Optimizing Encapsulation: Comparative Analysis of Spray-Drying and Freeze-Drying for Sustainable Recovery of Bioactive Compounds from Citrus x paradisi L. Peels
Abstract
1. Introduction
2. Materials and Methods
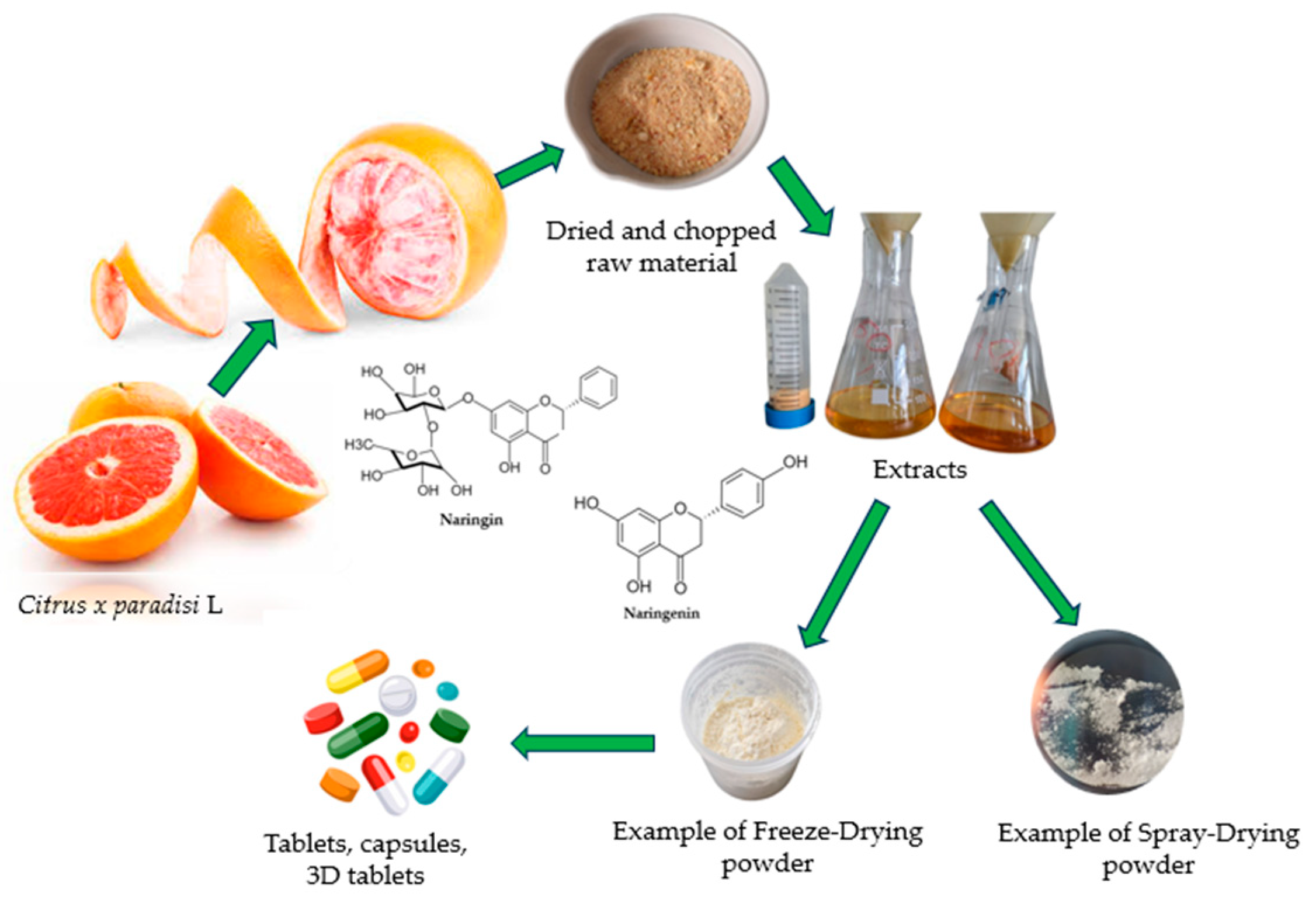
2.1. Citrus x paradisi.L Extracts Preparation
2.2. Formulation of Emulsion for Spray-Drying and Freeze-Drying Processes
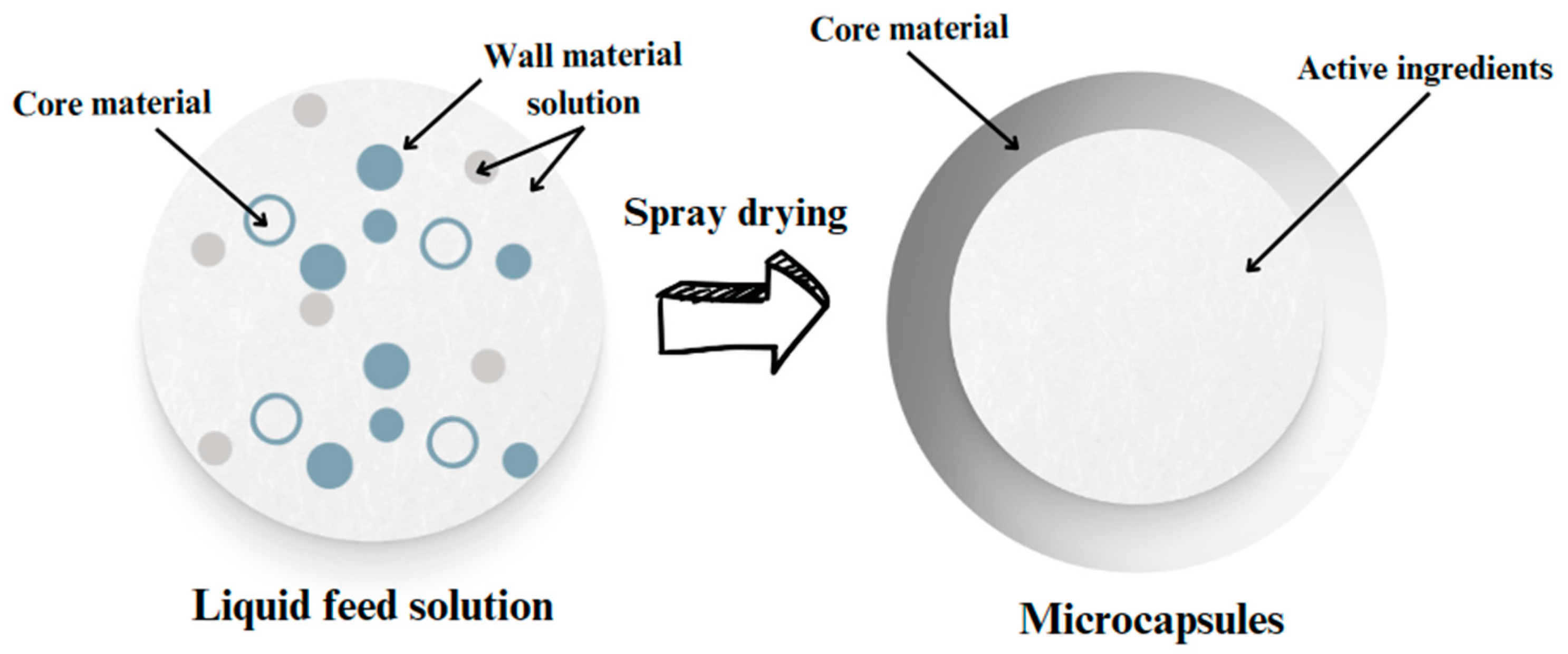
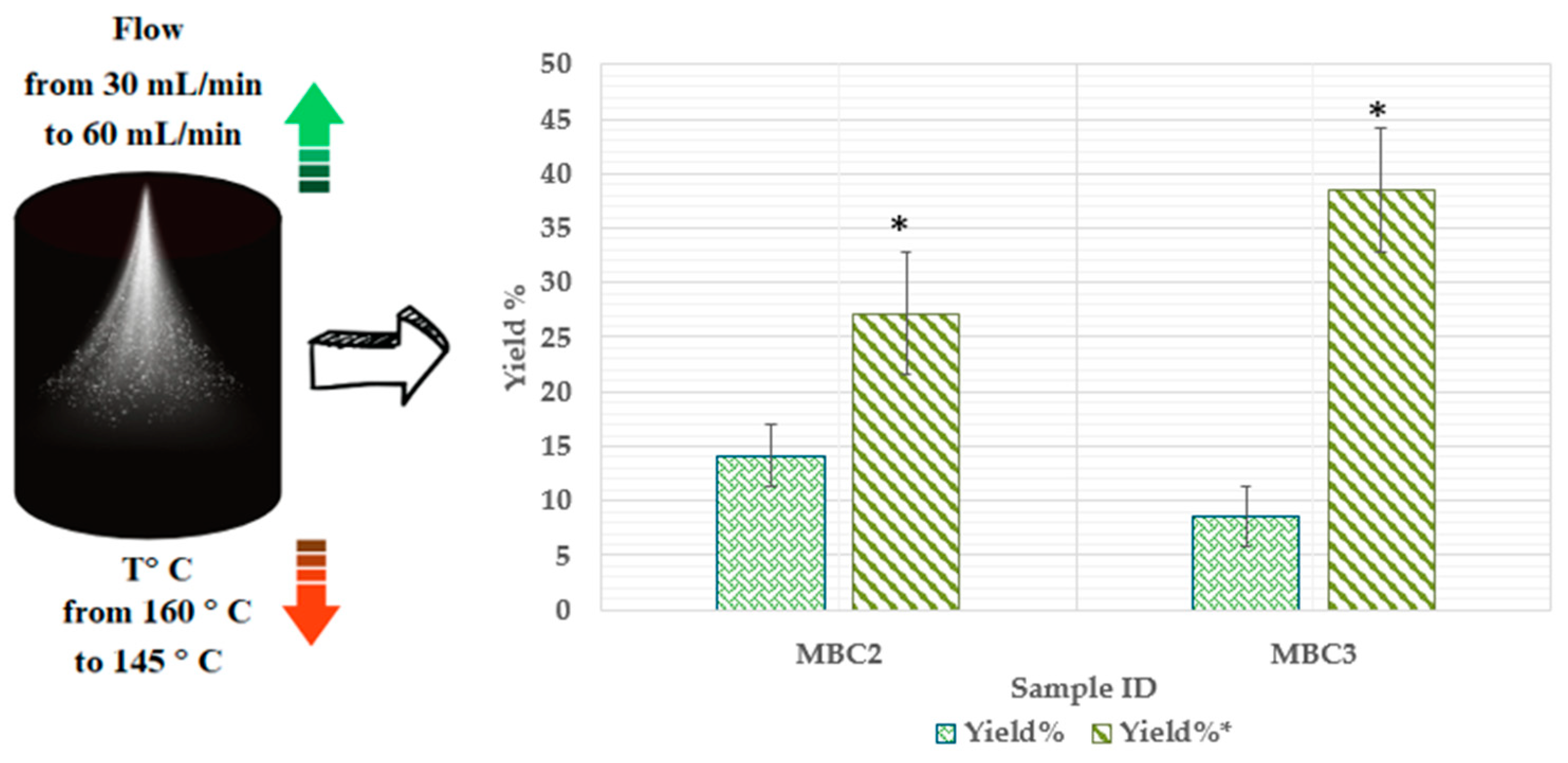
2.3. Parameters for Spray-Drying Process
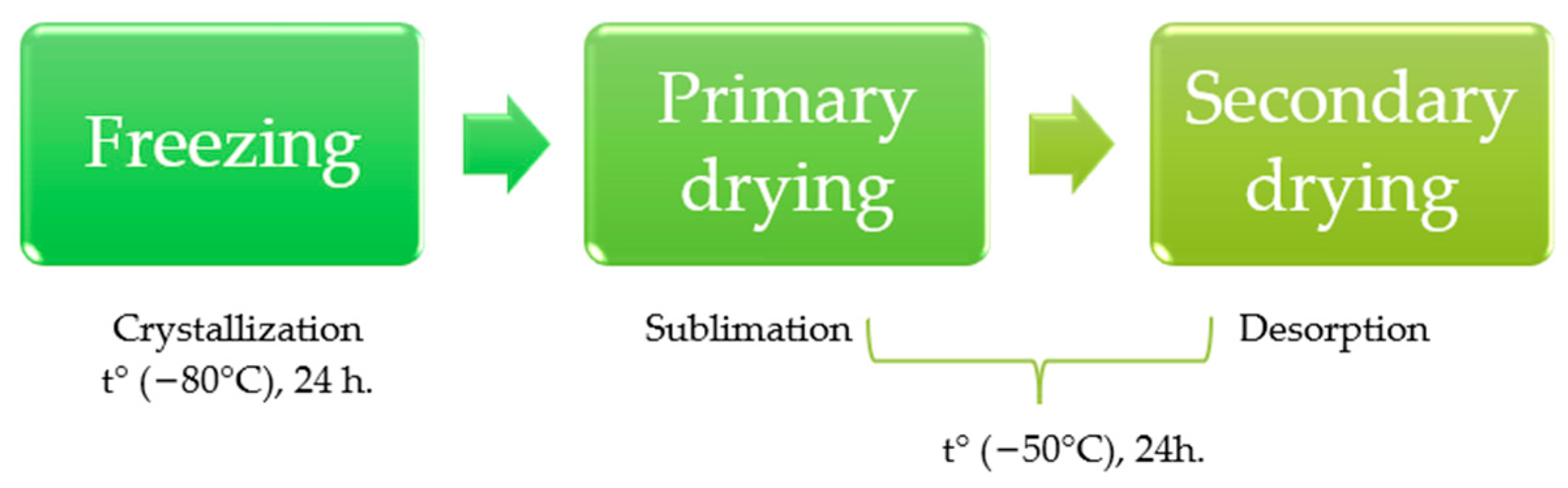
2.4. Freeze-Drying Procedure
2.5. Characterizations of the Microcapsules
2.5.1. Determination of Moisture Content
2.5.2. Wettability Analysis of Spray-Dried and Freeze-Dried Powders
2.5.3. SEM Analysis of Microcapsules: Morphological Evaluation
2.5.4. Assessment of Process Yield (Y%)
2.5.5. Measurement of Bulk and Tapped Volumes for Spray-Dried and Freeze-Dried Powders
2.5.6. Solubility Assessments
2.5.7. Quantification of Total and Surface Phenolic Content in Powdered Samples
3. Results and Discussion
3.1. Influences of Different Conditions of Microencapsulation on the Physicochemical Properties
3.2. Impact of Wall Material Composition on the Physicochemical Characteristics of Microcapsules
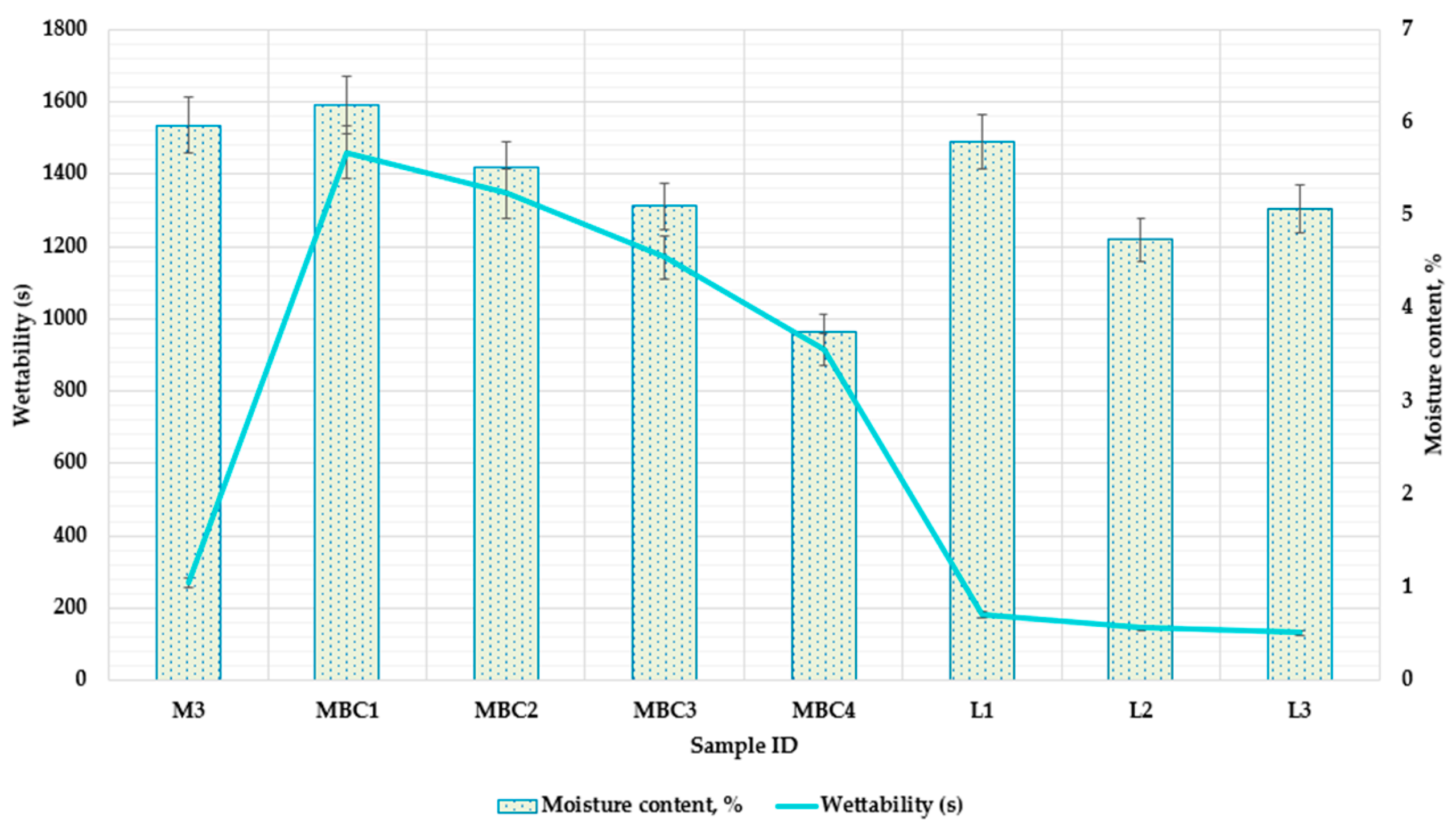
3.2.1. Examining Moisture Content and Wettability in Microcapsule Formulations
3.2.2. Impacts of Composition and Drying Methods on the Flowability of Microencapsulated Powders
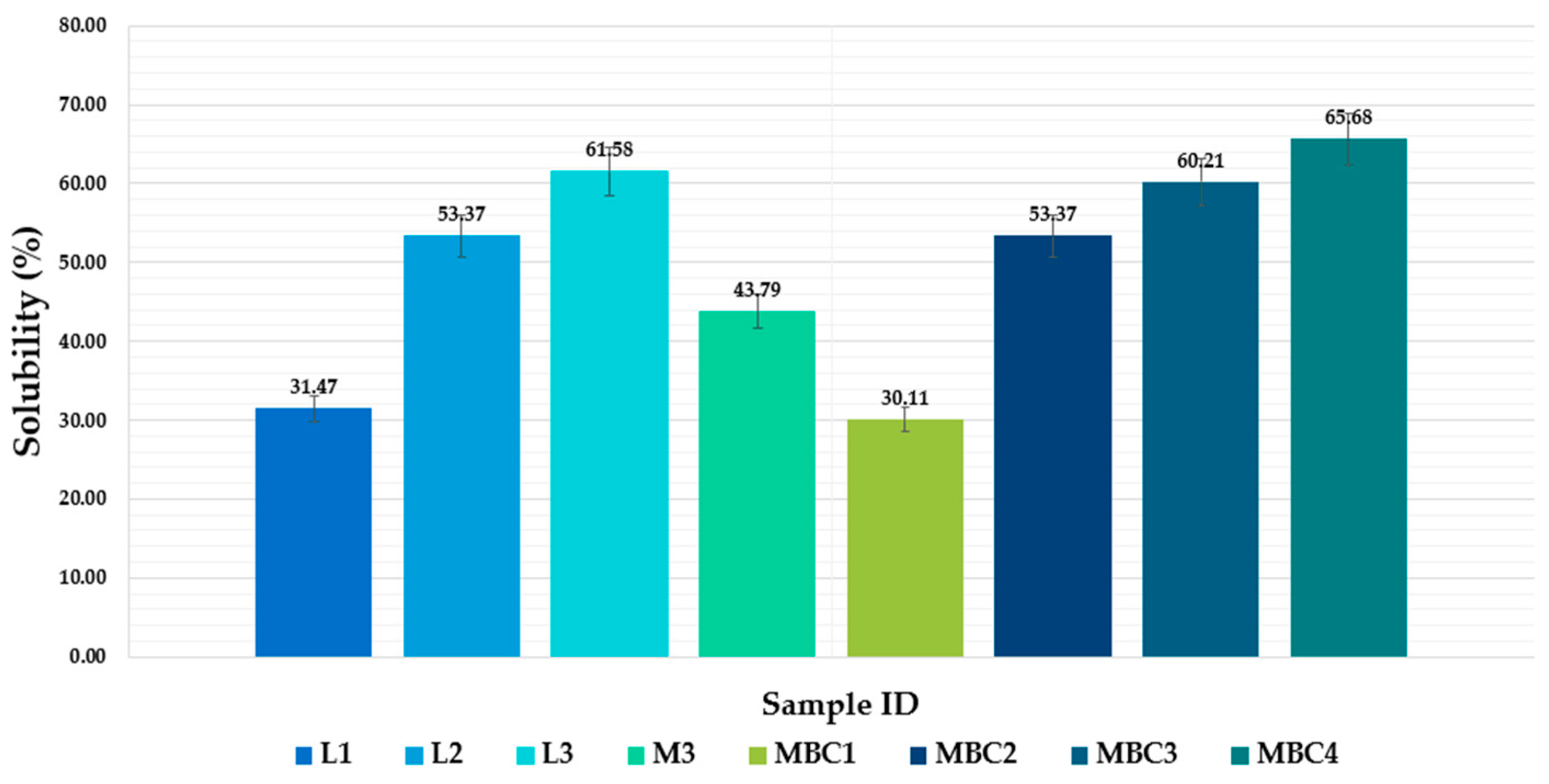
3.2.3. Optimization of Solubility and Release Profiles in Microencapsulated Phenolic Compounds
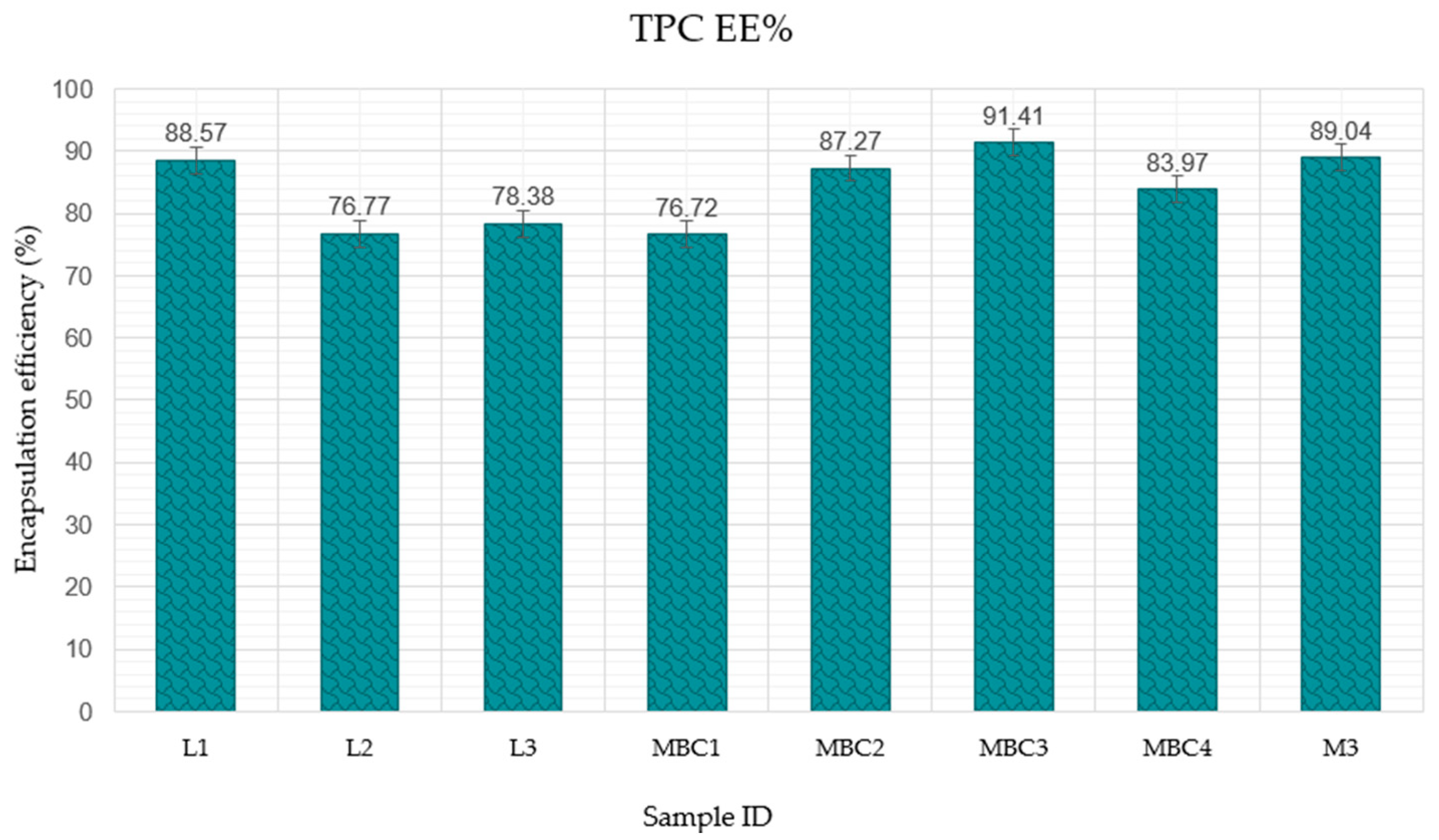
3.2.4. The Impact of Wall Material Composition on the Encapsulation Efficiency of Active Ingredients
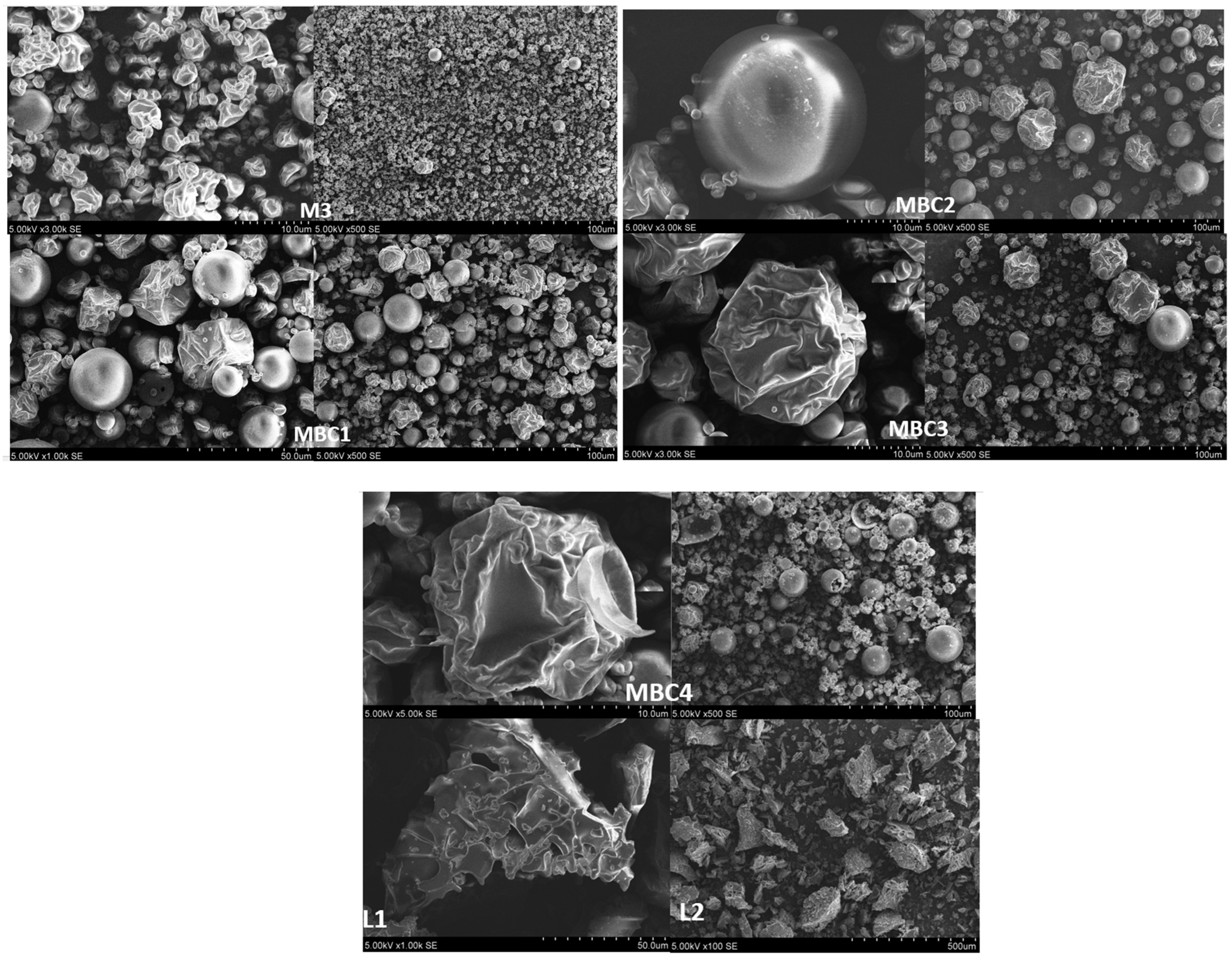
3.2.5. Scanning Electron Microscopy of Spray-Dried and Freeze-Dried Powders
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stabrauskiene, J.; Marksa, M.; Ivanauskas, L.; Bernatoniene, J. Optimization of Naringin and Naringenin Extraction from Citrus × paradisi L. Using Hydrolysis and Excipients as Adsorbent. Pharmaceutics 2022, 14, 890. [Google Scholar] [CrossRef] [PubMed]
- Stabrauskiene, J.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Naringin and Naringenin: Their Mechanisms of Action and the Potential Anticancer Activities. Biomedicines 2022, 10, 1686. [Google Scholar] [CrossRef] [PubMed]
- Stabrauskiene, J.; Marksa, M.; Ivanauskas, L.; Viskelis, P.; Viskelis, J.; Bernatoniene, J. Citrus × paradisi L. Fruit Waste: The Impact of Eco-Friendly Extraction Techniques on the Phytochemical and Antioxidant Potential. Nutrients 2023, 15, 1276. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, M.; Lin, X.; Zheng, X.; Qi, H.; Chen, J.; Zeng, X.; Bai, W.; Xiao, G. Biological Activities and Solubilization Methodologies of Naringin. Foods 2023, 12, 2327. [Google Scholar] [CrossRef] [PubMed]
- Ravetti, S.; Garro, A.G.; Gaitán, A.; Murature, M.; Galiano, M.; Brignone, S.G.; Palma, S.D. Naringin: Nanotechnological Strategies for Potential Pharmaceutical Applications. Pharmaceutics 2023, 15, 863. [Google Scholar] [CrossRef] [PubMed]
- Pudziuvelyte, L.; Marksa, M.; Jakstas, V.; Ivanauskas, L.; Kopustinskiene, D.M.; Bernatoniene, J. Microencapsulation of Elsholtzia ciliata Herb Ethanolic Extract by Spray-Drying: Impact of Resistant-Maltodextrin Complemented with Sodium Caseinate, Skim Milk, and Beta-Cyclodextrin on the Quality of Spray-Dried Powders. Molecules 2019, 24, 1461. [Google Scholar] [CrossRef] [PubMed]
- Binesh, N.; Babaloo, H.; Farhadian, N. Chapter 14—Microencapsulation: Spray drying. In Principles of Biomaterials Encapsulation: Volume One; Sefat, F., Farzi, G., Mozafari, M., Eds.; Woodhead Publishing: Sawston, UK, 2023; Volume 1, pp. 271–296. ISBN 978-0-323-85947-9. [Google Scholar]
- Mehta, N.; Kumar, P.; Verma, A.K.; Umaraw, P.; Kumar, Y.; Malav, O.P.; Sazili, A.Q.; Domínguez, R.; Lorenzo, J.M. Microencapsulation as a Noble Technique for the Application of Bioactive Compounds in the Food Industry: A Comprehensive Review. Appl. Sci. 2022, 12, 1424. [Google Scholar] [CrossRef]
- Poshadri, A.; Kuna, A. Microencapsulation technology: A review. J. Res. ANGRAU 2010, 38, 86–102. [Google Scholar]
- Lukova, P.; Katsarov, P.; Pilicheva, B. Application of Starch, Cellulose, and Their Derivatives in the Development of Microparticle Drug-Delivery Systems. Polymers 2023, 15, 3615. [Google Scholar] [CrossRef]
- Safeer Abbas, M.; Afzaal, M.; Saeed, F.; Asghar, A.; Jianfeng, L.; Ahmad, A.; Ullah, Q.; Elahi, S.; Ateeq, H.; Shah, Y.A.; et al. Probiotic viability as affected by encapsulation materials: Recent updates and perspectives. Int. J. Food Prop. 2023, 26, 1324–1350. [Google Scholar] [CrossRef]
- Garg, A.; Chhipa, K.; Kumar, L. MICROENCAPSULATION. Available online: https://www.jetir.org/papers/JETIR2404766.pdf (accessed on 1 May 2024).
- Sánchez-Osorno, D.M.; López-Jaramillo, M.C.; Caicedo Paz, A.V.; Villa, A.L.; Peresin, M.S.; Martínez-Galán, J.P. Recent Advances in the Microencapsulation of Essential Oils, Lipids, and Compound Lipids through Spray Drying: A Review. Pharmaceutics 2023, 15, 1490. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Cheng, D.; Niu, B.; Wang, X.; Wu, X.; Wang, A. Properties of Poly (Lactic-co-Glycolic Acid) and Progress of Poly (Lactic-co-Glycolic Acid)-Based Biodegradable Materials in Biomedical Research. Pharmaceuticals 2023, 16, 454. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Júnior, M.E.; Araújo, M.V.R.L.; Martins, A.C.S.; Dos Santos Lima, M.; Da Silva, F.L.H.; Converti, A.; Maciel, M.I.S. Microencapsulation by spray-drying and freeze-drying of extract of phenolic compounds obtained from ciriguela peel. Sci. Rep. 2023, 13, 15222. [Google Scholar] [CrossRef] [PubMed]
- Shirsath, P.R.; Khandre, R.A. A Review: Microencapsulation. Int. J. Creat. Res. Thoughts (IJCRT) 2022, 10, 2320–2882. [Google Scholar]
- Mohammed, N.K.; Tan, C.P.; Manap, Y.A.; Muhialdin, B.J.; Hussin, A.S.M. Spray Drying for the Encapsulation of Oils—A Review. Molecules 2020, 25, 3873. [Google Scholar] [CrossRef]
- Gullifa, G.; Risoluti, R.; Mazzoni, C.; Barone, L.; Papa, E.; Battistini, A.; Martin Fraguas, R.; Materazzi, S. Microencapsulation by a Spray Drying Approach to Produce Innovative Probiotics-Based Products Extending the Shelf-Life in Non-Refrigerated Conditions. Molecules 2023, 28, 860. [Google Scholar] [CrossRef]
- Kandansamy, K.; Somasundaram, P.D. Microencapsulation of Colors by Spray Drying—A Review. Int. J. Food Eng. 2012, 18. [Google Scholar] [CrossRef]
- Sarabandi, K.; Tamjidi, F.; Akbarbaglu, Z.; Samborska, K.; Gharehbeglou, P.; Kharazmi, M.S.; Jafari, S.M. Modification of Whey Proteins by Sonication and Hydrolysis for the Emulsification and Spray Drying Encapsulation of Grape Seed Oil. Pharmaceutics 2022, 14, 2434. [Google Scholar] [CrossRef]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- Saftić Martinović, L.; Birkic, N.; Miletić, V.; Antolović, R.; Štanfel, D.; Wittine, K. Antioxidant Activity, Stability in Aqueous Medium and Molecular Docking/Dynamics Study of 6-Amino- and N-Methyl-6-amino-L-ascorbic Acid. Int. J. Mol. Sci. 2023, 24, 1410. [Google Scholar] [CrossRef]
- Aiassa, V.; Garnero, C.; Zoppi, A.; Longhi, M.R. Cyclodextrins and Their Derivatives as Drug Stability Modifiers. Pharmaceuticals 2023, 16, 1074. [Google Scholar] [CrossRef]
- Igual, M.; Cebadera, L.; Cámara, R.M.; Agudelo, C.; Martínez-Navarrete, N.; Cámara, M. Novel Ingredients Based on Grapefruit Freeze-Dried Formulations: Nutritional and Bioactive Value. Foods 2019, 8, 506. [Google Scholar] [CrossRef] [PubMed]
- Pudziuvelyte, L.; Marksa, M.; Sosnowska, K.; Winnicka, K.; Morkuniene, R.; Bernatoniene, J. Freeze-Drying Technique for Microencapsulation of Elsholtzia ciliata Ethanolic Extract Using Different Coating Materials. Molecules 2020, 25, 2237. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Castillo, E.; Bengoechea, C.; Felix, M.; Guerrero, A. Freeze-Drying versus Heat-Drying: Effect on Protein-Based Superabsorbent Material. Processes 2021, 9, 1076. [Google Scholar] [CrossRef]
- Kubbutat, P.; Tauchnitz, A.; Kulozik, U. Water Vapor Pathways during Freeze-Drying of Foamed Product Matrices Stabilized by Maltodextrin at Different Concentrations. Processes 2020, 8, 1463. [Google Scholar] [CrossRef]
- Silvestro, I.; Sergi, R.; Scotto D’Abusco, A.; Mariano, A.; Martinelli, A.; Piozzi, A.; Francolini, I. Chitosan scaffolds with enhanced mechanical strength and elastic response by combination of freeze gelation, photo-crosslinking and freeze-drying. Carbohydr. Polym. 2021, 267, 118156. [Google Scholar] [CrossRef]
- Brougham, C.M.; Levingstone, T.J.; Shen, N.; Cooney, G.M.; Jockenhoevel, S.; Flanagan, T.C.; O’Brien, F.J. Freeze-Drying as a Novel Biofabrication Method for Achieving a Controlled Microarchitecture within Large, Complex Natural Biomaterial Scaffolds. Adv. Healthc. Mater. 2017, 6, 1700598. [Google Scholar] [CrossRef] [PubMed]
- Thuong Nhan, N.P.; Tan Thanh, V.; Huynh Cang, M.; Lam, T.D.; Cam Huong, N.; Hong Nhan, L.T.; Thanh Truc, T.; Tran, Q.T.; Bach, L.G. Microencapsulation of Lemongrass (Cymbopogon citratus) Essential Oil Via Spray Drying: Effects of Feed Emulsion Parameters. Processes 2020, 8, 40. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Y.; Geng, F.; Shen, X. Characterization of Spray-Dried Microcapsules of Paprika Oleoresin Induced by Ultrasound and High-Pressure Homogenization: Physicochemical Properties and Storage Stability. Molecules 2023, 28, 7075. [Google Scholar] [CrossRef]
- Wang, Y.; Ghosh, S.; Nickerson, M.T. Microencapsulation of Flaxseed Oil by Lentil Protein Isolate-κ-Carrageenan and -ι-Carrageenan Based Wall Materials through Spray and Freeze Drying. Molecules 2022, 27, 3195. [Google Scholar] [CrossRef]
- Tomsone, L.; Galoburda, R.; Kruma, Z.; Durrieu, V.; Cinkmanis, I. Microencapsulation of Horseradish (Armoracia rusticana L.) Juice Using Spray-Drying. Foods 2020, 9, 1332. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Arboleda Mejia, J.A.; Versari, A.; Chiarello, E.; Bordoni, A.; Parpinello, G.P. Microencapsulation of polyphenolic compounds recovered from red wine lees: Process optimization and nutraceutical study. Food Bioprod. Process. 2022, 132, 1–12. [Google Scholar] [CrossRef]
- Díaz-Montes, E. Wall Materials for Encapsulating Bioactive Compounds via Spray-Drying: A Review. Polymers 2023, 15, 2659. [Google Scholar] [CrossRef] [PubMed]
- Emadzadeh, B.; Ghorani, B.; Naji-Tabasi, S.; Charpashlo, E.; Molaveisi, M. Fate of β-cyclodextrin-sugar beet pectin microcapsules containing garlic essential oil in an acidic food beverage. Food Biosci. 2021, 42, 101029. [Google Scholar] [CrossRef]
- Mohammadalinejhad, S.; Kurek, M.A. Microencapsulation of Anthocyanins—Critical Review of Techniques and Wall Materials. Appl. Sci. 2021, 11, 3936. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Zhao, Y.; Lv, H.; Liu, K. Encapsulation and Characterization of Proanthocyanidin Microcapsules by Sodium Alginate and Carboxymethyl Cellulose. Foods 2024, 13, 740. [Google Scholar] [CrossRef] [PubMed]
- Afshari, K.; Javanmard Dakheli, M.; Ramezan, Y.; Bassiri, A.; Ahmadi Chenarbon, H. Physicochemical and control releasing properties of date pit (Phoenix dactylifera L.) phenolic compounds microencapsulated through fluidized-bed method. Food Sci. Nutr. 2023, 11, 1367–1382. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Xia, J.; Zhao, Q.; Niu, Y.; Zhao, D. Maltodextrin as wall material for microcapsules: A review. Carbohydr. Polym. 2022, 298, 120113. [Google Scholar] [CrossRef]

| Microencapsulation Materials | Material Examples | Common Use |
|---|---|---|
| Polysaccharides | Dextrines (maltodextrin, cyclodextrins), Ethylcellulose, Methylcellulose, Hydroxypropyl methylcellulose, Carboxymethylcellulose, Carrageenan | Food, Pharmaceuticals, Nutraceuticals |
| Proteins | Gelatin, Casein, Whey protein, Skim milk, Egg white | Food, Pharmaceuticals, Nutraceuticals |
| Lipids | Waxes (beeswax, carnauba wax), Animal sources, Fats, and Plant sources | Food, Pharmaceuticals, Nutraceuticals |
| Synthetics | Poly-lactic-co-glycolic acid (PLGA) | Target drug delivery, Bioengineering |
| Inlet T (°C) | Outlet T (°C) | Flow Rate (mL/min) | Air Pressure | Yield (%) | Moisture Content (%) | Sample ID |
|---|---|---|---|---|---|---|
| 90 | 25 | 30 | 8 bars | 48.10 ± 2.40 | 7.60 ± 0.38 | M1 |
| 120 | 65 | 30 | 8 bars | 51.65 ± 2.58 | 6.57 ± 0.32 | M2 |
| 160 | 80 | 30 | 8 bars | 52.95 ± 2.64 | 5.97 ± 0.298 | M3 |
| 170 | 116 | 30 | 8 bars | 48.00 ± 2.40 | 5.31 ± 0.265 | M7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stabrauskiene, J.; Pudziuvelyte, L.; Bernatoniene, J. Optimizing Encapsulation: Comparative Analysis of Spray-Drying and Freeze-Drying for Sustainable Recovery of Bioactive Compounds from Citrus x paradisi L. Peels. Pharmaceuticals 2024, 17, 596. https://doi.org/10.3390/ph17050596
Stabrauskiene J, Pudziuvelyte L, Bernatoniene J. Optimizing Encapsulation: Comparative Analysis of Spray-Drying and Freeze-Drying for Sustainable Recovery of Bioactive Compounds from Citrus x paradisi L. Peels. Pharmaceuticals. 2024; 17(5):596. https://doi.org/10.3390/ph17050596
Chicago/Turabian StyleStabrauskiene, Jolita, Lauryna Pudziuvelyte, and Jurga Bernatoniene. 2024. "Optimizing Encapsulation: Comparative Analysis of Spray-Drying and Freeze-Drying for Sustainable Recovery of Bioactive Compounds from Citrus x paradisi L. Peels" Pharmaceuticals 17, no. 5: 596. https://doi.org/10.3390/ph17050596
APA StyleStabrauskiene, J., Pudziuvelyte, L., & Bernatoniene, J. (2024). Optimizing Encapsulation: Comparative Analysis of Spray-Drying and Freeze-Drying for Sustainable Recovery of Bioactive Compounds from Citrus x paradisi L. Peels. Pharmaceuticals, 17(5), 596. https://doi.org/10.3390/ph17050596








