Small Molecule Drugs Targeting Viral Polymerases
Abstract
1. Introduction
2. Viral Polymerases: An Overview
3. Targeting Viral Polymerases with Small Molecules
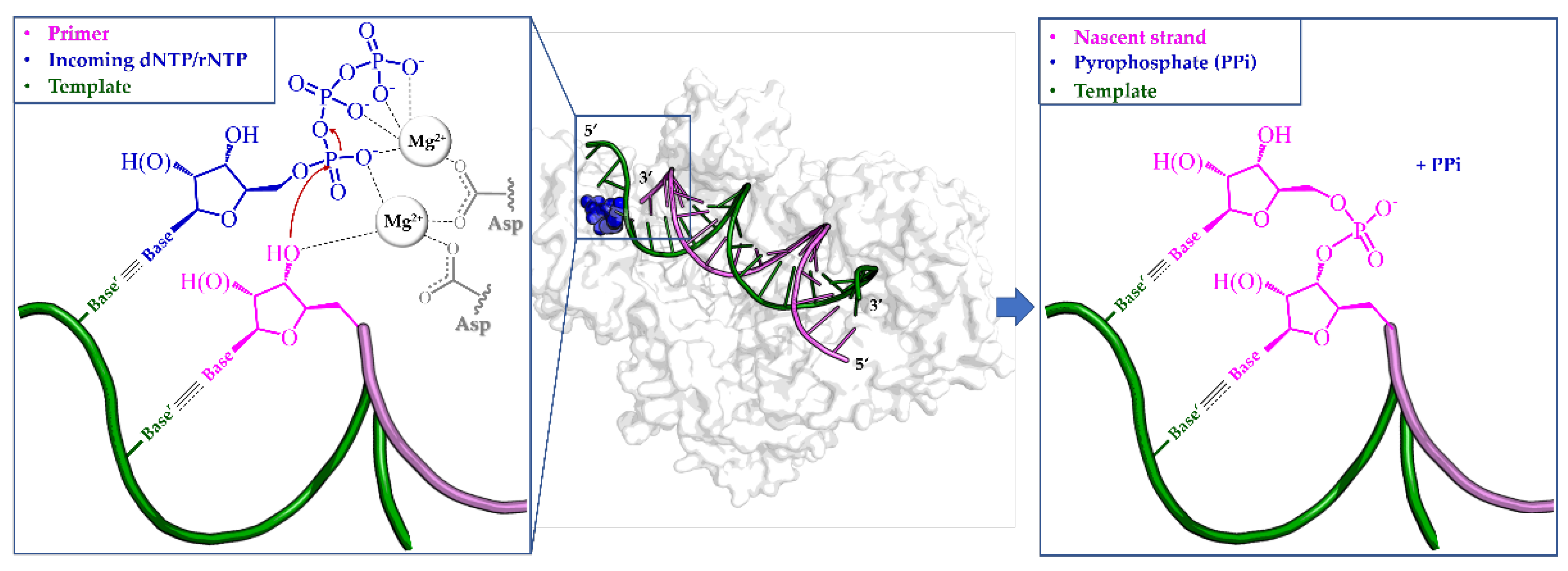
3.1. Nucleoside and Nucleotide Inhibitors (NIs)
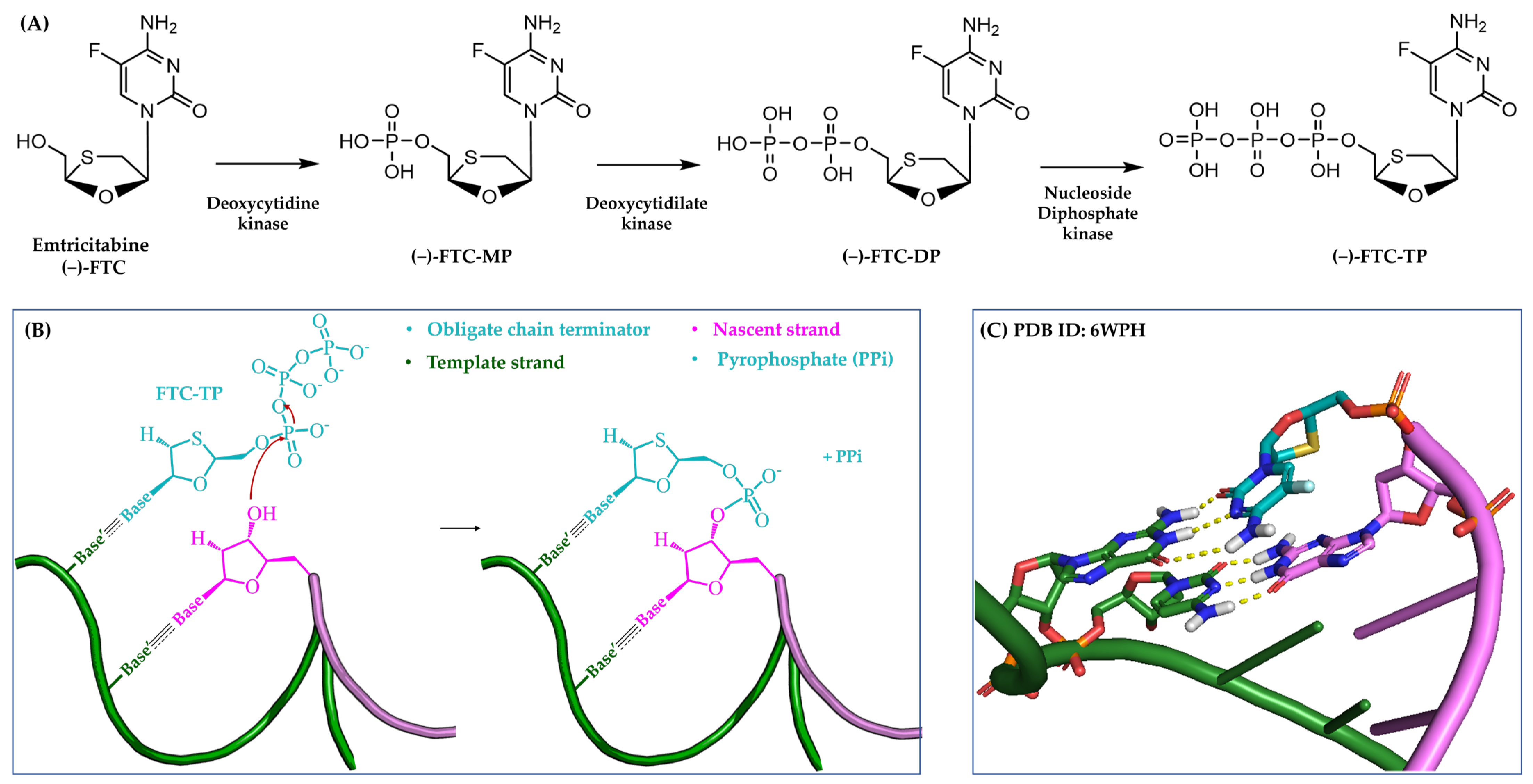
3.1.1. Obligate Chain Terminators
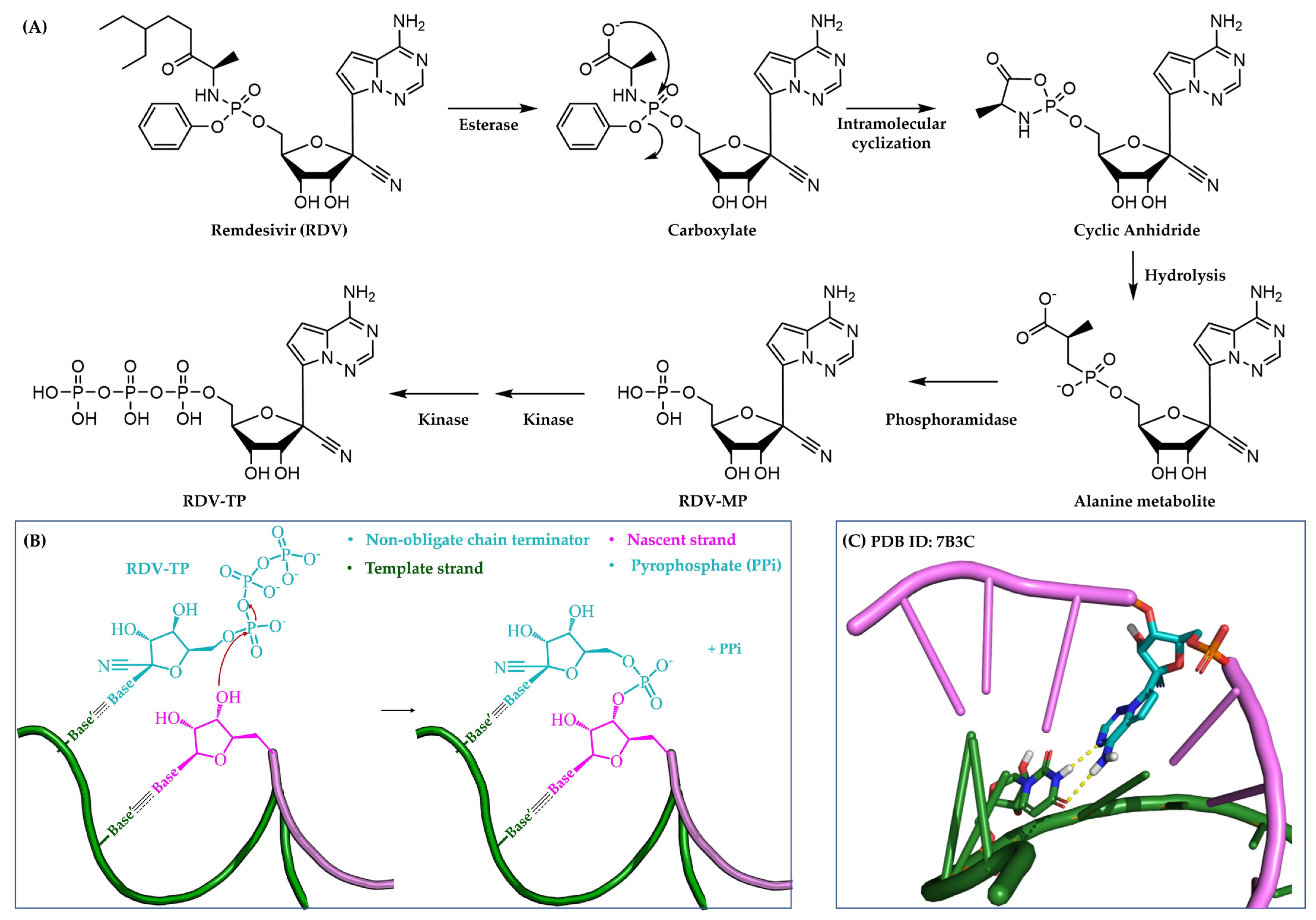
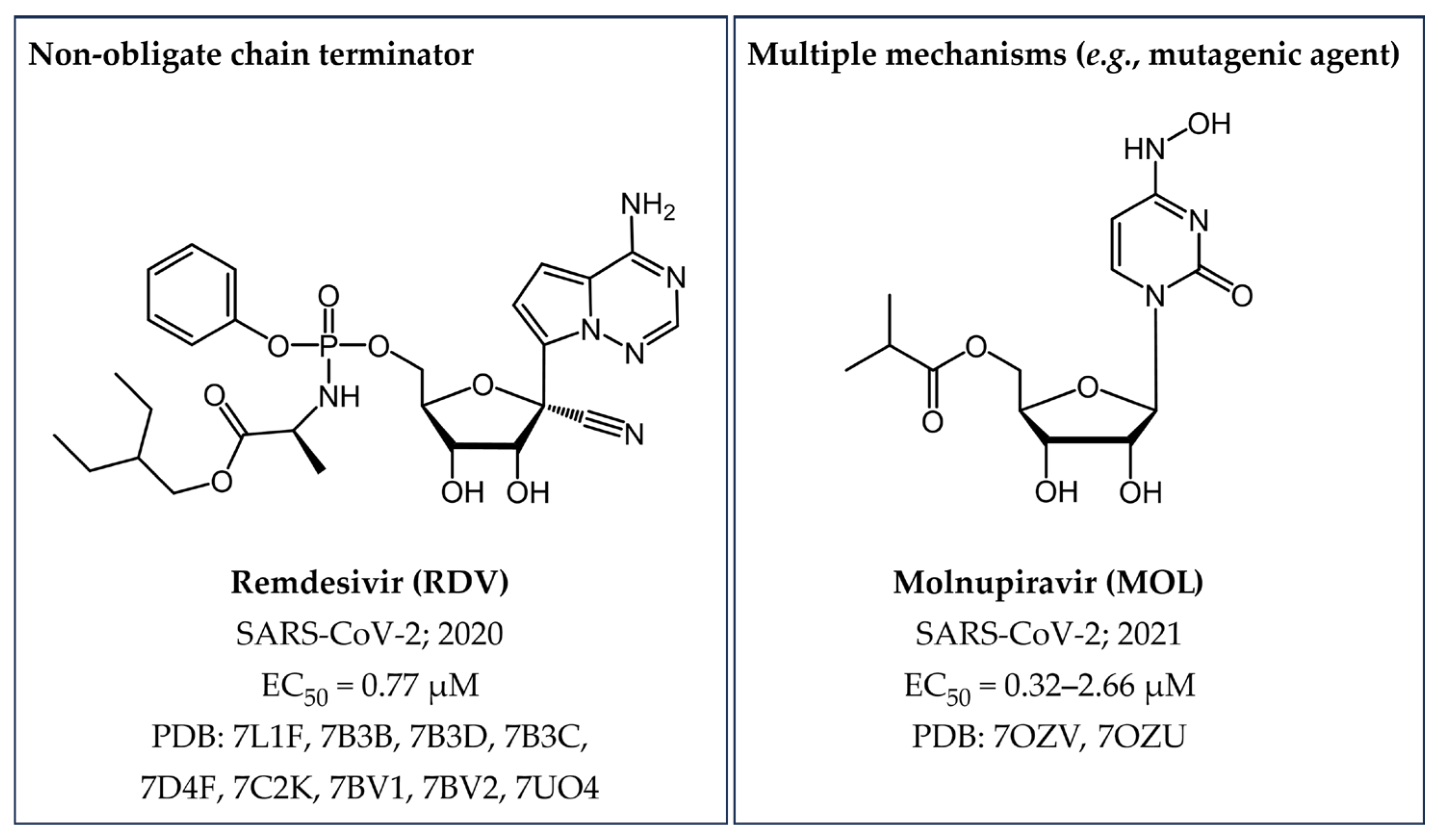
3.1.2. Non-Obligate Chain Terminators
3.2. Non-Nucleoside Inhibitors (NNIs)
3.2.1. Allosteric Inhibitors
3.2.2. PPi Analogues
3.3. Mutagenic Agents
4. Small Molecule Drugs Approved to Target Viral Polymerases
4.1. Small Molecules Targeting DNA Viruses
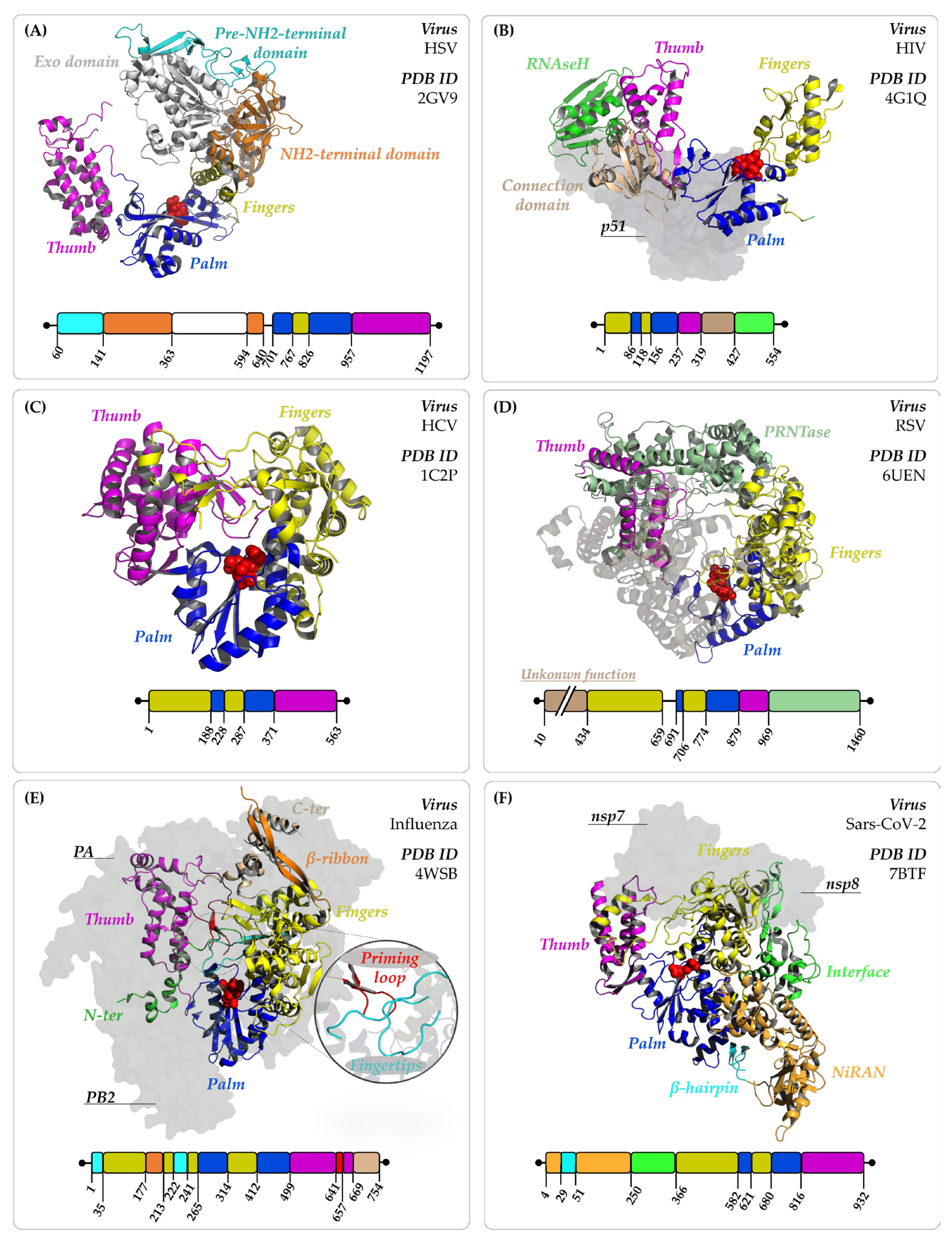
4.1.1. Herpes Simplex Virus (HSV), Human Cytomegalovirus (HCMV), and Varicella-Zoster Virus (VZV)
4.1.2. Hepatitis B Virus (HBV)
4.2. Small Molecules Targeting RNA Viruses
4.2.1. Human Immunodeficiency Virus (HIV)
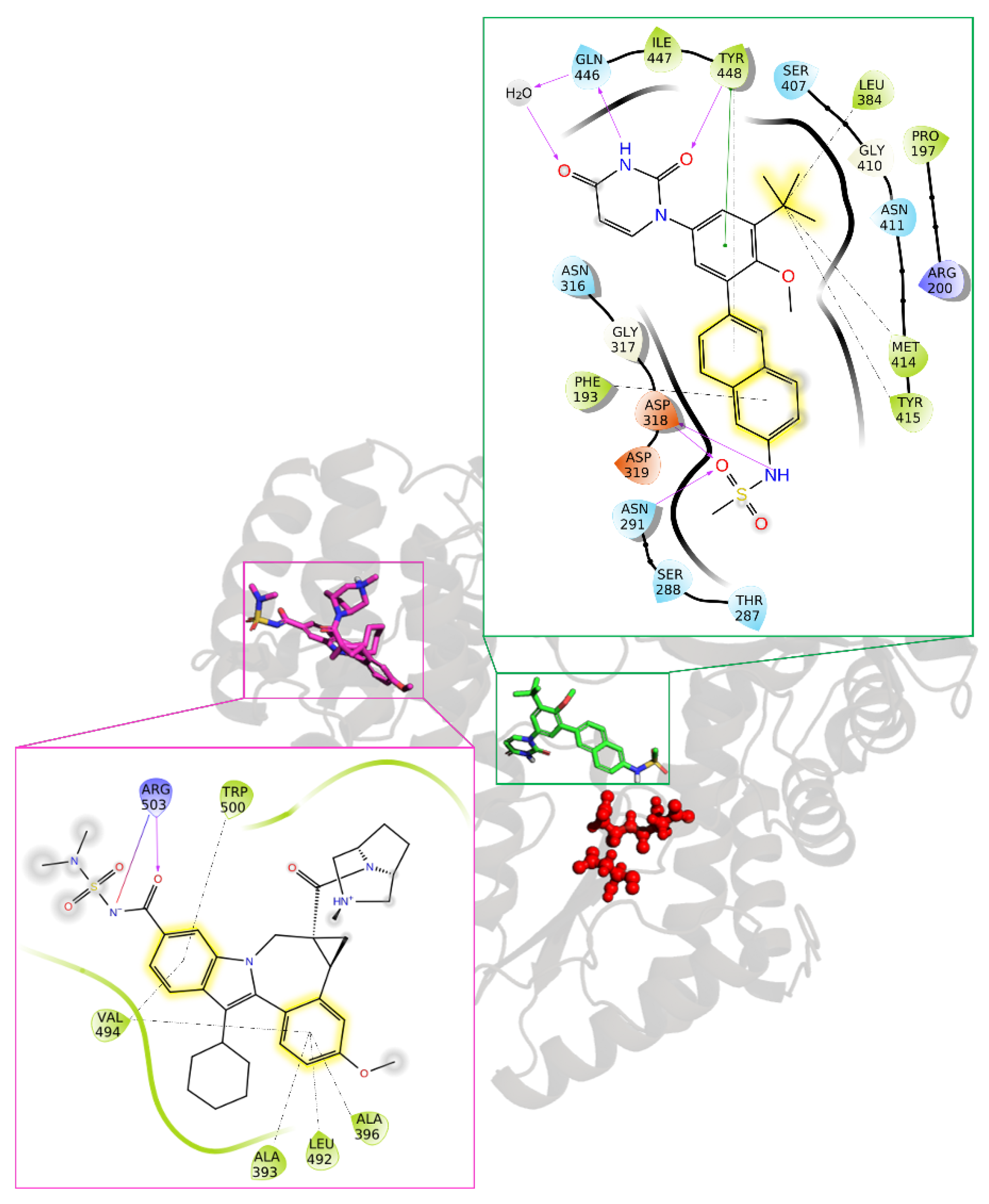
4.2.2. Hepatitis C Virus (HCV)
4.2.3. Respiratory Syncytial Virus (RSV)
4.2.4. Influenza Virus
4.2.5. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, G.; Hilgenfeld, R.; Whitley, R.; De Clercq, E. Therapeutic strategies for COVID-19: Progress and lessons learned. Nat. Rev. Drug Discov. 2023, 22, 449–475. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H. Viral polymerases. In Viral Molecular Machines; Book Series: Advances in Experimental Medicine and Biology (AEMB, volume 726); Springer: Boston, MA, USA, 2012; pp. 267–304. [Google Scholar] [CrossRef]
- Pathania, S.; Rawal, R.K.; Singh, P.K. RdRp (RNA-dependent RNA polymerase): A key target providing anti-virals for the management of various viral diseases. J. Mol. Struct. 2022, 1250, 131756. [Google Scholar] [CrossRef]
- Tsai, C.H.; Lee, P.Y.; Stollar, V.; Li, M.L. Antiviral therapy targeting viral polymerase. Curr. Pharm. Des. 2006, 12, 1339–1355. [Google Scholar] [CrossRef]
- De Clercq, E.; Li, G. Approved Antiviral Drugs over the Past 50 Years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef] [PubMed]
- Picarazzi, F.; Vicenti, I.; Saladini, F.; Zazzi, M.; Mori, M. Targeting the RdRp of Emerging RNA Viruses: The Structure-Based Drug Design Challenge. Molecules 2020, 25, 5695. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, R.J.; Aliota, M.T.; Bonnac, L.F. Broad-Spectrum Antiviral Strategies and Nucleoside Analogues. Viruses 2021, 13, 667. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yue, T.; Zhang, P.; Gu, W.; Gao, L.J.; Tan, L. Drug Discovery of Nucleos(t)ide Antiviral Agents: Dedicated to Prof. Dr. Erik De Clercq on Occasion of His 80th Birthday. Molecules 2021, 26, 923. [Google Scholar] [CrossRef] [PubMed]
- Tompa, D.R.; Immanuel, A.; Srikanth, S.; Kadhirvel, S. Trends and strategies to combat viral infections: A review on FDA approved antiviral drugs. Int. J. Biol. Macromol. 2021, 172, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Kausar, S.; Said Khan, F.; Ishaq Mujeeb Ur Rehman, M.; Akram, M.; Riaz, M.; Rasool, G.; Hamid Khan, A.; Saleem, I.; Shamim, S.; Malik, A. A review: Mechanism of action of antiviral drugs. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211002621. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Symons, J.A.; Deval, J. Innovation and trends in the development and approval of antiviral medicines: 1987–2017 and beyond. Antivir. Res. 2018, 155, 76–88. [Google Scholar] [CrossRef]
- Steitz, T.A. Structural biology—A mechanism for all polymerases. Nature 1998, 391, 231–232. [Google Scholar] [CrossRef] [PubMed]
- Delarue, M.; Poch, O.; Tordo, N.; Moras, D.; Argos, P. An attempt to unify the structure of polymerases. Protein Eng. 1990, 3, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Fralish, Z.; Chen, A.; Khan, S.; Zhou, P.; Reker, D. The landscape of small-molecule prodrugs. Nat. Rev. Drug Discov. 2024, 23, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Deval, J. Antimicrobial strategies: Inhibition of viral polymerases by 3′-hydroxyl nucleosides. Drugs 2009, 69, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Shannon, A.; Canard, B. Kill or corrupt: Mechanisms of action and drug-resistance of nucleotide analogues against SARS-CoV-2. Antivir. Res. 2023, 210, 105501. [Google Scholar] [CrossRef] [PubMed]
- Kokic, G.; Hillen, H.S.; Tegunov, D.; Dienemann, C.; Seitz, F.; Schmitzova, J.; Farnung, L.; Siewert, A.; Hobartner, C.; Cramer, P. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat. Commun. 2021, 12, 279. [Google Scholar] [CrossRef] [PubMed]
- Swanstrom, R.; Schinazi, R.F. Lethal mutagenesis as an antiviral strategy. Science 2022, 375, 497–498. [Google Scholar] [CrossRef] [PubMed]
- Perales, C.; Gallego, I.; de Avila, A.I.; Soria, M.E.; Gregori, J.; Quer, J.; Domingo, E. The increasing impact of lethal mutagenesis of viruses. Future Med. Chem. 2019, 11, 1645–1657. [Google Scholar] [CrossRef]
- Hadj Hassine, I.; Ben M'hadheb, M.; Menendez-Arias, L. Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity. Viruses 2022, 14, 841. [Google Scholar] [CrossRef]
- Siramshetty, V.B.; Grishagin, I.; Nguyen Eth, T.; Peryea, T.; Skovpen, Y.; Stroganov, O.; Katzel, D.; Sheils, T.; Jadhav, A.; Mathe, E.A.; et al. NCATS Inxight Drugs: A comprehensive and curated portal for translational research. Nucleic Acids Res. 2022, 50, D1307–D1316. [Google Scholar] [CrossRef]
- Lowe, D.M.; Alderton, W.K.; Ellis, M.R.; Parmar, V.; Miller, W.H.; Roberts, G.B.; Fyfe, J.A.; Gaillard, R.; Ertl, P.; Snowden, W.; et al. Mode of action of (R)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine against herpesviruses. Antimicrob. Agents Chemother. 1995, 39, 1802–1808. [Google Scholar] [CrossRef][Green Version]
- Andrei, G.; Sienaert, R.; McGuigan, C.; De Clercq, E.; Balzarini, J.; Snoeck, R. Susceptibilities of several clinical varicella-zoster virus (VZV) isolates and drug-resistant VZV strains to bicyclic furano pyrimidine nucleosides. Antimicrob. Agents Chemother. 2005, 49, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Prichard, M.N.; Quenelle, D.C.; Hartline, C.B.; Harden, E.A.; Jefferson, G.; Frederick, S.L.; Daily, S.L.; Whitley, R.J.; Tiwari, K.N.; Maddry, J.A.; et al. Inhibition of herpesvirus replication by 5-substituted 4'-thiopyrimidine nucleosides. Antimicrob. Agents Chemother. 2009, 53, 5251–5258. [Google Scholar] [CrossRef]
- Hobden, J.A.; Kumar, M.; Kaufman, H.E.; Clement, C.; Varnell, E.D.; Bhattacharjee, P.S.; Hill, J.M. In vitro synergism of trifluorothymidine and ganciclovir against HSV-1. Invest. Ophthalmol. Vis. Sci. 2011, 52, 830–833. [Google Scholar] [CrossRef]
- Wang, Z.; Zang, R.; Niu, Z.; Wang, W.; Wang, X.; Tang, Y. Synthesis and antiviral effect of phosphamide modified vidarabine for treating HSV 1 infections. Bioorg Med. Chem. Lett. 2021, 52, 128405. [Google Scholar] [CrossRef]
- Miwa, N.; Kurosaki, K.; Yoshida, Y.; Kurokawa, M.; Saito, S.; Shiraki, K. Comparative efficacy of acyclovir and vidarabine on the replication of varicella-zoster virus. Antivir. Res. 2005, 65, 49–55. [Google Scholar] [CrossRef]
- Xiong, X.; Smith, J.L.; Kim, C.; Huang, E.S.; Chen, M.S. Kinetic analysis of the interaction of cidofovir diphosphate with human cytomegalovirus DNA polymerase. Biochem. Pharmacol. 1996, 51, 1563–1567. [Google Scholar] [CrossRef] [PubMed]
- Piret, J.; Goyette, N.; Boivin, G. Novel Method Based on Real-Time Cell Analysis for Drug Susceptibility Testing of Herpes Simplex Virus and Human Cytomegalovirus. J. Clin. Microbiol. 2016, 54, 2120–2127. [Google Scholar] [CrossRef] [PubMed]
- Severini, A.; Liu, X.Y.; Wilson, J.S.; Tyrrell, D.L. Mechanism of inhibition of duck hepatitis B virus polymerase by (-)-beta-L-2′,3′-dideoxy-3′-thiacytidine. Antimicrob. Agents Chemother. 1995, 39, 1430–1435. [Google Scholar] [CrossRef][Green Version]
- Yin, G.Q.; Li, J.; Zhong, B.; Yang, Y.F.; Wang, M.R. New therapeutic options for persistent low-level viremia in patients with chronic hepatitis B virus infection: Increase of entecavir dosage. World J. Gastroenterol. 2021, 27, 666–676. [Google Scholar] [CrossRef]
- Callebaut, C.; Stepan, G.; Tian, Y.; Miller, M.D. In Vitro Virology Profile of Tenofovir Alafenamide, a Novel Oral Prodrug of Tenofovir with Improved Antiviral Activity Compared to That of Tenofovir Disoproxil Fumarate. Antimicrob. Agents Chemother. 2015, 59, 5909–5916. [Google Scholar] [CrossRef] [PubMed]
- Langley, D.R.; Walsh, A.W.; Baldick, C.J.; Eggers, B.J.; Rose, R.E.; Levine, S.M.; Kapur, A.J.; Colonno, R.J.; Tenney, D.J. Inhibition of hepatitis B virus polymerase by entecavir. J. Virol. 2007, 81, 3992–4001. [Google Scholar] [CrossRef] [PubMed]
- Arion, D.; Kaushik, N.; McCormick, S.; Borkow, G.; Parniak, M.A. Phenotypic mechanism of HIV-1 resistance to 3’-azido-3’-deoxythymidine (AZT): Increased polymerization processivity and enhanced sensitivity to pyrophosphate of the mutant viral reverse transcriptase. Biochemistry 1998, 37, 15908–15917. [Google Scholar] [CrossRef] [PubMed]
- Benbrik, E.; Chariot, P.; Bonavaud, S.; Ammi-Said, M.; Frisdal, E.; Rey, C.; Gherardi, R.; Barlovatz-Meimon, G. Cellular and mitochondrial toxicity of zidovudine (AZT), didanosine (ddI) and zalcitabine (ddC) on cultured human muscle cells. J. Neurol. Sci. 1997, 149, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Van Draanen, N.A.; Tisdale, M.; Parry, N.R.; Jansen, R.; Dornsife, R.E.; Tuttle, J.V.; Averett, D.R.; Koszalka, G.W. Influence of stereochemistry on antiviral activities and resistance profiles of dideoxycytidine nucleosides. Antimicrob. Agents Chemother. 1994, 38, 868–871. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Markland, W.; McQuaid, T.J.; Jain, J.; Kwong, A.D. Broad-Spectrum Antiviral Activity of the IMP Dehydrogenase Inhibitor VX-497: A Comparison with Ribavirin and Demonstration of Antiviral Additivity with Alpha Interferon. Antimicrob. Agents Chemother. 2000, 44, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Murakami, E.; Tolstykh, T.; Bao, H.; Niu, C.; Steuer, H.M.; Bao, D.; Chang, W.; Espiritu, C.; Bansal, S.; Lam, A.M.; et al. Mechanism of activation of PSI-7851 and its diastereoisomer PSI-7977. J. Biol. Chem. 2010, 285, 34337–34347. [Google Scholar] [CrossRef]
- Frediansyah, A.; Nainu, F.; Dhama, K.; Mudatsir, M.; Harapan, H. Remdesivir and its antiviral activity against COVID-19: A systematic review. Clin. Epidemiol. Glob. Health 2021, 9, 123–127. [Google Scholar] [CrossRef]
- Syed, Y.Y. Molnupiravir: First Approval. Drugs 2022, 82, 455–460. [Google Scholar] [CrossRef]
- Das, D.; Hong, J. Chapter 12—Herpesvirus Polymerase Inhibitors. In Viral Polymerases: Structures, Functions and Roles as Antiviral Drug Targets; Academic Press: Cambridge, MA, USA, 2019; pp. 333–356. [Google Scholar]
- Zarrouk, K.; Piret, J.; Boivin, G. Herpesvirus DNA polymerases: Structures, functions and inhibitors. Virus Res. 2017, 234, 177–192. [Google Scholar] [CrossRef]
- Luczkowiak, J.; Álvarez, M.; Sebastián-Martín, A.; Menéndez-Arias, L. DNA-Dependent DNA Polymerases as Drug Targets in Herpesviruses and Poxviruses. In Viral Polymerases; Elsevier: Amsterdam, The Netherlands, 2019; pp. 95–134. [Google Scholar]
- Appleton, B.A.; Brooks, J.; Loregian, A.; Filman, D.J.; Coen, D.M.; Hogle, J.M. Crystal structure of the cytomegalovirus DNA polymerase subunit UL44 in complex with the C terminus from the catalytic subunit. Differences in structure and function relative to unliganded UL44. J. Biol. Chem. 2006, 281, 5224–5232. [Google Scholar] [CrossRef]
- Liu, S.; Knafels, J.D.; Chang, J.S.; Waszak, G.A.; Baldwin, E.T.; Deibel, M.R., Jr.; Thomsen, D.R.; Homa, F.L.; Wells, P.A.; Tory, M.C.; et al. Crystal structure of the herpes simplex virus 1 DNA polymerase. J. Biol. Chem. 2006, 281, 18193–18200. [Google Scholar] [CrossRef]
- Berman, H.M.; Battistuz, T.; Bhat, T.N.; Bluhm, W.F.; Bourne, P.E.; Burkhardt, K.; Feng, Z.; Gilliland, G.L.; Iype, L.; Jain, S.; et al. The Protein Data Bank. Acta Crystallogr. D Biol. Crystallogr. 2002, 58, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Chemaly, R.F.; Hill, J.A.; Voigt, S.; Peggs, K.S. In vitro comparison of currently available and investigational antiviral agents against pathogenic human double-stranded DNA viruses: A systematic literature review. Antivir. Res. 2019, 163, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Majewska, A.; Mlynarczyk-Bonikowska, B. 40 Years after the Registration of Acyclovir: Do We Need New Anti-Herpetic Drugs? Int. J. Mol. Sci. 2022, 23, 3431. [Google Scholar] [CrossRef] [PubMed]
- Klysik, K.; Pietraszek, A.; Karewicz, A.; Nowakowska, M. Acyclovir in the Treatment of Herpes Viruses—A Review. Curr. Med. Chem. 2020, 27, 4118–4137. [Google Scholar] [CrossRef]
- O'Brien, J.J.; Campoli-Richards, D.M. Acyclovir. An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs 1989, 37, 233–309. [Google Scholar] [PubMed]
- Perry, C.M.; Faulds, D. Valaciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in herpesvirus infections. Drugs 1996, 52, 754–772. [Google Scholar] [CrossRef] [PubMed]
- Beutner, K.R. Valacyclovir: A review of its antiviral activity, pharmacokinetic properties, and clinical efficacy. Antivir. Res. 1995, 28, 281–290. [Google Scholar] [CrossRef]
- Kaufman, H.; Martola, E.L.; Dohlman, C. Use of 5-iodo-2′-deoxyuridine (IDU) in treatment of herpes simplex keratitis. Arch. Ophthalmol. 1962, 68, 235–239. [Google Scholar] [CrossRef]
- Prusoff, W.H. Synthesis and biological activities of iododeoxyuridine, an analog of thymidine. Biochim. Biophys. Acta 1959, 32, 295–296. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J. Overview of antiviral medications used in ophthalmology. Community Eye Health 2020, 33, 85–88. [Google Scholar] [PubMed]
- Kulikowski, T. Structure-activity relationships and conformational features of antiherpetic pyrimidine and purine nucleoside analogues. A review. Pharm. World Sci. 1994, 16, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Allaudeen, H.S.; Kozarich, J.W.; Bertino, J.R.; De Clercq, E. On the mechanism of selective inhibition of herpesvirus replication by (E)-5-(2-bromovinyl)-2′-deoxyuridine. Proc. Natl. Acad. Sci. USA 1981, 78, 2698–2702. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Discovery and development of BVDU (brivudin) as a therapeutic for the treatment of herpes zoster. Biochem. Pharmacol. 2004, 68, 2301–2315. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. The development of BVDU: An odyssey. Antivir. Chem. Chemother. 2023, 31, 20402066231152971. [Google Scholar] [CrossRef] [PubMed]
- Mancini, W.R.; De Clercq, E.; Prusoff, W.H. The relationship between incorporation of E-5-(2-Bromovinyl)-2'-deoxyuridine into herpes simplex virus type 1 DNA with virus infectivity and DNA integrity. J. Biol. Chem. 1983, 258, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Reefschlager, J.; Barwolff, D.; Herrmann, G.; Langen, P. Antiherpes activity of some novel analogues of (E)-5-(2-bromovinyl)-2′-deoxyuridine (E-BrVUdR) in two different cell lines. Acta Virol. 1984, 28, 1–10. [Google Scholar] [PubMed]
- Jones, A.S.; Rahim, S.G.; Walker, R.T.; De Clercq, E. Synthesis and antiviral properties of (Z)-5-(2-bromovinyl)-2′-deoxyuridine. J. Med. Chem. 1981, 24, 759–760. [Google Scholar] [CrossRef]
- Whitley, R.J.; Ch'ien, L.T.; Dolin, R.; Galasso, G.J.; Alford, C.A., Jr. Adenine arabinoside therapy of herpes zoster in the immunosuppressed. NIAID collaborative antiviral study. N. Engl. J. Med. 1976, 294, 1193–1199. [Google Scholar] [CrossRef]
- Brady, R.C.; Bernstein, D.I. Treatment of herpes simplex virus infections. Antivir. Res. 2004, 61, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Whitley, R.; Alford, C.; Hess, F.; Buchanan, R. Vidarabine: A preliminary review of its pharmacological properties and therapeutic use. Drugs 1980, 20, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Seley-Radtke, K.L.; Yates, M.K. The evolution of nucleoside analogue antivirals: A review for chemists and non-chemists. Part 1: Early structural modifications to the nucleoside scaffold. Antivir. Res. 2018, 154, 66–86. [Google Scholar] [CrossRef] [PubMed]
- Hamann, M.T.; Hill, R.; Roggo, S. Marine Natural Products. Key Advances to the Practical Application of this Resource in Drug Development. Chimia 2007, 61, 313. [Google Scholar] [CrossRef]
- Faulds, D.; Heel, R.C. Ganciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in cytomegalovirus infections. Drugs 1990, 39, 597–638. [Google Scholar] [CrossRef] [PubMed]
- Bacon, T.H.; Howard, B.A.; Spender, L.C.; Boyd, M.R. Activity of penciclovir in antiviral assays against herpes simplex virus. J. Antimicrob. Chemother. 1996, 37, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Cirelli, R.; Herne, K.; McCrary, M.; Lee, P.; Tyring, S.K. Famciclovir: Review of clinical efficacy and safety. Antiviral Res. 1996, 29, 141–151. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. The Elegance of the Acyclic Nucleoside Phosphonates (ANPs), Honorary Tribute to Antonin Holy, Who Passed Away on 16 July 2012, at the 10th Anniversary of His Death. Viruses 2022, 14, 1978. [Google Scholar] [CrossRef] [PubMed]
- Groaz, E.; De Jonghe, S. Overview of Biologically Active Nucleoside Phosphonates. Front. Chem. 2020, 8, 616863. [Google Scholar] [CrossRef]
- De Clercq, E.; Holy, A. Acyclic nucleoside phosphonates: A key class of antiviral drugs. Nat. Rev. Drug Discov. 2005, 4, 928–940. [Google Scholar] [CrossRef]
- Pradere, U.; Garnier-Amblard, E.C.; Coats, S.J.; Amblard, F.; Schinazi, R.F. Synthesis of nucleoside phosphate and phosphonate prodrugs. Chem. Rev. 2014, 114, 9154–9218. [Google Scholar] [CrossRef]
- Xiong, X.; Smith, J.L.; Chen, M.S. Effect of incorporation of cidofovir into DNA by human cytomegalovirus DNA polymerase on DNA elongation. Antimicrob. Agents Chemother. 1997, 41, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, J.M.; Sortino, K.; Sethna, P.; Bae, A.; Lanier, R.; Bambara, R.A.; Dewhurst, S. Cidofovir Diphosphate Inhibits Adenovirus 5 DNA Polymerase via both Nonobligate Chain Termination and Direct Inhibition, and Polymerase Mutations Confer Cidofovir Resistance on Intact Virus. Antimicrob. Agents Chemother. 2019, 63, 10–128. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.R.; Rutvisuttinunt, W.; Matsuura, S.E.; So, A.G.; Scott, W.A. Stable complexes formed by HIV-1 reverse transcriptase at distinct positions on the primer-template controlled by binding deoxynucleoside triphosphates or foscarnet. J. Mol. Biol. 2007, 369, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Zahn, K.E.; Tchesnokov, E.P.; Gotte, M.; Doublie, S. Phosphonoformic acid inhibits viral replication by trapping the closed form of the DNA polymerase. J. Biol. Chem. 2011, 286, 25246–25255. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, K.; Zhu, X.; Pham, V.D.; Goyette, N.; Piret, J.; Shi, R.; Boivin, G. Impact of Amino Acid Substitutions in Region II and Helix K of Herpes Simplex Virus 1 and Human Cytomegalovirus DNA Polymerases on Resistance to Foscarnet. Antimicrob. Agents Chemother. 2021, 65, e0039021. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Balzarini, J.; Miller, M.T.; Maguire, A.R.; DeStefano, J.J.; Arnold, E. Conformational States of HIV-1 Reverse Transcriptase for Nucleotide Incorporation vs Pyrophosphorolysis-Binding of Foscarnet. ACS Chem. Biol. 2016, 11, 2158–2164. [Google Scholar] [CrossRef] [PubMed]
- Buhlig, T.S.; Bowersox, A.F.; Braun, D.L.; Owsley, D.N.; James, K.D.; Aranda, A.J.; Kendrick, C.D.; Skalka, N.A.; Clark, D.N. Molecular, Evolutionary, and Structural Analysis of the Terminal Protein Domain of Hepatitis B Virus Polymerase, a Potential Drug Target. Viruses 2020, 12, 570. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.N.; Hu, J. Hepatitis B virus reverse transcriptase—Target of current antiviral therapy and future drug development. Antiviral Res. 2015, 123, 132–137. [Google Scholar] [CrossRef]
- Daga, P.R.; Duan, J.; Doerksen, R.J. Computational model of hepatitis B virus DNA polymerase: Molecular dynamics and docking to understand resistant mutations. Protein Sci. 2010, 19, 796–807. [Google Scholar] [CrossRef]
- Das, K.; Xiong, X.; Yang, H.; Westland, C.E.; Gibbs, C.S.; Sarafianos, S.G.; Arnold, E. Molecular modeling and biochemical characterization reveal the mechanism of hepatitis B virus polymerase resistance to lamivudine (3TC) and emtricitabine (FTC). J. Virol. 2001, 75, 4771–4779. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Thai, H.; Kitrinos, K.M.; Xia, G.; Gaggar, A.; Paulson, M.; Ganova-Raeva, L.; Khudyakov, Y.; Lara, J. Modeling the functional state of the reverse transcriptase of hepatitis B virus and its application to probing drug-protein interaction. BMC Bioinform. 2016, 17 (Suppl. S8), 280. [Google Scholar] [CrossRef] [PubMed]
- Tajwar, R.; Bradley, D.P.; Ponzar, N.L.; Tavis, J.E. Predicted structure of the hepatitis B virus polymerase reveals an ancient conserved protein fold. Protein Sci. 2022, 31, e4421. [Google Scholar] [CrossRef]
- Mokaya, J.; McNaughton, A.L.; Bester, P.A.; Goedhals, D.; Barnes, E.; Marsden, B.D.; Matthews, P.C. Hepatitis B virus resistance to tenofovir: Fact or fiction? A systematic literature review and structural analysis of drug resistance mechanisms. Wellcome Open Res. 2020, 5, 151. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Du, Y.; Liu, X.; Shen, Q.C.; Huang, A.L.; Zheng, M.Y.; Luo, X.M.; Jiang, H.L. Binding sensitivity of adefovir to the polymerase from different genotypes of HBV: Molecular modeling, docking and dynamics simulation studies. Acta Pharmacol. Sin. 2013, 34, 319–328. [Google Scholar] [CrossRef]
- van Hemert, F.J.; Berkhout, B.; Zaaijer, H.L. Differential binding of tenofovir and adefovir to reverse transcriptase of hepatitis B virus. PLoS ONE 2014, 9, e106324. [Google Scholar] [CrossRef] [PubMed]
- Tsai, E. Review of Current and Potential Treatments for Chronic Hepatitis B Virus Infection. Gastroenterol. Hepatol. 2021, 17, 367–376. [Google Scholar]
- Ranga, A.; Gupta, A.; Yadav, L.; Kumar, S.; Jain, P. Advancing beyond reverse transcriptase inhibitors: The new era of hepatitis B polymerase inhibitors. Eur. J. Med. Chem. 2023, 257, 115455. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Maag, H.; Alfredson, T. Prodrugs of nucleoside analogues for improved oral absorption and tissue targeting. J. Pharm. Sci. 2008, 97, 1109–1134. [Google Scholar] [CrossRef]
- Fung, J.; Lai, C.L.; Seto, W.K.; Yuen, M.F. Nucleoside/nucleotide analogues in the treatment of chronic hepatitis B. J. Antimicrob. Chemother. 2011, 66, 2715–2725. [Google Scholar] [CrossRef]
- Zheng, J.; Deng, M.; Qiu, X.; Chen, Z.; Li, D.; Deng, X.; Deng, Q.; Yu, Z. Rhabdomyolysis, lactic acidosis, and multiple organ failure during telbivudine treatment for hepatitis B: A case report and review of the literature. J. Med. Case Rep. 2017, 11, 331. [Google Scholar] [CrossRef] [PubMed]
- Domaoal, R.A.; McMahon, M.; Thio, C.L.; Bailey, C.M.; Tirado-Rives, J.; Obikhod, A.; Detorio, M.; Rapp, K.L.; Siliciano, R.F.; Schinazi, R.F.; et al. Pre-steady-state kinetic studies establish entecavir 5′-triphosphate as a substrate for HIV-1 reverse transcriptase. J. Biol. Chem. 2008, 283, 5452–5459. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, Y.; Tanaka, Y.; Kohgo, S.; Murakami, S.; Singh, K.; Das, D.; Venzon, D.J.; Amano, M.; Higashi-Kuwata, N.; Aoki, M.; et al. 4′-modified nucleoside analogs: Potent inhibitors active against entecavir-resistant hepatitis B virus. Hepatology 2015, 62, 1024–1036. [Google Scholar] [CrossRef] [PubMed]
- Yurdaydin, C. Entecavir: A step forward in combating hepatitis B disease. Expert. Opin. Pharmacother. 2008, 9, 3095–3109. [Google Scholar] [CrossRef] [PubMed]
- German Advisory Committee Blood (Arbeitskreis Blut), Subgroup ‘Assessment of Pathogens Transmissible by Blood’. Human Immunodeficiency Virus (HIV). Transfus. Med. Hemother 2016, 43, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Sarafianos, S.G.; Marchand, B.; Das, K.; Himmel, D.M.; Parniak, M.A.; Hughes, S.H.; Arnold, E. Structure and function of HIV-1 reverse transcriptase: Molecular mechanisms of polymerization and inhibition. J. Mol. Biol. 2009, 385, 693–713. [Google Scholar] [CrossRef]
- Beilhartz, G.L.; Gotte, M. HIV-1 Ribonuclease H: Structure, Catalytic Mechanism and Inhibitors. Viruses 2010, 2, 900–926. [Google Scholar] [CrossRef] [PubMed]
- Menendez-Arias, L.; Sebastian-Martin, A.; Alvarez, M. Viral reverse transcriptases. Virus Res. 2017, 234, 153–176. [Google Scholar] [CrossRef]
- Kuroda, D.G.; Bauman, J.D.; Challa, J.R.; Patel, D.; Troxler, T.; Das, K.; Arnold, E.; Hochstrasser, R.M. Snapshot of the equilibrium dynamics of a drug bound to HIV-1 reverse transcriptase. Nat. Chem. 2013, 5, 174–181. [Google Scholar] [CrossRef]
- Huang, H.; Chopra, R.; Verdine, G.L.; Harrison, S.C. Structure of a covalently trapped catalytic complex of HIV-1 reverse transcriptase: Implications for drug resistance. Science 1998, 282, 1669–1675. [Google Scholar] [CrossRef]
- Martin-Hernandez, A.M.; Domingo, E.; Menendez-Arias, L. Human immunodeficiency virus type 1 reverse transcriptase: Role of Tyr115 in deoxynucleotide binding and misinsertion fidelity of DNA synthesis. EMBO J. 1996, 15, 4434–4442. [Google Scholar] [CrossRef]
- Li, G.; Wang, Y.; De Clercq, E. Approved HIV reverse transcriptase inhibitors in the past decade. Acta Pharm. Sin. B 2022, 12, 1567–1590. [Google Scholar] [CrossRef]
- Holec, A.D.; Mandal, S.; Prathipati, P.K.; Destache, C.J. Nucleotide Reverse Transcriptase Inhibitors: A Thorough Review, Present Status and Future Perspective as HIV Therapeutics. Curr. HIV Res. 2017, 15, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Devineni, D.; Gallo, J.M. Zalcitabine. Clinical pharmacokinetics and efficacy. Clin. Pharmacokinet. 1995, 28, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Gumina, G.; Song, G.Y.; Chu, C.K. L-Nucleosides as chemotherapeutic agents. FEMS Microbiol. Lett. 2001, 202, 9–15. [Google Scholar] [PubMed]
- Andrade, C.H.; Freitas, L.M.d.; Oliveira, V.d. Twenty-six years of HIV science: An overview of anti-HIV drugs metabolism. Braz. J. Pharm. Sci. 2011, 47, 209–230. [Google Scholar] [CrossRef]
- Yeom, Y.H.; Remmel, R.P.; Huang, S.H.; Hua, M.; Vince, R.; Zimmerman, C.L. Pharmacokinetics and bioavailability of carbovir, a carbocyclic nucleoside active against human immunodeficiency virus, in rats. Antimicrob. Agents Chemother. 1989, 33, 171–175. [Google Scholar] [CrossRef]
- Wassner, C.; Bradley, N.; Lee, Y. A Review and Clinical Understanding of Tenofovir: Tenofovir Disoproxil Fumarate versus Tenofovir Alafenamide. J. Int. Assoc. Provid. AIDS Care 2020, 19, 2325958220919231. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, V.; Vanangamudi, M.; Kramer, V.G.; Kurup, S.; Zhan, P.; Liu, X.; Kongsted, J.; Byrareddy, S.N. The Journey of HIV-1 Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) from Lab to Clinic. J. Med. Chem. 2019, 62, 4851–4883. [Google Scholar] [CrossRef]
- Hsiou, Y.; Ding, J.; Das, K.; Clark, A.D., Jr.; Hughes, S.H.; Arnold, E. Structure of unliganded HIV-1 reverse transcriptase at 2.7 A resolution: Implications of conformational changes for polymerization and inhibition mechanisms. Structure 1996, 4, 853–860. [Google Scholar] [CrossRef]
- Adams, J.; Patel, N.; Mankaryous, N.; Tadros, M.; Miller, C.D. Nonnucleoside reverse transcriptase inhibitor resistance and the role of the second-generation agents. Ann. Pharmacother. 2010, 44, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release 2019-2, Maestro; Schrödinger, LLC: New York, NY, USA, 2019.
- Wolber, G.; Langer, T. LigandScout: 3-D pharmacophores derived from protein-bound ligands and their use as virtual screening filters. J. Chem. Inf. Model. 2005, 45, 160–169. [Google Scholar] [CrossRef] [PubMed]
- FDA-Approved HIV Medicines. Available online: https://hivinfo.nih.gov/understanding-hiv/fact-sheets/fda-approved-hiv-medicines (accessed on 30 March 2024).
- Barreca, M.L.; Iraci, N.; Manfroni, G.; Cecchetti, V. Allosteric inhibition of the hepatitis C virus NS5B polymerase: In silico strategies for drug discovery and development. Future Med. Chem. 2011, 3, 1027–1055. [Google Scholar] [CrossRef] [PubMed]
- Lesburg, C.A.; Cable, M.B.; Ferrari, E.; Hong, Z.; Mannarino, A.F.; Weber, P.C. Crystal structure of the RNA-dependent RNA polymerase from hepatitis C virus reveals a fully encircled active site. Nat. Struct. Biol. 1999, 6, 937–943. [Google Scholar] [PubMed]
- Brass, V.; Gouttenoire, J.; Wahl, A.; Pal, Z.; Blum, H.E.; Penin, F.; Moradpour, D. Hepatitis C Virus RNA Replication Requires a Conserved Structural Motif within the Transmembrane Domain of the NS5B RNA-Dependent RNA Polymerase. J. Virol. 2010, 84, 11580–11584. [Google Scholar] [CrossRef] [PubMed]
- Tomei, L.; Altamura, S.; Paonessa, G.; De Francesco, R.; Migliaccio, G. HCV antiviral resistance: The impact of in vitro studies on the development of antiviral agents targeting the viral NS5B polymerase. Antivir. Chem. Chemother. 2005, 16, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Sofia, M.J.; Chang, W.; Furman, P.A.; Mosley, R.T.; Ross, B.S. Nucleoside, nucleotide, and non-nucleoside inhibitors of hepatitis C virus NS5B RNA-dependent RNA-polymerase. J. Med. Chem. 2012, 55, 2481–2531. [Google Scholar] [CrossRef] [PubMed]
- Gotte, M.; Feld, J.J. Direct-acting antiviral agents for hepatitis C: Structural and mechanistic insights. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Johnson, K.A. Thumb Site 2 Inhibitors of Hepatitis C Viral RNA-dependent RNA Polymerase Allosterically Block the Transition from Initiation to Elongation. J. Biol. Chem. 2016, 291, 10067–10077. [Google Scholar] [CrossRef]
- Graci, J.D.; Cameron, C.E. Mechanisms of action of ribavirin against distinct viruses. Rev. Med. Virol. 2006, 16, 37–48. [Google Scholar] [CrossRef]
- Mathur, P.; Kottilil, S.; Wilson, E. Use of Ribavirin for Hepatitis C Treatment in the Modern Direct-acting Antiviral Era. J. Clin. Transl. Hepatol. 2018, 6, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Appleby, T.C.; Perry, J.K.; Murakami, E.; Barauskas, O.; Feng, J.; Cho, A.; Fox, D., 3rd; Wetmore, D.R.; McGrath, M.E.; Ray, A.S.; et al. Viral replication. Structural basis for RNA replication by the hepatitis C virus polymerase. Science 2015, 347, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Mantry, P.S.; Pathak, L. Dasabuvir (ABT333) for the treatment of chronic HCV genotype I: A new face of cure, an expert review. Expert. Rev. Anti Infect. Ther. 2016, 14, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, R.C.; Bourdet, D.L.; Brameld, K.A.; Chin, E.; de Vicente, J.; Fung, A.; Harris, S.F.; Lee, E.K.; Le Pogam, S.; Leveque, V.; et al. Discovery of a novel series of potent non-nucleoside inhibitors of hepatitis C virus NS5B. J. Med. Chem. 2013, 56, 8163–8182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gentles, R.G.; Ding, M.; Bender, J.A.; Bergstrom, C.P.; Grant-Young, K.; Hewawasam, P.; Hudyma, T.; Martin, S.; Nickel, A.; Regueiro-Ren, A.; et al. Discovery and preclinical characterization of the cyclopropylindolobenzazepine BMS-791325, a potent allosteric inhibitor of the hepatitis C virus NS5B polymerase. J. Med. Chem. 2014, 57, 1855–1879. [Google Scholar] [CrossRef] [PubMed]
- SeeSAR, version 13.0.1; BioSolveIT, GmbH: Sankt Augustin, Germany, 2023. Available online: www.biosolveit.de/SeeSAR (accessed on 30 March 2024).
- Cao, D.D.; Gao, Y.R.; Roesler, C.; Rice, S.; D'Cunha, P.; Zhuang, L.; Slack, J.; Domke, M.; Antonova, A.; Romanelli, S.; et al. Cryo-EM structure of the respiratory syncytial virus RNA polymerase. Nat. Commun. 2020, 11, 368. [Google Scholar] [CrossRef] [PubMed]
- Sutto-Ortiz, P.; Eleouet, J.F.; Ferron, F.; Decroly, E. Biochemistry of the Respiratory Syncytial Virus L Protein Embedding RNA Polymerase and Capping Activities. Viruses 2023, 15, 341. [Google Scholar] [CrossRef] [PubMed]
- Gilman, M.S.A.; Liu, C.; Fung, A.; Behera, I.; Jordan, P.; Rigaux, P.; Ysebaert, N.; Tcherniuk, S.; Sourimant, J.; Eleouet, J.F.; et al. Structure of the Respiratory Syncytial Virus Polymerase Complex. Cell 2019, 179, 193–204. [Google Scholar] [CrossRef]
- Kleiner, V.A.; T, O.F.; Howe, J.A.; Beshore, D.C.; Eddins, M.J.; Hou, Y.; Mayhood, T.; Klein, D.; Nahas, D.D.; Lucas, B.J.; et al. Conserved allosteric inhibitory site on the respiratory syncytial virus and human metapneumovirus RNA-dependent RNA polymerases. Commun. Biol. 2023, 6, 649. [Google Scholar] [CrossRef]
- Yu, X.; Abeywickrema, P.; Bonneux, B.; Behera, I.; Anson, B.; Jacoby, E.; Fung, A.; Adhikary, S.; Bhaumik, A.; Carbajo, R.J.; et al. Structural and mechanistic insights into the inhibition of respiratory syncytial virus polymerase by a non-nucleoside inhibitor. Commun. Biol. 2023, 6, 1074. [Google Scholar] [CrossRef]
- Cao, D.; Gao, Y.; Chen, Z.; Gooneratne, I.; Roesler, C.; Mera, C.; D’Cunha, P.; Antonova, A.; Katta, D.; Romanelli, S.; et al. Structures of the promoter-bound respiratory syncytial virus polymerase. Nature 2024, 625, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Groothuis, J.R.; Woodin, K.A.; Katz, R.; Robertson, A.D.; McBride, J.T.; Hall, C.B.; McWilliams, B.C.; Lauer, B.A. Early ribavirin treatment of respiratory syncytial viral infection in high-risk children. J. Pediatr. 1990, 117, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Reich, S.; Guilligay, D.; Pflug, A.; Malet, H.; Berger, I.; Crepin, T.; Hart, D.; Lunardi, T.; Nanao, M.; Ruigrok, R.W.; et al. Structural insight into cap-snatching and RNA synthesis by influenza polymerase. Nature 2014, 516, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Wandzik, J.M.; Kouba, T.; Cusack, S. Structure and Function of Influenza Polymerase. Cold Spring Harb. Perspect. Med. 2021, 11. [Google Scholar] [CrossRef]
- Lo, C.Y.; Tang, Y.S.; Shaw, P.C. Structure and Function of Influenza Virus Ribonucleoprotein. Subcell. Biochem. 2018, 88, 95–128. [Google Scholar]
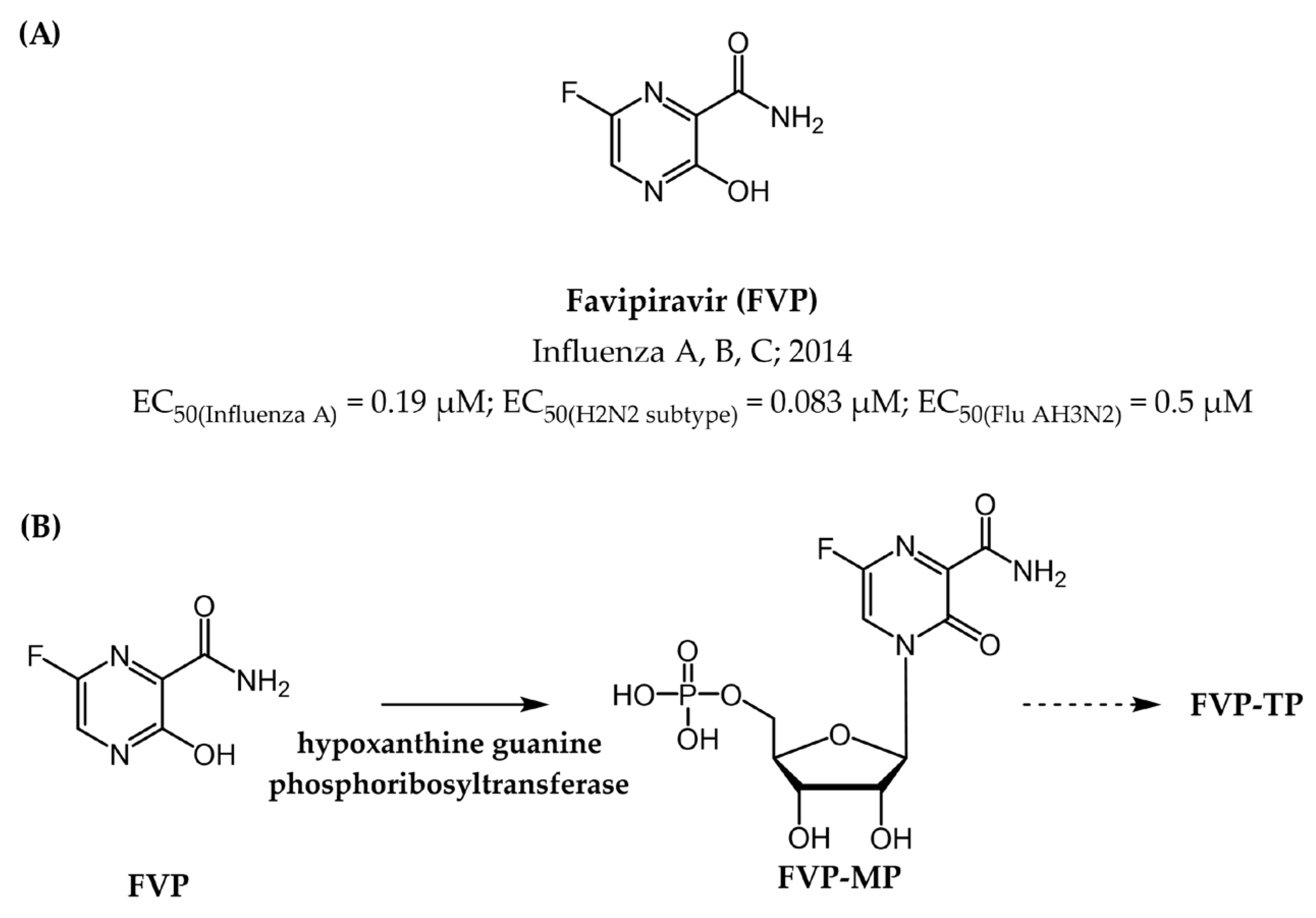
- Furuta, Y.; Komeno, T.; Nakamura, T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Furuta, Y.; Gowen, B.B.; Takahashi, K.; Shiraki, K.; Smee, D.F.; Barnard, D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antivir. Res. 2013, 100, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, K.; Daikoku, T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol. Ther. 2020, 209, 107512. [Google Scholar] [CrossRef]
- Shannon, A.; Selisko, B.; Le, N.T.; Huchting, J.; Touret, F.; Piorkowski, G.; Fattorini, V.; Ferron, F.; Decroly, E.; Meier, C.; et al. Rapid incorporation of Favipiravir by the fast and permissive viral RNA polymerase complex results in SARS-CoV-2 lethal mutagenesis. Nat. Commun. 2020, 11, 4682. [Google Scholar] [CrossRef]
- Baranovich, T.; Wong, S.S.; Armstrong, J.; Marjuki, H.; Webby, R.J.; Webster, R.G.; Govorkova, E.A. T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro. J. Virol. 2013, 87, 3741–3751. [Google Scholar] [CrossRef]
- Goldhill, D.H.; Langat, P.; Xie, H.; Galiano, M.; Miah, S.; Kellam, P.; Zambon, M.; Lackenby, A.; Barclay, W.S. Determining the Mutation Bias of Favipiravir in Influenza Virus Using Next-Generation Sequencing. J. Virol. 2019, 93, e01217-18. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Peng, R.; Yuan, B.; Wang, M.; Zhao, J.; Fu, L.; Qi, J.; Shi, Y. Structural Basis of SARS-CoV-2 Polymerase Inhibition by Favipiravir. Innovation 2021, 2, 100080. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern (vol 395, pg 470, 2020). Lancet 2020, 395, 496. [Google Scholar] [CrossRef]
- Dhama, K.; Khan, S.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Coronavirus Disease 2019-COVID-19. Clin Microbiol Rev 2020, 33, e00028-20. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19); StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, K.; Gao, W.; Lv, J.; Yu, C.; Wang, L.; Wang, Z.; Wang, B.; Liao, C.; Li, L. Asymptomatic and pre-symptomatic infection in Coronavirus Disease 2019 pandemic. Med. Rev. 2022, 2, 66–88. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.A.; Quandelacy, T.M.; Kada, S.; Prasad, P.V.; Steele, M.; Brooks, J.T.; Slayton, R.B.; Biggerstaff, M.; Butler, J.C. SARS-CoV-2 Transmission From People Without COVID-19 Symptoms. JAMA Netw. Open 2021, 4, e2035057. [Google Scholar] [CrossRef]
- Lei, S.; Chen, X.; Wu, J.; Duan, X.; Men, K. Small molecules in the treatment of COVID-19. Signal Transduct. Target. Ther. 2022, 7, 387. [Google Scholar] [CrossRef]
- Xiang, R.; Yu, Z.; Wang, Y.; Wang, L.; Huo, S.; Li, Y.; Liang, R.; Hao, Q.; Ying, T.; Gao, Y.; et al. Recent advances in developing small-molecule inhibitors against SARS-CoV-2. Acta Pharm. Sin. B 2022, 12, 1591–1623. [Google Scholar] [CrossRef]
- Kumari, M.; Lu, R.M.; Li, M.C.; Huang, J.L.; Hsu, F.F.; Ko, S.H.; Ke, F.Y.; Su, S.C.; Liang, K.H.; Yuan, J.P.; et al. A critical overview of current progress for COVID-19: Development of vaccines, antiviral drugs, and therapeutic antibodies. J. Biomed. Sci. 2022, 29, 68. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yan, L.; Huang, Y.; Liu, F.; Zhao, Y.; Cao, L.; Wang, T.; Sun, Q.; Ming, Z.; Zhang, L.; et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science 2020, 368, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Chen, C.Z.; Gorshkov, K.; Xu, M.; Lo, D.C.; Zheng, W. RNA-Dependent RNA Polymerase as a Target for COVID-19 Drug Discovery. SLAS Discov. 2020, 25, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Vicenti, I.; Zazzi, M.; Saladini, F. SARS-CoV-2 RNA-dependent RNA polymerase as a therapeutic target for COVID-19. Expert. Opin. Ther. Pat. 2021, 31, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yin, W.; Xu, H.E. RNA-dependent RNA polymerase: Structure, mechanism, and drug discovery for COVID-19. Biochem. Biophys. Res. Commun. 2021, 538, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Hillen, H.S. Structure and function of SARS-CoV-2 polymerase. Curr. Opin. Virol. 2021, 48, 82–90. [Google Scholar] [CrossRef]
- Hillen, H.S.; Kokic, G.; Farnung, L.; Dienemann, C.; Tegunov, D.; Cramer, P. Structure of replicating SARS-CoV-2 polymerase. Nature 2020, 584, 154–156. [Google Scholar] [CrossRef]
- Snijder, E.J.; Decroly, E.; Ziebuhr, J. The Nonstructural Proteins Directing Coronavirus RNA Synthesis and Processing. Adv. Virus Res. 2016, 96, 59–126. [Google Scholar]
- Askari, F.S.; Ebrahimi, M.; Parhiz, J.; Hassanpour, M.; Mohebbi, A.; Mirshafiey, A. Digging for the discovery of SARS-CoV-2 nsp12 inhibitors: A pharmacophore-based and molecular dynamics simulation study. Future Virol. 2022, 17, 743–759. [Google Scholar] [CrossRef]
- Gordon, C.J.; Tchesnokov, E.P.; Woolner, E.; Perry, J.K.; Feng, J.Y.; Porter, D.P.; Gotte, M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020, 295, 6785–6797. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.X.; et al. Compassionate Use of Remdesivir for Patients with Severe COVID-19. N. Engl. J. Med. 2020, 382, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Eastman, R.T.; Roth, J.S.; Brimacombe, K.R.; Simeonov, A.; Shen, M.; Patnaik, S.; Hall, M.D. Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19. ACS Cent. Sci. 2020, 6, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Mao, C.; Luan, X.; Shen, D.D.; Shen, Q.; Su, H.; Wang, X.; Zhou, F.; Zhao, W.; Gao, M.; et al. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science 2020, 368, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Masyeni, S.; Iqhrammullah, M.; Frediansyah, A.; Nainu, F.; Tallei, T.; Emran, T.B.; Ophinni, Y.; Dhama, K.; Harapan, H. Molnupiravir: A lethal mutagenic drug against rapidly mutating severe acute respiratory syndrome coronavirus 2—A narrative review. J. Med. Virol. 2022, 94, 3006–3016. [Google Scholar] [CrossRef] [PubMed]
- Zarenezhad, E.; Marzi, M. Review on molnupiravir as a promising oral drug for the treatment of COVID-19. Med. Chem. Res. 2022, 31, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Pang, Z.; Li, M.; Lou, F.; An, X.; Zhu, S.; Song, L.; Tong, Y.; Fan, H.; Fan, J. Molnupiravir and Its Antiviral Activity Against COVID-19. Front. Immunol. 2022, 13, 855496. [Google Scholar] [CrossRef] [PubMed]
- Kabinger, F.; Stiller, C.; Schmitzova, J.; Dienemann, C.; Kokic, G.; Hillen, H.S.; Hobartner, C.; Cramer, P. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat. Struct. Mol. Biol. 2021, 28, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; He, G.; Huang, W. A novel model of molnupiravir against SARS-CoV-2 replication: Accumulated RNA mutations to induce error catastrophe. Signal Transduct. Tar. 2021, 6, 410. [Google Scholar] [CrossRef]
- Ibrahim, M.A.A.; Shehata, M.N.I.; Moussa, N.A.M.; Hemia, R.R.A.; Abd Elhafez, H.S.M.; Abd El-Rahman, M.K.; Sayed, S.R.M.; Sidhom, P.A.; Dabbish, E.; Shoeib, T. Preferability of Molnupiravir, an Anti-COVID-19 Drug, toward Purine Nucleosides: A Quantum Mechanical Study. ACS Omega 2023, 8, 27553–27565. [Google Scholar] [CrossRef]
- Strasfeld, L.; Chou, S. Antiviral drug resistance: Mechanisms and clinical implications. Infect. Dis. Clin. N. Am. 2010, 24, 413–437. [Google Scholar] [CrossRef]
- Namba-Nzanguim, C.T.; Turon, G.; Simoben, C.V.; Tietjen, I.; Montaner, L.J.; Efange, S.M.N.; Duran-Frigola, M.; Ntie-Kang, F. Artificial intelligence for antiviral drug discovery in low resourced settings: A perspective. Front. Drug Discov. 2022, 2, 1013285. [Google Scholar] [CrossRef]
- Matthew, A.N.; Leidner, F.; Lockbaum, G.J.; Henes, M.; Zephyr, J.; Hou, S.; Rao, D.N.; Timm, J.; Rusere, L.N.; Ragland, D.A.; et al. Drug Design Strategies to Avoid Resistance in Direct-Acting Antivirals and Beyond. Chem. Rev. 2021, 121, 3238–3270. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Hu, X.; Menendez-Arias, L.; Zhan, P.; Liu, X. Target-based drug design strategies to overcome resistance to antiviral agents: Opportunities and challenges. Drug Resist. Updat. 2024, 73, 101053. [Google Scholar] [CrossRef] [PubMed]
- Barreca, M.L.; Iraci, N.; Manfroni, G.; Gaetani, R.; Guercini, C.; Sabatini, S.; Tabarrini, O.; Cecchetti, V. Accounting for target flexibility and water molecules by docking to ensembles of target structures: The HCV NS5B palm site I inhibitors case study. J. Chem. Inf. Model. 2014, 54, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.; Lo, C.W.; Einav, S. Preparing for the next viral threat with broad-spectrum antivirals. J. Clin. Investig. 2023, 133, e170236. [Google Scholar] [CrossRef]
- Felicetti, T.; Manfroni, G.; Cecchetti, V.; Cannalire, R. Broad-Spectrum Flavivirus Inhibitors: A Medicinal Chemistry Point of View. ChemMedChem 2020, 15, 2391–2419. [Google Scholar] [CrossRef] [PubMed]
- Malin, J.J.; Suarez, I.; Priesner, V.; Fatkenheuer, G.; Rybniker, J. Remdesivir against COVID-19 and Other Viral Diseases. Clin. Microbiol. Rev. 2020, 34, 10–128. [Google Scholar] [CrossRef] [PubMed]
- Ison, M.G.; Hayden, F.G.; Hay, A.J.; Gubareva, L.V.; Govorkova, E.A.; Takashita, E.; McKimm-Breschkin, J.L. Influenza polymerase inhibitor resistance: Assessment of the current state of the art—A report of the isirv Antiviral group. Antivir. Res. 2021, 194, 105158. [Google Scholar] [CrossRef]
- Vincent, F.; Nueda, A.; Lee, J.; Schenone, M.; Prunotto, M.; Mercola, M. Phenotypic drug discovery: Recent successes, lessons learned and new directions. Nat. Rev. Drug Discov. 2022, 21, 899–914. [Google Scholar] [CrossRef]
- Scarsi, K.K.; Swindells, S. The Promise of Improved Adherence With Long-Acting Antiretroviral Therapy: What Are the Data? J. Int. Assoc. Provid. AIDS Care 2021, 20, 23259582211009011. [Google Scholar] [CrossRef] [PubMed]
- Bekes, M.; Langley, D.R.; Crews, C.M. PROTAC targeted protein degraders: The past is prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.C.; Crews, C.M. Induced protein degradation: An emerging drug discovery paradigm. Nat. Rev. Drug Discov. 2017, 16, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Schapira, M.; Calabrese, M.F.; Bullock, A.N.; Crews, C.M. Targeted protein degradation: Expanding the toolbox. Nat. Rev. Drug Discov. 2019, 18, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.A.; Kumru, K. Extracellular targeted protein degradation: An emerging modality for drug discovery. Nat. Rev. Drug Discov. 2024, 23, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Gao, H.; Yang, Y.; He, M.; Wu, Y.; Song, Y.; Tong, Y.; Rao, Y. PROTACs: Great opportunities for academia and industry. Signal Transduct. Target. Ther. 2019, 4, 64. [Google Scholar] [CrossRef] [PubMed]
- de Wispelaere, M.; Du, G.; Donovan, K.A.; Zhang, T.; Eleuteri, N.A.; Yuan, J.C.; Kalabathula, J.; Nowak, R.P.; Fischer, E.S.; Gray, N.S.; et al. Small molecule degraders of the hepatitis C virus protease reduce susceptibility to resistance mutations. Nat. Commun. 2019, 10, 3468. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Xiao, H.; Wang, H.; Xu, X. Recent Advances in PROTACs for Drug Targeted Protein Research. Int. J. Mol. Sci. 2022, 23, 10328. [Google Scholar] [CrossRef] [PubMed]
- Kelm, J.M.; Pandey, D.S.; Malin, E.; Kansou, H.; Arora, S.; Kumar, R.; Gavande, N.S. PROTAC’ing oncoproteins: Targeted protein degradation for cancer therapy. Mol. Cancer 2023, 22, 62. [Google Scholar] [CrossRef]
- Qi, S.M.; Dong, J.; Xu, Z.Y.; Cheng, X.D.; Zhang, W.D.; Qin, J.J. PROTAC: An Effective Targeted Protein Degradation Strategy for Cancer Therapy. Front. Pharmacol. 2021, 12, 692574. [Google Scholar] [CrossRef]
- Ahmad, H.; Zia, B.; Husain, H.; Husain, A. Recent Advances in PROTAC-Based Antiviral Strategies. Vaccines 2023, 11, 270. [Google Scholar] [CrossRef] [PubMed]
- Crunkhorn, S. PROTAC induces HIV-1 Nef degradation. Nat. Rev. Drug Discov. 2024, 23, 340. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wu, Y.; Lan, K.; Dong, C.; Wu, S.; Li, S.; Zhou, H.B. Antiviral PROTACs: Opportunity borne with challenge. Cell Insight 2023, 2, 100092. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, A.; Yang, P.L. Targeted protein degradation as an antiviral approach. Antivir. Res. 2023, 210, 105480. [Google Scholar] [CrossRef] [PubMed]
- Mukerjee, N.; Ghosh, A. Revolutionizing viral disease treatment: PROTACs therapy could be the ultimate weapon of the future. J. Med. Virol. 2023, 95, e28981. [Google Scholar] [CrossRef] [PubMed]
- von Delft, A.; Hall, M.D.; Kwong, A.D.; Purcell, L.A.; Saikatendu, K.S.; Schmitz, U.; Tallarico, J.A.; Lee, A.A. Accelerating antiviral drug discovery: Lessons from COVID-19. Nat. Rev. Drug Discov. 2023, 22, 585–603. [Google Scholar] [CrossRef]
- Peng, S.; Wang, H.; Wang, Z.; Wang, Q. Progression of Antiviral Agents Targeting Viral Polymerases. Molecules 2022, 27, 7370. [Google Scholar] [CrossRef]
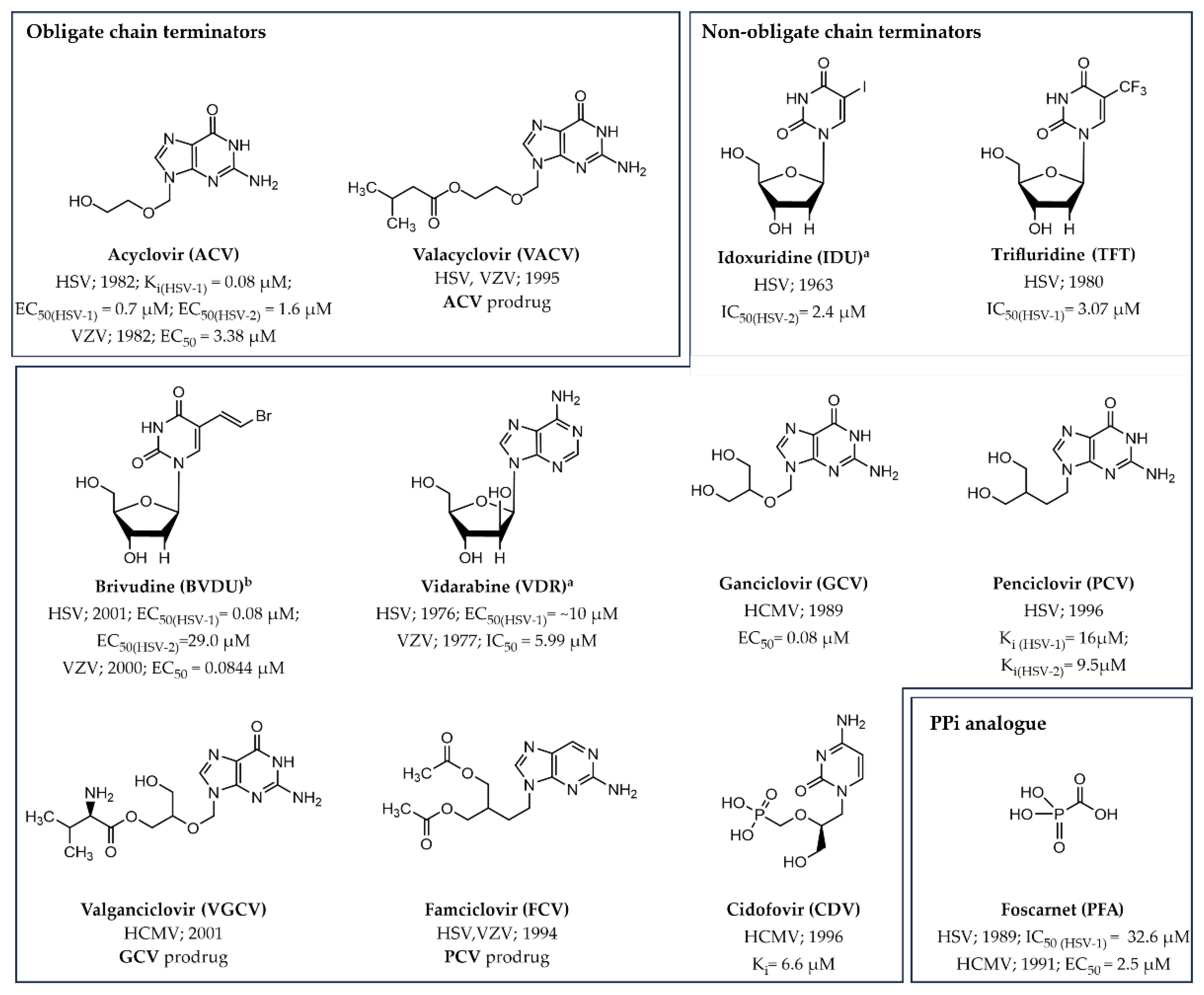


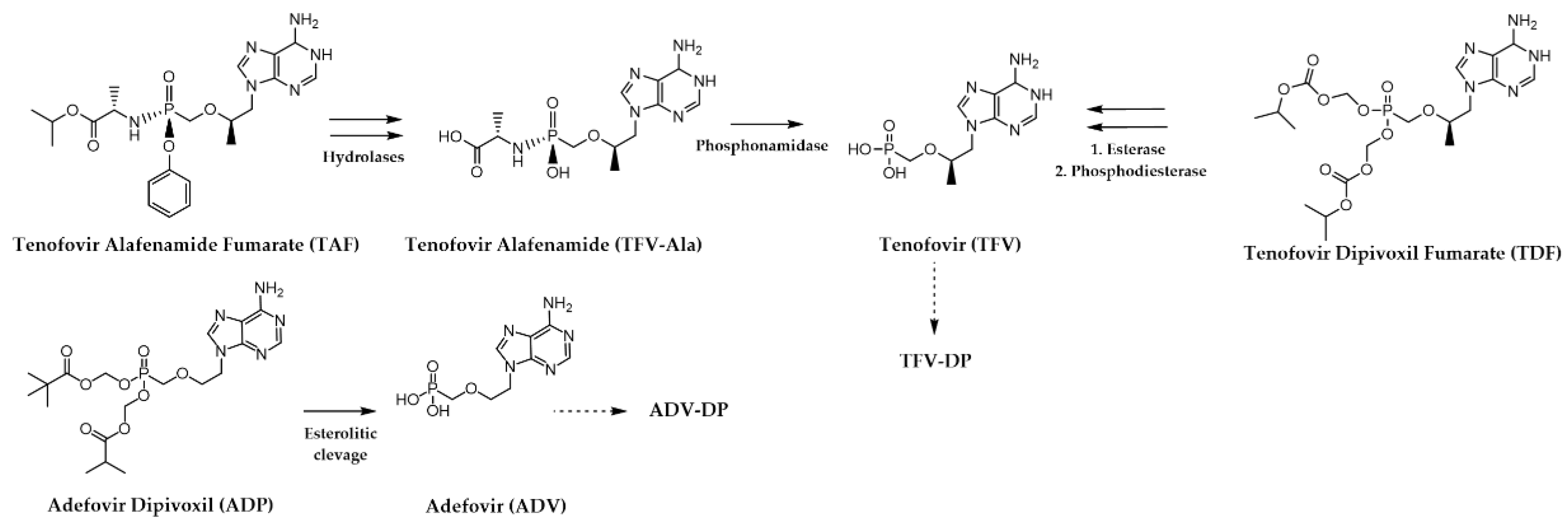


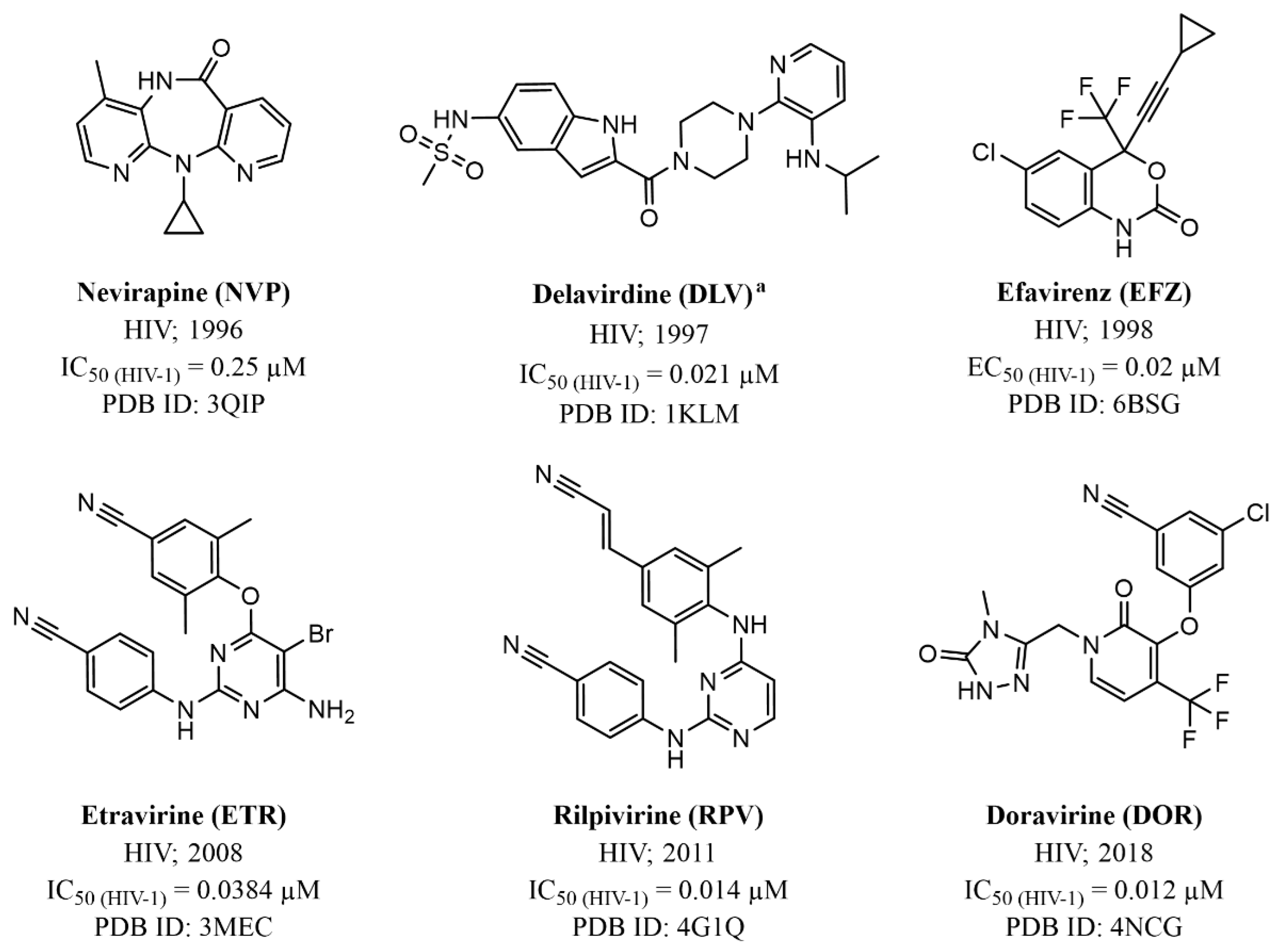



| Human Virus, Year of Discovery, Family | Baltimore Classification (Genome Organization) | Polymerase Class and Name | Mode of Transmission | Associated Diseases | |
|---|---|---|---|---|---|
| DNA | HCMV 1, 1956 Herpesviridae | I (linear, dsDNA) | DdDp, UL54 | Blood-borne, bodily fluids, maternal–neonatal | Retinitis, encephalitis, hepatitis, nephritis, mononucleosis-like syndrome, gastroenteritis |
| VZV 2, 1953 Herpesviridae | I (linear, dsDNA) | DdDp, ORF28 | Respiratory droplets, Maternal–neonatal | Varicella, Herpes zoster | |
| HSV 3, before 1900 Herpesviridae | I (linear, dsDNA) | DdDp, UL30 | Sexual or skin contacts, eye, maternal–neonatal | Labialis and genital herpes, keratinitis, pneumonia, encephalitis, meningitis | |
| HBV 4, 1963 Hepadnaviridae | VII (circular, (−) ssDNA/dsDNA) | RdDp/DdDp, RT | Blood-borne | Hepatitis B, hepatocellular carcinoma | |
| RNA | HCV 5, 1989 Flaviviridae | IV (linear, (+) ssRNA) | RdRp, NS5B | Blood-borne | Hepatitis C, cirrhosis, and liver cancer |
| RSV 6, 1957 Paramyxoviridae | V (linear, (−) ssRNA) | RdRp, L | Respiratory droplets | Respiratory illness | |
| SARS-CoV-2 7, 2019 Coronaviridae | IV ((+) ssRNA) | RdRp, Nsp12 | Respiratory droplets | COVID-19 | |
| HIV 8, 1983 Retroviridae | VI ((+) ssRNA-RT) | RdRp, RT | Blood-borne, bodily fluids, maternal–neonatal | AIDS | |
| Flu 9, 1933 Orthomyxoviridae | VI (linear, (+) ssRNA) | RdDp, Flu Pol | Blood-borne, bodily fluids, maternal–neonatal | Influenza |
| Drug Name (Abbreviation) | Targeted Virus | Biological Activity c | Figure |
|---|---|---|---|
| Acyclovir (ACV) | HSV | Ki (HSV-1) = 0.08 µM, EC50 (HSV-1) = 0.7 µM [22], EC50 (HSV-2) = 1.6 µM [22] | Figure 5 |
| VZV | EC50 (VZV) = 3.38 µM [23] | ||
| Valacyclovir (VACV) | HSV, VZV | ACV prodrug | Figure 5 |
| Idoxuridine a (IDU) | HSV | EC50 (HSV-2) = 2.4 µM [24] | Figure 5 |
| Trifluridine (TFT) | HSV | IC50(HSV-1) = 3.07 µM [25] | Figure 5 |
| Brivudine b (BVDU) | HSV VZV | EC50 (HSV-1) = 0.08 µM, EC50 (HSV-2) = 29.0 µM EC50 (VZV) = 0.0844 µM [23] | Figure 5 |
| Vidarabine a (VDR) | HSV VZV | EC50 (HSV-1) = ~10 µM [26] IC50 (VZV) = 5.99 µM [27] | Figure 5 |
| Ganciclovir (GCV) | HCMV | EC50 = 0.08 µM | Figure 5 |
| Penciclovir (PCV) | HSV | Ki (HSV-1) = 16.0 µM, Ki (HSV-2) = 9.5 µM | Figure 5 |
| Valganclovir (VGCV) | HCMV | GCV prodrug | Figure 5 |
| Famciclovir (FCV) | HSV, VZV | PCV prodrug | Figure 5 |
| Cidofovir (CDV) | HCMV | Ki = 6.6 µM [28] | Figure 5 |
| Foscarnet (PFA) | HSV HCMV | EC50 (HSV-1) = 32.6 µM [29] IC50 (HCMV) = 2.5 µM | Figure 5 |
| Lamivudine ((-)-3TC) | HBV HIV | IC50 (HBV) = 3.3 µM, Ki (HBV) =0.7 µM [30] IC50 (HIV-1) = 0.002 µM | Figure 7 |
| Tenofovir disoproxil fumarate (TDF) | HBV HIV | EC50 (HBV) = 0.49 µM EC50 (HIV-1) = 0.0033 µM, EC50 (HIV-2) = 0.0035 µM | Figure 7 |
| Adefovir dipivoxil (ADP) | HBV | Ki = 0.1 µM | Figure 7 |
| Tenofovir alafenamide fumarate (TAF) | HBV HIV | EC50 (HBV) = 0. 0866 µM [31] EC50 (HIV-1) = 0.0035 µM [32], EC50 (HIV-2) = 0.0018 µM [32] | Figure 7 |
| Telbivudine a (LdT) | HBV | IC50 = 1.3 µM | Figure 7 |
| Entecavir (ETV) | HBV | IC50 = 0.0005 µM [33] | Figure 7 |
| Zidovudine (AZT) | HIV | IC50 (HIV-1) = 0.1 µM [34], EC50 (HIV-1) = 0.01 µM | Figure 9 |
| Didanosine a (ddI) | HIV | IC50 = 0.49 µM [35] | Figure 9 |
| Zalcitabine a (ddC) | HIV | EC50 (HIV-1) = 2.2 µM [36] | Figure 9 |
| Stavudine a (d4T) | HIV | Ki (HIV-1) = 0.0083 µM | Figure 9 |
| Emtricitabine ((-)-FTC) | HIV | EC50 (HIV-1) = 0.31 µM | Figure 9 |
| Abacavir (ABC) | HIV | Ki (HIV-1) = 0.021 µM | Figure 9 |
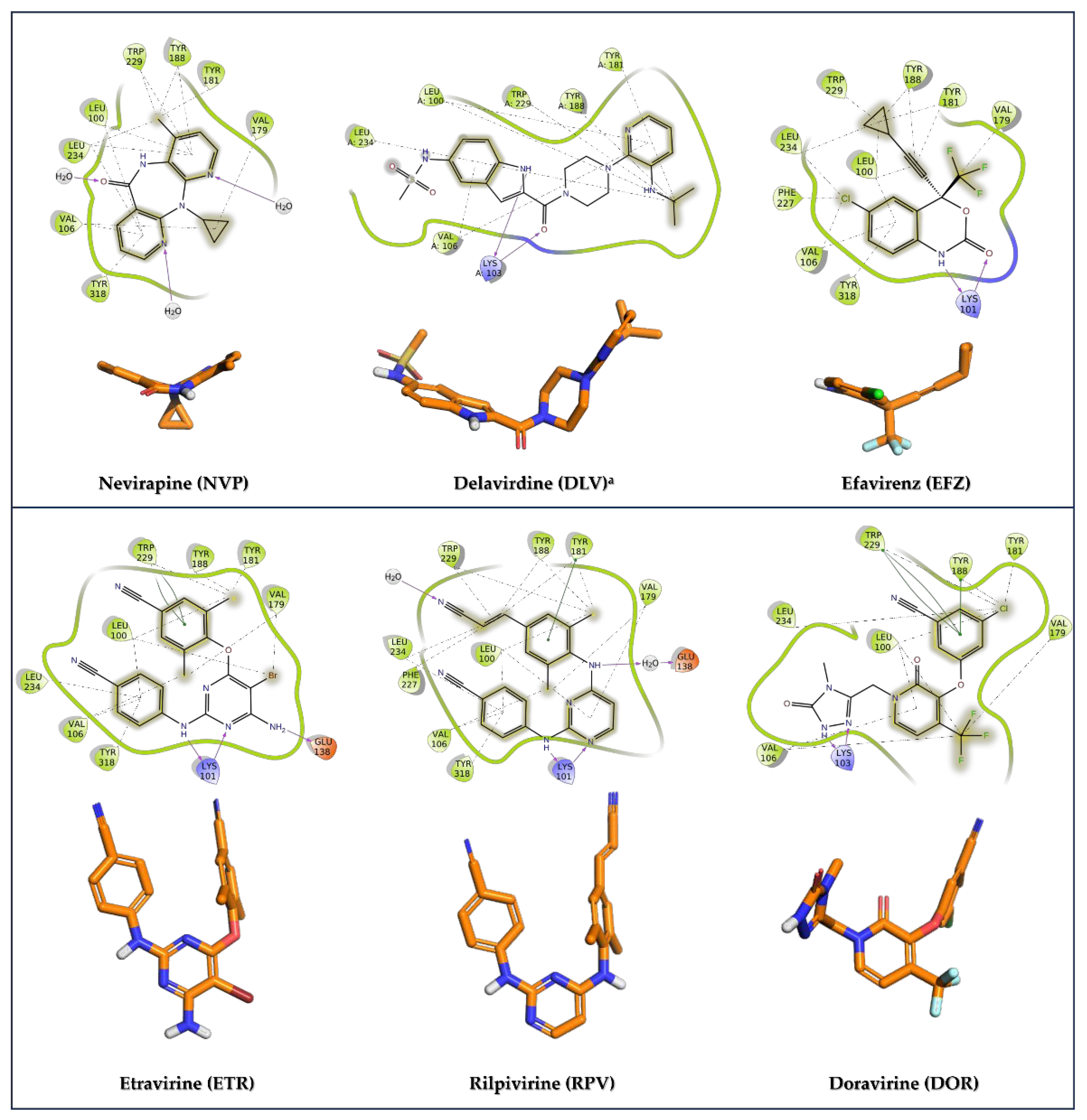
| Nevirapine (NVP) | HIV | IC50 (HIV-1) = 0.25 µM | Figure 11 |
| Delavirdine a (DLV) | HIV | IC50 (HIV-1) = 0.021 µM | Figure 11 |
| Efavirenz (EFV) | HIV | EC50 (HIV-1) = 0.02 µM | Figure 11 |
| Etravirine (ETR) | HIV | IC50 (HIV-1) = 0.0384 µM | Figure 11 |
| Rilpivirine (RPV) | HIV | IC50 (HIV-1) = 0.014 µM | Figure 11 |
| Doravirine (DOR) | HIV | IC50 (HIV-1) = 0.012 µM | Figure 11 |
| Ribavirin (RBV) | HCV, RSV | IC50 (RSV) = 20.9 µM [37] | Figure 14 |
| Sofosbuvir (SOF) | HCV | EC50 = 0.092 µM [38] | Figure 14 |
| Dasabuvir a (DSV) | HCV | IC50 = 0.0028 µM | Figure 14 |
| Beclabuvir b (BCV) | HCV | IC50 = 0.003 µM | Figure 14 |
| Favipiravir (FPV) | Influenza viruses-A, B, C | EC50 (influenza A) = 0.19 µM EC50 (H2N2 subtype) = 0.083 µM EC50 (Flu AH3N2) = 0.5 µM | Figure 17 |
| Remdesivir (RDV) | SARS-CoV-2 | EC50 = 0.77 µM [39] | Figure 18 |
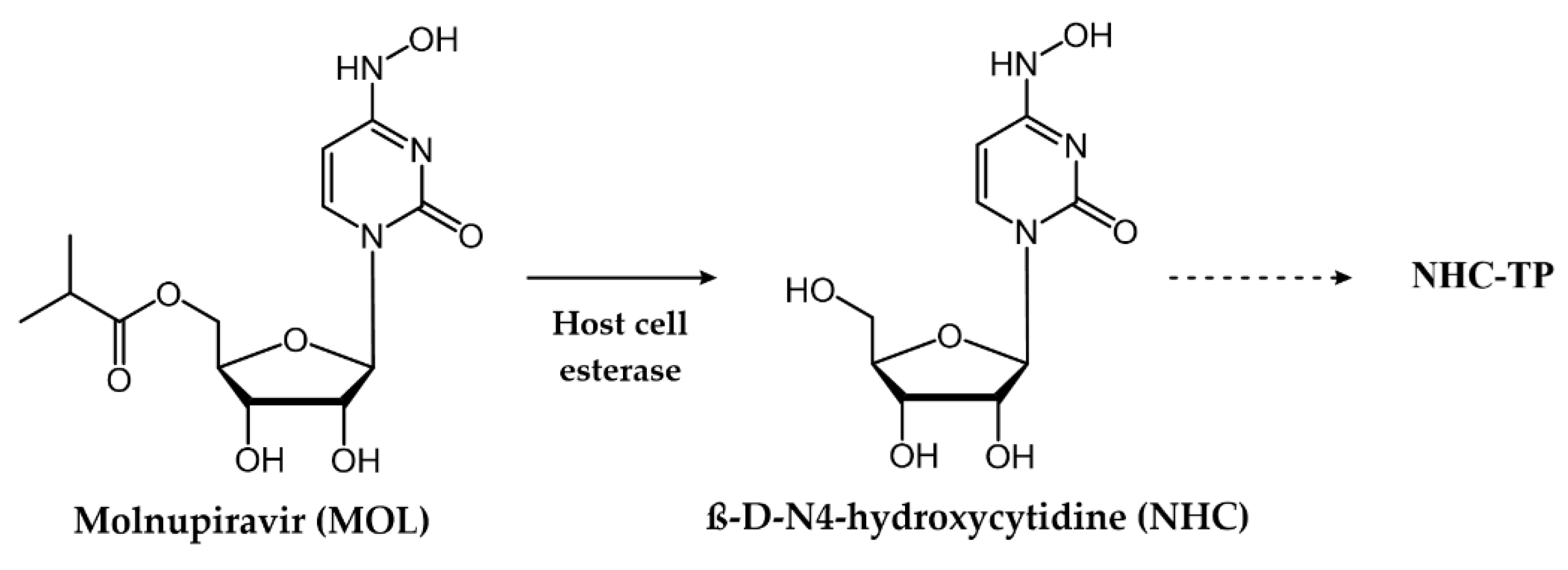
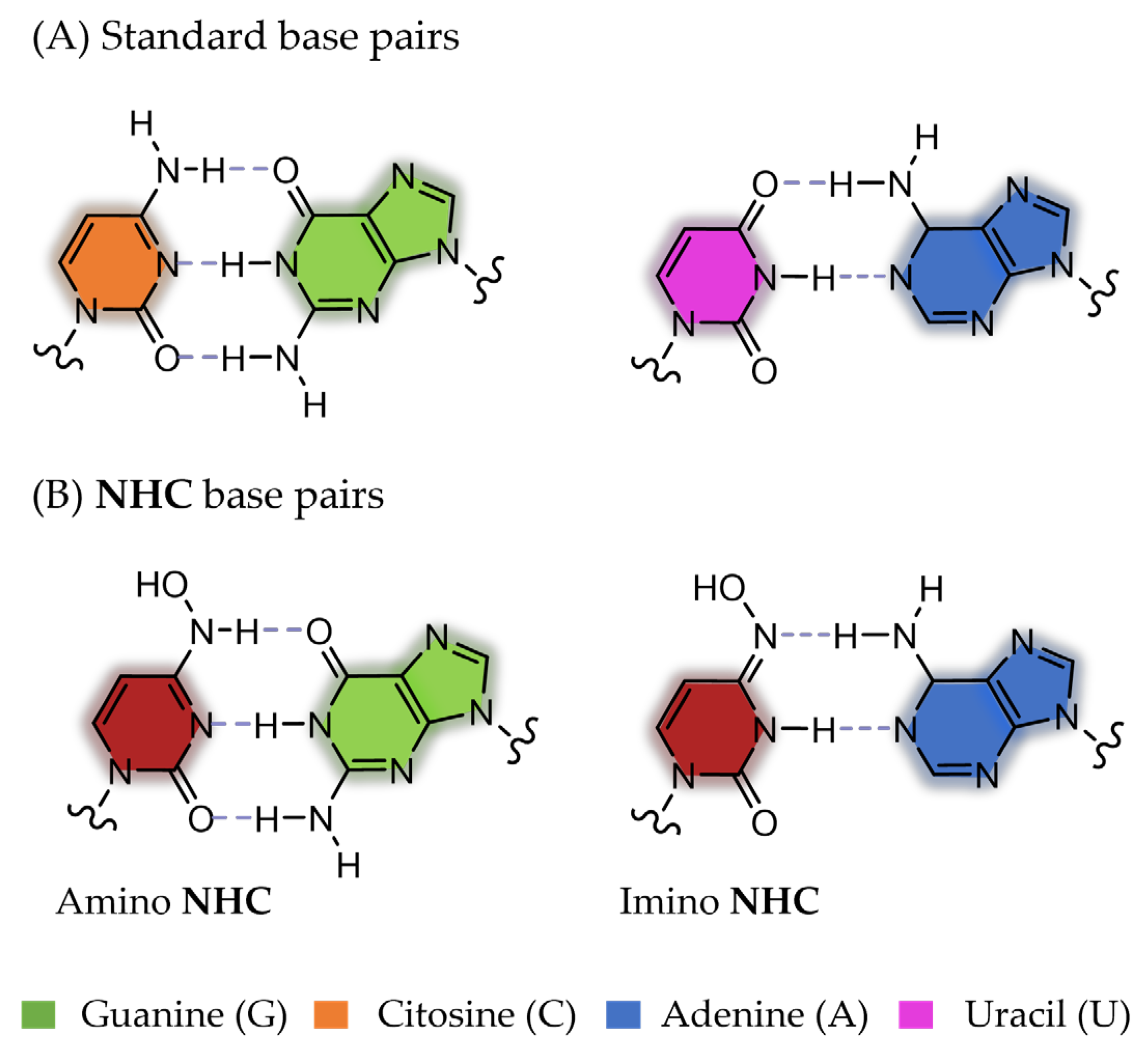
| Molnupiravir (MOL) | SARS-CoV-2 | EC50 = 0.32–2.66 µM [40] | Figure 18 |
| Drug | PDB Ligand Name | PDB IDs | Comments |
|---|---|---|---|
| Entecavir | ET9 | 5XN1, 6IKA, 6KDM | TP state |
| Foscarnet | PPF | 5HP1 | |
| Zidovudine | AZT | 3V4I, 5I42 | TP state |
| ATM | 5HP1, 1N5Y, 1N6Q, 3KLG, 3KLH, 3V4I, 3V6D, 3V81,5I3U | MP state | |
| Doravirine | 2KW | 4NCG, 7Z2H, 7Z2G | |
| Stavudine | D4T | 6AMO, 6AN2, 6AN8, 6ANQ, 6ASW, 6AVM, 6AVT, 6WPF, 6WPJ | TP state |
| D4M | 6WPF | MP state | |
| Lamivudine | 1RZ | 6KDJ, 6KDO, 6OUN, 6UJY | TP state |
| Emtricitabine | 43X | 6WPH | MP state |
| N8G | 6UJZ, 6OTZ | TP state, R | |
| 1RY | 6OR7, 6UIR, 6UJX, 7LRY | TP state, S | |
| Nevirapine | NVP | 1FKP, 1JLB, 1JLF, 1LW0, 1LWC, 1LWE, 1LWF, 1S1U, 1S1X, 1VRT, 2HND, 2HNY, 3HVT, 3LP0, 3LP1, 3QIP, 3V81, 4B3Q, 4PUO, 4PWD, 4Q0B, 5HBM, 7KJX, 7Z29, 7Z24 | |
| Delavirdine | SPP | 1KLM | |
| Efavirenz | EFZ | 7KJW, 1IKW, 1IKV, 4B3O, 1JKH, 1FKO, 1FK9, 6BSJ, 6BSI, 6BSH, 6BSG | |
| Etravirine | 65B | 1SV5, 3M8P, 3MEC, 3MED | |
| Rilpivirine | T27 | 2ZD1, 2ZE2, 3BGR, 3MEE, 3MEG, 3QLH, 4G1Q, 4ICL, 4ID5, 4IDK, 4IFV, 4IFY, 4IG3, 4KFB, 5CYM, 5CYQ, 6ELI, 7Z2D, 7Z2E, 8DX2, 8DX3, 8DX8, 8DXB, 8DXE, 8DXG, 8DXH, 8DXI, 8DXJ, 8DXK, 8DXL, 8DXM | |
| Penciclovir | HCU | 7DOK, 7DOI | MP state |
| Tenofovir | - | 1T03 | MP state |
| TFV | 1T05, 3JSM | DP state | |
| Sofosbuvir | 6SG | 4WTG | DP state |
| Beclabuvir | 2N7 | 4NLD | |
| Favipiravir | GE6 | 7AAP, 7CTT | TP state |
| 1RP | 7DFG | MP state | |
| Ribavirin | RBV | 3SFU | Unphosphorilated |
| RVP | 7DFH | MP state | |
| RTP | 2E9R | TP state | |
| Remdesivir | F86 | 7BV2 | MP state |
| NWX | 7UO4 | TP state | |
| - | 7L1F, 7B3B, 7B3D, 7B3C, 7D4F, 7C2K, 7BV1, 7BV2 | MP state | |
| Molnupiravir | - | 7OZV, 7OZU | MP state |
| Baltimore Class | Emerging Disease | Polymerase Name | Disease | Available Treatment(s) |
|---|---|---|---|---|
| V ((−) ssRNA) | EboV 1 | RdRp (L) | Fever, fatigue, muscle pain, headache and sore throat. | General supportive care management. FDA approved two monoclonal antibodies in adults and children. |
| IV ((+) ssRNA)) | COVID-19 2 | RdRp (nsp12) | Fever, cough, and fatigue; difficulty breathing or mild pneumonia. | |
| MERS-CoV 3 | RdRp (L) | No symptoms or mild respiratory symptoms to severe acute respiratory disease and death | General supportive care management | |
| SARS 4 | RdRp (nsp12) | Fever (>38 °C) and sometimes associated with chills, rigors, headache, malaise, and muscle pain. | Supportive treatment based on the symptoms. | |
| Zikv 5 | RdRp (NS5) | Fever, rash, conjunctivitis, muscle and joint pain, malaise, or headache. | Treatment of pain and fever with common medicines. | |
| V ((−) ssRNA)) | CCHF 6 | RdRp (L) | High fever, back, joint, and stomach pain. Red eyes, flushed face, red throat, and petechiae on the palate are common. In severe cases: jaundice, changes in mood, and sensory perception. | General supportive care management and ribavirin as an antiviral. |
| MV 7 | RdRp (L) | High fever, severe headache and malaise, muscle aches, severe watery diarrhea, abdominal pain, cramping, nausea, and vomiting. | General supportive care management and ribavirin as an antiviral. | |
| HeV 8 | RdRp (L) | Respiratory illness with severe flu-like signs and symptoms; encephalitis. | Cases are treated supportively in hospital or in intensive care. | |
| NiV 9 | RdRp (L4R) | Encephalitis and can cause mild to severe illness and even death. | Intensive supportive care is provided. | |
| V(ssRNA) | LASV 10 | RdRp (L) | Fever, general weakness, headache, sore throat, muscle, chest pain, nausea, vomiting, diarrhea, cough, and abdominal pain. | No specific treatment; generally supportive therapy. |
| RVF 11 | RdRp (L) | Fever, weakness, back pain, dizziness, ocular disease, encephalitis, or hemorrhagic fever. | Early supportive care. Ribavirin antiviral therapy seems effective. | |
| Disease X | A potential disease that could cause a serious global emergency. | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palazzotti, D.; Sguilla, M.; Manfroni, G.; Cecchetti, V.; Astolfi, A.; Barreca, M.L. Small Molecule Drugs Targeting Viral Polymerases. Pharmaceuticals 2024, 17, 661. https://doi.org/10.3390/ph17050661
Palazzotti D, Sguilla M, Manfroni G, Cecchetti V, Astolfi A, Barreca ML. Small Molecule Drugs Targeting Viral Polymerases. Pharmaceuticals. 2024; 17(5):661. https://doi.org/10.3390/ph17050661
Chicago/Turabian StylePalazzotti, Deborah, Martina Sguilla, Giuseppe Manfroni, Violetta Cecchetti, Andrea Astolfi, and Maria Letizia Barreca. 2024. "Small Molecule Drugs Targeting Viral Polymerases" Pharmaceuticals 17, no. 5: 661. https://doi.org/10.3390/ph17050661
APA StylePalazzotti, D., Sguilla, M., Manfroni, G., Cecchetti, V., Astolfi, A., & Barreca, M. L. (2024). Small Molecule Drugs Targeting Viral Polymerases. Pharmaceuticals, 17(5), 661. https://doi.org/10.3390/ph17050661








