Pharmacokinetic–Pharmacodynamic Correlation Analysis of Rhodiola crenulata in Rats with Myocardial Ischemia
Abstract
1. Introduction
2. Results
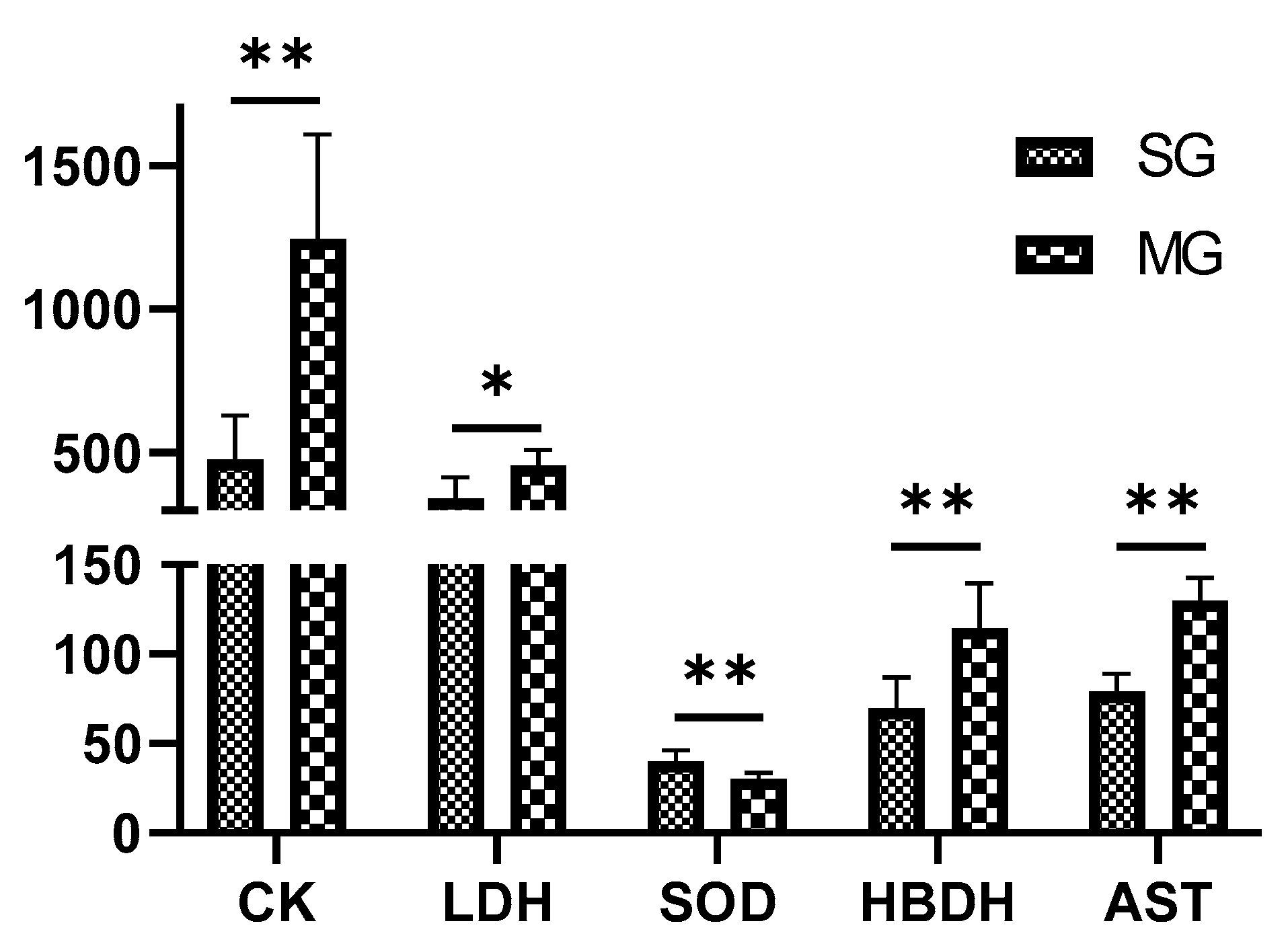
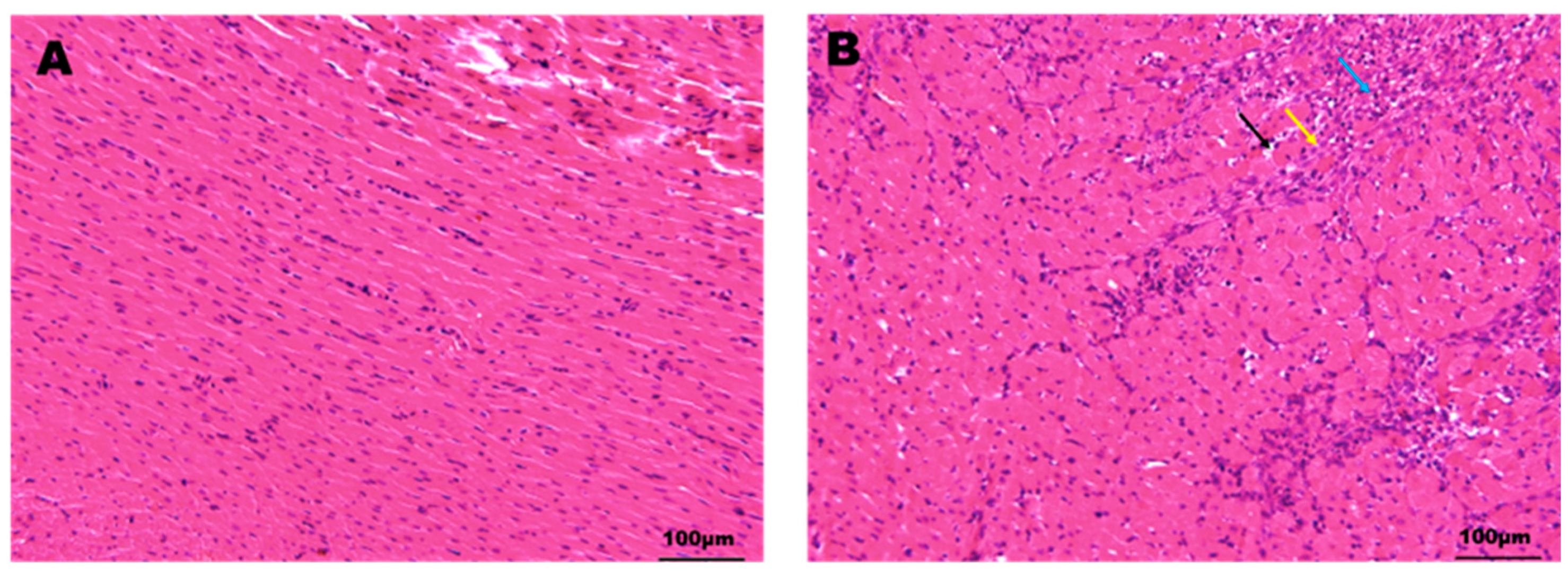
2.1. Characterization of the Myocardial Ischemia Model
2.2. Method Validation
2.2.1. Linearity, LOD, and LOQ
2.2.2. Specificity
2.2.3. Precision and Accuracy
2.2.4. Extraction Recovery and Matrix Effect
2.2.5. Stability
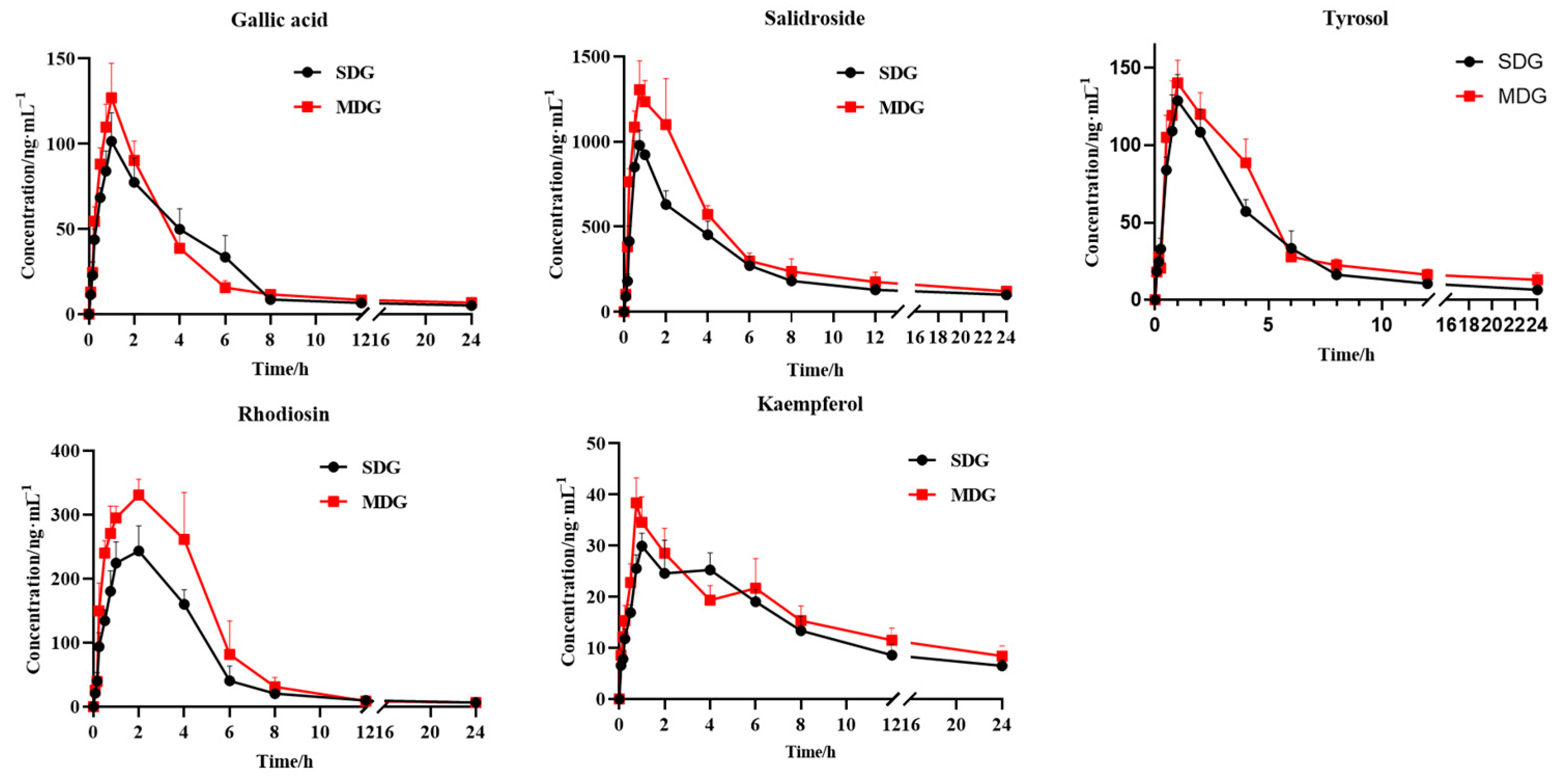
2.3. PK Profiles in Normal and Myocardial-Ischemia-Modeled Rats
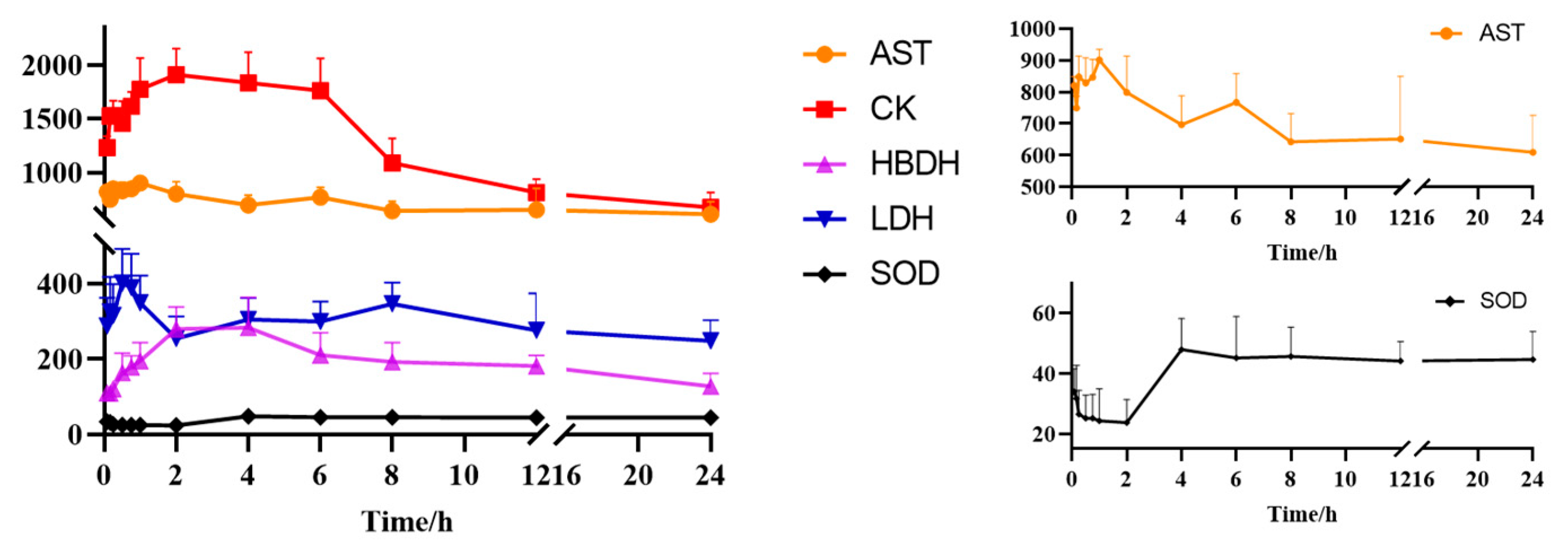
2.4. PD Study and PK-PD Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Instruments and Chromatographic Conditions
4.3. The Preparation of RC
4.4. Experimental Animals and Sample Collection
4.5. Biochemical Index Detection and Histopathology
4.6. The Preparation of Plasma Samples
4.7. Methodological Validation
4.8. PK Analysis
4.9. PD Study and PK-PD Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Antman, E.M.; Braunwald, E. Managing Stable Ischemic Heart Disease. N. Engl. J. Med. 2020, 382, 1468–1470. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, C.A.; Wereski, R.; Parsonage, W.; Cullen, L.; Khamis, R.; Foley, M.; Harrell, F.E., Jr.; Shun-Shin, M.J.; Mills, N.L.; Al-Lamee, R.K. Association Between High-Sensitivity Cardiac Troponin, Myocardial Ischemia, and Revascularization in Stable Coronary Artery Disease. J. Am. Coll. Cardiol. 2022, 79, 2185–2187. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; White, H.D. Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2007, 50, 2173–2195. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.L.; Zhang, M.Y.; Bai, R.Y.; Sun, L.K.; Li, W.H.; Yu, Y.L.; Zhang, Y.; Song, L.; Wang, Z.X.; Peng, Y.F.; et al. Anti-inflammatory effects of Rhodiola rosea L.: A review. Biomed. Pharmacother. 2020, 121, 109552. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tang, M.; Yuan, S.; Fu, S.; Li, Y.; Li, Y.; Wang, Q.; Cao, Y.; Liu, L.; Zhang, Q. Rhodiola rosea: A Therapeutic Candidate on Cardiovascular Diseases. Oxid. Med. Cell Longev. 2022, 2022, 1348795. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.; Qian, X.; Zheng, J.; Qian, J. The efficacy and safety of Rhodiola formulation for the treatment of ischemic heart disease: A protocol for systematic review and meta-analysis. Medicine 2022, 101, e31736. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Liu, M.; Xing, Z.; Chen, Z.; Zhu, M.; Wang, Y. Systematic Evaluation of Clinical Efficacy and Platelet Function of Sofren Injection in the Treatment of Angina Pectoris. Evid. Based Complement. Alternat. Med. 2021, 2021, 5591137. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Yang, S.Z.; Yang, X.Q. Effect and Mechanism of Sofren Injection in the Treatment of Stable Angina Petoris. Inf. Tradit. Chin. Med. 2017, 34, 56–60. [Google Scholar]
- Yang, Y.N.; Liu, Z.Z.; Feng, Z.M.; Jiang, J.S.; Zhang, P.C. Lignans from the root of Rhodiola crenulata. J. Agric. Food Chem. 2012, 60, 964–972. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Q.; Lou, Y.; Xu, J.; Feng, Z.; Chen, Y.; Tang, Q.; Zheng, G.; Zhang, Z.; Wu, Y.; et al. Salidroside attenuates neuroinflammation and improves functional recovery after spinal cord injury through microglia polarization regulation. J. Cell Mol. Med. 2018, 22, 1148–1166. [Google Scholar] [CrossRef]
- Guo, N.; Hu, Z.; Fan, X.; Zheng, J.; Zhang, D.; Xu, T.; Yu, T.; Wang, Y.; Li, H. Simultaneous determination of salidroside and its aglycone metabolite p-tyrosol in rat plasma by liquid chromatography-tandem mass spectrometry. Molecules 2012, 17, 4733–4754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, L.; Lin, L.; Liu, J.; Zhang, Z.; Xu, D.; Xiang, F. Pharmacokinetics, tissue distribution, and excretion of salidroside in rats. Planta Med. 2013, 79, 1429–1433. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Y.; Wang, P.; Zhang, J.; Chen, H.; Zhang, L.; Du, X.; Zhao, C.; Wu, D.; Liu, F.; et al. A comprehensive review of integrative pharmacology-based investigation: A paradigm shift in traditional Chinese medicine. Acta Pharm. Sin. B 2021, 11, 1379–1399. [Google Scholar] [CrossRef]
- Sun, J.; Li, R.; Zhang, J.; Huang, Y.; Lu, Y.; Liu, C.; Li, Y.; Liu, T. Analysis of compatibility mechanism of shenxiong glucose injection after multiple dosing based on differences of PK-PD correlation and cytochrome P450 enzyme. J. Pharm. Biomed. Anal. 2024, 239, 115899. [Google Scholar] [CrossRef]
- Rodríguez-Gascón, A.; Solinís, M.Á.; Isla, A. The Role of PK/PD Analysis in the Development and Evaluation of Antimicrobials. Pharmaceutics 2021, 13, 833. [Google Scholar] [CrossRef]
- Han, F.; Li, Y.; Ma, L.; Liu, T.; Wu, Y.; Xu, R.; Song, A.; Yin, R. A rapid and sensitive UHPLC-FT-ICR MS/MS method for identification of chemical constituents in Rhodiola crenulata extract, rat plasma and rat brain after oral administration. Talanta 2016, 160, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.S. Rhodiola rosea: A possible plant adaptogen. Altern. Med. Rev. 2001, 6, 293–302. [Google Scholar]
- Deng, R.; Wang, W.; Wu, H.; Zhang, Y.; Wang, W.; Dai, L.; Zhang, Z.; Fu, J.; Li, F. A Microdialysis in Adjuvant Arthritic Rats for Pharmacokinetics-Pharmacodynamics Modeling Study of Geniposide with Determination of Drug Concentration and Efficacy Levels in Dialysate. Molecules 2018, 23, 987. [Google Scholar] [CrossRef]
- Yan, R.; Yang, Y.; Chen, Y. Pharmacokinetics of Chinese medicines: Strategies and perspectives. Chin. Med. 2018, 13, 24. [Google Scholar] [CrossRef]
- Xu, L.; Liang, Y.; Chen, X.; Chen, B.; Han, Y.; Zhang, L. Hyperlipidemia affects the absorption, distribution and excretion of seven catechins in rats following oral administration of tea polyphenols. RSC Adv. 2015, 5, 97988–97994. [Google Scholar] [CrossRef]
- Milligan, P.A.; Brown, M.J.; Marchant, B.; Martin, S.W.; van der Graaf, P.H.; Benson, N.; Nucci, G.; Nichols, D.J.; Boyd, R.A.; Mandema, J.W.; et al. Model-based drug development: A rational approach to efficiently accelerate drug development. Clin. Pharmacol. Ther. 2013, 93, 502–514. [Google Scholar] [CrossRef]
- Gu, S.; Cao, B.; Sun, R.; Tang, Y.; Paletta, J.L.; Wu, X.; Liu, L.; Zha, W.; Zhao, C.; Li, Y.; et al. A metabolomic and pharmacokinetic study on the mechanism underlying the lipid-lowering effect of orally administered berberine. Mol. Biosyst. 2015, 11, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Holford, N.H.; Sheiner, L.B. Understanding the dose-effect relationship: Clinical application of pharmacokinetic-pharmacodynamic models. Clin. Pharmacokinet. 1981, 6, 429–453. [Google Scholar] [CrossRef] [PubMed]
- Toutain, P.L.; Cester, C.C.; Haak, T.; Laroute, V. A pharmacokinetic/pharmacodynamic approach vs. a dose titration for the determination of a dosage regimen: The case of nimesulide, a Cox-2 selective nonsteroidal anti-inflammatory drug in the dog. J. Vet. Pharmacol. Ther. 2001, 24, 43–55. [Google Scholar] [CrossRef]
- Lin, S.; Chu, J.; Zhang, L.; Chen, D.; Xiao, F.; Chen, H.; Lin, J.; Chen, Y.; Zhu, Y.; Peng, J. Protective effects of Shexiang Tongxin Dropping Pill on pituitrin-induced acute myocardial ischemia in rats. Mol. Med. Rep. 2017, 16, 3125–3132. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Zeng, Z.; Zhou, W.; Liu, J.; He, Z. Pharmacokinetics of ligustrazine ethosome patch in rats and anti-myocardial ischemia and anti-ischemic reperfusion injury effect. Int. J. Nanomed. 2011, 6, 1391–1398. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Zhang, R.; Li, W.; Du, H.Z.; Wu, S.S.; Xiong, R.; Hou, X.J.; Zhang, M.; Wang, X.J. Study on effects of different habitat processing methods of Salviae Miltiorrhizae Radix et Rhizoma in rats with acute myocardial ischemia. Zhongguo Zhong Yao Za Zhi 2020, 45, 5694–5700. [Google Scholar]
- Muders, F.; Neubauer, S.; Luchner, A.; Fredersdorf, S.; Ickenstein, G.; Riegger, G.A.; Horn, M.; Elsner, D. Alterations in myocardial creatinine kinase (CK) and lactate dehydrogenase (LDH) isoenzyme-distribution in a model of left ventricular dysfunction. Eur. J. Heart Fail. 2001, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mair, J. Progress in myocardial damage detection: New biochemical markers for clinicians. Crit. Rev. Clin. Lab. Sci. 1997, 34, 1–66. [Google Scholar] [CrossRef]
- Song, H.; Ren, J.; Yang, L.; Sun, H.; Yan, G.; Han, Y.; Wang, X. Elucidation for the pharmacological effects and mechanism of Shen Bai formula in treating myocardial injury based on energy metabolism and serum metabolomic approaches. J. Ethnopharmacol. 2023, 323, 117670. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, X.; Li, P.; Zhang, H.; Su, C.; Zeng, Z.; Wu, Y.; Xie, X.; Wang, Q.; Han, J.; et al. Si-Miao-Yong-An decoction ameliorates cardiac function through restoring the equilibrium of SOD and NOX2 in heart failure mice. Pharmacol. Res. 2019, 146, 104318. [Google Scholar] [CrossRef]
- Xing, N.; Qin, J.; Ren, D.; Du, Q.; Li, Y.; Mi, J.; Zhang, F.; Ai, L.; Zhang, S.; Zhang, Y.; et al. Integrating UPLC-Q-Exactive Orbitrap/MS, network pharmacology and experimental validation to reveal the potential mechanism of Tibetan medicine Rhodiola granules in improving myocardial ischemia-reperfusion injury. J. Ethnopharmacol. 2023, 314, 116572. [Google Scholar] [CrossRef]
- Qi, T.; Ge, B.K.; Zhao, L.; Ma, Y.; Li, X.R.; Xu, P.X.; Xue, M. Cytosolic β-glucosidase inhibition and renal blood flow suppression are leading causes for the enhanced systemic exposure of salidroside in hypoxic rats. RSC Adv. 2018, 8, 8469–8483. [Google Scholar] [CrossRef]
- Fan, F.; Yang, L.; Li, R.; Zou, X.; Li, N.; Meng, X.; Zhang, Y.; Wang, X. Salidroside as a potential neuroprotective agent for ischemic stroke: A review of sources, pharmacokinetics, mechanism and safety. Biomed. Pharmacother. 2020, 129, 110458. [Google Scholar] [CrossRef]
- He, Y.X.; Liu, X.D.; Wang, X.T.; Liu, X.; Wang, G.J.; Xie, L. Sodium-dependent Glucose Transporter Was Involved in Salidroside Absorption in Intestine of Rats. Chin. J. Nat. Med. 2009, 7, 444–448. [Google Scholar] [CrossRef]
- Park, J.W.; Choi, J.S. Role of kaempferol to increase bioavailability and pharmacokinetics of nifedipine in rats. Chin. J. Nat. Med. 2019, 17, 690–697. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Ma, Y.; Bai, L.; Li, Q.; Zhou, X.; Xu, P.; Li, X.; Xue, M. A UPLC-MS/MS method reveals the pharmacokinetics and metabolism characteristics of kaempferol in rats under hypoxia. Drug Metab. Pharmacokinet. 2022, 43, 100440. [Google Scholar] [CrossRef]
- Yu, Z.; Song, F.; Jin, Y.C.; Zhang, W.M.; Zhang, Y.; Liu, E.J.; Zhou, D.; Bi, L.L.; Yang, Q.; Li, H.; et al. Comparative Pharmacokinetics of Gallic Acid After Oral Administration of Gallic Acid Monohydrate in Normal and Isoproterenol-Induced Myocardial Infarcted Rats. Front. Pharmacol. 2018, 9, 328. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, B.J.; Yuan, G.Y.; Guo, R.C. Comparison of different pharmacodynamic models for pharmacokinetic-pharmacodynamic (PK-PD) modeling of carvedilol. Yao Xue Xue Bao. 2009, 44, 406–411. [Google Scholar]
- Chu, K.M.; Hu, O.Y.; Shieh, S.M. Cardiovascular effect and simultaneous pharmacokinetic and pharmacodynamic modeling of pimobendan in healthy normal subjects. Drug Metab. Dispos. 1999, 27, 701–709. [Google Scholar] [PubMed]
- Lan, K.; Jia, W. An integrated metabolomics and pharmacokinetics strategy for multi-component drugs evaluation. Curr. Drug Metab. 2010, 11, 105–114. [Google Scholar] [CrossRef]
- Tan, W.; Li, Y.; Wang, Y.; Zhang, Z.; Wang, T.; Zhou, Q.; Wang, X. Anti-coagulative and gastrointestinal motility regulative activities of Fructus Aurantii Immaturus and its effective fractions. Biomed. Pharmacother. 2017, 90, 244–252. [Google Scholar] [CrossRef]
- Chen, Q.; Wan, J.; Zhang, Y.; He, Y.; Bao, Y.; Yu, L.; Yang, J. Pharmacokinetic-pharmacodynamic modeling analysis for hydroxysafflor yellow A-calycosin in compatibility in normal and cerebral ischemic rats: A comparative study. Biomed. Pharmacother. 2022, 150, 112950. [Google Scholar] [CrossRef]

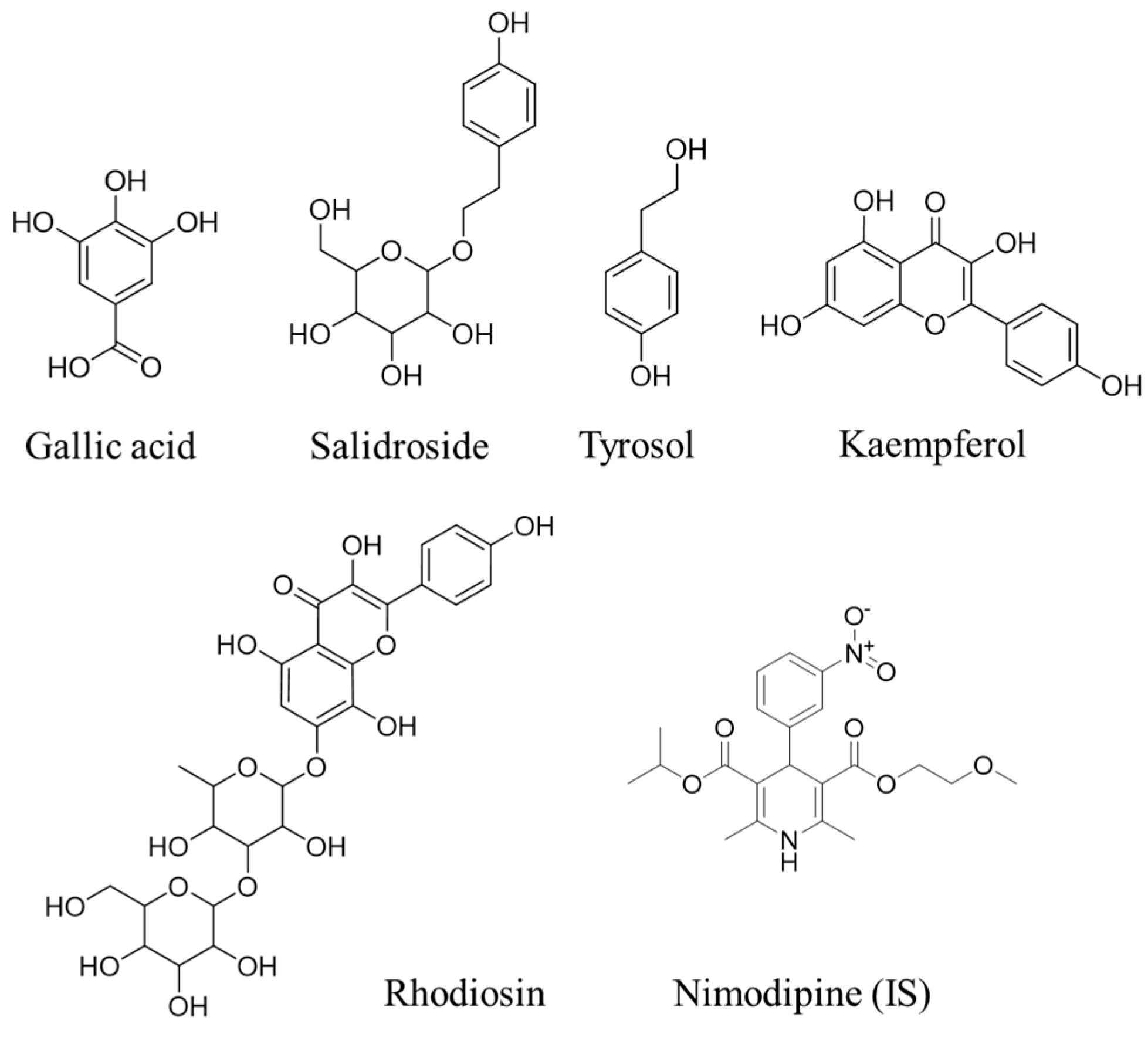
| Name | Curve | Correlation Coefficient (R) | LOQ (ng/mL) | LOD (ng/mL) |
|---|---|---|---|---|
| Gallic Acid | y = 0.3595x + 1.098 | 0.9996 | 0.5 | 0.2 |
| Salidroside | y = 0.0441x + 0.6128 | 0.9978 | 1 | 0.5 |
| Tyrosol | y = 0.0605x + 0.5116 | 0.9991 | 2 | 1 |
| Rhodiosin | y = 0.0708x − 0.1770 | 0.9992 | 2 | 1 |
| Kaempferol | y = 0.0532x − 0.1185 | 0.9998 | 1 | 0.5 |
| Analytes | Spiked Conc. (ng/mL) | Intra-Day | Inter-Day | ||
|---|---|---|---|---|---|
| RSD (%) | RE (%) | RSD (%) | RE (%) | ||
| Gallic Acid | 10 | 11.9 | 93.13 | 12.7 | 89.24 |
| 50 | 5.77 | 96.28 | 8.26 | 95.91 | |
| 400 | 5.62 | 99.88 | 3.11 | 97.25 | |
| Salidroside | 10 | 5.32 | 95.61 | 8.32 | 88.94 |
| 50 | 4.71 | 99.72 | 3.55 | 100.1 | |
| 400 | 6.89 | 101.3 | 5.28 | 98.12 | |
| Tyrosol | 10 | 9.74 | 96.54 | 14.7 | 86.31 |
| 50 | 8.09 | 91.09 | 9.55 | 91.83 | |
| 400 | 4.32 | 92.38 | 8.28 | 95.62 | |
| Rhodiosin | 10 | 13.2 | 85.71 | 14.1 | 86.22 |
| 50 | 9.85 | 89.92 | 6.12 | 90.14 | |
| 400 | 4.30 | 93.24 | 6.71 | 92.53 | |
| Kaempferol | 10 | 3.77 | 101.7 | 9.08 | 93.27 |
| 50 | 7.91 | 99.84 | 2.13 | 102.5 | |
| 400 | 6.32 | 98.52 | 5.56 | 97.95 |
| Analytes | Spiked Conc. (ng/mL) | Extraction Recovery | Matrix Effect | ||
|---|---|---|---|---|---|
| RSD (%) | RE (%) | RSD (%) | RE (%) | ||
| Gallic Acid | 10 | 12.7 | 88.62 | 14.1 | 89.92 |
| 50 | 6.58 | 95.73 | 8.40 | 92.33 | |
| 400 | 8.31 | 100.1 | 5.64 | 99.87 | |
| Salidroside | 10 | 5.32 | 90.53 | 6.90 | 104.2 |
| 50 | 6.43 | 92.74 | 3.38 | 102.3 | |
| 400 | 3.91 | 95.71 | 4.19 | 99.11 | |
| Tyrosol | 10 | 13.5 | 85.32 | 12.6 | 92.22 |
| 50 | 9.82 | 87.63 | 3.55 | 107.5 | |
| 400 | 8.28 | 89.27 | 6.39 | 98.30 | |
| Rhodiosin | 10 | 10.1 | 87.01 | 7.47 | 103.3 |
| 50 | 5.32 | 92.39 | 7.51 | 95.78 | |
| 400 | 6.29 | 90.01 | 3.22 | 97.85 | |
| Kaempferol | 10 | 5.11 | 95.52 | 5.36 | 99.01 |
| 50 | 4.37 | 99.03 | 2.77 | 103.7 | |
| 400 | 3.18 | 101.4 | 4.12 | 93.61 | |
| Analytes | Spiked Conc. (ng/mL) | 4 °C, 48 h | −80 °C, 10 Days | Three Freeze–Thaw Cycles | |||
|---|---|---|---|---|---|---|---|
| RSD (%) | RE (%) | RSD (%) | RE (%) | RSD (%) | RE (%) | ||
| Gallic Acid | 10 | 3.65 | 100.2 | 9.73 | 99.77 | 10.1 | 98.58 |
| 50 | 8.41 | 103.3 | 6.72 | 98.31 | 3.92 | 105.6 | |
| 400 | 7.22 | 99.11 | 7.69 | 96.65 | 4.44 | 90.26 | |
| Salidroside | 10 | 6.37 | 89.72 | 10.2 | 88.35 | 13.6 | 90.22 |
| 50 | 7.58 | 90.35 | 5.39 | 100.1 | 7.58 | 101.3 | |
| 400 | 4.39 | 98.56 | 6.23 | 97.63 | 8.31 | 99.82 | |
| Tyrosol | 10 | 12.1 | 85.44 | 13.0 | 99.52 | 12.7 | 94.31 |
| 50 | 9.56 | 90.28 | 9.32 | 93.68 | 7.32 | 96.93 | |
| 400 | 4.33 | 93.15 | 6.18 | 89.70 | 5.26 | 97.75 | |
| Rhodiosin | 10 | 5.42 | 103.8 | 6.27 | 92.37 | 8.24 | 88.83 |
| 50 | 7.26 | 95.62 | 6.11 | 100.9 | 3.71 | 98.28 | |
| 400 | 5.30 | 93.17 | 4.33 | 90.82 | 5.92 | 93.72 | |
| Kaempferol | 10 | 4.63 | 101.5 | 5.51 | 97.80 | 4.39 | 102.9 |
| 50 | 6.01 | 90.22 | 6.29 | 99.12 | 6.67 | 93.25 | |
| 400 | 3.99 | 92.55 | 3.72 | 100.4 | 5.10 | 94.57 | |
| PK Parameters | Gallic Acid | Salidroside | Tyrosol | Rhodiosin | Kaempferol | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| SDG | MDG | SDG | MDG | SDG | MDG | SDG | MDG | SDG | MDG | |
| AUC(0−t) (μg·h/L) | 474.84 ± 112.26 | 530.79 ± 60.89 | 5714.19 ± 320.74 | 7789.88 ± 1156.98 ** | 653.07 ± 63.25 | 837.42 ± 66.81 ** | 1190.07 ± 56.37 | 1736.61 ± 257.41 ** | 306.11 ± 30.50 | 354.36 ± 50.37 |
| AUC(0−∞) (μg·h/L) | 549.85 ± 168.90 | 664.23 ± 220.79 | 8347.26 ± 2313.72 | 10,049.064 ± 1918.20 | 728.65 ± 49.16 | 1102.46 ± 257.50 ** | 1208.73 ± 44.35 | 1754.66 ± 238.70 ** | 427.39 ± 95.37 | 645.08 ± 178.86 * |
| MRT(0−) (h) | 4.32 ± 1.26 | 5.53 ± 0.59 | 7.14 ± 0.51 | 6.70 ± 0.61 | 5.59 ± 0.50 | 6.54 ± 0.91 * | 4.28 ± 0.27 | 3.94 ± 0.34 | 8.54 ± 0.55 | 9.05 ± 0.70 |
| MRT(0−∞) (h) | 8.92 ± 5.98 | 13.64 ± 10.57 | 13.64 ± 10.57 | 16.71 ± 9.83 | 9.86 ± 5.68 | 16.81 ± 9.96 | 4.75 ± 1.08 | 4.3 ± 0.40 | 19.41 ± 9.37 | 30.85 ± 14.10 |
| t1/2z (h) | 10.25 ± 8.73 | 13.76 ± 10.86 | 19.89 ± 16.03 | 15.44 ± 11.21 | 10.02 ± 7.64 | 15.56 ± 0.79 | 4.18 ± 2.26 | 3.65 ± 2.87 | 14.11 ± 8.42 | 22.74 ± 10.65 |
| Tmax (h) | 0.92 ± 0.13 | 0.92 ± 0.13 | 0.75 ± 0.16 | 0.83 ± 0.13 | 1.17 ± 0.41 | 0.96 ± 0.10 | 1.83 ± 0.41 | 2.5 ± 1.23 | 1.67 ± 1.21 | 0.75 ± 0 |
| Vz/F (L/kg) | 116.47 ± 83.12 | 134.30 ± 68.90 | 15.21 ± 8.77 | 10.70 ± 7.46 | 97.04 ± 70.23 | 94.38 ± 44.37 | 25.06 ± 25.06 | 16.21 ± 14.31 | 224.28 ± 107.33 | 245.31 ± 67.03 |
| CLz/F (L/h/kg) | 9.88 ± 3.09 | 8.13 ± 2.21 | 0.64 ± 0.18 | 0.52 ± 0.12 | 6.89 ± 0.45 | 4.75 ± 1.12 ** | 4.14 ± 0.15 | 2.89 ± 0.39 ** | 12.17 ± 2.57 | 8.42 ± 2.93 * |
| Cmax (μg/L) | 102.79 ± 16.22 | 129.74 ± 19.93 * | 1010 ± 84.17 | 1348.87 ± 133.39 ** | 132.17 ± 12.64 | 143.38 ± 1.41 | 259.08 ± 30.04 | 336.63 ± 22.81 ** | 31.23 ± 1.53 | 38.40 ± 4.92 ** |
| Salidroside | Rhodiosin | Tyrosol | Gallic Acid | Kaempferol | |
|---|---|---|---|---|---|
| AST | E = 1091.001C0.233/(0.010.233 + C0.233) | E = 1084.02C0.397/(0.0110.397 + C0.397) | E = 1213.835C0.38/(0.010.38 + C0.38) | E = 1375.057C0.208/(0.010.208 + C0.208) | E = 1422.742C0.451/(0.010.451 + C0.451) |
| CK | E = 2288.494C0.717/(0.140.717 + C0.717) | E = 2289.293C0.814/(0.040.814 + C0.814) | E = 2289.882C0.904/(0.0170.904 + C0.904) | E = 2290.745C0.614/(0.0090.614 + C0.614) | E = 2736.472C0.997/(0.010.997 + C0.997) |
| HBDH | E = 4898.547C0.313/(5373.930.313 + C0.313) | E = 4335.008C0.318/(779.1670.318 + C0.318) | E = 7149.416C0.355/(579.0370.355 + C0.355) | E = 3540.625C0.304/(179.070.304 + C0.304) | E = 6565.084C0.0.405/(50.0030.405 + C0.405) |
| LDH | E = 482.587C0.184/(0.010.184 + C0.184) | E = 484.31C0.292/(0.010.292 + C0.292) | E = 483.057C0.481/(0.010.481 + C0.481) | E = 580.413C0.189/(0.010.189 + C0.189) | E = 599.812C0.467/(0.010.467 + C0.467) |
| SOD | E = 71.79C0/(0.010 + C0) | E = 72.933C0.01/(1.1730.01 + C0.01) | E = 68.804C0.078/(0.010.078 + C0.078) | E = 73.435C0.01/(1.4840.01 + C0.01) | E = 68.398C0.995/(0.010.995 + C0.995) |
| AST | CK | HBDH | LDH | SOD | ||
|---|---|---|---|---|---|---|
| salidroside | Emax | 1091.001 | 2288.494 | 4898.547 | 482.587 | 71.79 |
| ED50 | 0.01 | 0.14 | 5373.93 | 0.01 | 0.01 | |
| γ | 0.233 | 0.717 | 0.313 | 0.184 | 0 | |
| Ke0 | 2.821 | 2.698 | 0.46 | 2.476 | 0.025 | |
| R2 | 0.559 | 0.848 | 0.91 | 0.349 | 0 | |
| p | 0.153 | 0.007 | 0.002 | 0.429 | 1 | |
| rhodiosin | Emax | 1084.02 | 2289.293 | 4335.008 | 484.31 | 72.933 |
| ED50 | 0.011 | 0.04 | 779.167 | 0.01 | 1.173 | |
| γ | 0.397 | 0.814 | 0.318 | 0.292 | 0.01 | |
| Ke0 | 2.998 | 2.998 | 0.469 | 2.998 | 0.055 | |
| R2 | 0.614 | 0.857 | 0.901 | 0.401 | 0.001 | |
| p | 0.106 | 0.006 | 0.002 | 0.347 | 1 | |
| tyrosol | Emax | 1213.835 | 2289.882 | 7149.416 | 483.057 | 68.804 |
| ED50 | 0.01 | 0.017 | 579.037 | 0.01 | 0.01 | |
| γ | 0.38 | 0.904 | 0.355 | 0.481 | 0.078 | |
| Ke0 | 2.998 | 2.998 | 0.724 | 2.848 | 0.043 | |
| R2 | 0.614 | 0.861 | 0.941 | 0.384 | 0.005 | |
| p | 0.106 | 0.006 | 0 | 0.372 | 0.998 | |
| gallic acid | Emax | 1375.057 | 2290.745 | 3540.625 | 580.413 | 73.435 |
| ED50 | 0.01 | 0.009 | 179.07 | 0.01 | 1.484 | |
| γ | 0.208 | 0.614 | 0.304 | 0.189 | 0.01 | |
| Ke0 | 2.998 | 1.909 | 0.439 | 2.998 | 0.022 | |
| R2 | 0.656 | 0.834 | 0.903 | 0.407 | 0.009 | |
| p | 0.076 | 0.009 | 0.002 | 0.338 | 1 | |
| kaempferol | Emax | 1422.742 | 2736.472 | 6565.084 | 599.812 | 68.398 |
| ED50 | 0.01 | 0.01 | 50.003 | 0.01 | 0.01 | |
| γ | 0.451 | 0.997 | 0.405 | 0.467 | 0.995 | |
| Ke0 | 2.998 | 2.998 | 0.928 | 2.998 | 0.115 | |
| R2 | 0.417 | 0.816 | 0.902 | 0.333 | 0.415 | |
| p | 0.323 | 0.013 | 0.002 | 0.455 | 0.327 |
| Compound Name | Precursor Ion (m/z) | Product Ion (m/z) | Fragmentor (V) | Collision Energy (V) |
|---|---|---|---|---|
| Gallic acid | 169.0 | 125.1 | 90 | 15 |
| Salidroside | 299.1 | 119.0 | 115 | 30 |
| Tyrosol | 137.0 | 119.1 | 110 | 20 |
| Rhodiosin | 609.3 | 301.2 | 130 | 35 |
| Kaempferol | 285.2 | 151.1 | 160 | 20 |
| Nimodipine (IS) | 417.2 | 122.1 | 100 | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Z.; Zou, G.; Xie, Y.; Zhang, E.; Yimingjiang, M.; Cheng, X.; Fang, C.; Wei, F. Pharmacokinetic–Pharmacodynamic Correlation Analysis of Rhodiola crenulata in Rats with Myocardial Ischemia. Pharmaceuticals 2024, 17, 595. https://doi.org/10.3390/ph17050595
Jia Z, Zou G, Xie Y, Zhang E, Yimingjiang M, Cheng X, Fang C, Wei F. Pharmacokinetic–Pharmacodynamic Correlation Analysis of Rhodiola crenulata in Rats with Myocardial Ischemia. Pharmaceuticals. 2024; 17(5):595. https://doi.org/10.3390/ph17050595
Chicago/Turabian StyleJia, Zhixin, Guoming Zou, Yongyan Xie, Enning Zhang, Mureziya Yimingjiang, Xianlong Cheng, Cong Fang, and Feng Wei. 2024. "Pharmacokinetic–Pharmacodynamic Correlation Analysis of Rhodiola crenulata in Rats with Myocardial Ischemia" Pharmaceuticals 17, no. 5: 595. https://doi.org/10.3390/ph17050595
APA StyleJia, Z., Zou, G., Xie, Y., Zhang, E., Yimingjiang, M., Cheng, X., Fang, C., & Wei, F. (2024). Pharmacokinetic–Pharmacodynamic Correlation Analysis of Rhodiola crenulata in Rats with Myocardial Ischemia. Pharmaceuticals, 17(5), 595. https://doi.org/10.3390/ph17050595






