Long-Term Tumor-Targeting Effect of E. coli as a Drug Delivery System
Abstract
1. Introduction
2. Results and Discussion
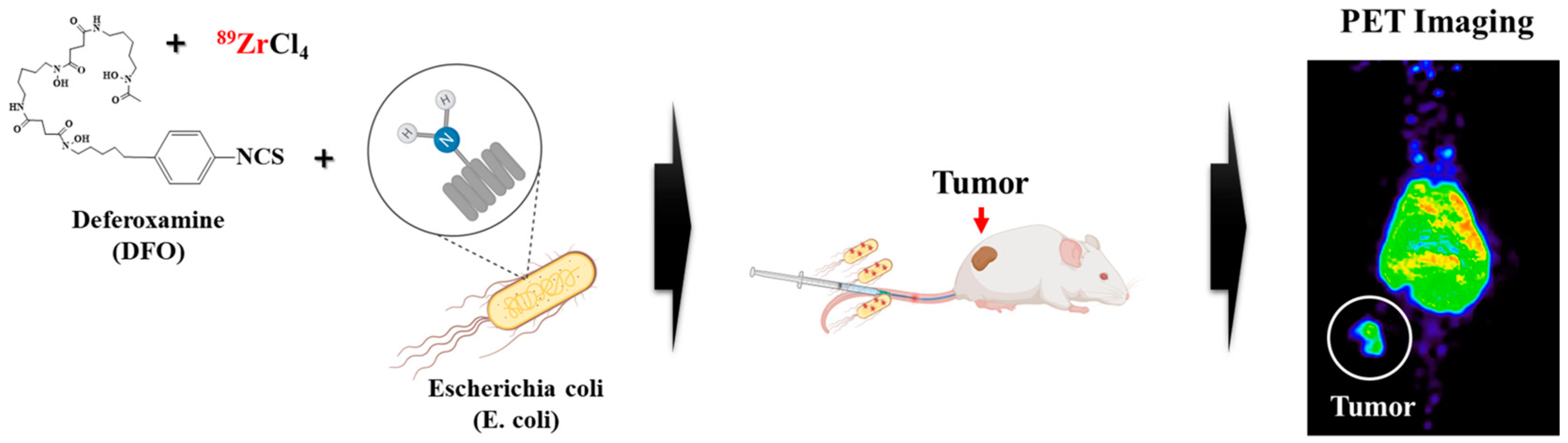
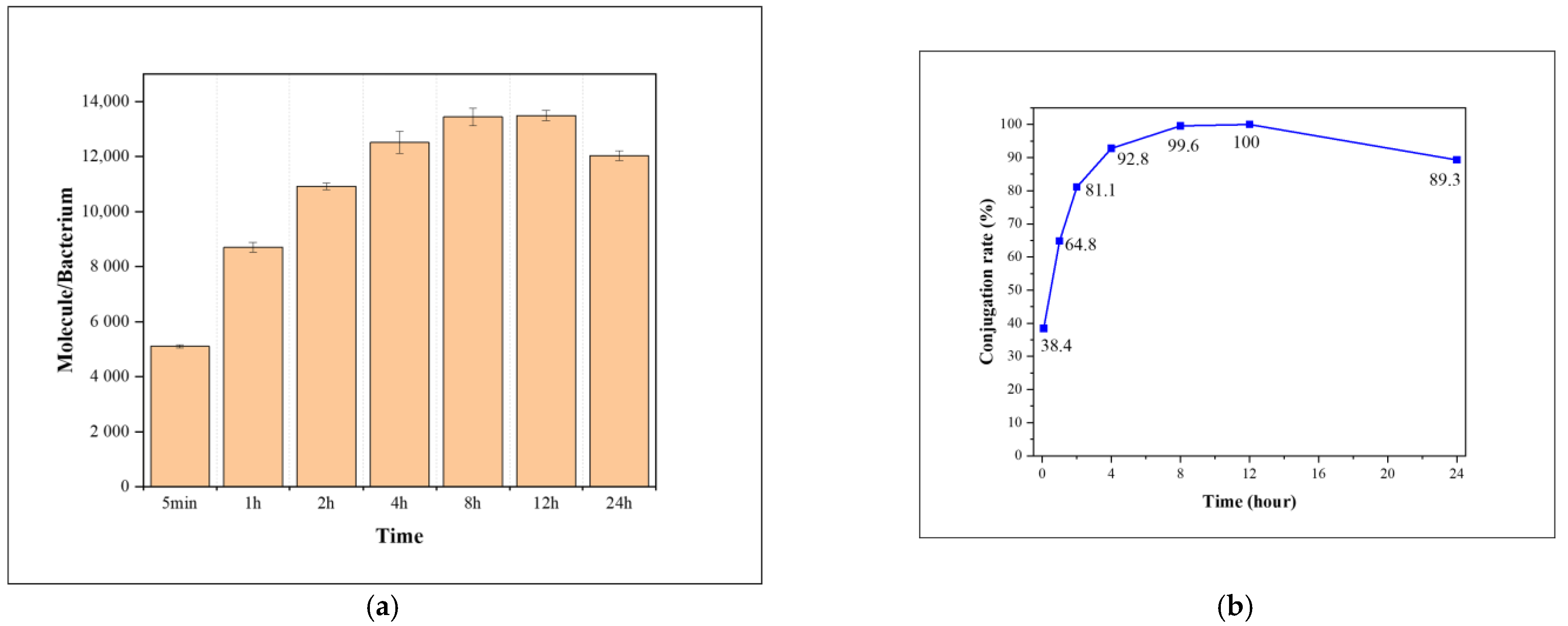
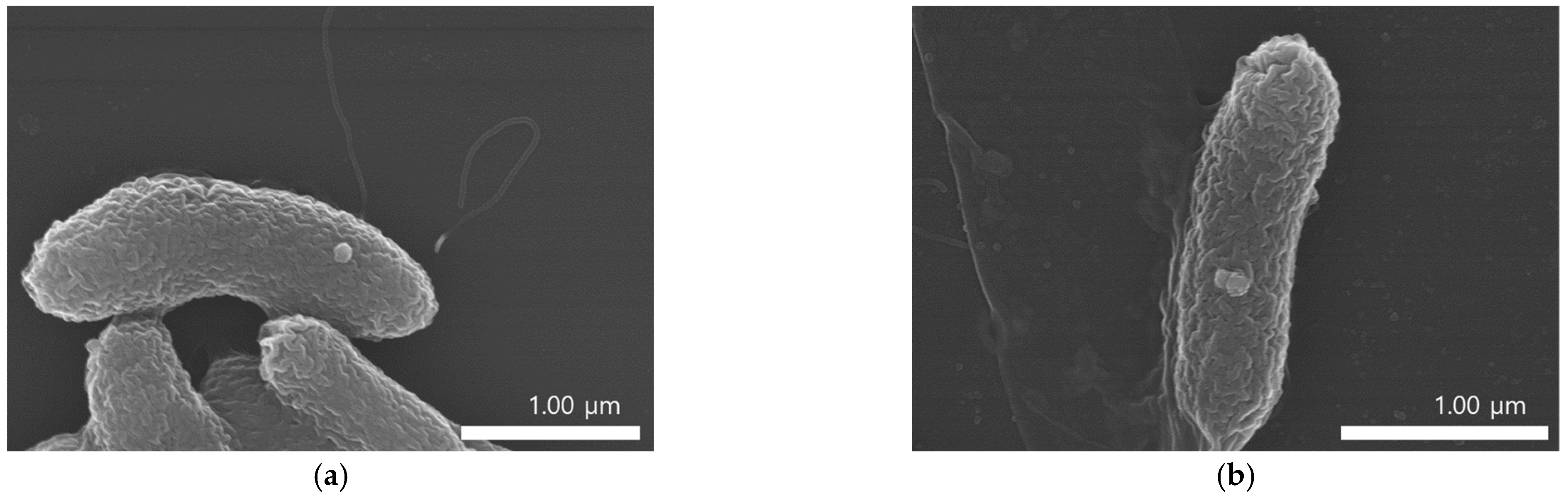
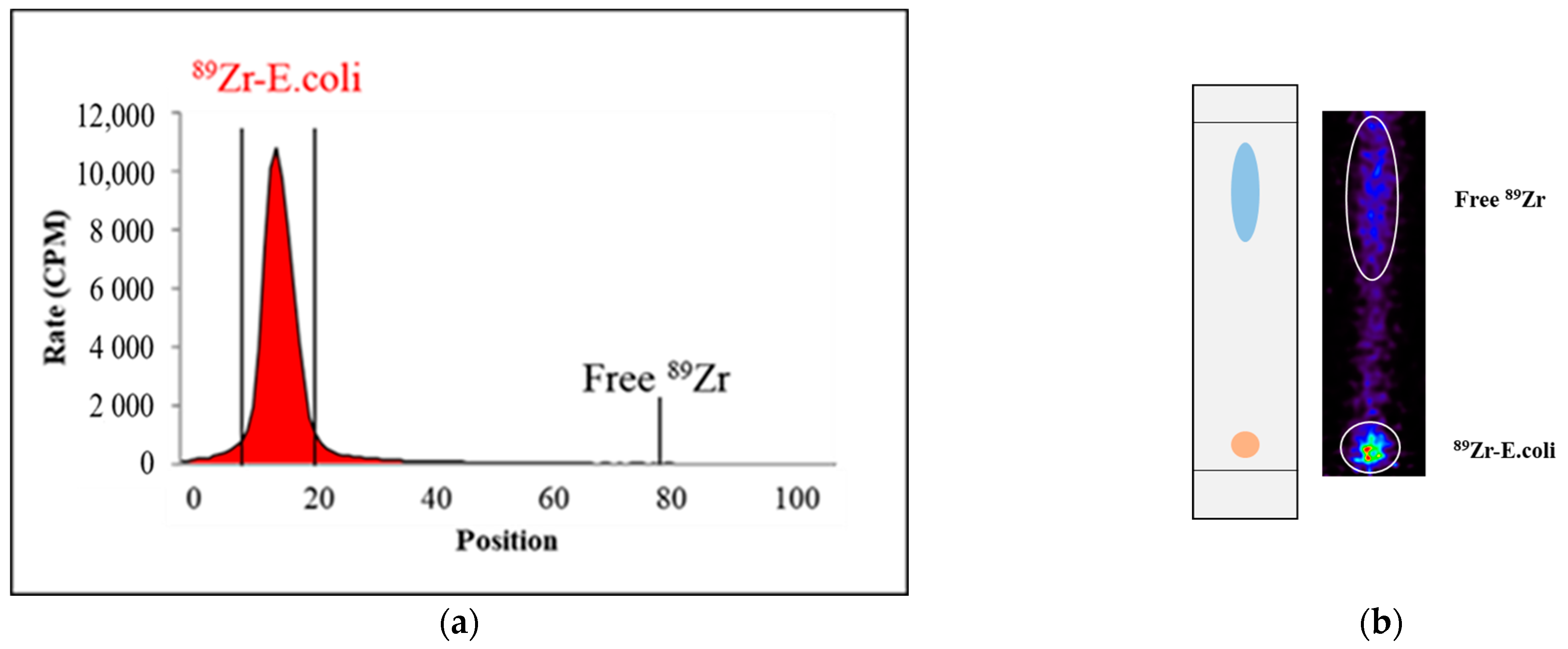
2.1. Synthesis
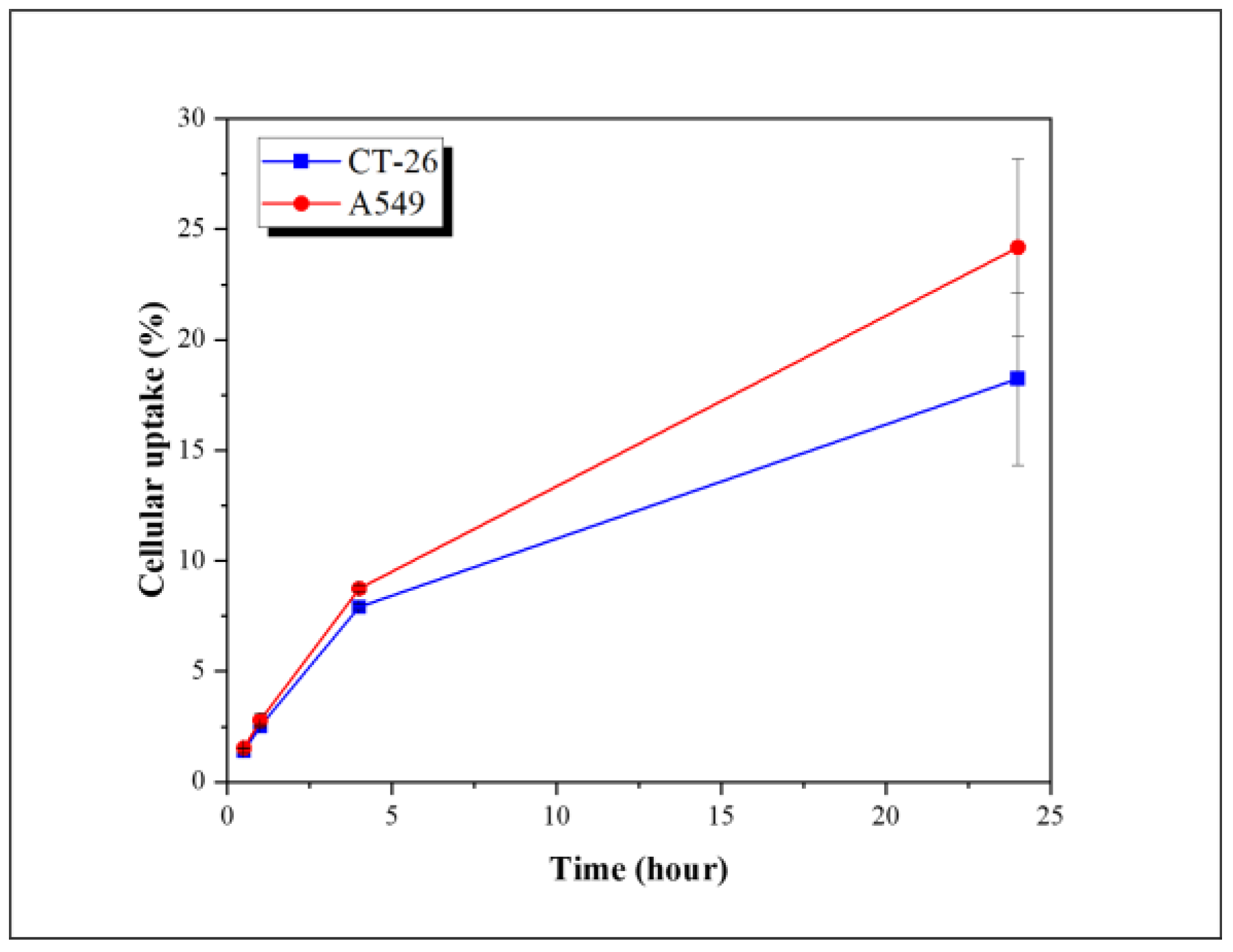
2.2. Cellular Uptake
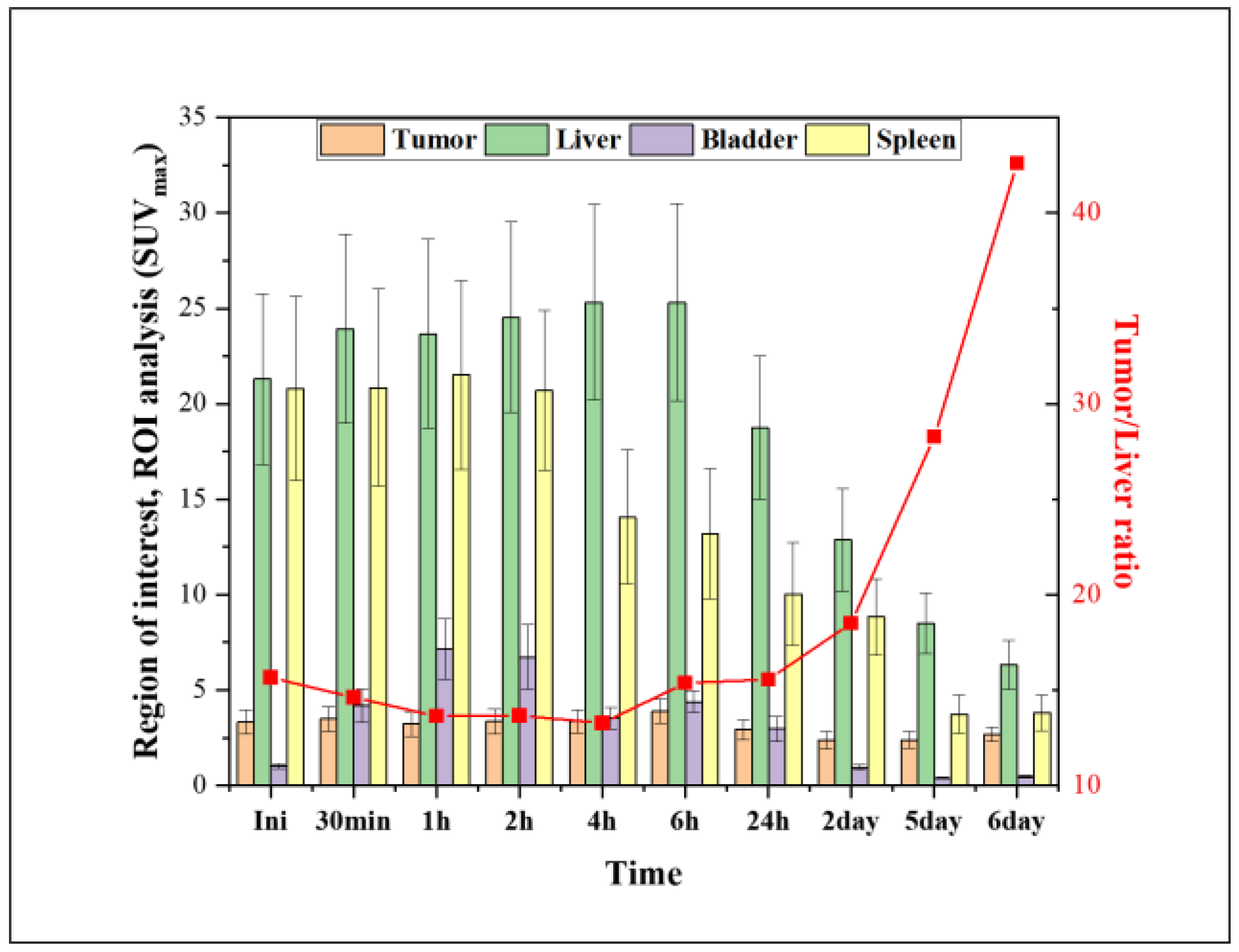
2.3. PET Studies
3. Materials and Methods
3.1. Materials
3.2. Preparation of FITC-Conjugated E. coli
3.3. Preparation of Zr-Conjugated E. coli
3.4. Preparation of 89Zr-Labeled E. coli
3.5. In Vitro Stability
3.6. Cellular Uptake
3.7. PET Studies and Ex Vivo Biodistribution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Bhutia, Y.D.; Yao, Q.; He, Z.; Sun, J.; Ganapathy, V. Transporter-Guided Delivery of Nanoparticles to Improve Drug Permeation across Cellular Barriers and Drug Exposure to Selective Cell Types. Front. Pharmacol. 2018, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Hosseinidoust, Z.; Mostaghaci, B.; Yasa, O.; Park, B.W.; Singh, A.V.; Sitti, M. Bioengineered and biohybrid bacteria-based systems for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Liu, J. Bacteria and bacterial derivatives as drug carriers for cancer therapy. J. Control. Release 2020, 326, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xie, N.; Zhang, H.; Zhou, W.; Ding, J. Bacterial Drug Delivery Systems for Cancer Therapy: “Why” and “How”. Pharmaceutics 2023, 15, 2214. [Google Scholar] [CrossRef] [PubMed]
- Claesen, J.; Fischbach, M.A. Synthetic microbes as drug delivery systems. ACS Synth. Biol. 2015, 4, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lin, C.; Yu, J.; Qi, Q.; Wang, Q. Bioengineered Escherichia coli Nissle 1917 for tumour-targeting therapy. Microb. Biotechnol. 2020, 13, 629–636. [Google Scholar] [CrossRef]
- Zhan, Y.; Fergusson, A.; McNally, L.R.; Davis, R.M.; Behkam, B. Robust and Repeatable Biofabrication of Bacteria-Mediated Drug Delivery Systems: Effect of Conjugation Chemistry, Assembly Process Parameters, and Nanoparticle Size. Adv. Intell. Syst. 2021, 4, 2100135. [Google Scholar] [CrossRef]
- Fan, J.X.; Li, Z.H.; Liu, X.H.; Zheng, D.W.; Chen, Y.; Zhang, X.Z. Bacteria-Mediated Tumor Therapy Utilizing Photothermally-Controlled TNF-alpha Expression via Oral Administration. Nano Lett. 2018, 18, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Shi, H.; Zhang, Y.; Fan, Y.; Wang, L.; Xiang, L.; Liu, Y.; Zhao, L.; Fu, S. Bacteria-driven hypoxia targeting delivery of chemotherapeutic drug proving outcome of breast cancer. J. Nanobiotechnol. 2022, 20, 178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Man, J.H.; Liang, B.; Zhou, T.; Wang, C.H.; Li, T.; Li, H.Y.; Li, W.H.; Jin, B.F.; Zhang, P.J.; et al. Tumor-targeted delivery of biologically active TRAIL protein. Cancer Gene Ther. 2010, 17, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Yang, W.; Shao, Z.; Zhao, Y. Genetically modified bacteria for targeted phototherapy of tumor. Biomaterials 2021, 272, 120809. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Pandey, M.K.; Demirhan, Y.E.; Nesbitt, J.J.; Crespo-Diaz, R.J.; Terzic, A.; Behfar, A.; DeGrado, T.R. Novel 89Zr cell labeling approach for PET-based cell trafficking studies. EJNMMI Res. 2015, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, R.; Hong, H.; Cai, W. Positron emission tomography image-guided drug delivery: Current status and future perspectives. Mol. Pharm. 2014, 11, 3777–3797. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.K.; Padmanabhan, P.; Yang, C.T.; Ng, D.C.E.; Palanivel, M.; Mishra, S.; Halldin, C.; Gulyas, B. Positron emission tomographic imaging in drug discovery. Drug Discov. Today 2022, 27, 280–291. [Google Scholar] [CrossRef]
- Lee, J.Y.; Vyas, C.K.; Kim, G.G.; Choi, P.S.; Hur, M.G.; Yang, S.D.; Kong, Y.B.; Lee, E.J.; Park, J.H. Red Blood Cell Membrane Bioengineered Zr-89 Labelled Hollow Mesoporous Silica Nanosphere for Overcoming Phagocytosis. Sci. Rep. 2019, 9, 7419. [Google Scholar] [CrossRef]
- Giesen, D.; Hooge, M.N.L.; Nijland, M.; Heyerdahl, H.; Dahle, J.; de Vries, E.G.E.; Pool, M. 89Zr-PET imaging to predict tumor uptake of 177Lu-NNV003 anti-CD37 radioimmunotherapy in mouse models of B cell lymphoma. Sci. Rep. 2022, 12, 6286. [Google Scholar] [CrossRef]
- Rosar, F.; Schaefer-Schuler, A.; Bartholoma, M.; Maus, S.; Petto, S.; Burgard, C.; Prive, B.M.; Franssen, G.M.; Derks, Y.H.W.; Nagarajah, J.; et al. [89Zr]Zr-PSMA-617 PET/CT in biochemical recurrence of prostate cancer: First clinical experience from a pilot study including biodistribution and dose estimates. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4736–4747. [Google Scholar] [CrossRef]
- Shi, S.; Li, T.; Wen, X.; Wu, S.Y.; Xiong, C.; Zhao, J.; Lincha, V.R.; Chow, D.S.; Liu, Y.; Sood, A.K.; et al. Copper-64 Labeled PEGylated Exosomes for In Vivo Positron Emission Tomography and Enhanced Tumor Retention. Bioconjug Chem. 2019, 30, 2675–2683. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Chae, J.H.; Hur, M.G.; Yang, S.D.; Kong, Y.B.; Lee, J.; Ju, J.S.; Choi, P.S.; Park, J.H. Theragnostic 64Cu/67Cu Radioisotopes Production With RFT-30 Cyclotron. Front. Med. 2022, 9, 889640. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Aboudzadeh, M.; Zali, A.; Zeinali, B. 86Y production via 86Sr(p,n) for PET imaging at a cyclotron. Appl. Radiat. Isot. 2009, 67, 1392–1396. [Google Scholar] [CrossRef] [PubMed]
- Brechbiel, T.K.N.a.M.W. 86Y Based PET Radiopharmaceuticals: Radiochemistry and Biological. Applications. Med. Chem. 2011, 7, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Eder, M.; Neels, O.; Muller, M.; Bauder-Wust, U.; Remde, Y.; Schafer, M.; Hennrich, U.; Eisenhut, M.; Afshar-Oromieh, A.; Haberkorn, U.; et al. Novel Preclinical and Radiopharmaceutical Aspects of [68Ga]Ga-PSMA-HBED-CC: A New PET Tracer for Imaging of Prostate Cancer. Pharmaceuticals 2014, 7, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Rodnick, M.E.; Sollert, C.; Stark, D.; Clark, M.; Katsifis, A.; Hockley, B.G.; Parr, D.C.; Frigell, J.; Henderson, B.D.; Abghari-Gerst, M.; et al. Cyclotron-based production of 68Ga, [68Ga]GaCl3, and [68Ga]Ga-PSMA-11 from a liquid target. EJNMMI Radiopharm. Chem. 2020, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, F.H.; Durack, J.C.; Pandit-Taskar, N.; Ulaner, G.A.; Lewis, J.S.; Morris, M.J.; Solomon, S.B. Long-Half-Life 89Zr-Labeled Radiotracers Can Guide Percutaneous Biopsy Within the PET/CT Suite Without Reinjection of Radiotracer. J. Nucl. Med. 2018, 59, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Choi, P.S.; Lee, J.Y.; Yang, S.D.; Park, J.H. Biological behavior of nanoparticles with Zr-89 for cancer targeting based on their distinct surface composition. J. Mater. Chem. B 2021, 9, 8237–8245. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Kang, Y.; Eom, Y.; Torati, S.R.; Kim, C. Functionalization of Biotinylated Polyethylene Glycol on Live Magnetotactic Bacteria Carriers for Improved Stealth Properties. Biology 2021, 10, 993. [Google Scholar] [CrossRef]
- Chomet, M.; Schreurs, M.; Bolijn, M.J.; Verlaan, M.; Beaino, W.; Brown, K.; Poot, A.J.; Windhorst, A.D.; Gill, H.; Marik, J.; et al. Head-to-head comparison of DFO* and DFO chelators: Selection of the best candidate for clinical 89Zr-immuno-PET. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 694–707. [Google Scholar] [CrossRef]
- Friberger, I.; Nilsson, J.N.; Lu, L.; Siikanen, J.; Ardenfors, O.; Milton, S.; Samen, E.; Goos, J.; Carlsten, M.; Holmin, S.; et al. Comparative in vivo biodistribution of cells labelled with [89Zr]Zr-(oxinate)4 or [89Zr]Zr-DFO-NCS using PET. EJNMMI Res. 2023, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.G.; Lee, J.Y.; Choi, P.S.; Kim, S.W.; Park, J.H. Tumor Targeting Effect of Triphenylphosphonium Cations and Folic Acid Coated with Zr-89-Labeled Silica Nanoparticles. Molecules 2020, 25, 2922. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Man, F.; Faruqu, F.N.; Kim, J.; Al-Salemee, F.; Carrascal-Minino, A.; Volpe, A.; Liam-Or, R.; Simpson, P.; Fruhwirth, G.O.; et al. PET Imaging of Small Extracellular Vesicles via [89Zr]Zr(oxinate)4 Direct Radiolabeling. Bioconjug. Chem. 2022, 33, 473–485. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.G.; Lee, H.; Jeong, D.B.; Kim, S.W.; So, J.-S. Long-Term Tumor-Targeting Effect of E. coli as a Drug Delivery System. Pharmaceuticals 2024, 17, 421. https://doi.org/10.3390/ph17040421
Kim GG, Lee H, Jeong DB, Kim SW, So J-S. Long-Term Tumor-Targeting Effect of E. coli as a Drug Delivery System. Pharmaceuticals. 2024; 17(4):421. https://doi.org/10.3390/ph17040421
Chicago/Turabian StyleKim, Gun Gyun, Hongje Lee, Dan Bi Jeong, Sang Wook Kim, and Jae-Seon So. 2024. "Long-Term Tumor-Targeting Effect of E. coli as a Drug Delivery System" Pharmaceuticals 17, no. 4: 421. https://doi.org/10.3390/ph17040421
APA StyleKim, G. G., Lee, H., Jeong, D. B., Kim, S. W., & So, J.-S. (2024). Long-Term Tumor-Targeting Effect of E. coli as a Drug Delivery System. Pharmaceuticals, 17(4), 421. https://doi.org/10.3390/ph17040421






