Change in Neurocognitive Function in Patients Who Receive CAR-T Cell Therapies: A Steep Hill to Climb
Abstract
1. Introduction
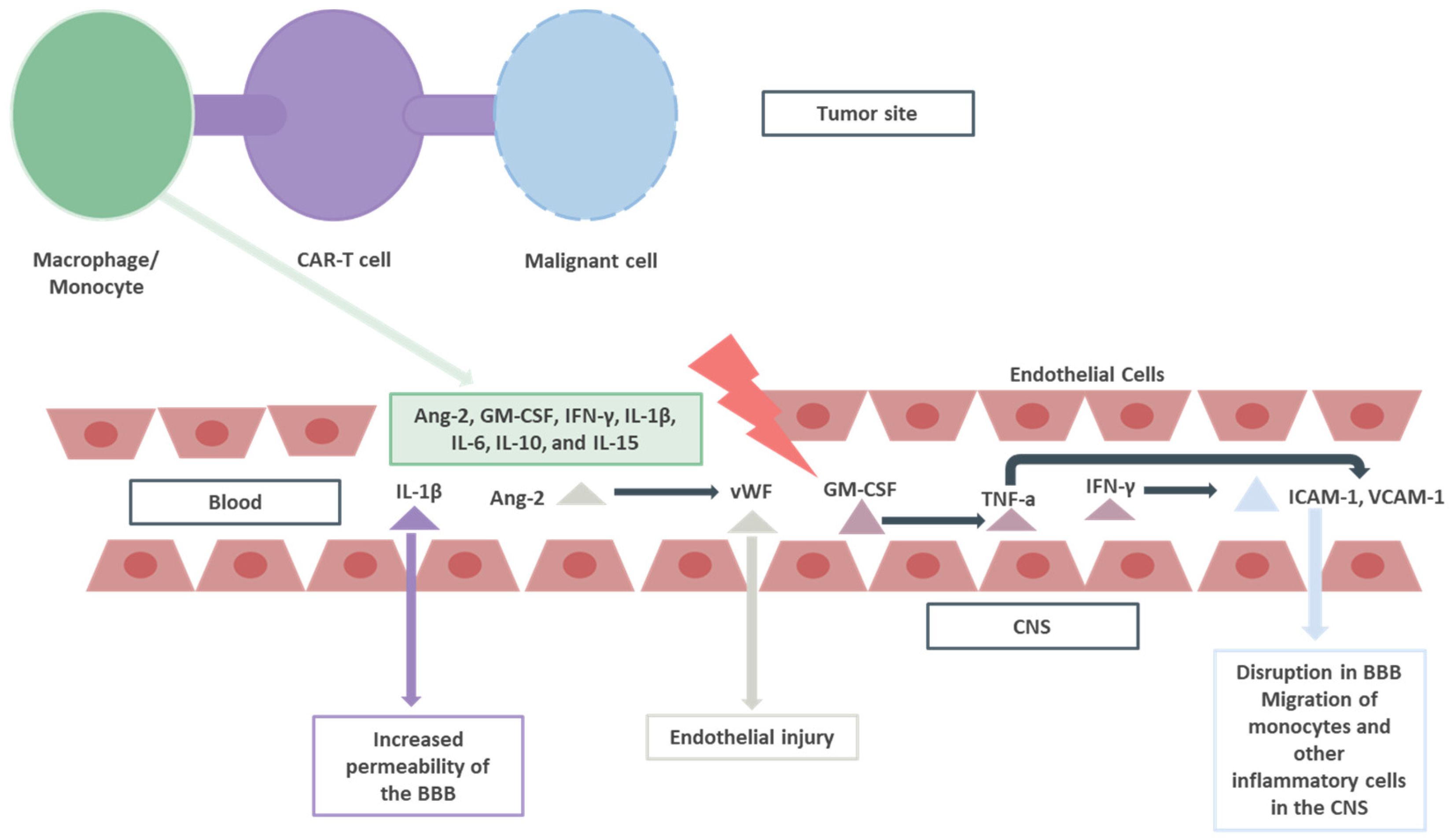
2. ICANS: Grading, Immunopathology, and Treatment Approach
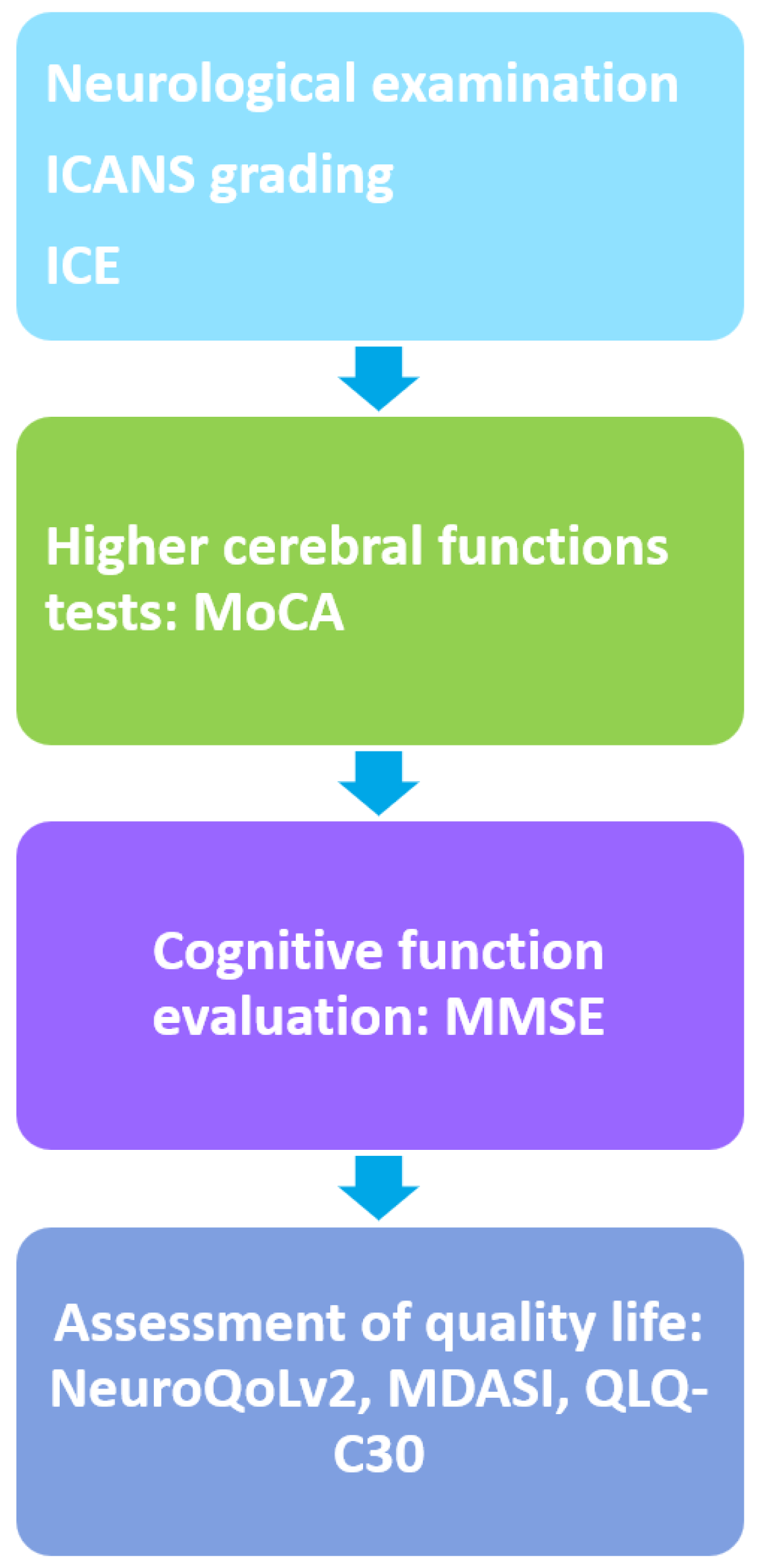
3. Cognitive Outcomes following CAR-T Cell Therapy
4. Risk Factors and Therapeutic Targets for Cognitive Impairment following CAR-T Cell Therapy: The Unanswered Question
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Sterner, R.C.; Sterner, R.M. CAR-T Cell Therapy: Current Limitations and Potential Strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- De Marco, R.C.; Monzo, H.J.; Ojala, P.M. CAR T Cell Therapy: A Versatile Living Drug. Int. J. Mol. Sci. 2023, 24, 6300. [Google Scholar] [CrossRef] [PubMed]
- Rendo, M.J.; Joseph, J.J.; Phan, L.M.; DeStefano, C.B. CAR T-Cell Therapy for Patients with Multiple Myeloma: Current Evidence and Challenges. Blood Lymphat. Cancer 2022, 12, 119–136. [Google Scholar] [CrossRef]
- Gavriilaki, E. Hematology: The Specialty with a Record Number of New Approvals. Front. Med. 2024, 11, 1385052. [Google Scholar] [CrossRef]
- Gavriilaki, E.; Sakellari, I.; Gavriilaki, M.; Anagnostopoulos, A. A New Era in Endothelial Injury Syndromes: Toxicity of CAR-T Cells and the Role of Immunity. Int. J. Mol. Sci. 2020, 21, 3886. [Google Scholar] [CrossRef]
- Gavriilaki, E.; Dolgyras, P.; Dimou-Mpesikli, S.; Poulopoulou, A.; Evangelidis, P.; Evangelidis, N.; Demosthenous, C.; Zachrou, E.; Siasios, P.; Mallouri, D.; et al. Risk Factors, Prevalence, and Outcomes of Invasive Fungal Disease Post Hematopoietic Cell Transplantation and Cellular Therapies: A Retrospective Monocenter Real-Life Analysis. Cancers 2023, 15, 3529. [Google Scholar] [CrossRef]
- Jain, T.; Olson, T.S.; Locke, F.L. How I Treat Cytopenias after CAR T-Cell Therapy. Blood 2023, 141, 2460–2469. [Google Scholar] [CrossRef]
- Sievers, S.; Watson, G.; Johncy, S.; Adkins, S. Recognizing and Grading CAR T-Cell Toxicities: An Advanced Practitioner Perspective. Front. Oncol. 2020, 10, 885. [Google Scholar] [CrossRef] [PubMed]
- van Doesum, J.A.; Salmanton-García, J.; Marchesi, F.; Di Blasi, R.; Falces-Romero, I.; Cabirta, A.; Farina, F.; Besson, C.; Weinbergerová, B.; Van Praet, J.; et al. Impact of SARS-CoV-2 Vaccination and Monoclonal Antibodies on Outcome Post-CD19-Directed CAR T-Cell Therapy: An EPICOVIDEHA Survey. Blood Adv. 2023, 7, 2645–2655. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Schmidts, A.; Wehrli, M.; Maus, M. V Toward Better Understanding and Management of CAR-T Cell-Associated Toxicity. Annu. Rev. Med. 2021, 72, 365–382. [Google Scholar] [CrossRef] [PubMed]
- Tallantyre, E.C.; Evans, N.A.; Parry-Jones, J.; Morgan, M.P.G.; Jones, C.H.; Ingram, W. Neurological Updates: Neurological Complications of CAR-T Therapy. J. Neurol. 2021, 268, 1544–1554. [Google Scholar] [CrossRef] [PubMed]
- Kazzi, C.; Kuznetsova, V.; Siriratnam, P.; Griffith, S.; Wong, S.; Tam, C.S.; Alpitsis, R.; Spencer, A.; O’Brien, T.J.; Malpas, C.B.; et al. Cognition Following Chimeric Antigen Receptor T-Cell Therapy: A Systematic Review. J. Autoimmun. 2023, 140, 103126. [Google Scholar] [CrossRef] [PubMed]
- Maziarz, R.T.; Schuster, S.J.; Romanov, V.V.; Rusch, E.S.; Li, J.; Signorovitch, J.E.; Maloney, D.G.; Locke, F.L. Grading of Neurological Toxicity in Patients Treated with Tisagenlecleucel in the JULIET Trial. Blood Adv. 2020, 4, 1440–1447. [Google Scholar] [CrossRef]
- Santomasso, B.D.; Park, J.H.; Salloum, D.; Riviere, I.; Flynn, J.; Mead, E.; Halton, E.; Wang, X.; Senechal, B.; Purdon, T.; et al. Clinical and Biological Correlates of Neurotoxicity Associated with CAR T-Cell Therapy in Patients with B-Cell Acute Lymphoblastic Leukemia. Cancer Discov. 2018, 8, 958–971. [Google Scholar] [CrossRef] [PubMed]
- YESCARTA® (Axicabtagene Ciloleucel). Available online: https://www.ema.europa.eu/en/documents/overview/yescarta-epar-medicine-overview_en.pdf (accessed on 27 March 2024).
- Frey, N.; Porter, D. Cytokine Release Syndrome with Chimeric Antigen Receptor T Cell Therapy. Biol. Blood Marrow Transplant. 2019, 25, e123–e127. [Google Scholar] [CrossRef] [PubMed]
- Siegler, E.L.; Kenderian, S.S. Neurotoxicity and Cytokine Release Syndrome After Chimeric Antigen Receptor T Cell Therapy: Insights Into Mechanisms and Novel Therapies. Front. Immunol. 2020, 11, 1973. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.N.; Krenciute, G. The Mechanisms of Altered Blood-Brain Barrier Permeability in CD19 CAR T-Cell Recipients. Int. J. Mol. Sci. 2024, 25, 644. [Google Scholar] [CrossRef]
- Gust, J.; Ponce, R.; Liles, W.C.; Garden, G.A.; Turtle, C.J. Cytokines in CAR T Cell-Associated Neurotoxicity. Front. Immunol. 2020, 11, 577027. [Google Scholar] [CrossRef] [PubMed]
- Silveira, C.R.F.; Corveloni, A.C.; Caruso, S.R.; Macêdo, N.A.; Brussolo, N.M.; Haddad, F.; Fernandes, T.R.; de Andrade, P.V.; Orellana, M.D.; Guerino-Cunha, R.L. Cytokines as an Important Player in the Context of CAR-T Cell Therapy for Cancer: Their Role in Tumor Immunomodulation, Manufacture, and Clinical Implications. Front. Immunol. 2022, 13, 947648. [Google Scholar] [CrossRef]
- Sterner, R.M.; Sakemura, R.; Cox, M.J.; Yang, N.; Khadka, R.H.; Forsman, C.L.; Hansen, M.J.; Jin, F.; Ayasoufi, K.; Hefazi, M.; et al. GM-CSF Inhibition Reduces Cytokine Release Syndrome and Neuroinflammation but Enhances CAR-T Cell Function in Xenografts. Blood 2019, 133, 697–709. [Google Scholar] [CrossRef]
- Yi, Y.; Chai, X.; Zheng, L.; Zhang, Y.; Shen, J.; Hu, B.; Tao, G. CRISPR-Edited CART with GM-CSF Knockout and Auto Secretion of IL6 and IL1 Blockers in Patients with Hematologic Malignancy. Cell Discov. 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Steiner, O.; Coisne, C.; Cecchelli, R.; Boscacci, R.; Deutsch, U.; Engelhardt, B.; Lyck, R. Differential Roles for Endothelial ICAM-1, ICAM-2, and VCAM-1 in Shear-Resistant T Cell Arrest, Polarization, and Directed Crawling on Blood–Brain Barrier Endothelium. J. Immunol. 2010, 185, 4846–4855. [Google Scholar] [CrossRef]
- Oluwole, O.O.; Kenderian, S.S.; Shiraz, P.; Karmali, R.; Reshef, R.; McCarthy, P.L.; Ghosh, N.; Lazaryan, A.; Filosto, S.; Poddar, S.; et al. ZUMA-19: A Phase 1/2 Study of Axicabtagene Ciloleucel Plus Lenzilumab in Patients With Relapsed or Refractory Large B-Cell Lymphoma. Blood 2022, 140, 10318–10320. [Google Scholar] [CrossRef]
- Gust, J.; Hay, K.A.; Hanafi, L.-A.; Li, D.; Myerson, D.; Gonzalez-Cuyar, L.F.; Yeung, C.; Liles, W.C.; Wurfel, M.; Lopez, J.A.; et al. Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017, 7, 1404–1419. [Google Scholar] [CrossRef]
- Melrose, J.; Tsurushita, N.; Liu, G.; Berg, E.L. IFN-Gamma Inhibits Activation-Induced Expression of E- and P-Selectin on Endothelial Cells. J. Immunol. 1998, 161, 2457–2464. [Google Scholar] [CrossRef]
- Benallegue, N.; Kebir, H.; Kapoor, R.; Crockett, A.; Li, C.; Cheslow, L.; Abdel-Hakeem, M.S.; Gesualdi, J.; Miller, M.C.; Wherry, E.J.; et al. The Hedgehog Pathway Suppresses Neuropathogenesis in CD4 T Cell-Driven Inflammation. Brain 2021, 144, 1670–1683. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, Y.; Fujikawa, S.; Serizawa, K.; Fujisawa, M.; Matsuo, K.; Nemoto, J.; Shimizu, F.; Sano, Y.; Tomizawa-Shinohara, H.; Miyake, S.; et al. New BBB Model Reveals That IL-6 Blockade Suppressed the BBB Disorder, Preventing Onset of NMOSD. Neurol. R Neuroimmunol. Neuroinflammation 2021, 8, e1076. [Google Scholar] [CrossRef]
- Norelli, M.; Camisa, B.; Barbiera, G.; Falcone, L.; Purevdorj, A.; Genua, M.; Sanvito, F.; Ponzoni, M.; Doglioni, C.; Cristofori, P.; et al. Monocyte-Derived IL-1 and IL-6 Are Differentially Required for Cytokine-Release Syndrome and Neurotoxicity Due to CAR T Cells. Nat. Med. 2018, 24, 739–748. [Google Scholar] [CrossRef]
- Le, R.Q.; Li, L.; Yuan, W.; Shord, S.S.; Nie, L.; Habtemariam, B.A.; Przepiorka, D.; Farrell, A.T.; Pazdur, R. FDA Approval Summary: Tocilizumab for Treatment of Chimeric Antigen Receptor T Cell-Induced Severe or Life-Threatening Cytokine Release Syndrome. Oncologist 2018, 23, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Hay, K.A.; Hanafi, L.-A.; Li, D.; Gust, J.; Liles, W.C.; Wurfel, M.M.; López, J.A.; Chen, J.; Chung, D.; Harju-Baker, S.; et al. Kinetics and Biomarkers of Severe Cytokine Release Syndrome after CD19 Chimeric Antigen Receptor–Modified T-Cell Therapy. Blood 2017, 130, 2295–2306. [Google Scholar] [CrossRef]
- Pennisi, M.; Sanchez-Escamilla, M.; Flynn, J.R.; Shouval, R.; Alarcon Tomas, A.; Silverberg, M.L.; Batlevi, C.; Brentjens, R.J.; Dahi, P.B.; Devlin, S.M.; et al. Modified EASIX Predicts Severe Cytokine Release Syndrome and Neurotoxicity after Chimeric Antigen Receptor T Cells. Blood Adv. 2021, 5, 3397–3406. [Google Scholar] [CrossRef]
- Gavriilaki, E.; Tzannou, I.; Vardi, A.; Tsonis, I.; Liga, M.; Girkas, K.; Ximeri, M.; Bousiou, Z.; Bouzani, M.; Sagiadinou, E.; et al. Easix Indices Predict CRS and Overall Survival in Adult CAR-T Cell Recipients. Blood 2023, 142, 6905. [Google Scholar] [CrossRef]
- Park, J.H.; Santomasso, B.; Riviere, I.; Senechal, B.; Wang, X.; Purdon, T.; Wang, Y.; Halton, E.; Diamonte, C.; Li, D.; et al. Baseline and Early Post-Treatment Clinical and Laboratory Factors Associated with Severe Neurotoxicity Following 19-28z CAR T Cells in Adult Patients with Relapsed B-ALL. J. Clin. Oncol. 2017, 35, 7024. [Google Scholar] [CrossRef]
- Santomasso, B.D.; Nastoupil, L.J.; Adkins, S.; Lacchetti, C.; Schneider, B.J.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated With Chimeric Antigen Receptor T-Cell Therapy: ASCO Guideline. J. Clin. Oncol. 2021, 39, 3978–3992. [Google Scholar] [CrossRef] [PubMed]
- Locke, F.L.; Neelapu, S.S.; Bartlett, N.L.; Lekakis, L.J.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; Timmerman, J.M.; et al. Preliminary Results of Prophylactic Tocilizumab after Axicabtageneciloleucel (Axi-Cel; KTE-C19) Treatment for Patients with Refractory, Aggressive Non-Hodgkin Lymphoma (NHL). Blood 2017, 130, 1547. [Google Scholar] [CrossRef]
- Belin, C.; Devic, P.; Ayrignac, X.; Dos Santos, A.; Paix, A.; Sirven-Villaros, L.; Simard, C.; Lamure, S.; Gastinne, T.; Ursu, R.; et al. Description of Neurotoxicity in a Series of Patients Treated with CAR T-Cell Therapy. Sci. Rep. 2020, 10, 18997. [Google Scholar] [CrossRef] [PubMed]
- Wudhikarn, K.; Pennisi, M.; Garcia-Recio, M.; Flynn, J.R.; Afuye, A.; Silverberg, M.L.; Maloy, M.A.; Devlin, S.M.; Batlevi, C.L.; Shah, G.L.; et al. DLBCL Patients Treated with CD19 CAR T Cells Experience a High Burden of Organ Toxicities but Low Nonrelapse Mortality. Blood Adv. 2020, 4, 3024–3033. [Google Scholar] [CrossRef]
- Torre, M.; Solomon, I.H.; Sutherland, C.L.; Nikiforow, S.; DeAngelo, D.J.; Stone, R.M.; Vaitkevicius, H.; Galinsky, I.A.; Padera, R.F.; Trede, N.; et al. Neuropathology of a Case With Fatal CAR T-Cell-Associated Cerebral Edema. J. Neuropathol. Exp. Neurol. 2018, 77, 877–882. [Google Scholar] [CrossRef]
- Rösler, W.; Bink, A.; Bissig, M.; Imbach, L.; Marques Maggio, E.; Manz, M.G.; Müller, T.; Roth, P.; Rushing, E.; Widmer, C.; et al. CAR T-Cell Infusion Following Checkpoint Inhibition Can Induce Remission in Chemorefractory Post-Transplant Lymphoproliferative Disorder of the CNS. Hemasphere 2022, 6, e733. [Google Scholar] [CrossRef]
- Nair, R.; Drillet, G.; Lhomme, F.; Le Bras, A.; Michel, L.; Rossi, J.; Sherman, M.; Xue, A.; Kerber, A.; Jittapiromsak, N.; et al. Acute Leucoencephalomyelopathy and Quadriparesis after CAR T-Cell Therapy. Haematologica 2021, 106, 1504–1506. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Zhang, C.; Chen, J.; Lou, X.; Chen, X.; Kang, L.; Xu, N.; Li, M.; Tan, J.; et al. Comparation of CART19 and Autologous Stem-Cell Transplantation for Refractory/Relapsed Non-Hodgkin’s Lymphoma. JCI Insight 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.E.; Grupp, S.A.; Pulsipher, M.A.; Dietz, A.C.; Rives, S.; Myers, G.D.; August, K.J.; Verneris, M.R.; Buechner, J.; Laetsch, T.W.; et al. Pooled Safety Analysis of Tisagenlecleucel in Children and Young Adults with B Cell Acute Lymphoblastic Leukemia. J. Immunother. Cancer 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Herr, M.M.; Chen, G.L.; Ross, M.; Jacobson, H.; McKenzie, R.; Markel, L.; Balderman, S.R.; Ho, C.M.; Hahn, T.; McCarthy, P.L. Identification of Neurotoxicity after Chimeric Antigen Receptor (CAR) T Cell Infusion without Deterioration in the Immune Effector Cell Encephalopathy (ICE) Score. Biol. Blood Marrow Transplant. 2020, 26, e271–e274. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.D.; Parekh, S.; Santomasso, B.D.; Gállego Pérez-Larraya, J.; van de Donk, N.W.C.J.; Arnulf, B.; Mateos, M.-V.; Lendvai, N.; Jackson, C.C.; De Braganca, K.C.; et al. Incidence and Management of CAR-T Neurotoxicity in Patients with Multiple Myeloma Treated with Ciltacabtagene Autoleucel in CARTITUDE Studies. Blood Cancer J. 2022, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Zhang, Y.; Xu, J. Sodium Oligomannate Combined with Rivastigmine May Improve Cerebral Blood Flow and Cognitive Impairment Following CAR-T Cell Therapy: A Case Report. Front. Oncol. 2022, 12, 902301. [Google Scholar] [CrossRef]
- Möhn, N.; Bonda, V.; Grote-Levi, L.; Panagiota, V.; Fröhlich, T.; Schultze-Florey, C.; Wattjes, M.P.; Beutel, G.; Eder, M.; David, S.; et al. Neurological Management and Work-up of Neurotoxicity Associated with CAR T Cell Therapy. Neurol. Res. Pract. 2022, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Sales, C.; Anderson, M.A.; Kuznetsova, V.; Rosenfeld, H.; Malpas, C.B.; Roos, I.; Dickinson, M.; Harrison, S.; Kalincik, T. Patterns of Neurotoxicity among Patients Receiving Chimeric Antigen Receptor T-Cell Therapy: A Single-Centre Cohort Study. Eur. J. Neurol. 2024, 31, e16174. [Google Scholar] [CrossRef] [PubMed]
- Aiello, E.N.; Gramegna, C.; Esposito, A.; Gazzaniga, V.; Zago, S.; Difonzo, T.; Maddaluno, O.; Appollonio, I.; Bolognini, N. The Montreal Cognitive Assessment (MoCA): Updated Norms and Psychometric Insights into Adaptive Testing from Healthy Individuals in Northern Italy. Aging Clin. Exp. Res. 2022, 34, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Shalabi, H.; Wolters, P.L.; Martin, S.; Toledo-Tamula, M.A.; Roderick, M.C.; Struemph, K.; Kane, E.; Yates, B.; Delbrook, C.; Mackall, C.L.; et al. Systematic Evaluation of Neurotoxicity in Children and Young Adults Undergoing CD22 Chimeric Antigen Receptor T-Cell Therapy. J. Immunother. 2018, 41, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Zelazo, P.D. The Dimensional Change Card Sort (DCCS): A Method of Assessing Executive Function in Children. Nat. Protoc. 2006, 1, 297–301. [Google Scholar] [CrossRef]
- Kavanaugh, B.C.; Cancilliere, M.K.; Fryc, A.; Tirrell, E.; Oliveira, J.; Oberman, L.M.; Wexler, B.E.; Carpenter, L.L.; Spirito, A. Measurement of Executive Functioning with the National Institute of Health Toolbox and the Association to Anxiety/Depressive Symptomatology in Childhood/Adolescence. Child. Neuropsychol. 2020, 26, 754–769. [Google Scholar] [CrossRef] [PubMed]
- Ciriegio, A.E.; Watson, K.H.; Pfalzer, A.C.; Hale, L.; Huitz, E.; Moroz, S.; Roth, M.C.; Snow, A.L.B.; Jones, M.T.; Guthrie, C.S.; et al. Inhibitory Control, Working Memory and Coping with Stress: Associations with Symptoms of Anxiety and Depression in Adults with Huntington’s Disease. Neuropsychology 2022, 36, 288–296. [Google Scholar] [CrossRef]
- Soto, T. Processing Speed Index. In Encyclopedia of Autism Spectrum Disorders; Springer: New York, NY, USA, 2013; pp. 2380–2381. [Google Scholar]
- Cella, D.; Lai, J.-S.; Nowinski, C.J.; Victorson, D.; Peterman, A.; Miller, D.; Bethoux, F.; Heinemann, A.; Rubin, S.; Cavazos, J.E.; et al. Neuro-QOL: Brief Measures of Health-Related Quality of Life for Clinical Research in Neurology. Neurology 2012, 78, 1860–1867. [Google Scholar] [CrossRef]
- Sidana, S.; Dueck, A.C.; Thanarajasingam, G.; Griffin, J.M.; Thompson, C.; Durani, U.; Burtis, M.; Warsame, R.; Paludo, J.; Gertz, M.A.; et al. Longitudinal Patient Reported Outcomes with CAR-T Cell Therapy Versus Autologous and Allogeneic Stem Cell Transplant. Transplant. Cell Ther. 2022, 28, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Barata, A.; Hoogland, A.I.; Kommalapati, A.; Logue, J.; Welniak, T.; Hyland, K.A.; Eisel, S.L.; Small, B.J.; Jayani, R.V.; Booth-Jones, M.; et al. Change in Patients’ Perceived Cognition Following Chimeric Antigen Receptor T-Cell Therapy for Lymphoma. Transplant. Cell Ther. 2022, 28, 401.e1–401.e7. [Google Scholar] [CrossRef] [PubMed]
- Sailors, M.H.; Bodurka, D.C.; Gning, I.; Ramondetta, L.M.; Williams, L.A.; Mendoza, T.R.; Agarwal, S.; Sun, C.C.; Cleeland, C.S. Validating the M. D. Anderson Symptom Inventory (MDASI) for Use in Patients with Ovarian Cancer. Gynecol. Oncol. 2013, 130, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Srour, S.A.; Whisenant, M.; Subbiah, I.M.; Chen, T.H.; Ponce, D.; Gonzalez, A.G.; Kamal, M.; Mendoza, T.; Cleland, C.S.; et al. Patient-Reported Symptom and Functioning Status during the First 12 Months after Chimeric Antigen Receptor T Cell Therapy for Hematologic Malignancies. Transplant. Cell Ther. 2021, 27, 930.e1–930.e10. [Google Scholar] [CrossRef]
- Ruark, J.; Mullane, E.; Cleary, N.; Cordeiro, A.; Bezerra, E.D.; Wu, V.; Voutsinas, J.; Shaw, B.E.; Flynn, K.E.; Lee, S.J.; et al. Patient-Reported Neuropsychiatric Outcomes of Long-Term Survivors after Chimeric Antigen Receptor T Cell Therapy. Biol. Blood Marrow Transplant. 2020, 26, 34–43. [Google Scholar] [CrossRef]
- Cheng, R.; Scippa, K.; Locke, F.L.; Snider, J.T.; Jim, H. Patient Perspectives on Health-Related Quality of Life in Diffuse Large B-Cell Lymphoma Treated with Car T-Cell Therapy: A Qualitative Study. Oncol. Ther. 2022, 10, 123–141. [Google Scholar] [CrossRef]
- Akinola, I.M.; Cusatis, R.; Pasquini, M.C.; Shaw, B.E.; Bollu, V.; Dalal, A.; Tesfaye, M.; Flynn, K.E. Multi-Stakeholder Qualitative Interviews to Inform Measurement of Patient Reported Outcomes After CAR-T. Transplant. Cell Ther. 2023, 29, 254.e1–254.e9. [Google Scholar] [CrossRef] [PubMed]
- Delforge, M.; Shah, N.; Miguel, J.S.F.; Braverman, J.; Dhanda, D.S.; Shi, L.; Guo, S.; Yu, P.; Liao, W.; Campbell, T.B.; et al. Health-Related Quality of Life with Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. Blood Adv. 2022, 6, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Maillet, D.; Belin, C.; Moroni, C.; Cuzzubbo, S.; Ursu, R.; Sirven-Villaros, L.; Di Blasi, R.; Thieblemont, C.; Carpentier, A.F. Evaluation of Mid-Term (6-12 Months) Neurotoxicity in B-Cell Lymphoma Patients Treated with CAR T Cells: A Prospective Cohort Study. Neuro Oncol. 2021, 23, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Ursu, R.; Maillet, D.; Belin, C.; Moroni, C.; Cuzzubbo, S.; Vernier, V.; Sirven-Villaros, L.; Carreau, C.; Di Blasi, R.; Thieblemont, C.; et al. Long-Term Neurologic Safety in Patients With B-Cell Lymphoma Treated With Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy. Neurology 2022, 99, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Ruchinskas, R. Wechsler Adult Intelligence Scale-4th Edition Digit Span Performance in Subjective Cognitive Complaints, Amnestic Mild Cognitive Impairment, and Probable Dementia of the Alzheimer Type. Clin. Neuropsychol. 2019, 33, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Wambach, D.; Lamar, M.; Swenson, R.; Penney, D.L.; Kaplan, E.; Libon, D.J. Digit Span. In Encyclopedia of Clinical Neuropsychology; Springer: New York, NY, USA, 2011; pp. 844–849. [Google Scholar]
- Grober, E.; Sanders, A.E.; Hall, C.; Lipton, R.B. Free and Cued Selective Reminding Identifies Very Mild Dementia in Primary Care. Alzheimer Dis. Assoc. Disord. 2010, 24, 284–290. [Google Scholar] [CrossRef]
- Teichmann, M.; Epelbaum, S.; Samri, D.; Levy Nogueira, M.; Michon, A.; Hampel, H.; Lamari, F.; Dubois, B. Free and Cued Selective Reminding Test-Accuracy for the Differential Diagnosis of Alzheimer’s and Neurodegenerative Diseases: A Large-Scale Biomarker-Characterized Monocenter Cohort Study (ClinAD). Alzheimers Dement. 2017, 13, 913–923. [Google Scholar] [CrossRef]
- Crawford, J.R.; Smith, G.; Maylor, E.A.; Della Sala, S.; Logie, R.H. The Prospective and Retrospective Memory Questionnaire (PRMQ): Normative Data and Latent Structure in a Large Non-Clinical Sample. Memory 2003, 11, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Llinàs-Reglà, J.; Vilalta-Franch, J.; López-Pousa, S.; Calvó-Perxas, L.; Torrents Rodas, D.; Garre-Olmo, J. The Trail Making Test. Assessment 2017, 24, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Bowie, C.R.; Harvey, P.D. Administration and Interpretation of the Trail Making Test. Nat. Protoc. 2006, 1, 2277–2281. [Google Scholar] [CrossRef] [PubMed]
- Ciolek, C.H.; Lee, S.Y. Cognitive Issues in the Older Adult. In Guccione’s Geriatric Physical Therapy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 425–452. [Google Scholar]
- Zhang, R.; Zhou, M.; Qi, J.; Miao, W.; Zhang, Z.; Wu, D.; Han, Y. Efficacy and Safety of Eculizumab in the Treatment of Transplant-Associated Thrombotic Microangiopathy: A Systematic Review and Meta-Analysis. Front. Immunol. 2020, 11, 564647. [Google Scholar] [CrossRef]
- Mailloux, Z. An Overview of Sensory Integration and Praxis Tests. Am. J. Occup. Ther. 1990, 44, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Hayward, R.W.; Naeser, M.A.; Zatz, L.M. Cranial Computed Tomography in Aphasia. Correlation of Anatomical Lesions with Functional Deficits. Radiology 1977, 123, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Tombaugh, T.N.; McIntyre, N.J. The Mini-Mental State Examination: A Comprehensive Review. J. Am. Geriatr. Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef]
- Creavin, S.T.; Wisniewski, S.; Noel-Storr, A.H.; Trevelyan, C.M.; Hampton, T.; Rayment, D.; Thom, V.M.; Nash, K.J.E.; Elhamoui, H.; Milligan, R.; et al. Mini-Mental State Examination (MMSE) for the Detection of Dementia in Clinically Unevaluated People Aged 65 and over in Community and Primary Care Populations. Cochrane Database Syst. Rev. 2016, 2016, CD011145. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; McMahon, K.L.; Copland, D.A.; Pourzinal, D.; Byrne, G.J.; Angwin, A.J.; O’Sullivan, J.D.; Dissanayaka, N.N. Semantic Fluency Deficits and Associated Brain Activity in Parkinson’s Disease with Mild Cognitive Impairment. Brain Imaging Behav. 2022, 16, 2445–2456. [Google Scholar] [CrossRef] [PubMed]
- Gisbert-Muñoz, S.; Quiñones, I.; Amoruso, L.; Timofeeva, P.; Geng, S.; Boudelaa, S.; Pomposo, I.; Gil-Robles, S.; Carreiras, M. MULTIMAP: Multilingual Picture Naming Test for Mapping Eloquent Areas during Awake Surgeries. Behav. Res. Methods 2021, 53, 918–927. [Google Scholar] [CrossRef]
- Hoogland, A.I.; Barata, A.; Logue, J.; Kommalapati, A.; Hyland, K.A.; Nelson, A.M.; Eisel, S.L.; Small, B.J.; James, B.W.; Christy, S.M.; et al. Change in Neurocognitive Performance Among Patients with Non-Hodgkin Lymphoma in the First Year after Chimeric Antigen Receptor T Cell Therapy. Transplant. Cell Ther. 2022, 28, 305.e1–305.e9. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, L.M.; Holbrook, L.; Rhoads, T.; Cerny, B.M.; Jennette, K.J.; Resch, Z.J.; Obolsky, M.A.; Ovsiew, G.P.; Soble, J.R. Examining Conners Continuous Performance Test-3 (CPT-3) Embedded Performance Validity Indicators in an Adult Clinical Sample Referred for ADHD Evaluation. Dev. Neuropsychol. 2021, 46, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Randolph, C.; Tierney, M.C.; Mohr, E.; Chase, T.N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary Clinical Validity. J. Clin. Exp. Neuropsychol. 1998, 20, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Scarpina, F.; Tagini, S. The Stroop Color and Word Test. Front. Psychol. 2017, 8, 241674. [Google Scholar] [CrossRef] [PubMed]
- Whitney, K.A.; Shepard, P.H.; Mariner, J.; Mossbarger, B.; Herman, S.M. Validity of the Wechsler Test of Adult Reading (WTAR): Effort Considered in a Clinical Sample of U.S. Military Veterans. Appl. Neuropsychol. 2010, 17, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Wefel, J.S.; Parsons, M.W.; Gondi, V.; Brown, P.D. Neurocognitive Aspects of Brain Metastasis. Handb. Clin. Neurol. 2018, 149, 155–165. [Google Scholar] [PubMed]
- Bell, M.L.; Dhillon, H.M.; Bray, V.J.; Vardy, J.L. Important Differences and Meaningful Changes for the Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog). J. Patient Rep. Outcomes 2018, 2, 48. [Google Scholar] [CrossRef]
- Musa, G.; Henríquez, F.; Muñoz-Neira, C.; Delgado, C.; Lillo, P.; Slachevsky, A. Utility of the Neuropsychiatric Inventory Questionnaire (NPI-Q) in the Assessment of a Sample of Patients with Alzheimer’s Disease in Chile. Dement. Neuropsychol. 2017, 11, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Strati, P.; Jallouk, A.; Deng, Q.; Li, X.; Feng, L.; Sun, R.; Adkins, S.; Johncy, S.; Cain, T.; Steiner, R.E.; et al. A Phase 1 Study of Prophylactic Anakinra to Mitigate ICANS in Patients with Large B-Cell Lymphoma. Blood Adv. 2023, 7, 6785–6789. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, C.A.; Rosenthal, A.C.; Arnason, J.; Agarwal, S.; Zhang, P.; Wu, W.; Amber, V.; Yared, J.A. A Phase 2 Trial of Defibrotide for the Prevention of Chimeric Antigen Receptor T-Cell–Associated Neurotoxicity Syndrome. Blood Adv. 2023, 7, 6790–6799. [Google Scholar] [CrossRef] [PubMed]
- Dowling, M.R.; Turtle, C.J. ICANS Prophylaxis: Potentially Transformative but Elusive. Blood Adv. 2023, 7, 6782–6784. [Google Scholar] [CrossRef] [PubMed]
- Mavrikou, I.; Chatzidimitriou, D.; Skoura, L.; Nikolousis, E.; Sakellari, I.; Gavriilaki, E. Molecular Advances in Sinusoidal Obstruction Syndrome/Veno-Occlusive Disease. Int. J. Mol. Sci. 2023, 24, 5620. [Google Scholar] [CrossRef] [PubMed]
- Gavriilaki, M.; Anyfanti, P.; Mastrogiannis, K.; Gavriilaki, E.; Lazaridis, A.; Kimiskidis, V.; Gkaliagkousi, E. Association between Ambulatory Blood Pressure Monitoring Patterns with Cognitive Function and Risk of Dementia: A Systematic Review and Meta-Analysis. Aging Clin. Exp. Res. 2023, 35, 745–761. [Google Scholar] [CrossRef]
- Koletsos, N.; Dipla, K.; Triantafyllou, A.; Dolgyras, P.; Aslanidis, S.; Zafeiridis, A.; Galanopoulou, V.; Douma, S.; Gkaliagkousi, E. Depression in Systemic Lupus Erythematosus: A Manifestation of Microcirculation Dysfunction? Lupus 2023, 32, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, R.J.; Soiza, R.L. Evidence of Endothelial Dysfunction in the Development of Alzheimer’s Disease: Is Alzheimer’s a Vascular Disorder? Am. J. Cardiovasc. Dis. 2013, 3, 197–226. [Google Scholar]
- Barata, A.; Dhawale, T.; Newcomb, R.A.; Amonoo, H.L.; Nelson, A.M.; Yang, D.; Karpinski, K.; Holmbeck, K.; Farnam, E.; Frigault, M.; et al. Quality of Life and Prognostic Awareness in Caregivers of Patients Receiving Chimeric Antigen Receptor T Cell Therapy. Transplant. Cell Ther. 2024, 30, 452.e1–452.e11. [Google Scholar] [CrossRef] [PubMed]
- Liang, E.C.; Rejeski, K.; Fei, T.; Albittar, A.; Huang, J.J.; Portuguese, A.J.; Wu, Q.; Raj, S.; Subklewe, M.; Shouval, R.; et al. Development and Validation of an Automated Computational Approach to Grade Immune Effector Cell-Associated Hematotoxicity. Bone Marrow Transplant. 2024. [Google Scholar] [CrossRef] [PubMed]
| First Author, Year of Publication | Type of Hematological Malignancy | Study Population (N) | Age of Study Participants | Tests for Cognition Evaluation | Outcomes |
|---|---|---|---|---|---|
| Ruark (2020) [61] | Relapsed/ refractory B-acute lymphoblastic leukemia, non-Hodgkin lymphoma, chronic lymphocytic leukemia | 40 | Median = 54 (range 22–74) | Self-reported questions on cognition | 37.5% of the participants reported one or more cognitive difficulties (35% memory, 30% word finding, 22.5% concentration, 12.5% problem solving) |
| Maillet et al. (2021) [65] | Relapsed/ refractory diffuse large B cell lymphomas | 56 | Mean = 58 (standard deviation ±14) | MMSE, BDAE, DO-80, semantic fluency, digit span, TMT, Stroop test, FCSRT, ROCF, praxis scale, PRMQ | Scores in tests for visuo-construction (p < 0.001), visuospatial ability (p < 0.001), and short-term memory (p = 0.002) were improved during follow-up. |
| Ursu et al. (2022) [66] | Relapsed non-Hodgkin lymphomas | 56 | Median = 69 (range 26–72) | MMSE, semantic fluency, DO-80, BDAE, digit span, TMT, Stroop test, FCSRT, ROCF, praxis scale, PRMQ | No statistically significant difference was reported between baseline and follow-up (2 years post-infusion) in any of the neuropsychological tests conducted. |
| Hoogland et al. (2022) [82] | Relapsed non-Hodgkin lymphomas | 117 | Mean = 60.92 (standard deviation ±11.57) | Color trails, WTAR, RBANS, CPT3, Stroop test | TNP and executive function decreased slightly from baseline to 3 months post-infusion and improved at 12 months (p < 0.04), while slight but significant linear declines in visuospatial ability were also reported (p = 0.03). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strongyli, E.; Evangelidis, P.; Sakellari, I.; Gavriilaki, M.; Gavriilaki, E. Change in Neurocognitive Function in Patients Who Receive CAR-T Cell Therapies: A Steep Hill to Climb. Pharmaceuticals 2024, 17, 591. https://doi.org/10.3390/ph17050591
Strongyli E, Evangelidis P, Sakellari I, Gavriilaki M, Gavriilaki E. Change in Neurocognitive Function in Patients Who Receive CAR-T Cell Therapies: A Steep Hill to Climb. Pharmaceuticals. 2024; 17(5):591. https://doi.org/10.3390/ph17050591
Chicago/Turabian StyleStrongyli, Evlampia, Paschalis Evangelidis, Ioanna Sakellari, Maria Gavriilaki, and Eleni Gavriilaki. 2024. "Change in Neurocognitive Function in Patients Who Receive CAR-T Cell Therapies: A Steep Hill to Climb" Pharmaceuticals 17, no. 5: 591. https://doi.org/10.3390/ph17050591
APA StyleStrongyli, E., Evangelidis, P., Sakellari, I., Gavriilaki, M., & Gavriilaki, E. (2024). Change in Neurocognitive Function in Patients Who Receive CAR-T Cell Therapies: A Steep Hill to Climb. Pharmaceuticals, 17(5), 591. https://doi.org/10.3390/ph17050591








