2-(4-Nitrophenyl)isothiazol-3(2H)-one: A Promising Selective Agent against Hepatocellular Carcinoma Cells
Abstract
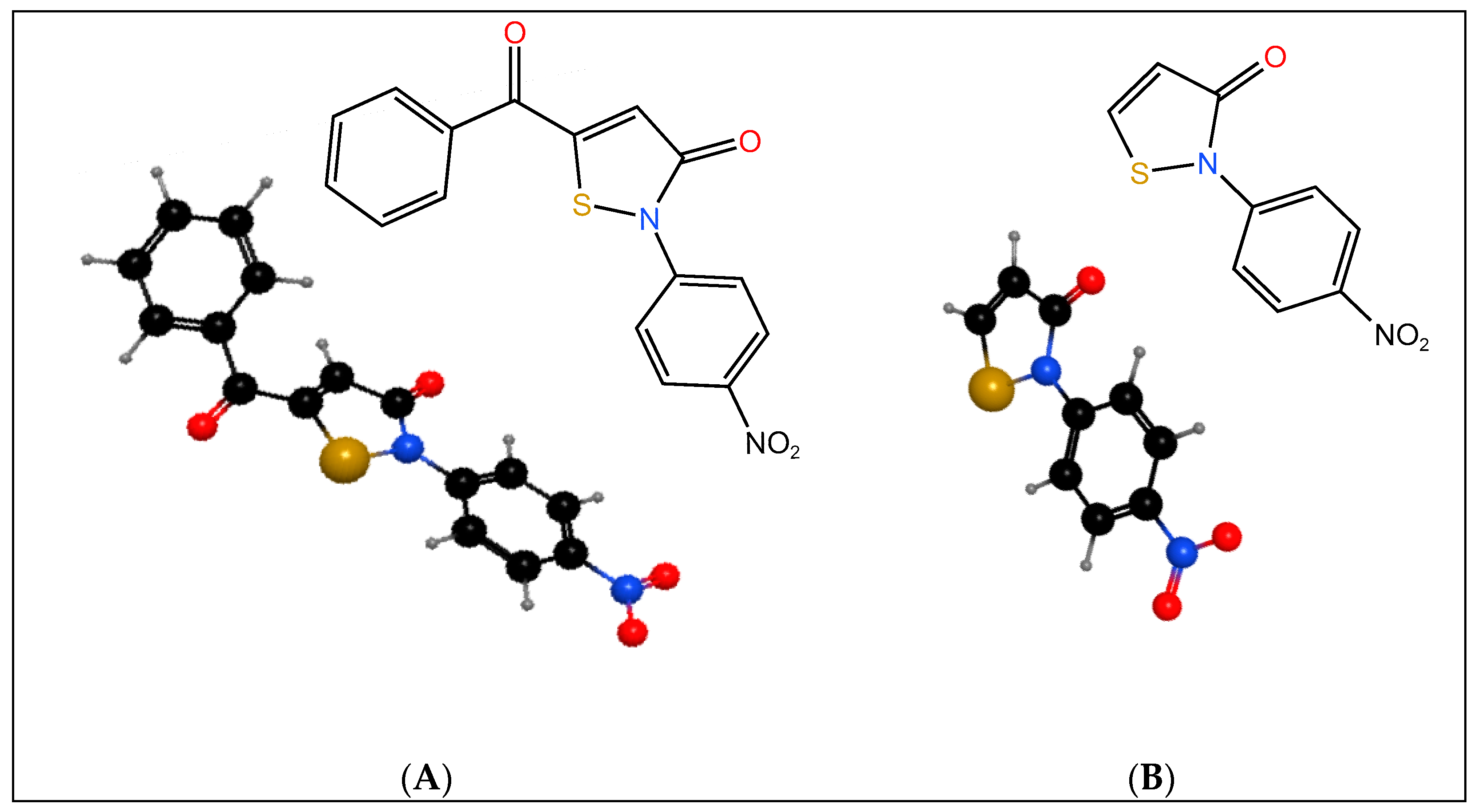
1. Introduction
2. Results
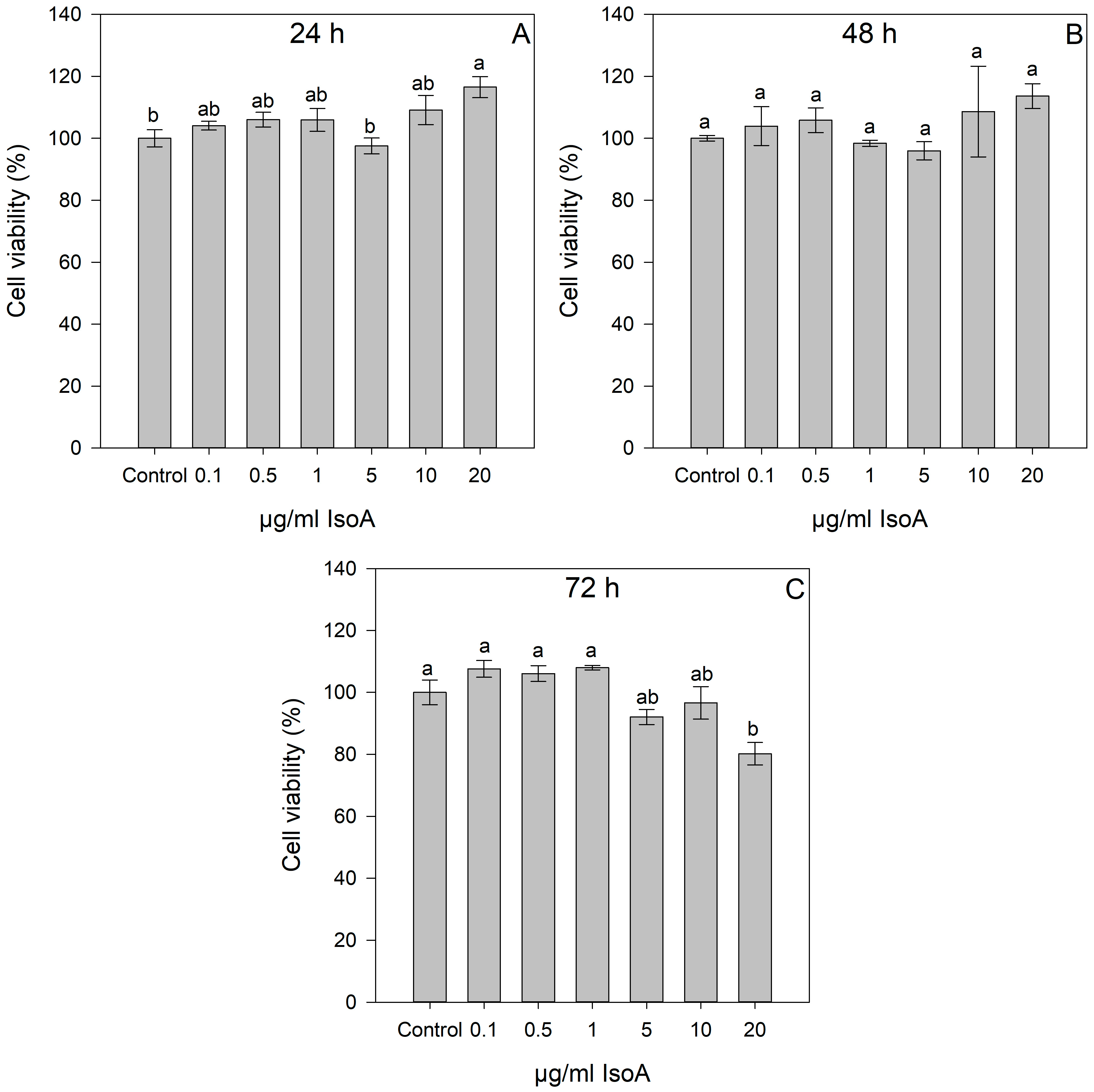
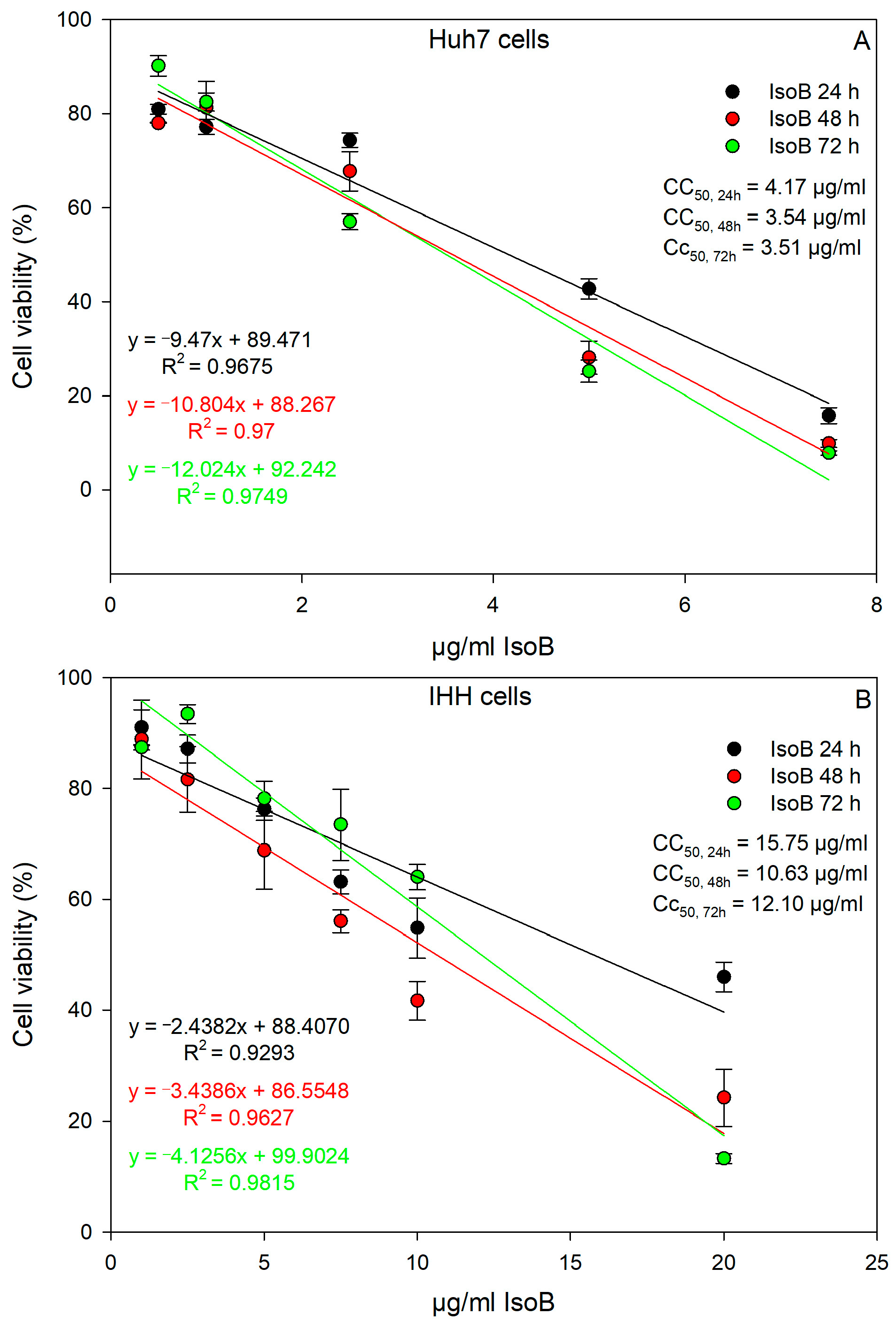
2.1. Dose- and Time-Dependent Cytotoxic Effects of IsoA, IsoB, and 5-FU on Huh7 Cells
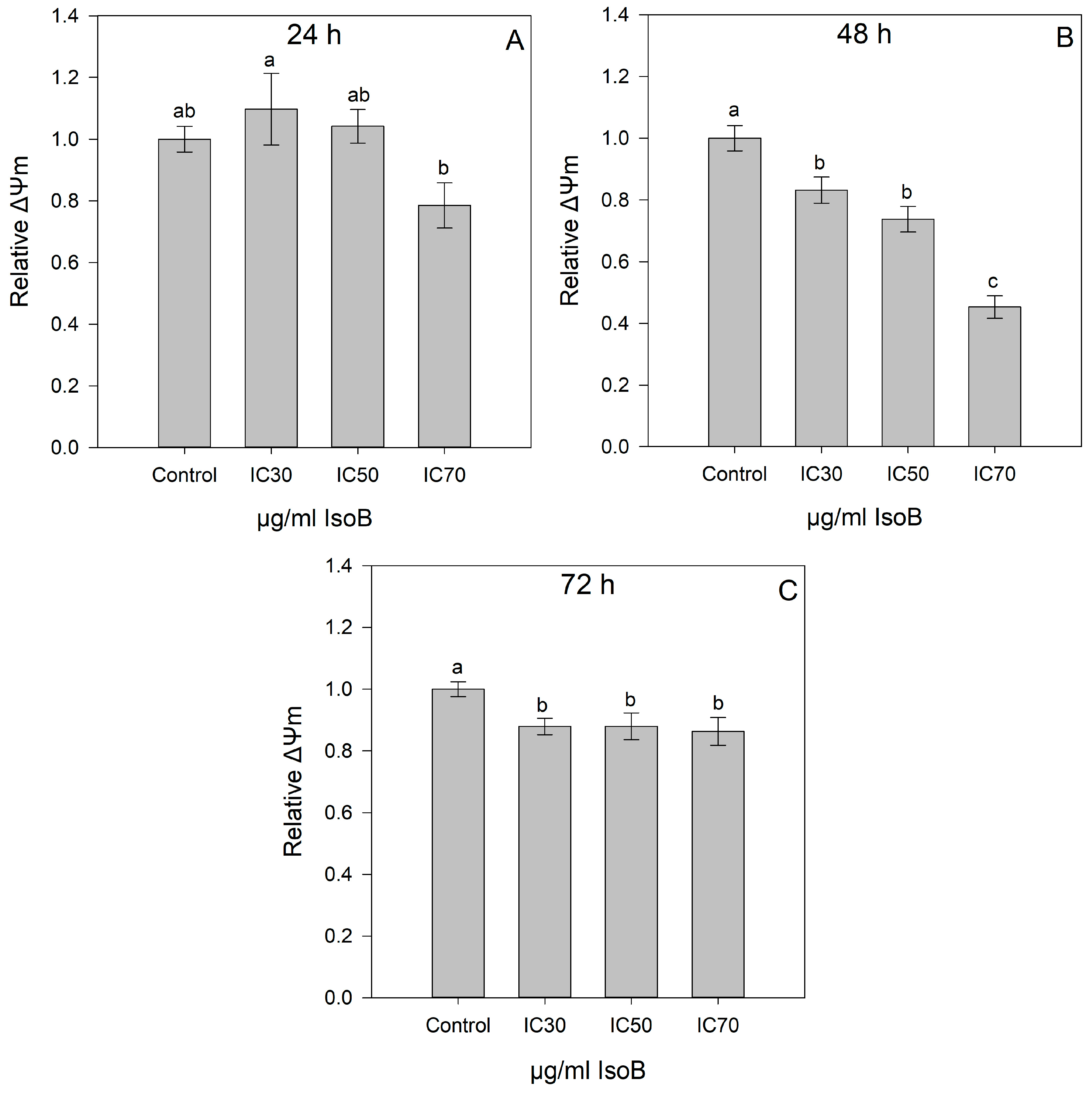
2.2. IsoB-Induced Effects on Membrane Potential and Mitochondrial Superoxide Production
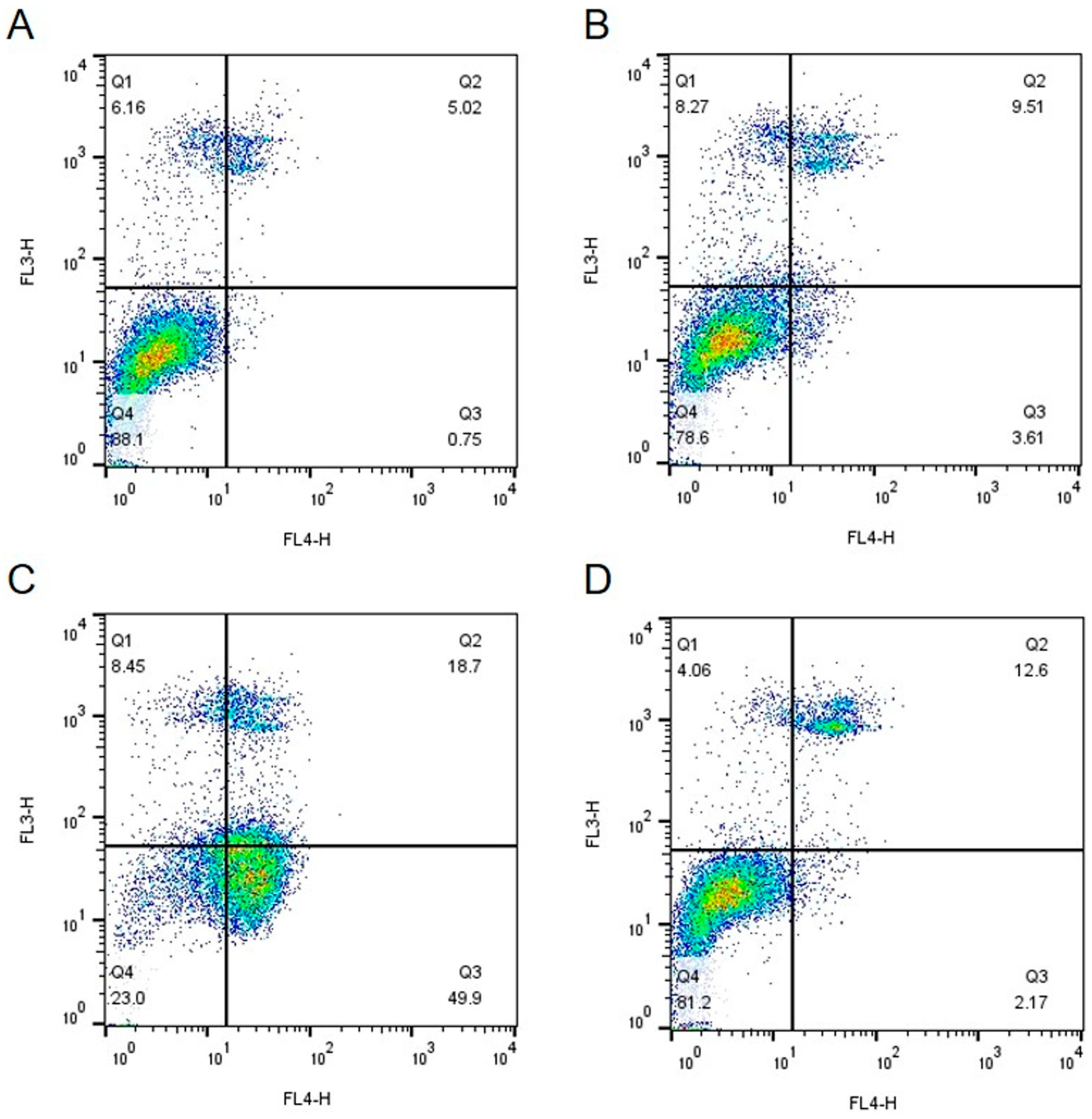
2.3. Gene Expression of IsoB-Treated Huh7 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.1.1. Isothiazolones
4.1.2. Cell Culture
4.1.3. Cell Viability Assessment
4.1.4. Fluorescence-Activated Cell Sorting (FACS) Analysis
4.1.5. Mitochondrial Membrane Potential (ΔΨm)
4.1.6. Mitochondrial Superoxide Levels
4.1.7. RNA Extraction and cDNA Synthesis
4.1.8. Real-Time Polymerase Chain Reaction (qPCR)
4.1.9. Data Analysis and Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maienfisch, P.; Edmunds, A.J.F. Chapter Three—Thiazole and Isothiazole Ring–Containing Compounds in Crop Protection. In Advances in Heterocyclic Chemistry; Scriven, E.F.V., Ramsden, C.A., Eds.; Heterocyclic Chemistry in the 21st Century; Academic Press: New York, NY, USA, 2017; Volume 121, pp. 35–88. ISBN 978-0-12-811174-1. [Google Scholar]
- Silva, M.G.D.; Cardoso, J.F.; Perasoli, F.B.; Branquinho, R.T.; Mourão, R.S.; Tavares, H.D.S.; Xocaira, M.L.C.T.; Guimarães, D.S.M.; Viana, G.H.R.; Varotti, F.D.P.; et al. Nanoemulsion Composed of 10-(4,5-Dihydrothiazol-2-Yl)Thio)Decan-1-Ol), a Synthetic Analog of 3-Alkylpiridine Marine Alkaloid: Development, Characterization, and Antimalarial Activity. Eur. J. Pharm. Sci. 2020, 151, 105382. [Google Scholar] [CrossRef]
- Khalaj, A.; Adibpour, N.; Shahverdi, A.R.; Daneshtalab, M. Synthesis and Antibacterial Activity of 2-(4-Substituted Phenyl)-3(2H)-Isothiazolones. Eur. J. Med. Chem. 2004, 39, 699–705. [Google Scholar] [CrossRef]
- Morley, J.O.; Kapur, A.J.O.; Charlton, M.H. Structure–Activity Relationships in 3-Isothiazolones. Org. Biomol. Chem. 2005, 3, 3713–3719. [Google Scholar] [CrossRef]
- Furdas, S.D.; Hoffmann, I.; Robaa, D.; Herquel, B.; Malinka, W.; Świątek, P.; Akhtar, A.; Sippl, W.; Jung, M. Pyrido- and Benzisothiazolones as Inhibitors of Histone Acetyltransferases (HATs). MedChemComm 2014, 5, 1856–1862. [Google Scholar] [CrossRef]
- Furdas, S.D.; Kannan, S.; Sippl, W.; Jung, M. Small Molecule Inhibitors of Histone Acetyltransferases as Epigenetic Tools and Drug Candidates. Arch. Pharm. 2012, 345, 7–21. [Google Scholar] [CrossRef]
- Hayakawa, N.; Nozawa, K.; Ogawa, A.; Kato, N.; Yoshida, K.; Akamatsu, K.; Tsuchiya, M.; Nagasaka, A.; Yoshida, S. Isothiazolone Derivatives Selectively Inhibit Telomerase from Human and Rat Cancer Cells in Vitro. Biochemistry 1999, 38, 11501–11507. [Google Scholar] [CrossRef]
- Mori, T.; Hidaka, M.; Lin, Y.-C.; Yoshizawa, I.; Okabe, T.; Egashira, S.; Kojima, H.; Nagano, T.; Koketsu, M.; Takamiya, M.; et al. A Dual Inhibitor against Prolyl Isomerase Pin1 and Cyclophilin Discovered by a Novel Real-Time Fluorescence Detection Method. Biochem. Biophys. Res. Commun. 2011, 406, 439–443. [Google Scholar] [CrossRef]
- Stimson, L.; Rowlands, M.G.; Newbatt, Y.M.; Smith, N.F.; Raynaud, F.I.; Rogers, P.; Bavetsias, V.; Gorsuch, S.; Jarman, M.; Bannister, A.; et al. Isothiazolones as Inhibitors of PCAF and P300 Histone Acetyltransferase Activity. Mol. Cancer Ther. 2005, 4, 1521–1532. [Google Scholar] [CrossRef]
- Nandakumar, N.; Gopinath, P.; Gopas, J.; Muraleedharan, K.M. Benzisothiazolone Derivatives Exhibit Cytotoxicity in Hodgkin’s Lymphoma Cells through NF-ΚB Inhibition and Are Synergistic with Doxorubicin and Etoposide. Anti-Cancer Agents Med. Chem.—Anti-Cancer Agents 2020, 20, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Gajer, J.M.; Furdas, S.D.; Gründer, A.; Gothwal, M.; Heinicke, U.; Keller, K.; Colland, F.; Fulda, S.; Pahl, H.L.; Fichtner, I.; et al. Histone Acetyltransferase Inhibitors Block Neuroblastoma Cell Growth in Vivo. Oncogenesis 2015, 4, e137. [Google Scholar] [CrossRef] [PubMed]
- Frosali, S.; Leonini, A.; Ettorre, A.; Di Maio, G.; Nuti, S.; Tavarini, S.; Di Simplicio, P.; Di Stefano, A. Role of Intracellular Calcium and S-Glutathionylation in Cell Death Induced by a Mixture of Isothiazolinones in HL60 Cells. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2009, 1793, 572–583. [Google Scholar] [CrossRef]
- Andreassi, M.; Ettorre, A.; Anselmi, C.; Stanghellini, E.; Tavarini, S.; Mariotti, G.; Andreassi, M.; Neri, P.; Stefano, A. A2. Cytotoxic Effects of a Mixture of Isothiazolinones on Normal Human Keratinocytes: In Vitro Induction of Apoptosis vs. Necrosis. J. Cosmet. Dermatol. 2002, 1, 105–110. [Google Scholar] [CrossRef]
- Zaharia, V.; Ignat, A.; Palibroda, N.; Ngameni, B.; Kuete, V.; Fokunang, C.N.; Moungang, M.L.; Ngadjui, B.T. Synthesis of Some p-Toluenesulfonyl-Hydrazinothiazoles and Hydrazino-Bis-Thiazoles and Their Anticancer Activity. Eur. J. Med. Chem. 2010, 45, 5080–5085. [Google Scholar] [CrossRef]
- Al-Salmi, F.A.; Alrohaimi, A.H.; Behery, M.E.; Megahed, W.; Abu Ali, O.A.; Elsaid, F.G.; Fayad, E.; Mohammed, F.Z.; Keshta, A.T. Anticancer Studies of Newly Synthesized Thiazole Derivatives: Synthesis, Characterization, Biological Activity, and Molecular Docking. Crystals 2023, 13, 1546. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Hopkins, B.D.; Goncalves, M.D.; Cantley, L.C. Obesity and Cancer Mechanisms: Cancer Metabolism. J. Clin. Oncol. 2016, 34, 4277–4283. [Google Scholar] [CrossRef]
- Viale, P.H. The American Cancer Society’s Facts & Figures: 2020 Edition. J. Adv. Pract. Oncol. 2020, 11, 135–136. [Google Scholar] [CrossRef]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global Burden of Primary Liver Cancer in 2020 and Predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef]
- Man, S.; Luo, C.; Yan, M.; Zhao, G.; Ma, L.; Gao, W. Treatment for Liver Cancer: From Sorafenib to Natural Products. Eur. J. Med. Chem. 2021, 224, 113690. [Google Scholar] [CrossRef]
- Niu, Z.-S.; Niu, X.-J.; Wang, W.-H. Genetic Alterations in Hepatocellular Carcinoma: An Update. World J. Gastroenterol. 2016, 22, 9069–9095. [Google Scholar] [CrossRef]
- Niu, M.; Yi, M.; Li, N.; Wu, K.; Wu, K. Advances of Targeted Therapy for Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 719896. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Yang, X.-R.; Chung, W.-Y.; Dennison, A.R.; Zhou, J. Targeted Therapy for Hepatocellular Carcinoma. Signal Transduct. Target. Ther. 2020, 5, 146. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Morizane, C.; Ueno, M.; Okusaka, T.; Ishii, H.; Furuse, J. Chemotherapy for Hepatocellular Carcinoma: Current Status and Future Perspectives. Jpn. J. Clin. Oncol. 2018, 48, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.Z.; Jubeen, F.; Sher, F. Future of 5-Fluorouracil in Cancer Therapeutics, Current Pharmacokinetics Issues and a Way Forward. J. Cancer Res. Pract. 2019, 6, 155. [Google Scholar] [CrossRef]
- Feng, M.Y.; Chan, L.L.; Chan, S.L. Drug Treatment for Advanced Hepatocellular Carcinoma: First-Line and Beyond. Curr. Oncol. 2022, 29, 5489–5507. [Google Scholar] [CrossRef] [PubMed]
- Hamilakis, S.; Kontonassios, D.; Tsolomitis, A. The Synthesis of N-Substituted Isothiazol-3(2H)-Ones from Nsubstituted 3-Benzoylpropionamides. J. Heterocycl. Chem. 2002, 39, 149–155. [Google Scholar] [CrossRef]
- Silva, V.; Silva, C.; Soares, P.; Garrido, E.M.; Borges, F.; Garrido, J. Isothiazolinone Biocides: Chemistry, Biological, and Toxicity Profiles. Molecules 2020, 25, 991. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, R.; Zhou, H.; Yang, S.; Tao, F.; Xie, Y.; Wang, H.; Yun, J. A Novel Benzothiazole Derivative Induces Apoptosis via the Mitochondrial Intrinsic Pathway Producing Antitumor Activity in Colorectal Cancer. Front. Pharmacol. 2023, 14, 1196158. [Google Scholar] [CrossRef] [PubMed]
- Kungyal, T.; Mutahar, A.Z.; Salimath, B.P. Computational, In Vitro and In Vivo Studies to Evaluate Anti-Cancer Activity of Benzisothiazole Derivative. Pharma Innov. 2019, 8, 272–278. [Google Scholar]
- Cao, L.; Wang, X.; Wang, Q.; Xue, P.; Jiao, X.; Peng, H.; Lu, H.; Zheng, Q.; Chen, X.; Huang, X.; et al. Rosiglitazone Sensitizes Hepatocellular Carcinoma Cell Lines to 5-Fluorouracil Antitumor Activity through Activation of the PPARγ Signaling Pathway. Acta Pharmacol. Sin. 2009, 30, 1316–1322. [Google Scholar] [CrossRef]
- Duan, X.; Xu, W.; Li, H.; Wang, M.; Wang, W.; Lu, H.; Zhang, Y.; Han, X. Nrf2-siRNA Enhanced the Anti-Tumor Effects of As2O3 in 5-Fluorouracil-Resistant Hepatocellular Carcinoma by Inhibiting HIF-1α/HSP70 Signaling. J. Hepatocell. Carcinoma 2022, 9, 1341–1352. [Google Scholar] [CrossRef]
- Kim, J.B.; Park, S.-Y.; Kim, H.R.; Ahn, Y.H.; Jee, H.-G.; Lee, J.-H.; Yu, S.J.; Lee, H.-S.; Lee, M.; Yoon, J.-H.; et al. JNK Signaling in Hepatocarcinoma Cells Is Associated with the Side Population upon Treatment with Anticancer Drugs. Mol. Med. Rep. 2015, 11, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Arning, J.; Dringen, R.; Schmidt, M.; Thiessen, A.; Stolte, S.; Matzke, M.; Bottin-Weber, U.; Caesar-Geertz, B.; Jastorff, B.; Ranke, J. Structure-Activity Relationships for the Impact of Selected Isothiazol-3-One Biocides on Glutathione Metabolism and Glutathione Reductase of the Human Liver Cell Line Hep G2. Toxicology 2008, 246, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Arning, J.; Matzke, M.; Stolte, S.; Nehen, F.; Bottin-Weber, U.; Böschen, A.; Abdulkarim, S.; Jastorff, B.; Ranke, J. Analyzing Cytotoxic Effects of Selected Isothiazol-3-One Biocides Using the Toxic Ratio Concept and Structure-Activity Relationship Considerations. Chem. Res. Toxicol. 2009, 22, 1954–1961. [Google Scholar] [CrossRef] [PubMed]
- Dekker, F.J.; Ghizzoni, M.; van der Meer, N.; Wisastra, R.; Haisma, H.J. Inhibition of the PCAF Histone Acetyl Transferase and Cell Proliferation by Isothiazolones. Bioorg. Med. Chem. 2009, 17, 460–466. [Google Scholar] [CrossRef]
- Gorsuch, S.; Bavetsias, V.; Rowlands, M.G.; Aherne, G.W.; Workman, P.; Jarman, M.; McDonald, E. Synthesis of Isothiazol-3-One Derivatives as Inhibitors of Histone Acetyltransferases (HATs). Bioorg. Med. Chem. 2009, 17, 467–474. [Google Scholar] [CrossRef]
- Sharma, S. Thiazoles: A Retrospective Study on Synthesis, Structure-Activity Relationship and Therapeutic Significance. Indian J. Pharm. Educ. Res. 2022, 56, 646–666. [Google Scholar] [CrossRef]
- Dawood, K.M.; Raslan, M.A.; Abbas, A.A.; Mohamed, B.E.; Abdellattif, M.H.; Nafie, M.S.; Hassan, M.K. Novel Bis-Thiazole Derivatives: Synthesis and Potential Cytotoxic Activity through Apoptosis with Molecular Docking Approaches. Front. Chem. 2021, 9, 694870. [Google Scholar] [CrossRef]
- Lu, H.; Rogowskyj, J.; Yu, W.; Venkatesh, A.; Khan, N.; Nakagawa, S.; Goossens, N.; Koh, A.P.; Higashi, T.; Gunasekaran, G.; et al. Novel Substituted Aminothiazoles as Potent and Selective Anti-Hepatocellular Carcinoma Agents. Bioorg. Med. Chem. Lett. 2016, 26, 5819–5824. [Google Scholar] [CrossRef]
- Babich, H.; Zuckerbraun, H.L.; Wurzburger, B.J.; Rubin, Y.L.; Borenfreund, E.; Blau, L. Benzoyl Peroxide Cytotoxicity Evaluated in Vitro with the Human Keratinocyte Cell Line, RHEK-1. Toxicology 1996, 106, 187–196. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Petrelli, D.; Vitali, L.A.; Bonacucina, G.; Cespi, M.; Vllasaliu, D.; Giorgioni, G.; Palmieri, G.F. Quaternary Ammonium Leucine-Based Surfactants: The Effect of a Benzyl Group on Physicochemical Properties and Antimicrobial Activity. Pharmaceutics 2019, 11, 287. [Google Scholar] [CrossRef]
- Morio, B.; Panthu, B.; Bassot, A.; Rieusset, J. Role of Mitochondria in Liver Metabolic Health and Diseases. Cell Calcium 2021, 94, 102336. [Google Scholar] [CrossRef]
- Grasso, D.; Zampieri, L.X.; Capelôa, T.; Van de Velde, J.A.; Sonveaux, P. Mitochondria in Cancer. Cell Stress 2020, 4, 114–146. [Google Scholar] [CrossRef]
- Ghosh, P.; Vidal, C.; Dey, S.; Zhang, L. Mitochondria Targeting as an Effective Strategy for Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 3363. [Google Scholar] [CrossRef]
- Kim, D.; Kim, E.-H.; Choi, S.; Lim, K.-M.; Tie, L.; Majid, A.; Bae, O.-N. A Commonly Used Biocide 2-N-Octyl-4-Isothiazolin-3-oneInduces Blood–Brain Barrier Dysfunction via Cellular Thiol Modification and Mitochondrial Damage. Int. J. Mol. Sci. 2021, 22, 2563. [Google Scholar] [CrossRef]
- Kim, D.; Shin, Y.; Kim, E.-H.; Lee, Y.; Kim, S.; Kim, H.S.; Kim, H.-C.; Leem, J.-H.; Kim, H.R.; Bae, O.-N. Functional and Dynamic Mitochondrial Damage by Chloromethylisothiazolinone/Methylisothiazolinone (CMIT/MIT) Mixture in Brain Endothelial Cell Lines and Rat Cerebrovascular Endothelium. Toxicol. Lett. 2022, 366, 45–57. [Google Scholar] [CrossRef]
- Park, E.-J.; Seong, E. Methylisothiazolinone Induces Apoptotic Cell Death via Matrix Metalloproteinase Activation in Human Bronchial Epithelial Cells. Toxicol. In Vitro 2020, 62, 104661. [Google Scholar] [CrossRef]
- Do, V.Q.; Seo, Y.-S.; Park, J.-M.; Yu, J.; Duong, M.T.H.; Nakai, J.; Kim, S.-K.; Ahn, H.-C.; Lee, M.-Y. A Mixture of Chloromethylisothiazolinone and Methylisothiazolinone Impairs Rat Vascular Smooth Muscle by Depleting Thiols and Thereby Elevating Cytosolic Zn2+ and Generating Reactive Oxygen Species. Arch. Toxicol. 2021, 95, 541–556. [Google Scholar] [CrossRef]
- Clarke, M.F. Clinical and Therapeutic Implications of Cancer Stem Cells. N. Engl. J. Med. 2019, 380, 2237–2245. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, H.; Hashemi, S.R.; Seow, H.F. 5-Fluorouracil Induce the Expression of TLR4 on HCT116 Colorectal Cancer Cell Line Expressing Different Variants of TLR4. Iran J. Pharm. Res. 2013, 12, 453–460. [Google Scholar] [PubMed]
- Wu, L.; Pan, C.; Wei, X.; Shi, Y.; Zheng, J.; Lin, X.; Shi, L. lncRNA KRAL Reverses 5-Fluorouracil Resistance in Hepatocellular Carcinoma Cells by Acting as a ceRNA against miR-141. Cell Commun. Signal. 2018, 16, 47. [Google Scholar] [CrossRef]
- Zhang, N.; Yin, Y.; Xu, S.-J.; Chen, W.-S. 5-Fluorouracil: Mechanisms of Resistance and Reversal Strategies. Molecules 2008, 13, 1551–1569. [Google Scholar] [CrossRef]
- Kiraz, Y.; Adan, A.; Kartal Yandim, M.; Baran, Y. Major Apoptotic Mechanisms and Genes Involved in Apoptosis. Tumor Biol. 2016, 37, 8471–8486. [Google Scholar] [CrossRef]
- Duffy, M.J.; Synnott, N.C.; O’Grady, S.; Crown, J. Targeting P53 for the Treatment of Cancer. Semin. Cancer Biol. 2022, 79, 58–67. [Google Scholar] [CrossRef]
- Jehan, S.; Zhong, C.; Li, G.; Zulqarnain Bakhtiar, S.; Li, D.; Sui, G. Thymoquinone Selectively Induces Hepatocellular Carcinoma Cell Apoptosis in Synergism with Clinical Therapeutics and Dependence of P53 Status. Front. Pharmacol. 2020, 11, 555283. [Google Scholar] [CrossRef]
- Kim, S.M.; Ha, S.E.; Lee, H.J.; Rampogu, S.; Vetrivel, P.; Kim, H.H.; Venkatarame Gowda Saralamma, V.; Lee, K.W.; Kim, G.S. Sinensetin Induces Autophagic Cell Death through P53-Related AMPK/mTOR Signaling in Hepatocellular Carcinoma HepG2 Cells. Nutrients 2020, 12, 2462. [Google Scholar] [CrossRef]
- Bressac, B.; Galvin, K.M.; Liang, T.J.; Isselbacher, K.J.; Wands, J.R.; Ozturk, M. Abnormal Structure and Expression of P53 Gene in Human Hepatocellular Carcinoma. Proc. Natl. Acad. Sci. USA 1990, 87, 1973–1977. [Google Scholar] [CrossRef]
- Muller, P.A.J.; Vousden, K.H. P53 Mutations in Cancer. Nat. Cell Biol. 2013, 15, 2–8. [Google Scholar] [CrossRef]
- Shen, Y.; White, E. P53-Dependent Apoptosis Pathways. Adv. Cancer Res. 2001, 82, 55–84. [Google Scholar] [CrossRef]
- Liu, F.; Liao, Z.; Zhang, Z. MYC in Liver Cancer: Mechanisms and Targeted Therapy Opportunities. Oncogene 2023, 42, 3303–3318. [Google Scholar] [CrossRef]
- Qin, X.-Y.; Gailhouste, L. Non-Genomic Control of Dynamic MYCN Gene Expression in Liver Cancer. Front. Oncol. 2021, 10, 618515. [Google Scholar] [CrossRef]
- Duffy, M.J.; O’Grady, S.; Tang, M.; Crown, J. MYC as a Target for Cancer Treatment. Cancer Treat. Rev. 2021, 94, 102154. [Google Scholar] [CrossRef]
- Li, H.; Ham, A.; Ma, T.C.; Kuo, S.-H.; Kanter, E.; Kim, D.; Ko, H.S.; Quan, Y.; Sardi, S.P.; Li, A.; et al. Mitochondrial Dysfunction and Mitophagy Defect Triggered by Heterozygous GBA Mutations. Autophagy 2019, 15, 113–130. [Google Scholar] [CrossRef]
- Polster, B.M.; Nicholls, D.G.; Ge, S.X.; Roelofs, B.A. Use of Potentiometric Fluorophores in the Measurement of Mitochondrial Reactive Oxygen Species. Methods Enzymol. 2014, 547, 225–250. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.L.; Moorman, A.F.M. Assumption-Free Analysis of Quantitative Real-Time Polymerase Chain Reaction (PCR) Data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef]





| Gene Symbol | Gene Name | Primer F (5′-3′) | Primer R (5′-3′) |
|---|---|---|---|
| ACTB | actin, beta | CTGTCCACCTTCCAGCAGATGT | AGCATTTGCGGTGGACGAT |
| BAX | BCL2-associated X protein | CGCCCTTTTCTACTTTGCCAG | GCCCATGATGGTTCTGATCAG |
| BID | BH3 interacting domain death agonist | TCCTTGCTCCGTGATGTCTTTC | AAGCTCCTCACGTAGGTGCGTA |
| BIRC3 | baculoviral IAP repeat containing 3 | TGCTTTTGCTGTGATGGTGG | CACTTGGCATGTTGAACCCA |
| CASP3 | caspase 3 | TGGAATTGATGCGTGATGTT | TGGCTCAGAAGCACACAAAC |
| CASP7 | caspase 7 | CTCTTCGCCTATTCCACGGTTC | TTGCACAAACCAGGAGCCTCT |
| CASP9 | caspase 9 | TGGACGACATCTTTGAGCAG | GCATTAGCGACCCTAAGCAG |
| CD44 | CD44 molecule | TCATCTTGGCATCCCTCTTG | CCATTGCCACTGTTGATCACTA |
| FAS | Fas cell surface death receptor | GTGAACACTGTGACCCTTGCAC | TTGGTGTTGCTGGTGAGTGTG |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase | TTGCCCTCAACGACCACTTT | CACCCTGTTGCTGTAGCCAAA |
| MYCN | MYCN proto-oncogene, bHLH transcription factor | CCCCTGGGTCTGCCCCGTTT | GCCGAAGTAGAAGTCATCTT |
| TP53 | tumor protein p53 | CCAGCCAAAGAAGAAACCACTG | TCAGCTCTCGGAACATCTCGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marka, S.; Zografaki, M.-E.; Tsolomiti, G.; Kalliampakou, K.I.; Tsolomitis, A.; Koumantou, C.; Smirlis, D.; Vassilaki, N.; Kintzios, S. 2-(4-Nitrophenyl)isothiazol-3(2H)-one: A Promising Selective Agent against Hepatocellular Carcinoma Cells. Pharmaceuticals 2024, 17, 673. https://doi.org/10.3390/ph17060673
Marka S, Zografaki M-E, Tsolomiti G, Kalliampakou KI, Tsolomitis A, Koumantou C, Smirlis D, Vassilaki N, Kintzios S. 2-(4-Nitrophenyl)isothiazol-3(2H)-one: A Promising Selective Agent against Hepatocellular Carcinoma Cells. Pharmaceuticals. 2024; 17(6):673. https://doi.org/10.3390/ph17060673
Chicago/Turabian StyleMarka, Sofia, Maria-Eleftheria Zografaki, Georgia Tsolomiti, Katerina I. Kalliampakou, Athanasios Tsolomitis, Christina Koumantou, Despina Smirlis, Niki Vassilaki, and Spyros Kintzios. 2024. "2-(4-Nitrophenyl)isothiazol-3(2H)-one: A Promising Selective Agent against Hepatocellular Carcinoma Cells" Pharmaceuticals 17, no. 6: 673. https://doi.org/10.3390/ph17060673
APA StyleMarka, S., Zografaki, M.-E., Tsolomiti, G., Kalliampakou, K. I., Tsolomitis, A., Koumantou, C., Smirlis, D., Vassilaki, N., & Kintzios, S. (2024). 2-(4-Nitrophenyl)isothiazol-3(2H)-one: A Promising Selective Agent against Hepatocellular Carcinoma Cells. Pharmaceuticals, 17(6), 673. https://doi.org/10.3390/ph17060673








