Updated Progress on Polysaccharides with Anti-Diabetic Effects through the Regulation of Gut Microbiota: Sources, Mechanisms, and Structure–Activity Relationships
Abstract
1. Introduction
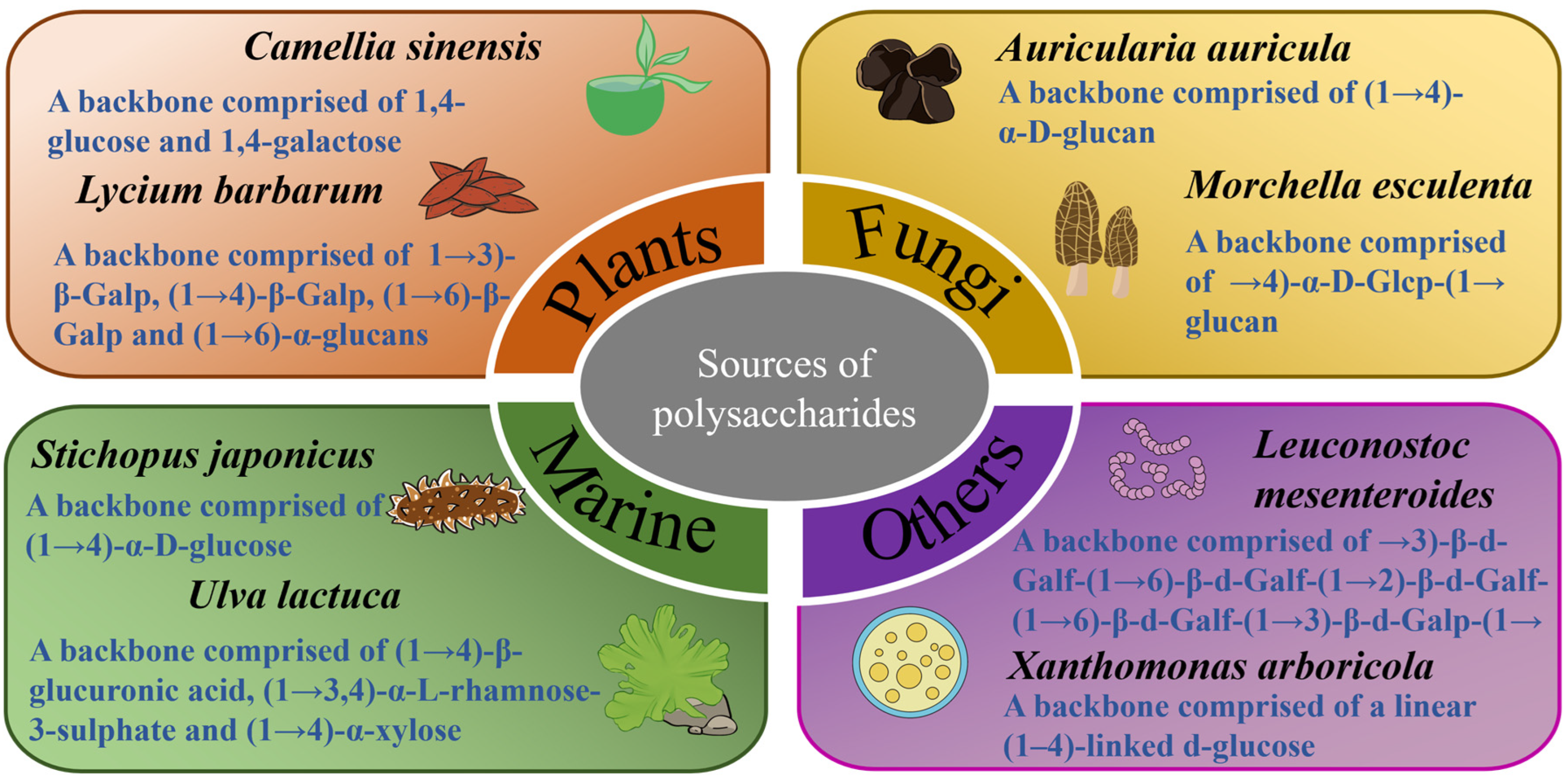
2. Sources of Polysaccharides and Their Anti-Diabetic Effects
2.1. Polysaccharides Extracted from Plants
2.2. Polysaccharides Extracted from Fungi
| Polysaccharide Source | Mw (kDa) | Monosaccharide Composition | Research Model | Anti-Diabetic Activity | Gut Microbiota Modulation | Reference |
|---|---|---|---|---|---|---|
| Apocynum venetum leaves | 289.2 | Man, Rha, GluA, GalA, Glu, Gal, and Ara with a ratio of 2.90:28.06:1.92:21.72:10.47:26.69:8.42; | HFD and STZ induced C57BL/6J male mice | ↓ liquid intake; liver and heart indexes; insulin resistance; GSP; TG; LDL-C; NEFA; ALT; AST; ↑ liver glycogen; glucose tolerance; β-cell function; CAT, SOD; GSH; SCFAs (acetate; butyrate); relieve the histopathological injuries of liver and pancreas | ↓ Firmicutes to Bacteroidetes ratio (P); Proteobacteria (P); Enterococcus (G); Klebsiella (G); Aerocuccus (G); ↑ Odoribacter (G); Anaeroplasma (G); Muribaculum (G); Parasutterella (G); | [10] |
| Astragalus membranaceus | 161.15 | Ara, Gal, Glu, Xyl, Man, GalA, and GluA with a ratio of 13.60:7.20:63.73:0.25:0.13:14.73:0.37 | HFD and STZ induced C57BL/6J male mice | ↓ body weight loss; food and water intake; FBG; GSP; FINs; TC; TG; LDL-C; LPS; TNF-α; IL-6; MDA; ALT; AST; hepatic lipid accumulation and steatosis; epididymal adipose; DAO; D-LA; ↑ glucose tolerance; HDL-C; IL-10; CAT; SOD; GSH; hepatic glycogen; relieve the histopathological injuries of pancreas and colon | ↓ Helicobacter (G); Cupriavidus (G); Halomonas (G); Bacteroides (G); Odoribacter (G); Erysipelotrichaceae_Clostridium (G); Enterococcus (G); Shigella (G); Akkermansia (G); Anaeroplasma (G); AF12 (G); [Prevotella] (G); Streptococcus (G); ↑ Allobaculum (G); Lactobacillus (G); | [88] |
| Berberis dasystachya | 102 | Man, Ara, Glu, Gal, Xyl, and Fru with a ratio of 113.59:89.07:69.46:59.55:7.48:2.33 | HFD and STZ induced Sprague Dawley male rats | ↓ food and water intake; weight loss; organ index (pancreas, liver, kidneys, and heart); FBG; insulin resistance; GSP; HbAlc; MDA; NO; NOS; ↑ glucose tolerance; insulin sensitivity index; GSH-Px; SOD; SCFAs (acetic, propionic, butyric, isobutyric, valeric, and isovaleric acids); relieve the histopathological injuries of pancreas, colon tissues | ↓ Bacteroidetes (P); Klebsiella (G); Ruminococcus torques group (G); Skermanella (G); Odoribacter (G); ↑ Firmicutes (P); Lactobacillus (G); Ruminococcaceae UCG-005 (G); Prevotellaceae NK3B31 group (G); Blautia (G); Ruminococcaceae NK4A214 group (G); Ruminococcus 2 (G); Eubacterium coprostanoligenes_group (G); Romboutsia (G); | [89] |
| Brasenia schreberi | 50–100 | - | HFD and STZ induced C57BL/6 male mice | ↓ FBG; insulin resistance; TC; LDL-C; ↑ glycogen level; regulate PI3K/Akt signal pathway | ↓ Firmicutes to Bacteroidetes ratio (P); Romboutsia; Desulfovibrio; ↑ Allopravotella; Lactobacillus (G); Bacteroides (G) | [90] |
| Camellia sinensis | 289.734 | Rha, Rib, Ara, Man, Glu, and Gal with a ratio of 1.26:3.18:4.08:1.00:1.52:3.92 | HFD and STZ induced male Wistar male rats | ↓ FBG; insulin resistance; TC; TG; LDL-C; FFA; Bax protein; colonic pH value; ↑ glucose tolerance; ADP; GLP-1; HDL-C; Bcl-2 protein; SCFAs (acetic acid; propionic acid; n-butyric acid; i-butyric acid and n-valeric acid); relieve the histopathological injury of pancreas | ↓ Bacteroidetes (P); ↑ Proteobacteria (P); Fluviicola (G); Roseburia (G); Victivallis (G); Lachnospira (G); | [91] |
| Coix seed | 13.285 | Fuc, Rha, Ara, Gal, Glu, Xyl, Man, Fru, Rib, GalA, GulA, GluA, and ManA with a ratio of 0.25:1.05:2.79:3.86:79.64:2.75:3.54:0.31:0.08:4.26:0.31:0.81:0.18 | HFD and STZ induced C57BL/6J male mice | ↓ FBG; body weight loss; food intake; insulin resistance; TC; TG; LDL-C; ↑ glucose tolerance; HDL-C; SCFAs; ZO-1 expression; relieve the histopathological injury of colon; regulate IGF1/PI3K/AKT signaling pathway | ↓ Firmicutes (P); Helicobacter; ↑ Bacteroidetes (P); Lactobacillus (G), Akkermansia (G), Bacteroides (G); Bifidobacterium (G); | [92] |
| Lycium barbarum | 98.0 | Rha, Ara, Xyl, Man, Glu, Gal, GluA, and GalA with a ratio of 0.23:1.90:0.26:0.20:1.0:1.26:0.44:1.49 | HFD and STZ induced C57BL/6 male mice | ↓ body weight loss; food and water intake; FBG; insulin resistance; HbA1c; GSP; insulin; TC; TG; LDL-C; ALT; AST; MDA; IL-6; IL-1b; TNF-α; LPS; ↑ glucose tolerance; insulin sensitivity; GLP-1; PYY; TBA; HDL-C; CAT; SOD; GSH-Px; TAOC; β cell function; glycogen; SCFAs (acetate, propionate, butyrate, isobutyrate, valerate, iso-valerate, and isovalerate); relieve the histopathological injuries of pancreas, liver, and skeletal muscle | ↓ Firmicutes (P); Allobaculum (G); Dubosiella (G); Romboutsia (G); ↑ Bacteroidetes (P); Bacteroides (G); Ruminococcaceae_UCG-014 (G); Mucispirillum (G); Intestinimonas (G); Ruminococcaceae_UCG-009 (G); | [93] |
| Cyclocarya paliuru | 2.584 | Glu, Ara, Gal, Man, Xyl, Rha, GalA, GluA, Fuc, and Rib in a ratio of 27.90:9.68:7.67:1.93:1.67:1.26:0.72:0.66:0.17:0.16 | HFD and STZ induced Sprague-Dawley male rats | ↓ FBG; insulin resistance; TC; TG; LDL-C; ↑ glucose intolerance; HDL-C; GLP-1; PYY; CAZyme subtypes; SCFAs (malonic acid, propionic acid, isobutyric acid; glutaric acid); SCFAs derivates (D-3-hydroxybutyricacid; D (-)-beta-hydroxy butyric acid and 3-hydroxycapric acid) | ↓ Spirochaetes (P); Proteobacteria (P); Enterococcus_faecium (S) ↑ Firmicutes(P); Ruminococcaceae (F); Eubacteriaceae (F); Lachnospiraceae (F); Ruminococcus_bromii (S); Anaerotruncus_colihominis (S); Clostridium_methylpentosum (S); Roseburia_intestinalis (S); Roseburia_hominis (S); Clostridium_asparagiforme (S); Pseudoflavonifractor_capillosus (S); Intestinimonas_butyriciproducens (S); Intestinimonas_sp._GD2 (S); Oscillibacter_valericigenes (S); Oscillibacter_ruminantium (S) | [22] |
| Nigella sativa seed | - | - | HFD and STZ induced Kunming male mice | ↓ FBG; GSP; body weight loss; TC; TG; LDL-C; MDA; IL-6; TNF-α; IL-1β; ↑ insulin; HDL-C; T-AOC; SOD; CAT; p-AKT; GLUT4; SCFAs (↑propionic acid; ↓acetic acid); relieve the histopathological injuries of liver and pancreas | ↓ Firmicutes (P); Lachnospiraceae_NK4A136_group (G); f_Lachnospiraceae_Unclassified (G); ↑ Bacteroidetes (P); Bacteroides (G); f_Muribaculaceae_Unclassified (G); Lactobacillus (G); | [94] |
| Moutan Cortex | 164 | Glu and Ara with a ratio of 3.31:2.25 | high-fat and high-sugar diet, and STZ induced SD male rats | ↓ HbA1c; insulin resistance; renal function index (UP/24 h, Scr, BUN, UACR); IL-6; isovaleric acid; ↑ GLP-1; expression of tight junction proteins (ZO-1, Claudin-1, Occludin); IL-10; SCFAs (acetic acid, propionic acid, butyric acid); relieve the histopathological injuries of kidney, ileum, colon | ↑ Verrucomicrobia (P); Mollicutes (G), Bacteroidia (G); Lactobacillus (G); Akkermansia (G); Ruminococcaceae_UCG-014 (G); Muribaculaceae_unclassified (G) | [95] |
| Setaria italica | - | Man, Rha, Gal, Xyl, and Ara in a ratio of 0.72:0.59:76.26:1.03:0.83 | HFD and STZ induced Kunming male rats | ↓ body weight loss; FBG; TC; TG; LDL-C; MDA ↑ glucose tolerance; HDL-C; CAT; SOD; GSH-Px; SCFAs (acetic acid, propionic acid, butyric acid); relieve the histopathological injuries of liver and pancreas | ↓ Firmicutes (P); Verrucomicrobiota (P); Peptostreptococcales-Tissierellales (O); Lachnospirales (O); Romboutsia (O); Bacteroides (O); ↑ Proteobacteria (P); Pseudomonadales (O); Pseudomonas (G); Alloprevotella (G); Akkermansia (G); Alistipes (G) | [11] |
| Polysaccharide Source | Mw (kDa) | Monosaccharide Composition | Research Model | Anti-Diabetic Activity | Gut Microbiota Modulation | Reference |
|---|---|---|---|---|---|---|
| Auricularia auricula-judae | - | Man, Glu, Gal, Rha, Xyl, and Fru in a ratio of 62:12.6:4:1.31:4: 3.8 | HFD and STZ induced C57BL/6 male mice | ↓ relative epididymal fat weight; FBG; insulin resistance; TC; TG; LDL-C; lipid accumulation; ALT; AST; TNF-α; IL-6; ↑ glucose tolerance; GLP-1; HDL-C; relieve the histopathological injuries of liver and pancreas; regulate the AKT/AMPK signaling pathways; enrich KEGG pathways | ↓ Firmicutes to Bacteroidetes ratio (P); Proteobacteria (P); Alistipes; Allobaculum; unidentified_Lachnospiraceae; Clostridium; ↑ Lactobacillus; Oscillospira; Rikenella; Bacteroides; Lactococcus; Odoribacter; Ruminococcus; Anaerotruncus; | [96] |
| Ganoderma lucidum | 11.079 | Ara, Gal, Glu, Xyl, Man, Rib, and Rha in a ratio of 5.32:5.47:57.63:0.84:25.41:1.95:3.38 | HFD and STZ induced Kunming male mice | ↓ body weight loss; liver and kidney weight; FBG; insulin resistance; LDL-C; TC; TG; ALT; AST; MDA; fat accumulation; ↑ HDL-C; GSH-Px; SOD; liver glycogen; relieve the histopathological injuries of liver and pancreas | ↓ Firmicutes (P); Proteobacteria (P); Desulfovibrionaceae (F); Bacteroidaceae (F); Lachnospiraceae (F); Lactobacillaceae (F); _f__Desulfovibrionaceae (G); Acetatifactor (G); Lactobacillus (G); ↑ Bacteroidetes (P); Epsilonbacteraeota (P); Muribaculaceae (F); Helicobacteraceae (F); Peptococcaceae (F); Lactobacillaceae (F); Ruminococcaceae (F); Prevotellaceae(F); Alloprevotella (G); Ruminiclostridium_5 (G); f__Peptococcaceae (G); Tyzzerella (G); | [97] |
| Cordyceps militaris | 87.8 | Man, Gal, and Glu in a ratio of 2.2:15.1:1 | HFD and STZ induced C57BL/6 male mice | ↓ food and water intake; FBG; insulin resistance; LEP; TC; TG; ALT; AST; BUN; Cr; LPS; TNF-α; IL-1β; IL-6; ↑ glucose tolerance; GLP-1; ADP; colon tight junction proteins (Claudin1, Occludin, and ZO-1); relieve the histopathological injuries of liver, kidney, pancreas and colon; inhibit TLR4/NF-κB pathway | ↓ Firmicutes/Bacteroidetes ratio (P); Verrucomicrobiota (P); Proteobacteria (P); Desulfobacterota (F); Escherichia-Shigella (G); Enterococcus (G); ↑ Bacteroidota (P); Campilobacterota (F); Actinobacteriota (F); norank_f_Muribaculaceae (G); Lachnospiraceae_NK4A136_group (G); norank_o__Clostridia_UCG-014 (G); Alistipes (G), Helicobacter (G); Eubacterium_xylanophilum_group (G) | [98] |
| Grifola frondosa | 12,600 | Ara, Man and Glu in a ratio of 3.79:1.00:49.70. | high-fat, high-sugar diet and STZ induced ICR male mice | ↓ FBG; HbA1c; expression of JNK1/2; ↑ glucose tolerance; β-cells function; expression of IRS1and PI3K; GLUT4; relieve the histopathological injuries of liver and kidney | ↓ Firmicutes (P); Proteobacteria (P); ↑ Bacteroidetes (P); Porphyromonas gingivalis (S); Akkermansia muciniphila (S); Lactobacillus acidophilus (S); Tannerella forsythia (S); Bacteroides acidifaciens (S); Roseburia intestinalis (S) | [99] |
| Morchella esculenta | - | Man, Rib, Rha, GluA, GalA, Glu, Gal, Ara, and Fuc in a ratio of 5.77:0.263:0.018:0.036:0.006:81.35:3.543:8.99:0.016 | HFD and STZ induced BALB/c male mice | ↓ body weight loss; FBG; insulin resistance; IL-6; IL-1β; TNF-α; LPS; ↑ glucose tolerance; colon tight junction proteins (ZO-1, occludin, and claudin-1); MUC2 protein; relieve the histopathological injuries of colon; regulate the KEGG pathways | ↓ Firmicutes (P); Corynebacterium (G); Facklamia (G); Corynebacteriaceae (F); Actinomyceletes (C); Staphylococcaceae (S); ↑ Actinobacteria (P); Lactobacillus (G); Lactobacillaceae (F); Lachnospiraceae (F); Enterobacteriaceae (F); Lactobacilliaceae (S) | [100] |
2.3. Polysaccharides Extracted from Marine Organisms
| Polysaccharide Source | Mw (kDa) | Monosaccharide Composition | Research Model | Anti-Diabetic Activity | Gut Microbiota Modulation | Reference |
|---|---|---|---|---|---|---|
| Dictyopteris divaricata | 63.06 | Man, Rib, Rha, GluA, Glu, Gal, Xyl, Ara, and Fuc in a ratio of 15.02:9.90:1.28:17.54:1.86:17.19:4.54:0.55 | high sugar diet and STZ induced Balb/c male mice | ↓ body weight loss; food and water intake; FBG; PBG-2h; insulin resistance; TC; TG; LDL-C; IL-1β; IL-2; IL-6, TNF-α; IFN-γ; MDA; ↑ glucose tolerance; β cell function; HDL-C; SOD; MUC-2; ZO-1; tight junction proteins (Occludin; Claudin-1); IRS-1; relieve histopathological injury of colon | ↓ Bacteroidetes (P); Proteobacteria (P); Actinobacteria (P); S24-7 (F); Paraprevotellaceae (F); Odoribacteraceae (F); Corynebacteriaceae (F); Bacteroides (G); Corynebacterium (G); Ruminococcus (G); Parabacteroides (G); ↑ Firmicutes (P); Lactobacillus (G); Prevotella (G); Oscillospira (G); Lactobacillaceae (F); Ruminococaceae (F); Lachnospiraceae (F); Rikenellaceae (F) | [117] |
| Holothuria leucospilota | 52.8 | Rha, Fuc, Glua, galactose, Glu, and Xyl in a ratio of 39.1:35.7:10.7:8.4:4.2:1.8 | GK male rats and age-matched Wistar rats | ↓ FBG; TC; TG; LDL-C; insulin; LEP; CD36; Bax; ↑ glucose tolerance; HDL-C; adiponectin; GLP-1; PI3K; AKT; PPAR-α; GLUT4; Bcl-2; SCFAs (acetic, butyric acid, pentanoic acid); relieve histopathological injuries of pancreas, colon | ↓ Firmicutes (P); Proteobacteria (P); Spirochaetes (P); Actinobacteria (P); Bilophila (G); Bifidobacterium (G); Mucispirillum (G); Colinsella (G); Gemella (G); Treponema (G); Anaerobiospirillum (G); Aggregatibacter (G); Facklamia (G); Lactobacillus (G); ↑ Bacteroidetes (P); TM7 (P); Cyanobacteria (P); Tenericutes (P); Ruminococcus (G); Holdemania (G); Clostridium (G); Helicobacter (G); Turicibacter (G); Paraprevotella (G); Bacteroides (G); Faecalibacterium (G) | [118] |
| Ulva lactuca | 224 | Rha, GluA, Gal, and Xyl in a ratio of 32.75:22.83:1.07:6.46 | high-fat high sugar diet and STZ induced ICR male mice | ↓ FBG; body weight loss; MDA; ↑ glucose tolerance; CAT; SOD; GSH-PX; relieve the histopathological injury of liver; regulate JAK/STAT3 pathway | ↓ Firmicutes (P); ↑ Bacteroidetes (P); Actinobacteria (P); s_weissella_cibaria (G); g_Candidatus_Saccharimonas (G); f_Saccharimonadaceae (G); c_Saccharimonadia (G); o_Saccharimonadales (G) | [119] |
| Macrocystis pyrifera | 342.1 | Gal, Fuc, Man, and GluA in ratio of 29.29:27.59:21.24: 16.99 | high-fat, high-sugar and STZ induced Sprague Dawley male rats | ↓ body weight loss; glucose; HbA1c; insulin resistance; TG; TC; LDL-C; AST; ALT; BUN; Cr; TNF-α; IL-6; MDA ↑ glucose tolerance; GSH-Px; | ↓ Escherichia–Shigella (G); ↑ Muribaculaceae_norank (G); Akkermansia (G); Bifidobacterium (G); Lactobacillus (G); Olsenella (G); Lachnospiraceae_NK4A136_group (G); Ruminococcaceae_UCG-014 (G); Ruminococcus_1 (G); Eubacterium_coprostanoligenes_group (G) | [120] |
| Onchidium struma | 8–14 | Ara, Man and Glu in a ratio of 3.79:1.00:49.70. | high-sucrose high-fat diet and STZ induced Kungming male mice | ↓ body weight loss; FBG; blood glucose; FIN level; HOMA-IRI; TC; TG; LDL-C; GSP; IL-6; LPS; TNF-α; GSK-3β; ↑ daily intake; glucose tolerance; FER value; HOMA-ISI; HOMA-β; HDL-C; IL-10; mRNA expression (PI3K, AKT-1, mTOR, GLUT-2); SCFAs (acetate, propionate, isobutyrate, butyrate, isovalerate, valerate); relieve the histopathological injury of liver | ↓ Firmicutes to Bacteroidetes (P); Lachnoclostridium; Parabacteroides; ↑ Alipipes; Lactobacillus | [121] |
3. Mechanism of the Anti-Diabetic Effects of Polysaccharides through Regulating Gut Microbiota
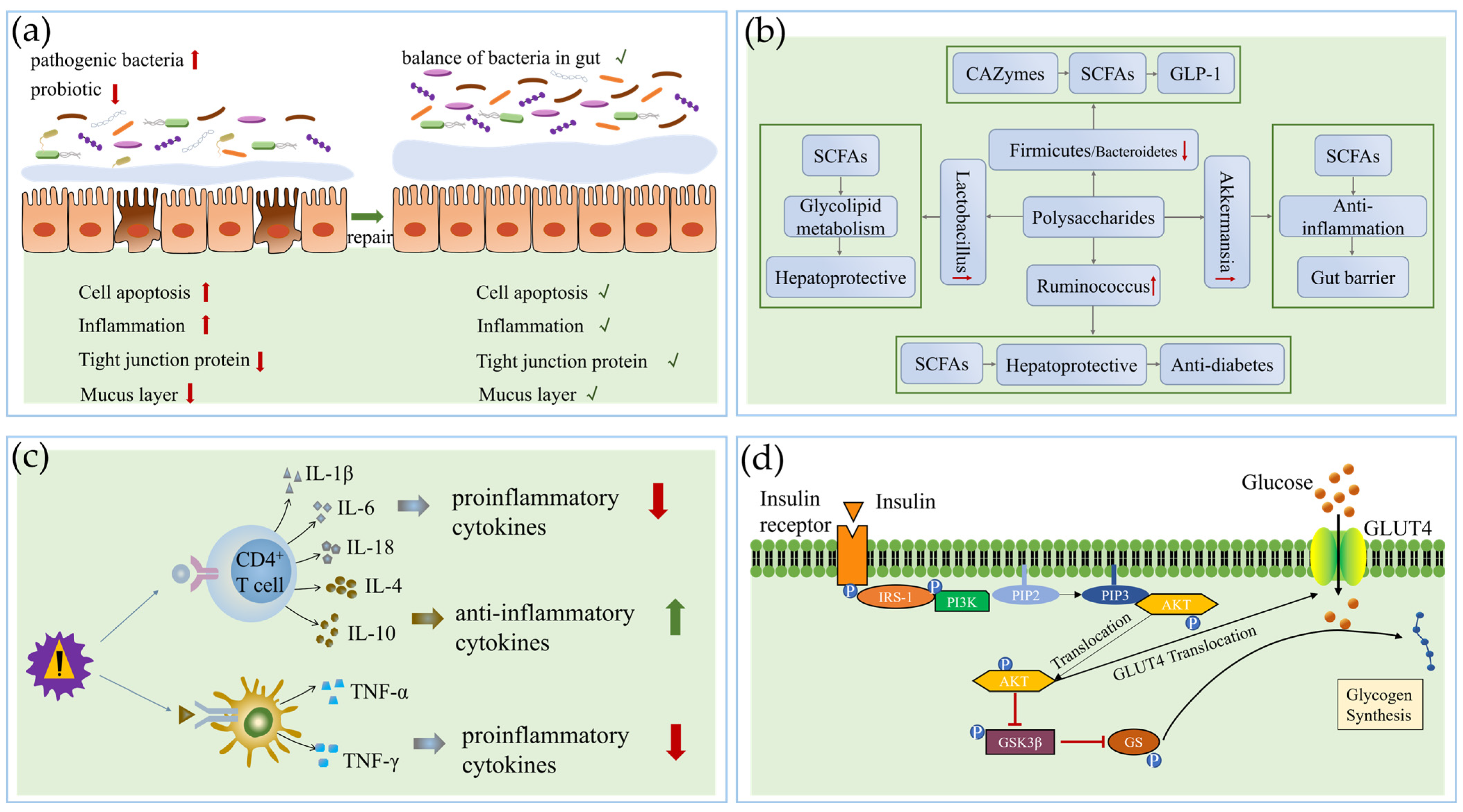
3.1. Repairing the Gut Barrier
3.2. Changing Gut Microbiota Composition and Metabolites
3.3. Regulating Anti-Inflammatory Activity and Immune Function
3.4. Regulating the Signal Pathway
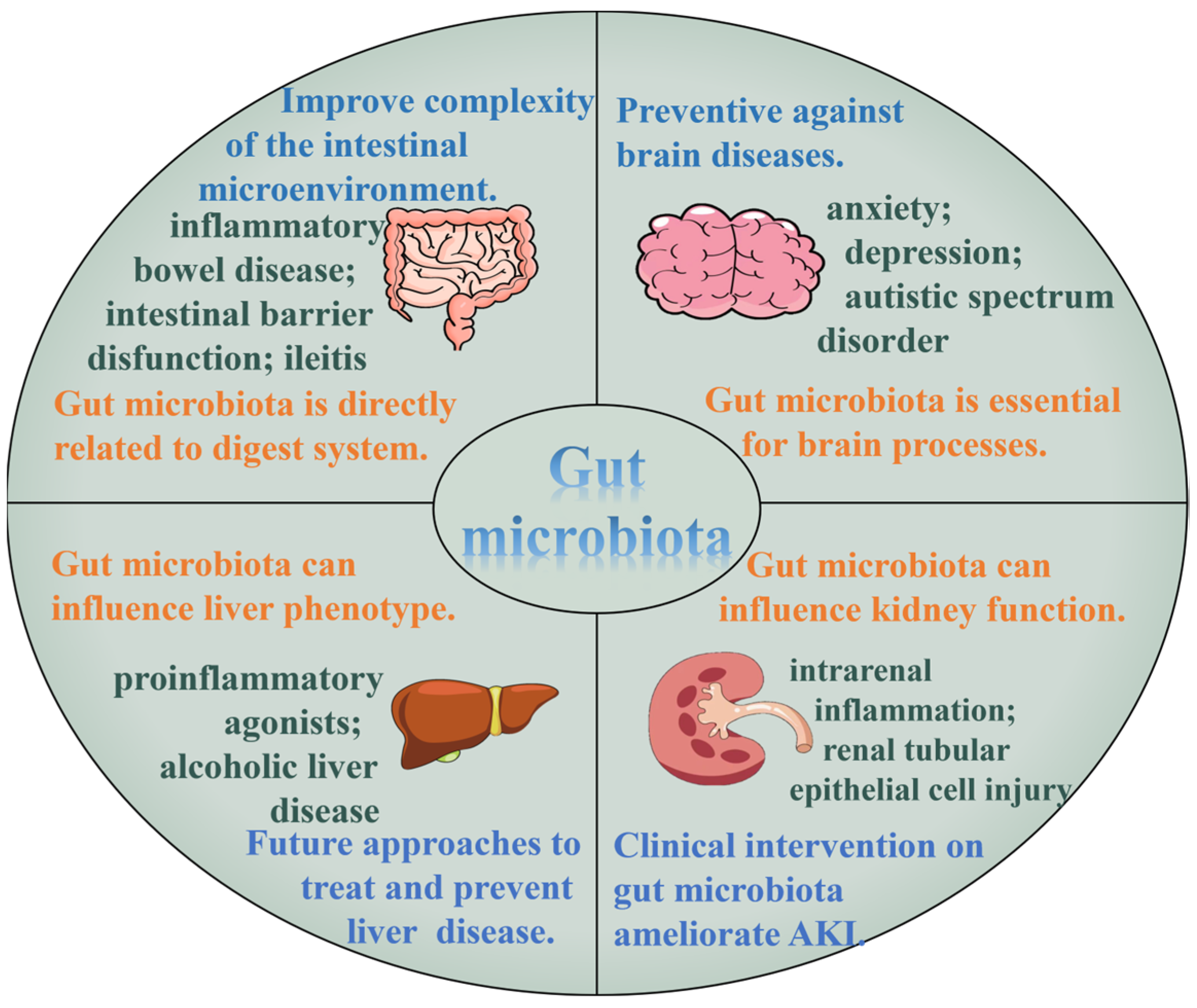
3.5. Action on Related Tissue and Organs
4. Structure–Activity Relationship of the Anti-Diabetic Effects of Polysaccharides through Regulating Gut Microbiota
4.1. Monosaccharide Composition
4.2. Molecular Weight
4.3. Types of Glycosidic Linkage
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef] [PubMed]
- Craciun, C.I.; Neag, M.A.; Catinean, A.; Mitre, A.O.; Rusu, A.; Bala, C.; Roman, G.; Buzoianu, A.D.; Muntean, D.M.; Craciun, A.E. The Relationships between Gut Microbiota and Diabetes Mellitus, and Treatments for Diabetes Mellitus. Biomedicines 2022, 10, 308. [Google Scholar] [CrossRef]
- Giovannini, P.; Howes, M.J.R.; Edwards, S.E. Medicinal plants used in the traditional management of diabetes and its sequelae in Central America: A review. J. Ethnopharmacol. 2016, 184, 58–71. [Google Scholar] [CrossRef]
- Baek, S.M.; Kim, K.; Kim, S.; Son, Y.; Hong, H.S.; Yu, S.Y. SP prevents T2DM complications by immunomodulation. Sci. Rep. 2020, 10, 16753. [Google Scholar] [CrossRef]
- Yi, X.R.; Dong, M.S.; Guo, N.F.; Tian, J.L.; Lei, P.; Wang, S.; Yang, Y.F.; Shi, Y. Flavonoids improve type 2 diabetes mellitus and its complications: A review. Front. Nutr. 2023, 10, 1192131. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zeng, W.Z.; Zhang, X.; Yang, J.F. Characterization of Acidic Tea Polysaccharides from Yellow Leaves of Wuyi Rock Tea and Their Hypoglycemic Activity via Intestinal Flora Regulation in Rats. Foods 2022, 11, 617. [Google Scholar] [CrossRef]
- Perreault, L.; Pan, Q.; Aroda, V.R.; Barrett-Connor, E.; Dabelea, D.; Dagogo-Jack, S.; Hamman, R.F.; Kahn, S.E.; Mather, K.J.; Knowler, W.C.; et al. Exploring residual risk for diabetes and microvascular disease in the Diabetes Prevention Program Outcomes Study (DPPOS). Diabet. Med. 2017, 34, 1747–1755. [Google Scholar] [CrossRef]
- Pagano, G.; Polychronis, S.; Wilson, H.; Giordano, B.; Ferrara, N.; Niccolini, F.; Politis, M. Diabetes mellitus and Parkinson disease. Neurology 2018, 90, e1654–e1662. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.M. Diabetes Mellitus and Cardiovascular Disease Emerging Therapeutic Approaches. Arter. Throm. Vas. 2019, 39, 558–568. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, J.; Zheng, Y.; Xu, Z.; Li, Y.; Zhou, S.; Zhang, C. Beneficial effects of polysaccharide-rich extracts from Apocynum venetum leaves on hypoglycemic and gut microbiota in type 2 diabetic mice. Biomed. Pharmacother. 2020, 127, 110182. [Google Scholar] [CrossRef]
- Zhang, J.H.; Wang, W.J.; Guo, D.Y.; Bai, B.Q.; Bo, T.; Fan, S.H. Antidiabetic Effect of Millet Bran Polysaccharides Partially Mediated via Changes in Gut Microbiome. Foods 2022, 11, 3406. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.F.; Ye, H.; Zhu, Y.J.; Li, Y.P.; Wang, J.F.; Wang, P. Characterization and Hypoglycemic Activity of a Rhamnan-Type Sulfated Polysaccharide Derivative. Mar. Drugs 2019, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- Kholiya, F.; Chatterjee, S.; Bhojani, G.; Sen, S.; Barkume, M.; Kasinathan, N.K.; Kode, J.; Meena, R. Seaweed polysaccharide derived bioaldehyde nanocomposite: Potential application in anticancer therapeutics. Carbohydr. Polym. 2020, 240, 116282. [Google Scholar] [CrossRef] [PubMed]
- Roszczyk, A.; Turlo, J.; Zagozdzon, R.; Kaleta, B. Immunomodulatory Properties of Polysaccharides from Lentinula edodes. Int. J. Mol. Sci. 2022, 23, 8980. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Shi, Y.Q.; Yuan, Z.X.; Huang, Z.N.; Cai, F.H.; Zhu, J.F.; Zhang, W.W.; Li, J.; Xiong, Q.P.; Wang, Y.P.; et al. Isolation, Identification, and Anti-Inflammatory Activity of Polysaccharides of Typha angustifolia. Biomacromolecules 2021, 22, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.K.; Yu, H.L.; Liu, B.; Wang, H.; Luo, Q.; Ding, X.G. Antioxidative mechanism of Lycium barbarum polysaccharides promotes repair and regeneration following cavernous nerve injury. Neural Regen. Res. 2016, 11, 1312–1321. [Google Scholar] [PubMed]
- Fan, Y.M.; Zhou, X.F.; Huang, G.L. Preparation, structure, and properties of tea polysaccharide. Chem. Biol. Drug Des. 2022, 99, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Khalil, A.A.; Shaukat, F.; Song, Y.D. Sources, Extraction and Biomedical Properties of Polysaccharides. Foods 2019, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Alba, K.; Dimopoulou, M.; Kontogiorgos, V. Baobab polysaccharides as emulsifiers. LWT 2021, 144, 111235. [Google Scholar] [CrossRef]
- Sun, B.N.; Yu, S.; Zhao, D.Y.; Guo, S.H.; Wang, X.H.; Zhao, K. Polysaccharides as vaccine adjuvants. Vaccine 2018, 36, 5226–5234. [Google Scholar] [CrossRef]
- Yang, Z.J.; Liu, W.W.; Liu, H.M.; Li, R.; Chang, L.; Kan, S.N.; Hao, M.; Wang, D.X. The applications of polysaccharides in dentistry. Front. Bioeng. Biotech. 2022, 10, 970041. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yan, L.J.; Chen, H.; Wu, N.; Wang, W.B.; Wang, D.S. Cyclocarya paliurus polysaccharides alleviate type 2 diabetic symptoms by modulating gut microbiota and short-chain fatty acids. Phytomedicine 2020, 77, 153268. [Google Scholar] [CrossRef] [PubMed]
- Blandino, G.; Inturri, R.; Lazzara, F.; Di Rosa, M.; Malaguarnera, L. Impact of gut microbiota on diabetes mellitus. Diabetes Metab. 2016, 42, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Hermann-Bank, M.L.; Skovgaard, K.; Stockmarr, A.; Larsen, N.; Molbak, L. The Gut Microbiotassay: A high-throughput qPCR approach combinable with next generation sequencing to study gut microbial diversity. BMC Genom. 2013, 14, 788. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.J.; Wang, Y.Z.; Gong, T. The interplay between oral microbiota, gut microbiota and systematic diseases. J. Oral. Microbiol. 2023, 15, 2213112. [Google Scholar] [CrossRef] [PubMed]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; FitzGerald, M.G.; Fulton, R.S.; et al. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Li, S.; Gan, R.Y.; Zhou, T.; Xu, D.P.; Li, H.B. Impacts of Gut Bacteria on Human Health and Diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef]
- Fan, H.X.; Liu, X.C.; Ren, Z.Y.; Fei, X.N.; Luo, J.; Yang, X.Y.; Xue, Y.Y.; Zhang, F.F.; Liang, B. Gut microbiota and cardiac arrhythmia. Front. Cell Infect. Microbiol. 2023, 13, 1147687. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.W.; Dai, L.L.; Zhao, Q.; Zhang, X. A review on the effect of gut microbiota on metabolic diseases. Arch. Microbiol. 2022, 204, 192. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kirabo, A. Salt and Gut Microbiota in Heart Failure. Curr. Hypertens. Rep. 2023, 25, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Mulak, A.; Bonaz, B. Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroenterol. 2015, 21, 10609–10620. [Google Scholar] [CrossRef] [PubMed]
- AL-Ishaq, R.K.; Samuel, S.M.; Büsselberg, D. The Influence of Gut Microbial Species on Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 8118. [Google Scholar] [CrossRef]
- Bajinka, O.; Tan, Y.R.; Darboe, A.; Ighaede-Edwards, I.G.; Abdelhalim, K.A. The gut microbiota pathway mechanisms of diabetes. Amb. Express 2023, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Xiao, X.Y.; Wang, S.; Shi, F.X.; Liu, N.; Chen, X.F.; Yang, W.J.; Wang, L.; Chen, F.X. Hypoglycemic effect of astragaloside IV via modulating gut microbiota and regulating AMPK/SIRT1 and PI3K/AKT pathway. J. Ethnopharmacol. 2021, 281, 114558. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.T.; Tian, S.H.; Sun, J.; Pang, X.Y.; Hu, Q.B.; Li, X.F.; Lu, Y.J. Broccoli microgreens have hypoglycemic effect by improving blood lipid and inflammatory factors while modulating gut microbiota in mice with type 2 diabetes. J. Food Biochem. 2022, 46, e14145. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Wang, X.; Jiang, H.; Cai, C.; Li, G.Y.; Hao, J.J.; Yu, G.L. Marine polysaccharides attenuate metabolic syndrome by fermentation products and altering gut microbiota: An overview. Carbohydr. Polym. 2018, 195, 601–612. [Google Scholar] [CrossRef]
- Xie, G.X.; Zhong, W.; Zheng, X.J.; Li, Q.; Qiu, Y.P.; Li, H.K.; Chen, H.Y.; Zhou, Z.X.; Jia, W. Chronic Ethanol Consumption Alters Mammalian Gastrointestinal Content Metabolites. J. Proteome Res. 2013, 12, 3297–3306. [Google Scholar] [CrossRef] [PubMed]
- Sam, Q.H.; Ling, H.; Yew, W.S.; Tan, Z.H.; Ravikumar, S.; Chang, M.W.; Chai, L.Y.A. The Divergent Immunomodulatory Effects of Short Chain Fatty Acids and Medium Chain Fatty Acids. Int. J. Mol. Sci. 2021, 22, 6453. [Google Scholar] [CrossRef]
- Alvarez-Mercado, A.I.; Plaza-Diaz, J. Dietary Polysaccharides and Gut Microbiota Ecosystem. Nutrients 2022, 14, 4285. [Google Scholar] [CrossRef]
- Riedl, R.A.; Atkinson, S.N.; Burnett, C.M.L.; Grobe, J.L.; Kirby, J.R. The Gut Microbiome, Energy Homeostasis, and Implications for Hypertension. Curr. Hypertens. Rep. 2017, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, R.L.; Xue, Z.H.; Li, S.Q.; Zhou, J.N.; Liu, J.Y.; Zhang, M.; Panichayupakaranant, P.; Chen, H.X. Mulberry leaf polysaccharides ameliorate obesity through activation of brown adipose tissue and modulation of the gut microbiota in high-fat diet fed mice. Food Funct. 2022, 13, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.H.; Ma, Q.Q.; Chen, Y.; Lu, Y.P.; Wang, Y.J.; Jia, Y.N.; Zhang, M.; Chen, H.X. Structure characterization of soluble dietary fiber fractions from mushroom Lentinula edodes (Berk.) Pegler and the effects on fermentation and human gut microbiota in vitro. Food Res. Int. 2020, 129, 108870. [Google Scholar] [CrossRef] [PubMed]
- Jiao, R.; Liu, Y.X.; Gao, H.; Xiao, J.; So, K.F. The Anti-Oxidant and Antitumor Properties of Plant Polysaccharides. Am. J. Chin. Med. 2016, 44, 463–488. [Google Scholar] [CrossRef]
- Ge, Y.Z.; Ahmed, S.; Yao, W.Z.; You, L.J.; Zheng, J.X.; Hileuskaya, K. Regulation effects of indigestible dietary polysaccharides on intestinal microflora: An overview. J. Food Biochem. 2021, 45, e13564. [Google Scholar] [CrossRef]
- Xu, X.F.; Xu, P.P.; Ma, C.W.; Tang, J.; Zhang, X.W. Gut microbiota, host health, and polysaccharides. Biotechnol. Adv. 2013, 31, 318–337. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Liu, W.; Lin, Y.X.; Zhang, S.B.; Zou, B.R.; Xiao, D.; Lin, L.; Zhong, Y.P.; Zheng, H.H.; Liao, Q.F.; et al. Compound polysaccharides ameliorate experimental colitis by modulating gut microbiota composition and function. J. Gastroenterol. Hepatol. 2019, 34, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Bo, R.; Liu, Z.; Zhang, J.; Gu, P.; Ou, N.; Sun, Y.; Hu, Y.; Liu, J.; Wang, D. Mechanism of Lycium barbarum polysaccharides liposomes on activating murine dendritic cells. Carbohydr. Polym. 2019, 205, 540–549. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, H.; Zong, X.; Li, S.; Wang, J.; Wang, Y.; Jin, M. Polysaccharides from Auricularia auricula: Preparation, structural features and biological activities. Carbohydr. Polym. 2020, 247, 116750. [Google Scholar] [CrossRef]
- Gong, P.X.; Wu, Y.C.; Liu, Y.; Lv, S.Z.; You, Y.; Zhou, Z.L.; Chen, X.; Li, H.J. Structure and hypoglycemic effect of a neutral polysaccharide isolated from sea cucumber Stichopus japonicus. Int. J. Biol. Macromol. 2022, 216, 14–23. [Google Scholar] [CrossRef]
- Guidara, M.; Yaich, H.; Amor, I.B.; Fakhfakh, J.; Gargouri, J.; Lassoued, S.; Blecker, C.; Richel, A.; Attia, H.; Garna, H. Effect of extraction procedures on the chemical structure, antitumor and anticoagulant properties of ulvan from Ulva lactuca of Tunisia coast. Carbohydr. Polym. 2021, 253, 117283. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of xanthan gum as polysaccharide in tissue engineering: A review. Carbohydr. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.V.; Zhang, X.; Huttunen, E.; Widmalm, G. Structural studies of the capsular polysaccharide produced by Leuconostoc mesenteroides ssp. cremoris PIA2. Biomacromolecules 2011, 12, 2496–2501. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.; Zhang, Y.; Jin, X.; Zhu, Y.; Li, L.; Huang, X.; Wang, D.; Lin, Z. Structure and hepatoprotective activity of Usp10/NF-kappaB/Nrf2 pathway-related Morchella esculenta polysaccharide. Carbohydr. Polym. 2023, 303, 120453. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.H.; Xie, F.; Tan, J.; Yuan, Y.; Mei, H.; Zheng, Y.; Sheng, R. Extraction, structure and pharmacological effects of the polysaccharides from Cordyceps sinensis: A review. J. Funct. Foods 2022, 89, 104909. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, X.X.; Chen, T.T.; Chen, X.Q. A review of the antibacterial activity and mechanisms of plant polysaccharides. Trends Food Sci. Tech. 2022, 123, 264–280. [Google Scholar] [CrossRef]
- Zhou, J.N.; Li, R.L.; Jia, Y.A.; Wang, Y.J.; Liu, J.Y.; Panichayupakaranant, P.; Chen, H.X. Recent Progress in Natural Anticancer Agents Discovery from Tea (Camellia sinensis): A Review. Recent. Pat. Anti-Cancer 2022, 17, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.X.; Chen, Z.Y.; Zhou, H.; Yu, C.; Han, Z.; Shao, S.R.; Hu, X.C.; Wei, X.L.; Wang, Y.F. Effects of extraction methods on physicochemical properties and hypoglycemic activities of polysaccharides from coarse green tea. Glycoconj. J. 2020, 37, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Alba, K.; Offiah, V.; Laws, A.P.; Falade, K.O.; Kontogiorgos, V. Baobab polysaccharides from fruits and leaves. Food Hydrocoll. 2020, 106, 105874. [Google Scholar] [CrossRef]
- Xu, L.L.; Chen, Y.; Chen, Z.Q.; Gao, X.D.; Wang, C.L.; Panichayupakaranant, P.; Chen, H.X. Ultrafiltration isolation, physicochemical characterization, and antidiabetic activities analysis of polysaccharides from green tea, oolong tea, and black tea. J. Food Sci. 2020, 85, 4025–4032. [Google Scholar] [CrossRef]
- Guilbaud, A.; Howsam, M.; Niquet-Léridon, C.; Delguste, F.; Fremont, M.; Lestavel, S.; Maboudou, P.; Garat, A.; Schraen, S.; Onraed, B.; et al. The Effect of Lactobacillus fermentum ME-3 Treatment on Glycation and Diabetes Complications. Mol. Nutr. Food Res. 2020, 64, e1901018. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Zhang, Y.C.; Zhang, Y.; Liu, Y.; Wang, S.Q.; Dong, X.M.; Wang, Y.Y.; Zhang, H.P. Screening of potential probiotic properties of Lactobacillus fermentum isolated from traditional dairy products. Food Control 2010, 21, 695–701. [Google Scholar] [CrossRef]
- Saavedra, J.M.; Bauman, N.A.; Perman, J.A.; Yolken, R.H.; Saavedra, J.M.; Bauman, N.A.; Oung, I. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet 1994, 344, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Makras, L.; Triantafyllou, V.; Fayol-Messaoudi, D.; Adriany, T.; Zoumpopoulou, G.; Tsakalidou, E.; Servin, A.; De Vuyst, L. Kinetic analysis of the antibacterial activity of probiotic lactobacilli towards Salmonella enterica serovar Typhimurium reveals a role for lactic acid and other inhibitory compounds. Res. Microbiol. 2006, 157, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Tan, F.; Yi, R.K.; Mu, J.F.; Zhao, X.; Yang, Z.N. Effects of Lactobacillus on Mice with Diabetes Induced by High-Fat Diet with Streptozotocin (STZ). Appl. Sci. 2018, 8, 1249. [Google Scholar] [CrossRef]
- Din, A.U.; Hassan, A.; Zhu, Y.; Zhang, K.; Wang, Y.; Li, T.; Wang, Y.; Wang, G. Inhibitory effect of Bifidobacterium bifidum ATCC 29521 on colitis and its mechanism. J. Nutr. Biochem. 2020, 79, 108353. [Google Scholar] [CrossRef] [PubMed]
- Fernando, W.M.A.D.B.; Flint, S.H.; Ranaweera, K.K.D.S.; Bamunuarachchi, A.; Johnson, S.K.; Brennan, C.S. The potential synergistic behaviour of inter- and intra-genus probiotic combinations in the pattern and rate of short chain fatty acids formation during fibre fermentation. Int. J. Food Sci. Nutr. 2018, 69, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xiao, D.; Liu, W.; Song, Y.; Zou, B.; Li, L.; Li, P.; Cai, Y.; Liu, D.; Liao, Q.; et al. Intake of Ganoderma lucidum polysaccharides reverses the disturbed gut microbiota and metabolism in type 2 diabetic rats. Int. J. Biol. Macromol. 2020, 155, 890–902. [Google Scholar] [CrossRef]
- Li, S.; Qi, Y.; Chen, L.; Qu, D.; Li, Z.; Gao, K.; Chen, J.; Sun, Y. Effects of Panax ginseng polysaccharides on the gut microbiota in mice with antibiotic-associated diarrhea. Int. J. Biol. Macromol. 2019, 124, 931–937. [Google Scholar] [CrossRef]
- Yang, L.N.; Huang, J.H.; Wu, X.H.; Li, L.; Cai, W.Q.; Zhu, L.J.; Wang, S.N.; Song, H.; Zhu, D.S.; Ma, T.; et al. Interactions between gut microbiota and soy hull polysaccharides regulate the air-liquid interfacial activity. Food Hydrocoll. 2021, 119, 106704. [Google Scholar] [CrossRef]
- Turroni, F.; Milani, C.; Duranti, S.; Mahony, J.; van Sinderen, D.; Ventura, M. Glycan Utilization and Cross-Feeding Activities by Bifidobacteria. Trends Microbiol. 2018, 26, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Guo, L.X.; Hu, W.H.; Peng, Z.T.; Wang, C.; Chen, Z.C.; Liu, E.Y.L.; Dong, T.T.X.; Wang, T.J.; Tsim, K.W.K. Polysaccharide from tuberous roots of Ophiopogon japonicus regulates gut microbiota and its metabolites during alleviation of high-fat diet-induced type-2 diabetes in mice. J. Funct. Foods 2019, 63, 103593. [Google Scholar] [CrossRef]
- Li, J.; Pang, B.; Yan, X.; Shang, X.; Hu, X.; Shi, J. Prebiotic properties of different polysaccharide fractions from Artemisia sphaerocephala Krasch seeds evaluated by simulated digestion and in vitro fermentation by human fecal microbiota. Int. J. Biol. Macromol. 2020, 162, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.L.; Lu, J.M.; Wang, Y.F.; Gu, W.; Yang, X.X.; Yu, J. Intake of total saponins and polysaccharides from Polygonatum kingianum affects the gut microbiota in diabetic rats. Phytomedicine 2017, 26, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.J.; Zhang, M.N.; Wang, X.N.; Ren, Y.C.; Yue, T.L.; Wang, Z.L.; Gao, Z.P. Edible fungal polysaccharides, the gut microbiota, and host health. Carbohydr. Polym. 2021, 273, 118558. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.A.; Li, L.; Wu, X.H.; Cai, W.Q.; Lin, Q.; Zhu, D.S. The Effect of Natural Soluble Polysaccharides on the Type 2 Diabetes through Modulating Gut Microbiota: A Review. Curr. Med. Chem. 2021, 28, 5368–5385. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.U.; Khan, A.I.; Xin, Y.; Liang, W. Morchella esculenta polysaccharide attenuate obesity, inflammation and modulate gut microbiota. Amb. Express 2022, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Meng, C.; Chen, W.J.; Chen, H.M.; Chen, W.X. Ganoderma amboinense polysaccharide prevents obesity by regulating gut microbiota in high-fat-diet mice. Food Biosci. 2021, 42, 101107. [Google Scholar] [CrossRef]
- Su, A.X.; Ma, G.X.; Ma, N.; Pei, F.; Yang, W.J.; Hu, Q.H. Effects of Flammulina velutipes polysaccharides on gut microbiota composition and metabolism in vitro fermentation. Food Sci. Biotechnol. 2023, 32, 361–369. [Google Scholar] [CrossRef]
- Zhong, L.; Ma, N.; Zheng, H.H.; Ma, G.X.; Zhao, L.Y.; Hu, Q.H. Tuber indicum polysaccharide relieves fatigue by regulating gut microbiota in mice. J. Funct. Foods 2019, 63, 103580. [Google Scholar] [CrossRef]
- Yu, R.X.; Luo, J.M.; Liu, L.; Peng, X.C.; Gan, R.Y.; Wu, D.T.; Hu, Y.C. Hypoglycemic Effect of Edible Fungi Polysaccharides Depends on Their Metabolites from the Fermentation of Human Fecal Microbiota. Foods 2024, 13, 103580. [Google Scholar] [CrossRef]
- Fuller, R.; Moore, M.V.; Lewith, G.; Stuart, B.L.; Ormiston, R.V.; Fisk, H.L.; Noakes, P.S.; Calder, P.C. Yeast-derived β-1,3/1,6 glucan, upper respiratory tract infection and innate immunity in older adults. Nutrition 2017, 39–40, 30–35. [Google Scholar] [CrossRef]
- Gudi, R.; Perez, N.; Johnson, B.M.; Sofi, M.H.; Brown, R.; Quan, S.; Karumuthil-Melethil, S.; Vasu, C. Complex dietary polysaccharide modulates gut immune function and microbiota, and promotes protection from autoimmune diabetes. Immunology 2019, 157, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.L.; Deng, J.C.; Pan, Y.Y.; Xu, J.X.; Hong, J.L.; Shi, F.F.; Liu, G.L.; Qian, M.; Bai, W.D.; Zhang, W.; et al. Hypoglycemic and hypolipidemic activities of Grifola frondosa polysaccharides and their relationships with the modulation of intestinal microflora in diabetic mice induced by high-fat diet and streptozotocin. Int. J. Biol. Macromol. 2020, 153, 1231–1240. [Google Scholar] [CrossRef]
- Ouyang, Y.Z.; Nie, J.P.; Gao, X.X.; Zhao, C. Grifola Frondosa Polysaccharide Ameliorates Hyperglycemia and Gut Microbiota in Type 2 Diabetic Mice. Free Radic. Biol. Med. 2022, 180, s65. [Google Scholar] [CrossRef]
- Fu, C.J.; Ye, K.; Ma, S.; Du, H.J.; Chen, S.G.; Liu, D.H.; Ma, G.X.; Xiao, H. Simulated gastrointestinal digestion and gut microbiota fermentation of polysaccharides from Agaricus bisporus. Food Chem. 2023, 418, 135849. [Google Scholar] [CrossRef]
- Tsung-Ru, W.; Chuan-Sheng, L.; Chih-Jung, C.; Tzu-Lung, L.; Jan, M.; Yun-Fei, K.; David, M.O.; Chia-Chen, L.; John, D.Y.; Hsin-Chih, L. Gut commensal Parabacteroides goldsteiniiplays plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut 2019, 68, 248. [Google Scholar]
- Chen, X.X.; Chen, C.; Fu, X. Hypoglycemic effect of the polysaccharides from Astragalus membranaceus on type 2 diabetic mice based on the “gut microbiota-mucosal barrier”. Food Funct. 2022, 13, 10121–10133. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Han, L.J.; Raza, S.H.A.; Yue, Q.M.; Sun, S.N.; Zhao, Y.X.; Lv, L.F.; Deng, Y.R.; Yuan, Z.Z.; Alsharif, I.; et al. Polysaccharides in Berberis dasystachya improve intestinal flora depending on the molecular weight and ameliorate type 2 diabetes in rats. J. Funct. Foods 2023, 100, 105381. [Google Scholar] [CrossRef]
- Liu, G.D.; Feng, S.M.; Yan, J.D.; Luan, D.; Sun, P.L.; Shao, P. Antidiabetic potential of polysaccharides from Brasenia schreberi regulating insulin signaling pathway and gut microbiota in type 2 diabetic mice. Curr. Res. Food Sci. 2022, 5, 1465–1474. [Google Scholar] [CrossRef]
- Li, H.S.; Fang, Q.Y.; Nie, Q.X.; Hu, J.L.; Yang, C.; Huang, T.; Li, H.; Nie, S.P. Hypoglycemic and Hypolipidemic Mechanism of Tea Polysaccharides on Type 2 Diabetic Rats via Gut Microbiota and Metabolism Alteration. J. Agric. Food Chem. 2020, 68, 10015–10028. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Liu, C.S.; Hu, Y.N.; Luo, Z.Y.; Chen, F.L.; Yuan, L.X.; Tan, X.M. Coix seed polysaccharides alleviate type 2 diabetes mellitus via gut microbiota-derived short-chain fatty acids activation of IGF1/PI3K/ AKT signaling. Food Res. Int. 2021, 150, 110717. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.Y.; Zhai, R.H.; Xie, X.Q.; Chen, T.; Zhang, Z.Q.; Liu, H.C.; Nie, C.X.; Yuan, X.J.; Tu, A.B.; Tian, B.M.; et al. Hypoglycemic Effects of Lycium barbarum Polysaccharide in Type 2 Diabetes Mellitus Mice Modulating Gut Microbiota. Front. Nutr. 2022, 9, 916271. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Liang, Q.X.; Niu, Y.; Jiang, S.J.; Zhou, L.; Wang, J.M.; Ma, C.Y.; Kang, W.Y. Effects of Nigella sativa seed polysaccharides on type 2 diabetic mice and gut microbiota. Int. J. Biol. Macromol. 2020, 159, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, L.C.; Zhu, M.M.; Yang, B.; Yang, Y.J.; Jia, X.B.; Feng, L. Moutan Cortex polysaccharide ameliorates diabetic kidney disease via modulating gut microbiota dynamically in rats. Int. J. Biol. Macromol. 2022, 206, 849–860. [Google Scholar] [CrossRef]
- Xu, N.; Zhou, Y.J.; Lu, X.Y.; Chang, Y.N. Auricularia auricula-judae (Bull.) polysaccharides improve type 2 diabetes in HFD/STZ-induced mice by regulating the AKT/AMPK signaling pathways and the gut microbiota. J. Food Sci. 2021, 86, 5479–5494. [Google Scholar] [CrossRef]
- Shao, W.M.; Xiao, C.; Yong, T.Q.; Zhang, Y.F.; Hu, H.P.; Xie, T.; Liu, R.J.; Huang, L.H.; Li, X.M.; Xie, Y.Z.; et al. A polysaccharide isolated from Ganoderma lucidum ameliorates hyperglycemia through modulating gut microbiota in type 2 diabetic mice. Int. J. Biol. Macromol. 2022, 197, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, M.; Liu, L.; Li, D.; Zhao, L.; Wu, Z.; Zhou, M.; Jia, L.; Yang, F. Cordyceps militaris polysaccharide alleviates diabetic symptoms by regulating gut microbiota against TLR4/NF-κB pathway. Int. J. Biol. Macromol. 2023, 230, 123241. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Liu, D.; Wang, D.Y.; Lai, S.S.; Zhong, R.T.; Liu, Y.Y.; Yang, C.F.; Liu, B.; Sarker, M.R.; Zhao, C. Hypoglycemic activity and gut microbiota regulation of a novel polysaccharide from Grifola frondosa in type 2 diabetic mice. Food Chem. Toxicol. 2019, 126, 295–302. [Google Scholar] [CrossRef]
- Rehman, A.U.; Siddiqui, N.Z.; Farooqui, N.A.; Alam, G.; Gul, A.; Ahmad, B.; Asim, M.; Khan, A.I.; Xin, Y.; Zexu, W.; et al. Morchella esculenta mushroom polysaccharide attenuates diabetes and modulates intestinal permeability and gut microbiota in a type 2 diabetic mice model. Front. Nutr. 2022, 9, 984695. [Google Scholar] [CrossRef]
- Xue, Z.H.; Li, R.L.; Liu, J.Y.; Zhou, J.N.; Zhang, X.Y.; Zhang, T.T.; Zhang, M.; Yang, Y.; Chen, H.X. Preventive and synbiotic effects of the soluble dietary fiber obtained from Lentinula edodes byproducts and Lactobacillus plantarum against dextran sulfate sodium-induced colitis in mice. J. Sci. Food Agric. 2023, 103, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Oh, S.; Kim, S.Y.; Ahn, H.; Son, M.; Heo, S.J.; Byun, K.; Jeon, Y.J. Anti-obesity effects of Ishophloroglucin A from the brown seaweed Ishige okamurae (Yendo) regulation of leptin signal in ob/ob mice. Algal Res. 2022, 61, 102533. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, M.; Park, M.H. Diphlorethohydroxycamalol isolated from Ishige okamurae prevents H2O2-induced oxidative damage via BMP2/Runx2 signaling in osteoblastic MC3T3-E1 cells. Fitoterapia 2021, 152, 104921. [Google Scholar] [CrossRef]
- Xiao, Z.B.; Yang, S.T.; Liu, Y.; Zhou, C.X.; Hong, P.Z.; Sun, S.L.; Qian, Z.J. A novel glyceroglycolipid from brown algae Ishige okamurae improve photoaging and counteract inflammation in UVB-induced HaCaT cells. Chem.-Biol. Interact. 2022, 351, 109737. [Google Scholar] [CrossRef]
- Yang, H.W.; Fernando, K.H.N.; Oh, J.Y.; Li, X.; Jeon, Y.J.; Ryu, B. Anti-Obesity and Anti-Diabetic Effects of Ishige okamurae. Mar. Drugs 2019, 17, 202. [Google Scholar] [CrossRef] [PubMed]
- Usoltseva, R.V.; Anastyuk, S.D.; Shevchenko, N.M.; Surits, V.V.; Silchenko, A.S.; Isakov, V.V.; Zvyagintseva, T.N.; Thinh, P.D.; Ermakova, S.P. Polysaccharides from brown algae Sargassum duplicatum: The structure and anticancer activity. Carbohydr. Polym. 2017, 175, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.W.; Zhou, T.S.; Qiu, W.H.; Wang, Y.K.; Xu, Q.L.; Ke, S.Z.; Wang, S.J.; Jin, W.H.; Chen, J.W.; Zhang, H.W.; et al. Characterization and hypoglycemic effects of sulfated polysaccharides derived from brown seaweed. Food Chem. 2021, 341, 128148. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Cha, J.D.; Choi, K.M.; Lee, K.Y.; Han, K.M.; Jang, Y.S. Fucoidan inhibits LPS-induced inflammation in vitro and during the acute response in vivo. Int. Immunopharmacol. 2017, 43, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Pang, D.R.; Wen, L.R.; You, L.J.; Huang, R.M.; Kulikouskaya, V. In vitro digestibility and prebiotic activities of a sulfated polysaccharide from Gracilaria Lemaneiformis. J. Funct. Foods 2020, 64, 103652. [Google Scholar] [CrossRef]
- Mou, J.J.; Li, Q.; Shi, W.W.; Qi, X.H.; Song, W.G.; Yang, J. Chain conformation, physicochemical properties of fucosylated chondroitin sulfate from sea cucumber Stichopus chloronotus and its in vitro fermentation by human gut microbiota. Carbohydr. Polym. 2020, 228, 115359. [Google Scholar] [CrossRef]
- Seong, H.; Bae, J.H.; Seo, J.S.; Kim, S.A.; Kim, T.J.; Han, N.S. Comparative analysis of prebiotic effects of seaweed polysaccharides laminaran, porphyran, and ulvan using in vitro human fecal fermentation. J. Funct. Foods 2019, 57, 408–416. [Google Scholar] [CrossRef]
- Pratap, K.; Majzoub, M.E.; Taki, A.C.; Hernandez, S.M.; Magnusson, M.; Glasson, C.R.K.; de Nys, R.; Thomas, T.; Lopata, A.L.; Kamath, S.D. The Algal Polysaccharide Ulvan and Carotenoid Astaxanthin Both Positively Modulate Gut Microbiota in Mice. Foods 2022, 11, 565. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Yan, C.H.; Lin, X.P.; Ai, C.Q.; Dong, X.P.; Shao, L.; Wang, S.T.; Song, S.; Zhu, B.W. Responses of the gut microbiota and metabolite profiles to sulfated polysaccharides from sea cucumber in humanized microbiota mice. Food Funct. 2022, 13, 4171–4183. [Google Scholar] [CrossRef]
- Zhu, Z.J.; Zhu, B.W.; Sun, Y.J.; Ai, C.Q.; Wang, L.L.; Wen, C.R.; Yang, J.F.; Song, S.; Liu, X.L. Sulfated Polysaccharide from Sea Cucumber and its Depolymerized Derivative Prevent Obesity in Association with Modification of Gut Microbiota in High-Fat Diet-Fed Mice. Mol. Nutr. Food Res. 2018, 62, e1800446. [Google Scholar] [CrossRef]
- Wei, B.; Zhong, Q.-W.; Ke, S.-Z.; Zhou, T.-S.; Xu, Q.-L.; Wang, S.-J.; Chen, J.-W.; Zhang, H.-W.; Jin, W.-H.; Wang, H. Sargassum fusiforme Polysaccharides Prevent High-Fat Diet-Induced Early Fasting Hypoglycemia and Regulate the Gut Microbiota Composition. Mar. Drugs 2020, 18, 444. [Google Scholar] [CrossRef]
- Lin, H.T.; Zhang, J.W.; Li, S.Y.; Zheng, B.D.; Hu, J.M. Polysaccharides isolated from Laminaria japonica attenuates gestational diabetes mellitus by regulating the gut microbiota in mice. Food Front. 2021, 2, 208–217. [Google Scholar] [CrossRef]
- Siddiqui, N.Z.; Rehman, A.U.; Yousuf, W.; Khan, A.I.; Farooqui, N.A.; Zang, S.Z.; Xin, Y.; Wang, L. Effect of crude polysaccharide from seaweed, Dictyopteris divaricata (CDDP) on gut microbiota restoration and anti-diabetic activity in streptozotocin (STZ)-induced T1DM mice. Gut Pathog. 2022, 14, 39. [Google Scholar] [CrossRef]
- Zhao, F.Q.; Liu, Q.B.; Cao, J.; Xu, Y.S.; Pei, Z.S.; Fan, H.F.; Yuan, Y.Q.; Shen, X.R.; Li, C. A sea cucumber (Holothuria leucospilota) polysaccharide improves the gut microbiome to alleviate the symptoms of type 2 diabetes mellitus in Goto-Kakizaki rats. Food Chem. Toxicol. 2020, 135, 110886. [Google Scholar] [CrossRef]
- Ruan, Q.L.; Chen, Y.H.; Wen, J.H.; Qiu, Y.H.; Huang, Y.J.; Zhang, Y.; Farag, M.A.; Zhao, C. Regulatory mechanisms of the edible alga Ulva lactuca polysaccharide via modulation of gut microbiota in diabetic mice. Food Chem. 2023, 409, 135287. [Google Scholar] [CrossRef]
- Jia, R.B.; Li, Z.R.; Lin, L.Z.; Luo, D.H.; Chen, C.; Zhao, M.M. The potential mechanisms of Macrocystis pyrifera polysaccharides mitigating type 2 diabetes in rats. Food Funct. 2022, 13, 7918–7929. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Song, P.L.; Yin, S.; Fan, T.Y.; Li, F.W.; Ge, X.D.; Liu, T.T.; Xu, W.; Xu, S.; Chen, L.G. Onchidium struma polysaccharides exhibit hypoglycemic activity and modulate the gut microbiota in mice with type 2 diabetes mellitus. Food Funct. 2023, 14, 1937–1951. [Google Scholar] [CrossRef]
- Zhao, L.P.; Zhang, F.; Ding, X.Y.; Wu, G.J.; Lam, Y.Y.; Wang, X.J.; Fu, H.Q.; Xue, X.H.; Lu, C.H.; Ma, J.L.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Sun, Z.Y.; Yu, S.; Tian, Y.; Han, B.Q.; Zhao, Y.; Li, Y.Q.; Wang, Y.; Sun, Y.J.; Shen, W. Chestnut polysaccharides restore impaired spermatogenesis by adjusting gut microbiota and the intestinal structure. Food Funct. 2022, 13, 425–436. [Google Scholar] [CrossRef]
- Martínez-López, Y.E.; Esquivel-Hernández, D.A.; Sánchez-Castañeda, J.P.; Neri-Rosario, D.; Guardado-Mendoza, R.; Resendis-Antonio, O. Type 2 diabetes, gut microbiome, and systems biology: A novel perspective for a new era. Gut Microbes 2022, 14, 2111952. [Google Scholar] [CrossRef]
- Yang, J.P.; Summanen, P.H.; Henning, S.M.; Hsu, M.; Lam, H.; Huang, J.J.; Tseng, C.H.; Dowd, S.E.; Finegold, S.M.; Heber, D.; et al. Xylooligosaccharide supplementation alters gut bacteria in both healthy and prediabetic adults: A pilot study. Front. Physiol. 2015, 6, 216. [Google Scholar] [CrossRef]
- Santilli, A.; Stefanopoulos, S.; Cresci, G.A.M. The gut barrier and chronic diseases. Curr. Opin. Clin. Nutr. 2022, 25, 178–185. [Google Scholar] [CrossRef]
- Wang, Y.J.; Chen, Y.; Zhang, X.Y.; Lu, Y.P.; Chen, H.X. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: A review. J. Funct. Foods 2020, 75, 104248. [Google Scholar] [CrossRef]
- Fang, J.Y.; Lin, Y.; Xie, H.L.; Farag, M.A.; Feng, S.M.; Li, J.J.; Shao, P. Dendrobium officinale leaf polysaccharides ameliorated hyperglycemia and promoted gut bacterial associated SCFAs to alleviate type 2 diabetes in adult mice. Food Chem. X 2022, 13, 100207. [Google Scholar] [CrossRef]
- Macho-González, A.; Garcimartín, A.; Redondo, N.; Cofrades, S.; Bastida, S.; Nova, E.; Benedí, J.; Sánchez-Muniz, F.J.; Marcos, A.; López-Oliva, M.E. Carob fruit extract-enriched meat, as preventive and curative treatments, improves gut microbiota and colonic barrier integrity in a late-stage T2DM model. Food Res. Int. 2021, 141, 110124. [Google Scholar] [CrossRef]
- Ma, G.X.; Ma, S.; Du, H.J.; Li, X.Y.; Tao, Q.; Hu, Q.H.; Xiao, H. Interactions between intestinal microbial fermentation products of Pleurotus eryngii polysaccharide with gut mucus. Food Funct. 2024, 15, 1476–1488. [Google Scholar] [CrossRef]
- Jiang, G.Y.; Lei, A.T.; Chen, Y.; Yu, Q.; Xie, J.H.; Yang, Y.; Yuan, T.J.; Su, D. The protective effects of the Ganoderma atrum polysaccharide against acrylamide-induced inflammation and oxidative damage in rats. Food Funct. 2021, 12, 397–407. [Google Scholar] [CrossRef]
- Bai, Y.F.; Yue, Z.L.; Wang, Y.N.; Li, Y.D.; Li, C.; Liu, X.T.; Shi, R.H.; Huo, N.N.; Li, D.D.; Gao, S.; et al. Synergistic effect of polysaccharides and flavonoids on lipid and gut microbiota in hyperlipidemic rats. Food Funct. 2023, 14, 921–933. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.Y.; Shi, P.P.; Li, P.P.; Fu, Y.; Tan, G.F.; Zhou, J.J.; Zeng, J.G.; Huang, P. Modulation of Acute Intestinal Inflammation by Dandelion Polysaccharides: An In-Depth Analysis of Antioxidative, Anti-Inflammatory Effects and Gut Microbiota Regulation. Int. J. Mol. Sci. 2024, 25, 1429. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, C.R.; Li, J.S.; Li, T.T.; Zhang, Y.; Liang, Y.X.; Mei, Y.X. Phellinus linteus polysaccharide extract improves insulin resistance by regulating gut microbiota composition. FASEB J. 2020, 34, 1065–1078. [Google Scholar] [CrossRef]
- van Muijlwijk, G.H.; van Mierlo, G.; Jansen, P.W.T.C.; Vermeulen, M.; Bleumink-Pluym, N.M.C.; Palm, N.W.; van Putten, J.P.M.; de Zoete, M.R. Identification of Allobaculum mucolyticum as a novel human intestinal mucin degrader. Gut Microbes 2021, 13, 1966278. [Google Scholar] [CrossRef]
- Zhou, W.T.; Yang, T.T.; Xu, W.Q.; Huang, Y.J.; Ran, L.W.; Yan, Y.M.; Mi, J.; Lu, L.; Sun, Y.; Zeng, X.X.; et al. The polysaccharides from the fruits of Lycium barbarum L. confer anti-diabetic effect by regulating gut microbiota and intestinal barrier. Carbohydr. Polym. 2022, 291, 119626. [Google Scholar] [CrossRef]
- Cordeiro, L.M.C.; Reinhardt, V.D.; Baggio, C.H.; Werner, M.F.D.; Burci, L.M.; Sassaki, G.L.; Iacomini, M. Arabinan and arabinan-rich pectic polysaccharides from quinoa (Chenopodium quinoa) seeds: Structure and gastroprotective activity. Food Chem. 2012, 130, 937–944. [Google Scholar] [CrossRef]
- Wang, M.X.; Chen, Y.X.; Wang, Y.Y.; Li, Y.; Zheng, H.H.; Ma, F.L.; Ma, C.W.; Zhang, X.J.; Lu, B.Y.; Xie, Z.Y.; et al. The effect of probiotics and polysaccharides on the gut microbiota composition and function of weaned rats. Food Funct. 2018, 9, 1864–1877. [Google Scholar] [CrossRef]
- Guo, C.L.; Zhang, S.H.; Wang, Y.Q.; Li, M.X.; Ding, K. Isolation and structure characterization of a polysaccharide from Crataegus pinnatifida and its bioactivity on gut microbiota. Int. J. Biol. Macromol. 2020, 154, 82–91. [Google Scholar] [CrossRef]
- Song, Q.B.; Cheng, S.W.; Li, D.; Cheng, H.Y.; Lai, Y.S.; Han, Q.B.; Wu, H.Y.; Shaw, P.C.; Zuo, Z. Gut microbiota mediated hypoglycemic effect of Astragalus membranaceus polysaccharides in db/db mice. Front. Pharmacol. 2022, 13, 1043527. [Google Scholar] [CrossRef]
- Granado-Serrano, A.B.; Martín-Garí, M.; Sánchez, V.; Solans, M.R.; Berdún, R.; Ludwig, I.A.; Rubió, L.; Vilaprinyó, E.; Portero-Otín, M.; Serrano, J.C.E. Faecal bacterial and short-chain fatty acids signature in hypercholesterolemia. Sci. Rep. 2019, 9, 1772. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Chen, H.; Zhang, K.F.; Lu, Y.M.; Wu, Q.Z.; Chen, J.L.; Li, Y.; Wu, Q.X.; Chen, Y. Extraction, purification, structural characterization, and gut microbiota relationship of polysaccharides: A review. Int. J. Biol. Macromol. 2022, 213, 967–986. [Google Scholar] [CrossRef]
- Soverini, M.; Turroni, S.; Biagi, E.; Quercia, S.; Brigidi, P.; Candela, M.; Rampelli, S. Variation of Carbohydrate-Active Enzyme Patterns in the Gut Microbiota of Italian Healthy Subjects and Type 2 Diabetes Patients. Front. Microbiol. 2017, 8, 299497. [Google Scholar] [CrossRef]
- Li, Q.; Hu, J.; Nie, Q.; Chang, X.; Fang, Q.; Xie, J.; Li, H.; Nie, S. Hypoglycemic mechanism of polysaccharide from Cyclocarya paliurus leaves in type 2 diabetic rats by gut microbiota and host metabolism alteration. Sci. China Life Sci. 2021, 64, 117–132. [Google Scholar] [CrossRef]
- Deng, J.; Zhong, J.; Long, J.; Zou, X.Y.; Wang, D.; Song, Y.; Zhou, K.; Liang, Y.X.; Huang, R.M.; Wei, X.Q.; et al. Hypoglycemic effects and mechanism of different molecular weights of konjac glucomannans in type 2 diabetic rats. Int. J. Biol. Macromol. 2020, 165, 2231–2243. [Google Scholar] [CrossRef]
- Zhong, L.; Peng, X.J.; Wu, C.T.; Li, Q.; Chen, Y.F.; Wang, M.; Li, Y.T.; He, K.Y.; Shi, Y.; Bie, C.Q.; et al. Polysaccharides and flavonoids from cyclocarya paliurus modulate gut microbiota and attenuate hepatic steatosis, hyperglycemia, and hyperlipidemia in nonalcoholic fatty liver disease rats with type 2 diabetes mellitus. Int. J. Diabetes Dev. Ctries. 2023, 43, 317–327. [Google Scholar] [CrossRef]
- Collado, M.C.; Derrien, M.; Isolauri, E. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl. Environ. Microb. 2007, 73, 7767–7770. [Google Scholar] [CrossRef]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef]
- Deng, Q.H.; Wang, W.J.; Zhang, L.Y.; Chen, L.L.; Zhang, Q.F.; Zhang, Y.; He, S.C.; Li, J.E. Gougunao tea polysaccharides ameliorate high-fat diet-induced hyperlipidemia and modulate gut microbiota. Food Funct. 2023, 14, 703–719. [Google Scholar] [CrossRef]
- Huo, J.Y.; Lei, M.; Li, F.F.; Hou, J.J.; Zhang, Z.J.; Long, H.L.; Zhong, X.C.; Liu, Y.M.; Xie, C.; Wu, W.Y. Structural Characterization of a Polysaccharide from Gastrodia elata and Its Bioactivity on Gut Microbiota. Molecules 2021, 26, 4443. [Google Scholar] [CrossRef]
- Wu, H.Q.; Ma, Z.L.; Zhang, D.X.; Wu, P.; Guo, Y.H.; Yang, F.; Li, D.Y. Sequential Extraction, Characterization, and Analysis of Pumpkin Polysaccharides for Their Hypoglycemic Activities and Effects on Gut Microbiota in Mice. Front. Nutr. 2021, 8, 769181. [Google Scholar] [CrossRef]
- Fu, Z.F.; Han, L.F.; Zhang, P.; Mao, H.P.; Zhang, H.; Wang, Y.F.; Gao, X.M.; Liu, E.W. Cistanche polysaccharides enhance echinacoside absorption in vivo and affect the gut microbiota. Int. J. Biol. Macromol. 2020, 149, 732–740. [Google Scholar] [CrossRef]
- Milton-Laskibar, I.; Cuevas-Sierra, A.; Portillo, M.P.; Martínez, J.A. Effects of Resveratrol Administration in Liver Injury Prevention as Induced by an Obesogenic Diet: Role of Ruminococcaceae. Biomedicines 2022, 10, 1797. [Google Scholar] [CrossRef]
- Li, L.; Guo, W.L.; Zhang, W.; Xu, J.X.; Qian, M.; Bai, W.D.; Zhang, Y.Y.; Rao, P.F.; Ni, L.; Lv, X.C. Grifola frondosa polysaccharides ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet fed rats. Food Funct. 2019, 10, 2560–2572. [Google Scholar] [CrossRef]
- Dziarski, R.; Park, S.Y.; Kashyap, D.R.; Dowd, S.E.; Gupta, D. Pglyrp-Regulated Gut Microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii Enhance and Attenuates Colitis in Mice. PLoS ONE 2016, 11, e0146162. [Google Scholar] [CrossRef]
- Gao, X.X.; Liu, D.; Gao, L.Y.; Ouyang, Y.Z.; Wen, Y.X.; Ai, C.; Chen, Y.Q.; Zhao, C. Health benefits of Grifola frondosa polysaccharide on intestinal microbiota in type 2 diabetic mice. Food Sci. Hum. Well 2022, 11, 68–73. [Google Scholar] [CrossRef]
- Xu, X.F.; Zhang, X.W. Lentinula edodes-Derived Polysaccharide Alters the Spatial Structure of Gut Microbiota in Mice. PLoS ONE 2015, 10, e0115037. [Google Scholar] [CrossRef]
- Liu, Q.; An, X.; Chen, Y.; Deng, Y.X.; Niu, H.L.; Ma, R.S.; Zhao, H.A.; Cao, W.; Wang, X.R.; Wang, M. Effects of Auricularia auricula Polysaccharides on Gut Microbiota and Metabolic Phenotype in Mice. Foods 2022, 11, 2700. [Google Scholar] [CrossRef]
- Su, L.; Xin, C.X.; Yang, J.T.; Dong, L.R.; Mei, H.R.B.; Dai, X.J.; Wang, Q. A polysaccharide from Inonotus obliquus ameliorates intestinal barrier dysfunction in mice with type 2 diabetes mellitus. Int. J. Biol. Macromol. 2022, 214, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Huang, G.X.; Li, X.A.; Leong, W.; Xia, W.R.; Hsiao, W.L.W. Mushroom polysaccharides from Ganoderma lucidum and Poria cocos reveal prebiotic functions. J. Funct. Foods 2018, 41, 191–201. [Google Scholar] [CrossRef]
- Christovich, A.; Luo, X.M. Gut Microbiota, Leaky Gut, and Autoimmune Diseases. Front. Immunol. 2022, 13, 946248. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.L.; Yap, Y.A.; McLeod, K.H.; Mackay, C.R.; Mariño, E. Dietary metabolites and the gut microbiota: An alternative approach to control inflammatory and autoimmune diseases. Clin. Transl. Immunol. 2016, 5, e82. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, M.Y.; Jin, H.Y.; Yang, J.B.; Kang, S.; Liu, Y.; Yang, S.; Ma, S.C.; Ni, J. Effects of Lycium barbarum Polysaccharides on Immunity and the Gut Microbiota in Cyclophosphamide-Induced Immunosuppressed Mice. Front. Microbiol. 2021, 12, 701566. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.G.; Sefik, E.; Geva-Zatorsky, N.; Kua, L.; Naskar, D.; Teng, F.; Pasman, L.; Ortiz-Lopez, A.; Jupp, R.; Wu, H.J.J.; et al. Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal Th17 cells in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E8141–E8150. [Google Scholar] [CrossRef] [PubMed]
- Bunker, J.J.; Drees, C.; Watson, A.R.; Plunkett, C.H.; Nagler, C.R.; Schneewind, O.; Eren, A.M.; Bendelac, A. B cell superantigens in the human intestinal microbiota. Sci. Transl. Med. 2019, 11, eaau9356. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Tang, F.; Huang, X.Y.; Ma, J.J.; Wang, F.M.; Zhang, P. Polysaccharide Isolated from Agaricus blazei Murill Alleviates Intestinal Ischemia/Reperfusion Injury through Regulating Gut Microbiota and Mitigating Inflammation in Mice. J. Agric. Food Chem. 2024, 72, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Ge, Y.M.; Du, H.; Li, Q.; Xu, X.M.; Yi, H.; Wu, X.Y.; Kuang, T.T.; Fan, G.; Zhang, Y. Berberis kansuensis extract alleviates type 2 diabetes in rats by regulating gut microbiota composition. J. Ethnopharmacol. 2021, 273, 113995. [Google Scholar] [CrossRef]
- Xia, W.; Li, X.; Su, L.; Khan, I.; Leong, W.K.; Yin, L.; Bian, X.; Su, J.; Huang, G.; Hsiao, W.L.W. Corrigendum to “Lycium Berry Polysaccharides Strengthen Gut Microenvironment and Modulate Gut Microbiota of the Mice”. Evid. Based Complement. Altern. Med. 2020, 2020, 4840656. [Google Scholar] [CrossRef]
- Zhang, F.H.; Wang, M.; Yang, J.J.; Xu, Q.; Liang, C.; Chen, B.; Zhang, J.M.; Yang, Y.; Wang, H.L.; Shang, Y.F.; et al. Response of gut microbiota in type 2 diabetes to hypoglycemic agents. Endocrine 2019, 66, 485–493. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.J.; Li, Y.M.; Sun, L.Y.; Xiao, Y.; Gao, W.G.; Zhang, Z.S. Mogroside derivatives exert hypoglycemics effects by decreasing blood glucose level in HepG2 cells and alleviates insulin resistance in T2DM rats. J. Funct. Foods 2019, 63, 103566. [Google Scholar] [CrossRef]
- Jiang, Q.H.; Chen, L.; Wang, R.; Chen, Y.; Deng, S.G.; Shen, G.X.; Liu, S.L.; Xiang, X.W. Hypoglycemic mechanism of Tegillarca granosa polysaccharides on type 2 diabetic mice by altering gut microbiota and regulating the PI3K-akt signaling pathway. Food Sci. Hum. Well 2024, 13, 842–855. [Google Scholar] [CrossRef]
- Zhong, R.T.; Chen, L.B.; Liu, Y.Y.; Xie, S.X.; Li, S.M.; Liu, B.; Zhao, C. Anti-diabetic effect of aloin via JNK-IRS1/PI3K pathways and regulation of gut microbiota. Food Sci. Hum. Well 2022, 11, 189–198. [Google Scholar] [CrossRef]
- Li, D.; Feng, G.L.; Li, Y.; Pan, H.; Luo, P.; Liu, B.; Ding, T.; Wang, X.; Xu, H.B.; Zhao, Y.F.; et al. Benefits of Huang Lian mediated by gut microbiota on HFD/STZ-induced type 2 diabetes mellitus in mice. Front. Endocrinol. 2023, 14, 1120221. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.J.; Bai, Z.Y.; Wan, Y.J.; Shi, H.F.; Huang, X.J.; Nie, S.P. Antidiabetic effects of polysaccharide from azuki bean (Vigna angularis) in type 2 diabetic rats via insulin/PI3K/AKT signaling pathway. Food Hydrocoll. 2020, 101, 105456. [Google Scholar] [CrossRef]
- Yang, C.F.; Lai, S.S.; Chen, Y.H.; Liu, D.; Liu, B.; Ai, C.; Wan, X.Z.; Gao, L.Y.; Chen, X.H.; Zhao, C. Anti-diabetic effect of oligosaccharides from seaweed Sargassum confusum via JNK-IRS1/PI3K signalling pathways and regulation of gut microbiota. Food Chem. Toxicol. 2019, 131, 110562. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Liu, P.; Zhang, X.; Bao, T.; Wang, T.; Guo, L.; Li, Y.; Dong, X.; Li, X.; Dong, Y.; et al. Inulin and Lycium barbarum polysaccharides ameliorate diabetes by enhancing gut barrier via modulating gut microbiota and activating gut mucosal TLR2+ intraepithelial γδ T cells in rats. J. Funct. Foods 2021, 79, 104407. [Google Scholar] [CrossRef]
- Zhang, C.H.; Sheng, J.Q.; Sarsaiya, S.; Shu, F.X.; Liu, T.T.; Tu, X.Y.; Ma, G.Q.; Xu, G.L.; Zheng, H.X.; Zhou, L.F. The anti-diabetic activities, gut microbiota composition, the anti-inflammatory effects of Scutellaria-coptis herb couple against insulin resistance-model of diabetes involving the toll-like receptor 4 signaling pathway. J. Ethnopharmacol. 2019, 237, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Quintela, A.; Macarulla, M.T.; Gómez-Zorita, S.; González, M.; Milton-Laskibar, I.; Portillo, M.P. Relationship between changes in microbiota induced by resveratrol and its anti-diabetic effect on type 2 diabetes. Front. Nutr. 2023, 9, 1084702. [Google Scholar] [CrossRef]
- Chassaing, B.; Etienne-Mesmin, L.; Gewirtz, A.T. Microbiota-Liver Axis in Hepatic Disease. Hepatology 2014, 59, 328–339. [Google Scholar] [CrossRef]
- de Clercq, N.C.; Frissen, M.N.; Groen, A.K.; Nieuwdorp, M. Gut Microbiota and the Gut-Brain Axis: New Insights in the Pathophysiology of Metabolic Syndrome. Psychosom. Med. 2017, 79, 874–879. [Google Scholar] [CrossRef]
- Dumas, A.; Bernard, L.; Poquet, Y.; Lugo-Villarino, G.; Neyrolles, O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell Microbiol. 2018, 20, e12966. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Xin, W.; Xiong, J.C.; Yao, M.Y.; Zhang, B.; Zhao, J.H. The Intestinal Microbiota and Metabolites in the Gut-Kidney-Heart Axis of Chronic Kidney Disease. Front. Pharmacol. 2022, 13, 837500. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.J.; Li, Z.Q.; Liu, X.Y.; Ge, X.D.; Zhao, R.F.; Liu, B.; Zhao, L.A.; Zhao, C. Laminaria japonica polysaccharide alleviates type 2 diabetes by regulating the microbiota-gut-liver axis: A multi-omics mechanistic analysis. Int. J. Biol. Macromol. 2024, 258, 128853. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.M.; Wen, J.M.; Xiao, N.; Cai, Y.T.; Xiao, J.; Dai, W.H.; Chen, J.P.; Zeng, K.W.; Liu, F.S.; Du, B.; et al. Anti-diabetic and gut microbiota modulation effects of sacha inchi (Plukenetia volubilis L.) leaf extract in streptozotocin-induced type 1 diabetic mice. J. Sci. Food Agric. 2022, 102, 4304–4312. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.Y.; Huang, X.J.; Wu, G.J.; Ye, H.; Huang, W.Q.; Nie, Q.X.; Chen, H.H.; Yin, J.Y.; Chen, Y.; Nie, S.P. Polysaccharides from red kidney bean alleviating hyperglycemia and hyperlipidemia in type 2 diabetic rats via gut microbiota and lipid metabolic modulation. Food Chem. 2023, 404, 134598. [Google Scholar] [CrossRef]
- Chen, C.; You, L.J.; Huang, Q.; Fu, X.; Zhang, B.; Liu, R.H.; Li, C. Modulation of gut microbiota by mulberry fruit polysaccharide treatment of obese diabetic db/db mice. Food Funct. 2018, 9, 3732–3742. [Google Scholar] [CrossRef]
- Ding, W.; Wang, Y.N.; Zhou, J.F.; Shi, B. Effect of structure features of polysaccharides on properties of dialdehyde polysaccharide tanning agent. Carbohydr. Polym. 2018, 201, 549–556. [Google Scholar] [CrossRef]
- Silva, I.M.V.; Machado, F.; Moreno, M.J.; Nunes, C.; Coimbra, M.A.; Coreta-Gomes, F. Polysaccharide Structures and Their Hypocholesterolemic Potential. Molecules 2021, 26, 4559. [Google Scholar] [CrossRef]
- Ji, X.L.; Guo, J.H.; Cao, T.Z.; Zhang, T.T.; Liu, Y.Q.; Yan, Y.Z. Review on mechanisms and structure-activity relationship of hypoglycemic effects of polysaccharides from natural resources. Food Sci. Hum. Well 2023, 12, 1969–1980. [Google Scholar] [CrossRef]
- Ji, N.; Liu, P.; Zhang, N.; Yang, S.Y.; Zhang, M.S. Comparison on Bioactivities and Characteristics of Polysaccharides from Four Varieties of Gastrodia elata Blume. Front. Chem. 2022, 10, 956724. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.T.; Li, H.L.; Hou, Y.T.; Zhang, P.J.; Tan, M.Q. Plant polysaccharides: Sources, structures, and antidiabetic effects. Curr. Opin. Food Sci. 2023, 51, 101013. [Google Scholar] [CrossRef]
- Dou, Z.M.; Chen, C.; Fu, X. Digestive Property and Bioactivity of Blackberry Polysaccharides with Different Molecular Weights. J. Agric. Food Chem. 2019, 67, 12428–12440. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Lazarte, A.; Kachrimanidou, V.; Villamiel, M.; Rastall, R.A.; Moreno, F.J. In vitro fermentation properties of pectins and enzymatic-modified pectins obtained from different renewable bioresources. Carbohydr. Polym. 2018, 199, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.Z.; Li, S.; Orfila, C.; Shen, X.M.; Zhou, S.Y.; Linhardt, R.J.; Ye, X.Q.; Chen, S.G. Depolymerized RG-I-enriched pectin from citrus segment membranes modulates gut microbiota, increases SCFA production, and promotes the growth of Bifidobacterium spp., Lactobacillus spp. and Faecalibaculum spp. Food Funct. 2019, 10, 7828–7843. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, W.Z.; Gao, Q.Y.; Zou, Y.X. Hypoglycemic Effect of Chinese Yam (Dioscorea opposita rhizoma) Polysaccharide in Different Structure and Molecular Weight. J. Food Sci. 2017, 82, 2487–2494. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.Y.; Yao, L.; Zhang, L.; Zhang, Y.; Luo, Q.; Qiu, S.Y.; Zeng, X.Y.; Chen, S.G.; Ye, X.Q. In Vitro Digestion and Fecal Fermentation of Peach Gum Polysaccharides with Different Molecular Weights and Their Impacts on Gut Microbiota. Foods 2022, 11, 3970. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.Y.; Xu, H.T.; Fang, F.; Liu, J.C.; Wu, K.Z.; Zhang, Y.W.; Wu, J.H.; Gao, J. In vitro effects of two polysaccharide fractions from Laminaria japonica on gut microbiota and metabolome. Food Funct. 2023, 14, 3379–3390. [Google Scholar] [CrossRef]
- Kim, K.T.; Rioux, L.E.; Turgeon, S.L. Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry 2014, 98, 27–33. [Google Scholar] [CrossRef]
- Harris, H.C.; Edwards, C.A.; Morrison, D.J. Impact of Glycosidic Bond Configuration on Short Chain Fatty Acid Production from Model Fermentable Carbohydrates by the Human Gut Microbiota. Nutrients 2017, 9, 26. [Google Scholar] [CrossRef]
- Fang, F.; Xiao, C.Q.; Wan, C.; Li, Y.Q.; Lu, X.Y.; Lin, Y.; Gao, J. Two Laminaria japonica polysaccharides with distinct structure characterization affect gut microbiota and metabolites in hyperlipidemic mice differently. Food Res. Int. 2022, 159, 111615. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, J.; Zhang, T.; Li, S.; Liu, J.; Li, M.; Lu, J.; Zhang, M.; Chen, H. Updated Progress on Polysaccharides with Anti-Diabetic Effects through the Regulation of Gut Microbiota: Sources, Mechanisms, and Structure–Activity Relationships. Pharmaceuticals 2024, 17, 456. https://doi.org/10.3390/ph17040456
Zhang X, Wang J, Zhang T, Li S, Liu J, Li M, Lu J, Zhang M, Chen H. Updated Progress on Polysaccharides with Anti-Diabetic Effects through the Regulation of Gut Microbiota: Sources, Mechanisms, and Structure–Activity Relationships. Pharmaceuticals. 2024; 17(4):456. https://doi.org/10.3390/ph17040456
Chicago/Turabian StyleZhang, Xiaoyu, Jia Wang, Tingting Zhang, Shuqin Li, Junyu Liu, Mingyue Li, Jingyang Lu, Min Zhang, and Haixia Chen. 2024. "Updated Progress on Polysaccharides with Anti-Diabetic Effects through the Regulation of Gut Microbiota: Sources, Mechanisms, and Structure–Activity Relationships" Pharmaceuticals 17, no. 4: 456. https://doi.org/10.3390/ph17040456
APA StyleZhang, X., Wang, J., Zhang, T., Li, S., Liu, J., Li, M., Lu, J., Zhang, M., & Chen, H. (2024). Updated Progress on Polysaccharides with Anti-Diabetic Effects through the Regulation of Gut Microbiota: Sources, Mechanisms, and Structure–Activity Relationships. Pharmaceuticals, 17(4), 456. https://doi.org/10.3390/ph17040456