Chemical Behavior and Bioactive Properties of Spinorphin Conjugated to 5,5′-Dimethyl- and 5,5′-Diphenylhydantoin Analogs
Abstract
1. Introduction
2. Results and Discussion
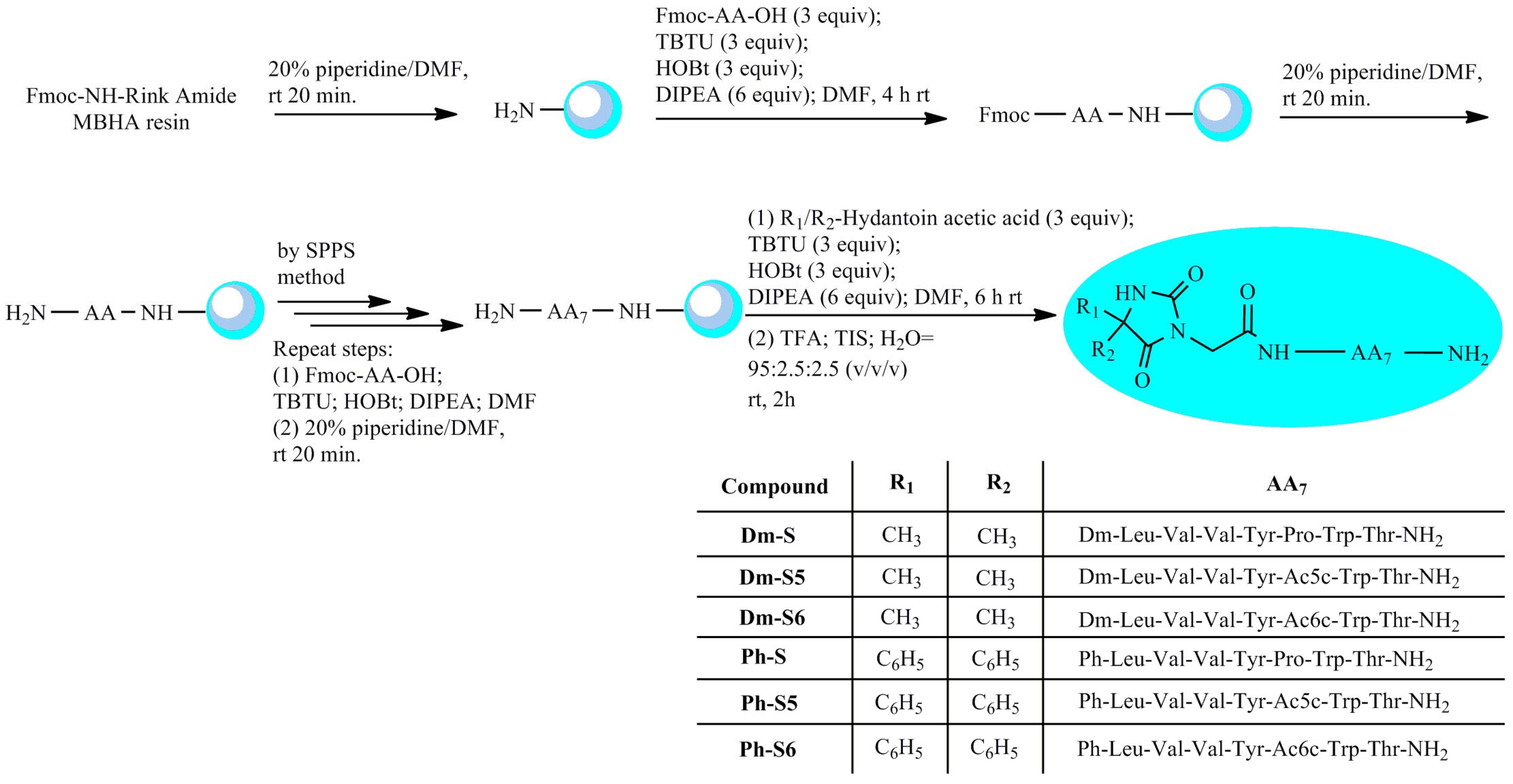
2.1. Chemistry of Spinorphine Derivatives
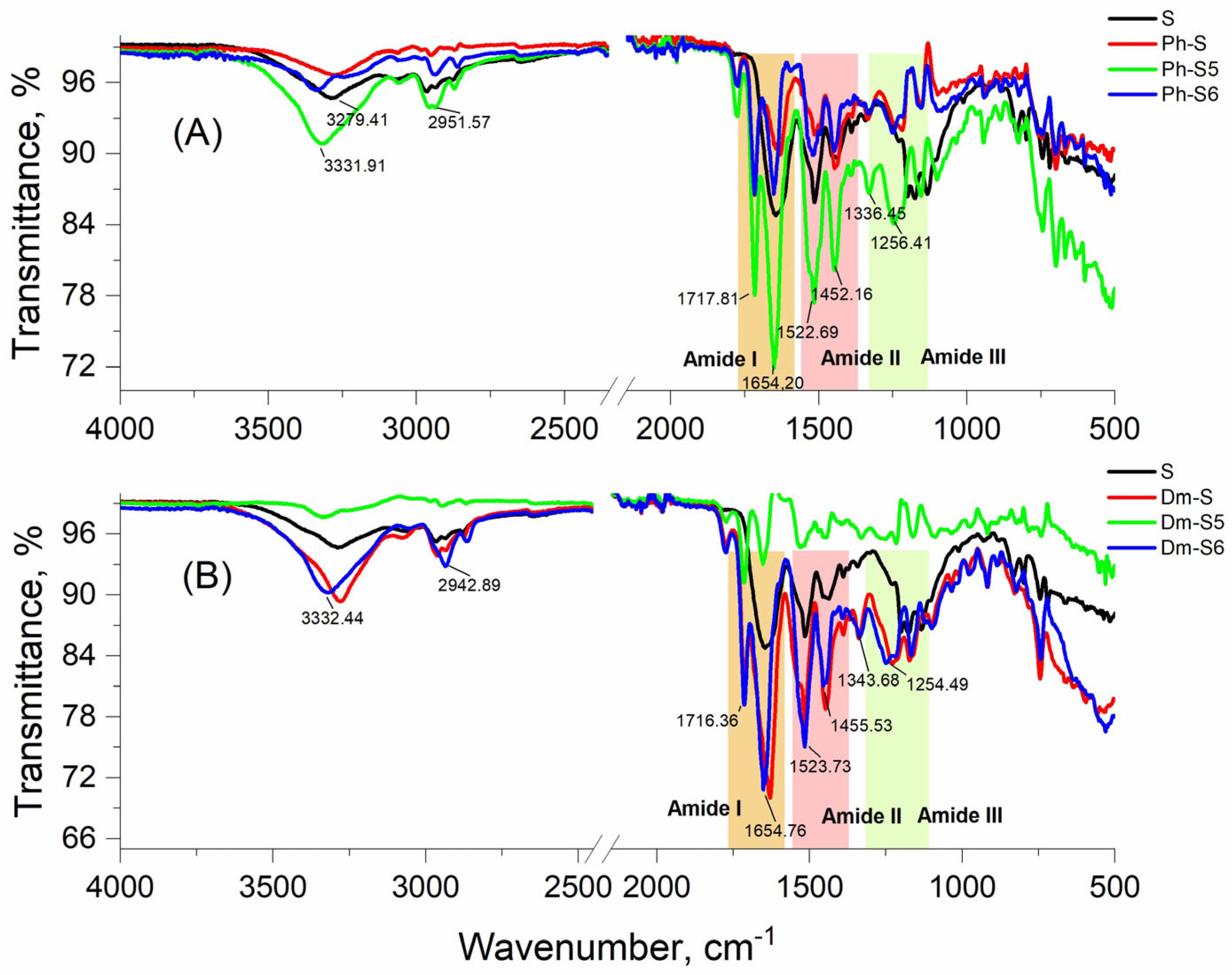
2.2. Analytical Characteristics
2.3. Determination of Hydrolytic Stability
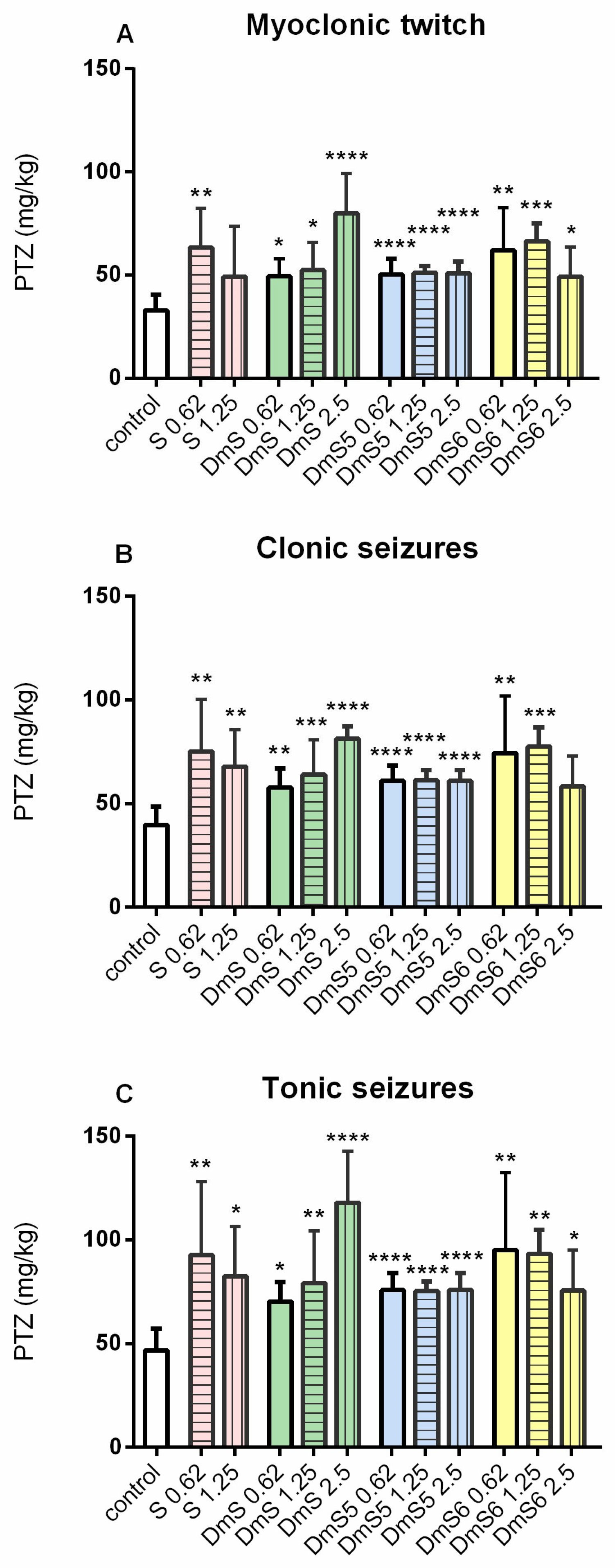
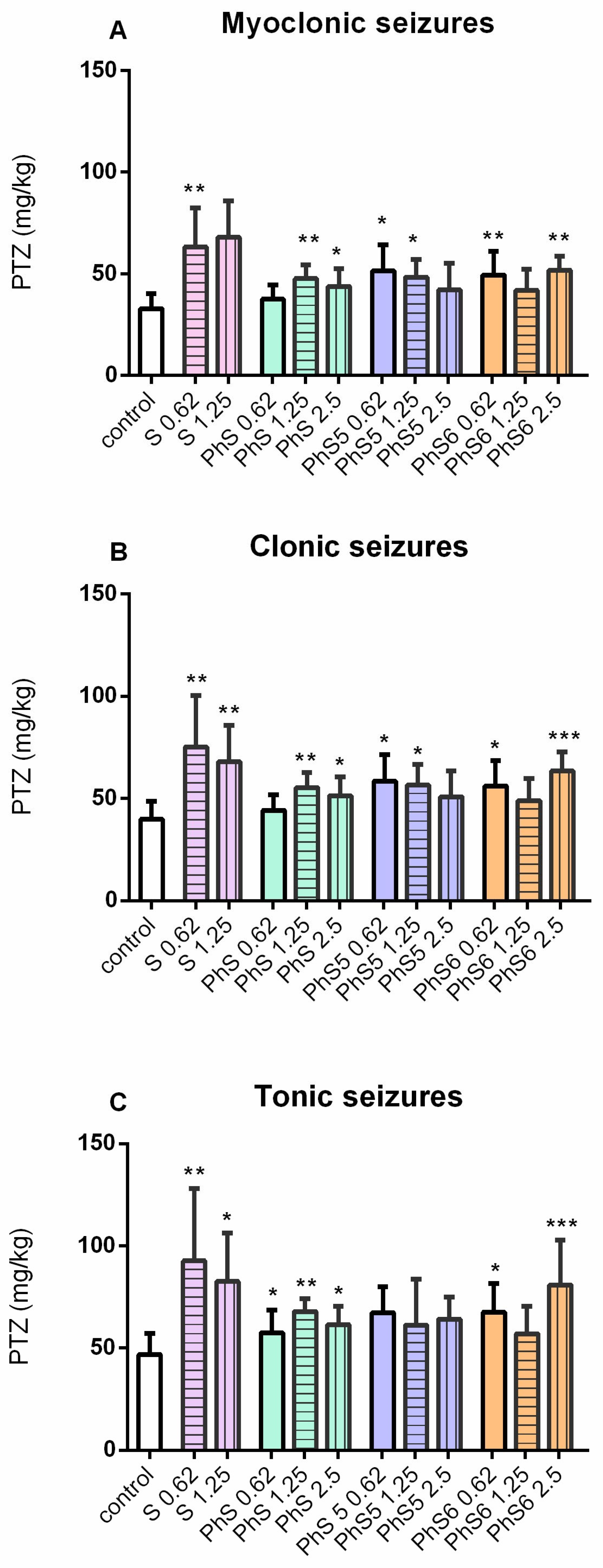
2.4. Results for Anticonvulsant Activity
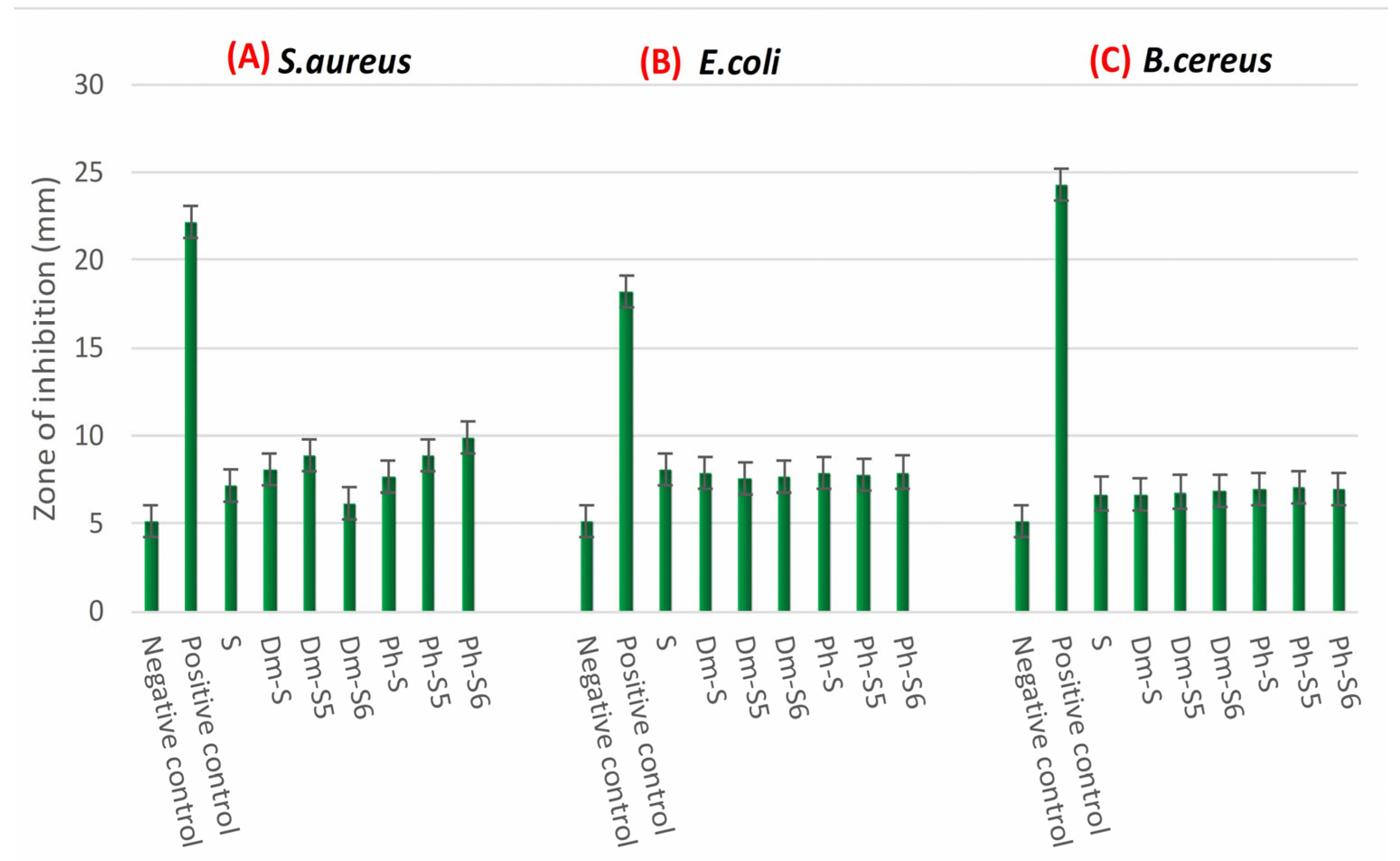
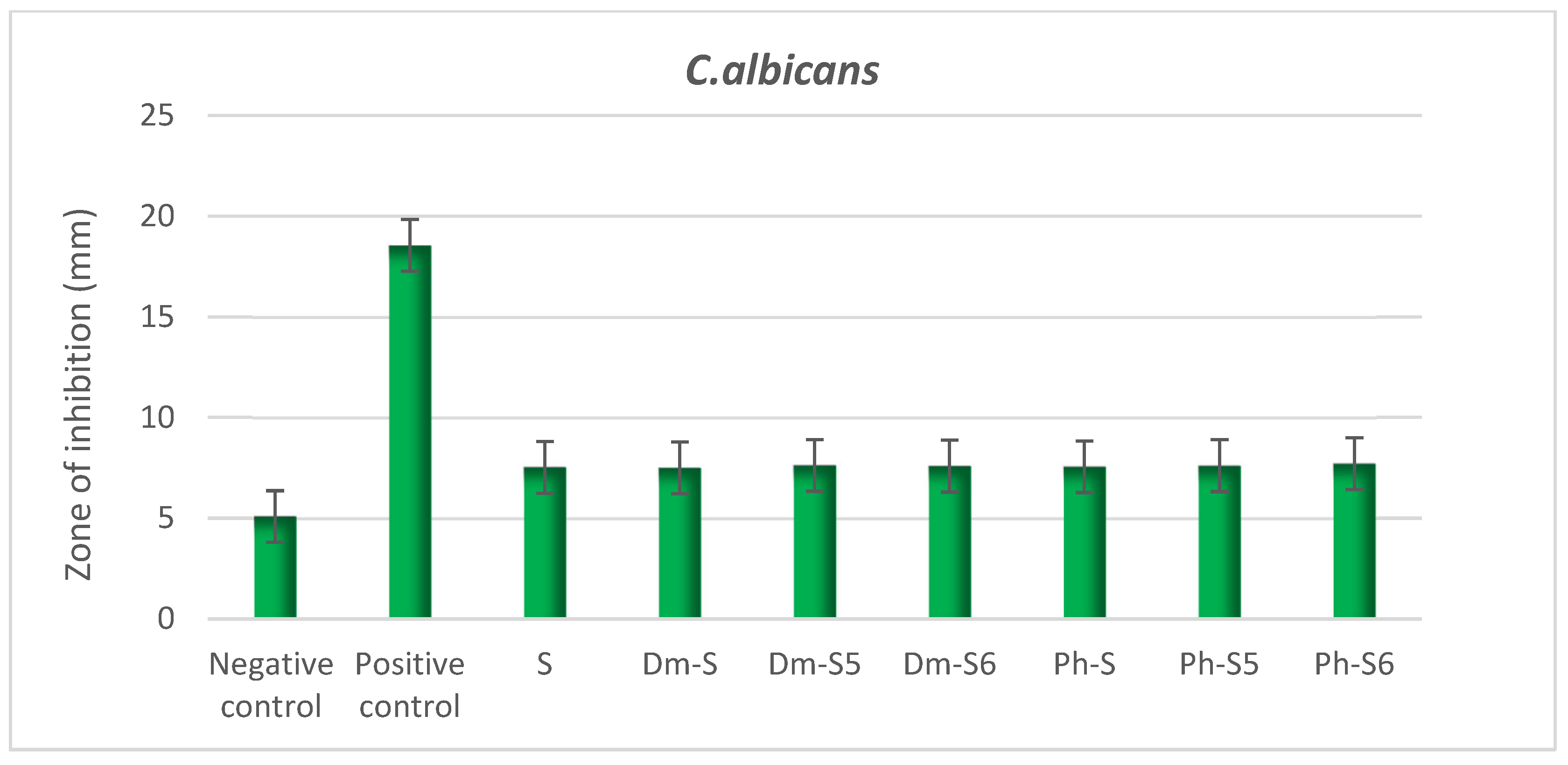
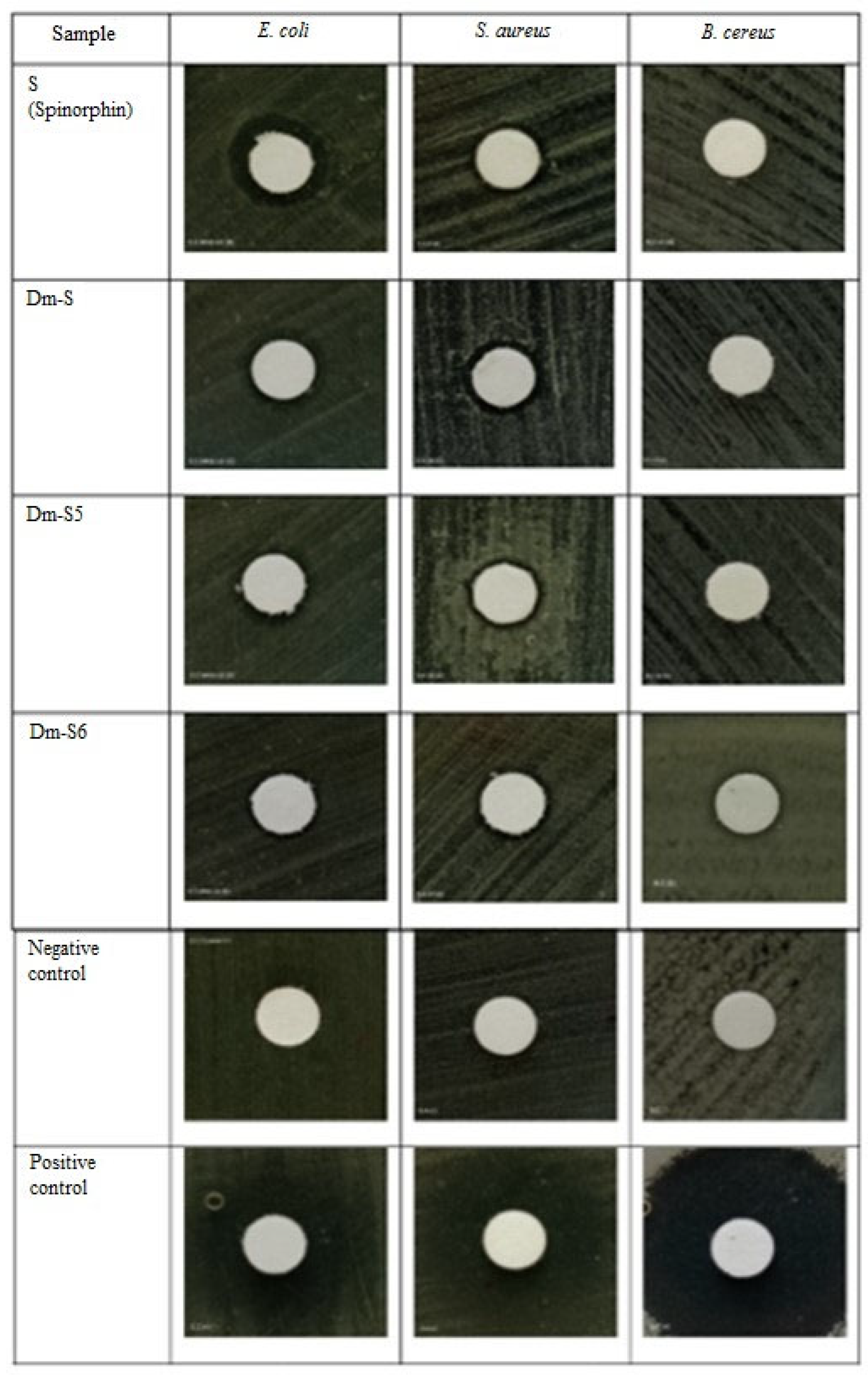
2.5. Antioxidant Activity Test
3. Materials and Methods
3.1. Synthesis of Peptide Analogues
General Procedure for the Peptide Synthesis of Compounds (Dm-S, Dm-S5, Dm-S6, Ph-S, Ph-S5, and Ph-S6)
3.2. Physicochemical Characterization
3.2.1. Determination of Partition Coefficient
3.2.2. Voltamperometric Analysis
3.2.3. Hydrolytic Activity Testing
3.2.4. pK and pI Determination
3.2.5. Spectral Characterizations
3.3. Study of Bioactive Properties
3.3.1. Pharmacology: In Vivo Experiments
- Animals:
- Drugs and dosage
- Seizure tests
- Maximal electroshock Test (MES test).
- Rota-rod test
- Statistical analysis
3.3.2. Microbiological Analyses: Antibacterial Activity, Antifungal, and Antioxidant Test
- Antibacterial Activity Test
- Antifungal Activity
- Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamley, I.W. Small bioactive peptides for biomaterials design and therapeutics. Chem. Rev. 2017, 117, 14015–14041. [Google Scholar] [CrossRef] [PubMed]
- Karami, Z.; Akbari-Adergani, B. Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties. J. Food Sci. Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, D.; Rafiq, S.; Gat, Y.; Gat, P.; Waghmare, R.; Kumar, V. A review on bioactive peptides: Physiological functions, bioavailability and safety. Int. J. Pept. Res. Ther. 2020, 26, 139–150. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Oh, D.H.; Lee, B.H. Bioactive peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Gentilucci, L. New Trends in the Development of Opioid Peptide Analogues as Advanced Remedies for Pain Relief. Curr. Top. Med. Chem. 2004, 4, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, G.; Gentilucci, L.; Tolomelli, A.; Spinosa, R.; Calienni, M.; Qasem, A.R.; Spampinato, S. Synthesis and evaluation of the affinity toward μ-opioid receptors of atypical, lipophilic ligands based on the sequence c [-Tyr-Pro-Trp-Phe-Gly-]. J. Med. Chem. 2004, 47, 5198–5203. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, J.V.; McLaughlin, J.P. Opioid peptides: Potential for drug development. Drug Discov. Today Technol. 2012, 9, e23–e31. [Google Scholar] [CrossRef] [PubMed]
- Zarzhitsky, S.; Jiang, A.; EStanley, E.; HHecht, M. Harnessing synthetic biology to enhance heterologous protein expression. Protein Sci. 2020, 29, 1698–1706. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Todorov, P.; Georgieva, S.; Tchekalarova, J.; Subaer, S.; Peneva, P.; Hartati, H. Synthesis, Characterization and Biological Investigation of New N-Modified Spinorphin Analogs. Pharmaceuticals 2022, 15, 1251. [Google Scholar] [CrossRef]
- Liang, T.S.; Gao, J.L.; Fatemi, O.; Lavigne, M.; Leto, T.L.; Murphy, P.M. The endogenous opioid spinorphin blocks fMet-Leu-Phe-induced neutrophil chemotaxis by acting as a specific antagonist at the N-formylpeptide receptor subtype FPR. J. Immunol. 2001, 167, 6609–6614. [Google Scholar] [CrossRef]
- Nishimura, K.; Hazato, T. Spinorphin, a new inhibitor of enkephalin-degrading enzymes derived from the bovine spinal cord. Masui. Jpn. J. Anesthesiol. 1993, 42, 1497–1503. [Google Scholar] [PubMed]
- Nishimura, K.; Hazato, T. Isolation and identification of an endogenous inhibitor of enkephalin-degrading enzymes from bovine spinal cord. Biochem. Biophys. Res. Commun. 1993, 194, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Tadahiro, K.; Mariko, S.; Masaaki, U.; Tadahiko, H. Inhibitory effects of spinorphin, a novel endogenous regulator, on chemotaxis, O− 2 generation, and exocytosis by N-formylmethionyl-leucyl-phenylalanine (FMLP)-stimulated neutrophils. Biochem. Pharmacol. 1997, 54, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Matsunaga, S.; Inoue, M.; Yamamoto, Y.; Hazato, T. Complete inhibition of purinoceptor agonist-induced nociception by spinorphin, but not by morphine. Peptides 2000, 21, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H. In vivo molecular signal transduction of peripheral mechanisms of pain. Jpn. J. Pharmacol. 1999, 79, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Ono, H.; Ueda, A.; Shimamura, M.; Nishimura, K.; Hazato, T. Spinorphin as an endogenous inhibitor of enkephalin-degrading enzymes: Roles in pain and inflammation. Curr. Protein Pept. Sci. 2002, 3, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Okutsu, H.; Matsuura, T.; Miyagi, T.; Yamamoto, Y.; Hazato, T.; Ono, H. Spinorphin, an endogenous inhibitor of enkephalin-degrading enzymes, potentiates leu-enkephalin-induced anti-allodynic and antinociceptive effects in mice. Jpn. J. Pharmacol. 2001, 87, 261–267. [Google Scholar] [CrossRef]
- Todorov, P.; Peneva, P.; Georgieva, S.; Tchekalarova, J.; Rangelov, M.; Todorova, N. Synthesis and characterization of new 5, 5′-dimethyl-and 5,5′-diphenylhydantoin-conjugated hemorphin derivatives designed as potential anticonvulsant agents. New J. Chem. 2022, 46, 2198–2217. [Google Scholar] [CrossRef]
- Bartollino, S.; Chiosi, F.; di Staso, S.; Uva, M.; Pascotto, A.; Rinaldi, M.; Hesselink, J.M.K.; Costagliola, C. The retinoprotective role of phenytoin. Drug Des. Dev. Ther. 2018, 12, 3485–3489. [Google Scholar] [CrossRef]
- Guerrab, W.; Lgaz, H.; Kansiz, S.; Mague, J.T.; Dege, N.; Ansar, M.; Marzouki, R.; Taoufik, J.; Ali, I.H.; Chung, I.M.; et al. Synthesis of a novel phenytoin derivative: Crystal structure, Hirshfeld surface analysis and DFT calculations. J. Mol. Struct. 2020, 1205, 127630. [Google Scholar] [CrossRef]
- Todorov, P.T.; Peneva, P.N.; Georgieva, S.I.; Rusew, R.I.; Shivachev, B.L.; Georgiev, A.H. Photochromic and molecular switching behaviour of new Schiff bases containing hydantoin rings: Synthesis, characterization and crystal structures. New J. Chem. 2019, 43, 2740. [Google Scholar] [CrossRef]
- Todorov, P.; Peneva, P.; Tchekalarova, J.; Georgieva, S. Potential anticonvulsant activity of novel VV-hemorphin-7 analogues containing unnatural amino acids: Synthesis and characterization. Amino Acids 2020, 52, 567–585. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Rao, R.B.; Balaram, P. Contrasting solution conformations of peptides containing α, α-dialkylated residues with linear and cyclic side chains. Biopolym. Orig. Res. Biomol. 1995, 35, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Adochitei, A.; Drochioiu, G. Rapid characterization of peptide secondary structure by FT-IR spectroscopy. Rev. Roum. Chim. 2011, 56, 783–791. [Google Scholar]
- Hincapié, O.; Giraldo, P.; Orduz, S. In silico design of polycationic antimicrobial peptides active against Pseudomonas aeruginosa and Staphylococcus aureus. Antonie Van Leeuwenhoek 2018, 111, 1871–1882. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.A. Interpretation of α-synuclein UV absorption spectra in the peptide bond and the aromatic regions. J. Photochem. Photobiol. B Biol. 2020, 212, 112022. [Google Scholar] [CrossRef] [PubMed]
- Ivanović, V.; Rančić, M.; Arsić, B.; Pavlović, A. Lipinski’s rule of five, famous extensions and famous exceptions. Pop. Sci. Artic. 2020, 3, 171–177. [Google Scholar] [CrossRef]
- Enache, T.; Oliveira-Brett, A. Peptide methionine sulfoxide reductase A(MsrA): Direct electrochemical oxidation on carbon electrodes. Bioelectrochemistry 2013, 89, 11e18. [Google Scholar] [CrossRef] [PubMed]
- Diculescu, V.; Enache, T. Electrochemical evaluation of Abelson tyrosineprotein kinase 1 activity and inhibition by imatinib mesylate and danusertib. Anal. Chim. Acta 2014, 845, 23e29. [Google Scholar] [CrossRef]
- Enache, T.; Oliveira-Brett, A. Phenol and para-substituted phenols electrochemical oxidation pathways. J. Electroanal. Chem. 2011, 655, 9e16. [Google Scholar] [CrossRef]
- Enache, T.; Oliveira-Brett, A. Pathways of electrochemical oxidation of indolic compounds. Electroanalysis 2011, 23, 1337e1344. [Google Scholar] [CrossRef]
- Enache, T.A.; Chiorcea-Paquim, A.M.; Oliveira-Brett, A.M. Amyloid–β peptides time-dependent structural modifications: AFM and voltammetric characterization. Anal. Chim. Acta 2016, 926, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Kerman, K.; Saito, M.; Nagatani, N.; Takamura, Y.; Tamiya, E. A rapid label-free electrochemical detection and kinetic study of Alzheimer’s amyloid beta aggregation. J. Am. Chem. Soc. 2005, 127, 11892e11893. [Google Scholar] [CrossRef] [PubMed]
- Krall, R.; Penry, J.; White, B.; Kupferberg, H.; Swinyard, E. Antiepileptic drug development: II. Anticonvulsant drug screening. Epilepsia 1978, 19, 409–428. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Plant protein-derived antioxidant peptides: Isolation, identification, mechanism of action and application in food systems: A review. Trends Food Sci. Technol. 2020, 105, 308–322. [Google Scholar] [CrossRef]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive peptides: Synthesis, sources, applications, and proposed mechanisms of action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Juárez, I.; Blancas-Luciano, B.E.; García-Contreras, R.; Fernández-Presas, A.M. Antimicrobial peptides properties beyond growth inhibition and bacterial killing. PeerJ 2022, 10, e12667. [Google Scholar] [CrossRef]
- Jung, W.K.; Rajapakse, N.; Kim, S.K. Antioxidative activity of a low molecular weight peptide derived from the sauce of fermented blue mussel, Mytilus edulis. Eur. Food Res. Technol. 2005, 220, 535–539. [Google Scholar] [CrossRef]
- Peredo-Lovillo, A.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Romero-Luna, H.E. Conventional and in silico approaches to select promising food-derived bioactive peptides: A review. Food Chem. X 2022, 13, 100183. [Google Scholar] [CrossRef]
- Todorov, P.; Georgieva, S.; Tchekalarova, J. Recent Synthesis, Characterization, and Pharmacological Evaluation of Multifunctional Hemorphins Containing Non-Natural Amino Acids with Potential Biological Importance. Pharmaceuticals 2022, 15, 1425. [Google Scholar] [CrossRef]
- Murray, P.R.; Baron, E.J.; Jorgensen, J.H.; Landry, M.L.; Pfaller, M.A. Antibacterial agents and susceptibility test methods. In Manual of Clinical Microbiology, 9th ed.; Murray, P.R., Barron, E.J., Jorgensen, J.H., Landry, M.L., Pfaller, M.A., Eds.; ASM Press: Washington, DC, USA, 2007; pp. 1160–1162. [Google Scholar]
- Kuete, V.; Nana, F.; Ngameni, B.; Mbaveng, A.T.; Keumedjio, F.; Ngadjui, B.T. Antimicrobial activity of the crude extract, fractions and compounds from stem bark of Ficus ovata (Moraceae). J. Ethnopharmacol. 2009, 124, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Khamsah, S.M.; Akowuah, G.; Zhari, I. Antioxidant activity and phenolic content of Orthosiphon stamineus Benth from different geographic. J. Sustain. Sci. Manag. 2006, 1, 14–20. [Google Scholar]

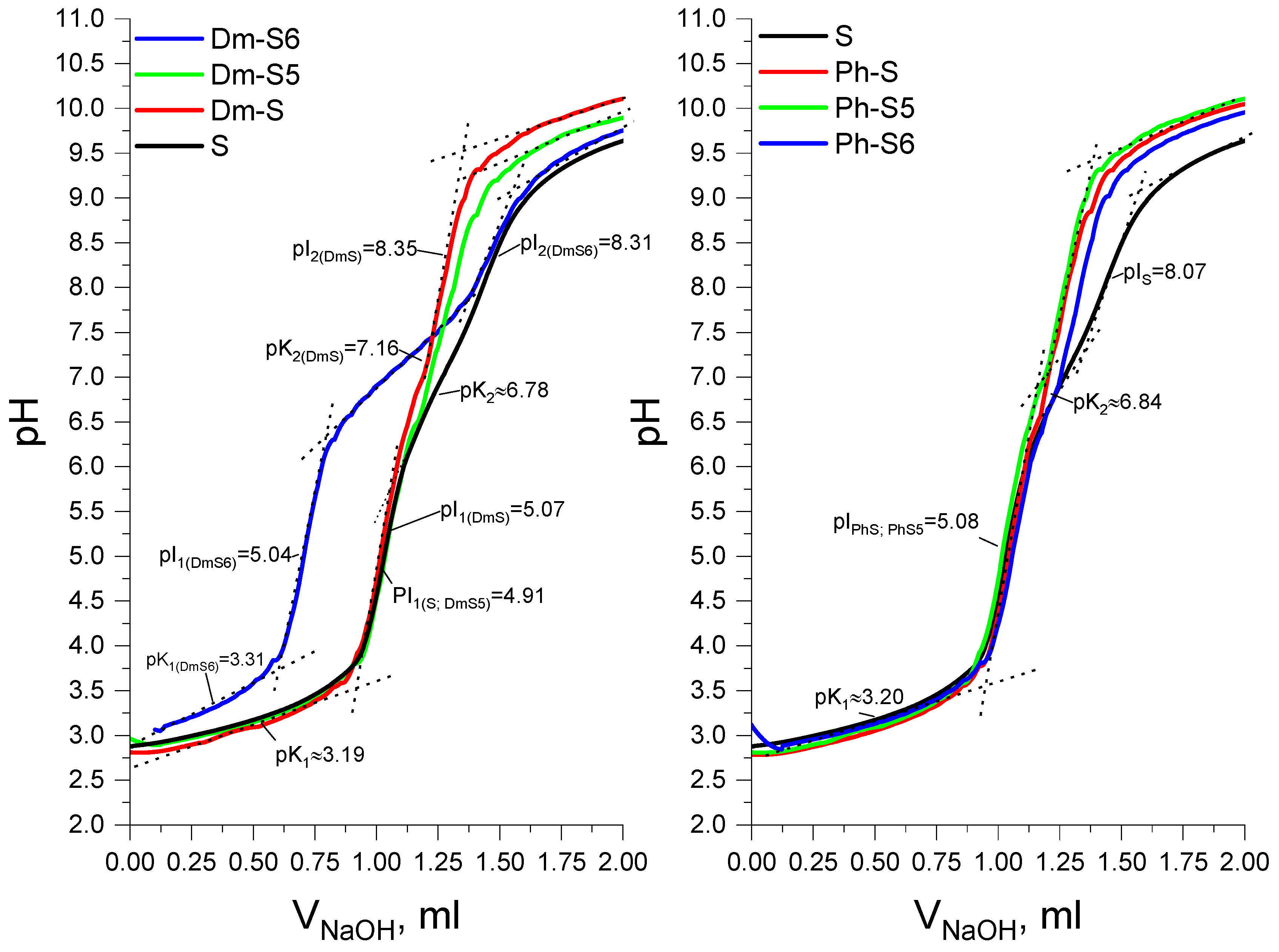
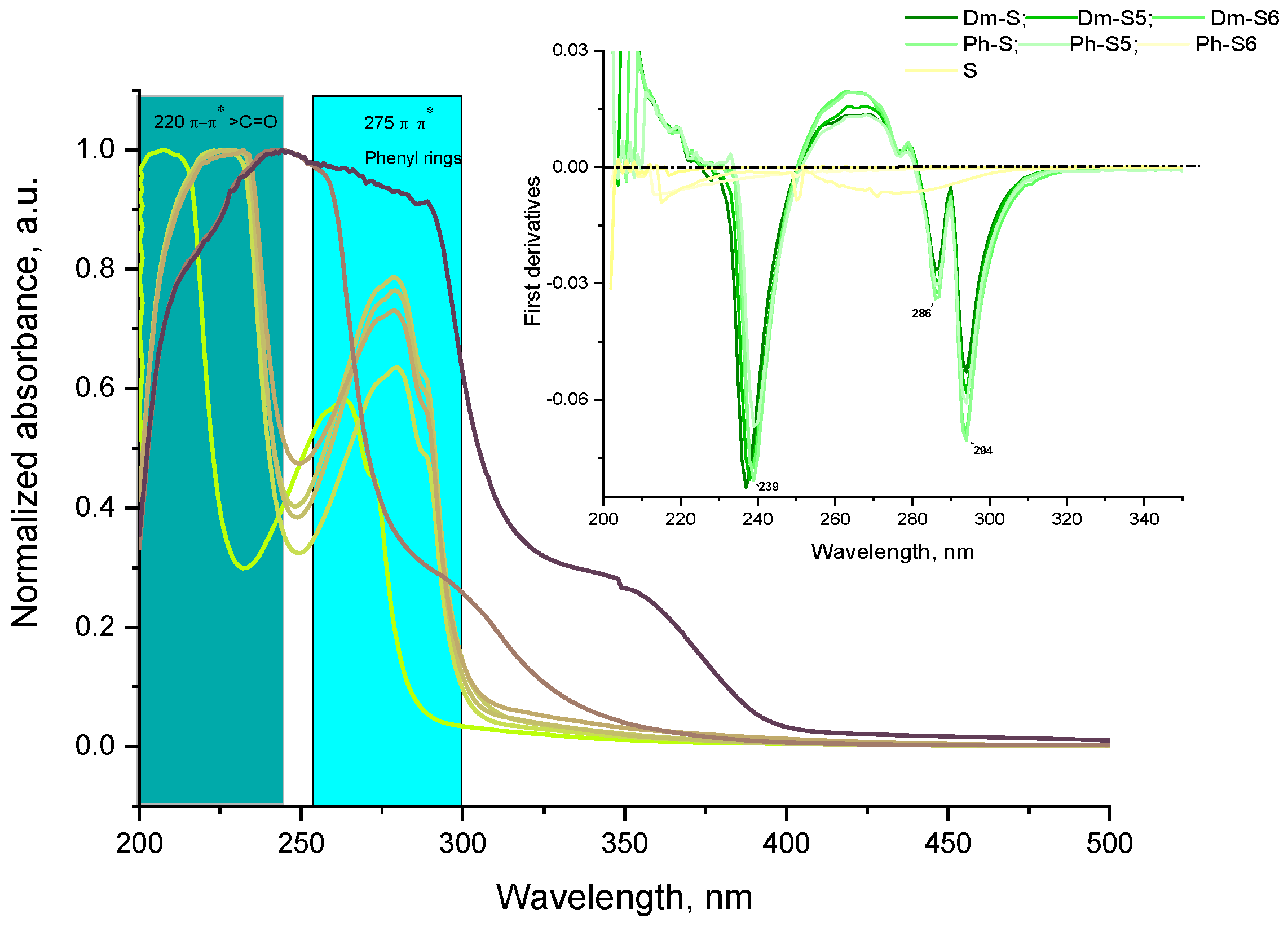

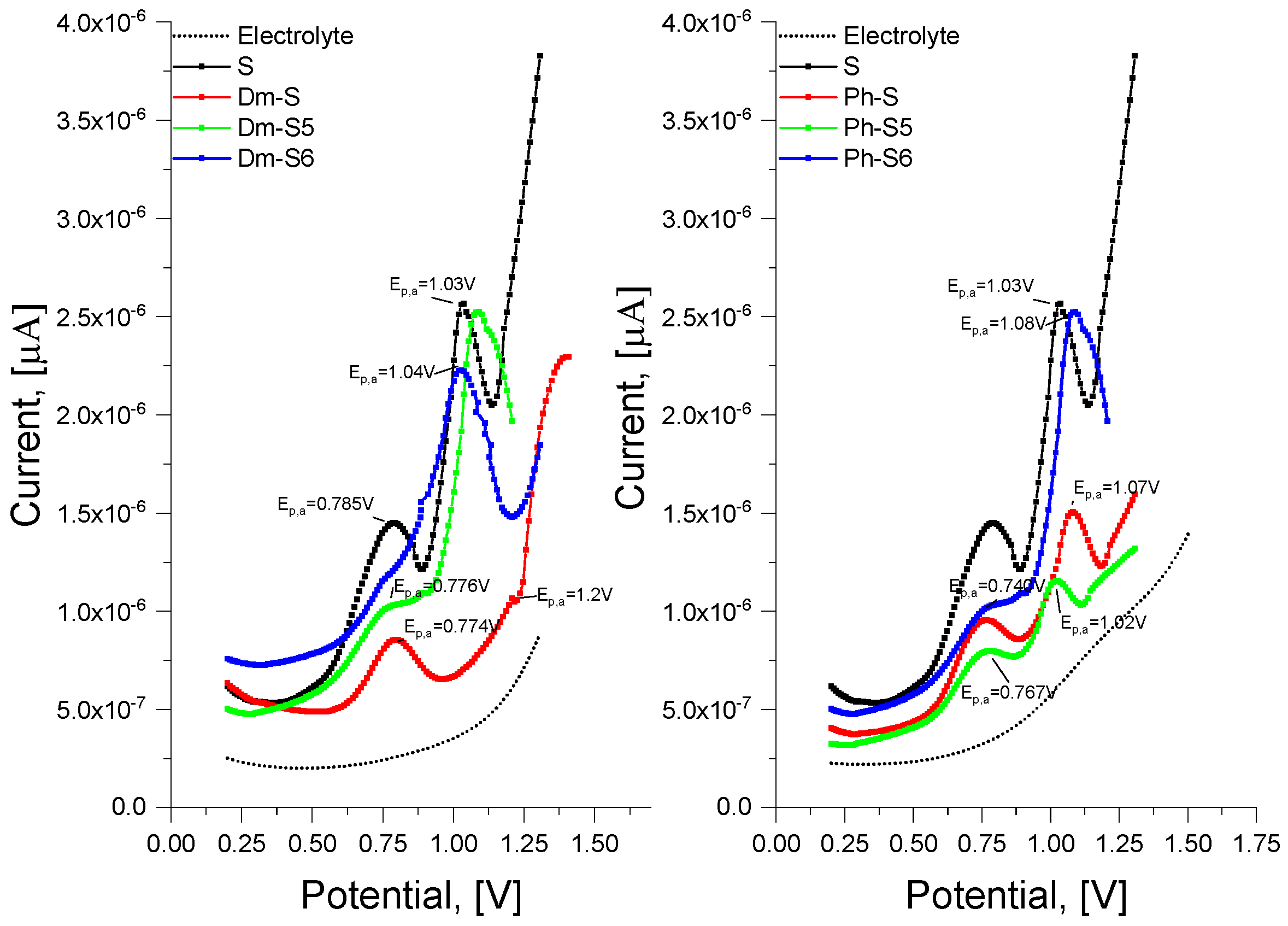
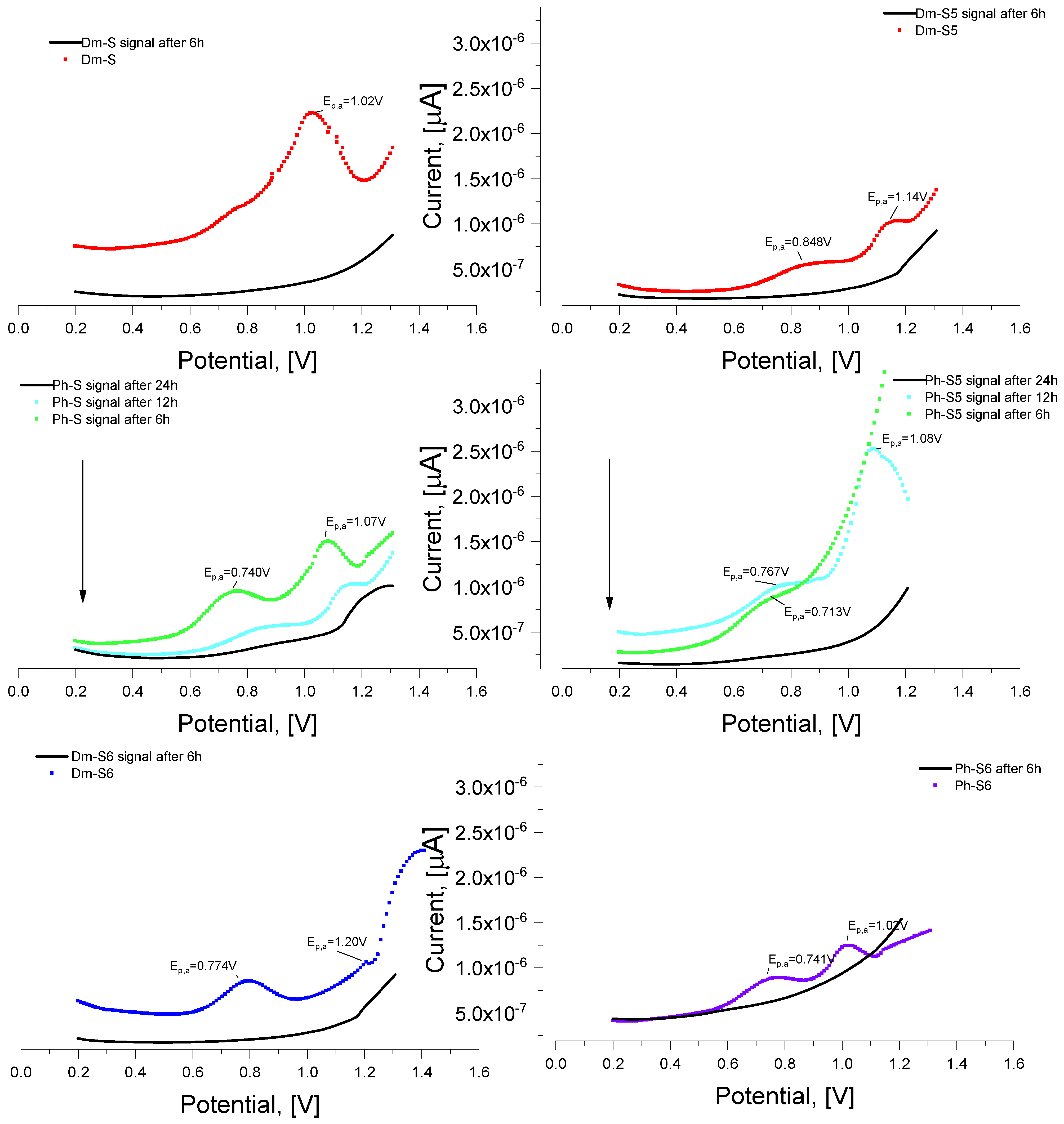

| № | Peptide | a [α]54620 (o) | Molecular Formula | b tR, min | c [MH] + Calculated | c [MH] + Observed | LogP | pKa1 | pKa2 | pI1; pI2 |
|---|---|---|---|---|---|---|---|---|---|---|
| S | H-Leu-Val-Val-Tyr-Pro-Trp-Thr-NH2 | −74 | C45H65N9O9 | 20.04 | 876.0525 | 876.4970 | 0.421 | 3.15 | 6.78 | 4.91; 8.31 |
| Dm-S | Dm-Leu-Val-Val-Tyr-Pro-Trp-Thr-NH2 | −70 | C52H73N11O12 | 26.88 | 1044.2025 | 1044.5501 | 1.16 | 3.18 | 7.16 | 5.07; 8.35 |
| Dm-S5 | Dm-Leu-Val-Val-Tyr-Ac5c-Trp-Thr-NH2 | −20 | C53H75N11O12 | 33.76 | 1058.2291 | 1058.5654 | 1.08 | 3.19 | - | 4.91; 8.30 |
| Dm-S6 | Dm-Leu-Val-Val-Tyr-Ac6c-Trp-Thr-NH2 | −32 | C54H77N11O12 | 36.63 | 1072.2557 | 1072.5814 | 0.934 | 3.31 | 7.23 | 5.04; 8.31 |
| Ph-S | Ph-Leu-Val-Val-Tyr-Pro-Trp-Thr-NH2 | −68 | C62H77N11O12 | 38.64 | 1168.3413 | 1168.5799 | 1.01 | 3.20 | - | 5.06 |
| Ph-S5 | Ph-Leu-Val-Val-Tyr-Ac5c-Trp-Thr-NH2 | −72 | C63H79N11O12 | 45.72 | 1182.3679 | 1182.5957 | 1.24 | 3.22 | - | 5.09 |
| Ph-S6 | Ph-Leu-Val-Val-Tyr-Ac6c-Trp-Thr-NH2 | −20 | C64H81N11O12 | 45.00 | 1196.3944 | 1196.6118 | 1.35 | 3.21 | 6.84 | 5.06; 8.06 |
| Group | Dose µg/10 µL | No. of Animals Protected/No. of Animals Tested | % Protection |
|---|---|---|---|
| control | 0 | 0/8 | 0% |
| S | 0.62 | 3/6 | 50% |
| 1.25 | 3/6 | 50% | |
| 2.5 | 4/6 | 67% * | |
| Dm-S | 0.62 | 2/6 | 33% |
| 1.25 | 2/6 | 33% | |
| 2.5 | 2/6 | 33% | |
| Dm-S5 | 0.62 | 2/5 | 40% |
| 1.25 | 2/5 | 40% | |
| 2.5 | 3/6 | 50% | |
| Dm-S6 | 0.62 | 1/6 | 17% |
| 1.25 | 3/6 | 50% | |
| 2.5 | 3/6 | 50% |
| Group | Dose µg/10 µL | No. of Animals Protected/No. of Animals Tested | % Protection |
|---|---|---|---|
| control | 0 | 0/8 | 0% |
| Ph-S | 0.62 | 4/6 | 33% |
| 1.25 | 4/6 | 67% * | |
| 2.5 | 2/6 | 67% * | |
| Ph-S5 | 0.62 | 1/6 | 17% |
| 1.25 | 3/6 | 50% | |
| 2.5 | 3/6 | 50% | |
| Ph-S6 | 0.62 | 1/6 | 17% |
| 1.25 | 3/6 | 50% | |
| 2.5 | 4/6 | 67% * |
| Group | Dose µg/10 µL | No. of Animals Protected/No. of Animals Tested | % Protection | % Mortality |
|---|---|---|---|---|
| control | 0 | 0/8 | 0% | 30% |
| S | 0.62 | 2/6 | 40% | 0% |
| 1.25 | 2/6 | 40% | 30% | |
| 2.5 | 3/6 | 50% | 50% | |
| Dm-S | 0.62 | 0/6 | 0% | 83% |
| 1.25 | 1/6 | 17% | 33% | |
| 2.5 | 2/6 | 33% | 50% | |
| Dm-S5 | 0.62 | 0/6 | 0% | 33% |
| 1.25 | 1/6 | 17% | 33% | |
| 2.5 | 2/6 | 33% | 33% | |
| Dm-S6 | 0.62 | 1/6 | 17% | 33% |
| 1.25 | 2/6 | 33% | 50% | |
| 2.5 | 4/6 | 67% * | 0% |
| Group | Dose µg/10 µL | No. of Animals Protected/No. of Animals Tested | % Protection | % Mortality |
|---|---|---|---|---|
| control | 0 | 0/8 | 0% | 30% |
| Ph-S | 0.62 | 2/6 | 33% | 0% |
| 1.25 | 3/6 | 50% | 50% | |
| 2.5 | 3/6 | 50% | 17% | |
| Ph-S5 | 0.62 | 2/6 | 33% | 50% |
| 1.25 | 3/6 | 50% | 33% | |
| 2.5 | 4/6 | 67% * | 17% | |
| Ph-S6 | 0.62 | 2/6 | 40% | 60% |
| 1.25 | 3/6 | 50% | 67% | |
| 2.5 | 4/6 | 67% * | 17% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgieva, S.; Todorov, P.; Tchekalarova, J.; Subaer, S.; Peneva, P.; Chakarov, K.; Hartati, H.; Faika, S. Chemical Behavior and Bioactive Properties of Spinorphin Conjugated to 5,5′-Dimethyl- and 5,5′-Diphenylhydantoin Analogs. Pharmaceuticals 2024, 17, 770. https://doi.org/10.3390/ph17060770
Georgieva S, Todorov P, Tchekalarova J, Subaer S, Peneva P, Chakarov K, Hartati H, Faika S. Chemical Behavior and Bioactive Properties of Spinorphin Conjugated to 5,5′-Dimethyl- and 5,5′-Diphenylhydantoin Analogs. Pharmaceuticals. 2024; 17(6):770. https://doi.org/10.3390/ph17060770
Chicago/Turabian StyleGeorgieva, Stela, Petar Todorov, Jana Tchekalarova, Subaer Subaer, Petia Peneva, Kalin Chakarov, Hartati Hartati, and Sitti Faika. 2024. "Chemical Behavior and Bioactive Properties of Spinorphin Conjugated to 5,5′-Dimethyl- and 5,5′-Diphenylhydantoin Analogs" Pharmaceuticals 17, no. 6: 770. https://doi.org/10.3390/ph17060770
APA StyleGeorgieva, S., Todorov, P., Tchekalarova, J., Subaer, S., Peneva, P., Chakarov, K., Hartati, H., & Faika, S. (2024). Chemical Behavior and Bioactive Properties of Spinorphin Conjugated to 5,5′-Dimethyl- and 5,5′-Diphenylhydantoin Analogs. Pharmaceuticals, 17(6), 770. https://doi.org/10.3390/ph17060770










