Titanium-45 (45Ti) Radiochemistry and Applications in Molecular Imaging
Abstract
1. Introduction
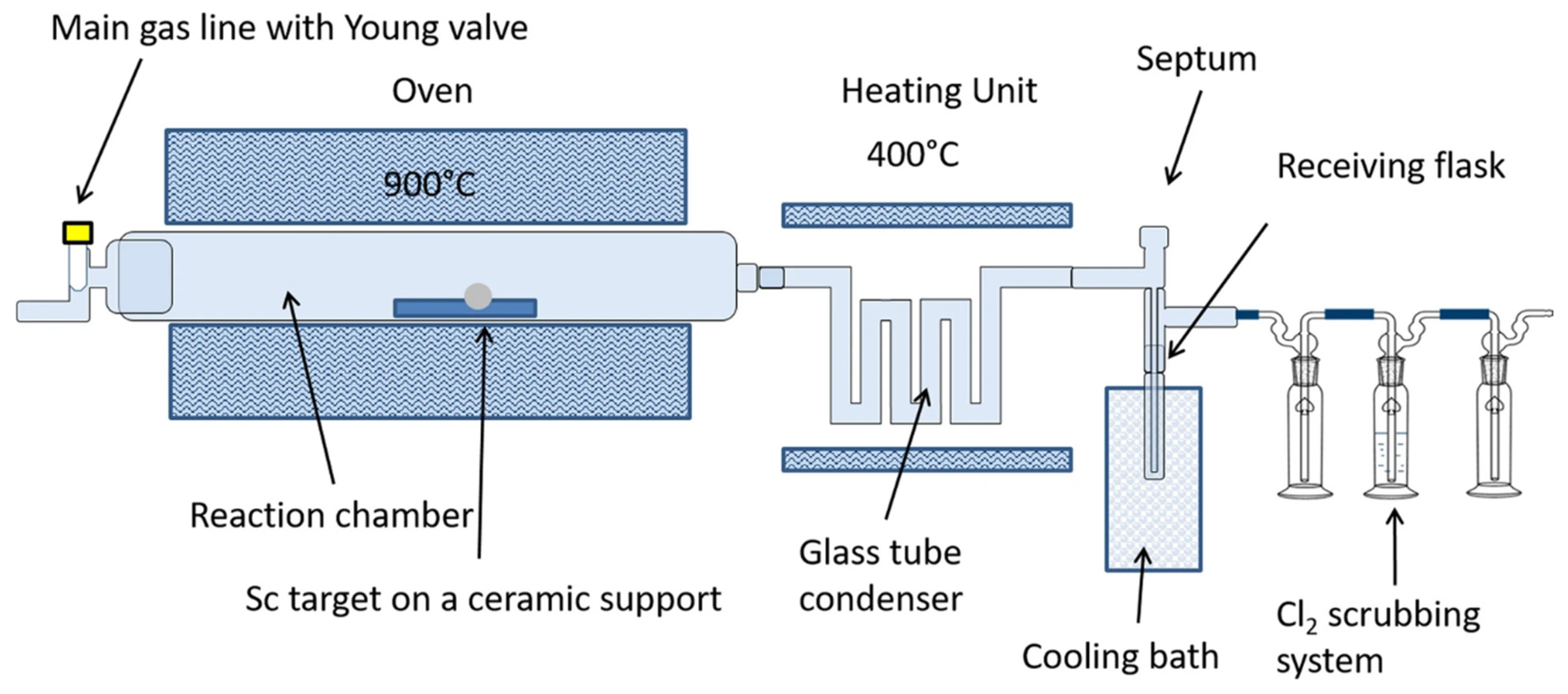
2. Production and Purification
Production of Titanium-45
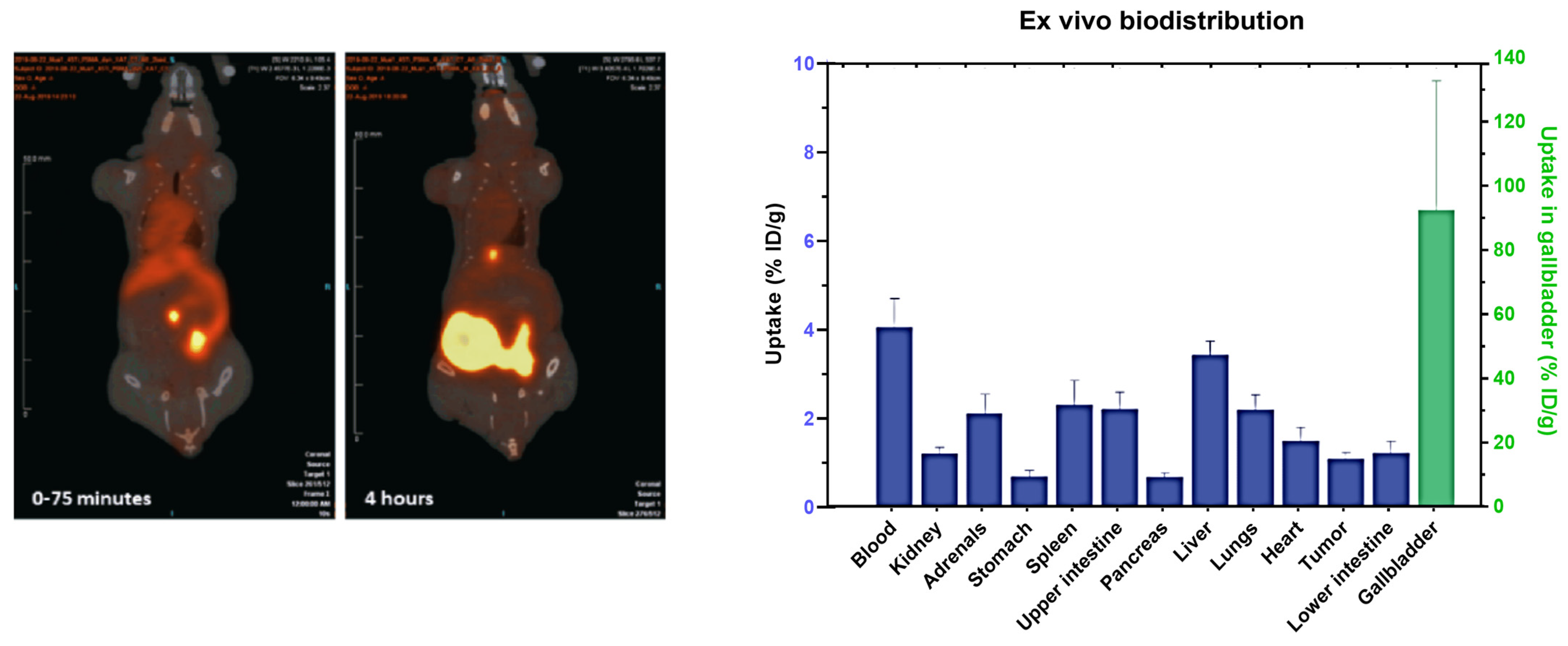
3. Imaging Applications and Chelation Chemistry of Titanium-45
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lyu, S.; Wei, X.; Chen, J.; Wang, C.; Wang, X.; Pan, D. Titanium as a beneficial element for crop production. Front. Plant Sci. 2017, 8, 237149–237167. [Google Scholar] [CrossRef] [PubMed]
- Valentine, A.M. Titanium: Inorganic & Coordination Chemistry. In Encyclopedia of Inorganic Chemistry; John Wiley & Sons. Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Waghmode, M.S.; Gunjal, A.B.; Mulla, J.A.; Patil, N.N.; Nawani, N.N. Studies on the titanium dioxide nanoparticles: Biosynthesis, applications and remediation. SN Appl. Sci. 2019, 1, 310. [Google Scholar] [CrossRef]
- Saxena, M.; Loza-Rosas, S.A.; Gaur, K.; Sharma, S.; Otero, S.C.P.; Tinoco, A.D. Exploring titanium (IV) chemical proximity to iron (III) to elucidate a function for Ti (IV) in the human body. Coord. Chem. Rev. 2018, 363, 109–125. [Google Scholar] [CrossRef]
- Caruso, F.; Rossi, M. Antitumor titanium compounds. Mini Rev. Med. Chem. 2004, 4, 49–60. [Google Scholar] [PubMed]
- Ellahioui, Y.; Prashar, S.; Gomez-Ruiz, S. Anticancer applications and recent investigations of metallodrugs based on gallium, tin and titanium. Inorganics 2017, 5, 4. [Google Scholar] [CrossRef]
- Kaluđerović, G.N.; Tayurskaya, V.; Paschke, R.; Prashar, S.; Fajardo, M.; Gómez-Ruiz, S. Synthesis, characterization and biological studies of alkenyl-substituted titanocene (IV) carboxylate complexes. Appl. Organomet. Chem. 2010, 24, 656–662. [Google Scholar] [CrossRef]
- Ceballos-Torres, J.; Caballero-Rodríguez, M.J.; Prashar, S.; Paschke, R.; Steinborn, D.; Kaluđerović, G.N.; Gómez-Ruiz, S. Synthesis, characterization and in vitro biological studies of titanocene (IV) derivatives containing different carboxylato ligands. J. Organomet. Chem. 2012, 716, 201–207. [Google Scholar] [CrossRef]
- Barroso, S.; Coelho, A.M.; Gómez-Ruiz, S.; Calhorda, M.J.; Žižak, Ž.; Kaluđerović, G.N.; Martins, A.M. Synthesis, cytotoxic and hydrolytic studies of titanium complexes anchored by a tripodal diamine bis (phenolate) ligand. Dalton Trans. 2014, 43, 17422–17433. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Quintanilla, D.; Gómez-Ruiz, S.; Žižak, Ž.; Sierra, I.; Prashar, S.; del Hierro, I.; Fajardo, M.; Juranić, Z.D.; Kaluđerović, G.N. A new generation of anticancer drugs: Mesoporous materials modified with titanocene complexes. Chem. A Eur. J. 2009, 15, 5588–5597. [Google Scholar] [CrossRef]
- Kaluđerović, G.N.; Pérez-Quintanilla, D.; Sierra, I.; Prashar, S.; del Hierro, I.; Žižak, Ž.; Juranić, Z.D.; Fajardo, M.; Gómez-Ruiz, S. Study of the influence of the metal complex on the cytotoxic activity of titanocene-functionalized mesoporous materials. J. Mater. Chem. 2010, 20, 806–814. [Google Scholar] [CrossRef]
- Kaluđerović, G.N.; Pérez-Quintanilla, D.; Žižak, Ž.; Juranić, Z.D.; Gómez-Ruiz, S. Improvement of cytotoxicity of titanocene-functionalized mesoporous materials by the increase of the titanium content. Dalton Trans. 2010, 39, 2597–2608. [Google Scholar] [CrossRef] [PubMed]
- García-Peñas, A.; Gómez-Ruiz, S.; Pérez-Quintanilla, D.; Paschke, R.; Sierra, I.; Prashar, S.; del Hierro, I.; Kaluđerović, G.N. Study of the cytotoxicity and particle action in human cancer cells of titanocene-functionalized materials with potential application against tumors. J. Inorg. Biochem. 2012, 106, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Roesch, F. Scandium-44: Benefits of a long-lived PET radionuclide available from the 44Ti/44Sc generator system. Curr. Radiopharm. 2012, 5, 187–201. [Google Scholar] [CrossRef]
- Gajecki, L.; Marino, C.M.; Cutler, C.S.; Sanders, V.A. Evaluation of hydroxamate-based resins towards a more clinically viable 44Ti/44Sc radionuclide generator. Appl. Radiat. Isot. 2023, 192, 110588–110595. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Singh, B. Nuclear Structure and Decay Data for A = 44 Isobars. Nucl. Data Sheets 2023, 190, 1–318. [Google Scholar] [CrossRef]
- Benabdallah, N.; Zhang, H.; Unnerstall, R.; Fears, A.; Summer, L.; Fassbender, M.; Rodgers, B.E.; Abou, D.; Radchenko, V.; Thorek, D.L. Engineering a modular 44Ti/44Sc generator: Eluate evaluation in preclinical models and estimation of human radiation dosimetry. EJNMMI Res. 2023, 13, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Klouda, J.; Fassbender, M.E.; Mocko, V. A combined inorganic-organic titanium-44/scandium-44g radiochemical generator. J. Chromatogr. A 2023, 1711, 464438–464446. [Google Scholar] [CrossRef]
- Lederer, C.; Shirley, V. Table of Isotopes; John Wiley & Sons. Inc.: New York, NY, USA, 1978. [Google Scholar]
- Merrill, J.; Lambrecht, R.; Wolf, A. Cyclotron isotopes and radiopharmaceuticals. 24. Titanium-45. Int. J. Appl. Radiat. Isot. 1978, 29, 115–116. [Google Scholar] [CrossRef]
- Conti, M.; Eriksson, L. Physics of pure and non-pure positron emitters for PET: A review and a discussion. EJNMMI Phys. 2016, 3, 8. [Google Scholar] [CrossRef]
- Banerjee, S.R.; Pomper, M.G. Clinical applications of Gallium-68. Appl. Radiat. Isot. 2013, 76, 2–13. [Google Scholar] [CrossRef]
- Vāvere, A.L.; Laforest, R.; Welch, M.J. Production, processing and small animal PET imaging of titanium-45. Nucl. Med. Biol. 2005, 32, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Chaple, I.F.; Thiele, K.; Thaggard, G.; Fernandez, S.; Boros, E.; Lapi, S.E. Optimized methods for production and purification of Titanium-45. Appl. Radiat. Isot. 2020, 166, 109398. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D.W.; Shefer, R.E.; Klinkowstein, R.E.; Bass, L.A.; Margeneau, W.H.; Cutler, C.S.; Anderson, C.J.; Welch, M.J. Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl. Med. Biol. 1997, 24, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Goud, N.S.; Bhattacharya, A.; Joshi, R.K.; Nagaraj, C.; Bharath, R.D.; Kumar, P. Carbon-11: Radiochemistry and target-based PET molecular imaging applications in oncology, cardiology, and neurology. J. Med. Chem. 2021, 64, 1223–1259. [Google Scholar] [CrossRef] [PubMed]
- Boscutti, G.; Huiban, M.; Passchier, J. Use of carbon-11 labelled tool compounds in support of drug development. Drug Discov. Today Technol. 2017, 25, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.J.; Redvanly, C.S. Handbook of Radiopharmaceuticals: Radiochemistry and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Kumar, K. The current status of the production and supply of Gallium-68. Cancer Biother. Radiopharm. 2020, 35, 163–166. [Google Scholar] [CrossRef]
- Kinsey, R.; Dunford, C.; Tuli, J.; Burrows, T. The NUDAT/PCNUDAT program for nuclear data. In Proceedings of the 9th International Symposium on Capture Gamma-Ray Spectroscopy and Related Topics, Budapest, Hungary, 8–12 October 1996. [Google Scholar]
- Vavere, A.L.; Welch, M.J. Preparation, biodistribution, and small animal PET of 45Ti-transferrin. J. Nucl. Med. 2005, 46, 683–690. [Google Scholar] [PubMed]
- Gagnon, K.; Severin, G.; Barnhart, T.; Engle, J.; Valdovinos, H.; Nickles, R. 45Ti extraction using hydroxamate resin. In Proceedings of the AIP Conference Proceedings, Playa del Carmen, Mexico, 26–29 August 2012; pp. 211–214. [Google Scholar]
- Chen, F.; Valdovinos, H.F.; Hernandez, R.; Goel, S.; Barnhart, T.E.; Cai, W. Intrinsic radiolabeling of Titanium-45 using mesoporous silica nanoparticles. Acta Pharmacol. Sin. 2017, 38, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.S.; Imbrogno, J.; Fonslet, J.; Lusardi, M.; Jensen, K.F.; Zhuravlev, F. Liquid–liquid extraction in flow of the radioisotope titanium-45 for positron emission tomography applications. React. Chem. Eng. 2018, 3, 898–904. [Google Scholar] [CrossRef]
- Giesen, K.; Spahn, I.; Neumaier, B. Thermochromatographic separation of 45Ti and subsequent radiosynthesis of [45Ti] salan. J. Radioanal. Nucl. Chem. 2020, 326, 1281–1287. [Google Scholar] [CrossRef]
- Søborg Pedersen, K.; Baun, C.; Michaelsen Nielsen, K.; Thisgaard, H.; Ingemann Jensen, A.; Zhuravlev, F. Design, synthesis, computational, and preclinical evaluation of natTi/45Ti-labeled urea-based glutamate PSMA ligand. Molecules 2020, 25, 1104. [Google Scholar] [CrossRef] [PubMed]
- Chaple, I.F.; Houson, H.A.; Koller, A.; Pandey, A.; Boros, E.; Lapi, S.E. 45Ti targeted tracers for PET imaging of PSMA. Nucl. Med. Biol. 2022, 108, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Koller, A.J.; Saini, S.; Chaple, I.F.; Joaqui-Joaqui, M.A.; Paterson, B.M.; Ma, M.T.; Blower, P.J.; Pierre, V.C.; Robinson, J.R.; Lapi, S.E. A General Design Strategy Enabling the Synthesis of Hydrolysis-Resistant, Water-Stable Titanium (IV) Complexes. Angew. Chem. Int. Ed. 2022, 61, e202201211. [Google Scholar] [CrossRef] [PubMed]
- Koller, A.J.; Wang, L.; Deluca, M.; Glaser, O.; Robis, M.J.; Mixdorf, J.C.; Chernysheva, M.N.; Guzei, I.A.; Aluicio-Sarduy, E.; Barnhart, T.E. De Novo Approaches to the Solid-Phase Separation of Titanium (IV) and Scandium (III): Translating Speciation Data to Selective on-Bead Chelation toward Applications in Nuclear Medicine. Inorg. Chem. 2023, 62, 20655–20665. [Google Scholar] [CrossRef] [PubMed]
- Zerkin, V.; Pritychenko, B. The experimental nuclear reaction data (EXFOR): Extended computer database and Web retrieval system. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2018, 888, 31–43. [Google Scholar] [CrossRef]
- Nelson, F.; Murase, T.; Kraus, K.A. Ion exchange procedures: I. Cation exchange in concentration HCl and HClO4 solutions. J. Chromatogr. A 1964, 13, 503–535. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Enferadi, M.; Nadi, H. 45Ti, a candidate for positron emission tomography: Study of the cyclotron production. Radiochemistry 2011, 53, 411–414. [Google Scholar] [CrossRef]
- Severin, G.W.; Nielsen, C.H.; Jensen, A.I.; Fonslet, J.; Kjær, A.; Zhuravlev, F. Bringing radiotracing to titanium-based antineoplastics: Solid phase radiosynthesis, pet and ex vivo evaluation of antitumor agent [45Ti](salan) Ti (dipic). J. Med. Chem. 2015, 58, 7591–7595. [Google Scholar] [CrossRef]
- Strecker, J.; Wachten, T.; Neumaier, B.; Spahn, I. Radiochemical isolation of 45Ti using ion chromatography. J. Radioanal. Nucl. Chem. 2024, 1–7. [Google Scholar] [CrossRef]
- Price, R.; Sheil, R.; Scharli, R.; Chan, S.; Gibbons, P.; Jeffery, C.; Morandeau, L. Titanium-45 as a candidate for PET imaging: Production, processing & applications. In Proceedings of the 15th International Workshop on Targetry and Target Chemistry, Prague, Czech Republic, 18–21 August 2015. [Google Scholar]
- Holland, J.P.; Sheh, Y.; Lewis, J.S. Standardized methods for the production of high specific-activity zirconium-89. Nucl. Med. Biol. 2009, 36, 729–739. [Google Scholar] [CrossRef]
- Tsoodol, Z.; Aikawa, M.; Ichinkhorloo, D.; Khishigjargal, T.; Norov, E.; Komori, Y.; Haba, H.; Takács, S.; Ditrói, F.; Szűcs, Z. Production cross sections of 45Ti in the deuteron-induced reaction on 45Sc up to 24 MeV. Appl. Radiat. Isot. 2021, 168, 109448–109452. [Google Scholar] [CrossRef] [PubMed]
- Hermanne, A.; Rebeles, R.A.; Tarkanyi, F.; Takacs, S.; Takacs, M.; Csikai, J.; Ignatyuk, A. Cross sections of deuteron induced reactions on 45Sc up to 50 MeV: Experiments and comparison with theoretical codes. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2012, 270, 106–115. [Google Scholar] [CrossRef]
- Ishiwata, K.; Ido, T.; Monma, M.; Murakami, M.; Fukuda, H.; Kameyama, M.; Yamada, K.; Endo, S.; Yoshioka, S.; Sato, T. Potential radiopharmaceuticals labeled with titanium-45. Int. J. Radiat. Appl. Instrum. Part A Appl. Radiat. Isot. 1991, 42, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Kagaya, Y.; Nozaki, E.; Ishide, N.; Maruyama, Y.; Takishima, T.; Takahashi, T.; Ishiwata, K.; Watanuki, S.; Ido, T. Myocardial imaging using 11C-CoQ10 with positron emission tomography. Int. J. Radiat. Appl. Instrum. Part B Nucl. Med. Biol. 1987, 14, 1–6. [Google Scholar] [CrossRef]
- Saini, S.; Mullen, G.E.; Blower, P.J.; Lapi, S.E. Radiochemistry and In Vivo Imaging of [45Ti] Ti-THP-PSMA. Mol. Pharm. 2024, 21, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Afaq, A.; Payne, H.; Davda, R.; Hines, J.; Cook, G.J.; Meagher, M.; Priftakis, D.; Warbey, V.S.; Kelkar, A.; Orczyk, C. A Phase II, Open-Label Study to Assess Safety and Management Change Using 68Ga-THP PSMA PET/CT in Patients with High-Risk Primary Prostate Cancer or Biochemical Recurrence After Radical Treatment: The PRONOUNCED Study. J. Nucl. Med. 2021, 62, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Yekany, L.V.; Chiniforoush, T.A.; Fazaeli, Y.; Aboudzadeh, M.; Sadeghi, M. Preparation and quality control of a new porphyrin complex labeled with 45Ti for PET imaging. Appl. Radiat. Isot. 2023, 193, 110650. [Google Scholar] [CrossRef] [PubMed]
- Carbo-Bague, I.; Saini, S.; Cingoranelli, S.J.; Davey, P.R.; Tosato, M.; Lapi, S.E.; Ramogida, C.F. Evaluation of a novel hexadentate 1,2-hydroxypyridinone-based acyclic chelate, HOPO-O6-C4, for 43Sc/47Sc, 68Ga, and 45Ti radiopharmaceuticals. Nucl. Med. Biol. 2023, 128–129, 108872. [Google Scholar] [CrossRef]
- Messori, L.; Orioli, P.; Banholzer, V.; Pais, I.; Zatta, P. Formation of titanium (IV) transferrin by reaction of human serum apotransferrin with titanium complexes. Febs Lett. 1999, 442, 157–161. [Google Scholar] [CrossRef]
- Guo, M.; Sun, H.; McArdle, H.J.; Gambling, L.; Sadler, P.J. TiIV uptake and release by human serum transferrin and recognition of TiIV-transferrin by cancer cells: Understanding the mechanism of action of the anticancer drug titanocene dichloride. Biochemistry 2000, 39, 10023–10033. [Google Scholar] [CrossRef]
- Kratz, F.; Hartmann, M.; Keppler, B.; Messori, L. The binding properties of two antitumor ruthenium (III) complexes to apotransferrin. J. Biol. Chem. 1994, 269, 2581–2588. [Google Scholar] [CrossRef] [PubMed]
- Nuevo-Ordoñez, Y.; Montes-Bayón, M.; Blanco González, E.; Sanz-Medel, A. Titanium preferential binding sites in human serum transferrin at physiological concentrations. Metallomics 2011, 3, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Kratz, F.; Beyer, U. Serum proteins as drug carriers of anticancer agents: A review. Drug Deliv. 1998, 5, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Roat-Malone, R.M. Bioinorganic Chemistry: A Short Course; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Buettner, K.M.; Valentine, A.M. Bioinorganic chemistry of titanium. Chem. Rev. 2012, 112, 1863–1881. [Google Scholar] [CrossRef]
- Chen, F.; Goel, S.; Valdovinos, H.F.; Luo, H.; Hernandez, R.; Barnhart, T.E.; Cai, W. In vivo integrity and biological fate of chelator-free zirconium-89-labeled mesoporous silica nanoparticles. ACS Nano 2015, 9, 7950–7959. [Google Scholar] [CrossRef]



| Isotope | Half-Life (t1/2) | Decay | Mean β+ Energy (keV) | Reaction | Target Abundance | Cost | References |
|---|---|---|---|---|---|---|---|
| 45Ti | 184 min | β+ (85%) | 439 | 45Sc(p,n)45Ti | 100% | $ | [23,24] |
| 64Cu | 12.7 h | β+ (17.8%) | 278 | 64Ni(p,n)64Cu | 1.16% | $$$ | [25] |
| 11C | 20.3 min | β+ (100%) | 386 | 14N(p,α)11C | 99.63% | [26,27] | |
| 18F | 110 min | β+ (97%) | 250 | 18O(p,n)18F | 0.2% | $$ | [28] |
| 68Ga | 68 min | β+ (88%) | 830 | 68Ge generator | N/A | $$$ | [29] |
| Resin | Target | Elution | Elution Volume | Radiochemical Yield | Reference |
|---|---|---|---|---|---|
| Dowex 1-X8 (100–200 mesh) | Foil | 8 M HCl | 0.1 mL | 30% | [20] |
| AG 50W x 8 (100–200 mesh) | Foil | 6 M HCl | 2 mL | 75–90% | [41] |
| AG 50W x 8 (100–200 mesh) | Foil | 6 M HCl | 6 × 6 mL | 92.5% | [23] |
| AG 50W x 8 (100–200 mesh) | Sc2O3 | 6 M HCl | NA | N/A | [42] |
| HypoGel 200 | Foil | 3 mL | ~30% | [43] | |
| Hydroxamate resin | Foil | Oxalic acid (1 M) | 5 mL | N/A | [32] |
| Liquid–liquid extraction | Foil | guaiacol/anisole | N/A | [34] | |
| Solvent-free | Foil | TiCl4 | NA | 53% | [35] |
| Hydroxamate resin | Foil | citric acid (1 M) | 3 mL | 78 ± 8% | [24] |
| CA-Def * | Foil | HCl (6 M) | 0.4 mL | 80% | [39] |
| Hydroxamate resin | Foil | Oxalic acid (0.1 M, pH 3) | 2.5 mL | 61 ± 8% | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saini, S.; Lapi, S.E. Titanium-45 (45Ti) Radiochemistry and Applications in Molecular Imaging. Pharmaceuticals 2024, 17, 479. https://doi.org/10.3390/ph17040479
Saini S, Lapi SE. Titanium-45 (45Ti) Radiochemistry and Applications in Molecular Imaging. Pharmaceuticals. 2024; 17(4):479. https://doi.org/10.3390/ph17040479
Chicago/Turabian StyleSaini, Shefali, and Suzanne E. Lapi. 2024. "Titanium-45 (45Ti) Radiochemistry and Applications in Molecular Imaging" Pharmaceuticals 17, no. 4: 479. https://doi.org/10.3390/ph17040479
APA StyleSaini, S., & Lapi, S. E. (2024). Titanium-45 (45Ti) Radiochemistry and Applications in Molecular Imaging. Pharmaceuticals, 17(4), 479. https://doi.org/10.3390/ph17040479







