Comprehensive Analysis of Bioactive Compounds in Wild Ganoderma applanatum Mushroom from Kerala, South India: Insights into Dietary Nutritional, Mineral, Antimicrobial, and Antioxidant Activities
Abstract
1. Introduction
2. Results
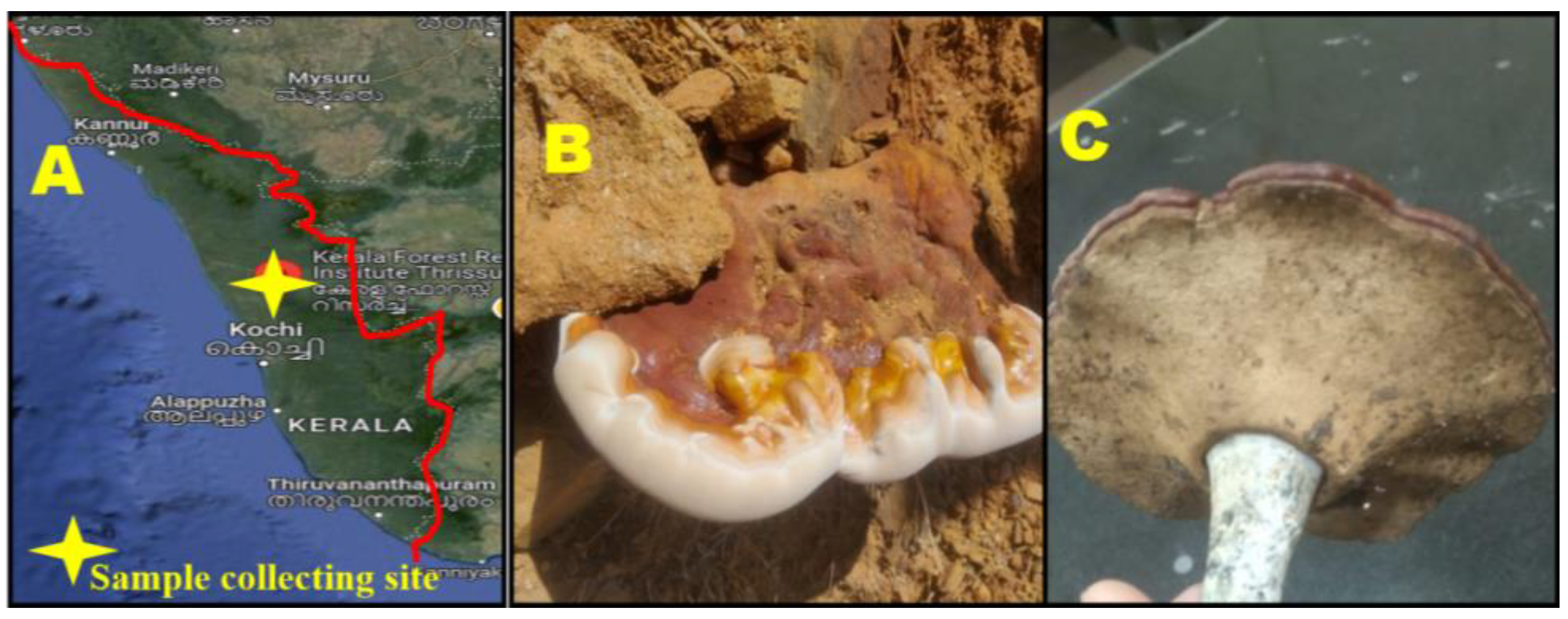
2.1. Collection of Wild Mushroom G. applanatum
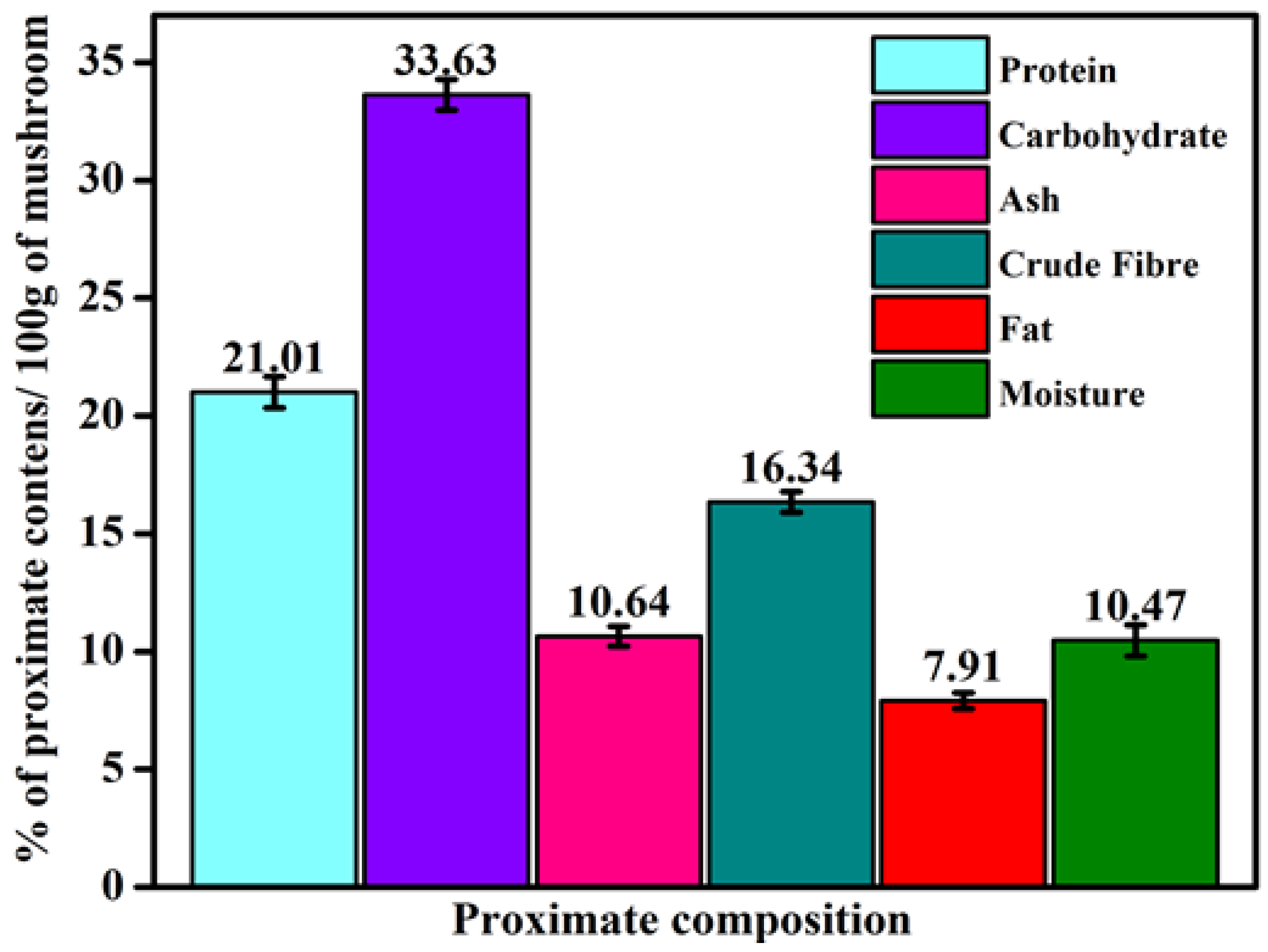
2.2. Proximate Composition of G. applanatum Mushroom
2.3. Qualitative Analysis of Phytochemicals from G. applanatum
2.4. Quantitative Analysis of Phytochemicals from G. applanatum
2.5. Minerals Constitution in G. applanatum Mushroom
2.6. Elucidation and Characterization of Compounds from G. applanatum Mushroom Extracts
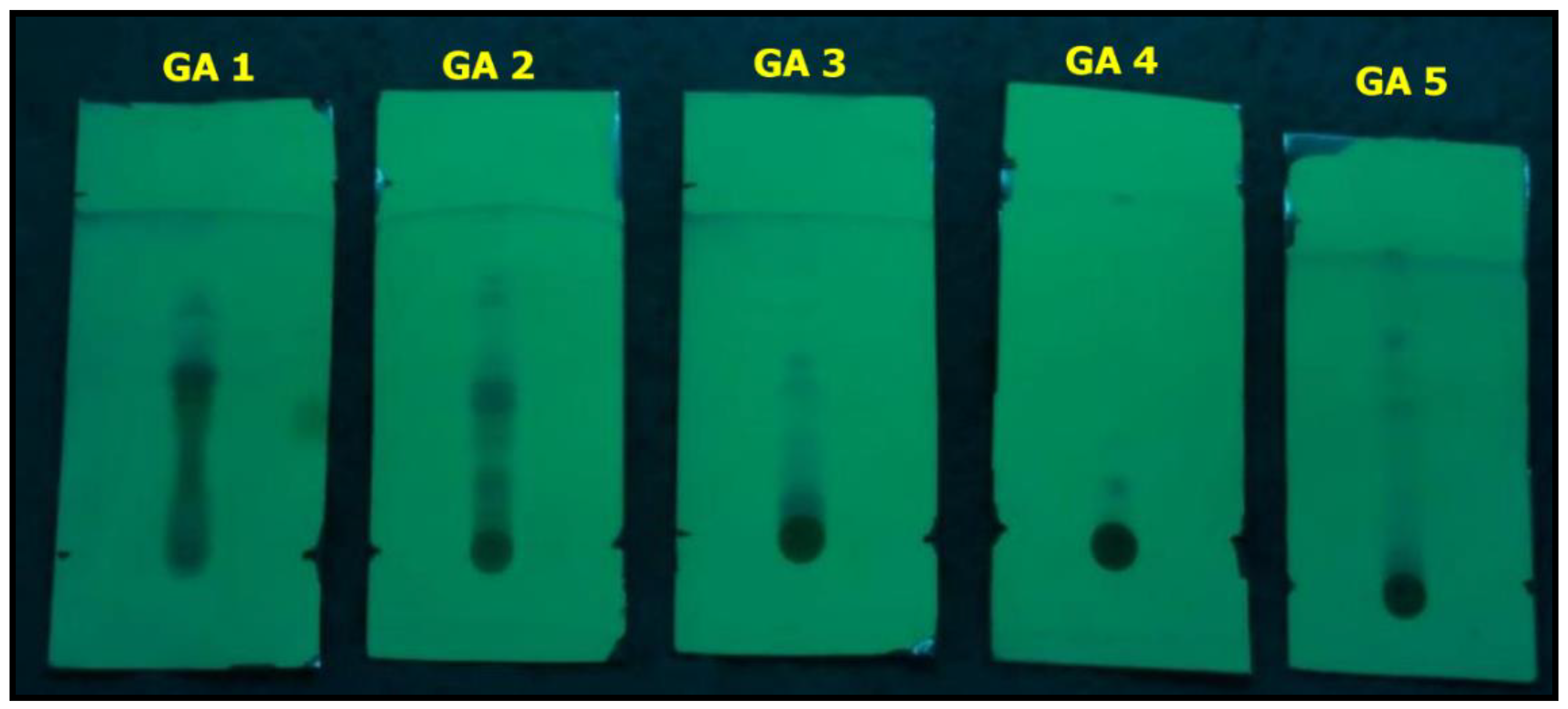
2.6.1. Thin-Layer Chromatography (TLC) Profile
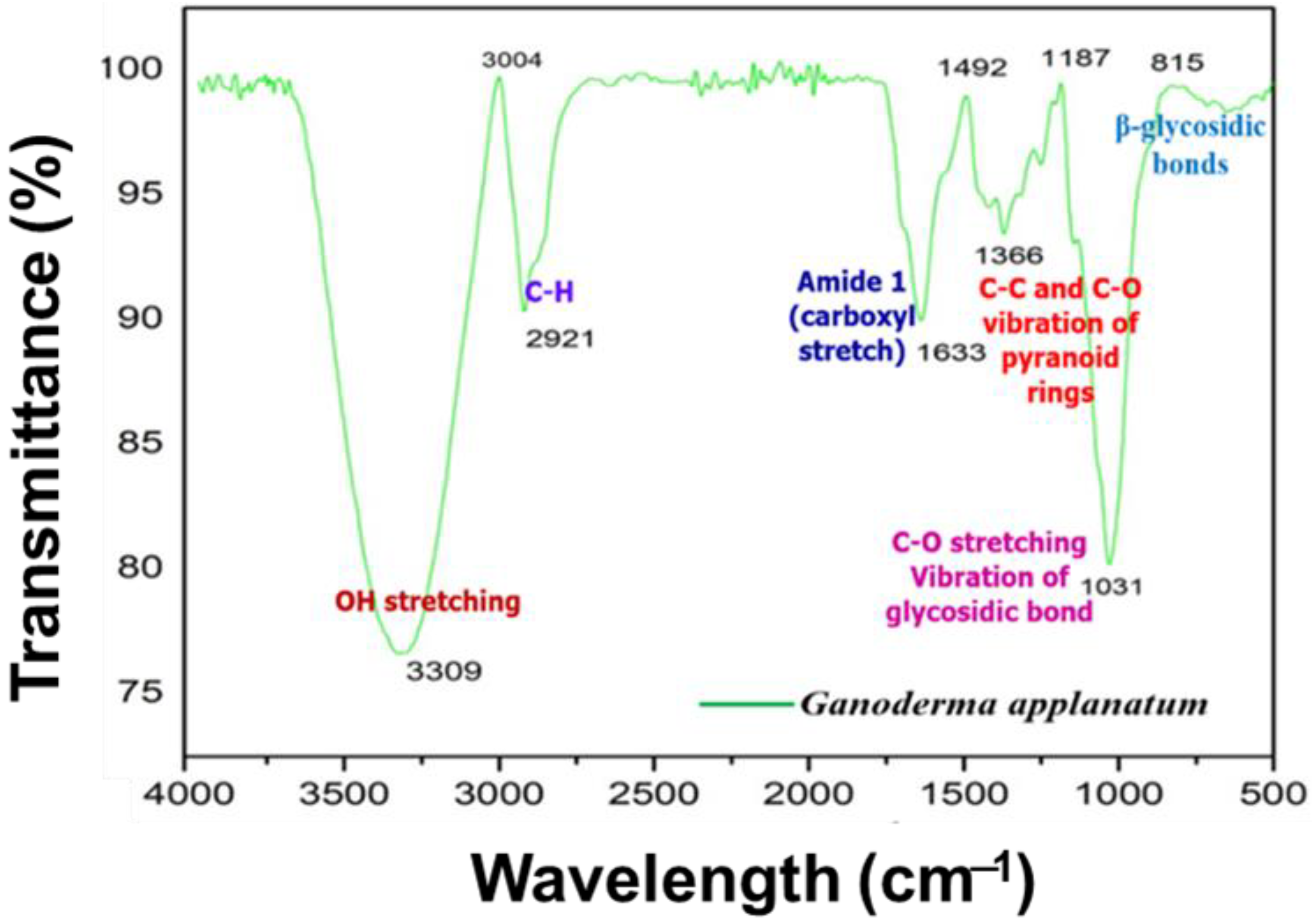
2.6.2. FTIR Analysis
2.6.3. GC-MS Chromatogram
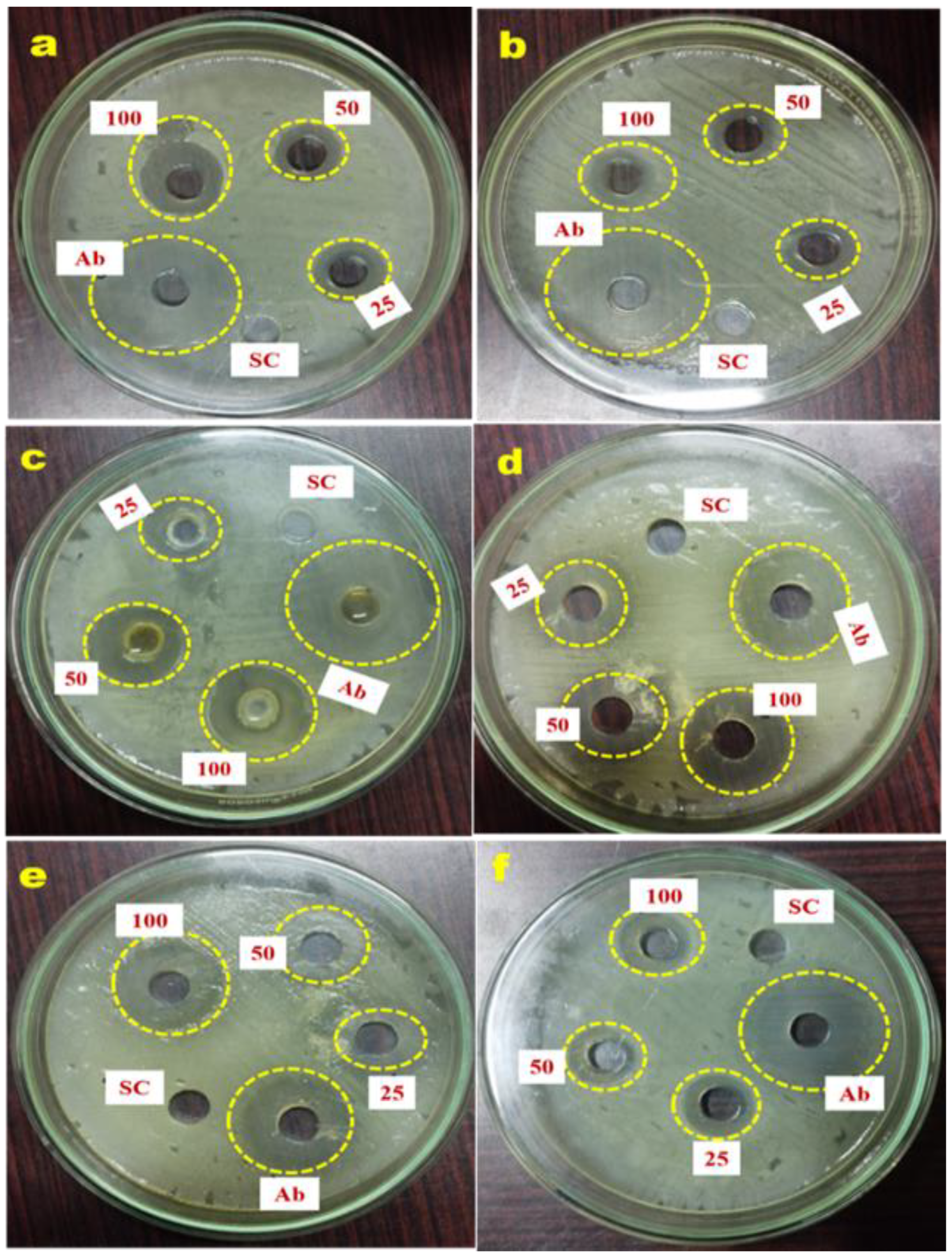
2.7. Antimicrobial Potential of G. applanatum Mushroom
2.8. Antioxidant Activity of G. applanatum Mushroom Extract
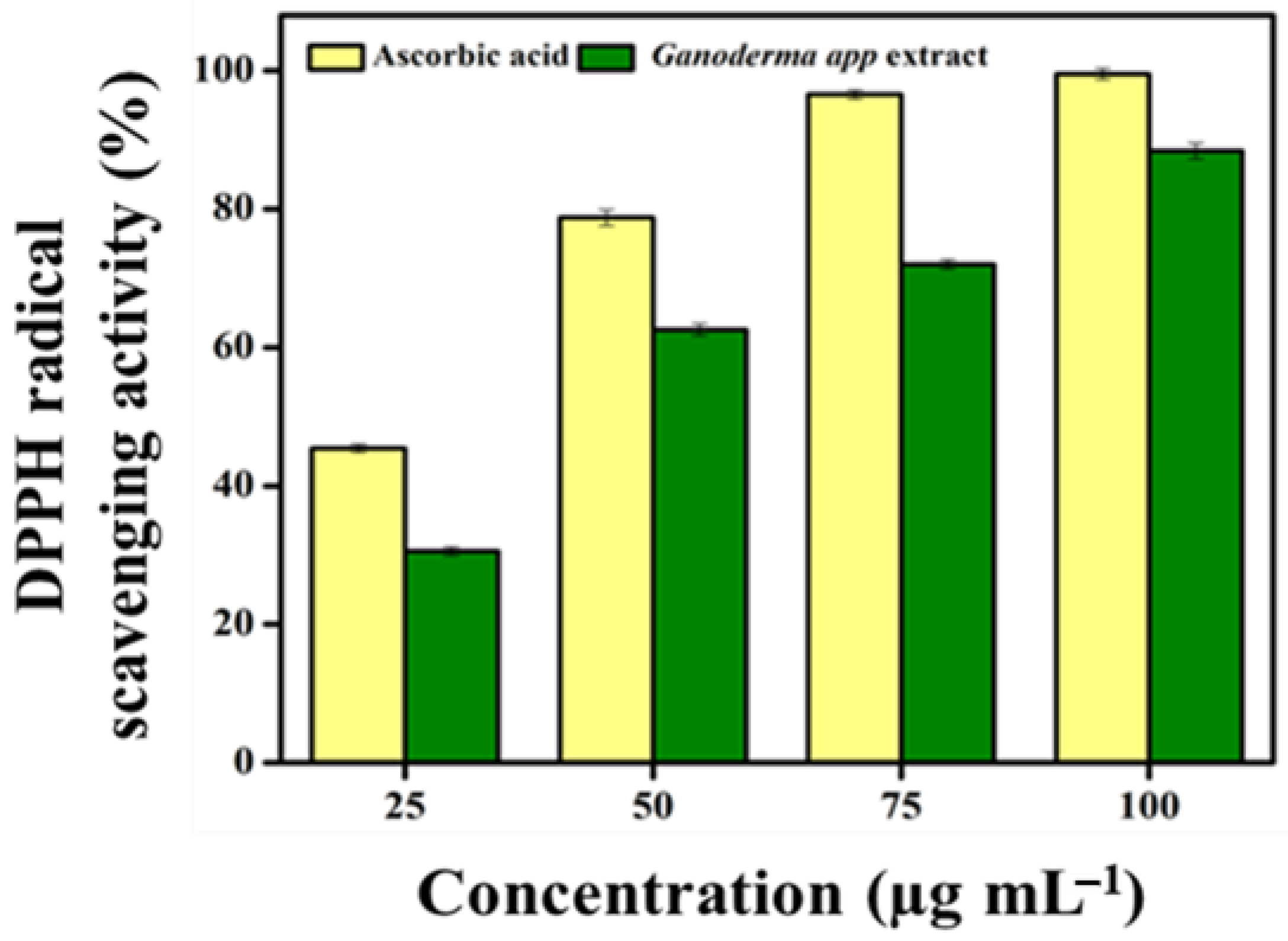
2.8.1. Radical Scavenging Activity
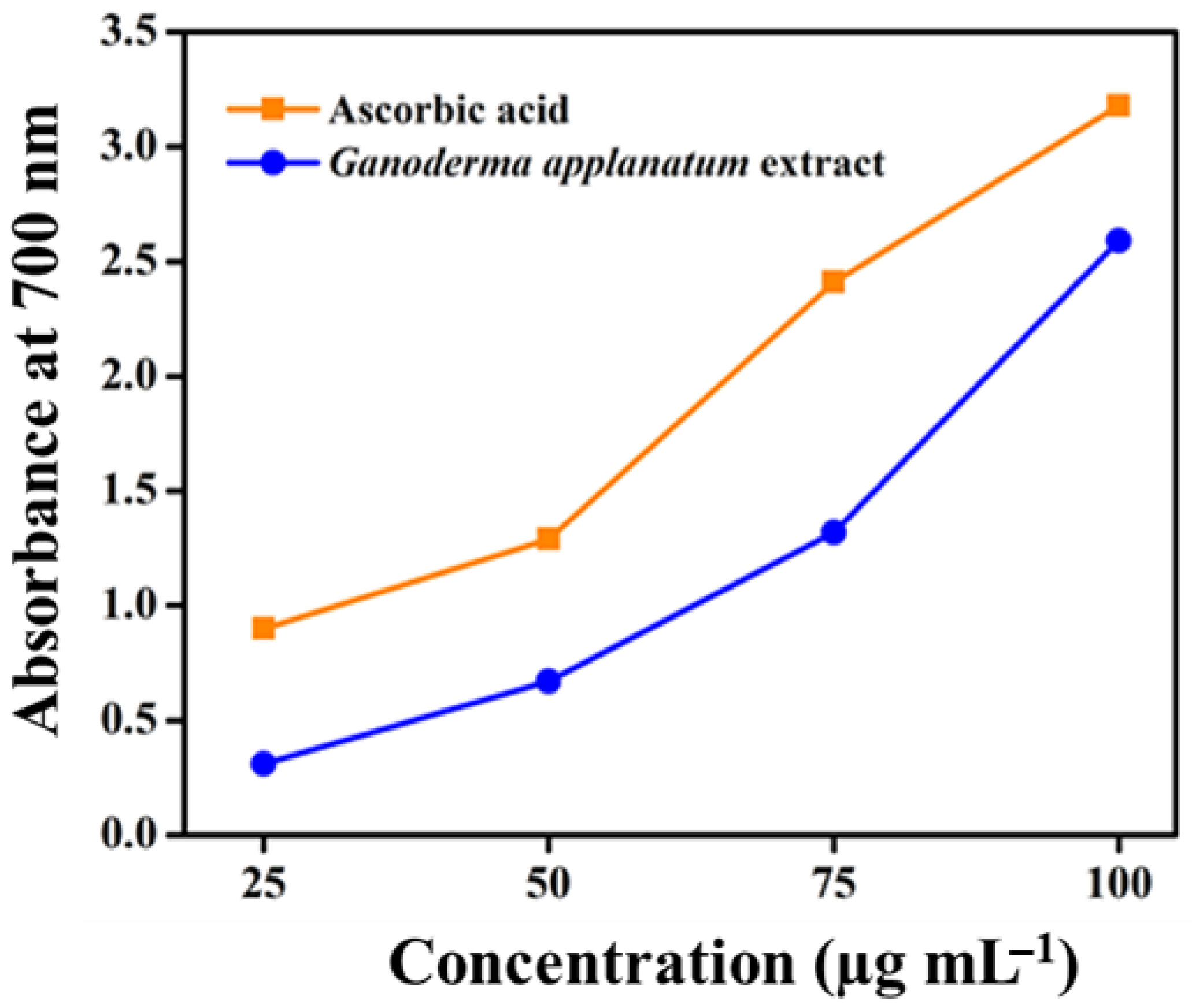
2.8.2. Reducing Power Activity
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Methanol Extraction from G. applanatum
4.3. Proximate Analysis of G. applanatum
4.3.1. Estimation of Carbohydrate
4.3.2. Estimation of Protein
4.3.3. Estimation of Moisture
4.3.4. Estimation of Fat
4.3.5. Estimation of Ash Content
4.3.6. Estimation of Crude Fiber
4.3.7. Estimation of Energy Values
4.4. Preliminary Phytochemical Analysis Using Biochemical Methods
4.4.1. Phenol Test (Lead Acetate Test)
4.4.2. Flavonoid Test (Alkaline Reagent Test)
4.4.3. Terpenoid Test (Salkowski Test)
4.4.4. Steroid Test
4.4.5. Alkaloid Test (Mayer’s Test)
4.4.6. Test for Tannins (Ferric Chloride Test)
4.4.7. Saponin Test (Frothing Test)
4.5. Quantitative Estimation of Phytochemicals from G. applanatum Mushroom Extracts
4.5.1. Total Amount of Flavonoid
4.5.2. Total Amount of Terpenoid
4.5.3. Total Amount of Phenolics
4.5.4. Total Saponin Content
4.6. Elemental Analysis
4.7. Compound Elucidation and Characterization
4.7.1. Thin-Layer Chromatography (TLC)
4.7.2. Column Chromatography
4.7.3. Fourier-Transform Infrared Spectroscopy (FTIR)
4.7.4. Gas Chromatography–Mass Spectrometry (GC-MS)
4.8. Antimicrobial Activity of Methanolic G. applanatum Mushroom Extract
4.9. Antioxidant Activity of Methanolic G. applanatum Mushroom Extract
4.9.1. Free Radical Scavenging Assay
100, where Ab517 (control) and Ab517 (sample) is the absorbance of DPPH.
4.9.2. Reducing Power Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singdevsachan, S.K.; Patra, J.K.; Tayung, K.; Thatoi, H. Chemical Constituents, Antioxidative and Antibacterial Properties of Medicinal Mushrooms Collected from Similipal Biosphere Reserve, Odisha, India. Proc. Natl. Acad. Sci. India Sect. B-Biol. Sci. 2017, 87, 559–570. [Google Scholar] [CrossRef]
- Menaga, D.; Mahalingam, P.U.; Rajakumar, S.; Ayyasamy, P.M. Evaluation of Phytochemical Characteristics and Antimicrobial Activity of Pleurotus Florida Mushroom. Asian J. Pharm. Clin. Res. 2012, 5, 102–106. [Google Scholar]
- Chandran Priyadarshni, K.; Krishnamoorthi, R.; Mumtha, C.; Ulagan Mahalingam, P. Biochemical Analysis of Cultivated Mushroom, Pleurotus Florida and Synthesis of Silver Nanoparticles for Enhanced Antimicrobial Effects on Clinically Important Human Pathogens. Inorg. Chem. Commun. 2022, 142, 109673. [Google Scholar] [CrossRef]
- Krishnamoorthi, R.; Srinivash, M.; Mahalingam, P.U.; Malaikozhundan, B. Dietary Nutrients in Edible Mushroom, Agaricus Bisporus and Their Radical Scavenging, Antibacterial, and Antifungal Effects. Process Biochem. 2022, 121, 10–17. [Google Scholar] [CrossRef]
- Antunes, F.; Marçal, S.; Taofiq, O.; Morais, A.M.M.B.; Freitas, A.C.; Ferreira, I.C.F.R.; Pintado, M. Value-Added Compounds and Potential Applications. Molecules 2020, 1, 1–40. [Google Scholar]
- Ugbogu, E.A.; Emmanuel, O.; Salem, A.Z.M.; Elghandour, M.M.M.Y. Nutritional Composition of Termitomyces Robustus (Agaricomycetes) and Lentinus Squarrosulus (Mont.) Singer in South East Nigeria. Agrofor. Syst. 2020, 94, 1291–1300. [Google Scholar] [CrossRef]
- Kalač, P. A Review of Chemical Composition and Nutritional Value of Wild-Growing and Cultivated Mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef]
- Rahman, M.A.; Abdullah, N.; Aminudin, N. Interpretation of Mushroom as a Common Therapeutic Agent for Alzheimer’s Disease and Cardiovascular Diseases. Crit. Rev. Biotechnol. 2016, 36, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef]
- Tang, C.; Hoo, P.C.X.; Tan, L.T.H.; Pusparajah, P.; Khan, T.M.; Lee, L.H.; Goh, B.H.; Chan, K.G. Golden Needle Mushroom: A Culinary Medicine with Evidenced-Based Biological Activities and Health Promoting Properties. Front. Pharmacol. 2016, 7, 474. [Google Scholar] [CrossRef]
- Erdoğan Eliuz, E.A. Antibacterial Activity and Antibacterial Mechanism of Ethanol Extracts of Lentinula Edodes (Shiitake) and Agaricus Bisporus (Button Mushroom). Int. J. Environ. Health Res. 2021, 32, 1828–1841. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.A.; Sato, S.; Konishi, T. Obesity Preventive Function of Novel Edible Mushroom, Basidiomycetes-X (Echigoshirayukidake): Manipulations of Insulin Resistance and Lipid Metabolism. J. Tradit. Complement. Med. 2020, 10, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Khinsar, K.H.; Abdul, S.; Hussain, A.; Ud Din, R.; Lei, L.; Cao, J.; Abbasi, M.; Ur Rehman, A.; Farooqui, N.; Yi, X.; et al. Anti-Tumor Effect of Polysaccharide from Pleurotus Ostreatus on H22 Mouse Hepatoma Ascites in-Vivo and Hepatocellular Carcinoma in-Vitro Model. AMB Express 2021, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z.; Liu, H.; Wang, D.; Wang, J.; Liu, M.; Yang, Y.; Zhong, S. A Natural Selenium Polysaccharide from Pleurotus Ostreatus: Structural Elucidation, Anti-Gastric Cancer and Anti-Colon Cancer Activity in Vitro. Int. J. Biol. Macromol. 2022, 201, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Morris-Natschke, S.; Yang, X.; Huang, R.; Zhou, T.; Wu, S.F.; Shi, Q.; Itokawa, H. Recent Progress of Research on Medicinal Mushrooms, Foods, and Other Herbal Products Used in Traditional Chinese Medicine. J. Tradit. Complement. Med. 2012, 2, 1–12. [Google Scholar] [CrossRef]
- Wu, Q.; Dai, T.; Song, J.; Liu, X.; Song, S.; Li, L.; Liu, J.; Pugazhendhi, A.; Jacob, J.A. Effects of Herbal and Mushroom Formulations Used in Traditional Chinese Medicine on in Vitro Human Cancer Cell Lines at the Preclinical Level: An Empirical Review of the Cell Killing Mechanisms. Process Biochem. 2020, 94, 136–142. [Google Scholar] [CrossRef]
- Rathore, H.; Prasad, S.; Sharma, S. Mushroom Nutraceuticals for Improved Nutrition and Better Human Health: A Review. PharmaNutrition 2017, 5, 35–46. [Google Scholar] [CrossRef]
- Ajith, T.A.; Janardhanan, K.K. Indian Medicinal Mushrooms as a Source of Antioxidant and Antitumor Agents. J. Clin. Biochem. Nutr. 2007, 40, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Selvi, S.; Umadevi, P.; Murugan, S.; Giftson Senapathy, J. Anticancer Potential Evoked by Pleurotus Florida and Calocybe Indica Using T 24 Urinary Bladder Cancer Cell Line. African J. Biotechnol. 2011, 10, 7279–7285. [Google Scholar] [CrossRef]
- Bulam, S.; Üstün, N.Ş.; Pekşen, A. Health Benefits of Ganoderma Lucidum as a Medicinal Mushroom. Turkish J. Agric.-Food Sci. Technol. 2019, 7, 84–93. [Google Scholar] [CrossRef]
- Peng, X.R.; Wang, Q.; Su, H.G.; Zhou, L.; Xiong, W.Y.; Qiu, M.H. Anti-Adipogenic Lanostane-Type Triterpenoids from the Edible and Medicinal Mushroom Ganoderma Applanatum. J. Fungi 2022, 8, 331. [Google Scholar] [CrossRef]
- Martínez-Montemayor, M.M.; Ling, T.; Suárez-Arroyo, I.J.; Ortiz-Soto, G.; Santiago-Negrón, C.L.; Lacourt-Ventura, M.Y.; Valentín-Acevedo, A.; Lang, W.H.; Rivas, F. Identification of Biologically Active Ganoderma Lucidum Compounds and Synthesis of Improved Derivatives That Confer Anti-Cancer Activities in Vitro. Front. Pharmacol. 2019, 10, 115. [Google Scholar] [CrossRef]
- Lau, M.F.; Chua, K.H.; Sabaratnam, V.; Kuppusamy, U.R. In Vitro and in Silico Anticancer Evaluation of a Medicinal Mushroom, Ganoderma Neo-Japonicum Imazeki, against Human Colonic Carcinoma Cells. Biotechnol. Appl. Biochem. 2021, 68, 902–917. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Alsayegh, A.A.; Ahmad, F.A.; Akhtar, M.S.; Alavudeen, S.S.; Bantun, F.; Wahab, S.; Ahmed, A.; Ali, M.; Elbendary, E.Y.; et al. Ganoderma Lucidum: Insight into Antimicrobial and Antioxidant Properties with Development of Secondary Metabolites. Heliyon 2024, 10, e25607. [Google Scholar] [CrossRef] [PubMed]
- Karadeniz, M.; Bakır, T.K.; Ünal, S. Investigation of The Antioxidant Activity And Total Phenolic Substance of Fomes Fomentarius And Ganoderma Applanatum Mushrooms Showing Therapeutic Properties. Bilge Int. J. Sci. Technol. Res. 2024, 8, 14–18. [Google Scholar] [CrossRef]
- Kiddane, A.T.; Kang, M.J.; Ho, T.C.; Getachew, A.T.; Patil, M.P.; Chun, B.S.; Kim, G. Do Anticancer and Apoptotic Activity in Cervical Adenocarcinoma HeLa Using Crude Extract of Ganoderma Applanatum. Curr. Issues Mol. Biol. 2022, 44, 1012–1026. [Google Scholar] [CrossRef]
- Prabhu, N.; Karunakaran, S.; Devi, S.K.; Ravya, R.; Subashree, P.; Senthil Vadivu, M. Biogenic Synthesis of Myconanoparticles From Mushroom Extracts and Its Medical Applications: A Review. Int. J. Pharm. Sci. Res. 2019, 10, 2108–2118. [Google Scholar] [CrossRef]
- Ao, T.; Seb, J.; Ajungla, T.; Deb, C.R. Diversity of Wild Mushrooms in Nagaland, India. Open J. For. 2016, 06, 404–419. [Google Scholar] [CrossRef][Green Version]
- Ao, T.; Deb, C.R. Nutritional and Antioxidant Potential of Some Wild Edible Mushrooms of Nagaland, India. J. Food Sci. Technol. 2019, 56, 1084–1089. [Google Scholar] [CrossRef]
- Manoharachary, C.; Sridhar, K.; Singh, R.; Adholeya, A.; Suryanarayanan, T.S.; Rawat, S.; Johri, B.N. Fungal Biodiversity: Distribution, Conservation and Prospecting of Fungi from India. Curr. Sci. 2005, 89, 58–71. [Google Scholar]
- Verma, N.; Bhalla, A.; Kumar, S.; Dhiman, R.K.; Chawla, Y.K. Wild Mushroom Poisoning in North India: Case Series with Review of Literature. J. Clin. Exp. Hepatol. 2014, 4, 361–365. [Google Scholar] [CrossRef]
- Upadhyay, M.K.; Shukla, A.; Yadav, P.; Srivastava, S. A Review of Arsenic in Crops, Vegetables, Animals and Food Products. Food Chem. 2019, 276, 608–618. [Google Scholar] [CrossRef]
- Chumbhale, D.S.; Upasani, C.D. Pharmacognostic Standardization of Stems of Thespesia Lampas (Cav.) Dalz & Gibs. Asian Pac. J. Trop. Biomed. 2012, 2, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Nakalembe, I.; Kabasa, J.D.; Olila, D. Comparative Nutrient Composition of Selected Wild Edible Mushrooms from Two Agro-Ecological Zones, Uganda. Springerplus 2015, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mleczek, M.; Budka, A.; Siwulski, M.; Mleczek, P.; Gąsecka, M.; Jasińska, A.; Kalač, P.; Sobieralski, K.; Niedzielski, P.; Proch, J.; et al. Investigation of Differentiation of Metal Contents of Agaricus Bisporus, Lentinula Edodes and Pleurotus Ostreatus Sold Commercially in Poland between 2009 and 2017. J. Food Compos. Anal. 2020, 90, 103488. [Google Scholar] [CrossRef]
- Krishnamoorthi, R.; Bharathakumar, S.; Malaikozhundan, B.; Mahalingam, P.U. Mycofabrication of Gold Nanoparticles: Optimization, Characterization, Stabilization and Evaluation of Its Antimicrobial Potential on Selected Human Pathogens. Biocatal. Agric. Biotechnol. 2021, 35, 102107. [Google Scholar] [CrossRef]
- Kombrink, A.; Tayyrov, A.; Essig, A.; Stöckli, M.; Micheller, S.; Hintze, J.; van Heuvel, Y.; Dürig, N.; Lin, C.W.; Kallio, P.T.; et al. Induction of Antibacterial Proteins and Peptides in the Coprophilous Mushroom Coprinopsis Cinerea in Response to Bacteria. ISME J. 2019, 13, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Meikle, T.G.; Dyett, B.P.; Strachan, J.B.; White, J.; Drummond, C.J.; Conn, C.E. Preparation, Characterization, and Antimicrobial Activity of Cubosome Encapsulated Metal Nanocrystals. ACS Appl. Mater. Interfaces 2020, 12, 6944–6954. [Google Scholar] [CrossRef]
- Boonsong, S.; Klaypradit, W.; Wilaipun, P. Antioxidant Activities of Extracts from Five Edible Mushrooms Using Different Extractants. Agric. Nat. Resour. 2016, 50, 89–97. [Google Scholar] [CrossRef]
- Moore, D.; Gange, A.C.; Gange, E.G.; Boddy, L. Chapter 5 Fruit Bodies: Their Production and Development in Relation to Environment. Br. Mycol. Soc. Symp. Ser. 2008, 28, 79–103. [Google Scholar] [CrossRef]
- Gbolagade, J.; Ajayi, A.; Oku, I.; Wankasi, D. Nutritive Value of Common Wild Edible Mushrooms from Southern Nigeria. Glob. J. Biotechnol. Biochem. 2006, 1, 16–21. [Google Scholar]
- Adeniyi, M.; Odeyemi, Y.; Odeyemi, O. Ecology, Diversity and Seasonal Distribution of Wild Mushrooms in a Nigerian Tropical Forest Reserve. Biodiversitas 2018, 19, 285–295. [Google Scholar] [CrossRef]
- Singh, R.; Kaur, N.; Shri, R.; Singh, A.P.; Dhingra, G.S. Proximate Composition and Element Contents of Selected Species of Ganoderma with Reference to Dietary Intakes. Environ. Monit. Assess. 2020, 192, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Shri, R.; Singh, A.P.; Dhingra, G.S. Valorization of Ganoderma Species: Chemical Characterization and Antidepressant-Like Activity. Waste and Biomass Valorization 2021, 12, 2025–2036. [Google Scholar] [CrossRef]
- Nagaraj, K.; Mallikarjun, N.; Naika, R.; Venugopal, T.M. Antioxdative Activities of Wild Macro Fungi Ganoderma Applanatum (PERS.) PAT. Asian J. Pharm. Clin. Res. 2014, 7, 166–171. [Google Scholar]
- Kolayli, S.; Sahin, H.; Aliyazicioglu, R.; Sesli, E. Phenolic Components and Antioxidant Activity of Three Edible Wild Mushrooms from Trabzon, Turkey. Chem. Nat. Compd. 2012, 48, 137–140. [Google Scholar] [CrossRef]
- Ibiam, U.A.; Uti, D.E.; Ejeogo, C.C.; Orji, O.U.; Aja, P.M.; Nwamaka, E.N.; Alum, E.U.; Chukwu, C.; Aloke, C.; Chinedum, K.E.; et al. In Vivo and in Silico Assessment of Ameliorative Effects of Xylopia Aethiopica on Testosterone Propionate-Induced Benign Prostatic Hyperplasia. Pharm. Front. 2023, 5, e64–e76. [Google Scholar] [CrossRef]
- Mallikarjuna, S.E.; Ranjini, A.; Haware, D.J.; Vijayalakshmi, M.R.; Shashirekha, M.N.; Rajarathnam, S. Mineral Composition of Four Edible Mushrooms. Hindawi 2012, 2013, 805284. [Google Scholar]
- Matute, R.G.; Figlas, D.; Curvetto, N. Agaricus Blazei Production on Non-Composted Substrates Based on Sunflower Seed Hulls and Spent Oyster Mushroom Substrate. World J. Microbiol. Biotechnol. 2011, 27, 1331–1339. [Google Scholar] [CrossRef]
- Pradhan, D.; Biswasroy, P.; Kapil; Kajol; Jatin; Pradhan, R. Qualitative and Quantitative Analysis of Caffeine in Some Commercial Brands of Tea Consumed in India. J. Ayurvedic Herb. Med. 2017, 3, 200–204. [Google Scholar] [CrossRef]
- Kristanti, H.; Tunjung, W.A.S. Detection of alkaloid, flavonoid, and terpenoid compounds in bread (Artocarpus Communis Forst.) leaves and pulps. KnE Life Sci. 2015, 2, 129. [Google Scholar] [CrossRef]
- Leela, K.; Anchana Devi, C. Isolation, Purification and Application of Secondary Metabolites From Lichen Parmelia Perlata. Biosci. Biotechnol. Res. Asia 2017, 14, 1413–1428. [Google Scholar] [CrossRef]
- Sonam, M.; Singh, R.P.; Saklani, P. Phytochemical Screening and TLC Profiling of Various Extracts of Reinwardtia Indica. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 8125. [Google Scholar] [CrossRef]
- Barman, S.; Chakraborty, U.; Chakraborty, B. GC-MS Analyses of Biochemical Constituents in Fruit Body of Agaricus Bisporus. J. Mycopathol. Res. 2021, 59, 47–61. [Google Scholar]
- Kaseleht, K.; Leitner, E.; Paalme, T. Determining Aroma-Active Compounds in Kama Flour Using SPME-GC/MS and GC-Olfactometry. Flavour Fragr. J. 2011, 26, 122–128. [Google Scholar] [CrossRef]
- Udu-Ibiam, O.E.; Ogbu, O.; Ibiam, U.A.; Nnachi, A.U.; Agah, M.V.; Ukaegbu, C.O.; Chukwu, O.S.; Agumah, N.B.; Ogbu, K.I. Phytochemical and Antioxidant Analyses of Selected Edible Mushrooms, Ginger and Garlic from Ebonyi State, Nigeria. IOSR J. Pharm. Biol. Sci. 2014, 9, 86–91. [Google Scholar] [CrossRef]
- Pal, A.; Ray, R.; Acharya, K.; Paul, S. Assessment of the Anti-Leukemic and Antioxidant Potential of the Methanol Extract of a Wild, Edible, and Novel Mushroom, Astraeus Hygrometricus, and Unraveling Its Metabolomic Profile. J. Adv. Biotechnol. Exp. Ther. 2021, 4, 388–404. [Google Scholar] [CrossRef]
- Pal, A.; Ray, R.; Chouni, A.; Hajra, S.; Paul, S. Novel Wild Edible Mushroom Astraeus Hygrometricus Induces Robust Apoptosis on Human Acute Lymphoblastic Leukemia Cells through a RONS-Subsisted Mitochondria-Dependent Pathway. J. Tradit. Chinese Med. Sci. 2023, 11, 67–77. [Google Scholar] [CrossRef]
- Arora, A. In-Vitro Antibacterial Activity of Some Ganoderma Species: A Review. Qeios 2023, 1–18. [Google Scholar] [CrossRef]
- Krishnamoorthi, R.; Ulagan Mahalingam, P.; Malaikozhundan, B. Edible Mushroom Extract Engineered Ag NPs as Safe Antimicrobial and Antioxidant Agents with No Significant Cytotoxicity on Human Dermal Fibroblast Cell. Inorg. Chem. Commun. 2022, 139, 109362. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Vunduk, J.; Zizak, Z.; Niksic, M.; Jakovljevic, D.; Vrvic, M.M.; Van Griensven, L.J.L.D. Nutraceutical Properties of the Methanolic Extract of Edible Mushroom Cantharellus Cibarius (Fries): Primary Mechanisms. Food Funct. 2015, 6, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Jeena, G.S.; Punetha, H.; Prakash, O.; Chandra, M.; Kushwaha, K.P.S. Study on in Vitro Antioxidant Potential of Some Cultivated Pleurotus Species (Oyster Mushroom). Indian J. Nat. Prod. Resour. 2014, 5, 56–61. [Google Scholar]
- Akata, I.; Zengin, G.; Picot, C.M.N.; Mahomoodally, M.F. Enzyme Inhibitory and Antioxidant Properties of Six Mushroom Species from the Agaricaceae Family. South African J. Bot. 2019, 120, 95–99. [Google Scholar] [CrossRef]
- Herawati, E.; Ramadhan, R.; Ariyani, F.; Marjenah; Kusuma, I.W.; Suwinarti, W.; Mardji, D.; Amirta, R.; Arung, E.T. Phytochemical Screening and Antioxidant Activity of Wild Mushrooms Growing in Tropical Regions. Biodiversitas 2021, 22, 4716–4721. [Google Scholar] [CrossRef]
- Campos, C.; Guzmán, R.; López-Fernández, E.; Casado, Á. Evaluation of the Copper(II) Reduction Assay Using Bathocuproinedisulfonic Acid Disodium Salt for the Total Antioxidant Capacity Assessment: The CUPRAC-BCS Assay. Anal. Biochem. 2009, 392, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A. Phenolic Compounds and Their Antioxidant Activity in Plants Growing under Heavy Metal Stress. Polish J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Suseem, S.R.; Saral, M.; Reddy, N.; Gregory, M. Evaluation of the Analgesic Activity of Ethyl Acetate, Methanol and Aqueous Extracts of Pleurotus Eous Mushroom. Asian Pac. J. Trop. Med. 2011, 4, 117–120. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Method of Analysis: Association of Analytical Chemists; AOAC: Washington, DC, USA, 2012. [Google Scholar]
- Shabbir, M.; Khan, M.R.; Saeed, N. Assessment of Phytochemicals, Antioxidant, Anti-Lipid Peroxidation and Anti-Hemolytic Activity of Extract and Various Fractions of Maytenus Royleanus Leaves. BMC Complement. Altern. Med. 2013, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of Total Flavonoid Content by Aluminum Chloride Assay: A Critical Evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Ogidi, O.I.; Oguoma, L.M.O.; Adigwe, P.C.; Anthony, B.B. Phytochemical Properties and In-Vitro Antimicrobial Potency of Wild Edible Mushrooms (Pleurotus Ostreatus) Obtained from Yenagoa, Nigeria. J. Phytopharm. 2021, 10, 180–184. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin-Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Altaf, U.; Lalotra, P.; Sharma, Y.P. Nutritional and Mineral Composition of Four Wild Edible Mushrooms from Jammu and Kashmir, India. Indian Phytopathol. 2020, 73, 313–320. [Google Scholar] [CrossRef]
- Cai, H.; Liu, X.; Chen, Z.; Liao, S.; Zou, Y. Isolation, Purification and Identification of Nine Chemical Compounds from Flammulina Velutipes Fruiting Bodies. Food Chem. 2013, 141, 2873–2879. [Google Scholar] [CrossRef] [PubMed]

| Qualitative Analysis of Phyto Compounds | |||
|---|---|---|---|
| Sample No. | Biochemical Test | Compound | G. applanatum |
| 1 | Salkowski test | Triterpenoids | + |
| 2 | FeCl3 test | Tannins | − |
| 3 | Mayer’s test | Alkaloids | + |
| 4 | Alkaline reagent | Flavonoids | + |
| 5 | Lead Acetate | Phenol | + |
| 6 | Frothing test | Saponins | + |
| 7 | Keller–Kiliani test | Glycosides | + |
| Sample No. | Biochemical Test | Amount of Phytochemicals per Dry Weight of Mushroom |
|---|---|---|
| 1 | Phenols | 22.9 mg GAE per gram |
| 2 | Flavonoid | 15.84 mg QE per gram |
| 3 | Saponin | 22.19 µg per milligram |
| 4 | Terpenoid | 0.351 mg per gram |
| Sample No. | Minerals | Mineral Contents (mg kg−1) |
|---|---|---|
| 1 | Copper (Cu) | 0.781 ± 0.88 |
| 2 | Calcium (Ca) | 283 ± 3.12 |
| 3 | Zinc (Zn) | 10.34 ± 1.27 |
| 4 | Potassium (K) | 23.9 ± 0.66 |
| 5 | Manganese (Mn) | 90.73 ± 1.22 |
| 6 | Magnesium (Mg) | 4.6 ± 0.66 |
| 7 | Iron (Fe) | 4.83 ± 0.33 |
| 8 | Sodium (Na) | 190 ± 2.76 |
| 9 | Phosphorous (P) | 117 ± 1.12 |
| Lane | Distance Traveled by the Solute (cm) | Distance Traveled by the Solvent (cm) | Band Colour Observed under UV Light | Rf Value | Expected Compound |
|---|---|---|---|---|---|
| GA1 | 2.3 | 4.3 | Green | 0.534 | Alkaloid |
| GA2 | 3.4 | 4.2 | Blue | 0.809 | Flavonoid |
| GA3 | 2.3 | 4.1 | Blue | 0.560 | Terpenoid |
| GA4 | 0.7 | 4.5 | Blue | 0.155 | Glycoside |
| GA5 | 3.1 | 4.3 | Blue | 0.720 | Phenolic |
| Peak | Retention Time (RT) | Peak Area (%) | Name | Molecular Weight (g mol−1) | Molecular Formula |
|---|---|---|---|---|---|
| 1 | 13.91 | 5.53 | 2,3,4,4-tretrapropyl-1-(trimethylsilyl)-1-(trimethylsilyloxy)-1,3-diaza-2,4-diborabutane | UN | UN |
| 2 | 17.527 | 2.5 | 2-cyclobuten-1-one, 4-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-2,3-dimethoxy-4-(3-phenyl-1-propynyl)- | UN | UN |
| 3 | 18.34 | 5.84 | 2-tert-butyl-4-(1,1,3,3-tetramethylbutyl)phenol | 262.4 | C18H30O |
| 4 | 19.942 | 29.53 | 1,2-benzenedicarboxylic acid, diethyl ester | 222.24 | C12H14O4 |
| 5 | 20.766 | 1.57 | 1-(3,4-ditrimethylsiloxyphenyl)-2-isopropylaminoethanol | UN | UN |
| 6 | 22.083 | 1.04 | Isopropenyl dodecanoate | 334 | C22H38O2 |
| 7 | 24.904 | 3.04 | 1,2-benzenedicarboxylic acid, dicyclohexyl ester | 330.4 | C20H26O4 |
| 8 | 26.493 | 3.26 | Furo[2,3-c]pyridine, 2,3-dihydro-2,7-dimethyl- | 149.19 | C9H11NO |
| 9 | 26.59 | 0.97 | 2H-3,11c-(epoxymethano)phenanthro[10,1-bc]pyran, picras-3-en-21-oic acid derivative | 548.6 | C28H36O11 |
| 10 | 37.46 | 23.99 | 1,4-benzenedicarboxylic acid, bis(2-ethylhexyl)ester | 390.6 | C24H38O4 |
| 11 | 37.783 | 0.94 | 3-pyridinemethanol, 4-[(5H-dibenzo[a,d]cyclohepten-5-ylimino)methyl]-5-hydroxy-6-methyl-,(e)- | UN | UN |
| 12 | 38.145 | 0.86 | Silane, trimethyl[2-methylene-4,4-bis(phenylsulfonyl)butyl] | UN | UN |
| 13 | 38.273 | 3.95 | 2-propanone, 1,1,1-tris(ethylthio)-3-(4-methoxyphenyl)-3-[(trimethylsilyl)oxy]- | UN | UN |
| 14 | 38.325 | 5.13 | 3-bromo-4-(difluoromethyl)pyridine | 208 | C6H4BrF2N |
| 15 | 38.48 | 2.2 | 7-oxabicyclo [2.2.1]hept-2-ene, 5,6-bis(chloromethyl)-2,3-dimethyl-, (exo,exo)- | UN | UN |
| 16 | 38.615 | 4.99 | 12-azabicyclo(9.2.1)tetradeca-1(14)-ene-13-one | UN | UN |
| 17 | 38.648 | 1.46 | 12-azabicyclo(9.2.1)tetradeca-1(14)-ene-13-one | UN | UN |
| 18 | 38.86 | 1.11 | 12-azabicyclo(9.2.1)tetradeca-1(14)-ene-13-one | UN | UN |
| 19 | 38.9 | 1.63 | Cyclotrisiloxane, hexamethyl- | 222.46 | C6H18O3Si3 |
| 20 | 40.116 | 0.48 | 1H-Furo[3,4-c]pyrrole-4-carboxylic acid, 6-(2-furanyl)hexahydro-1,3-dioxo-4-phenyl-, methyl ester, (3a.alpha.,4.beta.,6.beta.,) | UN | UN |
| Pathogens | Zone of Inhibition (mm) (Well Size 6 mm) | ||||
|---|---|---|---|---|---|
| Mushroom Extract (µg mL−1) | Antibiotic Control | Solvent Control | |||
| 25 | 50 | 100 | (30 µg mL−1) | 50 µL | |
| Gram-positive bacterial pathogens | |||||
| S. pyogenes | 10.22 ± 0.33 | 11.33 ± 0.88 | 17.32 ± 0.33 | 26.12 ± 0.33 | NA |
| E. faecalis | 9.98 ± 0.88 | 10.64 ± 0.66 | 11.21 ± 0.88 | 25.9 ± 0.12 | NA |
| Gram-negative bacterial pathogens | |||||
| K. pneumoniae | 9.78 ± 0.88 | 13.58 ± 0.33 | 18.29 ± 1.12 | 26.4 ± 0.33 | NA |
| S. flexneri | 11.56 ± 0.33 | 14.27 ± 0.66 | 19.98 ± 0.88 | 21.28 ± 0.33 | NA |
| Fungal pathogens | |||||
| C. albicans | 9.1 ± 0.33 | 12.75 ± 0.88 | 16.54 ± 1.12 | 18.21 ± 0.66 | NA |
| A. fumigatus | 8.97 ± 0.33 | 9.58 ± 0.66 | 11.27 ± 0.33 | 23.9 ± 0.88 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rijia, A.; Krishnamoorthi, R.; Rasmi, M.; Mahalingam, P.U.; Kim, K.-s. Comprehensive Analysis of Bioactive Compounds in Wild Ganoderma applanatum Mushroom from Kerala, South India: Insights into Dietary Nutritional, Mineral, Antimicrobial, and Antioxidant Activities. Pharmaceuticals 2024, 17, 509. https://doi.org/10.3390/ph17040509
Rijia A, Krishnamoorthi R, Rasmi M, Mahalingam PU, Kim K-s. Comprehensive Analysis of Bioactive Compounds in Wild Ganoderma applanatum Mushroom from Kerala, South India: Insights into Dietary Nutritional, Mineral, Antimicrobial, and Antioxidant Activities. Pharmaceuticals. 2024; 17(4):509. https://doi.org/10.3390/ph17040509
Chicago/Turabian StyleRijia, Akbar, Raman Krishnamoorthi, Madhusoodhanan Rasmi, Pambayan Ulagan Mahalingam, and Kwang-sun Kim. 2024. "Comprehensive Analysis of Bioactive Compounds in Wild Ganoderma applanatum Mushroom from Kerala, South India: Insights into Dietary Nutritional, Mineral, Antimicrobial, and Antioxidant Activities" Pharmaceuticals 17, no. 4: 509. https://doi.org/10.3390/ph17040509
APA StyleRijia, A., Krishnamoorthi, R., Rasmi, M., Mahalingam, P. U., & Kim, K.-s. (2024). Comprehensive Analysis of Bioactive Compounds in Wild Ganoderma applanatum Mushroom from Kerala, South India: Insights into Dietary Nutritional, Mineral, Antimicrobial, and Antioxidant Activities. Pharmaceuticals, 17(4), 509. https://doi.org/10.3390/ph17040509