Wound Healing Efficacy of Cucurbitaceae Seed Oils in Rats: Comprehensive Phytochemical, Pharmacological, and Histological Studies Tackling AGE/RAGE and Nrf2/Ho-1 Cue
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of Cucurbitaceae Seed Oils
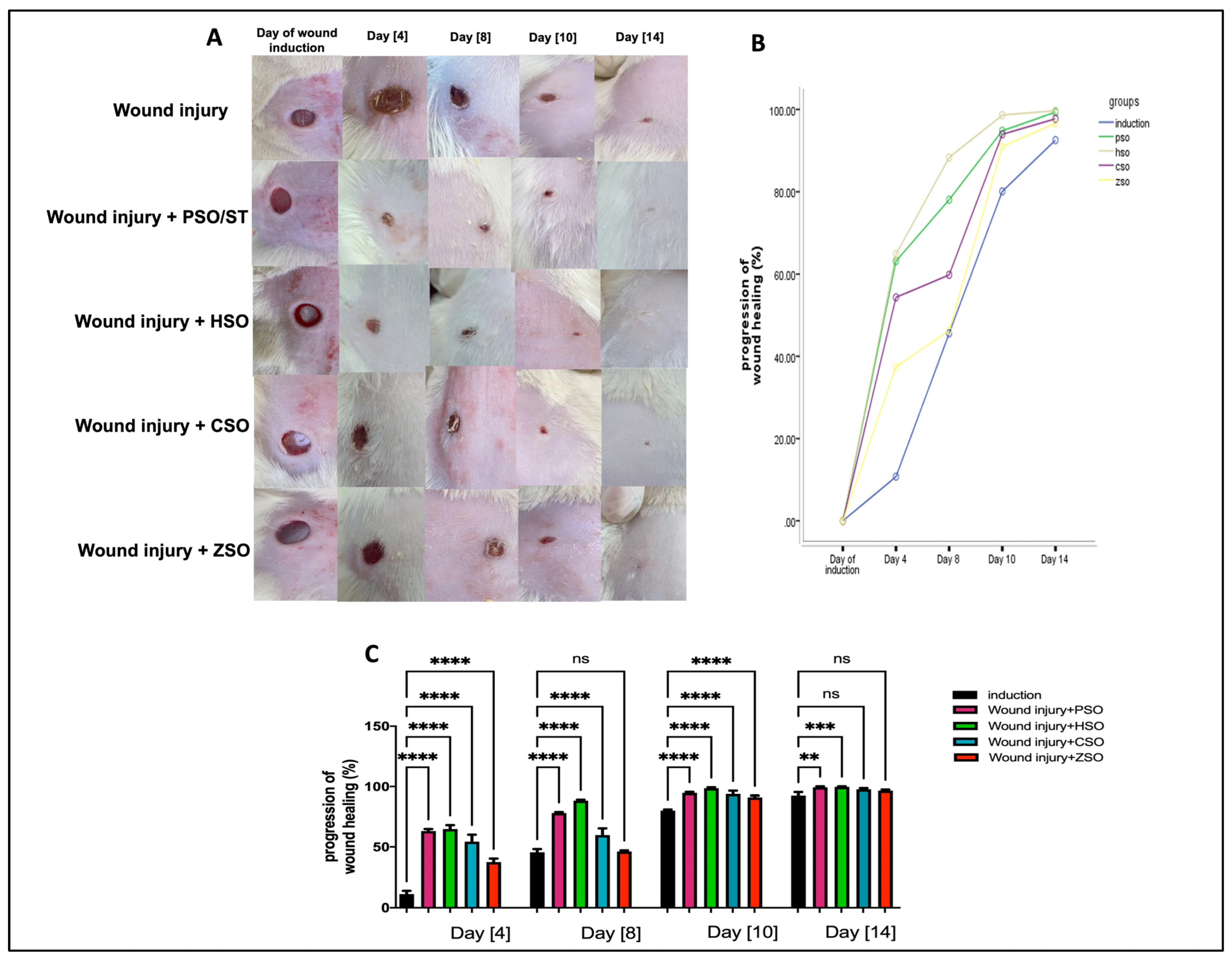
2.2. Macroscopic Quantitative Assessment of Wound Healing Progression
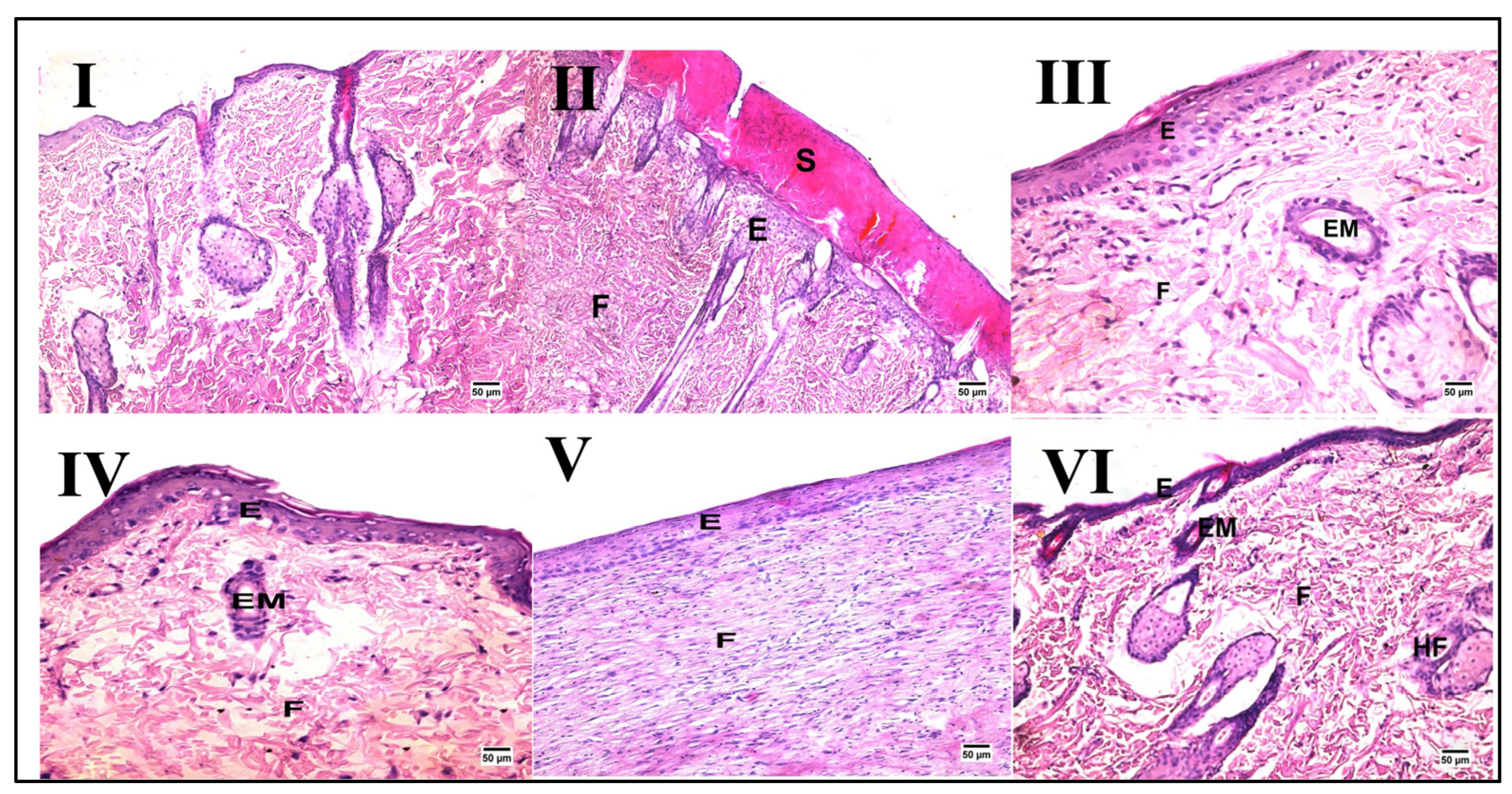
2.3. Effect of Different Oils on Histopathological Alterations after Wound Induction
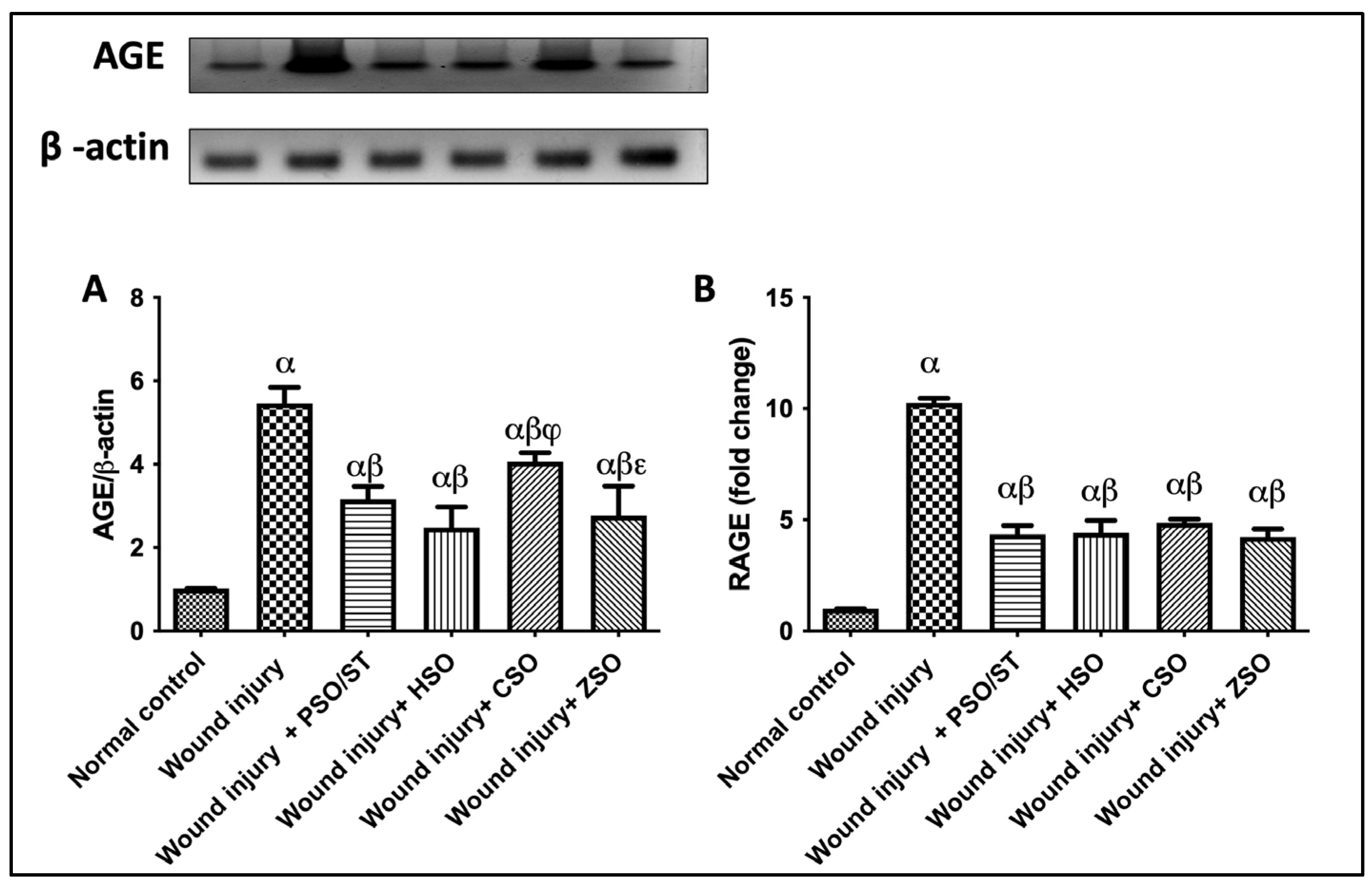
2.4. Effect of Different Oils on the Protein Expression of AGE and RAGE as Signaling Cue/Genes
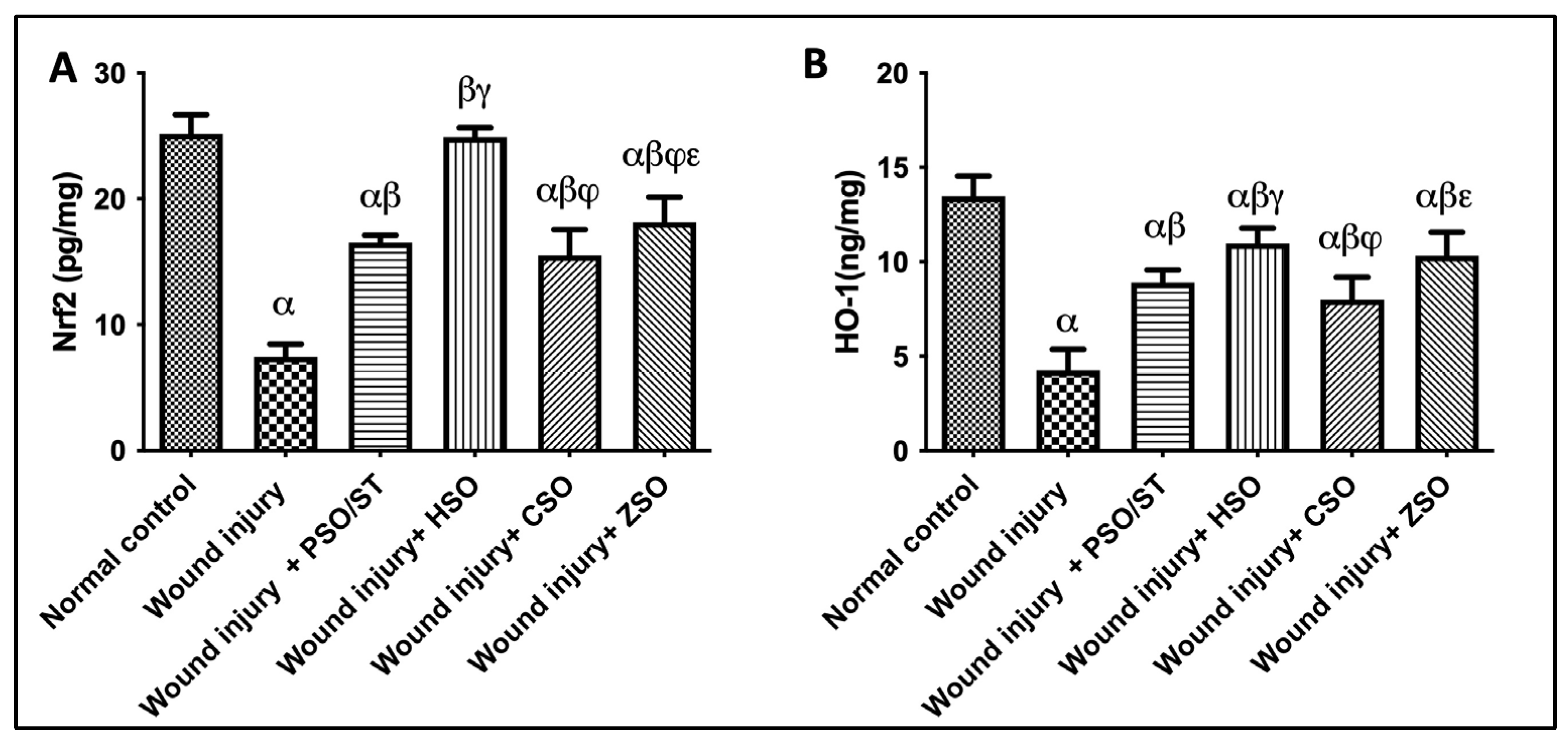
2.5. Effect of Different Oils on the Tissue Contents of Nrf2/HO-1 as Antioxidant Signaling Molecules
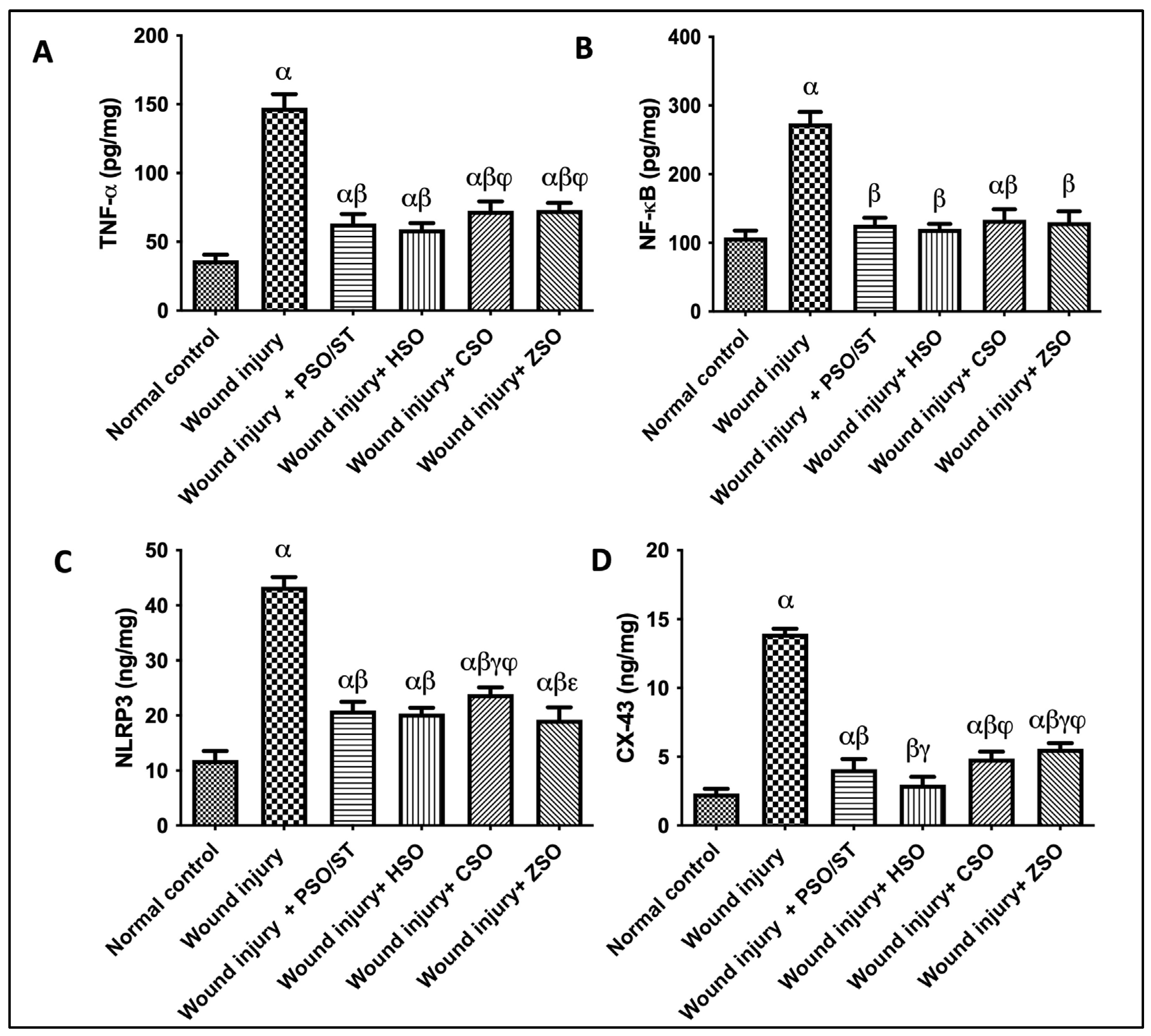
2.6. Effect of Different Oils on TNF-α, NF-κB, and NLRP3 as Inflammatory Markers in Addition to CX-43 as Skin Integral Signaling Protein
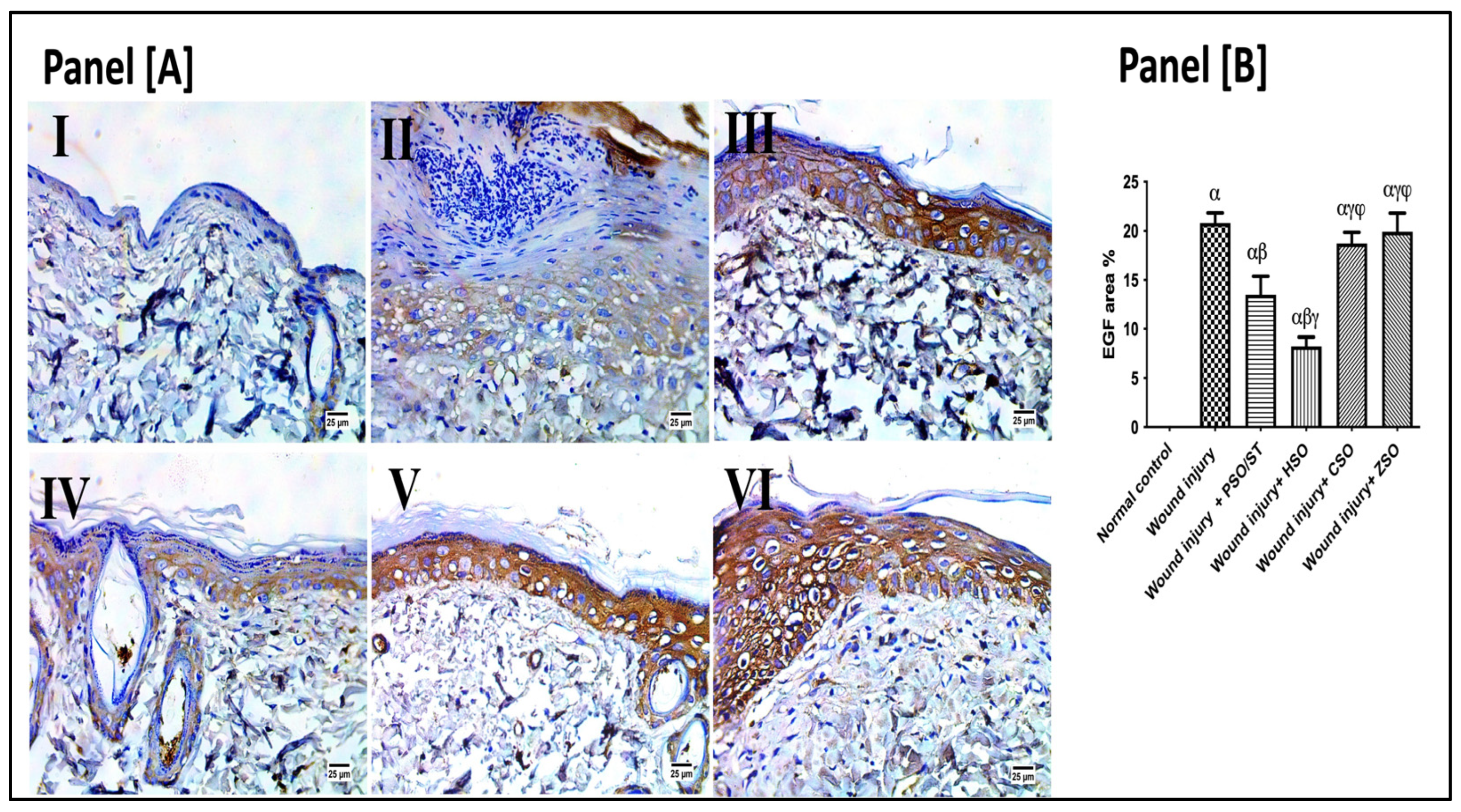
2.7. Effect of Different Oils on Immunohistochemistry Expression of Epidermal Growth Factor (EGF) after Wound Induction
3. Discussion
4. Material and Methods
4.1. Plant Material
4.2. Oil Extraction
4.3. Chemical Analysis of the Oils
4.3.1. Sample Derivatizations
4.3.2. Gas Chromatography Analysis
4.4. In Vivo Wound Healing Model
4.4.1. Animals
4.4.2. Creation of Excision Wounds
4.4.3. Experiment Protocol
4.4.4. Tissue Collection
4.4.5. Quantitative Real-Time Polymerase Chain Reaction (RT-PCR)
4.4.6. Western Blotting (WB)
4.4.7. Determination of Tissue Antioxidant Stress Markers, Inflammatory Factors, and Connexin 43
4.4.8. Histopathology Examination
4.4.9. Immunohistochemistry (IHC) Examination
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ambrozova, N.; Ulrichova, J.; Galandakova, A. Models for the study of skin wound healing. The role of Nrf2 and NF-κB. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 2017, 161, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, G.S.; Greenwell-Wild, T.; Horan, M.A.; Wahl, S.M.; Ferguson, M.W. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am. J. Pathol. 1999, 155, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Werner, S. Cancer as an overhealing wound: An old hypothesis revisited. Nat. Rev. Mol. Cell Biol. 2008, 9, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Brett, J.; Schmidt, A.M.; Yan, S.D.; Zou, Y.S.; Weidman, E.; Pinsky, D.; Nowygrod, R.; Neeper, M.; Przysiecki, C.; Shaw, A. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am. J. Pathol. 1993, 143, 1699–1712. [Google Scholar] [PubMed]
- Schmidt, A.M.; Stern, D.M. Receptor for age (RAGE) is a gene within the major histocompatibility class III region: Implications for host response mechanisms in homeostasis and chronic disease. Front. Biosci.-Landmark 2001, 6, 1151–1160. [Google Scholar]
- Xie, J.; Méndez, J.D.; Méndez-Valenzuela, V.; Hernandez, M.M.A. Cellular signalling of the receptor for advanced glycation end products (RAGE). Cell. Signal. 2013, 25, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Sorci, G.; Riuzzi, F.; Giambanco, I.; Donato, R. RAGE in tissue homeostasis, repair and regeneration. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2013, 1833, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Thangapazham, R.L.; Sharad, S.; Maheshwari, R.K. Phytochemicals in wound healing. Adv. Wound Care 2016, 5, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Criollo-Mendoza, M.S.; Contreras-Angulo, L.A.; Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Jiménez-Ortega, L.A.; Heredia, J.B. Wound healing properties of natural products: Mechanisms of action. Molecules 2023, 28, 598. [Google Scholar] [CrossRef]
- Ogunwa, K.I.; Ofodile, S.; Achugasim, O. Feasibility study of melon seed oil as a source of biodiesel. J. Power Energy Eng. 2015, 3, 24–27. [Google Scholar] [CrossRef]
- Rezig, L.; Gharsallah, K.; Mahfoudhi, N. Bioactive Compounds of Cucurbitaceae Seed Oils as Nutraceuticals and Health-Promoting Substances. In Handbook of Research on Advanced Phytochemicals and Plant-Based Drug Discovery; IGI Global: Hershey, PA, USA, 2022; pp. 292–313. [Google Scholar]
- Bardaa, S.; Moalla, D.; Ben Khedir, S.; Rebai, T.; Sahnoun, Z. The evaluation of the healing proprieties of pumpkin and linseed oils on deep second-degree burns in rats. Pharm. Biol. 2016, 54, 581–587. [Google Scholar] [CrossRef]
- Poljšak, N.; Kreft, S.; Glavač, N.K. Vegetable butters and oils in skin wound healing: Scientific evidence for new opportunities in dermatology. Phytother. Res. 2020, 34, 254–269. [Google Scholar] [CrossRef]
- Jorge, N.; da Silva, A.C.; Malacrida, C.R. Physicochemical characterisation and radical-scavenging activity of Cucurbitaceae seed oils. Nat. Prod. Res. 2015, 29, 2313–2317. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Loizzo, M.R.; Gortzi, O.; Çankaya, İ.T.; Tundis, R.; Suntar, İ.; Shirooie, S.; Zengin, G.; Devkota, H.P.; Reboredo-Rodríguez, P.; et al. The potential of pumpkin seed oil as a functional food—A comprehensive review of chemical composition, health benefits, and safety. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4422–4446. [Google Scholar] [CrossRef] [PubMed]
- Bardaa, S.; Ben Halima, N.; Aloui, F.; Ben Mansour, R.; Jabeur, H.; Bouaziz, M.; Sahnoun, Z. Oil from pumpkin (Cucurbita pepo L.) seeds: Evaluation of its functional properties on wound healing in rats. Lipids Health Dis. 2016, 15, 73. [Google Scholar]
- Ribeiro Barros Cardoso, C.; Aparecida Souza, M.; Amália Vieira Ferro, E.; Favoreto, S., Jr.; Deolina Oliveira Pena, J. Influence of topical administration of n-3 and n-6 essential and n-9 nonessential fatty acids on the healing of cutaneous wounds. Wound Repair Regener. 2004, 12, 235–243. [Google Scholar] [CrossRef]
- Theilla, M.; Singer, P.; Cohen, J.; DeKeyser, F. A diet enriched in eicosapentanoic acid, gamma-linolenic acid and antioxidants in the prevention of new pressure ulcer formation in critically ill patients with acute lung injury: A randomized, prospective, controlled study. Clin. Nutr. 2007, 26, 752–757. [Google Scholar] [CrossRef]
- Pereira, L.M.; Hatanaka, E.; Martins, E.F.; Oliveira, F.; Liberti, E.A.; Farsky, S.H.; Curi, R.; Pithon-Curi, T.C. Effect of oleic and linoleic acids on the inflammatory phase of wound healing in rats. Cell Biochem. Funct. Cell. Biochem. Its Modul. Act. Agents Dis. 2008, 26, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.-H.; El Omari, N.; Sheikh, R.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Health Benefits and Pharmacological Properties of Stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef]
- Saputra, W.D.; Shono, H.; Ohsaki, Y.; Sultana, H.; Komai, M.; Shirakawa, H. Geranylgeraniol Inhibits Lipopolysaccharide-Induced Inflammation in Mouse-Derived MG6 Microglial Cells via NF-κB Signaling Modulation. Int. J. Mol. Sci. 2021, 22, 10543. [Google Scholar] [CrossRef]
- Wear–Maggitti, K.; Lee, J.; Conejero, A.; Schmidt, A.M.; Grant, R.; Breitbart, A. Use of topical sRAGE in diabetic wounds increases neovascularization and granulation tissue formation. Ann. Plast. Surg. 2004, 52, 519–521. [Google Scholar] [CrossRef] [PubMed]
- Hiebert, P.; Werner, S. Regulation of wound healing by the NRF2 transcription factor—More than cytoprotection. Int. J. Mol. Sci. 2019, 20, 3856. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef]
- Grochot-Przeczek, A.; Lach, R.; Mis, J.; Skrzypek, K.; Gozdecka, M.; Sroczynska, P.; Dubiel, M.; Rutkowski, A.; Kozakowska, M.; Zagorska, A.; et al. Heme oxygenase-1 accelerates cutaneous wound healing in mice. PLoS ONE 2009, 4, e5803. [Google Scholar] [CrossRef] [PubMed]
- Hanselmann, C.; Mauch, C.; Werner, S. Haem oxygenase-1: A novel player in cutaneous wound repair and psoriasis? Biochem. J. 2001, 353, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Mosser, D. Macrophage activation by endogenous danger signals. J. Pathol. J. Pathol. Soc. Great Br. Irel. 2008, 214, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regener. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Ding, X.; Zhang, H.; Li, S.; Yang, P.; Tan, Q. Relevance of NLRP3 Inflammasome-Related Pathways in the Pathology of Diabetic Wound Healing and Possible Therapeutic Targets. Oxid. Med. Cell. Longev. 2022, 2022, 9687925. [Google Scholar] [CrossRef] [PubMed]
- Cárdeno, A.; Aparicio-Soto, M.; Montserrat-de la Paz, S.; Bermúdez, B.; Muriana, F.J.; Alarcón-de-la-Lastra, C. Squalene targets pro-and anti-inflammatory mediators and pathways to modulate over-activation of neutrophils, monocytes and macrophages. J. Funct. Foods 2015, 14, 779–790. [Google Scholar] [CrossRef]
- Sutcliffe, J.; Chin, K.; Thrasivoulou, C.; Serena, T.; O’Neil, S.; Hu, R.; White, A.; Madden, L.; Richards, T.; Phillips, A.; et al. Abnormal connexin expression in human chronic wounds. Br. J. Dermatol. 2015, 173, 1205–1215. [Google Scholar] [CrossRef]
- Tarzemany, R.; Jiang, G.; Jiang, J.X.; Larjava, H.; Häkkinen, L. Connexin 43 hemichannels regulate the expression of wound healing-associated genes in human gingival fibroblasts. Sci. Rep. 2017, 7, 14157. [Google Scholar] [CrossRef] [PubMed]
- Grek, C.L.; Prasad, G.; Viswanathan, V.; Armstrong, D.G.; Gourdie, R.G.; Ghatnekar, G.S. Topical administration of a connexin43-based peptide augments healing of chronic neuropathic diabetic foot ulcers: A multicenter, randomized trial. Wound Repair Regener. 2015, 23, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Ghatnekar, G.; Gourdie, R.G.; Potts, J.D. Impact of the controlled release of a connexin 43 peptide on corneal wound closure in an STZ model of type I diabetes. PLoS ONE 2014, 9, e86570. [Google Scholar] [CrossRef]
- Abd El-Aal, S.A.; El-Sayyad, S.M.; El-Gazar, A.A.; Ibrahim, S.S.; Essa, M.A.; Abostate, H.M.; Ragab, G.M. Boswellic acid and apigenin alleviate methotrexate-provoked renal and hippocampal alterations in rats: Targeting autophagy, NOD-2/NF-κB/NLRP3, and connexin-43. Int. Immunopharmacol. 2024, 134, 112147. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, R.J. Epidermal growth factor and epidermal growth factor receptor: The Yin and Yang in the treatment of cutaneous wounds and cancer. Adv. Wound Care 2013, 2, 24–29. [Google Scholar] [CrossRef]
- Magdalon, J.; Vinolo, M.A.R.; Rodrigues, H.G.; Paschoal, V.A.; Torres, R.P.; Mancini-Filho, J.; Calder, P.C.; Hatanaka, E.; Curi, R. Oral administration of oleic or linoleic acids modulates the production of inflammatory mediators by rat macrophages. Lipids 2012, 47, 803–812. [Google Scholar] [CrossRef]
- Christie, W.W. Lipid Analysis; Pergamon Press: Oxford, UK, 1982; Volume 207. [Google Scholar]
- Elosaily, A.H.; Mahrous, E.A.; Salama, A.A.; Salama, A.M.; Elzalabani, S.M. Composition, anti-inflammatory, and antioxidant activities of avocado oil obtained from Duke and Fuerte cultivars. J. Am. Oil Chem. Soc. 2022, 99, 181–186. [Google Scholar] [CrossRef]
- Baky, M.H.; Farag, M.A.; Rasheed, D.M. Metabolome-based analysis of herbal cough preparations via headspace solid-phase microextraction GC/MS and multivariate data analyses: A prospect for its essential oil equivalency. ACS Omega 2020, 5, 31370–31380. [Google Scholar] [CrossRef] [PubMed]
- Refai, H.; El-Gazar, A.A.; Ragab, G.M.; Hassan, D.H.; Ahmed, O.S.; Hussein, R.A.; Shabana, S.; Waffo-Téguo, P.; Valls, J.; Al-Mokaddem, A.K.; et al. Enhanced Wound Healing Potential of Spirulina platensis Nanophytosomes: Metabolomic Profiling, Molecular Networking, and Modulation of HMGB-1 in an Excisional Wound Rat Model. Mar. Drugs 2023, 21, 149. [Google Scholar] [CrossRef]
- Mori, H.-M.; Kawanami, H.; Kawahata, H.; Aoki, M. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement. Altern. Med. 2016, 16, 144. [Google Scholar] [CrossRef]
- Mounir, R.; Alshareef, W.A.; El Gebaly, E.A.; El-Haddad, A.E.; Ahmed, A.M.S.; Mohamed, O.G.; Enan, E.T.; Mosallam, S.; Tripathi, A.; Selim, H.M.R.M.; et al. Unlocking the Power of Onion Peel Extracts: Antimicrobial and Anti-Inflammatory Effects Improve Wound Healing through Repressing Notch-1/NLRP3/Caspase-1 Signaling. Pharmaceuticals 2023, 16, 1379. [Google Scholar] [CrossRef] [PubMed]
- El-Gazar, A.A.; Emad, A.M.; Ragab, G.M.; Rasheed, D.M. Mentha pulegium L. (Pennyroyal, Lamiaceae) Extracts Impose Abortion or Fetal-Mediated Toxicity in Pregnant Rats; Evidenced by the Modulation of Pregnancy Hormones, MiR-520, MiR-146a, TIMP-1 and MMP-9 Protein Expressions, Inflammatory State, Certain Related Signaling Pathways, and Metabolite Profiling via UPLC-ESI-TOF-MS. Toxins 2022, 14, 347. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.B.; Ibrahim, S.S.; Alsherbiny, M.A.; Sheta, E.; El-Shiekh, R.A.; Ashour, R.M.; El-Gazar, A.A.; Ragab, G.M.; El-Gayed, S.H.; Li, C.G.; et al. Gastroprotective potential of red onion (Allium cepa L.) peel in ethanol-induced gastric injury in rats: Involvement of Nrf2/HO-1 and HMGB-1/NF-κB trajectories. J. Ethnopharmacol. 2024, 319, 117115. [Google Scholar] [CrossRef] [PubMed]
- Van De Vyver, M.; Boodhoo, K.; Frazier, T.; Hamel, K.; Kopcewicz, M.; Levi, B.; Maartens, M.; Machcinska, S.; Nunez, J.; Pagani, C.; et al. Histology scoring system for murine cutaneous wounds. Stem Cells Dev. 2021, 30, 1141–1152. [Google Scholar] [CrossRef]
- Kim, Y.J.; Cribbie, R.A. ANOVA and the variance homogeneity assumption: Exploring a better gatekeeper. Br. J. Math. Stat. Psychol. 2018, 71, 1–12. [Google Scholar] [CrossRef]

| Name | Area Sum Percentage % | |||
|---|---|---|---|---|
| CSO | HSO | PSO | ZSO | |
| Undecanoic acid | - | - | 0.12 | - |
| Lauric acid | 1.59 | 0.18 | 0.29 | 0.20 |
| Tridecanoic acid | 1.88 | 0.23 | 0.29 | 0.23 |
| Myristic acid | 0.90 | 0.15 | 0.27 | 0.18 |
| Palmitic acid | 9.87 | 10.33 | 12.94 | 12.48 |
| Palmitoleic acid | 0.08 | 0.12 | 0.17 | 0.08 |
| Stearic acid | 5.11 | 5.28 | 7.96 | 7.72 |
| Oleic acid | 14.17 | 16.25 | 36.85 | 29.37 |
| Linoleic acid | 65.60 | 65.90 | 39.26 | 48.55 |
| Linolenic acid | 0.28 | 0.23 | 0.18 | 0.16 |
| Arachidic acid | 0.20 | 0.24 | 0.59 | 0.41 |
| Behenic acid | 0.10 | 0.05 | 0.18 | 0.12 |
| Nervonic acid | 0.07 | 0.91 | 0.24 | 0.32 |
| Saturated fatty acids | 19.65 | 16.46 | 22.64 | 21.34 |
| Monounsaturated fatty acids | 14.32 | 17.28 | 37.26 | 29.77 |
| Polyunsaturated fatty acids | 65.88 | 66.13 | 39.44 | 48.71 |
| Total % | 99.85 | 99.87 | 99.34 | 99.82 |
| Name | Area Sum Percentage % | |||
|---|---|---|---|---|
| CSO | HSO | PSO | ZSO | |
| Squalene | 15.07 | 16.65 | 54.12 | 12.27 |
| Longipinene epoxide | 2.01 | - | - | 1.39 |
| γ-Tocopherol | - | - | 0.61 | - |
| α-Tocopherol | - | - | 0.34 | - |
| Geranylgeranyl alcohol | 9.14 | 13.23 | - | 0.92 |
| 21,25-Dihydroxy-vitamin D3 | 1.99 | - | - | 47.38 |
| Stigmasterol | 48.51 | 34.40 | 1.51 | 6.43 |
| β-Sitosterol | 7.88 | 14.89 | 14.88 | 9.59 |
| Desmosterol | - | - | 10.70 | - |
| α-Amyrin | - | - | 4.66 | - |
| Simiarenol | - | - | 7.65 | - |
| Lupeol | - | - | 0.30 | - |
| Sterols | 56.39 | 49.29 | 27.09 | 16.02 |
| Terpenoidal compounds | 26.22 | 29.88 | 66.73 | 14.58 |
| Vitamins | 1.99 | - | 0.95 | 47.38 |
| %Total identified | 84.59 | 79.17 | 94.77 | 77.99 |
| Group | Re-Epithelization | Epidermal Thickness |
|---|---|---|
| Normal control | 2 | 2 |
| Wound injury | 1 | 0 |
| Wound injury + PSO/ST | 2 | 1 |
| Wound injury + HSO | 2 | 2 |
| Wound injury + CSO | 2 | 1 |
| Wound injury + ZSO | 2 | 2 |
| RAGE | forward: 5′-CAGGGTCACAGAAACCGG-3′ reverse: 5′-ATTCAGCTCTGCACGTTCCT-3′ |
| β-actin | forward: 5′-AGGCCAACCGTGAAAAGATG 3′ reverse: 5′-ACCAGAGGCATACAGGGACAA3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emad, A.M.; Mahrous, E.A.; Rasheed, D.M.; Gomaa, F.A.M.; Hamdan, A.M.E.; Selim, H.M.R.M.; Yousef, E.M.; Abo-Zalam, H.B.; El-Gazar, A.A.; Ragab, G.M. Wound Healing Efficacy of Cucurbitaceae Seed Oils in Rats: Comprehensive Phytochemical, Pharmacological, and Histological Studies Tackling AGE/RAGE and Nrf2/Ho-1 Cue. Pharmaceuticals 2024, 17, 733. https://doi.org/10.3390/ph17060733
Emad AM, Mahrous EA, Rasheed DM, Gomaa FAM, Hamdan AME, Selim HMRM, Yousef EM, Abo-Zalam HB, El-Gazar AA, Ragab GM. Wound Healing Efficacy of Cucurbitaceae Seed Oils in Rats: Comprehensive Phytochemical, Pharmacological, and Histological Studies Tackling AGE/RAGE and Nrf2/Ho-1 Cue. Pharmaceuticals. 2024; 17(6):733. https://doi.org/10.3390/ph17060733
Chicago/Turabian StyleEmad, Ayat M., Engy A. Mahrous, Dalia M. Rasheed, Fatma Alzahraa M. Gomaa, Ahmed Mohsen Elsaid Hamdan, Heba Mohammed Refat M. Selim, Einas M. Yousef, Hagar B. Abo-Zalam, Amira A. El-Gazar, and Ghada M. Ragab. 2024. "Wound Healing Efficacy of Cucurbitaceae Seed Oils in Rats: Comprehensive Phytochemical, Pharmacological, and Histological Studies Tackling AGE/RAGE and Nrf2/Ho-1 Cue" Pharmaceuticals 17, no. 6: 733. https://doi.org/10.3390/ph17060733
APA StyleEmad, A. M., Mahrous, E. A., Rasheed, D. M., Gomaa, F. A. M., Hamdan, A. M. E., Selim, H. M. R. M., Yousef, E. M., Abo-Zalam, H. B., El-Gazar, A. A., & Ragab, G. M. (2024). Wound Healing Efficacy of Cucurbitaceae Seed Oils in Rats: Comprehensive Phytochemical, Pharmacological, and Histological Studies Tackling AGE/RAGE and Nrf2/Ho-1 Cue. Pharmaceuticals, 17(6), 733. https://doi.org/10.3390/ph17060733






