Protective Effects of Pear Extract on Skin from In Vitro and In Vivo UVA-Induced Damage
Abstract
1. Introduction
2. Results
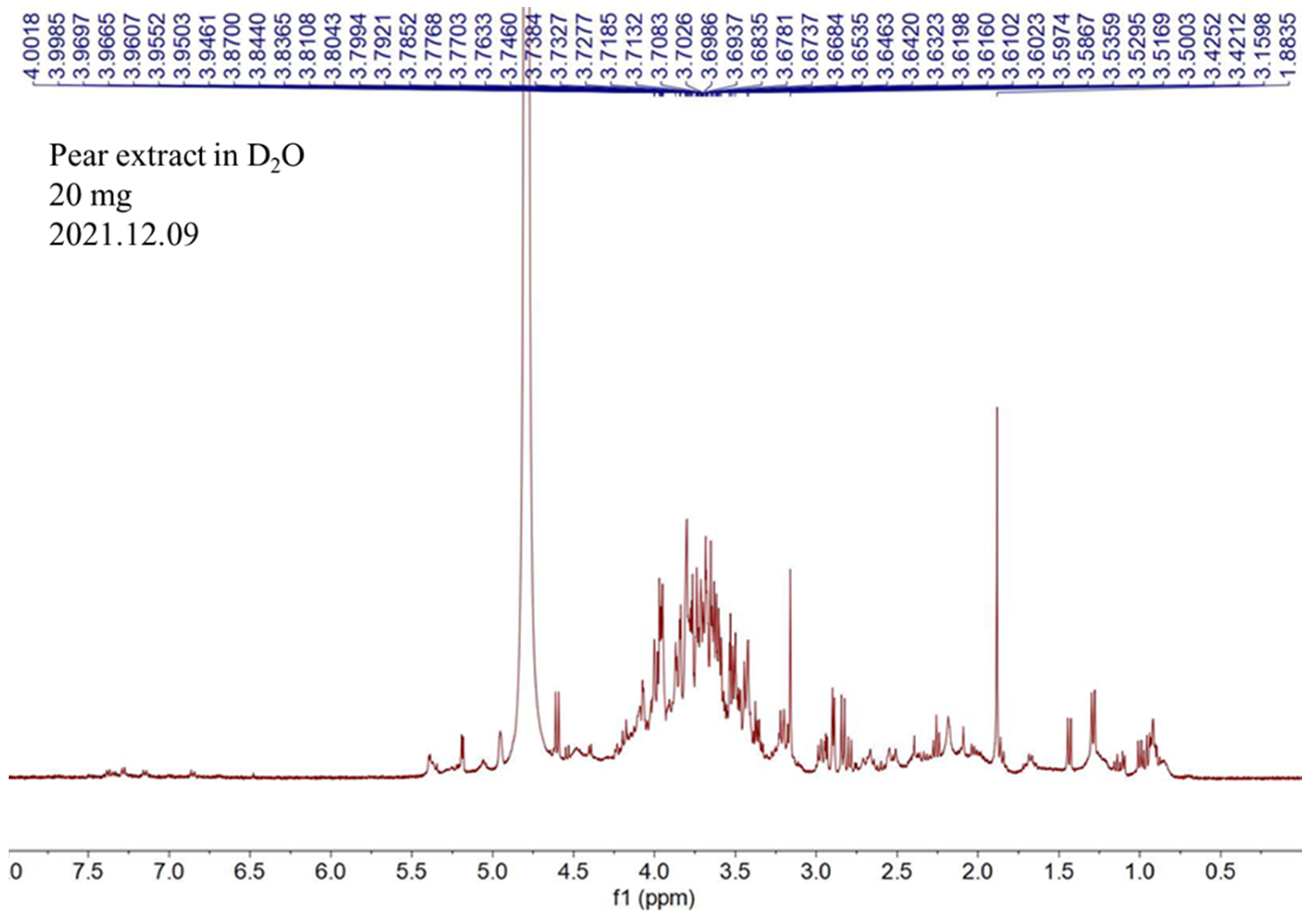
2.1. NMR Analysis of Pear Water Extract
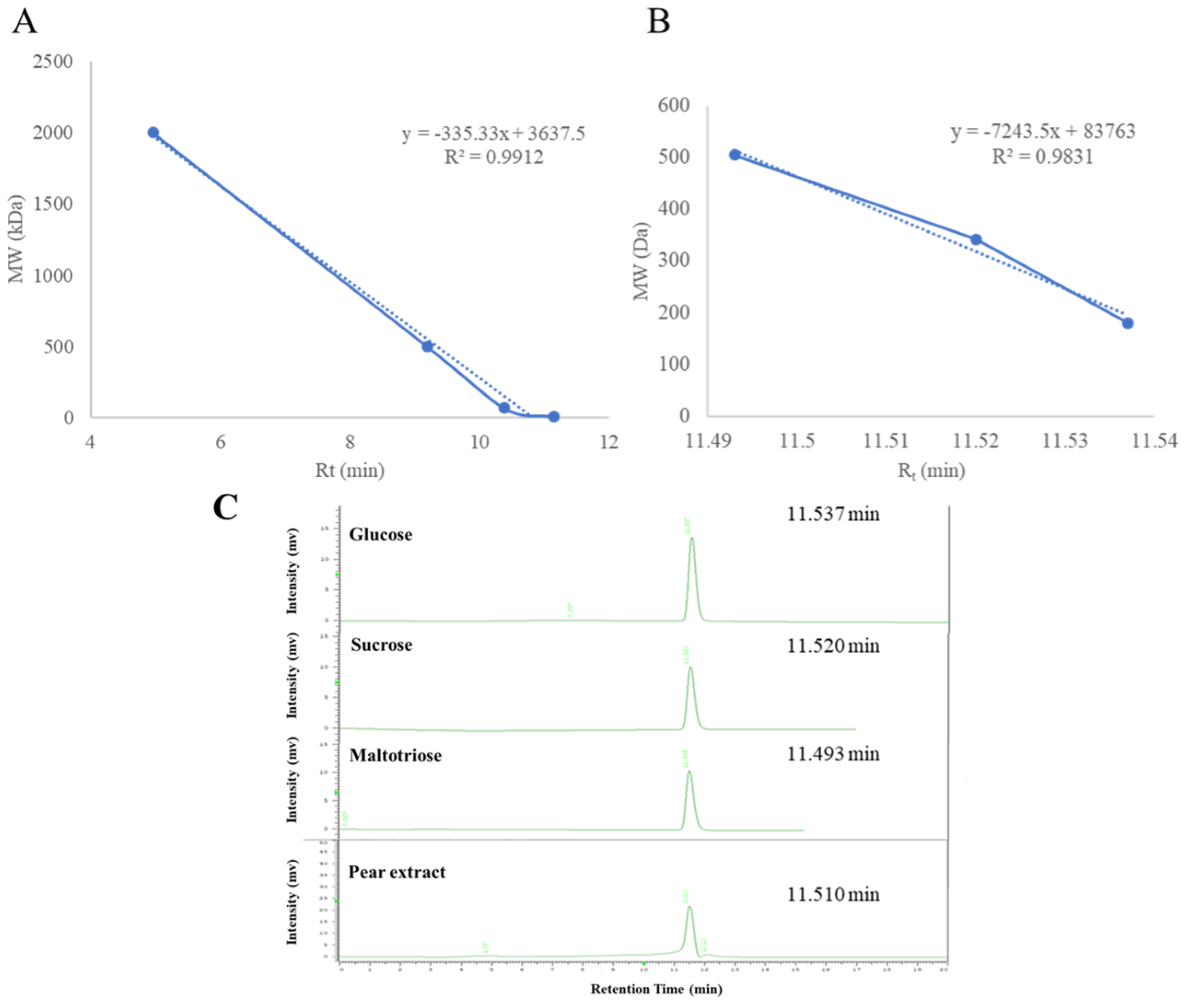
2.2. Determination of Molecular Weight of Pear Water Extract
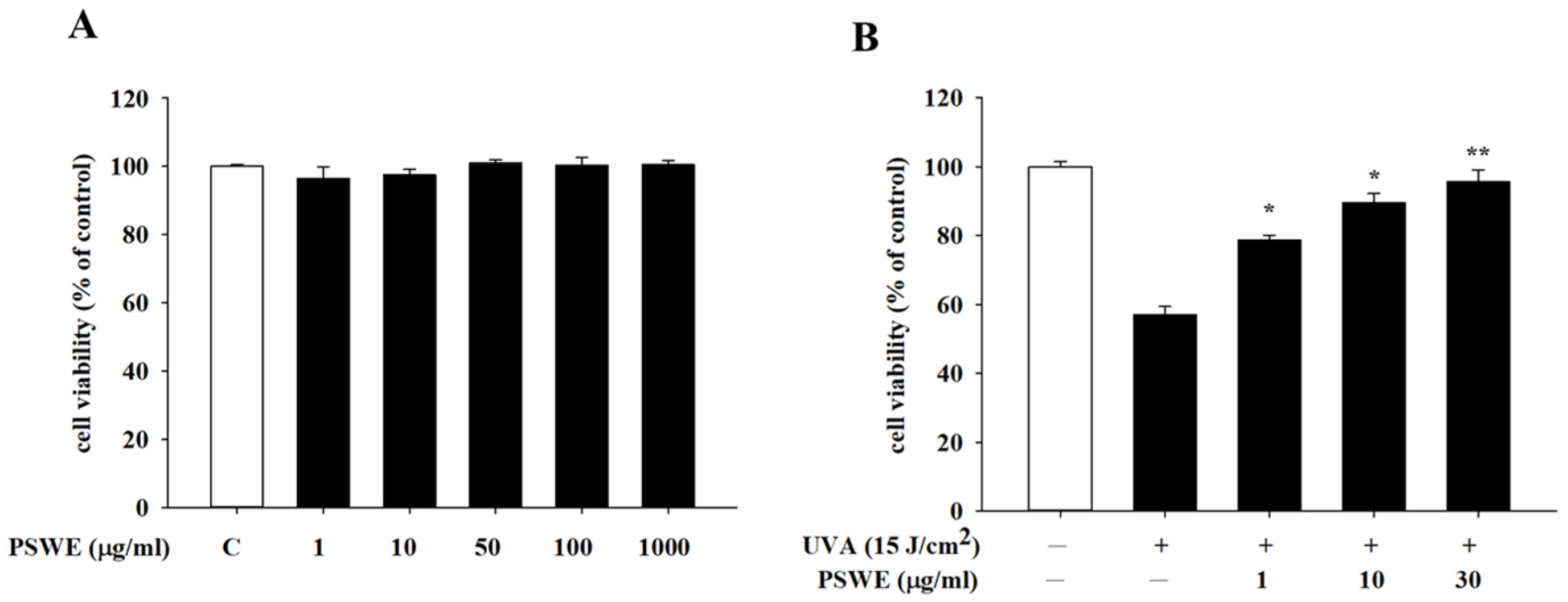
2.3. Cytotoxicity and Protective Effects of PSWE against UVA on HaCaT Cells
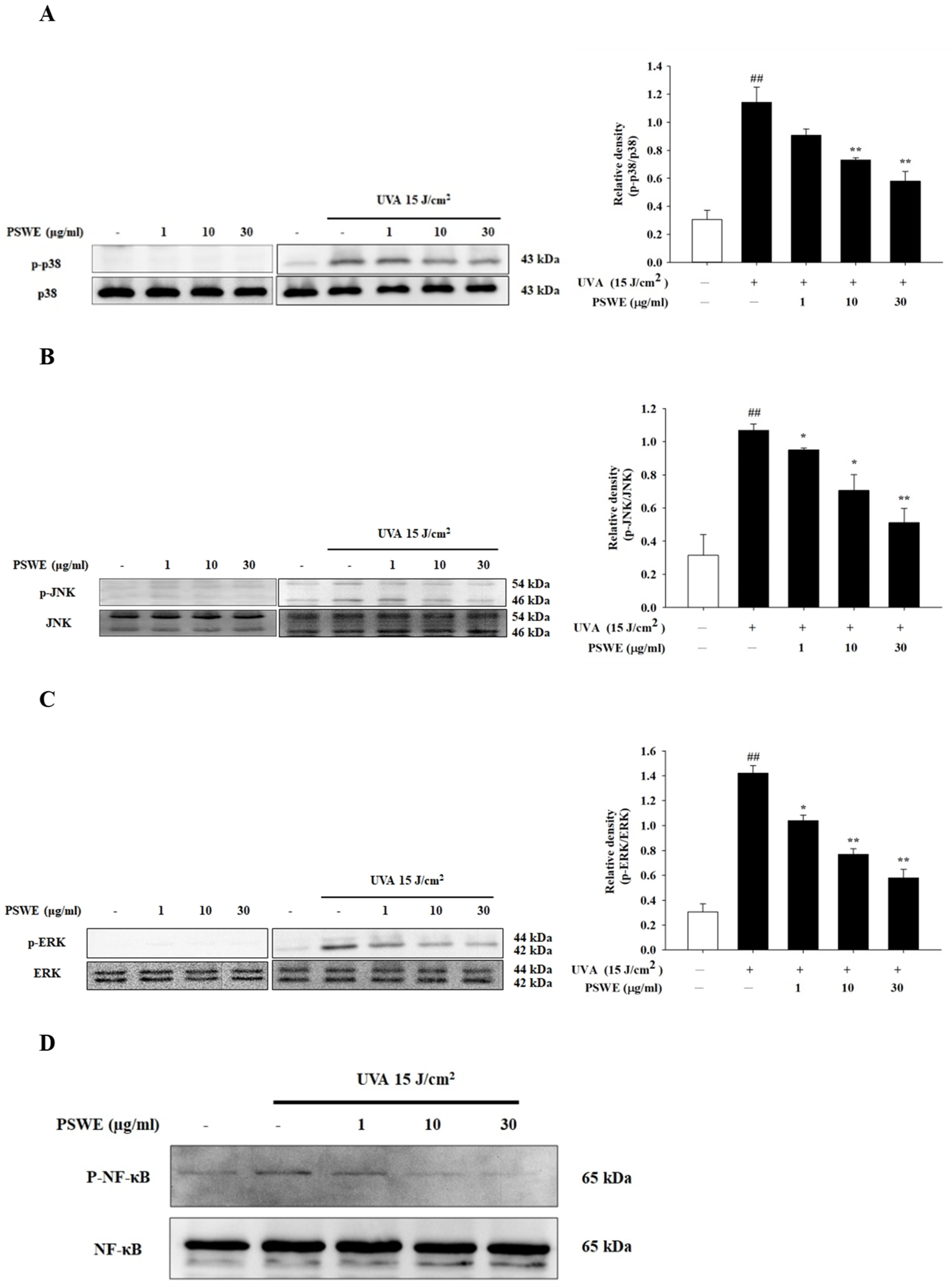
2.4. PSWE Inhibits the Phosphorylation of UVA-Induced MAP Kinase and NF-κB Pathway in HaCaT
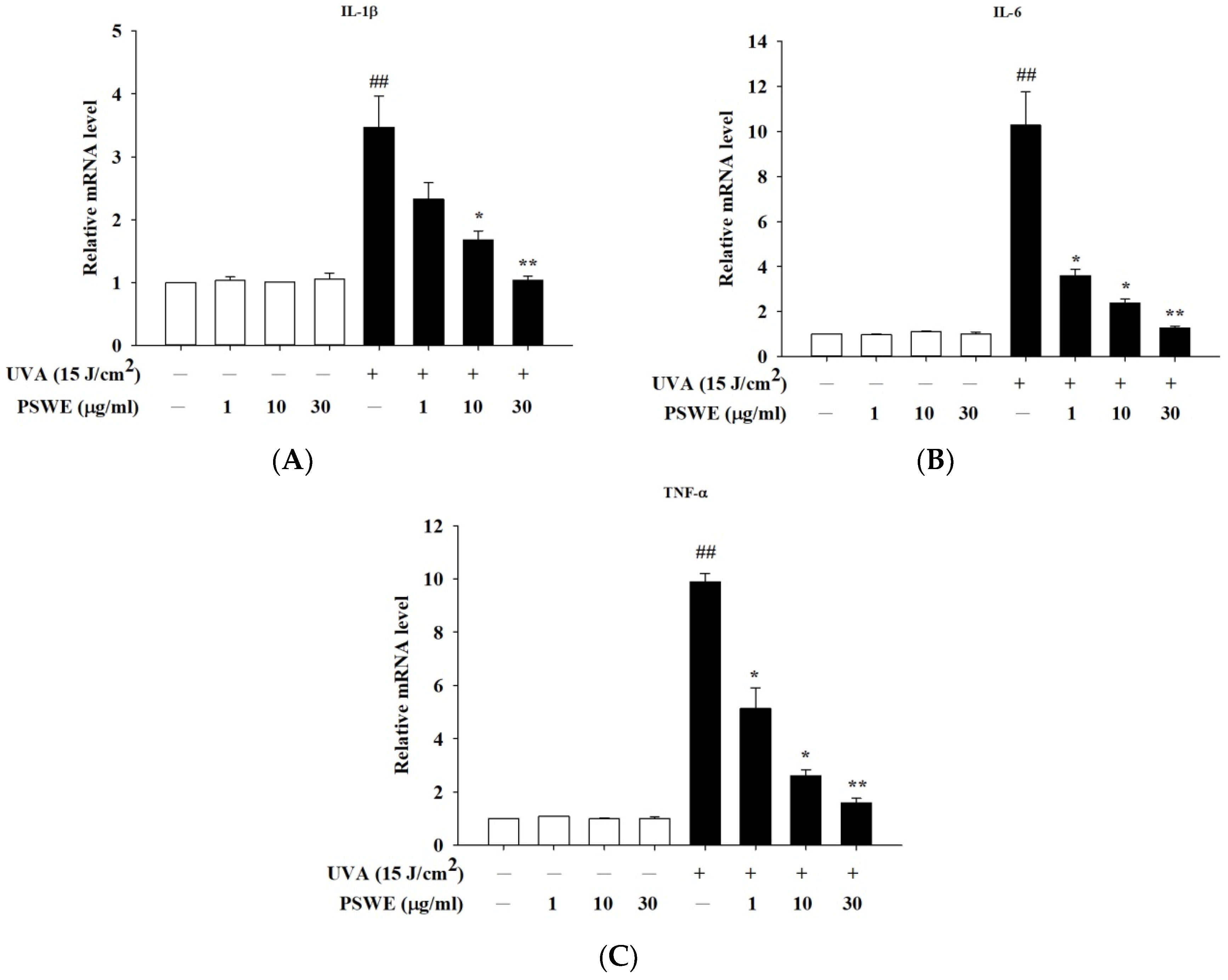
2.5. PSWE Down-Regulated the mRNA Expressions of Pro-Inflammatory Hormones IL-1β, IL-6, and TNF-α Produced by HaCaT Cells Stimulated by UVA
2.6. PSWE Inhibits the Effect of UVA-Induced ROS Production in HaCaT Cells
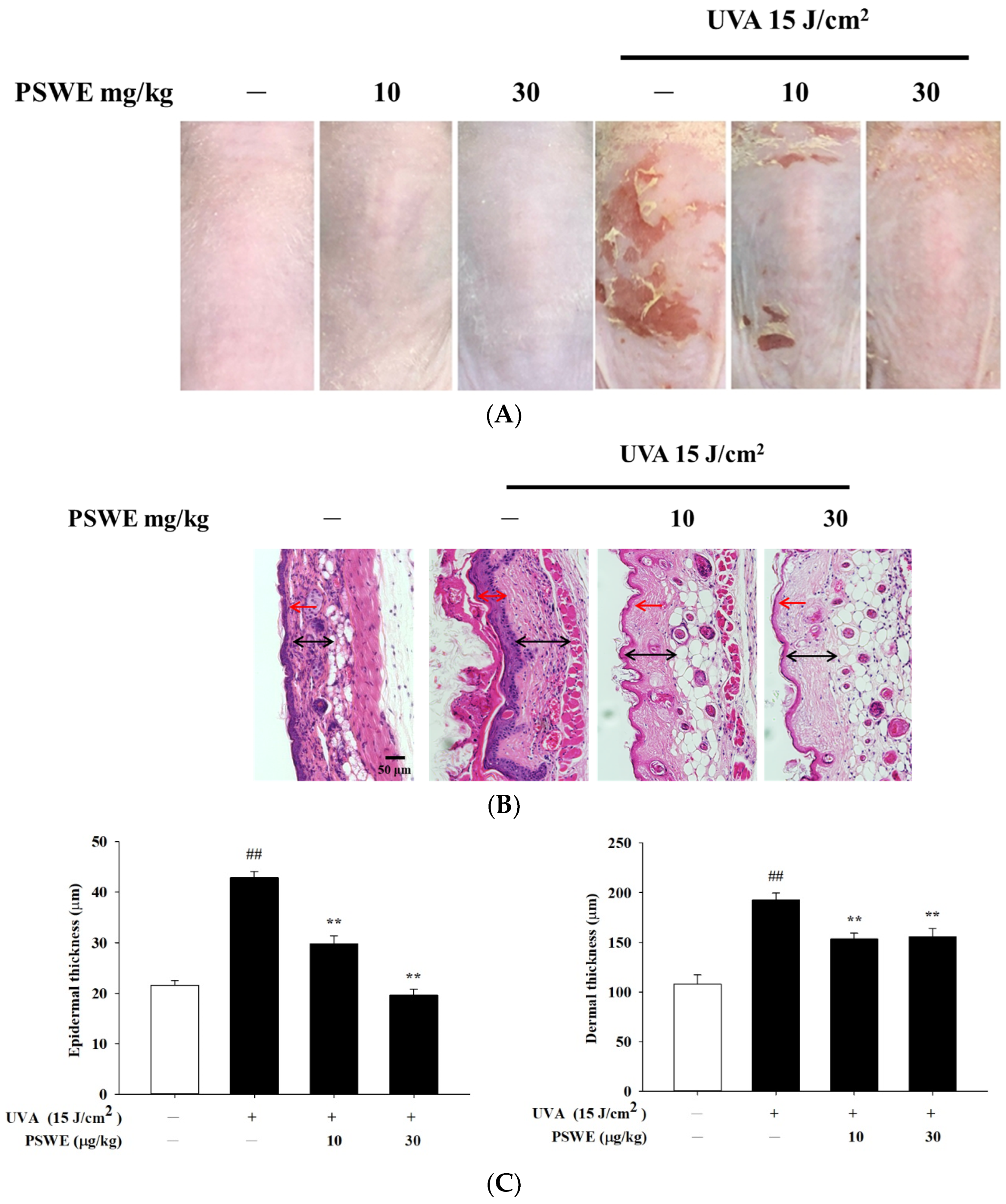
2.7. The Protective Effect of PSWE on Skin Damage Caused by UVA
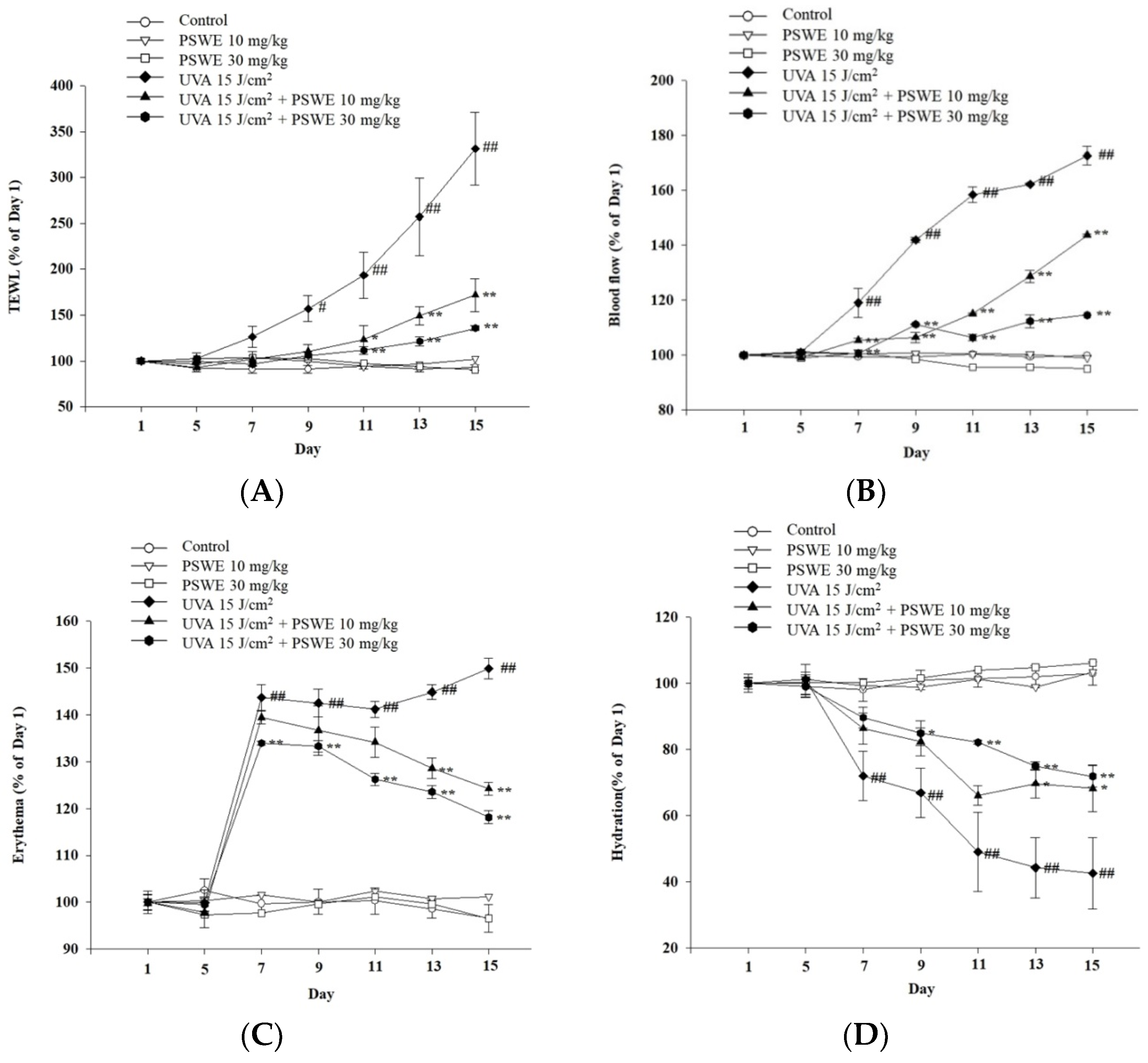
2.8. PSWE Improved UVA-Induced Skin Inflammation in Hairless Mice
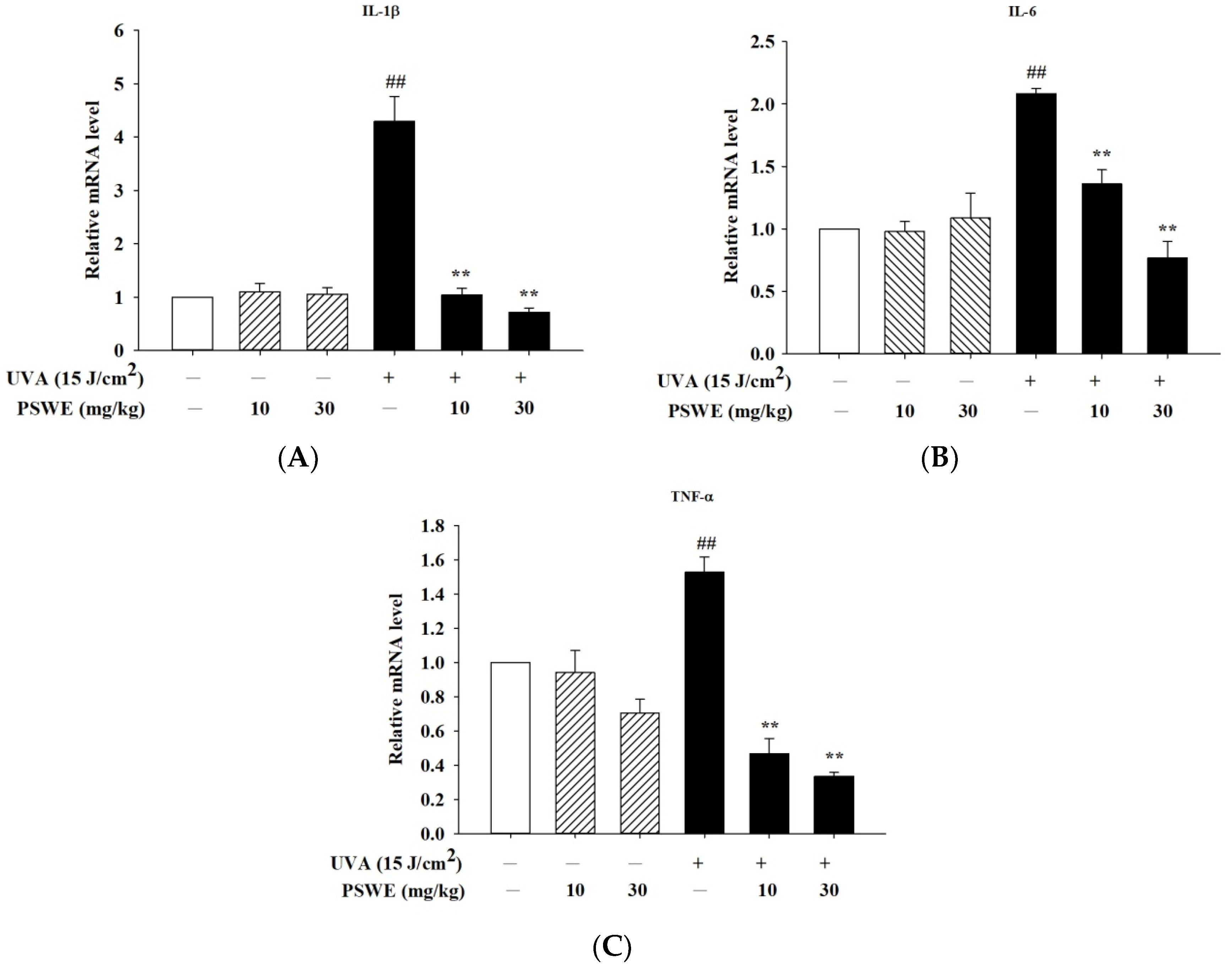
2.9. PSWE Reduces the Expression of mRNA That Promotes Inflammatory Cytokines IL-1β, IL-6, and TNF-α Induced by UVA in Hairless Mice
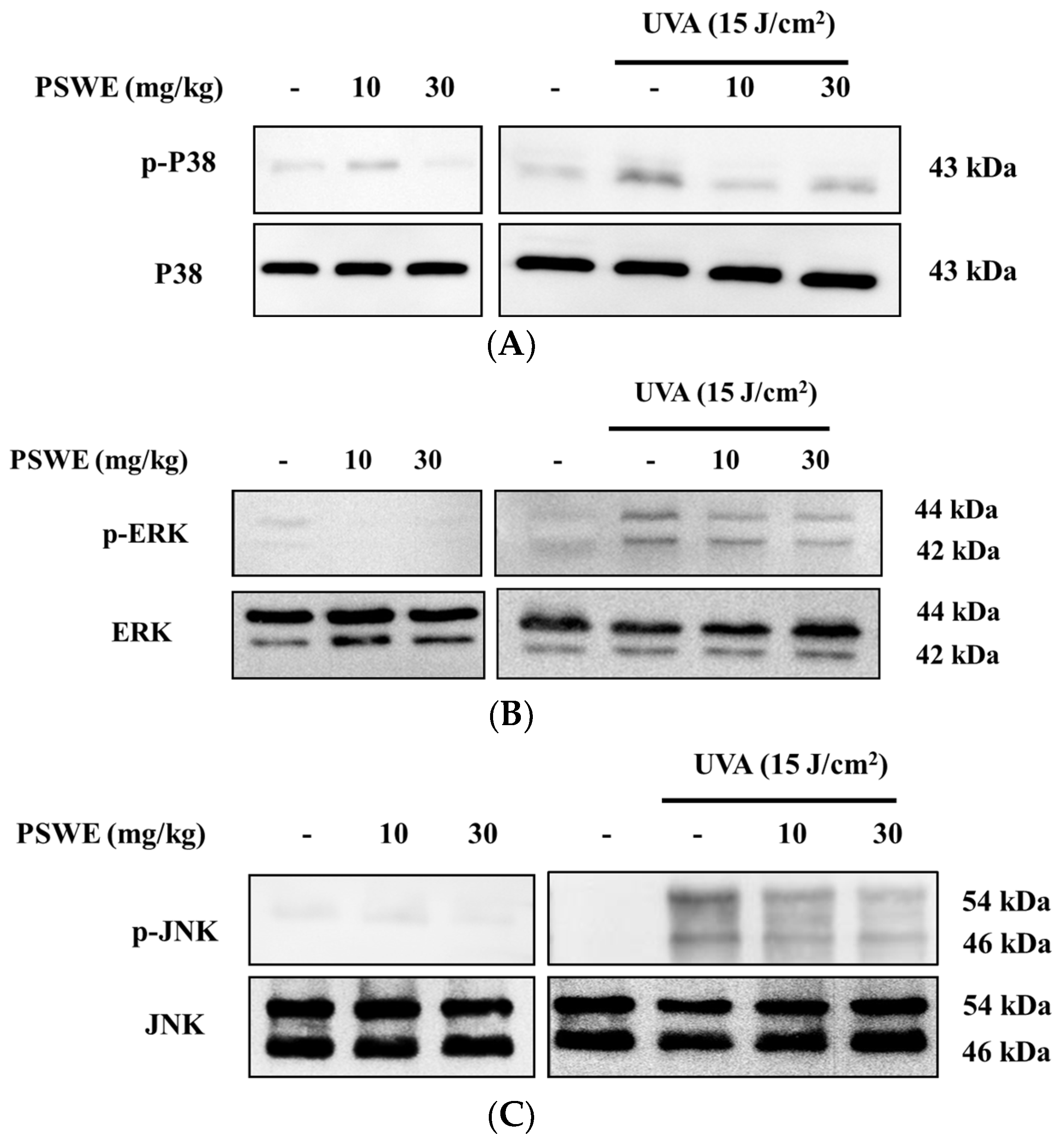
2.10. PSWE Down-Regulates Phosphorylation of the MAP Kinase Pathway in Hairless Mice Induced by Ultraviolet A
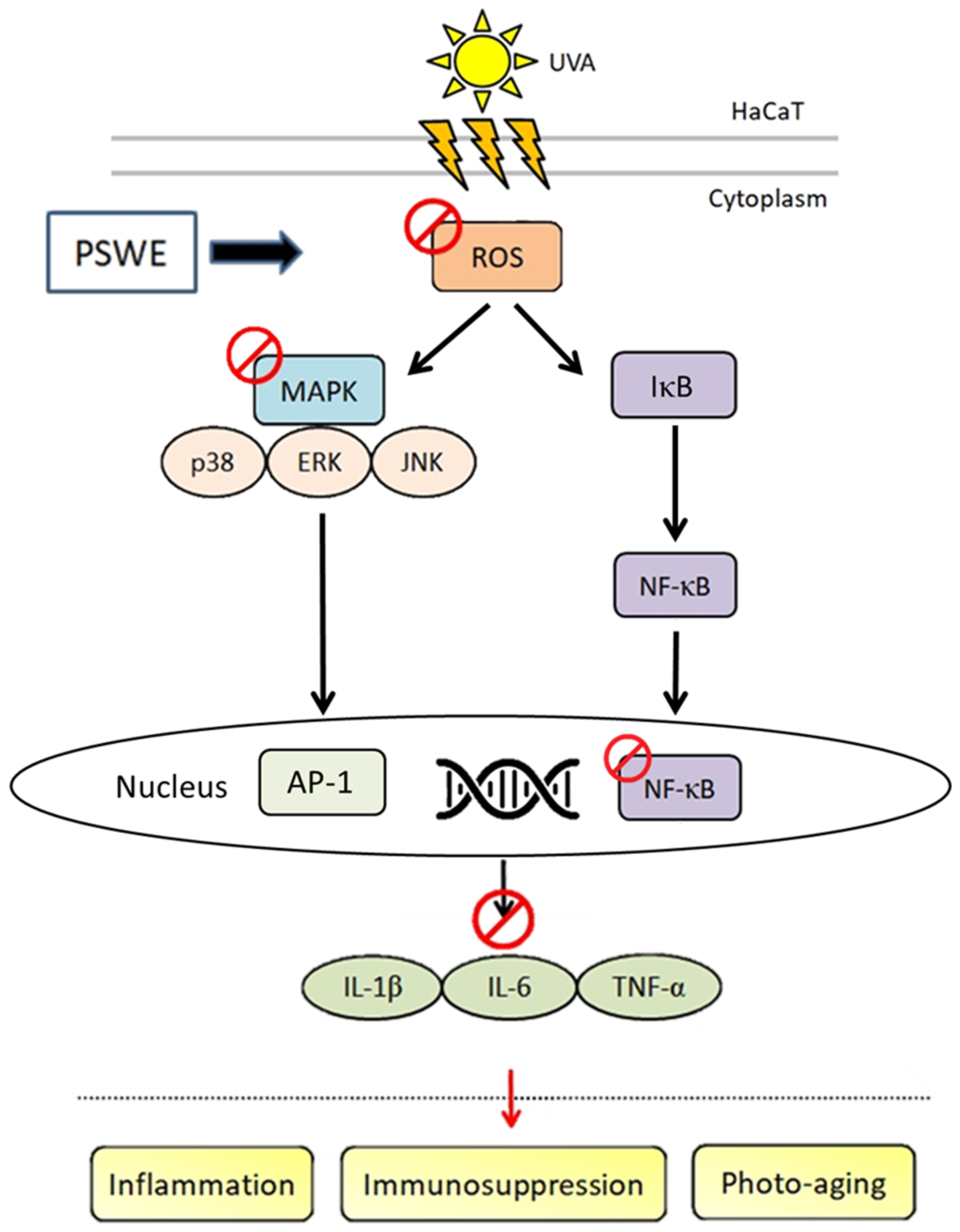
3. Discussion
4. Materials and Methods
4.1. Analysis of the Chemical Content of Pear Water Extract
4.2. Cell Line Culture
4.3. Ultraviolet A (UVA) Irradiation
4.4. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium (MTT) Assay
4.5. 2′,7′-Dichlorofluorescein Diacetate (DCFH-DA) Assay
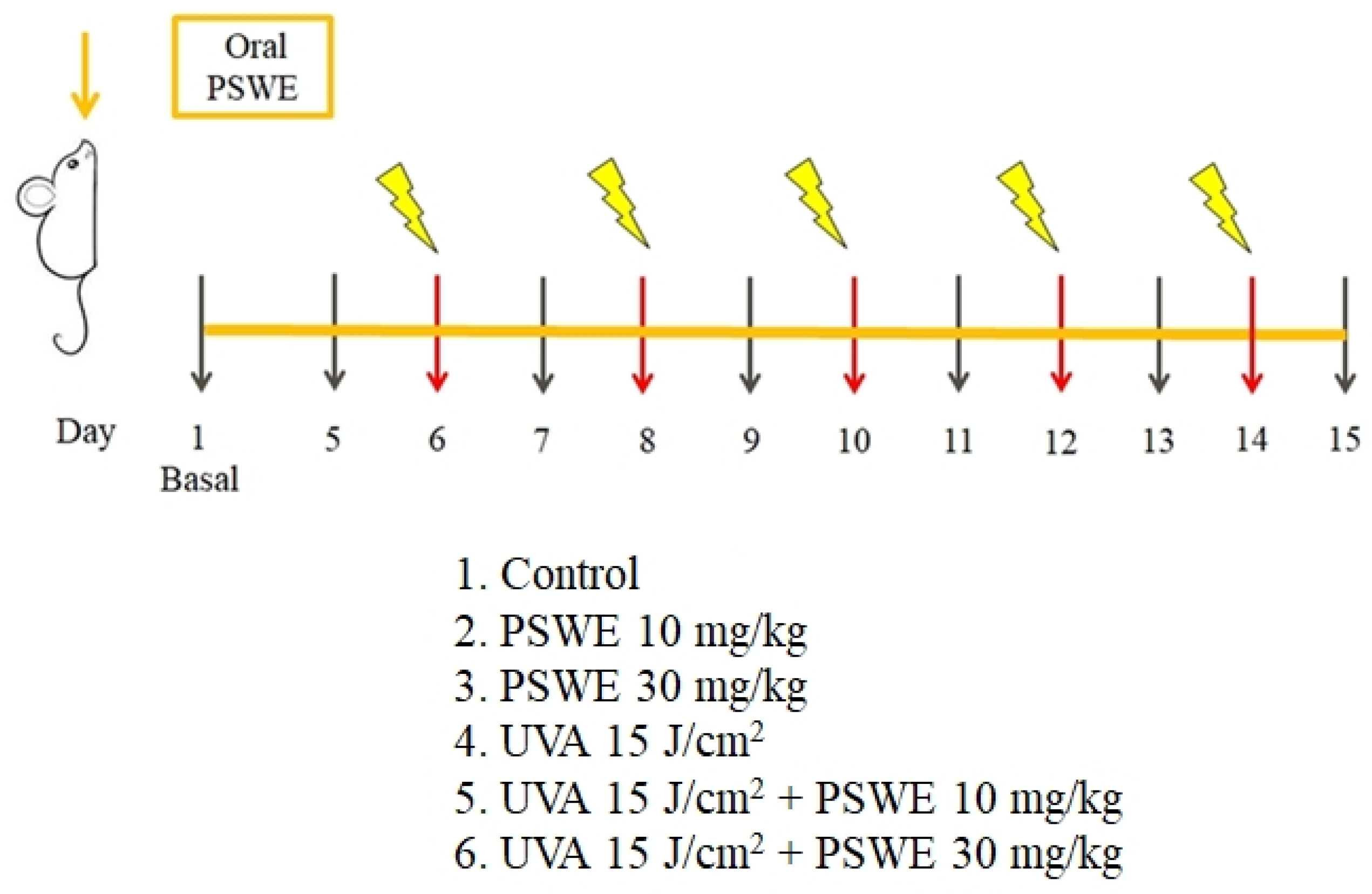
4.6. In Vivo Experiments
4.7. Histopathological Analysis
4.8. Quantitative Polymerization Chain Reaction Technology (RT-qPCR)
4.9. Western Blot Analysis Test
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guan, L.L.; Lim, H.W.; Mohammad, T.F. Sunscreens and Photoaging: A Review of Current Literature. Am. J. Clin. Dermatol. 2021, 22, 819–828. [Google Scholar] [CrossRef]
- Shetty, N.; Schalka, S.; Lim, H.W.; Mohammad, T.F. The effects of UV filters on health and the environment. Photochem. Photobiol. Sci. 2023, 22, 2463–2471. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Jiratchayamaethasakul, C.; Lee, S.H. Protocatechuic Aldehyde Attenuates UVA-Induced Photoaging in Human Dermal Fibroblast Cells by Suppressing MAPKs/AP-1 and NF-κB Signaling Pathways. Int. J. Mol. Sci. 2020, 21, 4619. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Q.; Travers, J.B.; Kemp, M.G. Roles of UVA radiation and DNA damage responses in melanoma pathogenesis. Environ. Mol. Mutagen. 2018, 59, 438–460. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef]
- Bachelor, M.A.; Bowden, G.T. UVA-mediated activation of signaling pathways involved in skin tumor promotion and progression. Semin. Cancer Biol. 2004, 14, 131–138. [Google Scholar] [CrossRef]
- Ichihashi, M.; Ueda, M.; Budiyanto, A.; Bito, T.; Oka, M.; Fukunaga, M.; Tsuru, K.; Horikawa, T. UV-induced skin damage. Toxicology 2003, 189, 21–39. [Google Scholar] [CrossRef]
- Silvers, A.L.; Bachelor, M.A.; Bowden, G.T. The role of JNK and p38 MAPK activities in UVA-induced signaling pathways leading to AP-1 activation and c-Fos expression. Neoplasia 2003, 5, 319–329. [Google Scholar] [CrossRef]
- Hong, J.; Mu, T.; Sun, H.; Blecker, C.; Richel, A. Photoprotective effects of sweet potato leaf polyphenols and caffeic acid against UV-induced skin-damage in BALB/C nude mice. Food Funct. 2022, 13, 7075–7087. [Google Scholar] [CrossRef] [PubMed]
- Rabe, J.H.; Mamelak, A.J.; McElgunn, P.J.; Morison, W.L.; Sauder, D.N. Photoaging: Mechanisms and repair. J. Am. Acad. Dermatol. 2006, 55, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Rebholz, B.; Kehe, K.; Ruzicka, T.; Rupec, R.A. Role of NF-kappaB/RelA and MAPK pathways in keratinocytes in response to sulfur mustard. J. Investig. Dermatol. 2008, 128, 1626–1632. [Google Scholar] [CrossRef][Green Version]
- You, M.; Wang, Z.; Kim, H.J.; Lee, Y.H.; Kim, H.A. Pear pomace alleviated atopic dermatitis in NC/Nga mice and inhibited LPS-induced inflammation in RAW 264.7 macrophages. Nutr. Res. Pract. 2022, 16, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Cheng, H.; Cheng, Y.; Zhao, J.; He, J.; Li, N.; Wang, J.; Guan, J. Chinese Traditional Pear Paste: Physicochemical Properties, Antioxidant Activities and Quality Evaluation. Foods 2023, 12, 187. [Google Scholar] [CrossRef]
- Hong, S.Y.; Lansky, E.; Kang, S.S.; Yang, M. A review of pears (Pyrus spp.), ancient functional food for modern times. BMC Complement. Med. Ther. 2021, 21, 219. [Google Scholar] [CrossRef]
- Cui, T.; Nakamura, K.; Ma, L.; Li, J.Z.; Kayahara, H. Analyses of arbutin and chlorogenic acid, the major phenolic constituents in Oriental pear. J. Agric. Food Chem. 2005, 53, 3882–3887. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Parveen, A.; Kang, M.C.; Subedi, L.; Lee, J.H.; Park, S.Y.; Jin, M.R.; Yoon, H.; Son, Y.K.; Kim, S.Y. Pyrus ussuriensis Maxim. leaves extract ameliorates DNCB-induced atopic dermatitis-like symptoms in NC/Nga mice. Phytomedicine 2018, 48, 76–83. [Google Scholar] [CrossRef]
- Chang, H.H.; Ko, H.H.; Lu, T.M.; Lin, J.Y.; Chang, D.C.; Chu, T.W.; Hung, C.F. Inhibition of UVA Damage on Human Skin Dermis Fibroblasts by the Isoflavonoid Intermediate Deoxybenzoin-3A. Chem. Res. Toxicol. 2021, 34, 1133–1139. [Google Scholar] [CrossRef]
- Oh, J.H.; Joo, Y.H.; Karadeniz, F.; Ko, J.; Kong, C.S. Syringaresinol Inhibits UVA-Induced MMP-1 Expression by Suppression of MAPK/AP-1 Signaling in HaCaT Keratinocytes and Human Dermal Fibroblasts. Int. J. Mol. Sci. 2020, 21, 3981. [Google Scholar] [CrossRef]
- Li, Q.; Bai, D.; Qin, L.; Shao, M.; Zhang, S.; Yan, C.; Yu, G.; Hao, J. Protective effect of d-tetramannuronic acid tetrasodium salt on UVA-induced photo-aging in HaCaT cells. Biomed. Pharmacother. 2020, 126, 110094. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Chae, M.; Son, E.D.; Bae, I.H.; Cho, E.G.; Kim, H.J.; Jung, J.Y. UVB-dependent inhibition of lipin-1 protects against proinflammatory responses in human keratinocytes. Exp. Mol. Med. 2020, 52, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Matsui, M.S.; Elmets, C.A.; Mukhtar, H. Polyphenolic antioxidant (-)-epigallocatechin-3-gallate from green tea reduces UVB-induced inflammatory responses and infiltration of leukocytes in human skin. Photochem. Photobiol. 1999, 69, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Takasawa, R.; Nakamura, H.; Mori, T.; Tanuma, S. Differential apoptotic pathways in human keratinocyte HaCaT cells exposed to UVB and UVC. Apoptosis 2005, 10, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, T.; Zhou, B.; Gao, W.; Cao, J.; Huang, L. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.). Food Chem. 2014, 152, 531–538. [Google Scholar] [CrossRef]
- Jiang, G.H.; Yim, S.H.; Eun, J.B. Physicochemical characteristics and antioxidant activities of new Asian pear cultivars. J. Appl. Biol. Chem. 2016, 59, 337–343. [Google Scholar] [CrossRef]
- Nisar, M.F.; Liu, T.; Wang, M.; Chen, S.; Chang, L.; Karisma, V.W.; Weixu; Diao, Q.; Xue, M.; Tang, X.; et al. Eriodictyol protects skin cells from UVA irradiation-induced photodamage by inhibition of the MAPK signaling pathway. J. Photochem. Photobiol. B 2022, 226, 112350. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Li, H.J.; Wu, N.L.; Hsiao, C.Y.; Lin, C.N.; Chang, H.H.; Hung, C.F. Photoprotective Effects of Cycloheterophyllin against UVA-Induced Damage and Oxidative Stress in Human Dermal Fibroblasts. PLoS ONE 2016, 11, e0161767. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Hung, Y.L.; Li, H.J.; Lin, Y.F.; Wang, S.J.; Chang, D.C.; Pu, C.M.; Hung, C.F. Quercetin inhibits histamine-induced calcium influx in human keratinocyte via histamine H4 receptors. Int. Immunopharmacol. 2021, 96, 107620. [Google Scholar] [CrossRef]
- Park, H.H.; Lee, S.; Son, H.Y.; Park, S.B.; Kim, M.S.; Choi, E.J.; Singh, T.S.; Ha, J.H.; Lee, M.G.; Kim, J.E.; et al. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch. Pharm. Res. 2008, 31, 1303–1311. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Wu, T.; Liu, R.; Loewen, S.; Tsao, R. Microwave-assisted extraction of phenolics with maximal antioxidant activities in tomatoes. Food Chem. 2012, 130, 928–936. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Majhi, S.; Saha, B.P.; Mukherjee, P.K. Chlorogenic acid-phospholipid complex improve protection against UVA induced oxidative stress. J. Photochem. Photobiol. B 2014, 130, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.H.; Jung, J.H.; Lee, J.E.; Jeon, E.J.; Kim, W.; Park, C.S. Biotechnological production of arbutins (α- and β-arbutins), skin-lightening agents, and their derivatives. Appl. Microbiol. Biotechnol. 2012, 95, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Harnly, J.M. Phenolic compounds and chromatographic profiles of pear skins (Pyrus spp.). J. Agric. Food Chem. 2008, 56, 9094–9101. [Google Scholar] [CrossRef]
- Li, Q.; Wang, D.; Bai, D.; Cai, C.; Li, J.; Yan, C.; Zhang, S.; Wu, Z.; Hao, J.; Yu, G. Photoprotective effect of Astragalus membranaceus polysaccharide on UVA-induced damage in HaCaT cells. PLoS ONE 2020, 15, e0235515. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Huang, J.; Pei, S.; Ouyang, Y.; Ding, Y.; Jiang, L.; Lu, J.; Kang, L.; Huang, L.; Xiang, H.; et al. Ganoderma lucidum polysaccharide inhibits UVB-induced melanogenesis by antagonizing cAMP/PKA and ROS/MAPK signaling pathways. J. Cell Physiol. 2019, 234, 7330–7340. [Google Scholar] [CrossRef] [PubMed]
- Fourtanier, A.; Moyal, D.; Seite, S. UVA filters in sun-protection products: Regulatory and biological aspects. Photochem. Photobiol. Sci. 2012, 11, 81–89. [Google Scholar] [CrossRef]
- He, H.; Li, A.; Li, S.; Tang, J.; Li, L.; Xiong, L. Natural components in sunscreens: Topical formulations with sun protection factor (SPF). Biomed. Pharmacother. 2021, 134, 111161. [Google Scholar] [CrossRef]
- Kawałkiewicz, W.; Matthews-Kozanecka, M.; Janus-Kubiak, M.; Kubisz, L.; Hojan-Jezierska, D. Instrumental diagnosis of facial skin—A necessity or a pretreatment recommendation in esthetic medicine. J. Cosmet. Dermatol. 2021, 20, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Rajneesh; Pathak, J.; Chatterjee, A.; Singh, S.P.; Sinha, R.P. Detection of Reactive Oxygen Species (ROS) in Cyanobacteria Using the Oxidant-sensing Probe 2′,7′-Dichlorodihydrofluorescein Diacetate (DCFH-DA). Bio-Protocol 2017, 7, e2545. [Google Scholar] [CrossRef] [PubMed]
- Green, M.R.; Sambrook, J. Amplification of cDNA Generated by Reverse Transcription of mRNA: Two-Step Reverse Transcription-Polymerase Chain Reaction (RT-PCR). Cold Spring Harb. Protoc. 2019, 2019, pdb-rot095190. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Hung, Y.L.; Ko, W.C.; Tsai, Y.J.; Chang, J.F.; Liang, C.W.; Chang, D.C.; Hung, C.F. Effect of Neferine on DNCB-Induced Atopic Dermatitis in HaCaT Cells and BALB/c Mice. Int. J. Mol. Sci. 2021, 22, 8237. [Google Scholar] [CrossRef]
- Huang, C.-C.; Lo, Y.-H.; Hsu, Y.-J.; Cheng, Y.-B.; Kung, C.-C.; Liang, C.-W.; Chang, D.-C.; Wang, K.-L.; Hung, C.-F. Anti-Atopic Dermatitis Activity of Epi-Oxyzoanthamine Isolated from Zoanthid. Mar. Drugs 2023, 21, 447. [Google Scholar] [CrossRef]

| Genes | Primers | Sequence (5′-3′) |
|---|---|---|
| Mouse GAPDH | Forward Reverse | ACCCAGAAGACTGTGGATGG CACATTGGGGGTAGGAACAC |
| Mouse IL-1® | Forward Reverse | TGGACCTTCCAGGATGAGGACA GTTCATCTCGGAGCCTGTAGTG |
| Mouse IL-6 | Forward Reverse | AGTTGCCTTCTTGGGACTGA TCCACGATTTCCCAGAGAAC |
| Mouse TNF-α | Forward Reverse | GGTGCCTATGTCTCAGCCTCTTTT GCCATAGAACTGATGAGAGGGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, T.W.; Ho, C.-C.; Hsu, Y.-J.; Lo, Y.-H.; Wu, N.-L.; Cheng, Y.-B.; Hong, M.-X.; Chang, D.-C.; Hung, C.-F. Protective Effects of Pear Extract on Skin from In Vitro and In Vivo UVA-Induced Damage. Pharmaceuticals 2024, 17, 583. https://doi.org/10.3390/ph17050583
Chu TW, Ho C-C, Hsu Y-J, Lo Y-H, Wu N-L, Cheng Y-B, Hong M-X, Chang D-C, Hung C-F. Protective Effects of Pear Extract on Skin from In Vitro and In Vivo UVA-Induced Damage. Pharmaceuticals. 2024; 17(5):583. https://doi.org/10.3390/ph17050583
Chicago/Turabian StyleChu, Thomas W., Ching-Chih Ho, Yu-Jou Hsu, Yuan-Hsin Lo, Nan-Lin Wu, Yuan-Bin Cheng, Mao-Xuan Hong, Der-Chen Chang, and Chi-Feng Hung. 2024. "Protective Effects of Pear Extract on Skin from In Vitro and In Vivo UVA-Induced Damage" Pharmaceuticals 17, no. 5: 583. https://doi.org/10.3390/ph17050583
APA StyleChu, T. W., Ho, C.-C., Hsu, Y.-J., Lo, Y.-H., Wu, N.-L., Cheng, Y.-B., Hong, M.-X., Chang, D.-C., & Hung, C.-F. (2024). Protective Effects of Pear Extract on Skin from In Vitro and In Vivo UVA-Induced Damage. Pharmaceuticals, 17(5), 583. https://doi.org/10.3390/ph17050583








