Human ABC and SLC Transporters: The Culprit Responsible for Unspecific PSMA-617 Uptake?
Abstract
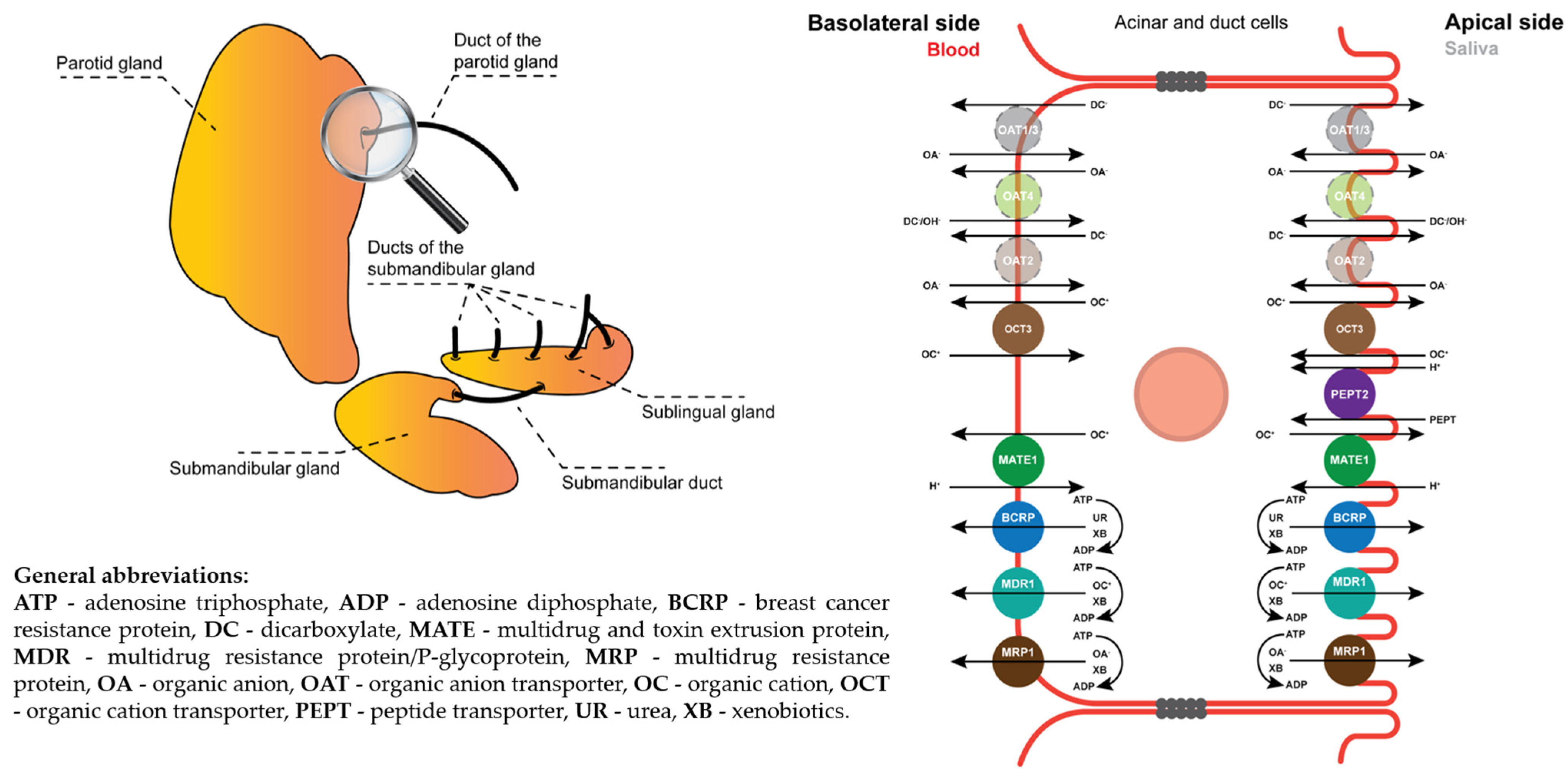
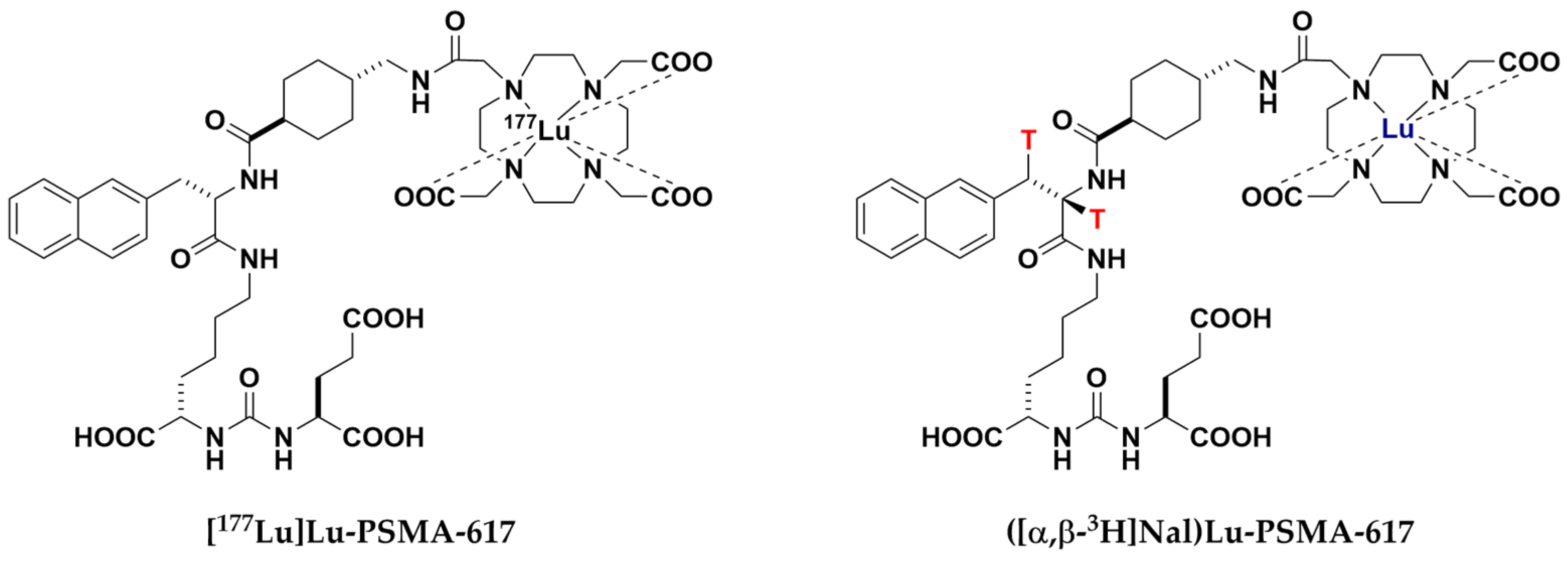
1. Introduction
2. Results
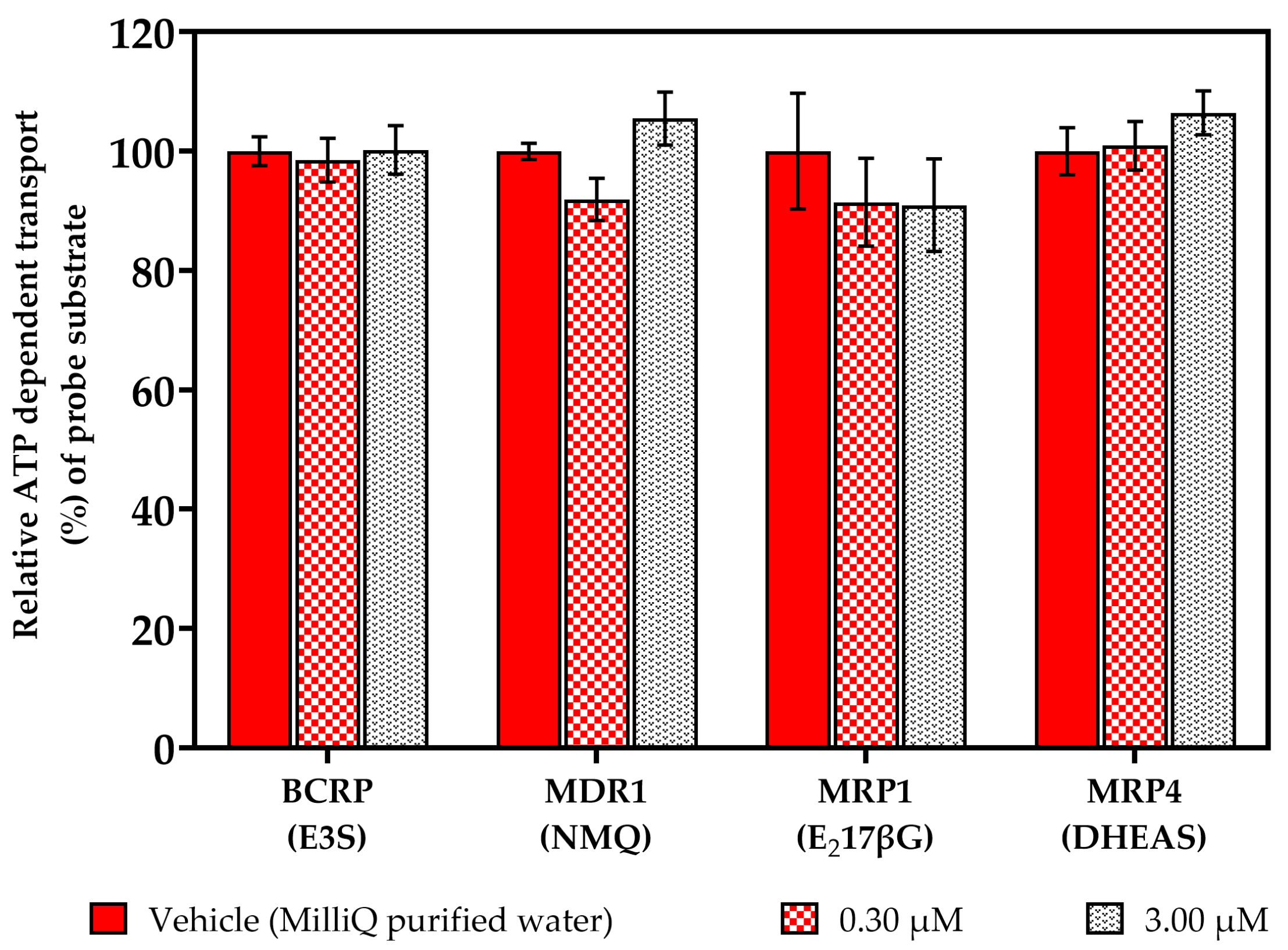
2.1. Vesicular Transport Inhibition (ABC) Assays
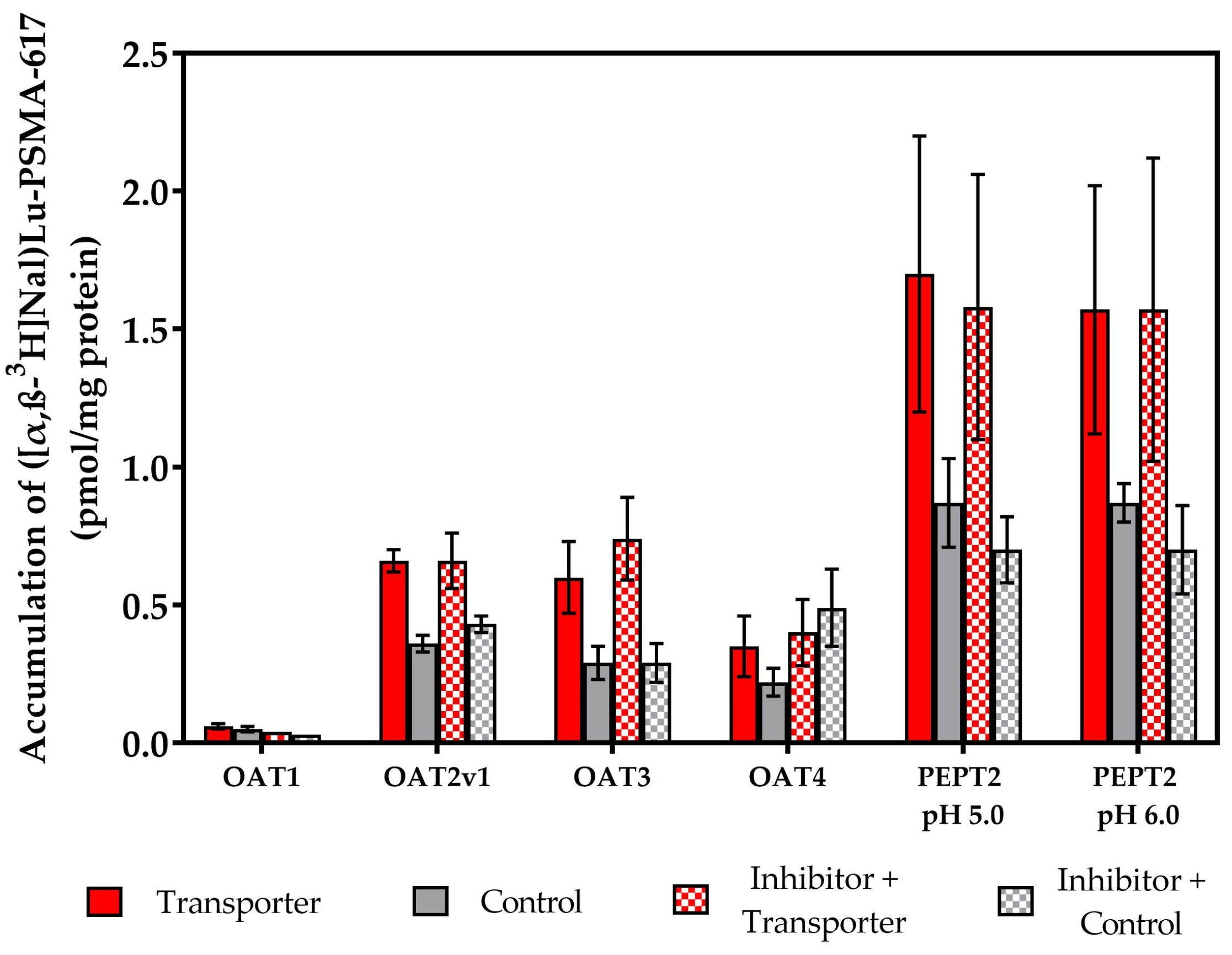
2.2. Vesicular Transporter Substrate (SLC) Assays
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents and Instruments
4.2. Cell Culture
4.3. Synthesis and Test Substance Sample Preparation
4.4. Experimental Methods for the Vesicular Transport Inhibition Assays (ABC Transporter)
4.5. Experimental Methods for the Transporter Substrate Assays (SLC Transporter)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Czerwińska, M.; Bilewicz, A.; Kruszewski, M.; Wegierek-Ciuk, A.; Lankoff, A. Targeted Radionuclide Therapy of Prostate Cancer-from Basic Research to Clinical Perspectives. Molecules 2020, 25, 1743. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef]
- Sweat, S.D.; Pacelli, A.; Murphy, G.P.; Bostwick, D.G. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology 1998, 52, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Mannweiler, S.; Amersdorfer, P.; Trajanoski, S.; Terrett, J.A.; King, D.; Mehes, G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol. Oncol. Res. 2009, 15, 167–172. [Google Scholar] [CrossRef]
- Maurer, T.; Eiber, M.; Schwaiger, M.; Gschwend, J.E. Current use of PSMA-PET in prostate cancer management. Nat. Rev. Urol. 2016, 13, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Benešová, M.; Schäfer, M.; Bauder-Wüst, U.; Afshar-Oromieh, A.; Kratochwil, C.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Preclinical Evaluation of a Tailor-Made DOTA-Conjugated PSMA Inhibitor with Optimized Linker Moiety for Imaging and Endoradiotherapy of Prostate Cancer. J. Nucl. Med. 2015, 56, 914–920. [Google Scholar] [CrossRef]
- Benešová, M.; Bauder-Wüst, U.; Schäfer, M.; Klika, K.D.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Linker Modification Strategies to Control the Prostate-Specific Membrane Antigen (PSMA)-Targeting and Pharmacokinetic Properties of DOTA-Conjugated PSMA Inhibitors. J. Med. Chem. 2016, 59, 1761–1775. [Google Scholar] [CrossRef]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schäfers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar]
- Mullard, A. FDA approves first PSMA-targeted radiopharmaceutical. Nat. Rev. Drug Discov. 2022, 21, 327. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Kuratsukuri, K.; Landas, S.; Imaida, K.; Rovito, P.M.; Wang, C.Y.; Haas, G.P. Expression of prostate-specific membrane antigen in normal and malignant human tissues. World J. Surg. 2006, 30, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Sheikhbahaei, S.; Afshar-Oromieh, A.; Eiber, M.; Solnes, L.B.; Javadi, M.S.; Ross, A.E.; Pienta, K.J.; Allaf, M.E.; Haberkorn, U.; Pomper, M.G.; et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2117–2136. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Reinhardt, S.; Ilhan, H.; Delker, A.; Böning, G.; Gildehaus, F.J.; Stief, C.; Bartenstein, P.; Gratzke, C.; Lehner, S.; et al. Preliminary experience with dosimetry, response and patient reported outcome after 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer. Oncotarget 2017, 8, 3581–3590. [Google Scholar] [CrossRef] [PubMed]
- Kalidindi, T.M.; Lee, S.-G.; Jou, K.; Chakraborty, G.; Skafida, M.; Tagawa, S.T.; Bander, N.H.; Schoder, H.; Bodei, L.; Pandit-Taskar, N.; et al. A simple strategy to reduce the salivary gland and kidney uptake of PSMA-targeting small molecule radiopharmaceuticals. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2642–2651. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. 177Lu-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Heynickx, N.; Herrmann, K.; Vermeulen, K.; Baatout, S.; Aerts, A. The salivary glands as a dose limiting organ of PSMA- targeted radionuclide therapy: A review of the lessons learnt so far. Nucl. Med. Biol. 2021, 98–99, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Rupp, N.J.; Umbricht, C.A.; Pizzuto, D.A.; Lenggenhager, D.; Töpfer, A.; Müller, J.; Muehlematter, U.J.; Ferraro, D.A.; Messerli, M.; Morand, G.B.; et al. First Clinicopathologic Evidence of a Non-PSMA-Related Uptake Mechanism for 68Ga-PSMA-11 in Salivary Glands. J. Nucl. Med. 2019, 60, 1270–1276. [Google Scholar] [CrossRef]
- Tönnesmann, R.; Meyer, P.T.; Eder, M.; Baranski, A.-C. [177Lu]Lu-PSMA-617 Salivary Gland Uptake Characterized by Quantitative in Vitro Autoradiography. Pharmaceuticals 2019, 12, 18. [Google Scholar] [CrossRef]
- Troyer, J.K.; Beckett, M.L.; Wright, G.L. Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int. J. Cancer 1995, 62, 552–558. [Google Scholar]
- Langbein, T.; Chaussé, G.; Baum, R.P. Salivary Gland Toxicity of PSMA Radioligand Therapy: Relevance and Preventive Strategies. J. Nucl. Med. 2018, 59, 1172–1173. [Google Scholar] [CrossRef] [PubMed]
- Vasiliou, V.; Vasiliou, K.; Nebert, D.W. Human ATP-binding cassette (ABC) transporter family. Hum. Genom. 2009, 3, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Zamek-Gliszczynski, M.J.; Taub, M.E.; Chothe, P.P.; Chu, X.; Giacomini, K.M.; Kim, R.B.; Ray, A.S.; Stocker, S.L.; Unadkat, J.D.; Wittwer, M.B.; et al. Transporters in Drug Development: 2018 ITC Recommendations for Transporters of Emerging Clinical Importance. Clin. Pharmacol. Ther. 2018, 104, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Catalán, M.A.; Nakamoto, T.; Melvin, J.E. The salivary gland fluid secretion mechanism. J. Med. Investig. 2009, 56, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Roussa, E. Channels and transporters in salivary glands. Cell Tissue Res. 2011, 343, 263–287. [Google Scholar] [CrossRef] [PubMed]
- Lapczuk-Romanska, J.; Busch, D.; Gieruszczak, E.; Drozdzik, A.; Piotrowska, K.; Kowalczyk, R.; Oswald, S.; Drozdzik, M. Membrane Transporters in Human Parotid Gland-Targeted Proteomics Approach. Int. J. Mol. Sci. 2019, 20, 4825. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.-F.; Sun, Q.-H.; Du, J.; Wang, S. Differential gene expression profiles of normal human parotid and submandibular glands. Oral Dis. 2008, 14, 500–509. [Google Scholar] [CrossRef]
- Nishimura, M.; Naito, S. Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab. and Pharmacokinet. 2005, 20, 452–477. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, T.; Yamaoka, M.; Matsuura, T.; Doto, R.; Hotomi, H.; Yamada, A.; Hasumi-Nakayama, Y.; Kayamoto, D. P-glycoprotein expression in human major and minor salivary glands. Arch. Oral Biol. 2001, 46, 521–527. [Google Scholar] [CrossRef]
- Uematsu, T.; Yamaoka, M.; Doto, R.; Tanaka, H.; Matsuura, T.; Furusawa, K. Expression of ATP-binding cassette transporter in human salivary ducts. Arch. Oral Biol. 2003, 48, 87–90. [Google Scholar] [CrossRef]
- Ikarashi, R.; Shibasaki, K.; Yamaguchi, A. Immunohistochemical studies of organic anion transporters and urate transporter 1 expression in human salivary gland. Acta Odontol. Scand. 2013, 71, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Duan, H.; Hebert, M.F.; Liang, C.J.; Rice, K.M.; Wang, J. Taste of a pill: Organic cation transporter-3 (OCT3) mediates metformin accumulation and secretion in salivary glands. J. Biol. Chem. 2014, 289, 27055–27064. [Google Scholar] [CrossRef] [PubMed]
- Ivanyuk, A.; Livio, F.; Biollaz, J.; Buclin, T. Renal Drug Transporters and Drug Interactions. Clin. Pharmacokinet. 2017, 56, 825–892. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Shi, B.; Zeng, T.; Zhang, Y.; Huang, B.; Ouyang, B.; Cai, Z.; Liu, M. Drug Transporters in the Kidney: Perspectives on Species Differences, Disease Status, and Molecular Docking. Front. Pharmacol. 2021, 12, 746208. [Google Scholar] [CrossRef]
- Basit, A.; Radi, Z.; Vaidya, V.S.; Karasu, M.; Prasad, B. Kidney Cortical Transporter Expression across Species Using Quantitative Proteomics. Drug Metab. Dispos. 2019, 47, 802–808. [Google Scholar] [CrossRef]
- Bauder-Wüst, U.; Schäfer, M.; Winter, R.; Remde, Y.; Roscher, M.; Breyl, H.; Poethko, T.; Tömböly, C.; Benešová-Schäfer, M. Synthesis of tritium-labeled Lu-PSMA-617: Alternative tool for biological evaluation of radiometal-based pharmaceuticals. Appl. Radiat. Isot. 2023, 197, 110819. [Google Scholar] [CrossRef]
- van Kalmthout, L.W.M.; Lam, M.G.E.H.; de Keizer, B.; Krijger, G.C.; Ververs, T.F.T.; de Roos, R.; Braat, A.J.A.T. Impact of external cooling with icepacks on 68Ga-PSMA uptake in salivary glands. EJNMMI Res. 2018, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Langbein, T.; Singh, A.; Shahinfar, M.; Schuchardt, C.; Volk, G.F.; Kulkarni, H. Injection of Botulinum Toxin for Preventing Salivary Gland Toxicity after PSMA Radioligand Therapy: An Empirical Proof of a Promising Concept. Nucl. Med. Mol. Imaging 2018, 52, 80–81. [Google Scholar] [CrossRef]
- Pillarsetty, N.; Kalidindi, T.; Carlin, S.; Easwaramoorthy, B.; Abbasi, A.; Larson, S.; Joseph, O.; Wolfgang, W. Effect of specific activity on the uptake of [68Ga]-DKFZ-PSMA11 in tumor and other organs. J. Nucl. Med. 2016, 57, 528. [Google Scholar]
- Heynickx, N.; Segers, C.; Coolkens, A.; Baatout, S.; Vermeulen, K. Characterization of Non-Specific Uptake and Retention Mechanisms of 177LuLu-PSMA-617 in the Salivary Glands. Pharmaceuticals 2023, 16, 692. [Google Scholar] [CrossRef]
- Nedelcovych, M.T.; Dash, R.P.; Wu, Y.; Choi, E.Y.; Lapidus, R.S.; Majer, P.; Abou, D.; Penet, M.-F.; Nikolopoulou, A.; Amor-Coarasa, A.; et al. JHU-2545 Selectively Shields Salivary Glands and Kidneys during PSMA-Targeted Radiotherapy; bioRxiv 2018, 457085.
- Kurth, J.; Krause, B.J.; Schwarzenböck, S.M.; Stegger, L.; Schäfers, M.; Rahbar, K. External radiation exposure, excretion, and effective half-life in 177Lu-PSMA-targeted therapies. EJNMMI Res. 2018, 8, 32. [Google Scholar] [CrossRef]
- Lu, X.; Chan, T.; Xu, C.; Zhu, L.; Zhou, Q.T.; Roberts, K.D.; Chan, H.-K.; Li, J.; Zhou, F. Human oligopeptide transporter 2 (PEPT2) mediates cellular uptake of polymyxins. J. Antimicrob. Chemother. 2016, 71, 403–412. [Google Scholar] [CrossRef]
- Uddin, M.E.; Talebi, Z.; Chen, S.; Jin, Y.; Gibson, A.A.; Noonan, A.M.; Cheng, X.; Hu, S.; Sparreboom, A. In Vitro and in Vivo Inhibition of MATE1 by Tyrosine Kinase Inhibitors. Pharmaceutics 2021, 13, 2004. [Google Scholar] [CrossRef]
- Food and Drug Administration. In Vitro Drug Interaction Studies—Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions-Guidance for Industry. Available online: https://www.fda.gov/media/134582/download (accessed on 1 November 2023).
- de Oliveira, L.M.; Diel, J.D.A.C.; Nunes, A.; da Silva Dal Pizzol, T. Prevalence of drug interactions in hospitalised elderly patients: A systematic review. Eur. J. Hosp. Pharm. 2021, 28, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.L., Jr.; Grob, B.M.; Haley, C.; Grossman, K.; Newhall, K.; Petrylak, D.; Troyer, J.; Konchuba, A.; Schellhammer, P.F.; Moriarty, R. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology 1996, 48, 326–334. [Google Scholar] [CrossRef]
- Joseph, S.; Nicolson, T.J.; Hammons, G.; Word, B.; Green-Knox, B.; Lyn-Cook, B. Expression of drug transporters in human kidney: Impact of sex, age, and ethnicity. Biol. Sex Differ. 2015, 6, 4. [Google Scholar] [CrossRef]
- Wen, J.; Zeng, M.; Shu, Y.; Guo, D.; Sun, Y.; Guo, Z.; Wang, Y.; Liu, Z.; Zhou, H.; Zhang, W. Aging increases the susceptibility of cisplatin-induced nephrotoxicity. AGE 2015, 37, 112. [Google Scholar] [CrossRef]
- Grundmann, O.; Mitchell, G.C.; Limesand, K.H. Sensitivity of salivary glands to radiation: From animal models to therapies. J. Dent. Res. 2009, 88, 894–903. [Google Scholar] [CrossRef]
- Vorlova, B.; Knedlik, T.; Tykvart, J.; Konvalinka, J. GCPII and its close homolog GCPIII: From a neuropeptidase to a cancer marker and beyond. Front. Biosci. (Landmark Ed.) 2019, 24, 648–687. [Google Scholar] [CrossRef]
- Bryant, A.K.; Nelson, T.J.; McKay, R.R.; Kader, A.K.; Parsons, J.K.; Einck, J.P.; Kane, C.J.; Sandhu, A.P.; Mundt, A.J.; Murphy, J.D.; et al. Impact of age on treatment response in men with prostate cancer treated with radiotherapy. BJUI Compass 2022, 3, 243–250. [Google Scholar] [CrossRef]
- Sathekge, M.; Lengana, T.; Maes, A.; Vorster, M.; Zeevaart, J.; Lawal, I.; Ebenhan, T.; van de Wiele, C. 68Ga-PSMA-11 PET/CT in primary staging of prostate carcinoma: Preliminary results on differences between black and white South-Africans. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Horoszewicz, J.S.; Kawinski, E.; Murphy, G.P. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. 1987, 7, 927–935. [Google Scholar] [PubMed]
- Troyer, J.K.; Beckett, M.L.; Wright, G.L. Location of prostate-specific membrane antigen in the LNCaP prostate carcinoma cell line. Prostate 1997, 30, 232–242. [Google Scholar] [CrossRef]
- Kahn, D.; Williams, R.D.; Manyak, M.J.; Haseman, M.K.; Seldin, D.W.; Libertino, J.A.; Maguire, R.T. 111Indium-capromab pendetide in the evaluation of patients with residual or recurrent prostate cancer after radical prostatectomy. The ProstaScint Study Group. J. Urol. 1998, 159, 2041–2047. [Google Scholar] [CrossRef] [PubMed]
- Petronis, J.D.; Regan, F.; Lin, K. Indium-111 capromab pendetide (ProstaScint) imaging to detect recurrent and metastatic prostate cancer. Clin. Nucl. Med. 1998, 23, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.H. PSMA specific antibodies and their diagnostic and therapeutic use. Expert Opin. Investig. Drugs 2001, 10, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Cimadamore, A.; Cheng, M.; Santoni, M.; Lopez-Beltran, A.; Battelli, N.; Massari, F.; Galosi, A.B.; Scarpelli, M.; Montironi, R. New Prostate Cancer Targets for Diagnosis, Imaging, and Therapy: Focus on Prostate-Specific Membrane Antigen. Front. Oncol. 2018, 8, 653. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Moy, P.; Kim, S.; Xia, Y.; Rajasekaran, A.; Navarro, V.; Knudsen, B.; Bander, N.H. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. 1997, 57, 3629–3634. [Google Scholar]
- Smith-Jones, P.M.; Vallabhajosula, S.; Navarro, V.; Bastidas, D.; Goldsmith, S.J.; Bander, N.H. Radiolabeled monoclonal antibodies specific to the extracellular domain of prostate-specific membrane antigen: Preclinical studies in nude mice bearing LNCaP human prostate tumor. J. Nucl. Med. 2003, 44, 610–617. [Google Scholar]
- Pandit-Taskar, N.; O’Donoghue, J.A.; Beylergil, V.; Lyashchenko, S.; Ruan, S.; Solomon, S.B.; Durack, J.C.; Carrasquillo, J.A.; Lefkowitz, R.A.; Gonen, M.; et al. ⁸⁹Zr-huJ591 immuno-PET imaging in patients with advanced metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2093–2105. [Google Scholar] [CrossRef]
- Milowsky, M.I.; Nanus, D.M.; Kostakoglu, L.; Vallabhajosula, S.; Goldsmith, S.J.; Bander, N.H. Phase I trial of yttrium-90-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for androgen-independent prostate cancer. JCO 2004, 22, 2522–2531. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J.; Pandit-Taskar, N.; Divgi, C.R.; Bender, S.; O’Donoghue, J.A.; Nacca, A.; Smith-Jones, P.; Schwartz, L.; Slovin, S.; Finn, R.; et al. Phase I evaluation of J591 as a vascular targeting agent in progressive solid tumors. Clin. Cancer Res. 2007, 13, 2707–2713. [Google Scholar] [CrossRef] [PubMed]
- Bander, N.H.; Milowsky, M.I.; Nanus, D.M.; Kostakoglu, L.; Vallabhajosula, S.; Goldsmith, S.J. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. JCO 2005, 23, 4591–4601. [Google Scholar] [CrossRef] [PubMed]
- Ballangrud, Å.M.; Yang, W.-H.; Charlton, D.E.; McDevitt, M.R.; Hamacher, K.A.; Panageas, K.S.; Ma, D.; Bander, N.H.; Scheinberg, D.A.; Sgouros, G. Response of LNCaP Spheroids after Treatment with an α-Particle Emitter (213Bi)-labeled Anti-Prostate-specific Membrane Antigen Antibody (J591)1. Cancer Res. 2001, 61, 2008–2014. [Google Scholar] [PubMed]
- Bandekar, A.; Zhu, C.; Jindal, R.; Bruchertseifer, F.; Morgenstern, A.; Sofou, S. Anti-prostate-specific membrane antigen liposomes loaded with 225Ac for potential targeted antivascular α-particle therapy of cancer. J. Nucl. Med. 2014, 55, 107–114. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Vallabhajosula, S.; Jhanwar, Y.; Ballman, K.V.; Hackett, A.; Emmerich, L.; Babich, J.; Sartor, A.O.; Harshman, L.C.; Beltran, H.; et al. Phase I dose-escalation study of 225 Ac-J591 for progressive metastatic castration resistant prostate cancer (mCRPC). JCO 2018, 36, TPS399. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Sun, M.; Sartor, A.O.; Thomas, C.; Singh, S.; Bissassar, M.; Fernandez, E.; Niaz, M.J.; Ho, B.; Vallabhajosula, S.; et al. Phase I study of 225 Ac-J591 for men with metastatic castration-resistant prostate cancer (mCRPC). JCO 2021, 39, 5015. [Google Scholar] [CrossRef]
- Oh, S.W.; Suh, M.; Cheon, G.J. Current Status of PSMA-Targeted Radioligand Therapy in the Era of Radiopharmaceutical Therapy Acquiring Marketing Authorization. Nucl. Med. Mol. Imaging 2022, 56, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Ruigrok, E.A.M.; van Vliet, N.; Dalm, S.U.; de Blois, E.; van Gent, D.C.; Haeck, J.; de Ridder, C.; Stuurman, D.; Konijnenberg, M.W.; van Weerden, W.M.; et al. Extensive preclinical evaluation of lutetium-177-labeled PSMA-specific tracers for prostate cancer radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1339–1350. [Google Scholar] [CrossRef]
- Lucaroni, L.; Georgiev, T.; Prodi, E.; Puglioli, S.; Pellegrino, C.; Favalli, N.; Prati, L.; Manz, M.G.; Cazzamalli, S.; Neri, D.; et al. Cross-reactivity to glutamate carboxypeptidase III causes undesired salivary gland and kidney uptake of PSMA-targeted small-molecule radionuclide therapeutics. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 957–961. [Google Scholar] [CrossRef]
- Lee, Z.; Heston, W.D.; Wang, X.; Basilion, J.P. GCP III is not the “off-target” for urea-based PSMA ligands. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2944–2946. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; DiFilippo, F.; Lindner, D.; Heston, W.D.W. Intriguing information from recent letter and article regarding unwanted targeting of salivary glands by PSMA ligands. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2950–2951. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taş, H.; Bakos, G.; Bauder-Wüst, U.; Schäfer, M.; Remde, Y.; Roscher, M.; Benešová-Schäfer, M. Human ABC and SLC Transporters: The Culprit Responsible for Unspecific PSMA-617 Uptake? Pharmaceuticals 2024, 17, 513. https://doi.org/10.3390/ph17040513
Taş H, Bakos G, Bauder-Wüst U, Schäfer M, Remde Y, Roscher M, Benešová-Schäfer M. Human ABC and SLC Transporters: The Culprit Responsible for Unspecific PSMA-617 Uptake? Pharmaceuticals. 2024; 17(4):513. https://doi.org/10.3390/ph17040513
Chicago/Turabian StyleTaş, Harun, Gábor Bakos, Ulrike Bauder-Wüst, Martin Schäfer, Yvonne Remde, Mareike Roscher, and Martina Benešová-Schäfer. 2024. "Human ABC and SLC Transporters: The Culprit Responsible for Unspecific PSMA-617 Uptake?" Pharmaceuticals 17, no. 4: 513. https://doi.org/10.3390/ph17040513
APA StyleTaş, H., Bakos, G., Bauder-Wüst, U., Schäfer, M., Remde, Y., Roscher, M., & Benešová-Schäfer, M. (2024). Human ABC and SLC Transporters: The Culprit Responsible for Unspecific PSMA-617 Uptake? Pharmaceuticals, 17(4), 513. https://doi.org/10.3390/ph17040513







