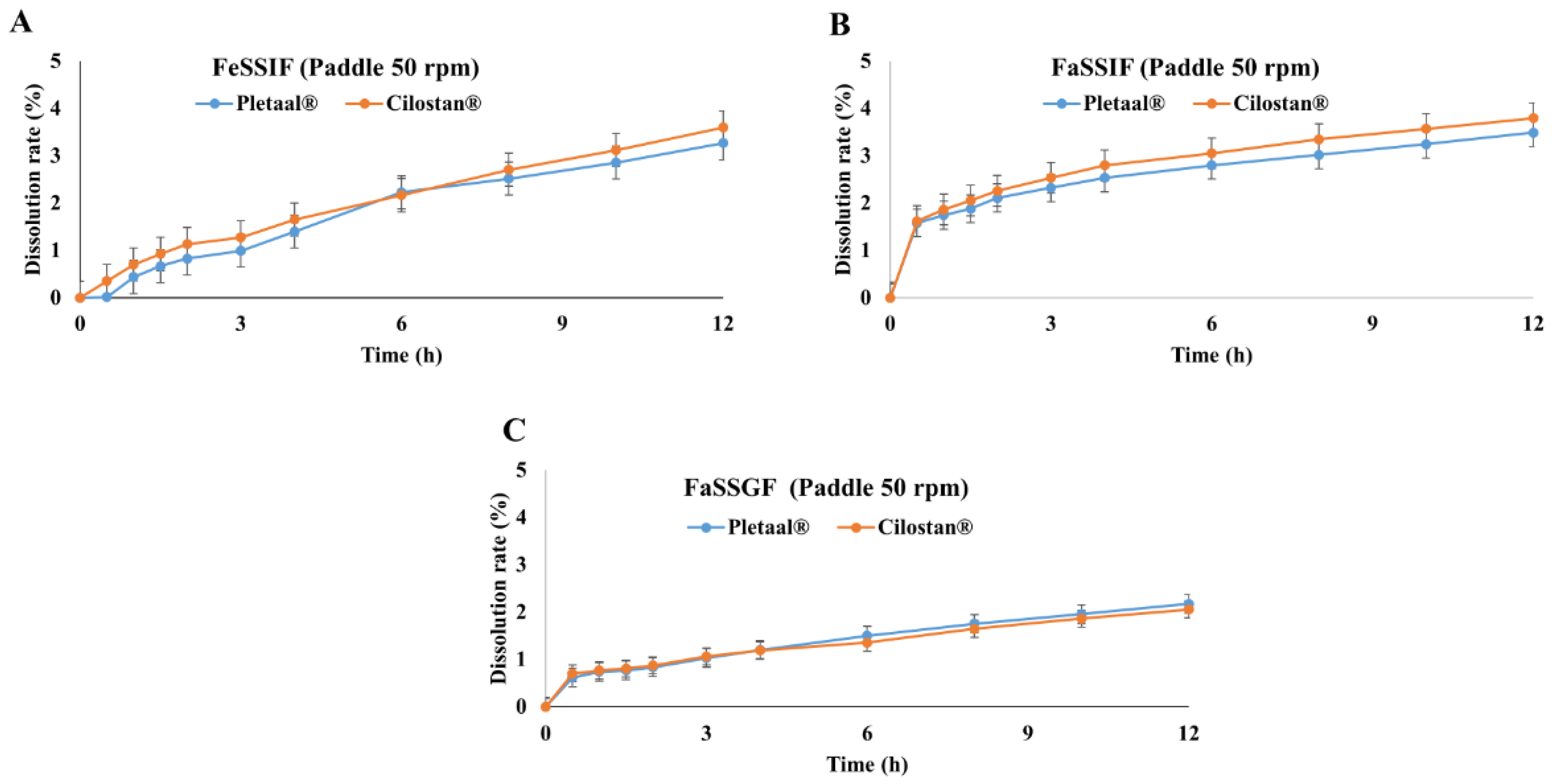
2.1. In Vitro Drug Release Profiling of Extended-Release Cilostazol Formulations in Different Medium Conditions
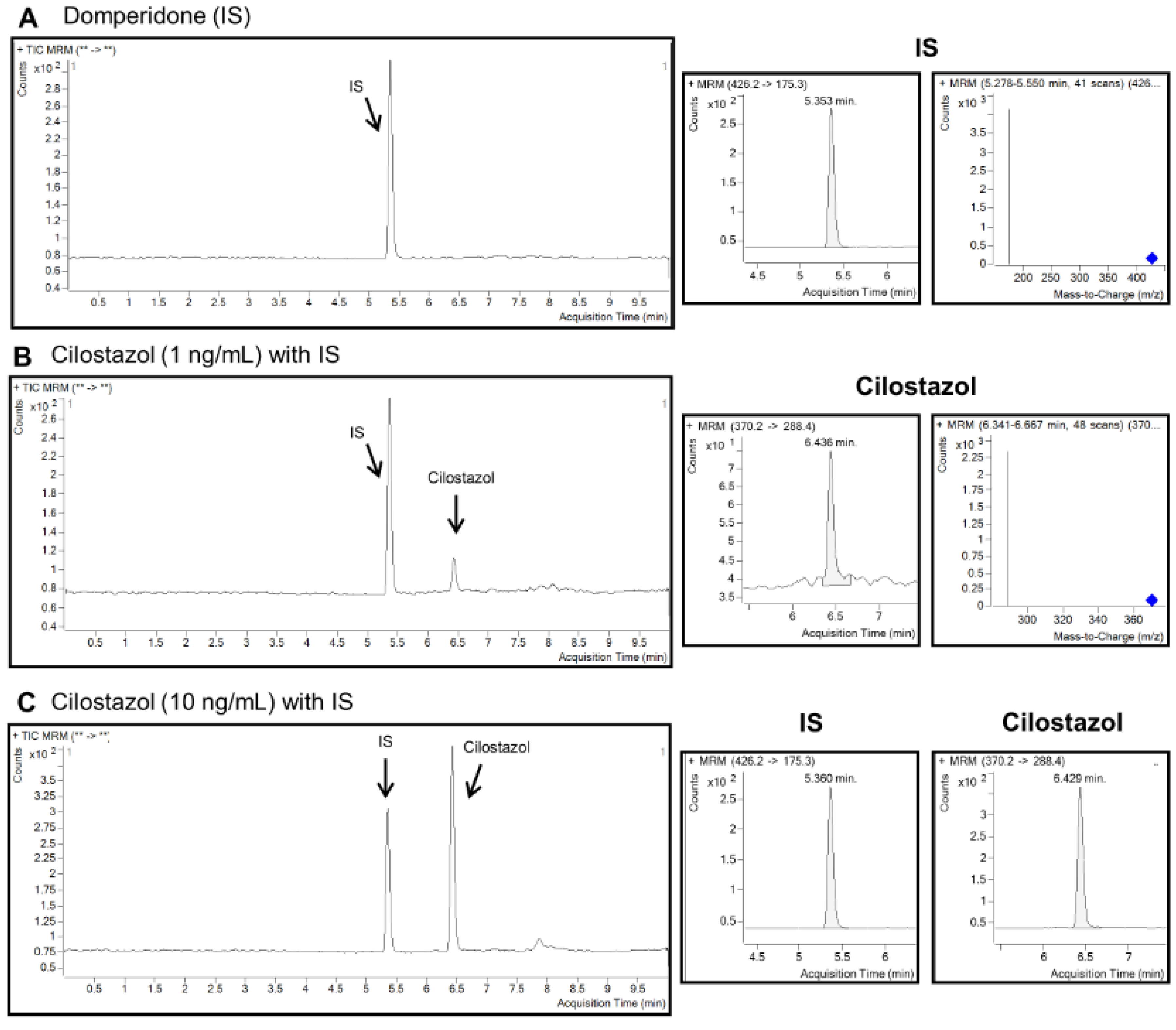
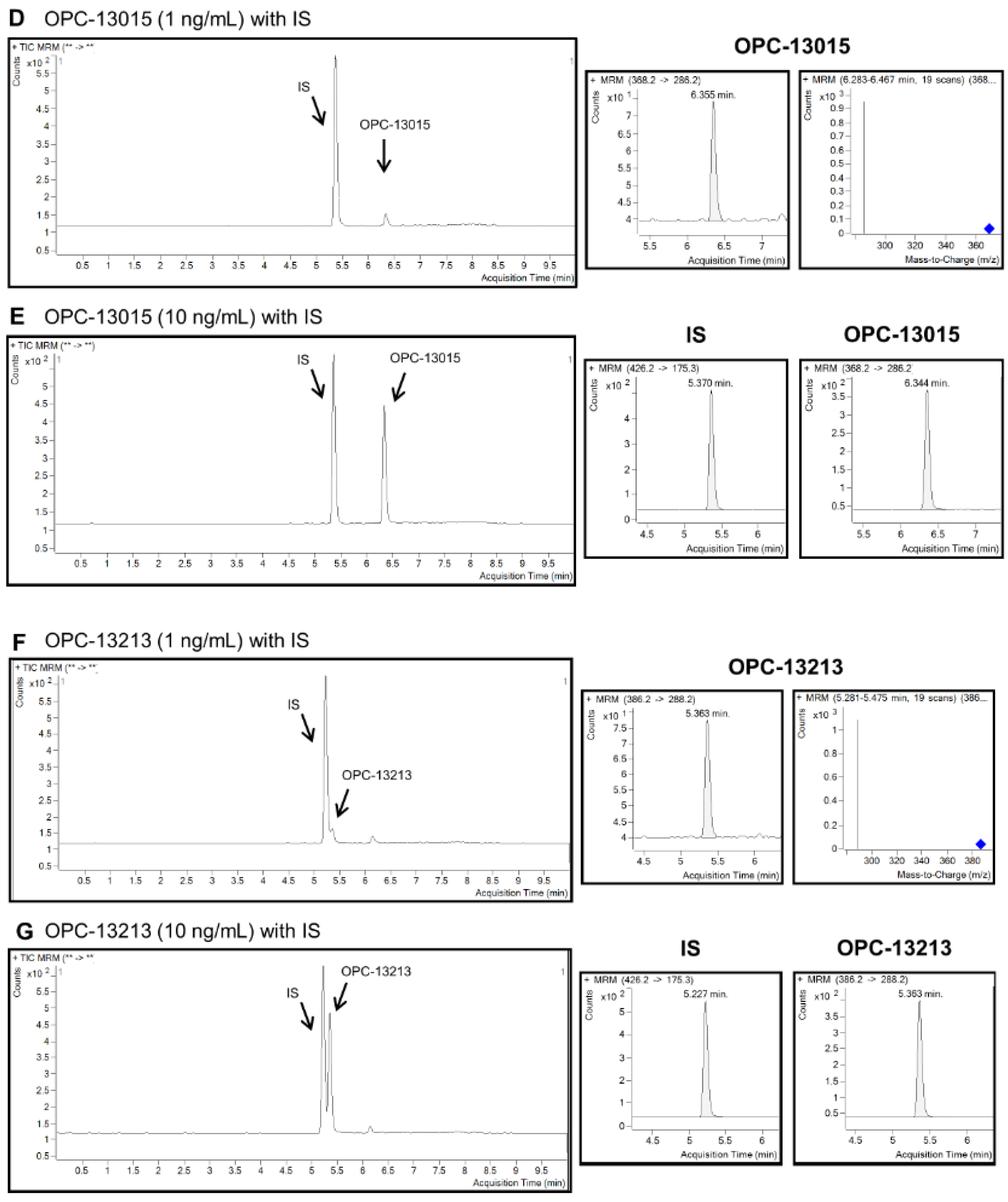
2.2. Quantitation of Cilostazol, OPC-13015, and OPC-13213 Using LC MS/MS
2.3. In Vivo PK Evaluation of Extended-Release Cilostazol Formulations under the Fasted and Fed Condition
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. In Vitro Release
4.3. Animal
4.4. Instrumentation: High-Performance Liquid Chromatography Coupled to Mass Spectrometry (LC MS/MS)
4.5. Standard Preparation and Method Validation
4.6. Two-Period Crossover Study
4.7. Analysis of Cilostazol, OPC-13015, and OPC-13213
4.8. PK Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanaka, T.; Ishikawa, T.; Hagiwara, M.; Onoda, K.; Itoh, H.; Hidaka, H. Effects of cilostazol, a selective cAMP phosphodiesterase inhibitor on the contraction of vascular smooth muscle. Pharmacology 1988, 36, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Reilly, M.P.; Mohler, E.R., III. Cilostazol: Treatment of intermittent claudication. Ann. Pharmacother. 2001, 35, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Pearce, L.; Ghosh, J.; Counsell, A.; Serracino-Inglott, F. Cilostazol and peripheral arterial disease. Expert Opin. Pharmacother. 2008, 9, 2683–2690. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shakur, Y.; Yoshitake, M.; Kambayashi, J.i. Cilostazol (Pletal®): A dual inhibitor of cyclic nucleotide phosphodiesterase type 3 and adenosine uptake. Cardiovasc. Drug Rev. 2001, 19, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, J.W.; Hirsh, J.; Spencer, F.A.; Baglin, T.P.; Weitz, J.I. Antiplatelet drugs: Antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012, 141, e89S–e119S. [Google Scholar] [CrossRef] [PubMed]
- Tjon, J.A.; Riemann, L.E. Treatment of intermittent claudication with pentoxifylline and cilostazol. Am. J. Health-Syst. Pharm. 2001, 58, 485–493. [Google Scholar] [CrossRef]
- Gomes, M.L.S.; da Silva Nascimento, N.; Borsato, D.M.; Pretes, A.P.; Nadal, J.M.; Novatski, A.; Gomes, R.Z.; Fernandes, D.; Farago, P.V.; Zanin, S.M.W. Long-lasting anti-platelet activity of cilostazol from poly (ε-caprolactone)-poly (ethylene glycol) blend nanocapsules. Mater. Sci. Eng. C 2019, 94, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choi, H.K. Enhancement of solubility and dissolution of cilostazol by solid dispersion technique. Arch. Pharm. Res. 2015, 38, 1336–1344. [Google Scholar] [CrossRef]
- Shin, K.-H.; Yoon, G.; Yoon, I.-S.; Park, J.W. Preparation and evaluation of oral controlled-release cilostazol formulation: Pharmacokinetics and antithrombotic efficacy in dogs and healthy male Korean participants. J. Pharm. Pharmacol. 2014, 66, 961–974. [Google Scholar] [CrossRef]
- Bibi, M.; ud Din, F.; Anwar, Y.; Alkenani, N.A.; Zari, A.T.; Mukhtiar, M.; Zeid, I.M.A.; Althubaiti, E.H.; Nazish, H.; Zeb, A. Cilostazol-loaded solid lipid nanoparticles: Bioavailability and safety evaluation in an animal model. J. Drug Deliv. Sci. Technol. 2022, 74, 103581. [Google Scholar] [CrossRef]
- Mahmoud, D.B.; Shukr, M.H.; Bendas, E.R. In vitro and in vivo evaluation of self-nanoemulsifying drug delivery systems of cilostazol for oral and parenteral administration. Int. J. Pharm. 2014, 476, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Dafe, A.; Etemadi, H.; Dilmaghani, A.; Mahdavinia, G.R. Investigation of pectin/starch hydrogel as a carrier for oral delivery of probiotic bacteria. Int. J. Biol. Macromol. 2017, 97, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Tarazi Riess, H.; Shani Levi, C.; Lesmes, U. Inclusion of phenolic bioactives in high amylose corn starch for gastro-intestinal delivery. Front. Nutr. 2022, 9, 981408. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.; Gani, A.; Jhan, F.; Shah, M.A.; ul Ashraf, Z. Ferulic acid loaded pickering emulsions stabilized by resistant starch nanoparticles using ultrasonication: Characterization, in vitro release and nutraceutical potential. Ultrason. Sonochem. 2022, 84, 105967. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Lim, S.T. Utilization of Enzyme-resistant Starch to Control Theophylline Release from Tablets. Starch-Stärke 2009, 61, 154–160. [Google Scholar] [CrossRef]
- Elgaied-Lamouchi, D.; Descamps, N.; Lefevre, P.; Rambur, I.; Pierquin, J.-Y.; Siepmann, F.; Siepmann, J.; Muschert, S. Starch-based controlled release matrix tablets: Impact of the type of starch. J. Drug Deliv. Sci. Technol. 2021, 61, 102152. [Google Scholar] [CrossRef]
- Henrist, D.; Van Bortel, L.; Lefebvre, R.A.; Remon, J.P. In vitro and in vivo evaluation of starch-based hot stage extruded double matrix systems. J. Control. Release 2001, 75, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Son, J.D.; Cho, S.M.; Choi, Y.W.; Kim, S.H.; Kwon, I.S.; Jin, E.-H.; Kim, J.W.; Hong, J.H. Pharmacokinetic characteristics of cilostazol 200 mg controlled-release tablet compared with two cilostazol 100 mg immediate-release tablets (Pletal) after single oral dose in healthy Korean male volunteers. Transl. Clin. Pharmacol. 2016, 24, 183–188. [Google Scholar] [CrossRef]
- Hiratsuka, M.; Hinai, Y.; Sasaki, T.; Konno, Y.; Imagawa, K.; Ishikawa, M.; Mizugaki, M. Characterization of human cytochrome p450 enzymes involved in the metabolism of cilostazol. Drug Metab. Dispos. 2007, 35, 1730–1732. [Google Scholar] [CrossRef]
- Okuda, Y.; Kimura, Y.; Yamashita, K. Cilostazol. Cardiovasc. Drug Rev. 1993, 11, 451–465. [Google Scholar] [CrossRef]
- Jinno, J.; Kamada, N.; Miyake, M.; Yamada, K.; Mukai, T.; Odomi, M.; Toguchi, H.; Liversidge, G.G.; Higaki, K.; Kimura, T. Effect of particle size reduction on dissolution and oral absorption of a poorly water-soluble drug, cilostazol, in beagle dogs. J. Control. Release 2006, 111, 56–64. [Google Scholar] [CrossRef]
- Shin, W.; Min-Kyoung, K.; Cho, D.-Y. Pharmacokinetic study of two extended-release formulations of cilostazol in healthy Korean subjects: A randomized, open-label, multiple-dose, two-period crossover study. Int. J. Clin. Pharmacol. Ther. 2019, 57, 408. [Google Scholar] [CrossRef]
- Jeon, S.-W.; Park, J.-H.; Kim, J.-E.; Park, Y.-J. Design of experiment (DoE)-based formulation design of bepotastine sustained-release tablet and in vitro-in vivo pharmacokinetic correlation. J. Pharm. Investig. 2023, 53, 407–416. [Google Scholar] [CrossRef]
- Shin, H.W.; Kim, J.E.; Park, Y.J. Nanoporous Silica Entrapped Lipid-Drug Complexes for the Solubilization and Absorption Enhancement of Poorly Soluble Drugs. Pharmaceutics 2021, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Park, Y.J. QbD Consideration for Developing a Double-Layered Tablet into a Single-Layered Tablet with Telmisartan and Amlodipine. Pharmaceutics 2022, 14, 377. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, J.K.; Jee, J.P.; Jang, D.J.; Park, Y.J.; Kim, J.E. Quality by Design (QbD) application for the pharmaceutical development process. J. Pharm. Investig. 2022, 52, 649–682. [Google Scholar] [CrossRef]
- Miyake, M.; Oka, Y.; Mukai, T. Food effect on meal administration time of pharmacokinetic profile of cilostazol, a BCS class II drug. Xenobiotica 2020, 50, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Osumi, T.; Niimi, K.; Nakagawa, K. Physico-chemical properties and stability of cilostazol. Arzneim.-Forsch. 1985, 35, 1117–1123. [Google Scholar]
- Toyobuku, H.; Tamai, I.; Ueno, K.; Tsuji, A. Limited influence of P-glycoprotein on small-intestinal absorption of cilostazol, a high absorptive permeability drug. J. Pharm. Sci. 2003, 92, 2249–2259. [Google Scholar] [CrossRef]
- Singh, B.N. Effects of food on clinical pharmacokinetics. Clin. Pharmacokinet. 1999, 37, 213–255. [Google Scholar] [CrossRef]
- Bramer, S.L.; Forbes, W.P. Relative bioavailability and effects of a high fat meal on single dose cilostazol pharmacokinetics. Clin. Pharmacokinet. 1999, 37 (Suppl. S2), 13–23. [Google Scholar] [CrossRef] [PubMed]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.K.; Kang, W.K.; Kwon, K.I. Pharmacokinetic and pharmacodynamic modeling of the antiplatelet and cardiovascular effects of cilostazol in healthy humans. Clin. Pharmacol. Ther. 2002, 71, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lim, L.A.; Jang, S.B.; Lee, Y.J.; Chung, J.Y.; Choi, J.R.; Kim, K.; Park, J.W.; Yoon, H.; Lee, J.; et al. Pharmacokinetic comparison of sustained- and immediate-release oral formulations of cilostazol in healthy Korean subjects: A randomized, open-label, 3-part, sequential, 2-period, crossover, single-dose, food-effect, and multiple-dose study. Clin. Ther. 2011, 33, 2038–2053. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Ghim, J.-L.; Jung, J.A.; Cho, S.-H.; Choe, S.; Choi, H.Y.; Bae, K.-S.; Lim, H.-S. Pharmacokinetic comparison of sustained-and immediate-release formulations of cilostazol after multiple oral doses in fed healthy male Korean volunteers. Drug Des. Dev. Ther. 2015, 9, 3571. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bramer, S.L.; Tata, P.N.; Vengurlekar, S.S.; Brisson, J.H. Method for the quantitative analysis of cilostazol and its metabolites in human plasma using LC/MS/MS. J. Pharm. Biomed. Anal. 2001, 26, 637–650. [Google Scholar] [CrossRef]
- Fu, C.J.; Tata, P.N.; Okada, K.; Akiyama, H.; Bramer, S.L. Simultaneous quantitative determination of cilostazol and its metabolites in human plasma by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1999, 728, 251–262. [Google Scholar] [CrossRef]
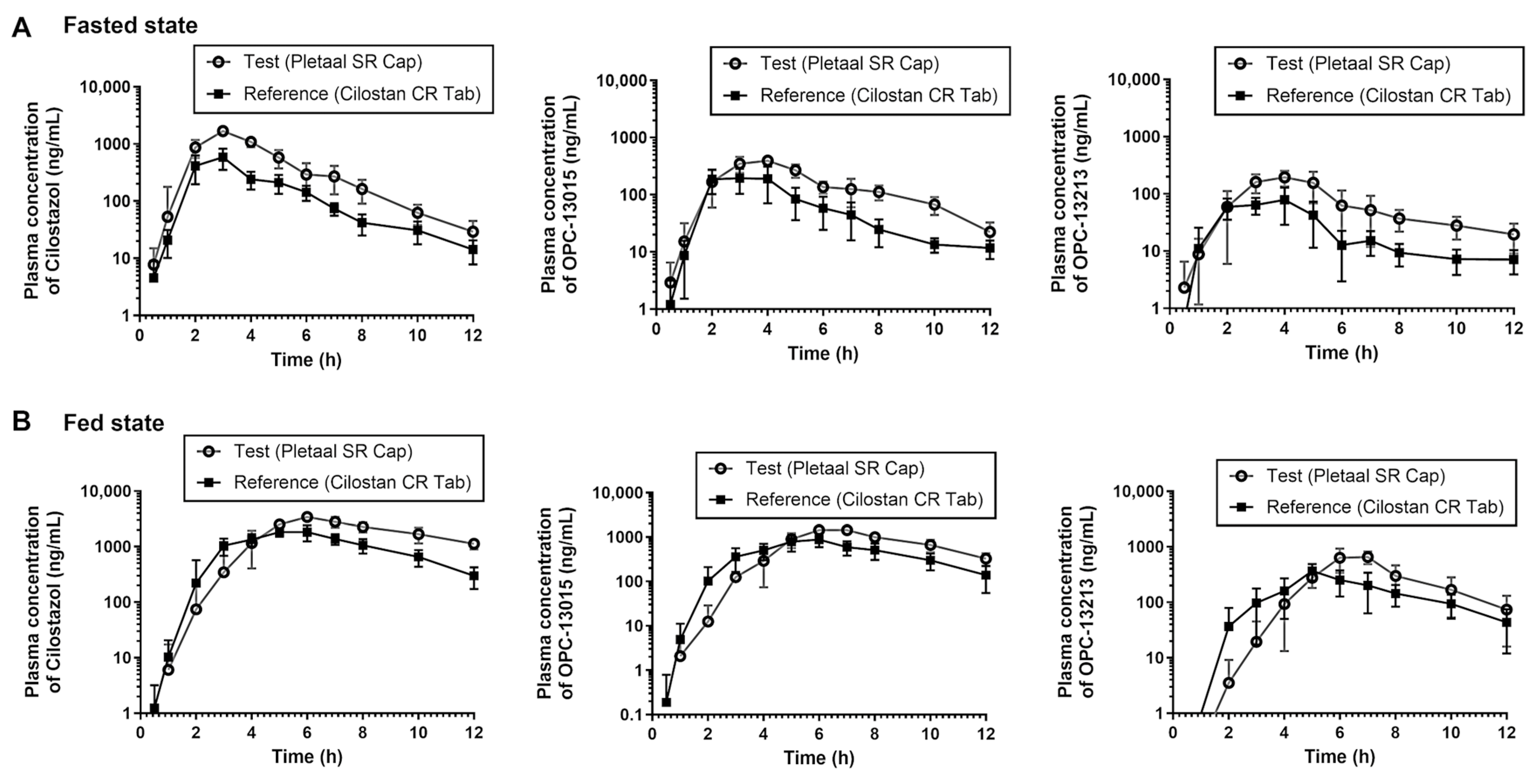
| Compound Type | PK Parameters under Fast State | Geometric Mean ± SD (CV, %) | Ratio (Pletaal® SR 200 mg Capsule/Cilostan® CR 200 mg Tablet) | p-Value | |
|---|---|---|---|---|---|
| Pletaal® SR 200 mg Capsule | Cilostan® CR 200 mg Tablet | ||||
| Cilostazol | Cmax (ng/mL) | 1656.83 ± 1.17 (16.4%) | 655.78 ± 1.19 (18.2%) | 2.53 | <0.001 |
| AUC0-t (h·ng/mL) | 5203.01 ± 1.06 (5.9%) | 1799.13 ± 1.19 (17.4%) | 2.89 | <0.001 | |
| AUC0-inf (h·ng/mL) | 5282.45 ± 1.07 (6.2%) | 1837.97 ± 1.19 (17.5%) | 2.87 | <0.001 | |
| OPC-13015 | Cmax (ng/mL) | 427.75 ± 1.17 (15.5%) | 277.25 ± 1.34 (25.7%) | 1.54 | <0.001 |
| AUC0-t (h·ng/mL) | 1769.29 ± 1.12 (12.3%) | 826.05 ± 1.23 (20.0%) | 2.14 | <0.001 | |
| AUC0-inf (h·ng/mL) | 1853.58 ± 1.13 (13.9%) | 872.19 ± 1.23 (19.5%) | 2.12 | <0.001 | |
| OPC-13213 | Cmax (ng/mL) | 241.29 ± 1.11 (10.0%) | 95.51 ± 1.35 (28.1%) | 2.52 | <0.001 |
| AUC0-t (h·ng/mL) | 817.66 ± 1.10 (9.7%) | 302.80 ± 1.37 (29.1%) | 2.70 | <0.001 | |
| AUC0-inf (h·ng/mL) | 937.21 ± 1.19 (17.9%) | 351.70 ± 1.30 (26.5%) | 2.66 | <0.001 | |
| Compound Type | PK Parameters under Fed State | Geometric Mean ± SD (CV, %) | Ratio (Pletaal® SR 200 mg Capsule/Cilostan® CR 200 mg Tablet) | p-Value | |
|---|---|---|---|---|---|
| Pletaal® SR 200 mg Capsule | Cilostan® CR 200 mg Tablet | ||||
| Cilostazol | Cmax (ng/mL) | 3477.43 ± 1.13 (11.2%) | 2144.69 ± 1.17 (16.7%) | 1.62 | <0.001 |
| AUC0-t (h·ng/mL) | 17,978.47 ± 1.19 (16.9%) | 10,776.83 ± 1.11 (9.7%) | 1.67 | <0.001 | |
| AUC0-inf (h·ng/mL) | 24,445.11 ± 1.18 (17.1%) | 11,966.19 ± 1.10 (9.9%) | 2.04 | <0.001 | |
| OPC-13015 | Cmax (ng/mL) | 1557.11 ± 1.17 (15.7%) | 1054.97 ± 1.22 (18.2%) | 1.48 | <0.001 |
| AUC0-t (h·ng/mL) | 7298.10 ± 1.11 (10.4%) | 4679.33 ± 1.18 (15.8%) | 1.56 | <0.001 | |
| AUC0-inf (h·ng/mL) | 8607.24 ± 1.17 (15.8%) | 5161.49 ± 1.22 (19.5%) | 1.67 | <0.001 | |
| OPC-13213 | Cmax (ng/mL) | 794.39 ± 1.22 (17.9%) | 412.49 ± 1.19 (15.3%) | 1.93 | <0.001 |
| AUC0-t (h·ng/mL) | 2445.29 ± 1.34 (27.9%) | 1544.16 ± 1.18 (16.8%) | 1.58 | <0.001 | |
| AUC0-inf (h·ng/mL) | 2687.73 ± 1.36 (30.7%) | 1732.12 ± 1.17 (15.8%) | 1.55 | <0.001 | |
| Compound Type | PK Parameters under Fed State | Fed/Fast Geometric Mean Ratio | |
|---|---|---|---|
| Pletaal® SR 200 mg Capsule | Cilostan® CR 200 mg Tablet | ||
| Cilostazol | Cmax (ng/mL) | 2.10 | 3.27 |
| AUC0-inf (h·ng/mL) | 4.63 | 6.51 | |
| OPC-13015 | Cmax (ng/mL) | 3.64 | 3.81 |
| AUC0-inf (h·ng/mL) | 4.64 | 5.92 | |
| OPC-13213 | Cmax (ng/mL) | 3.29 | 4.32 |
| AUC0-inf (h·ng/mL) | 2.87 | 4.92 | |
| Compound Type | PK Parameters | Geometric Mean Ratio (GMR) a (Pletaal® SR 200 mg Capsule/Cilostan® CR 200 mg Tablet) | 90% of CI for GMR b (Lower Limit–Upper Limit) | |
|---|---|---|---|---|
| Cilostazol | Under fasted state | Cmax (ng/mL) | 1.6214 | 2.1396–2.9833 |
| AUC0-t (h·ng/mL) | 1.6683 | 2.5665–3.2587 | ||
| AUC0-inf (h·ng/mL) | 2.0428 | 2.5568–3.2307 | ||
| Under fed state | Cmax (ng/mL) | 1.6214 | 1.4126–1.8611 | |
| AUC0-t (h·ng/mL) | 1.6683 | 1.4242–1.9542 | ||
| AUC0-inf (h·ng/mL) | 2.0428 | 1.7497–2.3851 | ||
| OPC-13015 | Under fasted state | Cmax (ng/mL) | 1.5428 | 1.3798–1.7251 |
| AUC0-t (h·ng/mL) | 2.1419 | 1.8130–2.5304 | ||
| AUC0-inf (h·ng/mL) | 2.1252 | 1.7915–2.5210 | ||
| Under fed state | Cmax (ng/mL) | 1.4760 | 1.2364–1.7619 | |
| AUC0-t (h·ng/mL) | 1.5596 | 1.4344–1.6959 | ||
| AUC0-inf (h·ng/mL) | 1.6676 | 1.5352–1.8114 | ||
| OPC-13213 | Under fasted state | Cmax (ng/mL) | 1.4760 | 1.2364–1.7619 |
| AUC0-t (h·ng/mL) | 1.5596 | 1.4344–1.6959 | ||
| AUC0-inf (h·ng/mL) | 1.6676 | 1.5352–1.8114 | ||
| Under fed state | Cmax (ng/mL) | 1.9258 | 1.6157–2.2956 | |
| AUC0-t (h·ng/mL) | 1.5836 | 1.2794–1.9600 | ||
| AUC0-inf (h·ng/mL) | 1.5517 | 1.2862–1.8720 | ||
| Product | Batch Number | Shelf-Life | Expiry Date | Ingredients |
|---|---|---|---|---|
| Pletaal® SR 200 mg capsule | PC215008B | 36 Months | 11 May 2024 | Cilostazol, Microcrystalline cellulose, Citric acid, Corn starch, pregelatinized starch, Polysorbate 80, Silicon dioxide, Hypromellose capsule |
| PC215009B | ||||
| PC215010B | ||||
| Cilostan® CR 200 mg tablet | 6125105 | 3 January 2024 | Cilostazol, Microcrystalline cellulose, Carbomer, Povidone, Magnesium stearate, Sodium Lauryl Sulfate, Silicon dioxide, Hypromellose | |
| 6125106 | 15 February 2024 | |||
| 6125113 | 5 April 2024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, K.A.; Kim, N.Y.; Jin, M.J.; Kim, D.; Ma, Y.; Karna, S.; Park, Y.-J. In Vitro/In Vivo Correlation of Two Extended-Release Cilostazol Formulations. Pharmaceuticals 2024, 17, 787. https://doi.org/10.3390/ph17060787
Min KA, Kim NY, Jin MJ, Kim D, Ma Y, Karna S, Park Y-J. In Vitro/In Vivo Correlation of Two Extended-Release Cilostazol Formulations. Pharmaceuticals. 2024; 17(6):787. https://doi.org/10.3390/ph17060787
Chicago/Turabian StyleMin, Kyoung Ah, Na Young Kim, Min Jeong Jin, Doyeon Kim, Yoonseo Ma, Sandeep Karna, and Young-Joon Park. 2024. "In Vitro/In Vivo Correlation of Two Extended-Release Cilostazol Formulations" Pharmaceuticals 17, no. 6: 787. https://doi.org/10.3390/ph17060787
APA StyleMin, K. A., Kim, N. Y., Jin, M. J., Kim, D., Ma, Y., Karna, S., & Park, Y.-J. (2024). In Vitro/In Vivo Correlation of Two Extended-Release Cilostazol Formulations. Pharmaceuticals, 17(6), 787. https://doi.org/10.3390/ph17060787






