The Therapeutic Potential of Harpagophytum procumbens and Turnera subulata and Advances in Nutraceutical Delivery Systems in Neurodegenerative Diseases
Abstract
1. Introduction
2. Therapeutic Properties of Harpagophytum procumbens and Turnera subulata
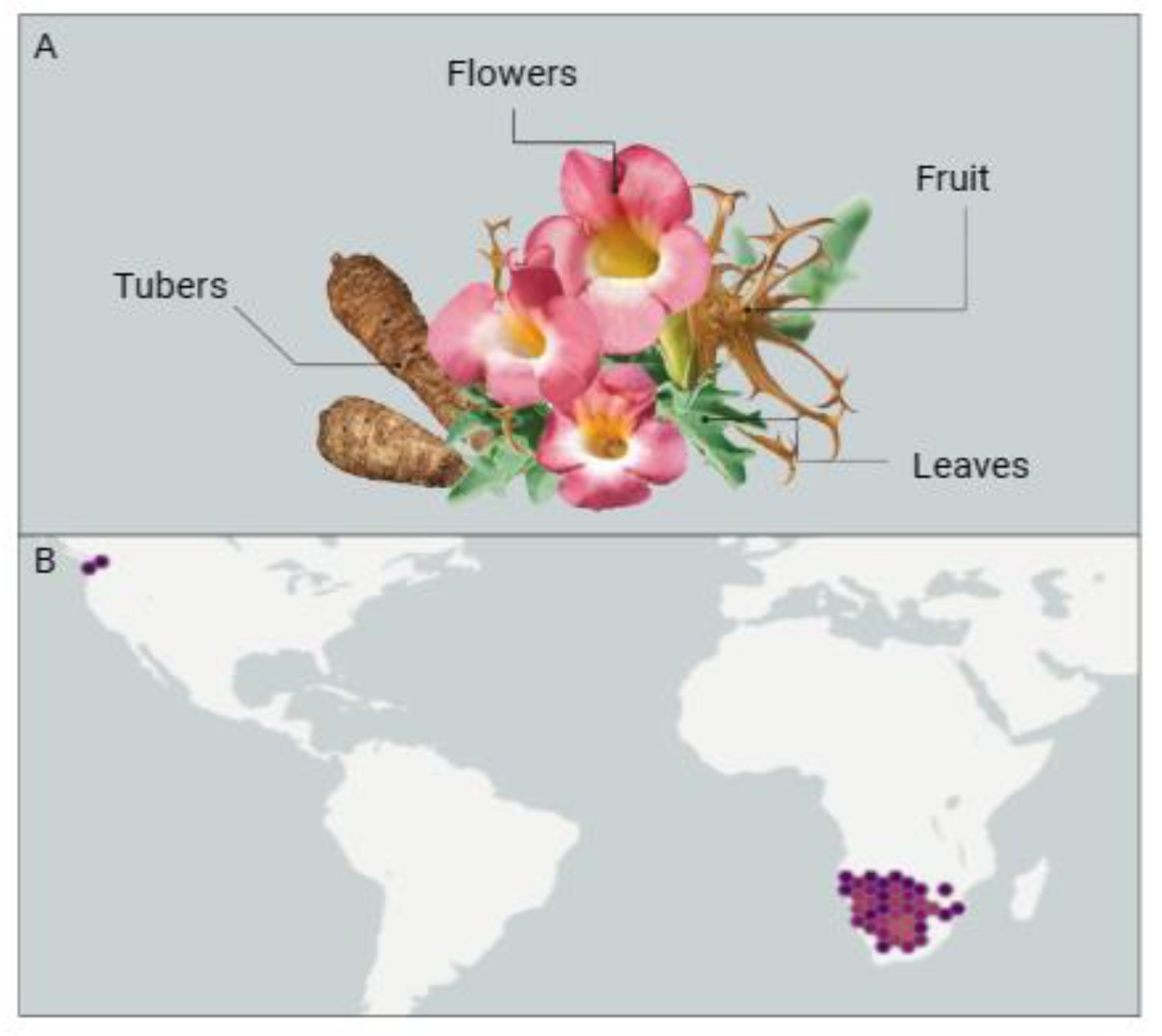
2.1. Harpagophytum procumbens
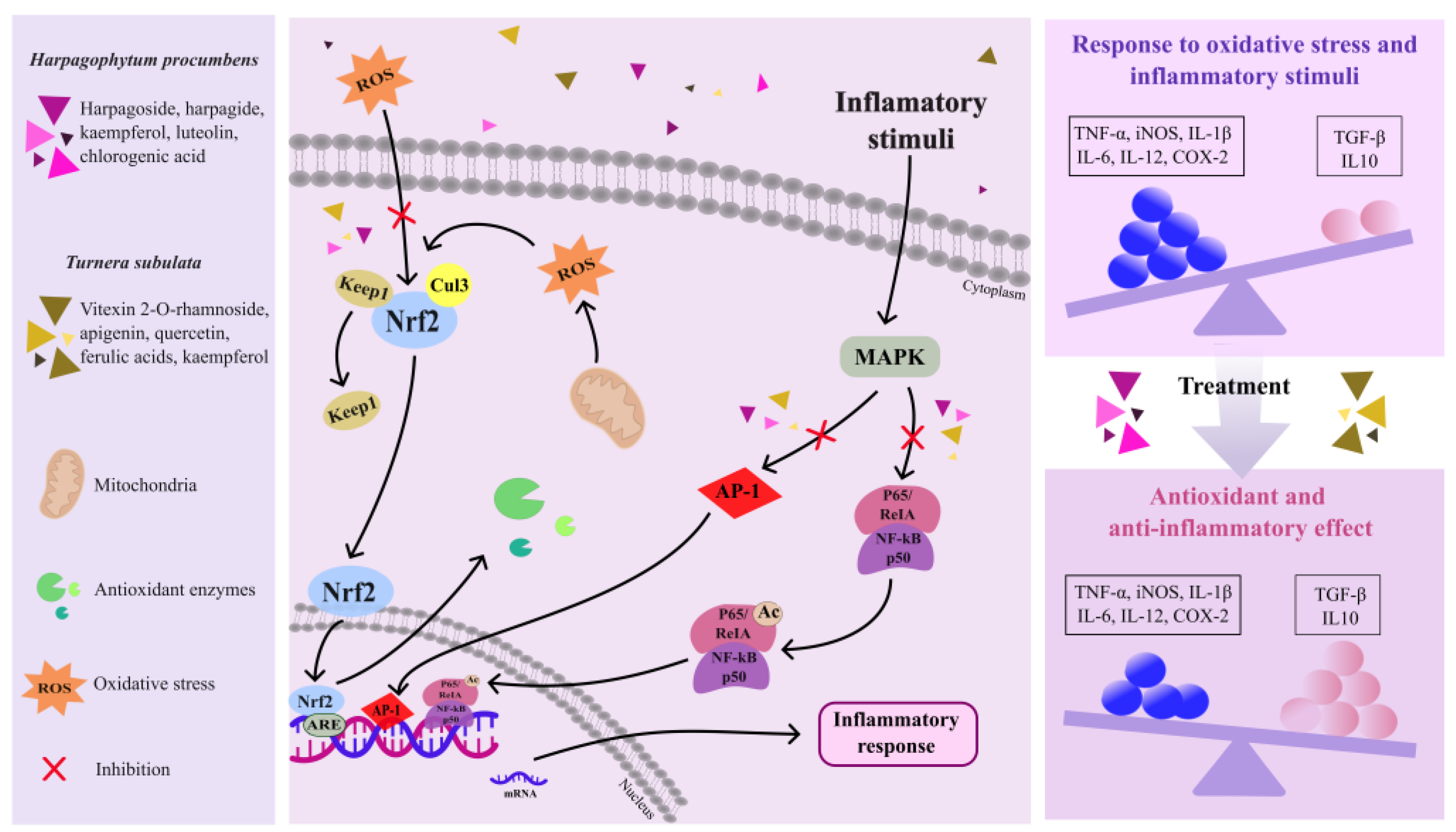
2.1.1. Anti-Inflammatory Activity
2.1.2. Antioxidant Activity
2.1.3. Neuroprotective Activity
2.2. Turnera subulata
3. Nutraceutical Delivery Systems
- Reduction in systemic inflammation: This involves neutralizing free radicals and suppressing proinflammatory cytokines in the body’s periphery, which, in turn, alleviates brain inflammation by acting on the BBB.
- Enhancement in BBB integrity: This aims to prevent the brain inflammation associated with increased BBB permeability.
- Ensuring penetration into the brain parenchyma: Enabling the targeting of glial cells to reduce brain inflammation.
- Gastrointestinal protection and regulation of the gut–brain axis: This includes minimizing limitations such as solubility, chemical instability, bitter taste, and unpleasant odor, as well as overcoming gastrointestinal membrane barriers, the influence of other chemical components, and gastric residence time.
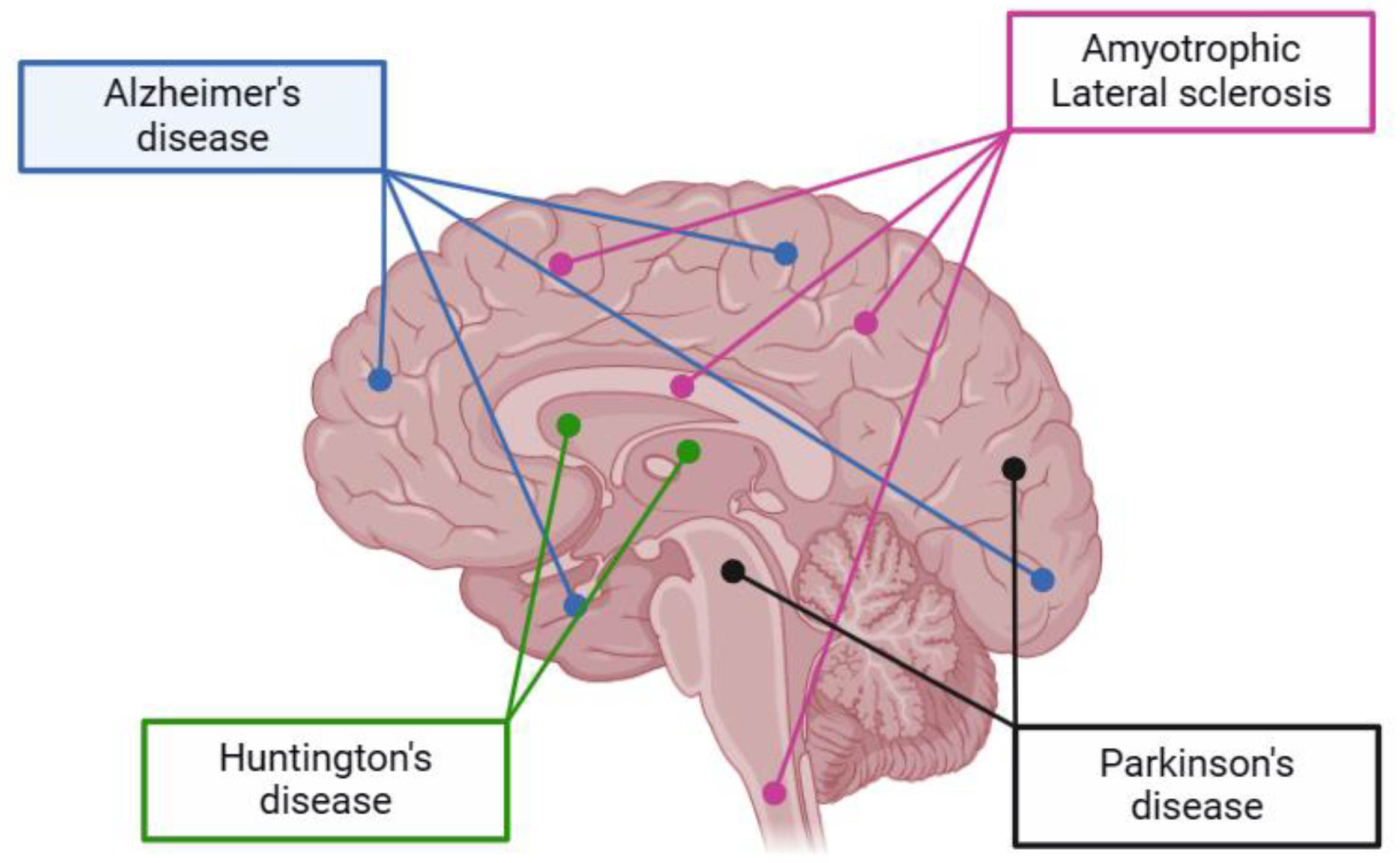
4. Impact of Nutraceuticals on Neurodegenerative Diseases
5. Nutraceuticals from Harpagophytum procumbens and Turnera subulata in Neurodegenerative Diseases: Challenges and Future
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adampourezare, M.; Hasanzadeh, M.; Nikzad, B. Recent Progress and Challenges in the Application of Molecularly Imprinted Polymers for Early-Stage Screening of Neurodegenerative Diseases-Related Protein Biomarkers. Microchem. J. 2023, 192, 108931. [Google Scholar] [CrossRef]
- Jog, N.R.; McClain, M.T.; Heinlen, L.D.; Gross, T.; Towner, R.; Guthridge, J.M.; Axtell, R.C.; Pardo, G.; Harley, J.B.; James, J.A. Epstein Barr Virus Nuclear Antigen 1 (EBNA-1) Peptides Recognized by Adult Multiple Sclerosis Patient Sera Induce Neurologic Symptoms in a Murine Model. J. Autoimmun. 2020, 106, 102332. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Gardner, L.; Douglas, J.; Meyers, L.; Lee, S.; Shin, Y. Neurodegeneration in Multiple Sclerosis Involves Multiple Pathogenic Mechanisms. Degener. Neurol. Neuromuscul. Dis. 2014, 49, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Sharma, N.; Khera, R.; Gupta, R.; Mehan, S. Guggulsterone Ameliorates Ethidium Bromide-Induced Experimental Model of Multiple Sclerosis via Restoration of Behavioral, Molecular, Neurochemical and Morphological Alterations in Rat Brain. Metab. Brain Dis. 2021, 36, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Hittle, M.; Culpepper, W.J.; Langer-Gould, A.; Marrie, R.A.; Cutter, G.R.; Kaye, W.E.; Wagner, L.; Topol, B.; LaRocca, N.G.; Nelson, L.M.; et al. Population-Based Estimates for the Prevalence of Multiple Sclerosis in the United States by Race, Ethnicity, Age, Sex, and Geographic Region. JAMA Neurol. 2023, 80, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Guo, M.-S.; Zhang, Y.; Yu, L.; Wu, J.-M.; Tang, Y.; Ai, W.; Zhu, F.-D.; Law, B.Y.-K.; Chen, Q.; et al. Dietary Plant Polyphenols as the Potential Drugs in Neurodegenerative Diseases: Current Evidence, Advances, and Opportunities. Oxid. Med. Cell. Longev. 2022, 2022, 5288698. [Google Scholar] [CrossRef]
- Hu, M.-L.; Pan, Y.-R.; Yong, Y.-Y.; Liu, Y.; Yu, L.; Qin, D.-L.; Qiao, G.; Law, B.Y.-K.; Wu, J.-M.; Zhou, X.-G.; et al. Poly (ADP-Ribose) Polymerase 1 and Neurodegenerative Diseases: Past, Present, and Future. Ageing Res. Rev. 2023, 91, 102078. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, M.; Senthilkumar, P.; Chinnadurai, V.; Murugesan Sakthivel, K.; Rajeshkumar, R.; Pugazhendhi, A. Antiangiogenic, Anti-Inflammatory and Their Antioxidant Activities of Turnera subulata Sm. (Turneraceae). Process Biochem. 2020, 89, 71–80. [Google Scholar] [CrossRef]
- Tsirigotis-Maniecka, M. Alginate-, Carboxymethyl Cellulose-, and κ-Carrageenan-Based Microparticles as Storage Vehicles for Cranberry Extract. Molecules 2020, 25, 3998. [Google Scholar] [CrossRef]
- Al Jayoush, A.R.; Hassan, H.A.F.M.; Asiri, H.; Jafar, M.; Saeed, R.; Harati, R.; Haider, M. Niosomes for Nose-to-Brain Delivery: A Non-Invasive Versatile Carrier System for Drug Delivery in Neurodegenerative Diseases. J. Drug Deliv. Sci. Technol. 2023, 89, 105007. [Google Scholar] [CrossRef]
- Mncwangi, N.; Chen, W.; Vermaak, I.; Viljoen, A.M.; Gericke, N. Devil’s Claw—A Review of the Ethnobotany, Phytochemistry and Biological Activity of Harpagophytum procumbens. J. Ethnopharmacol. 2012, 143, 755–771. [Google Scholar] [CrossRef]
- GBIF.org GBIF Occurrence Download 2023. Available online: https://www.gbif.org/ (accessed on 27 October 2023).
- Stewart, K.M.; Cole, D. The Commercial Harvest of Devil’s Claw (Harpagophytum Spp.) in Southern Africa: The Devil’s in the Details. J. Ethnopharmacol. 2005, 100, 225–236. [Google Scholar] [CrossRef]
- Cholet, J.; Decombat, C.; Vareille-Delarbre, M.; Gainche, M.; Berry, A.; Ogéron, C.; Ripoche, I.; Delort, L.; Vermerie, M.; Frai, D. Comparison of the Anti-Inflammatory and Immunomodulatory Mechanisms of Two Medicinal Herbs: Meadowsweet (Filipendula ulmaria) and Harpagophytum (Harpagophytum procumbens). Int. J. Plant Anim. Environ. Sci. 2019, 9, 145–163. [Google Scholar]
- Muzila, M.; Ekholm, A.; Nybom, H.; Widén, C.; Rumpunen, K. Harpagophytum Germplasm Varies in Tuber Peel and Pulp Content of Important Phenylpropanoids and Iridoids. S. Afr. J. Bot. 2018, 115, 153–160. [Google Scholar] [CrossRef]
- Mariano, A.; Bigioni, I.; Mattioli, R.; Di Sotto, A.; Leopizzi, M.; Garzoli, S.; Mariani, P.F.; Dalla Vedova, P.; Ammendola, S.; Scotto d’Abusco, A. Harpagophytum Procumbens Root Extract Mediates Anti-Inflammatory Effects in Osteoarthritis Synoviocytes through CB2 Activation. Pharmaceuticals 2022, 15, 457. [Google Scholar] [CrossRef]
- Rahimi, A.; Razmkhah, K.; Mehrnia, M.; Mohamadnia, A.; Sahebjamee, H.; Salehi, S.; Asl, E.A.; Tahmasebi, H.; Shandiz, S.A.S.; Davouodbeglou, F.; et al. Molecular Docking and Binding Study of Harpagoside and Harpagide as Novel Anti-Inflammatory and Anti-Analgesic Compound from Harpagophytum procumbens Based on Their Interactions with COX-2 Enzyme. Asian Pac. J. Trop. Dis. 2016, 6, 227–231. [Google Scholar] [CrossRef]
- Parenti, C.; Aricò, G.; Pennisi, M.; Venditti, A.; Scoto, G.M. Harpagophytum procumbens Extract Potentiates Morphine Antinociception in Neuropathic Rats. Nat. Prod. Res. 2016, 30, 1248–1255. [Google Scholar] [CrossRef]
- Gxaba, N.; Manganyi, M.C. The Fight against Infection and Pain: Devil’s Claw (Harpagophytum procumbens) a Rich Source of Anti-Inflammatory Activity: 2011–2022. Molecules 2022, 27, 3637. [Google Scholar] [CrossRef]
- Manon, L.; Béatrice, B.; Thierry, O.; Jocelyne, P.; Fathi, M.; Evelyne, O.; Alain, B. Antimutagenic Potential of Harpagoside and Harpagophytum procumbens against 1-Nitropyrene. Pharmacogn. Mag. 2015, 11, 29. [Google Scholar] [CrossRef]
- Bojnurd, I. Evaluation of in Vitro Anti-Inflammatory Activity of Harpagophytum procumbens and Urtica Dioica against the Denaturation of Protein. Plant Arch. 2018, 18, 161–166. [Google Scholar]
- Menghini, L.; Recinella, L.; Leone, S.; Chiavaroli, A.; Cicala, C.; Brunetti, L.; Vladimir-Knežević, S.; Orlando, G.; Ferrante, C. Devil’s Claw (Harpagophytum procumbens) and Chronic Inflammatory Diseases: A Concise Overview on Preclinical and Clinical Data. Phytother. Res. 2019, 33, 2152–2162. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Muñoz, E.; Rose, T.; Weiss, G.; McGregor, G.P. Molecular Targets of the Antiinflammatory Harpagophytum procumbens (Devil’s Claw): Inhibition of TNFα and COX-2 Gene Expression by Preventing Activation of AP-1. Phytother. Res. 2012, 26, 806–811. [Google Scholar] [CrossRef]
- Brendler, T. From Bush Medicine to Modern Phytopharmaceutical: A Bibliographic Review of Devil’s Claw (Harpagophytum Spp.). Pharmaceuticals 2021, 14, 726. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.M.J.; Bugert, J.; Denyer, S.P.; Heard, C.M. Anti-Inflammatory Activity of Punica granatum L. (Pomegranate) Rind Extracts Applied Topically to Ex Vivo Skin. Eur. J. Pharm. Biopharm. 2017, 112, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Cuspidi, C.; Sala, C.; Tadic, M.; Grassi, G.; Mancia, G. Systemic Hypertension Induced by Harpagophytum procumbens (Devil’s Claw): A Case Report. J. Clin. Hypertens. 2015, 17, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Mncwangi, N.; Chen, W.; Mulaudzi, N.; Vermaak, I.; Viljoen, A. Harpagophytum procumbens. In The South African Herbal Pharmacopoeia; Elsevier: Amsterdam, The Netherlands, 2023; pp. 211–246. [Google Scholar]
- Kriukova, A.; Lytkin, D.; Marchenko, M.; Vladymyrova, I. Development of Green Production Technology and Research of Harpagophytum procumbens Root Dry Extract. Sci. Pharm. Sci. 2021, 4, 43–49. [Google Scholar]
- Vreju, F.; Ciurea, P.; Rosu, A.; Chisalau, B.; Parvanescu, C.; Firulescu, S.; Stiolica, A.; Barbulescu, A.; Dinescu, S.; Dumitrescu, C.; et al. The Effect of Glucosamine, Chondroitin and Harpagophytum procumbens on Femoral Hyaline Cartilage Thickness in Patients with Knee Osteoarthritis–An MRI versus Ultrasonography Study. J. Mind Med. Sci. 2019, 6, 162–168. [Google Scholar] [CrossRef]
- Quarta, S.; Santarpino, G.; Carluccio, M.A.; Calabriso, N.; Scoditti, E.; Siculella, L.; Damiano, F.; Maffia, M.; Verri, T.; De Caterina, R.; et al. Analysis of the Anti-Inflammatory and Anti-Osteoarthritic Potential of Flonat Fast®, a Combination of Harpagophytum procumbens DC. Ex Meisn., Boswellia serrata Roxb., Curcuma longa L., Bromelain and Escin (Aesculus hippocastanum), Evaluated in In Vitro Models of Inflammation Relevant to Osteoarthritis. Pharmaceuticals 2022, 15, 1263. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.B.; Viana, A.R.; Santos, D.; Felipetto, N.; Mezzomo, N.F.; Zago, A.M.; Flores, E.M.M.; Machado, A.K.; Krause, A.; Peroza, L.R.; et al. Ethyl Acetate Fraction of Harpagophytum procumbens Prevents Oxidative Stress In Vitro and Amphetamine-Induced Alterations in Mice Behavior. Neurochem. Res. 2023, 48, 1716–1727. [Google Scholar] [CrossRef]
- Grąbkowska, R.; Matkowski, A.; Grzegorczyk-Karolak, I.; Wysokińska, H. Callus Cultures of Harpagophytum procumbens (Burch.) DC. Ex Meisn.; Production of Secondary Metabolites and Antioxidant Activity. S. Afr. J. Bot. 2016, 103, 41–48. [Google Scholar] [CrossRef]
- Abdulhussein, A.J.; HattabMutlag, S.; Khamees, A.H.; Sahib, H.; Ghazi, M.F. Evaluation of Antiangiogenic and Antioxidant Activity of Harpagophytum procumbens (Devil’s Claw). Drug Invent. Today 2018, 10, 3542–3545. [Google Scholar]
- Avato, P.; Argentieri, M.P. Quality Assessment of Commercial Spagyric Tinctures of Harpagophytum procumbens and Their Antioxidant Properties. Molecules 2019, 24, 2251. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, L.F.; de Freitas, C.M.; Chiapinotto Ceretta, A.P.; Peroza, L.R.; de Moraes Reis, E.; Krum, B.N.; Busanello, A.; Boligon, A.A.; Sudati, J.H.; Fachinetto, R.; et al. Harpagophytum procumbens Ethyl Acetate Fraction Reduces Fluphenazine-Induced Vacuous Chewing Movements and Oxidative Stress in Rat Brain. Neurochem. Res. 2016, 41, 1170–1184. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Are Polyphenols Antioxidants or Pro-Oxidants? What Do We Learn from Cell Culture and in Vivo Studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Abib, R.T.; Quincozes-Santos, A.; Zanotto, C.; Zeidán-Chuliá, F.; Lunardi, P.S.; Gonçalves, C.-A.; Gottfried, C. Genoprotective Effects of the Green Tea-Derived Polyphenol/Epicatechin Gallate in C6 Astroglial Cells. J. Med. Food 2010, 13, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.S.; Guimarães, A.G.; Santana, M.F.; Siqueira, R.S.; De Lima, A.D.C.B.; Dias, A.S.; Santos, M.R.V.; Onofre, A.S.C.; Quintans, J.S.S.; De Sousa, D.P.; et al. Anti-Inflammatory and Redox-Protective Activities of Citronellal. Biol. Res. 2011, 44, 363–368. [Google Scholar] [CrossRef]
- Quintans-Júnior, L.J.; Oliveira, M.G.B.; Santana, M.F.; Santana, M.T.; Guimarães, A.G.; Siqueira, J.S.; De Sousa, D.P.; Almeida, R.N. α-Terpineol Reduces Nociceptive Behavior in Mice. Pharm. Biol. 2011, 49, 583–586. [Google Scholar] [CrossRef]
- Peruru, R.; Usha Rani, R.; Thatiparthi, J.; Sampathi, S.; Dodoala, S.; Prasad, K.V.S.R.G. Devil’s Claw (Harpagophytum procumbens) Ameliorates the Neurobehavioral Changes and Neurotoxicity in Female Rats Exposed to Arsenic. Heliyon 2020, 6, e03921. [Google Scholar] [CrossRef]
- Hong, J.Y.; Kim, H.; Lee, J.; Jeon, W.-J.; Lee, Y.J.; Ha, I.-H. Harpagophytum procumbens Inhibits Iron Overload-Induced Oxidative Stress through Activation of Nrf2 Signaling in a Rat Model of Lumbar Spinal Stenosis. Oxid. Med. Cell. Longev. 2022, 2022, 1–18. [Google Scholar] [CrossRef]
- Jang, M.-H.; Lim, S.; Han, S.-M.; Park, H.-J.; Shin, I.; Kim, J.-W.; Kim, N.-J.; Lee, J.-S.; Kim, K.-A.; Kim, C.-J. Harpagophytum procumbens Suppresses Lipopolysaccharide-Stimulated Expressions of Cyclooxygenase-2 and Inducible Nitric Oxide Synthase in Fibroblast Cell Line L929. J. Pharmacol. Sci. 2003, 93, 367–371. [Google Scholar] [CrossRef]
- Göbel, H.; Heinze, A.; Ingwersen, M.; Niederberger, U.; Gerber, D. Harpagophytum-Extrakt LI 174 (Teufelskralle) Bei Der Behandlung Unspezifischer Rückenschmerzen. Der. Schmerz. 2001, 15, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, S.; Conradt, C.; Black, A. The Quality of Clinical Trials with Harpagophytum procumbens. Phytomedicine 2003, 10, 613–623. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rauf, A.; Badoni, H.; Abu-Izneid, T.; Olatunde, A.; Rahman, M.M.; Painuli, S.; Semwal, P.; Wilairatana, P.; Mubarak, M.S. Neuroinflammatory Markers: Key Indicators in the Pathology of Neurodegenerative Diseases. Molecules 2022, 27, 3194. [Google Scholar] [CrossRef] [PubMed]
- Hoozemans, J.; Rozemuller, J.; van Haastert, E.; Veerhuis, R.; Eikelenboom, P. Cyclooxygenase-1 and -2 in the Different Stages of Alzheimers Disease Pathology. Curr. Pharm. Des. 2008, 14, 1419–1427. [Google Scholar] [CrossRef]
- Hoozemans, J.J.M.; Rozemuller, A.J.M.; Janssen, I.; De Groot, C.J.A.; Veerhuis, R.; Eikelenboom, P. Cyclooxygenase Expression in Microglia and Neurons in Alzheimer’s Disease and Control Brain. Acta Neuropathol. 2001, 101, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Abdelouahab, N.; Heard, C. Effect of the Major Glycosides of Harpagophytum Procumbens (Devil’s Claw) on Epidermal Cyclooxygenase-2 (COX-2) In Vitro. J. Nat. Prod. 2008, 71, 746–749. [Google Scholar] [CrossRef]
- Anauate, M.C.; Torres, L.M.; de Mello, S.B.V. Effect of Isolated Fractions of Harpagophytum procumbens D.C. (Devil’s Claw) on COX-1, COX-2 Activity and Nitric Oxide Production on Whole-Blood Assay. Phytother. Res. 2010, 24, 1365–1369. [Google Scholar] [CrossRef]
- Chrubasik, S.; Sporer, F.; Dillmann-Marschner, R.; Friedmann, A.; Wink, M. Physicochemical Properties of Harpagoside and Its In Vitro Release from Harpagophytum procumbens Extract Tablets. Phytomedicine 2000, 6, 469–473. [Google Scholar] [CrossRef]
- Vlachojannis, J.; Roufogalis, B.D.; Chrubasik, S. Systematic Review on the Safety of Harpagophytum Preparations for Osteoarthritic and Low Back Pain. Phytother. Res. 2008, 22, 149–152. [Google Scholar] [CrossRef]
- Chantre, P.; Cappelaere, A.; Leblan, D.; Guedon, D.; Vandermander, J.; Fournie, B. Efficacy and Tolerance of Harpagophytum procumbens versus Diacerhein in Treatment of Osteoarthritis. Phytomedicine 2000, 7, 177–183. [Google Scholar] [CrossRef]
- Saravanan, M.; Senthilkumar, P.; Kalimuthu, K.; Chinnadurai, V.; Vasantharaj, S.; Pugazhendhi, A. Phytochemical and Pharmacological Profiling of Turnera subulata Sm., a Vital Medicinal Herb. Ind. Crops Prod. 2018, 124, 822–833. [Google Scholar] [CrossRef]
- Cordeiro, S.Z. Turnera subulata Sm. Herbário Prof. Jorge Pedro Pereira Carauta (huni) coleção didática do canto das flores 2020. Available online: https://www.unirio.br/ccbs/ibio/herbariohuni (accessed on 27 March 2024).
- Wu, J.; Wu, Y.; Yuan, Y.; Xia, C.; Saravanan, M.; Shanmugam, S.; Sabour, A.; Alshiekheid, M.; Brindhadevi, K.; Chi, N.T.L.; et al. Eco-Friendly, Green Synthesized Copper Oxide Nanoparticle (CuNPs) from an Important Medicinal Plant Turnera subulata Sm. and Its Biological Evaluation. Food Chem. Toxicol. 2022, 168, 113366. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.A. Special Issue—“Isolation, Structure Elucidation and Biological Activity of Natural Products”. Molecules 2023, 28, 5392. [Google Scholar] [CrossRef] [PubMed]
- Rebouças, E.L.; da Silva, A.W.; Rodrigues, M.C.; Ferreira, M.K.A.; Mendes, F.R.S.; Marinho, M.M.; Marinho, E.M.; Pereira, L.R.; de Araújo, J.I.F.; da Silva, J.Y.G.; et al. Antinociceptive, Anti-Inflammatory and Hypoglycemic Activities of the Ethanolic Turnera Subulata Sm. Flower Extract in Adult Zebrafish (Danio Rerio). J. Biomol. Struct. Dyn. 2022, 40, 13062–13074. [Google Scholar] [CrossRef] [PubMed]
- Cruz, N.G.; Cristaldo, P.F.; Bacci, L.; Almeida, C.S.; Camacho, G.P.; Santana, A.S.; Ribeiro, E.J.M.; Oliveira, A.P.; Santos, A.A.; Araújo, A.P.A. Variation in the Composition and Activity of Ants on Defense of Host Plant Turnera subulata (Turneraceae): Strong Response to Simulated Herbivore Attacks and to Herbivore’s Baits. Arthropod. Plant Interact. 2018, 12, 113–121. [Google Scholar] [CrossRef]
- Reyes-Becerril, M.; Ginera, P.; Silva-Jara, J.; Macias, A.; Velazquez-Carriles, C.; Alcaraz-Meléndez, L.; Angulo, C. Assessment of Chemical, Biological and Immunological Properties of “Damiana de California” Turnera diffusa Willd Extracts in Longfin Yellowtail (Seriola rivoliana) Leukocytes. Fish Shellfish Immunol. 2020, 100, 418–426. [Google Scholar] [CrossRef]
- Khushboo; Kumar, A.; Sharma, B. Biomedical Implications of Plant-Based Principles as Antidepressants: Prospects for Novel Drug Development. Mini-Rev. Med. Chem. 2022, 22, 904–926. [Google Scholar] [CrossRef] [PubMed]
- Souza, N.C.; de Oliveira, J.M.; Morrone, M.d.S.; Albanus, R.D.; Amarante, M.d.S.M.; Camillo, C.d.S.; Langassner, S.M.Z.; Gelain, D.P.; Moreira, J.C.F.; Dalmolin, R.J.S.; et al. Turnera subulata Anti-Inflammatory Properties in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. J. Med. Food 2016, 19, 922–930. [Google Scholar] [CrossRef] [PubMed]
- da Luz, J.R.D.; Barbosa, E.A.; do Nascimento, T.E.S.; de Rezende, A.A.; Ururahy, M.A.G.; Brito, A.d.S.; Araujo-Silva, G.; López, J.A.; Almeida, M.d.G. Chemical Characterization of Flowers and Leaf Extracts Obtained from Turnera subulata and Their Immunomodulatory Effect on LPS-Activated RAW 264.7 Macrophages. Molecules 2022, 27, 1084. [Google Scholar] [CrossRef]
- Annadurai, P.; Annadurai, V.; Yongkun, M.; Pugazhendhi, A.; Dhandayuthapani, K. Phytochemical Composition, Antioxidant and Antimicrobial Activities of Plecospermum Spinosum Trecul. Process Biochem. 2021, 100, 107–116. [Google Scholar] [CrossRef]
- Andrade-Pinheiro, J.C.; Sobral de Souza, C.E.; Ribeiro, D.A.; Silva, A.d.A.; da Silva, V.B.; dos Santos, A.T.L.; Juno Alencar Fonseca, V.; de Macêdo, D.G.; da Cruz, R.P.; Almeida-Bezerra, J.W.; et al. LC-MS Analysis and Antifungal Activity of Turnera subulata Sm. Plants 2023, 12, 415. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, S.; Rajesh, A.; Mathimani, T.; Melvin Samuel, S.; Shanmuganathan, R.; Brindhadevi, K. Efficacy of Crude Extracts of Clitoria Ternatea for Antibacterial Activity against Gram Negative Bacterium (Proteus mirabilis). Biocatal. Agric. Biotechnol. 2019, 21, 101328. [Google Scholar] [CrossRef]
- Fernandes, M.G.; Gomes, R.A.; Brito-Filho, S.G.; Silva-Filho, R.N.; Agra, M.F.; Falcão-Silva, V.S.; Siqueira-Junior, J.P.; Vieira, M.A.R.; Marques, M.O.M.; Souza, M.F.V. Characterization and Anti-Staphylococcal Activity of the Essential Oil from Turnera subulata Sm. Rev. Bras. Plantas Med. 2014, 16, 534–538. [Google Scholar] [CrossRef][Green Version]
- Freitas, C.L.A.; Santos, F.F.P.; Dantas-Junior, O.M.; Inácio, V.V.; Matias, E.F.F.; Quintans-Júnior, L.J.; Aguiar, J.J.S.; Coutinho, H.D.M. Enhancement of Antibiotic Activity by Phytocompounds of Turnera subulata. Nat. Prod. Res. 2020, 34, 2384–2388. [Google Scholar] [CrossRef] [PubMed]
- Marincaş, O.; Feher, I.; Magdas, D.A.; Puşcaş, R. Optimized and Validated Method for Simultaneous Extraction, Identification and Quantification of Flavonoids and Capsaicin, along with Isotopic Composition, in Hot Peppers from Different Regions. Food Chem. 2018, 267, 255–262. [Google Scholar] [CrossRef]
- Szewczyk, K.; Zidorn, C. Ethnobotany, Phytochemistry, and Bioactivity of the Genus Turnera (Passifloraceae) with a Focus on Damiana—Turnera diffusa. J. Ethnopharmacol. 2014, 152, 424–443. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Pisklak, M.A.; Kaliszewska, D.; Stolarczyk, M.; Kiss, A.K. Activity-Guided Isolation, Identification and Quantification of Biologically Active Isomeric Compounds from Folk Medicinal Plant Desmodium Adscendens Using High Performance Liquid Chromatography with Diode Array Detector, Mass Spectrometry and Multidimentional Nuclear Magnetic Resonance Spectroscopy. J. Pharm. Biomed. Anal. 2015, 102, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Reyes, R.; Ortiz-López, P.; Gutiérrez-Ortíz, J.; Martínez-Mota, L. Turnera diffusa Wild (Turneraceae) Recovers Sexual Behavior in Sexually Exhausted Males. J. Ethnopharmacol. 2009, 123, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, Y.; Chen, Z.; Leng, S.X. Connection between Systemic Inflammation and Neuroinflammation Underlies Neuroprotective Mechanism of Several Phytochemicals in Neurodegenerative Diseases. Oxid. Med. Cell. Longev. 2018, 2018, 1–16. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Chorilli, M. The Uses of Resveratrol for Neurological Diseases Treatment and Insights for Nanotechnology Based-Drug Delivery Systems. Int. J. Pharm. 2020, 589, 119832. [Google Scholar] [CrossRef]
- Mu, Y.; Gage, F.H. Adult Hippocampal Neurogenesis and Its Role in Alzheimer’s Disease. Mol. Neurodegener. 2011, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Pihlajamäki, M.; Soininen, H. Alzheimer’s Disease. In The Clinical Neurobiology of the Hippocampus; Oxford University Press: Oxford, UK, 2012; pp. 223–244. [Google Scholar]
- Goedert, M. Alzheimer’s and Parkinson’s Diseases: The Prion Concept in Relation to Assembled Aβ, Tau, and α-Synuclein. Science 2015, 349, 1255555. [Google Scholar] [CrossRef]
- Spires-Jones, T.L.; Attems, J.; Thal, D.R. Interactions of Pathological Proteins in Neurodegenerative Diseases. Acta Neuropathol. 2017, 134, 187–205. [Google Scholar] [CrossRef]
- Hussain, R.; Zubair, H.; Pursell, S.; Shahab, M. Neurodegenerative Diseases: Regenerative Mechanisms and Novel Therapeutic Approaches. Brain Sci. 2018, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Cookson, M.R.; Petrucelli, L.; La Spada, A.R. Converging Pathways in Neurodegeneration, from Genetics to Mechanisms. Nat. Neurosci. 2018, 21, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Klockgether, T. Parkinson’s Disease: Clinical Aspects. Cell Tissue Res. 2004, 318, 115–120. [Google Scholar] [CrossRef]
- Metzler-Baddeley, C.; Baddeley, R.J.; Lovell, P.G.; Laffan, A.; Jones, R.W. Visual Impairments in Dementia with Lewy Bodies and Posterior Cortical Atrophy. Neuropsychology 2010, 24, 35–48. [Google Scholar] [CrossRef]
- Shamsi, T.N.; Athar, T.; Parveen, R.; Fatima, S. A Review on Protein Misfolding, Aggregation and Strategies to Prevent Related Ailments. Int. J. Biol. Macromol. 2017, 105, 993–1000. [Google Scholar] [CrossRef]
- Vercruysse, P.; Vieau, D.; Blum, D.; Petersén, Å.; Dupuis, L. Hypothalamic Alterations in Neurodegenerative Diseases and Their Relation to Abnormal Energy Metabolism. Front. Mol. Neurosci. 2018, 11, 2. [Google Scholar] [CrossRef]
- Gorantla, S.; Wadhwa, G.; Jain, S.; Sankar, S.; Nuwal, K.; Mahmood, A.; Dubey, S.K.; Taliyan, R.; Kesharwani, P.; Singhvi, G. Recent Advances in Nanocarriers for Nutrient Delivery. Drug Deliv. Transl. Res. 2022, 12, 2359–2384. [Google Scholar] [CrossRef]
- Ganesan, P.; Ko, H.-M.; Kim, I.-S.; Choi, D.-K. Recent Trends in the Development of Nanophytobioactive Compounds and Delivery Systems for Their Possible Role in Reducing Oxidative Stress in Parkinson’s Disease Models. Int. J. Nanomed. 2015, 10, 6757. [Google Scholar] [CrossRef] [PubMed]
- Sommonte, F.; Arduino, I.; Racaniello, G.F.; Lopalco, A.; Lopedota, A.A.; Denora, N. The Complexity of the Blood-Brain Barrier and the Concept of Age-Related Brain Targeting: Challenges and Potential of Novel Solid Lipid-Based Formulations. J. Pharm. Sci. 2022, 111, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Ren, H.; Xie, Y.; Yu, M.; Chen, Y.; Yang, L. Engineering Advanced Nanomedicines against Central Nervous System Diseases. Mater. Today 2023, 69, 355–392. [Google Scholar] [CrossRef]
- Tarhan, O.; Spotti, M.J. Nutraceutical Delivery through Nano-Emulsions: General Aspects, Recent Applications and Patented Inventions. Colloids Surf. B-Biointerfaces 2021, 200, 111526. [Google Scholar] [CrossRef] [PubMed]
- Askarizadeh, A.; Barreto, G.E.; Henney, N.C.; Majeed, M.; Sahebkar, A. Neuroprotection by Curcumin: A Review on Brain Delivery Strategies. Int. J. Pharm. 2020, 585, 119476. [Google Scholar] [CrossRef] [PubMed]
- Nirale, P.; Paul, A.; Yadav, K.S. Nanoemulsions for Targeting the Neurodegenerative Diseases: Alzheimer’s, Parkinson’s and Prion’s. Life Sci. 2020, 245, 117394. [Google Scholar] [CrossRef] [PubMed]
- Gabal, Y.M.; Kamel, A.O.; Sammour, O.A.; Elshafeey, A.H. Effect of Surface Charge on the Brain Delivery of Nanostructured Lipid Carriers in Situ Gels via the Nasal Route. Int. J. Pharm. 2014, 473, 442–457. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M. Development of an Optimized Hyaluronic Acid-Based Lipidic Nanoemulsion Co-Encapsulating Two Polyphenols for Nose to Brain Delivery. Drug Deliv. 2016, 23, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Saffari, P.M.; Alijanpour, S.; Takzaree, N.; Sahebgharani, M.; Etemad-Moghadam, S.; Noorbakhsh, F.; Partoazar, A. Metformin Loaded Phosphatidylserine Nanoliposomes Improve Memory Deficit and Reduce Neuroinflammation in Streptozotocin-Induced Alzheimer’s Disease Model. Life Sci. 2020, 255, 117861. [Google Scholar] [CrossRef]
- Shalabalija, D.; Mihailova, L.; Crcarevska, M.S.; Karanfilova, I.C.; Ivanovski, V.; Nestorovska, A.K.; Novotni, G.; Dodov, M.G. Formulation and Optimization of Bioinspired Rosemary Extract Loaded PEGylated Nanoliposomes for Potential Treatment of Alzheimer’s Disease Using Design of Experiments. J. Drug Deliv. Sci. Technol. 2021, 63, 102434. [Google Scholar] [CrossRef]
- Bakrim, S.; Aboulaghras, S.; El Menyiy, N.; El Omari, N.; Assaggaf, H.; Lee, L.-H.; Montesano, D.; Gallo, M.; Zengin, G.; AlDhaheri, Y.; et al. Phytochemical Compounds and Nanoparticles as Phytochemical Delivery Systems for Alzheimer’s Disease Management. Molecules 2022, 27, 9043. [Google Scholar] [CrossRef] [PubMed]
- More, M.P.; Pardeshi, S.R.; Pardeshi, C.V.; Sonawane, G.A.; Shinde, M.N.; Deshmukh, P.K.; Naik, J.B.; Kulkarni, A.D. Recent Advances in Phytochemical-Based Nano-Formulation for Drug-Resistant Cancer. Med. Drug Discov. 2021, 10, 100082. [Google Scholar] [CrossRef]
- Alberti, T.B.; Coelho, D.S.; Maraschin, M. β-Caryophyllene Nanoparticles Design and Development: Controlled Drug Delivery of Cannabinoid CB2 Agonist as a Strategic Tool towards Neurodegeneration. Mater. Sci. Eng. C 2021, 121, 111824. [Google Scholar] [CrossRef]
- Fan, S.; Zheng, Y.; Liu, X.; Fang, W.; Chen, X.; Liao, W.; Jing, X.; Lei, M.; Tao, E.; Ma, Q.; et al. Curcumin-Loaded PLGA-PEG Nanoparticles Conjugated with B6 Peptide for Potential Use in Alzheimer’s Disease. Drug Deliv. 2018, 25, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, P. Lipid-Based Nanocarrier System for the Effective Delivery of Nutraceuticals. Molecules 2021, 26, 5510. [Google Scholar] [CrossRef]
- Alshawwa, S.Z.; Kassem, A.A.; Farid, R.M.; Mostafa, S.K.; Labib, G.S. Nanocarrier Drug Delivery Systems: Characterization, Limitations, Future Perspectives and Implementation of Artificial Intelligence. Pharmaceutics 2022, 14, 883. [Google Scholar] [CrossRef] [PubMed]
- Zolnik, B.S.; González-Fernández, A.; Sadrieh, N.; Dobrovolskaia, M.A. Minireview: Nanoparticles and the Immune System. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Pang, Y.; Zhou, H. The Interaction between Nanoparticles and Immune System: Application in the Treatment of Inflammatory Diseases. J. Nanobiotechnology 2022, 20, 127. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, M.; Kulsi, G.; Chakraborty, A.; Dinda, S. Therapeutic Potentials of Plant Iridoids in Alzheimer’s and Parkinson’s Diseases: A Review. Eur. J. Med. Chem. 2019, 169, 185–199. [Google Scholar] [CrossRef]
- Jiang, T.; Sun, Q.; Chen, S. Oxidative Stress: A Major Pathogenesis and Potential Therapeutic Target of Antioxidative Agents in Parkinson’s Disease and Alzheimer’s Disease. Prog. Neurobiol. 2016, 147, 1–19. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative Stress and the Amyloid Beta Peptide in Alzheimer’s Disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Tchekalarova, J.; Tzoneva, R. Oxidative Stress and Aging as Risk Factors for Alzheimer’s Disease and Parkinson’s Disease: The Role of the Antioxidant Melatonin. Int. J. Mol. Sci. 2023, 24, 3022. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Zaragoza, J.; Cuenca-Bermejo, L.; Almela, P.; Laorden, M.-L.; Herrero, M.-T. Could Small Heat Shock Protein HSP27 Be a First-Line Target for Preventing Protein Aggregation in Parkinson’s Disease? Int. J. Mol. Sci. 2021, 22, 3038. [Google Scholar] [CrossRef] [PubMed]
- Houldsworth, A. Role of Oxidative Stress in Neurodegenerative Disorders: A Review of Reactive Oxygen Species and Prevention by Antioxidants. Brain Commun. 2023, 6, fcad356. [Google Scholar] [CrossRef]
- McKeith, I.; Mintzer, J.; Aarsland, D.; Burn, D.; Chiu, H.; Cohen-Mansfield, J.; Dickson, D.; Dubois, B.; Duda, J.E.; Feldman, H.; et al. Dementia with Lewy Bodies. Lancet Neurol. 2004, 3, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Dalfó, E.; Portero-Otín, M.; Ayala, V.; Martínez, A.; Pamplona, R.; Ferrer, I. Evidence of Oxidative Stress in the Neocortex in Incidental Lewy Body Disease. J. Neuropathol. Exp. Neurol. 2005, 64, 816–830. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, L.F.; Peroza, L.R.; Boligon, A.A.; Athayde, M.L.; Alves, S.H.; Fachinetto, R.; Wagner, C. Harpagophytum procumbens Prevents Oxidative Stress and Loss of Cell Viability In Vitro. Neurochem. Res. 2013, 38, 2256–2267. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the Anti-Inflammatory and Antioxidant Activities of Luteolin, Kaempferol, Apigenin and Quercetin. S. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Slezak, J.; Kura, B.; LeBaron, T.W.; Singal, P.K.; Buday, J.; Barancik, M. Oxidative Stress and Pathways of Molecular Hydrogen Effects in Medicine. Curr. Pharm. Des. 2021, 27, 610–625. [Google Scholar] [CrossRef]
- Liu, D.; Wang, H.; Zhang, Y.; Zhang, Z. Protective Effects of Chlorogenic Acid on Cerebral Ischemia/Reperfusion Injury Rats by Regulating Oxidative Stress-Related Nrf2 Pathway. Drug Des. Devel. Ther. 2020, 14, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Sharapov, M.G.; Gudkov, S.V.; Lankin, V.Z. Hydroperoxide-Reducing Enzymes in the Regulation of Free-Radical Processes. Biochemistry 2021, 86, 1256–1274. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Sharma, P.; Gill, S.S.; Hasanuzzaman, M.; Khan, E.A.; Kachhap, K.; Mohamed, A.A.; Thangavel, P.; Devi, G.D.; Vasudhevan, P.; et al. Catalase and Ascorbate Peroxidase—Representative H2O2-Detoxifying Heme Enzymes in Plants. Environ. Sci. Pollut. Res. 2016, 23, 19002–19029. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; He, X.; Wu, L.; Yan, D.; Yan, S. Chlorogenic Acid, the Main Antioxidant in Coffee, Reduces Radiation-Induced Apoptosis and DNA Damage via NF-E2-Related Factor 2 (Nrf2) Activation in Hepatocellular Carcinoma. Oxid. Med. Cell Longev. 2022, 2022, 1–14. [Google Scholar] [CrossRef]
- Shi, A.; Shi, H.; Wang, Y.; Liu, X.; Cheng, Y.; Li, H.; Zhao, H.; Wang, S.; Dong, L. Activation of Nrf2 Pathway and Inhibition of NLRP3 Inflammasome Activation Contribute to the Protective Effect of Chlorogenic Acid on Acute Liver Injury. Int. Immunopharmacol. 2018, 54, 125–130. [Google Scholar] [CrossRef]

| Therapeutic Property | Description | References |
|---|---|---|
| Antioxidant potential | The compounds found in Harpagophytum procumbens, such as iridoid glycosides and phenolics, possess antioxidant properties that may help reduce oxidative stress, a factor associated with neurodegenerative diseases. | [40,41] |
| Possible inflammation reduction | The plant’s anti-inflammatory properties can aid in reducing chronic inflammation, which plays a role in the progression of neurodegenerative diseases such as Alzheimer’s and Parkinson’s. | [16] |
| Potential pain relief | Harpagophytum procumbens’s ability to alleviate pain can be beneficial for patients with neurodegenerative diseases who often suffer from chronic pain, improving their quality of life. | [30,42] |
| Complementary use | While not a cure for neurodegenerative diseases, the plant can be used as part of a complementary treatment regimen to alleviate symptoms like the muscle stiffness and discomfort commonly seen in these disorders. | [43] |
| Therapeutic Property | Description | References |
|---|---|---|
| Antioxidant activity | Reduces oxidative stress, protecting nerve cells against damage | [8] |
| GABA modulation | Regulates the neurotransmitter GABA, providing antianxiety and relaxation effects | [59,60] |
| Free radical inhibition | Effectively eliminates free radicals, reducing cell damage related to neurodegenerative diseases | [61,62] |
| Anti-inflammatory action | Reduces inflammation in the nervous system, decreasing the progression of neurodegenerative diseases | [62] |
| Antimicrobial activity | Combats infections that can worsen neurodegenerative conditions | [55,66,67] |
| Improved Cerebral Blood Flow | Increases blood flow to the brain, enhancing the supply of nutrients and oxygen | [71] |
| Potential for Memory Enhancement | May have a positive effect on cognition, including memory | [60] |
| Anxiolytic Properties | Reduces anxiety, alleviating psychological symptoms associated with neurodegenerative diseases | [57,58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vital Júnior, A.C.; da Silva, M.B.; Monteiro, S.S.; Pasquali, M.A.d.B. The Therapeutic Potential of Harpagophytum procumbens and Turnera subulata and Advances in Nutraceutical Delivery Systems in Neurodegenerative Diseases. Pharmaceuticals 2024, 17, 660. https://doi.org/10.3390/ph17050660
Vital Júnior AC, da Silva MB, Monteiro SS, Pasquali MAdB. The Therapeutic Potential of Harpagophytum procumbens and Turnera subulata and Advances in Nutraceutical Delivery Systems in Neurodegenerative Diseases. Pharmaceuticals. 2024; 17(5):660. https://doi.org/10.3390/ph17050660
Chicago/Turabian StyleVital Júnior, Antonio Carlos, Mikaelly Batista da Silva, Shênia Santos Monteiro, and Matheus Augusto de Bittencourt Pasquali. 2024. "The Therapeutic Potential of Harpagophytum procumbens and Turnera subulata and Advances in Nutraceutical Delivery Systems in Neurodegenerative Diseases" Pharmaceuticals 17, no. 5: 660. https://doi.org/10.3390/ph17050660
APA StyleVital Júnior, A. C., da Silva, M. B., Monteiro, S. S., & Pasquali, M. A. d. B. (2024). The Therapeutic Potential of Harpagophytum procumbens and Turnera subulata and Advances in Nutraceutical Delivery Systems in Neurodegenerative Diseases. Pharmaceuticals, 17(5), 660. https://doi.org/10.3390/ph17050660







